Abstract
Sparse labeling of individual cells is an important approach in neuroscience and many other fields of research. Various methods have been developed to sparsely label only a small population of cells; however, there is no simple and reproducible strategy for managing the probability of sparse labeling at desired levels. Here, we aimed to develop a novel methodology based on the Cre-lox system to regulate sparseness at desired levels, and we purely analyzed cleavage efficiencies of loxP mutants by Cre. We hypothesized that mutations in the loxP sequence reduce the recognition efficiency by Cre, which enables the regulation of the sparseness level of gene expression. In this research, we mutagenized the loxP sequence and analyzed a library of loxP variants. We evaluated more than 1000 mutant loxP sequences, including mutants with reduced excision efficiencies by Cre ranging from 0.51% to 59%. This result suggests that these mutant loxP sequences can be useful in regulating the sparseness of genetic labeling at desired levels.
Introduction
Sparse labeling is a genetic methodology that is used to label only a small number of cells in an overall population. Sparse labeling has impacted a variety of fields, and is especially important in neuroscience, because a massive diversity of neurons with unique morphologies is present in the nervous system [1, 2] and a tremendous number of neuronal cells exist in the brain; approximately 86 billion in the human brain and 100 million in the mouse brain [3, 4]. In addition, brains are tightly packed with neurons, and their mixed dendritic and axonal processes hamper the visualization of distinct morphologies. The paradigm in which one examines the characteristics of stochastically selected subsets of cells of the same type is particularly useful because it enables single-cell analysis to elucidate the functional logic of neuronal circuits. In this context, there is a great demand for stochastic gene expression for small populations of cells.
Several methodologies have been developed to achieve sparse labeling. One method consists in the use of an animal line with the desired expression patterns after screening transgenic lines with variegated gene expression [5–8]. Other methods have relied on site-specific recombinases (SSRs). In the methods using SSRs, the sparseness level can be regulated in several ways. First, the sparseness level can be regulated by a suitable tamoxifen dosage in a CreER-lox-mediated recombination system [9–15]. Second, viral injections of a low titer into Cre driver lines can be implemented [16, 17]. However, these methodologies have major problems in that it is difficult to determine the sparseness levels a priori with reproducibility, because they require very sophisticated experimental techniques or the time-consuming titration of chemical or gene induction conditions to limit the spatial and/or temporal expression of a recombinase.
Methodologies that can regulate sparseness at a predicted level with high reproducibility would be highly useful. In this situation, several methods have been developed in recent years. First, the mononucleotide repeat frameshift (MORF) method is a Cre-dependent sparse cell labeling approach based on mononucleotide repeat frameshifting as a stochastic translational switch. MORF can regulate sparseness levels with high reproducibility [18, 19]. The labeling rate is approximately ~1%– 5%, depending on the targeted cell type and Cre line used. Second, the mosaic analysis with a repressible cell marker (MARCM) and mosaic analysis with double markers (MADM) transgenic approaches have been established to sparsely label cells based on Cre-lox-mediated interchromosomal recombination, which occurs during mitosis [20–22]. The labeling rate is approximately ~1%– 5%, depending on the targeted cell type and Cre line used. These methods can label effectors with high reproducibility; however, the labeling rate is dependent on cell type and Cre line and cannot tune sparseness to a desired level. In addition, MADM and MARCM can be used only in cells undergoing mitosis. Third, sparse predictive activity through recombinase competition (SPARC) is a method that utilizes PhiC31 recombinase and two competing attP target sequences and an attB target sequence [23]. SPARC used three types of progressively truncated attP sequences to regulate the sparseness level. SPARC-D (Dense) labeled 48%– 51% of cells, SPARC-I (Intermediate) labeled 17%– 22% of cells, and SPARC-S (Sparse) labeled 3%– 7% of cells. Finally, stochastic gene activation with genetically regulated sparseness (STARS) [24, 25] was derived from Brainbow [26]. The Brainbow system is a method to stochastically label cells using two mutually exclusive lox sequences. STARS regulates the sparseness level of the effector by lengthening a spacer DNA sequence between lox2272 sequences, to regulate the excision efficiency by Cre. STARS transgenes that possess various lengths of spacers can regulate the sparseness level from 5% to 50% of the cell population. These methods yielded highly reproducible sparse labeling; however, it remains difficult to regulate the sparseness rate at desired levels. For example, SPARC can only adjust the sparseness level by three levels. STARS require a very long spacer DNA (e.g., > 10 kb) to achieve low stochastic labeling rate, which hampers the construction of transformants.
To overcome the difficulties of low reproducibility and low regulatability reported previously, we sought to develop a novel methodology that allows achieving sparseness at desired levels with high reproducibility by adapting the widely used Cre-lox recombination system [27–31]. In particular, we focused on the Brainbow system mentioned above. We hypothesized that the expression rate of a gene could be regulated by introducing a mutation in one of the lox sequences (lox2272 or loxP) to reduce the recognition rate by Cre [32–43]. We performed random mutagenesis on the loxP sequence, and obtained mutants with reduced excision rates by Cre. The loxP mutants acquired in this study could be used to label genes in a simple and reproducible way and potentially regulate gene expression in cell populations with a desired level of sparseness. This method is likely to be particularly effective because Cre can be applied to a wide range of organisms, including mice, flies, and worms, and can be employed in postmitotic cells [44–48].
Results
Strategy for achieving recombination efficiency at the desired rate
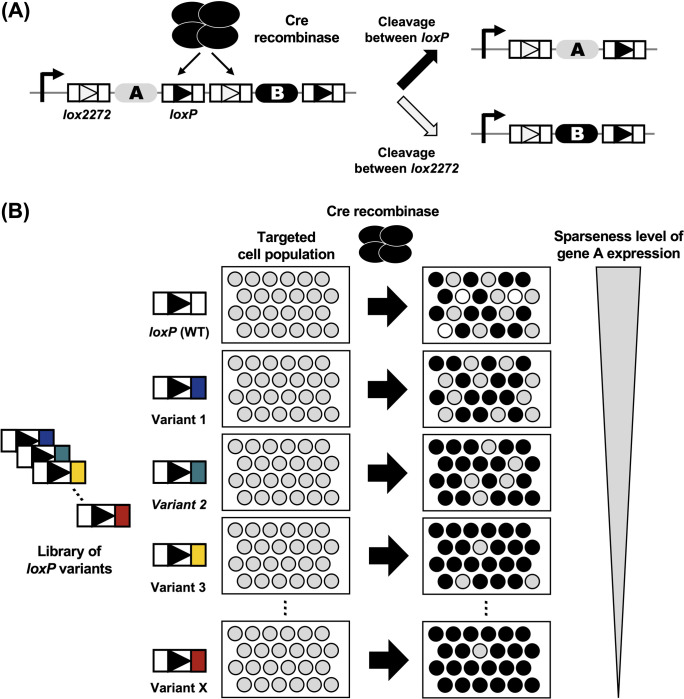
Cre-mediated recombination occurs between one of the two identical pairs of lox sites (a pair of loxP sequences and a pair of lox2272 sequences) in a mutually exclusive way in the Brainbow system [26]. Moreover, excision by one recombination event removes a lox site, which is required for the other recombination event to occur. In the genetic circuit in which two pairs of lox sequences are inserted alternately and gene A is inserted between loxP sequences and gene B is inserted between lox2272 sequences, the decision between the expression of gene A and gene B becomes stochastic and mutually exclusive (Fig 1A). The recognition efficiency of two pairs of lox sequences (loxP sequences and lox2272 sequences) is comparable [26]. Thus, the expression rates of gene A and gene B are expected to be approximately the same. To develop a method that can stochastically activate gene expression with a desired sparseness level, we first considered the reaction kinetics of Cre-lox-mediated intrachromosomal recombination. We hypothesized that the affinity of Cre for the mutagenized loxP sequence could be reduced relative to that for the lox2272 sequence (Fig 1B) and that we could regulate the expression rate of gene A and gene B using the mutagenized loxP sequences.
Fig 1. Schematic overview of our strategy.
(A) Stochastic excision of lox sequences by Cre. Gene A is excised when Cre cleaves lox2272 sequences and gene B is excised when Cre cleaves loxP sequences. Cre cleaves one of two pairs of lox sequences (a pair of lox2272 sequences or a pair of loxP sequences) in a mutually exclusive way, which leads to the exclusive expression of gene A or gene B. (B) Regulation of the sparse labeling rate using loxP variants. We hypothesized that mutations in loxP sequences affect the affinity of the loxP sequence against Cre, thus rendering them less likely to be cleaved by Cre.
Strategies for the construction and evaluation of a library of mutant loxP sequences
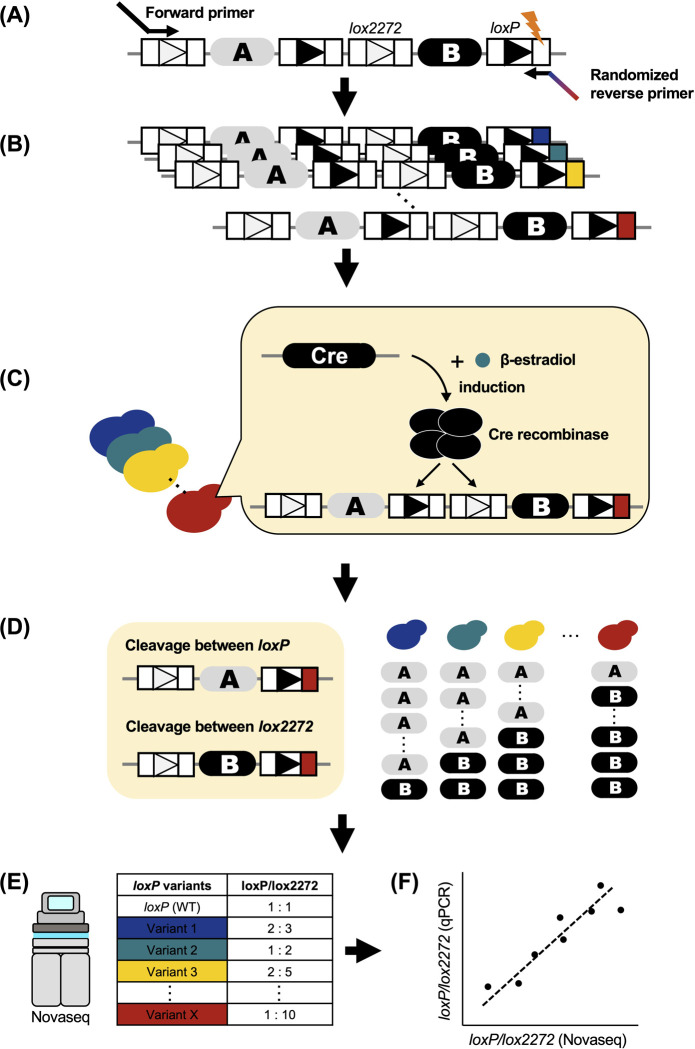
We designed a strategy to analyze the effect of a mutation in the loxP sequences on the excision rate by Cre in a high-throughput manner (Fig 2). First, we mutagenized one of the recombinase binding elements (RBEs) of the loxP sequence by PCR and constructed a library of mutant loxP sequences (Fig 2A and 2B). Next, the library was cloned into a centromere-type plasmid and introduced into Saccharomyces cerevisiae with a CreEBD system, which is a β-estradiol-inducible Cre expression construct [49]. Cre was then induced in yeast with the mutant loxP library (Fig 2C). After Cre induction, the library of mutant loxP sequences was extracted from the yeast (Fig 2D). Subsequently, the library of mutant loxP sequences was subjected to Illumina sequencing and the cleavage rate between loxP sequences was quantified (Fig 2E). Finally, to verify the accuracy of the Illumina sequencing results, we randomly selected loxP variants and quantified their cleavage rates by quantitative polymerase chain reaction (qPCR) (Fig 2F).
Fig 2. Workflow of this research.
Our workflow was divided into six steps. (A,B) Mutagenesis by PCR. We mutagenized one of the RBEs of the loxP sequence and established a library of mutant loxP sequences. (C) Transformation and Cre induction. The library of mutant loxP sequences was transformed into Saccharomyces cerevisiae and Cre was induced. (D) Extraction. After Cre induction, we extracted a library of mutant loxP sequences from S. cerevisiae. (E) Analysis. We analyzed the excision rate of the mutant loxP sequences by Novaseq. (F) Validation. We quantified the excision rate of loxP variants by qPCR, to validate the results of the Illumina sequencing analysis.
Design of a library of mutant loxP sequences
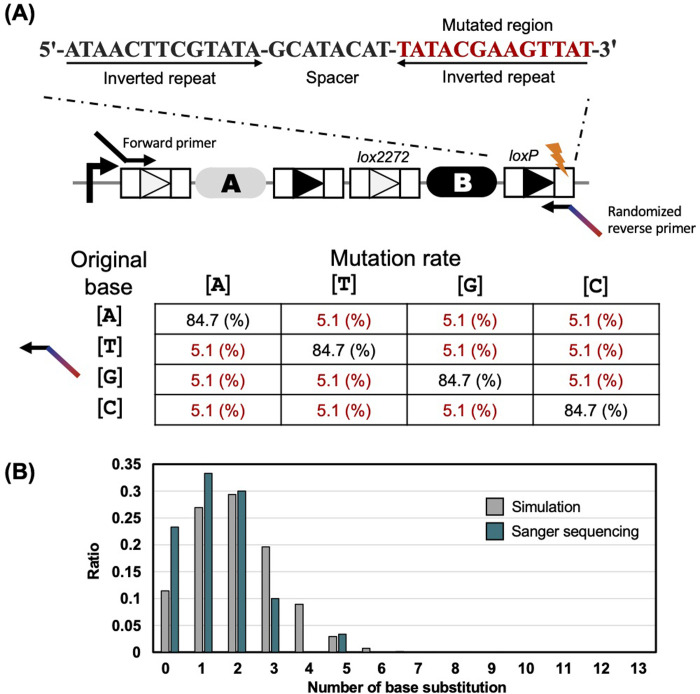
In Cre-lox recombination, Cre forms a complex with lox sequences by recognizing inverted repeats consisting of 13 bp on each side of the lox sequences, named RBEs [31]. In our study, we mutagenized 13 bp (5′- ATAACTTCGTATA-3′) of the right RBE of the loxP sequence. We predicted that an increase in the number of substitutions would result in a reduction of the affinity of loxP variants for Cre. Regarding the substitutions of 3 or more bases, it was difficult to obtain sufficient coverage rate because of the exponentially increasing number of combinations. Thus, the goal of this research was to evaluate most mutants with one or two nucleotide substitutions and a fraction of the mutants with substitutions of three or more nucleotides (Fig 3A). The right RBE of the loxP sequence was designed to preserve the original base at 84.7% probability, to obtain as many 2-base substitutions as possible. For example, if the original base was A, it was designed so that 84.7% remained as A, 5.1% as T, 5.1% as G, 5.1% as C. When the mutated rate in randomized primer is 15.3%, 2-base substitutions are most efficiently obtained. In this case, about 30% of all mutants of loxP sequences would have 2 base substitutions (Fig 3B). The RBE sequences of 30 samples after mutagenesis by PCR were confirmed by Sanger sequencing, and the mutations were successfully introduced at the desired position (S1 Table). As a result of the comparison of Sanger sequencing and simulation, we obtained the library of loxP variants with approximately the expected rate of the number of substitutions (Fig 3B).
Fig 3. Primer design to construct a library of loxP variants.
(A) Primer design to mutagenize the loxP sequence. We introduced a mutation into the right arm (13 bp) of the loxP sequence. The right arm of the loxP sequence is shown in red. We set the mutation rate of the primer to 15.3% to obtain as many 2-base substitutions as possible. When the mutated rate in randomized primer is 15.3%, 2-base substitutions are most efficiently obtained. (B) Validation of the distribution of the number of base substitutions by Sanger sequencing. Gray indicates the theoretical value (mutation rate = 15.3%). Green indicates the results of Sanger sequencing. The detailed results of Sanger sequencing are provided in S1 Table.
Analysis of the library of mutant loxP sequences by Illumina sequencing
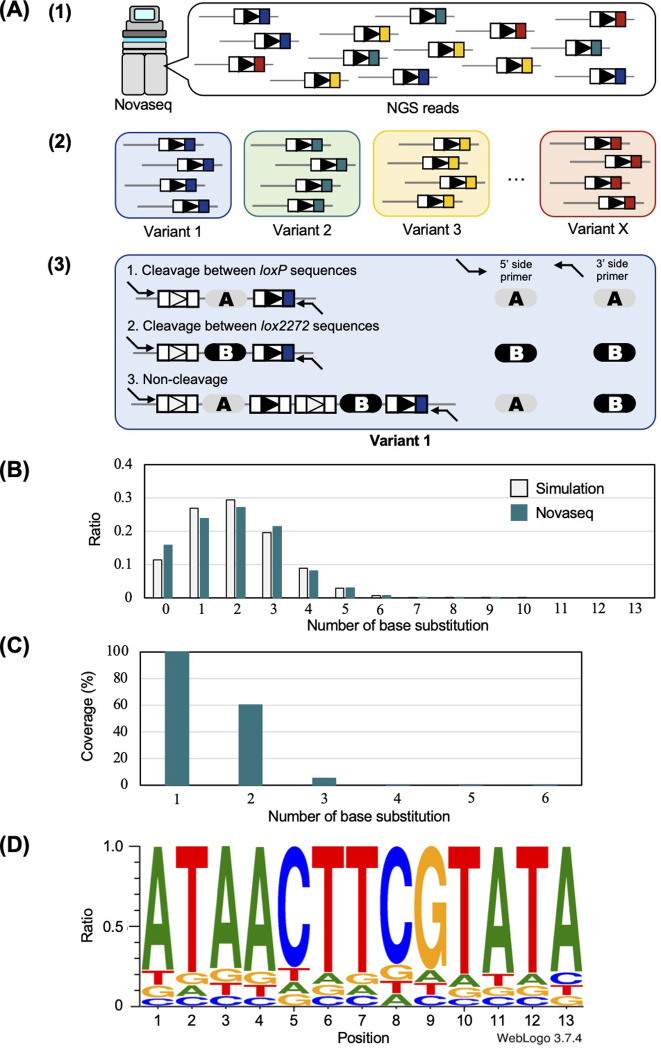
The library of mutagenized loxP sequences was analyzed by Illumina sequencing after a cleavage reaction that was carried out by inducing Cre using the CreEBD system in yeast. The workflow of this analysis is shown in Fig 4A. First, Illumina sequencing reads were classified according to the type of mutation of the loxP sequences (Fig 4A1 and 4A2). In total, we generated 16471 different mutants of loxP sequences. To obtain accurate data, we focused on 1111 loxP variants with a number of Illumina sequencing reads greater than 500 (S1 File). Next, we analyzed the cleavage rate of individual loxP mutant sequences using the combination of the Illumina sequencing reads acquired using a 5′ primer with those acquired using a 3′ primer (Fig 4A3). As a result of the calculation, the average non-cleavage rate was 3.5%, which confirmed that Cre was correctly induced. Before performing a detailed analysis of individual mutant loxP sequences, we confirmed the quality of the library of mutagenized loxP sequences. First, the distribution pattern of the number of base substitutions roughly corresponded to the theoretical one (Fig 4B), as previously suggested in Fig 3B using Sanger sequencing. The coverage rate of base substitution was examined. As a result, we acquired 100% (39/39 variants) of the single-base substitutions and 60.5% (425/702 variants) of the 2-base substitutions (Fig 4C). Third, we assessed the nucleotide bias at every 13 positions of the right RBE of the loxP sequence. The results showed that no bias existed in the type of bases that were introduced into each of the positions of the RBE (Fig 4D).
Fig 4. Analysis of the cleavage rate of loxP variants by Novaseq.
(A) Schematic overview of the Illumina sequencing analysis. We analyzed the Illumina sequencing data in three steps. (1) We extracted DNA sequences that possessed sequences of loxP variants. (2) All paired-end Illumina sequencing reads were classified according to the type of mutation in loxP sequences. (3) Using the paired-end Illumina sequencing reads obtained using the primer at the 5′ side and the primer at the 3′ side, we identified the patterns of recombination of Illumina sequencing reads for each loxP variant. If an Illumina sequencing read possessed sequence A, we determined that the plasmid was cleaved between loxP sequences by Cre. If an Illumina sequencing read possessed sequence B, we determined that the plasmid was cleaved between lox2272 sequences by Cre. If an Illumina sequencing read possessed sequences A and B, we determined that the plasmid was not cleaved. (B) Comparison of the distribution of the number of substituted bases between experimental values and theoretical values. The light-gray color indicates the theoretical values (substitution rate = 15.3%). Green indicates the experimental value. This method of calculation is provided in the Materials and Methods. (C) Coverage rate (%) of substitution. (D) Nucleotide rate at each position of loxP sequences.
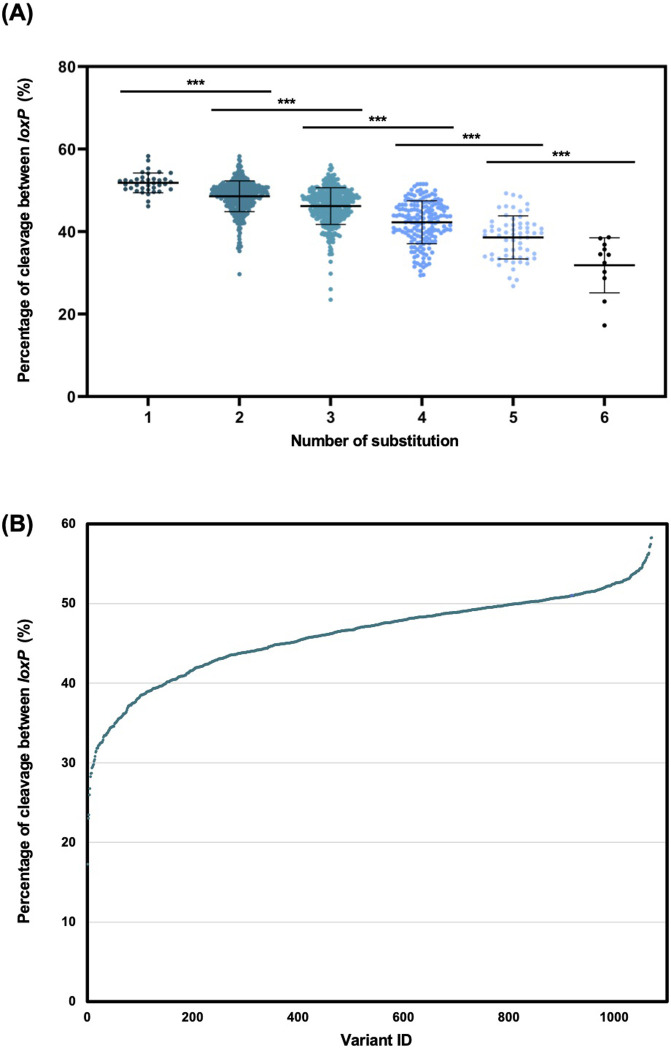
Subsequently, we quantified the cleavage rate of individual loxP sequences. As predicted, as the number of substitutions increased from a 1-base substitution to 6-base substitutions, the average cleavage rate between loxP sequences decreased monotonically (Fig 5A). As shown in Fig 5B, we obtained mutant loxP sequences with reduced recognition efficiencies by Cre in various proportions (Table 1). These results support our hypothesis that sparseness can be regulated by mutagenizing an RBE of lox sequences.
Fig 5. Illumina sequencing data analysis of the loxP variants.
(A) Cleavage rate of all loxP variants is classified according to the number of base substitutions. A significant difference was found between each number of substitutions. *** P < 0.001, two-tailed t-test. (B) Distribution of all loxP variants. The vertical axis indicates the cleavage rate between loxP sequences. loxP variants with reduced recognition rate by Cre were listed in order of the cleavage rate between loxP sequences. There was some overlap between the plots.
Table 1. List of top 50 mutant loxP sequences with the lowest recognition rate by Cre among evaluated loxP variants.
The underlined bases indicate mutated positions.
| Sequence of loxP variants | Number of substitutions | Cleavage rate between loxP sequences (%) | ||
|---|---|---|---|---|
| Left REB | Spacer | Right RBE | ||
| ATAACTTCGTATA | -GCATACAT- | GATGTCAAGATAG | 6 | 17.3 |
| ATAACTTCGTATA | -GCATACAT- | TGGAGCATGTCAT | 6 | 23 |
| ATAACTTCGTATA | -GCATACAT- | CGTACAAAGTTAT | 3 | 23.5 |
| ATAACTTCGTATA | -GCATACAT- | TCAACCAAGTTAT | 3 | 26 |
| ATAACTTCGTATA | -GCATACAT- | TGCAACAAGTCAT | 5 | 26.8 |
| ATAACTTCGTATA | -GCATACAT- | TCAACACATTTAT | 5 | 28.3 |
| ATAACTTCGTATA | -GCATACAT- | GATACTTATTGAC | 6 | 28.7 |
| ATAACTTCGTATA | -GCATACAT- | CATACCTCGTCAT | 5 | 28.7 |
| ATAACTTCGTATA | -GCATACAT- | AAATCGAAGTCAT | 4 | 29.4 |
| ATAACTTCGTATA | -GCATACAT- | AAAGCGGAGTTAT | 4 | 29.5 |
| ATAACTTCGTATA | -GCATACAT- | AAAACGAAGTTAT | 2 | 29.7 |
| ATAACTTCGTATA | -GCATACAT- | AAAACCAAGTTAT | 3 | 29.8 |
| ATAACTTCGTATA | -GCATACAT- | TTCACCCAGTTTC | 6 | 30.2 |
| ATAACTTCGTATA | -GCATACAT- | TCAACCAAGTAAT | 4 | 30.4 |
| ATAACTTCGTATA | -GCATACAT- | CTGACGGAGTGAT | 5 | 30.8 |
| ATAACTTCGTATA | -GCATACAT- | AAAACGATGTAAT | 4 | 31.3 |
| ATAACTTCGTATA | -GCATACAT- | TCTACATAGATAT | 4 | 31.5 |
| ATAACTTCGTATA | -GCATACAT- | TACATGACGCTAT | 4 | 31.8 |
| ATAACTTCGTATA | -GCATACAT- | TACATCACCTTAT | 5 | 31.9 |
| ATAACTTCGTATA | -GCATACAT- | TACATAAAGTCAT | 4 | 31.9 |
| ATAACTTCGTATA | -GCATACAT- | TACACTGAGTTAG | 4 | 32.2 |
| ATAACTTCGTATA | -GCATACAT- | CATCCCAAACTAT | 5 | 32.2 |
| ATAACTTCGTATA | -GCATACAT- | GATAAGACGTTGT | 4 | 32.3 |
| ATAACTTCGTATA | -GCATACAT- | GATTAGATGCTAC | 6 | 32.4 |
| ATAACTTCGTATA | -GCATACAT- | TGTAGGCAGATAG | 5 | 32.5 |
| ATAACTTCGTATA | -GCATACAT- | GATATGCAGTCAT | 4 | 32.5 |
| ATAACTTCGTATA | -GCATACAT- | TACACAAATTCAT | 4 | 32.5 |
| ATAACTTCGTATA | -GCATACAT- | GATAAAAAATTAC | 5 | 32.6 |
| ATAACTTCGTATA | -GCATACAT- | CATATGACGTTAT | 3 | 32.7 |
| ATAACTTCGTATA | -GCATACAT- | GATATCACGTTCT | 5 | 32.9 |
| ATAACTTCGTATA | -GCATACAT- | GGTACGCACTTCT | 5 | 33.2 |
| ATAACTTCGTATA | -GCATACAT- | CTTAGCCAGTTAT | 5 | 33.4 |
| ATAACTTCGTATA | -GCATACAT- | TTCACGGGATTAT | 5 | 33.4 |
| ATAACTTCGTATA | -GCATACAT- | GACACCAAGATAT | 4 | 33.5 |
| ATAACTTCGTATA | -GCATACAT- | TACACGGAACTAG | 5 | 33.5 |
| ATAACTTCGTATA | -GCATACAT- | TGTACTAAGTTCG | 4 | 33.5 |
| ATAACTTCGTATA | -GCATACAT- | TCTACTATTTTAA | 5 | 33.7 |
| ATAACTTCGTATA | -GCATACAT- | TGACCGACGTTAG | 5 | 33.7 |
| ATAACTTCGTATA | -GCATACAT- | CATACCAAATTCT | 4 | 33.8 |
| ATAACTTCGTATA | -GCATACAT- | TACACGTTATTCT | 5 | 34 |
| ATAACTTCGTATA | -GCATACAT- | CTTACCAAGTTTT | 4 | 34.1 |
| ATAACTTCGTATA | -GCATACAT- | TTTGTCACGTTAT | 5 | 34.2 |
| ATAACTTCGTATA | -GCATACAT- | TTCTGGAAGTTAT | 4 | 34.3 |
| ATAACTTCGTATA | -GCATACAT- | CGAGCGAACCTAT | 6 | 34.4 |
| ATAACTTCGTATA | -GCATACAT- | CATTCAAAAGTAT | 5 | 34.5 |
| ATAACTTCGTATA | -GCATACAT- | GATATGAAATTAT | 3 | 34.5 |
| ATAACTTCGTATA | -GCATACAT- | CATATGTTGTCAA | 6 | 34.5 |
| ATAACTTCGTATA | -GCATACAT- | TGTACGGAGTTCT | 3 | 34.5 |
| ATAACTTCGTATA | -GCATACAT- | GATACCAAGTCAT | 3 | 34.6 |
| ATAACTTCGTATA | -GCATACAT- | CATCGGAATTTAA | 5 | 34.6 |
Quantification of the cleavage rate of the mutant loxP sequences by qPCR
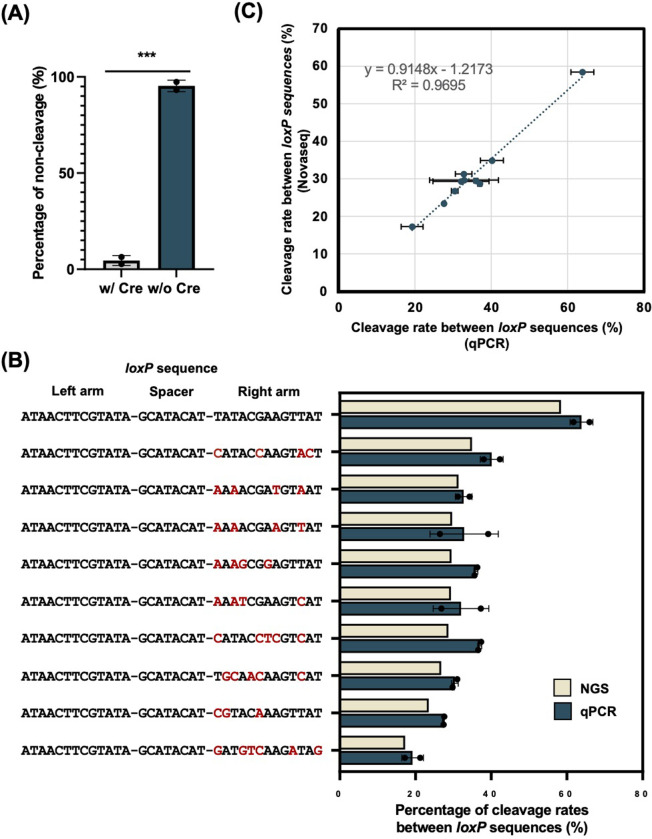
We assessed the cleavage rate of the mutagenized loxP sequences by qPCR to validate the accuracy of the results of the Illumina sequencing analysis. The results indicated that the addition of various mutations to the RBE can alter the cleavage efficiency of Cre at various rates. However, the sequencing results potentially have a certain bias because it requires PCR during sample preparation. To confirm that there is no significant bias in the sequencing results, we performed qPCR, which is low-throughput but can accurately quantify the cleavage rates by Cre. We quantified the non-cleavage rate and loxP cleavage rate in each mutant loxP sequence by qPCR. A comparison of non-cleavage rates in the absence or presence of Cre induction showed that the recombination events that occurred were dependent on Cre induction (Fig 6A). The cleavage rates of the randomly selected loxP variants were quantified using qPCR and compared with the results of the Illumina sequencing analysis (Fig 6B and S2 Table). These results showed that the cleavage rate between loxP sequences was highly correlated with that obtained by Illumina sequencing analysis (R2 = 0.9695) (Fig 6C). This qPCR result showed that the Illumina sequencing data are reliable.
Fig 6. Quantification of the cleavage rate of loxP variants by qPCR.
(A) Comparison of non-cleavage rates in the presence or absence of Cre induction. The error bars represent the standard deviation. A significant difference was found in the presence or absence of Cre induction (two-tailed t-test, N = 3). *** P < 0.001 (B) Comparison of the results of the Illumina sequencing analysis and qPCR. We randomly selected nine loxP variants and the WT loxP sequence for qPCR. We quantified the cleavage rate of the loxP sequence in each variant and WT (see details in the Materials and Methods). The mutated nucleotide is shown in red. The bar graph in light yellow shows the cleavage rate of loxP sequences quantified by Illumina sequencing, whereas green indicates the results of qPCR. Each plot was averaged across two independent experiments. The error bars represent the standard deviation. (C) Correlation of the cleavage rate between loxP sequences, as quantified by Illumina sequencing analysis and qPCR. Each plot was averaged across two independent experiments. The error bars represent the standard deviation.
Identification of mutant loxP sequences with less than 1% recognition efficiency
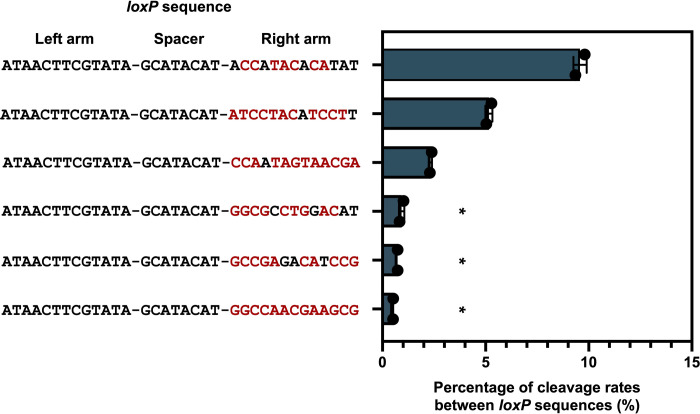
Although we analyzed 1111 variants and successfully identified variants with a recognition efficiency ranging from 17% to 59% (Fig 5B), the sparse labeling method requires mutant loxP sequences with a lower cleavage efficiency as low as 1%. Fig 5A indicates that the recognition rate by Cre drops as the number of base substitutions increase. To obtain mutant loxP sequences with lower recognition rates by Cre, we evaluated the recognition rate of mutant loxP sequences with more than 7-base substitutions. As a result of qPCR quantification, we obtain several mutant loxP sequences with recognition efficiencies of less than 1% (Fig 7 and S2 Table).
Fig 7. The quantification of loxP variants with more than 7-base substitutions by qPCR.
We randomly selected loxP mutants with more than 7-base substitutions. We quantified the cleavage rate of the each mutant loxP sequence using qPCR. The mutated nucleotide is shown in red. Mutants marked with an asterisk showed less than 1% of recognition rate by Cre. The bar graph in green indicates the results of qPCR. Each plot was averaged across two independent experiments. The error bars represent the standard deviation.
Discussion
The problems of sparseness labeling identified in previous studies included the low regulatability of the sparseness level of the effector with low reproducibility. In this study, we developed a novel sparseness labeling method that overcame the weakness of the previous studies. We aimed to obtain mutant loxP sequences by introducing random mutations into the right RBE sequence according to our hypothesis that loxP variants with reduced recognition efficiency by Cre can regulate the sparseness level desirably. We analyzed 1111 variants and successfully identified variants with a recognition efficiency ranging from 17% to 59% (Fig 5B).
This result supports our hypothesis that the efficiency of recognition of the loxP sequence by Cre can be regulated by precisely introducing mutations into the arm of the lox sequence. However, the mutated loxP sequences maintained a higher recognition efficiency by Cre than initially expected. Even in the case of three or more base substitutions, the recognition efficiency of loxP variants remained relatively high. If we adopt sparse labeling in dense tissues, such as the brain, a mutant that can achieve a high sparsity with a labeling rate of 1% or less is needed. As shown in Fig 5A, our experiments confirmed that the efficiency of recognition of loxP by Cre decreased as the number of substitutions introduced into the RBE of the loxP sequence increases. As shown in Fig 7, we evaluated the recognition rate of mutant loxP sequences with more than 7-base substitutions, and we obtained several mutant loxP sequences with recognition efficiencies of less than 1%. We also found that some mutants with fewer base substitutions may have lower cleavage rates than mutants with more base substitutions, as shown in Fig 7. This result indicates that the evaluation of individual mutant loxP sequences is vital to achieving sparse labeling at any desired rates.
As mentioned in the introduction, several previous studies evaluated the effect of mutations on Cre recombinase or loxP sequences [32–43]. Hartung, M. & Kisters-Woike, B. evaluated the effect of the mutation in Cre recombinase [41]. However, it is difficult to precisely regulate the sparseness level by introducing mutations into Cre recombinase. On the other hand, our approach can control the sparseness level only by selecting a mutant loxP sequence. Also, we can use existing Cre lines. Missirlis, P. I., et al. introduced mutations in the spacer region of the loxP sequences [34]. The spacer sequence determines the specificity. Hence, we think that many of the mutants would result in a complete loss of recognition by Cre. Thus, introducing mutations into the spacer region is not an appropriate approach to regulate the sparseness level. Sheren J et al. examined the effect of mutations on the spacer region and RBE of the loxP sequence, respectively. The method of evaluating mutant loxP sequences in our experiment is very different from that of Sheren J et al. In our study, the lox2272 sequence competes with the mutant loxP sequence. In contrast, Sheren J et al. evaluated mutant loxP sequences alone. In our experiment, Cre cannot clave the other lox sequence if Cre cleaves one lox sequence. On the other hand, if the mutant loxP sequence is present alone, the Cre can always cleave the loxP sequence while Cre is acting. Therefore, the cleavage rate of mutant loxP sequences measured by Sheren J et al. under competitive conditions with other lox sequences such as lox2272 is unclear. In addition, we have evaluated cleavage rates for over 1000 mutant loxP sequences in this study, allowing us to adjust sparse labeling rates very strictly ranging from 0.51%–59%. In summary, this research is the first large dataset for measuring the cleavage rate of mutant loxP sequences in competitive conditions with other lox sequences (lox2272 sequence in this study). Thus, our dataset may allow for regulating sparseness levels at the desired rate.
This study provides the proof of concept for establishing a novel method that allows sparse labeling at desired rates. The novel sparse labeling method proposed in this study has the potential to overcome some of the disadvantages of previous methods. The screening of transgenic lines with desired expression patterns [5–8], titration of a suitable tamoxifen dosage [9–15], or amount of viral injection [16, 17] require very sophisticated experimental techniques or the time-consuming titration of chemical or gene induction conditions to limit the spatial and temporal expression of a recombinase. In our method, screening or titrating the chemical and genetic induction conditions is unnecessary to achieve the desired sparseness because a mutant loxP sequence that can achieve a desired labeling rate is selected in advance. The MORF and MADM/MARCM methods have a disadvantage that the sparseness level cannot be controlled [18–22]. The SPARC and STARS methods have the problem of low controllable sparseness levels [23, 24]. On the other hand, our method can regulate the sparseness level in more than 1000 patterns ranging from 0.51% to 59% by appropriate mutant loxP sequence from the mutant loxP library.
The ability to regulate the sparseness level at the desired rate with high reproducibility is a major advantage of this method. One example is the combination of our method with the method that uses a cellomics approach [48]. The cellomics approach method applies the Cre-lox system to stochastically label opsin in a small population of neural networks. If the mutant loxP sequences can be used in the cellomics approach, we could intervene in the activity of a smaller number of neurons. In other examples, our method can be applied to generate a genetic mosaic for analyzing the population dosage of genes involved in sporadic genetic illnesses, as well as to promote cancer development. By applying this method to stepwise change normal cells into cells expressing cancer-inducing genes in a cell population at desired levels, it may be possible to mimic the environment of cell competition when a minority population of cancer cells is present in the majority of the normal cell population [50–55]. Taken together, as a purely genetic method, it has the potential to be adapted to a variety of fields of research.
Finally, we discuss the limitation of our sparse labeling method. The primary purpose of this study is to present data on the cleavage rate of mutant loxP sequencing, which is a proof-of-concept for establishing a novel methodology that allows regulating sparseness levels at the desired rate. We have not yet tested our methodology in neural tissues, and we also have not yet tested the labeling rate in different cell types. As a future experimental plan, it is necessary to test our method in several types of neural tissue and validate the possibility of regulating sparseness levels at the desired rates. Another potential limitation also exists. When combining the mutant loxP sequences with the Brainbow system, we can use existing mouse lines for the Cre expression system. For the Brainbow transgenic lines, we have to establish a new line for each mutant loxP sequence. There is currently no way to reduce this effort, and it will need to be solved in the future.
Materials and methods
Construction of a library of mutant loxP sequences
A library of mutant loxP sequences was constructed via PCR using the pRS416-lox plasmid as a template (S2 File). We mutagenized the loxP sequences using primer_No. 1 and primer_No. 2, as shown in S3 Table. The primers were synthesized commercially via solid-phase synthesis (Eurofin, Tokyo, Japan). The underlined 13-base sequence in primer_No. 2 indicates the mutagenized location of the loxP sequence. The underlined bases were synthesized using biased randomization with an 84.7% chance of retaining the original sequence (e.g., A means A: 84.7%, T: 5.1%, G: 5.1%, C: 5.1%). This is because this study aimed to evaluate loxP mutants with one or two nucleotide substitutions and a certain extent of mutants with three or more nucleotide substitutions. When x = 84.7, the two nucleotide substitutions are most efficiently obtained. We calculated the distribution of the number of base substitutions using the following formula:
In this formula, the retention rate [%] is represented by x and the number of base substitutions is represented by y.
The library was transformed in competent E. coli DH5α (F−, Φ80dlacZΔM15, Δ(lacZYA-argF)U169, deoR, recA1, endA1, hsdR17(rK-, mK+), phoA, supE44, λ−, thi-1, gyrA96, relA1). The transformed E. coli was cultured in a Luria-Bertani (LB) medium (1% [w/v] tryptone, 0.5% [w/v] yeast extract and 1% [w/v] sodium chloride) containing 100 μg/mL ampicillin. Then, plasmids were extracted using the FastGene Plasmid Minikit (NIPPON Genetics, Tokyo, Japan, FG-90502).
Cre induction in yeast
We constructed β-estradiol-inducible Cre-expressing yeast. The S. cerevisiae strain BY4741 (MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0) was used as the host. The pRS403-CreEBD plasmid (S2 File) was inserted genomically into the yeast his3Δ1 site. Using a Frozen EZ Yeast Transformation II Kit (Zymo Research, Irvine, CA, USA, T2001), yeast cells were transformed with the constructed plasmid. The transformants were screened on a synthetic defined (SD) solid medium without L-histidine. The components of the solid SD medium were 0.67% [w/v] yeast nitrogen base without amino acids, 2% [w/v] glucose, and 2% [w/v] agar with appropriate amino acids and a nucleobase (0.012% [w/v] L-leucine, 0.002% [w/v] L-methionine, and 0.002% [w/v] uracil). The mutagenized loxP library was transformed into a yeast strain possessing the CreEBD transgene. The transformants were screened on an SD solid medium without L-histidine and uracil. A total of more than 10,000 colonies were collected to prevent the loss of diversity in the loxP library. For 24 h, the colonies were precultured on liquid SD medium at 30°C and 250 rpm. The precultured yeast was inoculated into 10 mL of liquid SD medium containing D-galactose as a carbon source with an OD600 of 1. Yeast cells were cultured at 30°C and 250 rpm for 24 h.
Preparation of DNA samples for Illumina sequencing analysis
The loxP library after Cre induction was extracted using the ZymoprepTM Yeast Plasmid Miniprep II kit (ZYMO RESEARCH, D2004). The extracted samples were amplified by PCR using primer_No. 3 and primer_No. 4. The cycling parameters were as follows: 94°C for 2 min; followed by five cycles at 98°C for 10 s/55°C for 5 s/68°C for 30 s, 68°C for 7 min; and final hold at 4°C. The number of PCR cycles was set to five to reduce PCR bias as much as possible. The prepared samples were sequenced using Novaseq6000 (Illumina, San Diego, CA, USA) at the paired end. The quality check of samples, the addition of adaptor sequences, the addition of index sequences, and Illumina sequencing runs were performed using the services of MacroGen Japan (Tokyo, Japan).
Illumina sequencing data analysis
The Python script shown in S3 File was used to analyze the Illumina sequencing data. Briefly, using fastq files and the Python script, the sequences of loxP variants were extracted and the number of reads was counted. We set the cut-off for analysis at 500 to obtain reliable loxP cleavage rates. We classified the cleavage patterns of each Illumina sequencing read (non-cleavage, cleavage between loxP, and cleavage between lox2272) using the paired-end sequence information. We calculated the cleavage rate between loxP sequences based on the following formula:
The processed data are presented in S1 File.
Quantitative polymerase chain reaction (qPCR)
To confirm the accuracy of the results of the Illumina sequencing analysis, we conducted a validation experiment by quantitative polymerase chain reaction (qPCR). Nine loxP variants were selected randomly from sequences with a decreased cleavage rate compared with WT loxP. We constructed plasmids that possessed the target mutation via PCR using pRS416_Leu_Ura (S2 File) as a template. The primers used to amplify the DNA fragments are shown in S3 Table (primer_No. 5 to No. 15). Then, we prepared three sets of primers for qPCR: (1) primers to quantify the cleavage rate between lox2272 (primer_No. 16 and primer_No. 17), (2) primers to quantify the cleavage rate between loxP (primer_No. 18 and primer_No. 19), and (3) primers to quantify the non-cleavage rate (primer_No. 20 and primer_No. 21). In addition, we used pRS416_Leu_Ura, pRS416_Leu_Ura_ΔloxP, and pRS416_Leu_Ura_Δlox2272 (S2 File) for generating a calibration curve. A dilution series of 7 points in 10-fold increments from 1.0 × 107 copies/μL to 1 copy/μL was used for the calibration curve. The non-cleavage rate, the cleavage rate between loxP, and the cleavage rate between lox2272 of individual mutant loxP sequences were calculated using these calibration curves. The PCR mixture included: 17 μL of distilled water, 25 μL of FastStart SYBR Green Master (without ROX) (Roche, Basel, Switzerland, 04673484001), 1.5 μL of 10 pmol/μL forward primer, 1.5 μL of 10 pmol/μL reverse primer, and 5 μL of the template plasmid. PCR was carried out on a StepOnePlusTM instrument (Thermo Fisher Scientific, USA) using the following cycling conditions for all primer sets: 95°C for 10 min; followed by 40 cycles of 95°C for 15s, 60°C for 30s; and 1 cycle of 95°C for 15s, 60°C for 1 min, and 95°C for 10 s.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
The full sequences of the plasmids used in this study are shown.
(XLSX)
(TXT)
Data Availability
All relevant data are within the manuscript and its Supporting Information files except row Illumina sequencing data. Row Illumina sequencing data files are available from the NCBI database (NCBI SRA accession: SRR19749293).
Funding Statement
Y.Y. Grant No. 20J22603 KAKENHI, Japan Society for the Promotion of Science, https://www.jsps.go.jp/j-grantsinaid/20_tokushourei/index.html W.A. Grant No. JPMJPR16F1 Precursory research for embryonic science and technology, Japan Science and Technology, Japan https://www.jst.go.jp/kisoken/presto/index.html The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 2016 192. 2016;19: 335–346. doi: 10.1038/nn.4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson LW. Quest for the basic plan of nervous system circuitry. Brain Res Rev. 2007;55: 356–372. doi: 10.1016/j.brainresrev.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci U S A. 2012;109: 10661–10668. doi: 10.1073/pnas.1201895109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci U S A. 2006;103: 12138–12143. doi: 10.1073/pnas.0604911103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132: 2955–2967. doi: 10.1242/dev.01861 [DOI] [PubMed] [Google Scholar]
- 6.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28: 41–51. doi: 10.1016/s0896-6273(00)00084-2 [DOI] [PubMed] [Google Scholar]
- 7.Young P, Qiu L, Wang D, Zhao S, Gross J, Feng G. Single-neuron labeling with inducible Cre-mediated knockout in transgenic mice. Nat Neurosci 2008 116. 2008;11: 721–728. doi: 10.1038/nn.2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choong SC, Rosenberg SS, Fancy SPJ, Zhao C, Shen YAA, Hahn AT, et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109: 1299–1304. doi: 10.1073/pnas.1113540109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244: 305–318. doi: 10.1006/dbio.2002.0597 [DOI] [PubMed] [Google Scholar]
- 10.Badea TC, Wang Y, Nathans J. A Noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23: 2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotolo T, Smallwood PM, Williams J, Nathans J. Genetically-directed, cell type-specific sparse labeling for the analysis of neuronal morphology. PLoS One. 2008;3: e4099. doi: 10.1371/journal.pone.0004099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellisor D, Zervas M. Tamoxifen dose response and conditional cell marking: Is there regurate? Mol Cell Neurosci. 2010;45: 132–138. doi: 10.1016/J.MCN.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Casati ME, Murtie J, Taylor B, Corfas G. Cell-specific inducible gene recombination in postnatal inner ear supporting cells and glia. JARO J Assoc Res Otolaryngol. 2010;11: 19. doi: 10.1007/s10162-009-0191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Williams J, Nathans J. Morphologic diversity of cutaneous sensory afferents revealed by genetically directed sparse labeling. Elife. 2012;2012. doi: 10.7554/eLife.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badea TC, Hua ZL, Smallwood PM, Williams J, Rotolo T, Ye X, et al. New mouse lines for the analysis of neuronal morphology using CreER(T)/loxP-directed sparse labeling. PLoS One. 2009;4: e7859. doi: 10.1371/journal.pone.0007859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin R, Wang R, Yuan J, Feng Q, Zhou Y, Zeng S, et al. Cell-type-specific and projection-specific brain-wide reconstruction of single neurons. Nat Methods 2018 1512. 2018;15: 1033–1036. doi: 10.1038/s41592-018-0184-y [DOI] [PubMed] [Google Scholar]
- 17.Zingg B, Peng B, Huang J, Tao HW, Zhang LI. Synaptic specificity and application of anterograde transsynaptic AAV for probing neural circuitry. J Neurosci. 2020;40: 3250–3267. doi: 10.1523/JNEUROSCI.2158-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu XH, Yang XW. Genetically-directed sparse neuronal labeling in BAC transgenic mice through mononucleotide repeat frameshift. Sci Reports 2017 71. 2017;7: 1–10. doi: 10.1038/srep43915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldman MB, Park CS, Eyermann CM, Zhang JY, Zuniga-Sanchez E, Hirano AA, et al. Brainwide genetic sparse cell labeling to illuminate the morphology of neurons and glia with Cre-dependent MORF mice. Neuron. 2020;108: 111-127.e6. doi: 10.1016/j.neuron.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo L. Fly MARCM and mouse MADM: Genetic methods of labeling and manipulating single neurons. Brain Res Rev. 2007;55: 220–227. doi: 10.1016/J.BRAINRESREV.2007.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121: 479–492. doi: 10.1016/j.cell.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 22.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22: 451–461. doi: 10.1016/s0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- 23.Isaacman-Beck J, Paik KC, Wienecke CFR, Yang HH, Fisher YE, Wang IE, et al. SPARC enables genetic manipulation of precise proportions of cells. Nat Neurosci 2020 239. 2020;23: 1168–1175. doi: 10.1038/s41593-020-0668-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang SZ, Liu BH, Tao HW, Xia K, Zhang LI. A Genetic strategy for stochastic gene activation with regulated sparseness (STARS). PLoS One. 2009;4: 4200. doi: 10.1371/JOURNAL.PONE.0004200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim LA, Huang JJ, Wang SZ, Kim YJ, Zhang LI, Tao HW. Sparse labeling and neural tracing in brain circuits by STARS strategy: revealing morphological development of type II spiral ganglion neurons. Cereb Cortex. 2021;31: 2759–2772. doi: 10.1093/CERCOR/BHY154 [DOI] [PubMed] [Google Scholar]
- 26.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nat 2007 4507166. 2007;450: 56–62. doi: 10.1038/nature06293 [DOI] [PubMed] [Google Scholar]
- 27.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination: I. Recombination between loxP sites. J Mol Biol. 1981;150: 467–486. doi: 10.1016/0022-2836(81)90375-2 [DOI] [PubMed] [Google Scholar]
- 28.Hoess RH, Ziese M, Sternberg N. P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc Natl Acad Sci U S A. 1982;79: 3398. doi: 10.1073/PNAS.79.11.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Duyne GD. A structural view of Cre-loxP site-specific recombination. 2003;30: 87–104. doi: 10.1146/ANNUREV.BIOPHYS.30.1.87 [DOI] [PubMed] [Google Scholar]
- 30.Van Duyne GD. Cre Recombinase. Microbiol Spectr. 2015;3. doi: 10.1128/microbiolspec.MDNA3-0014-2014 [DOI] [PubMed] [Google Scholar]
- 31.Meinke G, Bohm A, Hauber J, Pisabarro MT, Buchholz F. Cre recombinase and other tyrosine recombinases. Chem Rev. 2016;116: 12785–12820. doi: 10.1021/acs.chemrev.6b00077 [DOI] [PubMed] [Google Scholar]
- 32.Lee G, Saito I. Role of nucleotide sequences of loxP spacer region in Cre-mediated recombination. Gene. 1998;216: 55–65. doi: 10.1016/S0378-1119(98)00325-4 [DOI] [PubMed] [Google Scholar]
- 33.Thomson JG, Rucker EB, Piedrahita JA. Mutational analysis of loxP sites for efficient Cre-mediated insertion into genomic DNA. genesis. 2003;36: 162–167. doi: 10.1002/GENE.10211 [DOI] [PubMed] [Google Scholar]
- 34.Missirlis PI, Smailus DE, Holt RA. A high-throughput screen identifying sequence and promiscuity characteristics of the loxP spacer region in Cre-mediated recombination. BMC Genomics. 2006;7. doi: 10.1186/1471-2164-7-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheren J, Langer SJ, Leinwand LA. A randomized library approach to identifying functional lox site domains for the Cre recombinase. Nucleic Acids Res. 2007;35: 5464–5473. doi: 10.1093/NAR/GKM604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel RW, Jain R, Bradbury A. Using an in vivo phagemid system to identify non-compatible loxP sequences. FEBS Lett. 2001;505: 467–473. doi: 10.1016/S0014-5793(01)02806-X [DOI] [PubMed] [Google Scholar]
- 37.Hoess RH, Wierzbicki A, Abremski K. The role of the loxP spacer region in P1 site-specific recombination. Nucleic Acids Res. 1986;14: 2287. doi: 10.1093/NAR/14.5.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauer B. Multiplex Cre/lox recombination permits selective site-specific DNA targeting to both a natural and an engineered site in the yeast genome. Nucleic Acids Res. 1996;24: 4608–4613. doi: 10.1093/NAR/24.23.4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albert H, Dale EC, Lee E, Ow DW. Site-specific integraten of DNA into wild-type and mutant lox sites placed in the plant genome. Plant J. 1995;7: 649–659. doi: 10.1046/J.1365-313X.1995.7040649.X [DOI] [PubMed] [Google Scholar]
- 40.Langer SJ, Ghafoori AP, Byrd M, Leinwand L. A genetic screen identifies novel non‐compatible loxP sites. Nucleic Acids Res. 2002;30: 3067–3077. doi: 10.1093/NAR/GKF421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartung M, Kisters-Woike B. Cre mutants with altered DNA binding properties. J Biol Chem. 1998;273: 22884–22891. doi: 10.1074/jbc.273.36.22884 [DOI] [PubMed] [Google Scholar]
- 42.Rüfer AW, Sauer B. Non-contact positions impose site selectivity on Cre recombinase. Nucleic Acids Res. 2002;30: 2764. doi: 10.1093/nar/gkf399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eroshenko N, Church GM. Mutants of Cre recombinase with improved accuracy. Nat Commun 2013 41. 2013;4: 1–10. doi: 10.1038/ncomms3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hadjieconomou D, Rotkopf S, Alexandre C, Bell DM, Dickson BJ, Salecker I. Flybow: genetic multicolor cell labeling for neural circuit analysis in Drosophila melanogaster. Nat Methods 2011 83. 2011;8: 260–266. doi: 10.1038/nmeth.1567 [DOI] [PubMed] [Google Scholar]
- 45.Hampel S, Chung P, McKellar CE, Hall D, Looger LL, Simpson JH. Drosophila Brainbow: a recombinase-based fluorescence labeling technique to subdivide neural expression patterns. Nat Methods 2011 83. 2011;8: 253–259. doi: 10.1038/nmeth.1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sethi S, Wang JW. A versatile genetic tool for post-translational regurate of gene expression in Drosophila melanogaster. Elife. 2017;6. doi: 10.7554/ELIFE.30327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kage-Nakadai E, Imae R, Suehiro Y, Yoshina S, Hori S, Mitani S. A conditional knockout toolkit for Caenorhabditis elegans based on the Cre/loxP recombination. PLoS One. 2014;9: e114680. doi: 10.1371/JOURNAL.PONE.0114680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aoki W, Matsukura H, Yamauchi Y, Yokoyama H, Hasegawa K, Shinya R, et al. Cellomics approach for high-throughput functional annotation of Caenorhabditis elegans neural network. Sci Reports 2018 81. 2018;8: 1–9. doi: 10.1038/s41598-018-28653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia B, Wu Y, Li BZ, Mitchell LA, Liu H, Pan S, et al. Precise regurate of SCRaMbLE in synthetic haploid and diploid yeast. Nat Commun 2018 91. 2018;9: 1–13. doi: 10.1038/s41467-018-03084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muzumdar MD, Luo L, Zong H. Modeling sporadic loss of heterozygosity in mice by using mosaic analysis with double markers (MADM). Proc Natl Acad Sci U S A. 2007;104: 4495–4500. doi: 10.1073/PNAS.0606491104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muzumdar MD, Dorans KJ, Chung KM, Robbins R, Tammela T, Gocheva V, et al. Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers. Nat Commun 2016 71. 2016;7: 1–13. doi: 10.1038/ncomms12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C, Sage JC, Miller MR, Verhaak RGW, Hippenmeyer S, Vogel H, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146: 209–221. doi: 10.1016/j.cell.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez PP, Kim J, Galvao RP, Cruickshanks N, Abounader R, Zong H. p53 and NF 1 loss plays distinct but complementary roles in glioma initiation and progression. Glia. 2018;66: 999–1015. doi: 10.1002/glia.23297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian A, Kang B, Li B, Qiu B, Jiang W, Shao F, et al. Oncogenic state and cell identity combinatorially dictate the susceptibility of cells within glioma development hierarchy to IGF1R targeting. Adv Sci. 2020;7: 2001724. doi: 10.1002/advs.202001724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao M, Ventura PB, Jiang Y, Rodriguez FJ, Wang L, Perry JSA, et al. Astrocytic trans-differentiation completes a multicellular paracrine feedback loop required for medulloblastoma tumor growth. Cell. 2020;180: 502-520.e19. doi: 10.1016/j.cell.2019.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]