Abstract
Glutathione peroxidase 1 (GPx1) is an important cellular antioxidant enzyme that is found in the cytoplasm and mitochondria of mammalian cells. Like most selenoenzymes, it has a single redox-sensitive selenocysteine amino acid that is important for the enzymatic reduction of hydrogen peroxide and soluble lipid hydroperoxides. Glutathione provides the source of reducing equivalents for its function. As an antioxidant enzyme, GPx1 modulates the balance between necessary and harmful levels of reactive oxygen species. In this review, we discuss how selenium availability and modifiers of selenocysteine incorporation alter GPx1 expression to promote disease states. We review the role of GPx1 in cardiovascular and metabolic health, provide examples of how GPx1 modulates stroke and provides neuroprotection, and consider how GPx1 may contribute to cancer risk. Overall, GPx1 is protective against the development and progression of many chronic diseases; however, there are some situations in which increased expression of GPx1 may promote cellular dysfunction and disease owing to its removal of essential reactive oxygen species.
Keywords: glutathione peroxidase 1, oxidative stress, selenium, selenocysteine, endothelial dysfunction, cardiac protection, angiogenesis, inflammation, atherosclerosis, stroke, neurodegeneration, cancer
Graphical Abstract
Glutathione peroxidase 1 (GPx1) is a member of a family of antioxidant enzymes that reduce hydrogen peroxide and soluble lipid hydroperoxides using glutathione (GSH) as a source of reducing equivalents1. Although not all GPxs are selenoproteins, GPx1 is a selenoprotein [1], containing the selenocysteine amino acid at its active site. Its identification as the first selenoenzyme, and the current understanding of its enzymology are discussed in a separate article in this issue (Flohé et al., special issue). This review highlights how GPx1, via its role as a selenocysteine-containing peroxidase, contributes to health and disease.
GPx1 is ubiquitously expressed in all tissues and can be found in the cytosol and mitochondria. It is one of many cellular antioxidant enzymes, including other GPxs, peroxiredoxins, and catalases, that reduce hydrogen peroxide [2]. At a cellular level, owing to its oxidation of cellular glutathione (GSH) and its reduction of cellular hydrogen peroxide, GPx1 influences the thiol redox state and maintains the balance between necessary and harmful levels of cellular oxidants. An accumulation of excess intracellular oxidants can promote redox-mediated signaling events, including antioxidant responses, such as the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) [3], as well as promote the activation of proinflammatory pathways that are mediated by redox-sensitive transcription factors, such as nuclear factor kappa B (NFκB) and activator protein 1 (AP-1) [4–6]. Furthermore, excess oxidants can cause cellular dysfunction owing, in part, to oxidative damage to proteins, lipids, and DNA to promote cardiovascular diseases, neurodegeneration, cancer, and other disease states [7, 8].
The term oxidative stress describes a condition in which the production of oxidants exceeds the antioxidant capacity of the cell. As illustrated by the examples discussed throughout this review, the consequences of oxidative stress can manifest in many forms, depending, in part, on the model system under study. Importantly, an accumulation of reactive oxygen species (ROS) can lead to the additional production of ROS by many different mechanisms. Thus, activation of NFκB by ROS (or other signaling pathways) upregulates the expression and activity of ROS generators, such as NADPH oxidase 1 and 2 (NOX1 and NOX2) that produce superoxide [9, 10]. ROS can also promote the subsequent production of superoxide or hydrogen peroxide from other enzymatic sources that are not normally ROS producers: hydrogen peroxide promotes the uncoupling of endothelial nitric oxide synthase (eNOS) to foster superoxide production rather than nitric oxide production [11, 12] and hydrogen peroxide can convert xanthine oxidoreductase to xanthine oxidase, a superoxide and hydrogen peroxide generator, either by reversible cysteine oxidation or by promoting irreversible proteolysis, [13]. Superoxide is spontaneously or enzymatically converted to hydrogen peroxide, which can be further reduced by Fe(II) to generate hydroxyl radicals (via the Fenton reaction). Non-enzymatic, hydroxyl radical-induced oxidation of free fatty acids results in the formation of isoprostanes, which are considered stable markers of oxidative stress. Similarly, the oxidation of lipids can lead to the formation of lipid aldehydes, such as 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA), that modify proteins to form other stable markers of oxidative stress. Oxidative modifications to proteins, lipids, and/or DNA can alter the function of these molecules and may result in the activation of ER stress, mitochondrial dysfunction, autophagy, and apoptosis, processes that themselves also generate ROS [14]. Thus, one consequence of oxidative damage to mitochondrial membranes and proteins is the uncoupling of electron transport and ATP production resulting in an increase in mitochondrial release of ROS (mostly as hydrogen peroxide, although some superoxide may also be released) [15]. Thus, by modulating hydrogen peroxide accumulation, GPx1 may also regulate the production of additional forms of ROS.
Unlike superoxide and hydroxyl radical, which have very short half-lives, hydrogen peroxide has a longer half-life and functions as a signaling molecule. Low levels ROS, including hydrogen peroxide, are needed for essential cellular processes, such as growth factor-mediated signaling, formation of necessary protein-disulfide bonds, and regulation of normal mitochondrial function [16, 17]. Thus, excess GPx1 may alter cellular function by limiting essential levels of hydrogen peroxide to create reductive stress, a condition with an altered redox balance that favors an excess of reducing equivalents that alters cellular redox functions. Chronic upregulation of GPx1 may also allow tumor cells to evade cell death in response to ROS-generating chemotherapeutic agents. Nonetheless, evidence suggests that, overall, GPx1 plays a protective role in mitigating disease development and progression.
Herein, we consider the role of GPx1 in maintaining normal physiological functions and protecting against the development and progression of disease (Figure 1). In particular, we discuss the role of GPx1 in cardiovascular disease and stroke, and provide examples for the role of GPx1 in neurodegenerative disease and in cancers. We review mechanistic studies in animals and in vitro systems that provide insights into the role of GPx1 in these biological systems and provide examples of how selenium deficiency and modifiers of selenocysteine incorporation alter GPx1 expression to promote disease states. Given the importance of ROS, including hydrogen peroxide, in many cellular events, we also discuss the consequences of excess or sustained GPx1 activity in biological systems. Thus, in some cases overexpression of GPx1 can result in a loss of necessary ROS to promote protein misfolding, alter vascular responses to hydrogen peroxide, attenuate growth factor-mediated signaling and insulin-mediated metabolism, and, as mentioned above, promote the survival of some cancer cells. In addition, we consider the role of functional polymorphisms in the human GPX1 gene on GPx1 expression and activity, and provide an update on the potential relationship between these polymorphisms and disease risk.
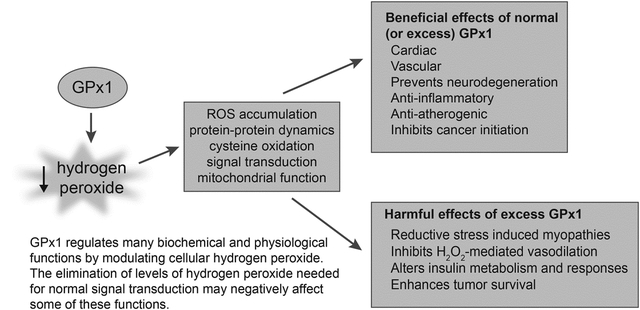
Figure 1.
Beneficial effects of GPx1. By attenuating the accumulation of cellular reactive oxygen species, GPx1 protects against dysfunction and disease in many biological systems. Arrows indicate that GPx1 expression is associated with these changes and that GPx1 deficiency attenuates these protective effects. Overexpression of GPx1 or use of ebselen, a GPx1 mimetic, has been shown to augment many of these protective effects of GPx1. Regarding metabolism, both the loss and the overexpression of GPx1 may promote metabolic dysfunction. Other examples where increased expression of GPx1 may alter normal function are discussed further in the text. I/R, ischemia/reperfusion; NO, nitric oxide; EPC, endothelial progenitor cells.
Selenium, GPx1, and Cardioprotection
GPx1 expression is sensitive to the concentration of the micronutrient selenium, decreasing with low levels of selenium. Not all selenoproteins are affected by selenium concentration to the same extent as GPx1; rather, there is a hierarchy of selenoprotein expression under low selenium conditions, with the expression of some selenoproteins preferentially preserved when selenium is limited [18, 19]. Recent studies in human cell lines suggest that the effects of selenium availability on selenoprotein expression vary in different cell types [20]. Interestingly, in human subjects, as well as in animal models, there appears to be a limited range for the beneficial effects of selenium: both too much and too little may have detrimental effects on health (reviewed in [21, 22]).
Keshan disease is an endemic cardiomyopathy that was originally found in regions of China with low selenium levels in the soil and food supply [23]. This cardiomyopathy is characterized by dysfunction of cardiac mitochondria, a decrease in plasma selenium, decreased cardiac GPx1 expression, increased oxidative stress in the myocardium as reflected by an increase in 8-hydroxy-2-deoxy-guanosine content of nuclei, and decreased whole blood GPx1 activity [24, 25]. Cardiomyocyte expression of thioredoxin reductase 1 was also shown to be decreased in cardiac tissue from Keshan disease patients. Owing to seasonal variations in the occurrence of this disease, it was suggested that a viral cofactor, such as myocarditic strains of the Coxsackie virus, may contribute to disease onset. Studies in mouse models provided evidence in support of this concept. In mice, Se supplementation was found to increase whole blood GPx1 activity and decrease mortality following infection with a myocarditic Coxsackie virus B3 [26]. Similarly, benign strains of Coxsackie virus (B3/0) induced myocarditis in selenium-deficient mice but not in mice on normal selenium diets [27]. Further studies linked the susceptibility to viral-induced myocarditis with a deficiency of GPx1, as GPx1 knockout (GPx1−/−) mice on a normal selenium diet were susceptible to viral-mediated myocarditis whereas wildtype (GPx1+/+) mice were protected from viral-induced cardiac damage [28]. Interestingly, GPx1 has been shown to decrease the severity of other viral infections, including those caused by influenza A virus [29].
In endemic regions with low soil selenium content, deficiency of selenium can be remedied by dietary supplementation to decrease the incidence of Keshan disease [30]. Furthermore, in patients with chronic Keshan disease, supplementation is associated with increased patient survival [31]. Presumably, selenium decreases disease severity, in part, via augmenting the expression and activity of selenoproteins, such as GPx1. Although the effect of selenium on the expression of GPx1 was not analyzed following treatment in the studies cited above, a study of children in this endemic area reported that selenium supplementation resulted in significant increases of GPx activity in plasma, red blood cells, and platelets [32]. The intracellular activity measured would mostly reflect GPx1 activity; whereas the plasma GPx activity is mostly due to GPx3, based on the relative expression levels of these GPx enzymes intracellularly and extracellularly and their relative sensitivity to selenium-mediated changes in expression compared with GPx4. The activity of other selenoproteins, such as the thioredoxin reductases, were not examined in this report. Selenium is an important micronutrient that maintains cardiovascular health; however, given the large range in global selenium distributions, only certain populations with low selenium intake may benefit from selenium supplementation [30, 33, 34], perhaps related to genetic context.
Additional studies have examined the role of genetics in Keshan disease. These findings suggest a nutrient-gene interaction between selenium and a functional polymorphism in the human GPX1 gene that may contribute to disease [35, 36]. The single nucleotide polymorphism, Pro198Leu (rs1050450), alters the normal Pro codon (CCC) to a Leu codon (CTC) in the variant GPX1 allele. In cultured cells, the variant Leu allele is less responsive to selenium-mediated increases in transcript levels and enzyme activity than the Pro allele [37]. Similarly, in a study of human subjects in New Zealand, selenium supplementation was found to increase GPx1 activity in patients with the Pro/Pro genotype compared to those with a Leu allele but only if they had a lower baseline plasma selenium concentration [38]. Consistent with a link between low selenium and the effects of the GPx1 polymorphisms, in regions endemic for Keshan disease, there was a significant difference in the distribution of alleles in cases and controls, with a greater risk for individuals with the Pro/Leu or Leu/Leu genotypes of developing Keshan disease [36].
Many other studies provide evidence that a deficiency of GPx1 can exacerbate cardiac dysfunction. Hearts from heterozygous (GPx1+/−) knockout mice were more susceptible to diastolic dysfunction following ischemia/reperfusion (I/R) injury than wildtype hearts [39] and GPx1−/− hearts (from male mice) showed both contractile and diastolic dysfunction following I/R, with an increase in oxidative stress as measured by an increase in protein carbonylation [40]. Similarly, mitochondria from GPx1−/− hearts showed increased production of DCF-detectable reactive oxygen species at baseline, whereas following hypoxia/reoxygenation, both DCF fluorescence and superoxide production (as measured by Mitotracker Red) was augmented. In addition, following ischemia/reperfusion, mitochondria from GPx1−/− hearts had increased levels of 8-hydroxy-2-deoxy guanosine, a DNA marker of oxidative stress. Mitochondria also showed dysfunction following ischemia reperfusion as characterized by decreased oxygen consumption rates, decreased ATP production, and increased mitochondrial membrane depolarization (ΔΨm) [41]. GPx1 deficiency also enhanced angiotensin II-mediated cardiac hypertrophy, increasing total cardiac mass, left ventricular mass, and left ventricular cardiomyocyte cross-sectional area compared to the changes found in wild type mice. Angiotensin II is a known vasoconstrictor that promotes cardiac hydrogen peroxide production; after 7 days of exposure, it also caused a significant reduction in cardiac shortening fraction only in the GPx1-deficient mice, an indication that GPx1 deficiency sensitized mice to angiotensin II-mediated left ventricular dysfunction [42]. Taken together, these data highlight the role of GPx1 in preventing cardiac injury in response to physiological stressors. GPx1 also has a beneficial role in decreasing the severity of cardiotoxicity caused by doxorubicin, a chemotherapeutic agent used to treat many cancers. In particular, exposure to doxorubicin augmented mitochondrial dysfunction in GPx1-deficient cardiomyocytes compared to normal cardiomyocytes, uncoupling electron transfer and oxidative phosphorylation. Furthermore, GPx1-deficient hearts had decreased cardiac contractility and worse diastolic dysfunction in response to doxorubicin compared to wild type hearts [43]. Similarly, hearts from transgenic mice overexpressing GPx1 had preserved mitochondrial and cardiac function in the presence of doxorubicin, with increased efficiency of mitochondrial respiration and better contractility and diastolic function compared wild type hearts exposed to doxorubicin [44]. Thus, in the context of a cardiotoxin known to produce superoxide, excess GPx1 mitigated ROS-mediated cardiac damage. Mitochondrial function is essential for regulating cardiac health, and by preserving mitochondrial function, GPx1 preserves cardiac contractility (Figure 2).
Figure 2.
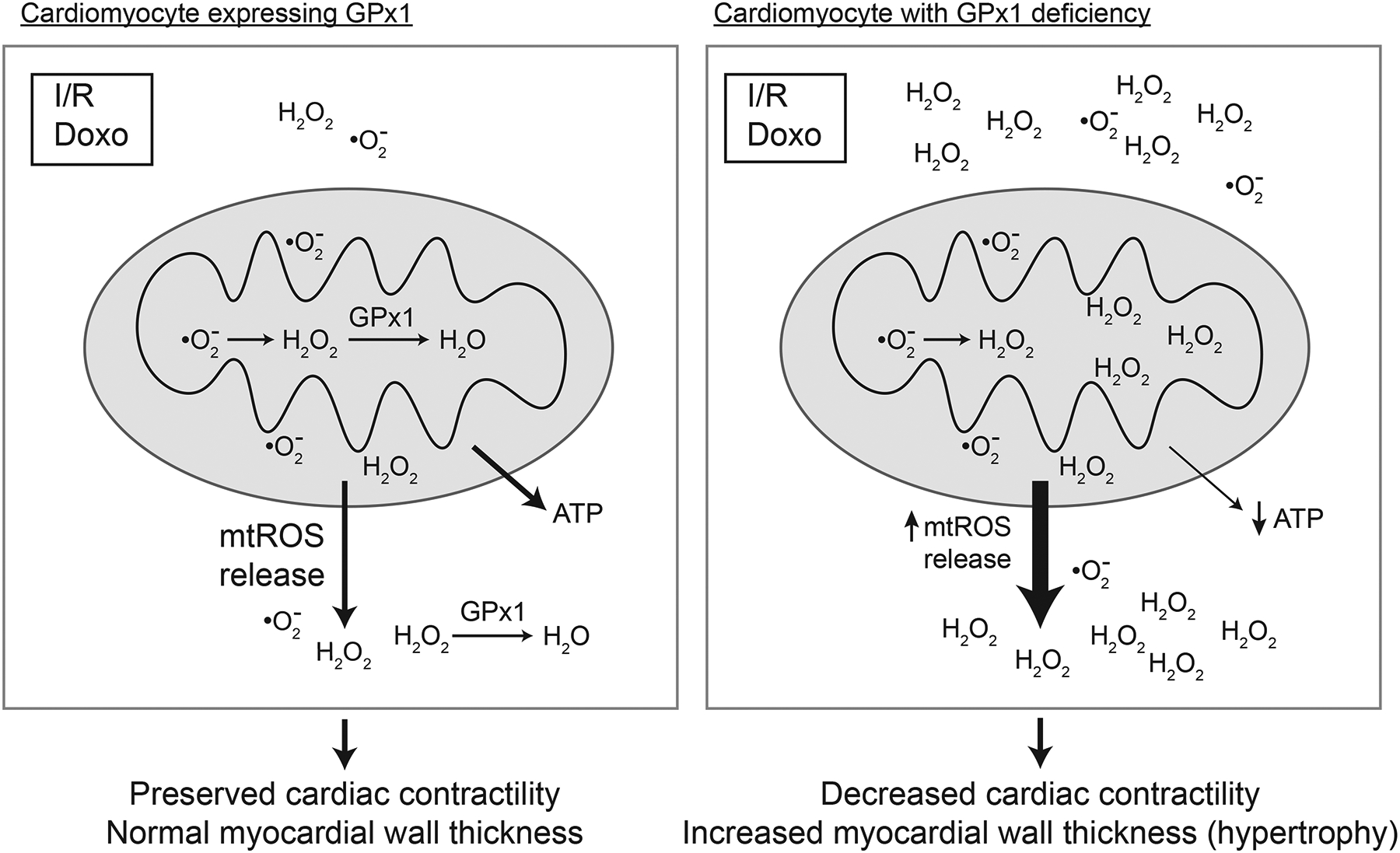
Dysfunction of cardiac mitochondria is augmented by GPx1 deficiency. Stressors such as ischemia reperfusion (I/R) and exposure to cardiotoxins, such as doxorubicin (Doxo), are associated with increased production of superoxide and/or hydrogen peroxide. In the absence of GPx1, ROS accumulates and promotes mitochondrial dysfunction. Oxidant mediated damage to mitochondrial lipids, proteins, and DNA enhances the accumulation of oxidants, in part, by uncoupling mitochondrial oxygen consumption and ATP production to produce more superoxide and increase mitochondrial release of ROS (mostly as hydrogen peroxide but superoxide may also be released). Mitochondrial dysfunction correlates with cardiac dysfunction including decreased cardiac contractility and hypertrophy, changes that are augmented with GPx1 deficiency. Note that in the context of doxorubicin exposure, transgenic mice overexpressing GPX1 showed less cardiac and mitochondrial dysfunction than wildtype hearts expressing normal levels of GPx1.
In contrast, in certain cardiomyopathies induced by protein aggregation, antioxidants, such as GPx1, are not beneficial. In humans, mutations in the small heat shock protein αB-crystallin was found to cause a desmin-like myopathy with protein aggregation [45]. Expression of the mutant human αB-crystallin in mice resulted in a cardiomyopathy, with progressive heart failure, and sudden, premature death. These mice had a reductive stress phenotype [46, 47], characterized, in part, by an increased activity of GPx1 and catalase as well as increased GSH/GSSG and a corresponding increased expression of enzymes involved in glutathione biosynthesis and GSSG recycling to GSH. The implication of these findings is that excess removal of hydrogen peroxide contributes to reductive stress that promotes protein misfolding and aggregation and the resulting cardiac dysfunction. Heat shock proteins were upregulated in this model, including Hsp25 and Hsp 27. In cell culture systems, the upregulation of these heat shock proteins similarly caused reductive stress with increased GSH and decreased ROS [48, 49]. In vivo, overexpression of Hsp27 resulted in cardiac hypertrophy, decreased cardiac contractility, and decreased diastolic function with increased GSH/GSSG and increased GPx1 expression and activity [50]. Cardiomyocytes from Hsp27 overexpressing mice also had altered histology and ultrastructure with evidence for increased cardiomyocyte diameter and protein aggregation. Electron microscopy showed a paucity of Z bands and myofilaments in some sections, vacuolation of the sarcoplasmic reticulum, and increased density of mitochondria in the HSP27 transgenic hearts compared to wildtype hearts. Cardiac ROS, as measured by DCF fluorescence in tissue homogenates, was reduced in HSP27 overexpressing mice. The importance of GPx1 activity in the pathogenesis of protein-aggregation-induced cardiomyopathy was shown by the improvement of cardiac function and hypertrophy in HSP27 overexpressing mice treated with mercaptosuccinate to inhibit GPx1 activity. Interestingly, in the context of the transgenic mice expressing the mutant human αB-crystallin, heterozygous knockout of Nrf2 lessened reductive stress decreasing the expression of the enzymes involved in GSH biosynthesis and recycling, resulting in a decrease in GSH concentration and the GSH/GSSG ratio. Lack of Nrf2 in this model also restored basal protein oxidation levels (measured by an increase in 4-hydroxynonenal modification, a byproduct of lipid peroxidation). Taken together, these changes suggest a return to a normal redox state with normal cellular levels of oxidant flux. Concurrently, lack of Nrf2 decreased protein aggregation and cardiac hypertrophy and improved cardiac contractility in the mice transgenic for the mutant human αB-crystallin, suggesting that the sustained upregulation of Nrf2 and subsequent increase in GSH-mediated removal of hydrogen peroxide contributes to the reductive stress phenotype [51].
Vascular reactivity and GPx1
Endothelial cells in the vessel wall regulate vascular homeostasis, in part, via the production of nitric oxide. Nitric oxide from endothelial cells attenuates thrombosis, inhibits the proliferation of vascular smooth muscle cells, prevents vascular remodeling, and maintains vascular tone. Vasodilators, such as acetylcholine or bradykinin, stimulate endothelial production of nitric oxide by activating endothelial nitric oxide synthase (eNOS). Nitric oxide released from endothelial cells stimulates soluble guanylyl cyclase in vascular smooth muscle cells to promote vasodilation. cGMP activates protein kinase G, which phosphorylates many target proteins involved in regulating contractile function, including those that modulate intracellular Ca2+ [52]. Depletion of bioavailable nitric oxide in the vasculature by excess oxidants causes endothelial dysfunction, which is characterized by a decrease in endothelium-dependent vasodilation while retaining the ability of vascular smooth muscle cells to vasodilate in response to nitric oxide donors, such as sodium nitroprusside [53]. Increased oxidant production can promote depletion of nitric oxide by many mechanisms, including the direct reaction of nitric oxide with superoxide to produce peroxynitrite. Interestingly, decreased availability of eNOS substrates, L-arginine or tetrahydrobiopterin (BH4), can promote enzymatic uncoupling of eNOS such that it generates superoxide instead of nitric oxide [54]. In addition, tetrahydrobiopterin is susceptible to hydrogen peroxide-mediated oxidation [12] as well as oxidation by other ROS [55]. The role of GPx1 in endothelial dysfunction is illustrated in Figure 3. As discussed further below, conditions that are associated with excess vascular ROS are associated with endothelial dysfunction. Excess oxidants in the vasculature also promote the pro-inflammatory activation of the endothelium to contribute to atherogenesis. Human subjects with elevated plasma homocysteine have endothelial dysfunction [56], and endothelial dysfunction is found in diabetics [57] and is thought to be an early marker of atherosclerosis [53].
Figure 3.
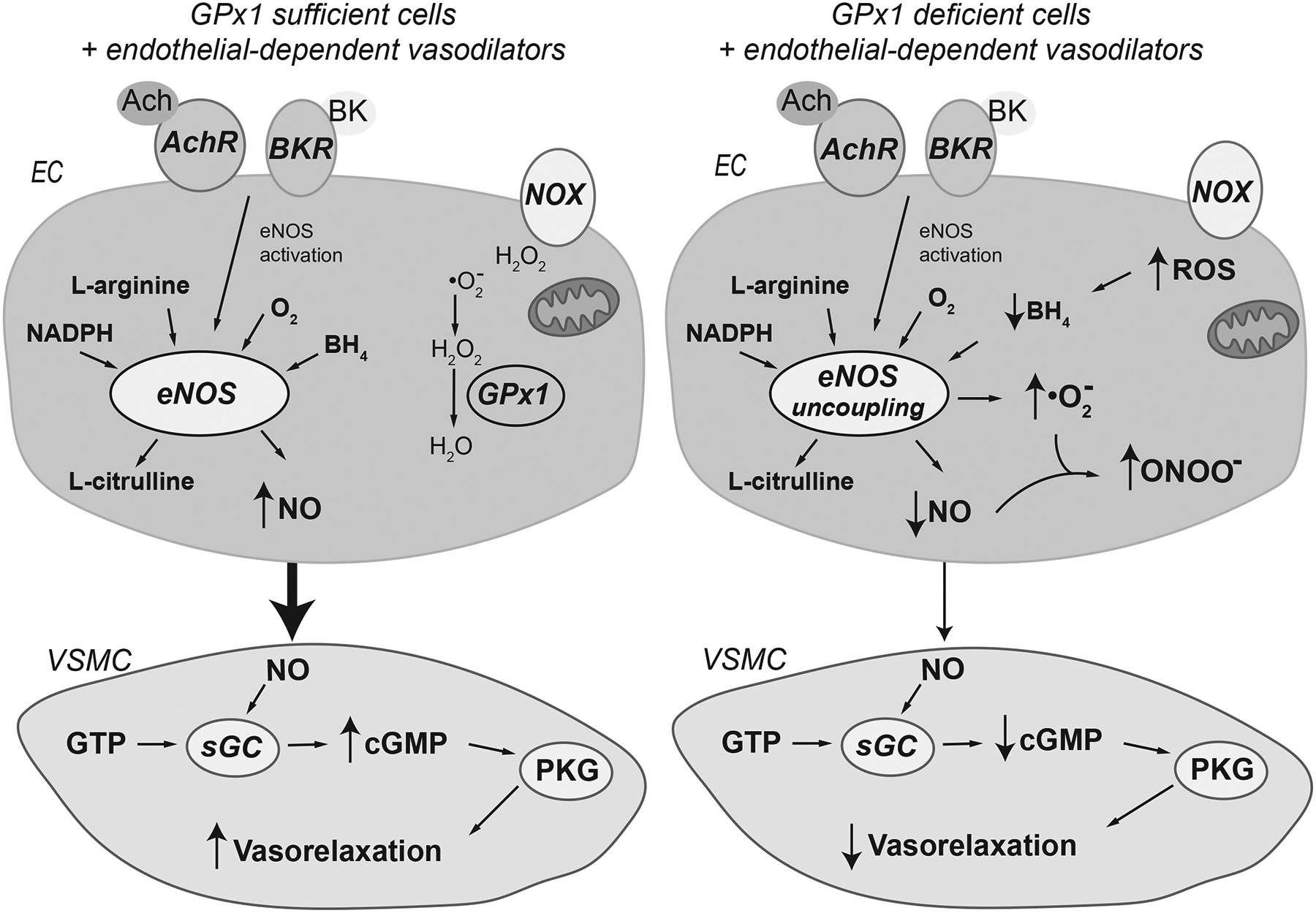
Endothelial dysfunction caused by GPx1 deficiency. The binding of the vasodilators acetylcholine (Ach) and bradykinin (BK) to their receptors, ACHR (muscarinic receptor) and BKR (bradykinin receptor), on endothelial cells (EC) activates endothelial nitric oxide synthase (eNOS) to promote the production of nitric oxide. Nitric oxide (NO) produced in endothelial cells (EC) promotes the relaxation of vascular smooth muscle cells (VSMC) via the cGMP-mediated activation of protein kinase G (PKG) to dilate blood vessels. Nitric oxide binding to the soluble guanylyl cyclase (sGC) stimulates the conversion of GTP to cGMP. In normal EC cells, basal NADPH oxidase (NOX) activity (primarily the hydrogen peroxide producing NOX4) and mitochondrial respiration provide most of the cellular ROS. GPx1 deficiency is associated with increased levels of ROS including increases in hydrogen peroxide and superoxide. Loss of GPx1 can promote the upregulation of superoxide generators, such as NOX2, and increased production and release of ROS from mitochondria, in part, due to ROS-mediated ROS production. ROS, including hydrogen peroxide, can oxidize the eNOS cofactor tetrahydrobiopterin (BH4) to uncouple eNOS. Uncoupling of eNOS results in substantially lower production of nitric oxide by eNOS and greater production of superoxide. The increased production of superoxide by eNOS promotes the formation of peroxynitrite, an oxidant that promotes additional oxidation of BH4, as well as the modification of protein tyrosine residues (3-nitrotyrosine modifications, not illustrated) and lipids. Note that decreased production of NO by EC results in diminished production of cGMP by sGC and a decrease in vasorelaxation and, in some cases, paradoxical vasoconstriction in response to agonist stimulation. GPx1 deficiency, however, does not affect the ability of VSMC to relax in response to nitric oxide donors, consistent with endothelial dysfunction.
We and others have shown a role for GPx1 in regulating endothelial function and pro-inflammatory activation. We found that the cardiovascular risk factor homocysteine decreased NO production in endothelial cells by a mechanism that involved decreased expression of GPx1 [58]. Our experiments in mouse models of mild hyperhomocysteinemia caused by a deficiency in cystathionine beta-synthase in CBS+/− (heterozygous deficient) mice found that increased plasma total homocysteine caused abnormal responses to endothelium-dependent vasodilators. Circulating levels of homocysteine in normal mice are 3–5 μM and heterozygous CBS deficiency in mice causes a modest increase in plasma homocysteine of approximately 1.5–2-fold [59, 60]. We found that this modest increase in homocysteine correlated with a decrease in endothelium-dependent vasodilation both in isolated aortic rings as well as in situ mesenteric micro-vessels from CBS+/− mice [59, 61]. Strikingly, in the mesenteric arterioles, the CBS+/− vessels showed paradoxical vasoconstriction to the endothelium-dependent vasodilators β-methacholine and bradykinin rather than the normal vasodilation found in wildtype vessels. These changes were found to be caused by endothelial cell dysfunction rather than a smooth muscle cell defect, as stimulation of mesenteric vessels with the NO donor sodium nitroprusside yielded the same vasodilatory response in CBS+/− and wildtype vessels. Furthermore, hyperhomocysteinemia correlated with decreased GPx1 activity and increased oxidative and nitrosative stress (as measured by 3-nitrotyrosine), consistent with a role for GPx1 in modulating endothelial superoxide production and the subsequent ROS-mediated formation of peroxynitrite. We found that these parameters were improved by treatment with the cysteine donor L-2-oxothiazolidine-4-carboxylic acid (OTC), which increased the GSH/GSSG ratio [61].
Interestingly, plasma and endothelial P-selectin, were increased in the CBS+/− mice compared to the wildtype mice. P-selectin is an adhesion molecule that contributes to leukocyte recruitment to endothelial cells, an early step in atherogenesis. Its cell surface localization from storage granules is caused by oxidative stress or exposure to pro-inflammatory signals, such as cytokines and endotoxin [62]. The soluble form is generated by alternative splicing that eliminates the cytoplasmic tail; the soluble form can also be shed from activated platelets that express membrane bound P-selectin [63]. L-2-oxothiazolidine-4-carboxylic acid (OTC) treatment attenuated plasma and tissue P-selectin levels, suggesting that the upregulation of P-selectin in hyperhomocysteinemic mice is also regulated by redox mechanisms [61]. Similarly, endothelium-mediated vasodilator responses were restored when a transgene for GPx1 was introduced into the CBS+/− mice by crossbreeding transgenic GPx1 overexpressing mice and CBS deficient mice, suggesting that increased expression of GPx1 could compensate for the negative effects of homocysteine on bioactive NO [60]. Consistent with these in vivo effects, we found that bradykinin-stimulated production of NO was enhanced by vector-mediated GPx1 overexpression in cultured endothelial cells at baseline and in the presence of exogenous homocysteine [60].
In our early studies we found that millimolar levels of homocysteine decreased GPx1 mRNA levels in endothelial cells [58]; however, micromolar levels of homocysteine are sufficient to decrease GPx1 activity. Thus, we hypothesized that homocysteine may alter GPx1 expression in a manner related to mechanisms of selenocysteine incorporation (Figure 4). The insertion of selenocysteine (Sec) during translation involves a unique set of specialized translational cofactors, including a Sec-specific elongation factor, a tRNASec that recognizes the UGA codon, and RNA binding proteins, such as SBP2 (SECIS binding protein 2) that bind to stem-loop structures in the 3’-untranslated region of the selenoprotein transcripts denoted selenocysteine incorporation sequence or SECIS (for review see [64, 65]). The biosynthesis of selenocysteine is also unique as selenocysteine is synthesized on the tRNASec after it isfirst amino-acylated with serine [66]. There are two major forms of the tRNASec that differ in the presence of a methyl group on the ribosyl moiety of position 34 (the wobble position of the anticodon) resulting in 5-methoxy-carbonylmethyl-2’-O-methyluridine (mcm5Um) or the 5-methoxy-carbonylmethyluridine (mcm5U) that lacks ribosyl methylation [67]. The methylation status of the tRNASec is dependent on selenium status, and the presence of the ribosyl methylation regulates the efficient translation of a subset of selenoprotein transcripts, including GPx1. Using mammalian cells in culture, we found that micromolar levels of homocysteine decreased GPx1 expression and activity without changing the levels of GPx1 mRNA [68]. Furthermore, by using a luciferase reporter system with a UGA codon inserted in the protein coding region of the luciferase reporter gene, we confirmed that selenium-dependent expression was independent of the GPx1 promoter [68]. Our subsequent studies in human endothelial cells showed that the homocysteine metabolite, S-adenosylhomocysteine, a potent inhibitor of many methyltransferases, decreased tRNASec methylation to suppress GPx1 expression and activity [69]. Decreased expression of GPx1, due to the cellular accumulation of S-adenosylhomocysteine, was found to promote oxidative stress, as hydrogen peroxide release from cells (measured by Amplex Red) as well as intracellular hydrogen peroxide production (measured by Hyper, a live-cell hydrogen peroxide probe) were enhanced by exposure of endothelial cells to increased amounts of S-adenosylhomocysteine. In addition, the increase in hydrogen peroxide production due to the suppression of GPx1 resulted in a proinflammatory activation of human endothelial cells, as characterized by the upregulation of the adhesion molecules ICAM1 and VCAM1. Treatment with N-acetyl-L-cysteine, an antioxidant, or adenovirus-mediated overexpression of GPx1 attenuated the upregulation of adhesion molecules, confirming the role for GPx-1 suppression and hydrogen peroxide accumulation in these proinflammatory responses.
Figure 4.
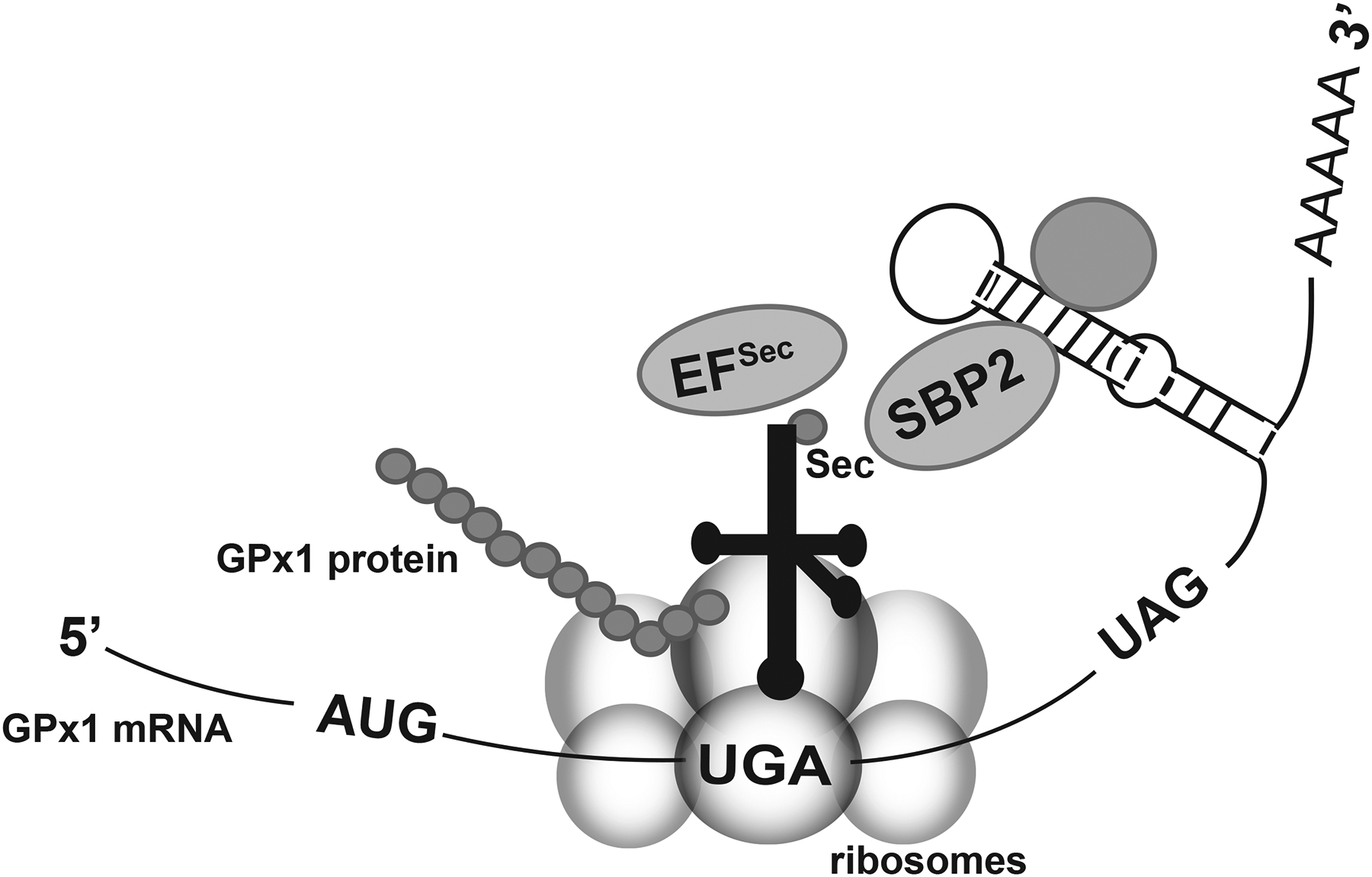
Incorporation of selenocysteine at a UGA codon during translation. Selenocysteine incorporation involves many unique cofactors to allow for the insertion of selenocysteine (Sec) at a UGA codon instead of terminating translation. The selenocysteine tRNA associates with the elongation factor for selenocysteine incorporation (EFSec) and the SBP2 protein. SBP2 also binds to the stem-loop structure (SECIS element) in the 3’ untranslated region of selenoprotein transcripts. Additional proteins may bind to the SECIS element (represented by the unlabeled shaded circle). Note that the codon that terminates GPx1 translation is UAG. (Modified from Lubos et al [2]).
Consistent with the link between homocysteine-mediated GPx1 suppression and endothelial dysfunction, endothelial dysfunction was also found in GPx1 knockout mice [39, 70, 71]. Thus, we found that mesenteric arterioles of homozygous mice (GPx1−/−) and older heterozygous (GPx1+/−) knockout mice had a loss of NO-dependent vasodilatory responses to β- methacholine and bradykinin, instead showing a vasoconstrictor response to these mediators that cause vasodilation in wildtype arterioles [39, 71]. Similarly, Dayal et al [70] found that GPx deficiency attenuated the acetylcholine-mediated relaxation-response of aortic rings. Our studies also showed a decrease in nitric oxide-mediated responses in aortic rings from GPx1+/− or GPx1−/− mice characterized by decreased accumulation of aortic cGMP following bradykinin treatment. In addition, aortic and plasma levels of the isoprostane iPF2α-III, a marker of oxidative stress and lipid peroxidation, were elevated in the GPx1 deficient mice [39, 71]. Furthermore, consistent with ROS-mediated inactivation of bioavailable NO in the absence of GPx1, aortic 3-nitrotyrosine staining, a marker of peroxynitrite formation, was augmented by GPx1 deficiency [71]. OTC treatment was found to restore endothelium-dependent vasodilator responses and return cGMP responses to normal while decreasing the levels of circulating isoprostanes in GPx1−/− mice [71]. Presumably, OTC-dependent increases in intracellular thiols improve cellular antioxidant defenses in the absence of GPx1. Interestingly, we found increased interstitial fibrosis and vascular abnormalities consistent with inflammation and fibrosis in coronary and aortic sections from older GPx1+/− mice [39], illustrating the detrimental effects of chronic oxidative stress on the vasculature. Another group similarly reported that vascular dysfunction and ROS levels were augmented in older GPx1−/− mice compared with age matched wildtype mice [72]. In addition, heterozygous GPx1+/− mice had augmented endothelial dysfunction, characterized by a decrease in acetylcholine-mediated vasodilator responses, in carotid arteries following exposure to angiotensin II, a known activator of NADPH-oxidase-dependent ROS generation [73].
The above findings suggest that GPx1 prevents endothelial dysfunction by limiting the damaging effects of excess ROS which preserves bioavailable NO and normal endothelial vasodilator responses; however, in some settings, hydrogen peroxide can promote (normal) vasodilation. Thus, in the cerebral arterioles, arachidonic acid promoted vasodilation that was dependent on hydrogen peroxide, and excess antioxidant enzymes, including GPx1, attenuated hydrogen peroxide-mediated vasodilation [74]. It has been proposed that hydrogen peroxide may mediate its effects by promoting oxidative modifications of redox-active cysteine residues that augment hyperpolarization of potassium channels in vascular smooth muscle cells resulting in vasodilation. Specifically, hydrogen peroxide was found to promote reversible disulfide bond formation between cGMP-dependent protein kinase (PKG) monomers to enhance kinase activity and promote K+-channel hyperpolarization [75]. Similarly other oxidative mechanisms, such as the hydrogen peroxide-dependent S-glutathionylation of 4-aminopyridine-sensitive Kv channels, have been suggested to contribute to the vasodilatory effects of hydrogen peroxide [76].
GPx1 also has a role in maintaining endothelial progenitor cell function. Endothelial progenitors are endothelial cell precursors that maintain vascular homeostasis by promoting vascular repair, neoangiogenesis, and revascularization. We found that endothelial progenitor cells from GPx1−/− mice were more susceptible to hydrogen peroxide-induced apoptosis compared to progenitors from wildtype mice. In addition, lack of GPx1 attenuated the ability of progenitors to form vascular networks in culture [77]. In vivo, GPx1−/− mice had a deficiency of progenitor cells as they failed to increase progenitor cell number following subcutaneous injection of vascular endothelial growth factor or in response to ischemic injury in the hindlimb ischemia model. Thus, GPx1−/− mice had impaired neovascularization in the hindlimb ischemia model with a decrease in capillary density and a decrease in the recovery of hindlimb blood flow following surgically-induced hindlimb ischemia compared to wildtype mice. In addition, administration of harvested endothelial progenitor cells from GPx1−/− mice failed to augment revascularization in wildtype mice, whereas the wildtype endothelial cells improved blood flow and capillary density in the GPx1−/− mice, suggesting that elevated levels of ROS in the GPx1-deficient cells are detrimental to endothelial progenitor cell function [77].
Atherogenesis and cardiovascular risk
In the presence of risk factors, such as high fat diet and diabetes, GPx1 deficiency was found to augment atherosclerosis in the context of apolipoprotein E deficient mice (GPx1−/−ApoE−/−) [78, 79]. Compared with ApoE−/− alone, GPx1−/−ApoE−/− double knockouts had increased oxidative stress in the vessel wall as measured by an increase in superoxide after 6 weeks on a high fat diet. Aortic rings and isolated heart mitochondria from the GPx1−/−ApoE mice also showed increased superoxide production compared to ApoE−/− mice [78]. Furthermore, acetylcholine-induced production of bioavailable nitric oxide was lower in aortic rings from GPx1−/−ApoE mice than ApoE−/− mice, and the double knockout mice had increased 3-nitrotyrosine staining in atheromatous plaques, similar to earlier findings of endothelial dysfunction in GPx1-deficient mice [39, 70, 71]. Plaques from the double knockout mice also had augmented macrophage infiltration at 12 weeks and increased smooth muscle cell content at 24 weeks compared with the lesions in the ApoE−/− mice [78]. These changes may account for the accelerated progression of atherosclerosis in the high fat diet fed GPx1−/−ApoE−/− mice. Similarly, in the context of diabetes, GPx1−/−ApoE−/− mice had augmented atherosclerosis with increased macrophage infiltration, increased nitrotyrosine levels, and increased expression of proinflammatory and profibrotic markers in mouse aorta [79]. In a separate analysis of ApoE−/− mice on an atherogenic diet for 12 weeks, GPx1 mRNA (detected by in situ hybridization) and GPx1 protein (detected by immunostaining) were highly concentrated in macrophage- and smooth muscle cell (SMC)-rich areas of atherosclerotic lesions of ApoE−/− aortas [80]. It is likely that GPx1 in macrophages and SMC may mitigate the effects of oxidized low-density lipoproteins (oxLDL) and inflammatory cytokines during atherogenesis, whereas loss of this protection in GPx1−/−ApoE−/− may accelerate lesion development. Consistent with this concept, isolated peritoneal macrophages from GPx1−/−ApoE−/− mice had enhanced proliferative responses to macrophage colony stimulating factor (MCSF) compared to macrophages from ApoE−/− mice [80]. The proliferative responses may be due, in part, to increased activation of extracellular signal-related kinase 1/2 (ERK1/2) pathways in the double knockout cells. Activation of ERK1/2, a mitogen-activated protein kinase (MAPK), is associated with cell proliferation and survival. Consistent with this function of ERK1/2, treatment with ebselen, a GPx mimetic and antioxidant, decreased ERK1/2 activation as well as the rate of macrophage cell proliferation [80]. These findings suggest that GPx1 modulates proliferation by limiting hydrogen peroxide-mediated effects, such as the activation of ERK1/2. In other cell culture studies that are discussed further below, loss of GPx1 caused excess activation of MAPK pathways to contribute to increased inflammatory responses in endothelial cells [6].
In diabetic ApoE−/− mice, ebselen treatment was also shown to be beneficial; it decreased total aortic lesion area, although lesion burden in the aortic sinus was not altered [81]. Ebselen also decreased 3-ntirotyrosine content in the lesions, as well as the expression of NOX2, and it altered the cellular composition of the lesions, decreasing the infiltration of macrophages. These findings suggest that increasing GPx activity with ebselen may mitigate some of the pro-atherogenic effects of diabetes. In cultured human aortic endothelial cells, ebselen attenuated hydrogen peroxide-mediated upregulation of pro-inflammatory pathways that are associated with atherogenesis, such as the activation of NFkB and JNK signaling and the increased expression of TNF-α and NOX2 [81]. Nonetheless, in a small crossover study of 26 human diabetic patients, 4 weeks of ebselen treatment did not alter plasma levels of oxidative stress markers, such as GSH/GSSG, isoprostanes, and nitrotyrosine, compared to placebo treatment [82]. It was suggested that the dose and timing of ebselen may not have been sufficient to reduce oxidative stress in these subjects. However, the patients treated in this study were a mix of type I and type II diabetics. A previous meta-analysis indicated that most studies using dietary antioxidant supplementation failed to improve vascular function in type 2 diabetics and that subpopulations of subjects without obesity may have better responses to antioxidant treatments [83].
In vitro analysis of primary endothelial cells lacking GPx1 finds that they also manifest increased oxidative stress. GPx1-deficient endothelial cells were more susceptible to the effects of inflammatory mediators, such as TNFα (tumor necrosis factor α) and lipopolysaccharide, than cells with normal levels of GPx1. Cells deficient in GPx1 generated more ROS, in the presence and absence of TNFα, as measured intracellularly by DCF fluorescence or extracellularly, as measured by Amplex Red detection of hydrogen peroxide in the media [6]. Additionally, oxidative stress and the enhanced proinflammatory activation of GPx1-deficient cells can be mitigated by improving antioxidant balance in the cells [6, 84, 85]. Thus, in human endothelial cells, we found that adhesion molecules, such as vascular adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1), are upregulated by the absence of GPx1 and the expression of adhesion molecules is further augmented with exposure to TNFα or lipopolysaccharide. Overexpression of GPx1 has the opposite effect, decreasing the expression of the adhesion molecules [6, 84]. Furthermore, enhanced inflammatory activation in GPx1-deficient cells is attenuated by the antioxidant N-acetyl-L-cysteine in the presence or absence of TNFα [6, 84]. In GPx1-deficient human endothelial cells, we found that NFκB mediated the inflammatory activation at baseline, although there is a role for NFκB and MAPK pathways (c-Jun N-terminal kinase or JNK and ERK1/2) in the augmented responses of these cells to TNFα as well [6]. Microarray and gene enrichment studies provided insights into novel TNFα targets that are modified by GPx1 deficiency. Specifically, GPx1 deficiency augmented TNFα suppression of the dual specific phosphatase DUSP4 to promote ERK1/2 activation, linking GPX1 and oxidant stress to MAPK regulation [6]. We also found evidence that GPx1 deficiency upregulated CD14 expression to enhance lipopolysaccharide-mediated endothelial cell activation [84]. Although many ROS generators in the cell contribute to the proinflammatory activation of endothelial cells, NADPH oxidase 4 (NOX4) appeared to have the greatest effect on hydrogen peroxide generation with knockdown of NOX4 significantly lowering oxidant accumulation in GPx1-deficient cells in the presence or absence of TNFα [6]. Consistent with our findings, studies with mouse aortic endothelial cells isolated from GPx1−/− and wildtype mice reported that GPx1-deficiency promotes ROS production (both superoxide and hydrogen peroxide), enhances MAPK and NFκB activation, and upregulates VCAM1 expression in response to TNFα [85]. These proinflammatory changes were shown to increase the binding of fluorescently-labeled leukocytes to the GPx1-deficient endothelial cells compared to the wildtype cells [85]. Leukocyte-binding to adhesion molecules on endothelial cells is an early event in atherogenesis, and the upregulation of leukocyte binding in GPx1-deficient cells is consistent with enhanced lesion development in atherosclerotic-susceptible mice. Ebselen pretreatment was found to be an effective means to suppress the upregulation of VCAM1, as well as the enhanced leukocyte adhesion caused by GPx1 deficiency in mouse endothelial cells [85].
Human studies also provide evidence that GPx1 is protective against cardiovascular disease. Thus, a prospective study on the risk of cardiovascular events in patients with coronary artery disease (CAD) established a link between erythrocyte GPx1 activity and the risk of future cardiovascular events. Patients in the highest quartile of GPx1 activity had a hazard ratio of 0.29 (95% CI, 0.15–0.58) compared to those in the lowest quartile [86]. In a follow-up study, homocysteine and GPx1 were the strongest predictors of future cardiovascular event risk, and those with low GPx-1 activity had a nearly 3-fold increase in risk if they also had homocysteine levels above the median [87]. Furthermore, a recent study in human subjects showed a significant association between quartiles of serum homocysteine levels and coronary microvascular endothelial dysfunction in patients with early coronary atherosclerosis [88], confirming the detrimental effect of homocysteine on endothelial function.
The association of the GPX1 Pro198Leu variant with coronary artery disease has been analyzed in human cohort studies. In a study of 265 unrelated CAD patients and age- and sex-matched controls from China, subjects with one or two copies of the Leu variant were found to have a significantly higher risk of CAD (adjusted OR, 2.02, 95%CI, 1.2–3.22) [89]. In a meta-analysis the above results were considered along with three other studies (2 of which were from China and one from India) to examine the association of the GPX1 rs1050450 Pro198Leu variant with coronary heart disease. Each of these studies individually reported an association between the Leu variant and coronary heart disease, with a final number of 1,560 CAD patients and 978 controls, resulting in a significant OR, 1.61 (95% CI, 1.25–2.07) [90]. Similarly, increases in intima-media thickness of the common carotid arteries, presence of coronary heart disease, and the presence of peripheral artery disease correlated with the Pro/Leu genotype compared to those homozygous for the Pro allele in a study of type 2 diabetic patients in Japan [91], consistent with a role for this polymorphism in cardiovascular risk. A recent study in Sri Lanka analyzed the association between the GPX1 rs1050450 Pro198Leu variant and coronary artery disease and reported that the variant was increased in patients compared to controls (OR, 2.84; 95% CI, 1.15–6.98). In these patients, GPx1 activity was inversely associated with disease severity, although there was no association between GPx1 genotypes and GPx1 activity [92]. In another study examining the risk of restenosis after stenting, carriers of the Leu allele had lower erythrocyte GPx1 activity than homozygous carriers of the Pro allele, and the Leu allele conferred an increased risk of restenosis (OR, 2,9; 95% CI, 1.23–6.82) [93]. Interestingly, a study of in stent-restenosis in ApoE−/− and GPx1−/−ApoE−/− mice found that lack of GPx1 promoted vascular remodeling by mechanisms that involved reductive stress-induced proliferation of vascular smooth muscle cells [94]. In this model, the deficiency of GPx1 caused excess vascular superoxide and decreased bioavailable nitric oxide, and vascular injury by balloon angioplasty and stenting increased the expression of GSH enzymes, with a corresponding increase in GSH and the GSH/GSSG ratio. In the context of GPx1 deficiency, the lack of GPx1 activity may contribute to the increase in GSH [95]. The enhanced proliferative response of vascular smooth muscle cells was shown to be caused by the inactivation of the SH2 domain-containing protein tyrosine phosphatase 2 (SHP-2 phosphatase) by glutathionylation to allow for the sustained phosphorylation and activation of the tyrosine kinase ROS1 (ROS proto-oncogene 1, receptor tyrosine kinase).
Metabolism and GPx1
Overall, the effects of GPx1 on metabolism and diabetes is complex. This subject has recently been reviewed by Huang and colleagues [96]. GPx1 knockout mice were shown to have improved sensitivity to insulin compared with GPx1 overexpressing mice. GPx1-deficient mice had excess generation of hydrogen peroxide in pancreatic islets that correlated with increased p53 and p38 MAPK phosphorylation, decreased islet cell mass, and hypoinsulinemia. Islets also showed impaired glucose-stimulated insulin secretion. In muscle of GPx1 knockout mice, Akt phosphorylation was increased following exposure of fasted mice to an insulin injection, presumably owing to increased production of hydrogen peroxide caused by GPx1 deficiency [97]. Nonetheless, in other studies, GPx1-deficient mice on a high fat diet were found to have hyperglycemia and attenuated insulin secretion due to defects in pancreatic β-cell function owing to increased oxidative stress [98]. In a model of hepatocyte-specific GPx1 deficiency, enhanced insulin sensitivity was found, as select hepatic tyrosine phosphatases showed increased oxidation compared to control mice. Furthermore, after 12 weeks on a high-fat diet, there was an increase in insulin-mediated phosphorylation of the insulin receptor and Akt in livers from the hepatocyte-specific GPx1 deficient mice [99]. In contrast, GPx1 overexpressing mice were found to have alterations in β-cell function that caused hyperinsulinemia. Insulin resistance in the GPx1 overexpressing mice altered glucose metabolism and caused a type 2 diabetes phenotype, owing, in part, to a decrease in cellular - hydrogen peroxide that normally oxidatively inactivates protein phosphatases [100, 101]. Excess phosphatase activity results in the attenuation of insulin-stimulated phosphorylation of the insulin receptor and Akt in liver and skeletal muscle in GPX1 overexpressing mice. Interestingly, in the context of ob/ob mice, β-cell overexpression of GPx1 is beneficial, decreasing hyperglycemia and preserving β-cell volume and the accumulation of insulin stores in granules [102]. In human subjects, several studies suggest that high selenium supplementation increases the risk of type 2 diabetes [103, 104], and, in mouse models, increased expression of selenoproteins as well as selenoprotein deficiency was also correlated with metabolic dysregulation [105]. Taken together with the studies in knockout and transgenic GPx1 models, these findings suggest a role for GPx1 in the regulation of insulin and glucose metabolism and suggest that modulation of selenoprotein expression, including GPx1, may influence metabolism in humans, as well.
Stroke and Neuroprotection
Oxidative stress plays a role in promoting neurological damage in stroke. Thus, absence of GPx1 in knockout mice was found to augment neuronal damage in the middle cerebral artery occlusion ischemia-reperfusion (MCAO) model of stroke, with increased infarct volume and increased apoptosis in brains of GPx1−/− mice compared with wildtype mice [106]. Additionally, GPx1 overexpression protected against cell death and decreased infiltration of inflammatory cells in the MCAO model [107]. Subsequent studies showed a role for upregulation of the pro-inflammatory mediator NFκB in the GPx1−/− brain following MCAO-induced injury, and found that NFκB contributes, in part, to the enhanced injury in the GPx1−/− mice compared to wildtype mice [108]. Additional studies showed that mice lacking GPx1 were more susceptibility to vascular complications in the MCAO model. Thus, following MCAO, GPx1−/− mice had decreased microvascular perfusion and increased vascular permeability compared to wildtype mice. Ebselen pretreatment restored microvascular perfusion and attenuated the increases in infarct size and vascular permeability found in GPx1−/− mice in this model of stroke [109], consistent with a role for ROS in this model.
In a human trial to test ebselen as a treatment for acute ischemic stroke, ebselen significantly improved outcomes when given within 24 h of stroke onset [110]; however, more studies are needed to confirm the usefulness of ebselen in human subjects. Regarding association studies between the GPX1 rs1050450 Pro198Leu variant and stroke, two published studies, one in Finnish/Swedish subjects and one in Turkish subjects, reported that there was no association of the GPX1 rs1050450 Pro198Leu variant and risk of stroke [111, 112]. Furthermore, the Finnish/Swedish study reported no difference in erythrocyte GPx1 activity among the various genotypes [112].
Oxidative stress is thought to play a major role in neurodegeneration caused by traumatic brain injury and exposure to neurotoxins, and may be a factor in the course of many chronic neurodegenerative diseases, including Alzheimer disease (AD). A recent study reported that traumatic brain injury decreased the antioxidant capacity of the brain through the downregulation of Nrf2 and the resulting decrease of antioxidant proteins, such as GPx1. A corresponding increase in the expression of oxidant generators, such as NOX1 and inducible nitric oxide synthase, also contributed to inflammation and apoptosis in this brain injury model [113]. Interestingly, in traumatic brain injury, GPx1 deficiency was associated with decreased mitochondrial bioenergetic capacity compared to wildtype mice, whereas transgenic GPx1 mice had no decrease in mitochondrial function [114] GPx1 deficiency also enhanced apoptosis and inflammation in a cold-induced brain injury model [115]. Similarly, several studies suggest a protective effect of GPx1 against neurotoxicity in response to a variety of harmful substances, including 6-hydroxydopamine and malonate (reviewed in [116]).
Oxidative stress is also thought to play a role in the seizure-inducing effects of kainic acid; however, GPx1 expression regulates the response to kainic acid in an unexpected manner: GPx1 knockout mice are more resistant to kainic acid-induced epileptic seizures than wildtype mice and transgenic GPx1 mice have augmented responses to kainic acid exposure [117, 118]. Compared to wildtype mice, GPx1 knockout mice have increased levels of oxidative stress in the brain as measured by increased DCF fluorescence before or after kainic acid exposure, and increased lipid peroxidation (measured by malondialdehyde), and increased protein carbonyl content following kainic acid treatment. Nonetheless, kainic acid-induced seizure activity and neurodegeneration were attenuated in the knockout mice. N-methyl-D-aspartate (NMDA) receptors have been found to regulate kainic acid-induced seizure activity and subsequent neuronal damage [119]. Previous studies have shown that oxidants can inhibit NMDA receptor activity by modifying redox-sensitive sulfhydryl groups [120]. GPx1-deficient mice had decreased activity of NMDA receptors, and consistent with an oxidative modification of thiol residues influencing this activity, DTT treatment enhanced NMDA activity, especially in the GPx1 knockout. In addition, there was a decrease of reduced sulfhydryl groups in the NR1 subunit of the NMDA receptor in the GPx1 knockout. By contrast, GPx1 overexpressing mouse showed increased seizures and neuronal cell death in response to kainic acid. Neurons from transgenic GPx1 mice had increased NMDA responses compared to those from wildtype mice, and DTT had no effect on the NMDA activity. Basal brain GSSG levels were decreased in transgenic mice compared to wildtype mice, possibly due to the excess GPx1 activity in overexpressing mice. Although it is not exactly clear how GPx1 upregulation is affecting NMDA activity following kainic acid, exposure of GPx1 overexpressing neurons grown in culture caused an accumulation of GSSG in the media, compared to the effects of kainic acid on wildtype neurons. GSSG has previously been shown to inhibit NMDA activity by fostering oxidation of the redox modulatory site of the NMDA receptor [121]; however, other studies suggest that GSSG may also function as a agonist of the NMDA receptor [122].
In Alzheimer disease (AD) model systems, GPx1 was shown to protect against ROS-mediated neurotoxicity caused by the toxic amyloid beta (Aβ) peptide. Cultured neurons isolated from GPx1−/− mice had increased Aβ peptide-induced cell death and apoptosis compared to wildtype neurons [123]. Furthermore, the increased toxicity to Aβ peptide exposure could be reversed by pretreatment with NAC or ebselen, illustrating the role of excess oxidants, such as hydrogen peroxide, in these responses. Similarly, in GPx1−/− mice, deficiency of GPx1 increased ROS (as measured by DCF fluorescence of brain homogenates), lipid peroxidation, and protein carbonylation in brains exposed to the Aβ peptide, whereas forced overexpression of GPx1 in the GPx1−/− mice attenuated these detrimental effects [124]. Interestingly, ERK1/2 signaling plays an important role in memory formation and retrieval [125], and brains exposed to the Aβ peptide have decreased activation of ERK1/2 due, in part, to a deficiency in protein kinase C βII (PKC βII)-mediated signaling [124]. These deficits in PKC βII/ERK signaling correlate with memory impairment and are augmented by GPx1 deficiency. In this model, excess ROS plays a role in decreasing the expression of PKC βII, as overexpression of GPx1 increases PKC βII expression and ERK1/2 activation. The mechanism by which ROS suppresses PKC βII protein expression is unclear. It is well known that some PKCs can become activated by ROS-induced modifications; however, inactivation of some PKCs by oxidative modifications has also been reported [126].
Two small case-control studies analyzed the association of the Pro198Leu (rs1050450) polymorphism with AD, with different results. In an Ecuadorian population, the Leu allele was found to be associated with AD risk [127], whereas in a Brazilian population, the Pro allele conferred increased risk of AD [128].
Multiple studies have shown that ERK1/2 suppression and memory impairment are attenuated by GPx1 upregulation or ebselen treatment [129, 130]. Nevertheless, excess GPx1 may also attenuate beneficial ERK1/2 activation that may be promoted, in some instances by ROS production. Thus, overexpression of GPx1 had a completely different effect on hypoxic preconditioning. Hypoxic preconditioning may protect the brain against damage caused by ischemia. Surprisingly, GPx1 overexpression caused a loss of the protective effect of hypoxic preconditioning that correlated with an attenuation of ERK1/2 activation in the brain [131]. It is not entirely clear if attenuation of ERK1/2 activation is the reason for the loss of the beneficial effects of hypoxic preconditioning; however, these findings highlight one of the complexities of using antioxidant therapies, as increased GPx1 activity was correlated with enhanced ERK1/2 activation when analyzing memory, whereas increased GPx1 correlated with a decrease in ERK1/2 activation in the hypoxic preconditioning study, presumably due to the loss of hydrogen peroxide-mediated ERK1/2 activation.
Cancer and GPx1
The role of GPx1 in cancer development and progression is complicated. Owing to its antioxidant properties, it has been suggested that GPx1 may inhibit cancer initiation by limiting ROS-mediated DNA damage; however; its ability to modulate ROS may also limit apoptosis and cell death in cancer cells to promote rampant cell growth and resistance to chemotherapy [see [132] for review]. Nonetheless, the forced overexpression of GPx1 has been shown to suppress cellular proliferation and migration of pancreatic cancer cells [133, 134], suggesting that GPx1 is a tumor suppressor in some cancers.
In many cancers, the increased expression and/or activity of GPx1 is associated with poor prognosis; however, the histopathology of the cancer is crucial to understanding the relationship between GPx1 expression and prognosis. Thus, upregulation of GPx1 was associated with poor prognosis in some but not all types of renal cell carcinoma: upregulation in kidney chromophobe cell carcinoma and renal clear cell carcinoma was an indicator of a poor prognosis, whereas in renal papillary cell carcinoma, upregulation of GPx1 was associated with better outcomes [135]. Consistent with a role for GPx1 in promoting the progression (of some forms) - of renal carcinoma, it was found that short hairpin RNA-mediated suppression of GPx1 expression decreased cell growth and colony formation in a number of renal cancer cell lines grown in culture [136]. Similarly, in laryngeal cancer, GPx1 upregulation was associated with lymph node metastasis and cancer stage and was an independent predictor of patient survival (HR, 2.101, 95%CI, 1.011–4.367) [137]. Elevated expression of GPx1 was also associated with poor survival for other cancers, including oral squamous cell carcinoma [138], acute myeloid leukemia [139], gliomas [140], and invasive ductal cell carcinoma [141].
Mechanistically, there may be a link between the expression of GPx1 and the PI3K/Akt pathway. Akt activation is associated with cell survival and growth. In pancreatic ductal carcinoma cells, suppression of GPx1 expression promoted ROS-induced Akt signaling (measured by Akt phosphorylation) to drive an epithelial-mesenchymal transition that is associated with enhanced tumor growth, whereas overexpression of GPx1 decreased the phenotypic changes in pancreatic ductal carcinoma cells by decreasing ROS-mediated pro-survival signaling in these cells [134]. Furthermore, treatment with N-acetyl-L-cysteine also decreased Akt phosphorylation in GPx1 deficient cells. In our studies in Chang liver cells, we similarly showed a dependence of cell growth on ROS-dependent pathways; excess GPx1 decreased growth factor stimulated cellular proliferation, and reduced growth factor mediated signal transduction and activation of Akt [16]. Overexpression of catalase similarly suppressed growth factor mediated signaling in these cells and GPx1 overexpression attenuated hydrogen peroxide-mediated increases in DCF-fluorescence, illustrating the effectiveness of GPx1 in preventing an accumulation of cellular oxidants. Interestingly in pancreatic carcinomas the survival advantage of low GPx1 was associated with resistance to gemcitabine, a chemotherapeutic agent, in patients, and enhanced resistance to cell death in cell culture as well as increased xenograph tumor growth in mice. Interestingly, in other cancers GPx1 upregulation is associated with resistance to chemotherapy. In non-small cell lung cancer, GPx1 overexpression promotes cisplatin resistance; however, unlike the studies in the pancreatic carcinoma cells, increased GPx1 expression in non-small cell cancer cells was associated with increased Akt activation and decreased apoptotic signaling [142]. Thus, increased cellular GPx1 activity provides a survival advantage in some cancer cells by eliminating harmful ROS generated by chemotherapy agents, such as cisplatin. Given the role of GPx1 in promoting cell survival pathways, the effectiveness of other chemotherapeutic agents, such as the isothiocyanate iberin, may be due, in part, to their ability to decrease GPx1 expression. In particular, iberin suppresses GPX1 gene transcription. Decreased expression of GPx1 by iberin results in an accumulation of detrimental concentrations of ROS (measured by DCF fluorescence) that inhibits cell proliferation, induces cell cycle arrest, and promotes apoptosis, whereas the forced upregulation of GPx1 or antioxidant treatment attenuates these therapeutic actions [143].
Mechanistic studies have uncovered numerous mechanisms by which GPx1 provides a survival advantage in various cancers. For example, in triple negative breast cancer cells, defined by lack of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor, GPx1 was associated with the autophosphorylation of focal adhesion kinase or FAK, a tyrosine kinase known to play a role in malignancy [144]. Loss of GPx1 downregulated FAK autophosphorylation and c-src activation and decreased the migration, invasiveness, adhesiveness, and spreading of the breast cancer cells. Based on these findings, it was suggested that protein-protein interactions between GPx1 and FAK may facilitate the protection of FAK from local hydrogen peroxide, but further examination is necessary to understand the precise mechanism by which FAK is inactivated by ROS. In other studies, it was found that GPx1 can also mitigate activation of the ROS-induced apoptosis signal-regulating kinase 1 (ASK1)/JNK-mediated apoptosis [145]. In unstressed cells, ASK1 is complexed with redox-active thioredoxin, but under many stress conditions, an accumulation of hydrogen peroxide oxidizes thioredoxin to release ASK1 [146]. Subsequently, ASK1 can then bind to tumor necrosis factor receptor-associated factors (TRAF2 and TRAF6) to potentiate the sustained activation of JNK which is necessary for apoptosis[147]. In a study of apoptotic responses to TNFα in cancer lines, GPx1 was shown to decrease thioredoxin oxidation to maintain the inactive thioredoxin-ASK1 complex by reducing ROS, as measured by DCF fluorescence. In addition, protein-protein interactions of GPx1 with TRAF prevents the formation of the active TRAF/ASK1 complex to prevent apoptosis [148] (Figure 5). Association of GPx1 with TRAF may also promote the local reduction of hydrogen peroxide to contribute to maintaining thioredoxin in a reduced state. Consistent with these findings, cancer cells with GPx1 knockdown had augmented apoptosis in response to TNF-α. This effect could be recapitulated in vivo: tumor volume of GPx1-deficient cells was lowered by exposure to TNF-α [148].
Figure 5.
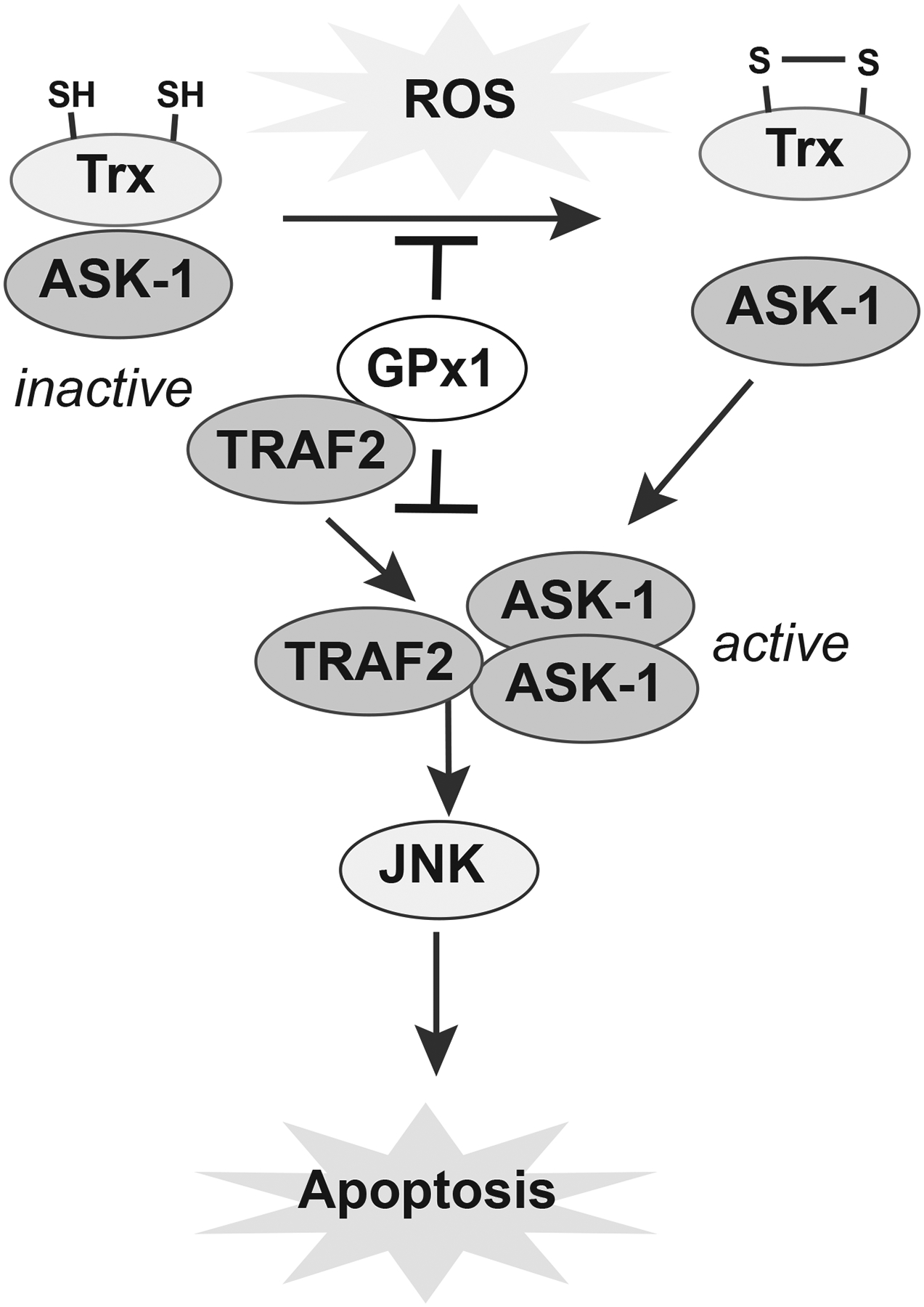
The antiapoptotic effect of GPx1 upregulation promotes cell survival in some cancers. ASK-1 is maintained in an inactive state by its association with the redox-sensitive thioredoxin (Trx). TNFα-induced oxidative stress can promote the oxidation of thioredoxin, leading to the association of TRAF2 and ASK1 oligomers. These active complexes promote JNK-mediated pathways of apoptosis. In some cancer cells, the presence of GPx1 can decrease the oxidation of Trx to keep ASK-1 in an inactive state. Furthermore, association of GPx1 with TRAF2 may prevent the formation of active ASK1/TRAF2 complexes to block JNK activation and apoptosis. Other mechanisms may promote cells survival in other types of cancer, as discussed in the text.
Many studies have examined the role of the GPX1 rs1050450 Pro198Leu variant and breast, lung, prostate, bladder, and other forms of cancer. As previously reviewed, (see [2, 149]), studies of the association of the rs1050450 SNP with cancer have yielded mixed results with some studies reporting an association of risk with the Leu variant or the Pro variant and other studies reporting no association between these alleles and cancer risk. Below we review some of the recent findings.
A meta-analysis performed in 2010 that included 5509 breast cancer patients and 6542 controls from 6 case-control studies found no association of the Leu allele with risk in the combined population, although there was an elevated risk in African American carriers of the Leu allele in this study [150]. Subsequent studies have found associations with different alleles. In a Danish cohort, carriers of the Leu/Leu genotype were found to have an increased risk of nonductal breast cancer (OR, 1.88; 95%CI, 1.08–3.28), whereas there was no risk associated with breast cancer in the combined analysis of 975 cases and controls or in the ductal breast cancer subgroup [151]. In contrast, a recent study in Polish women found, instead, a significant decrease of breast cancer risk in individuals with the Leu variant, with an adjusted OR of 0.61 (95%CI, 0.38–0.97) [152]. In this study of 136 breast cancer cases and 183 controls, erythrocyte GPx1 activity was also significantly higher in breast cancer cases compared to controls. The Breast and Prostate Cancer Cohort Consortium, which combined cohort studies from multiple studies in the US and European nations, examined 10,726 breast cancer cases with matched controls, found no association between the rs1050450 SNP and breast cancer [153]. In prostate cancer, the Consortium study examined 7532 prostate cancer cases and controls and found a weak inverse association between cancer risk and Leu carriers (OR 0.87, 95% XI 0.79–0.97). This finding, however, contrasts with other meta-analyses that concluded that the GPX1 rs1050450 Pro198Leu polymorphism was not associated with the risk of prostate cancer [154, 155]. In bladder cancer, the Leu allele was associated with increased risk in 2 overlapping meta-analyses [155, 156].
Drug-mediated regulation of GPx1 expression
In addition to iberin, which is discussed above, other chemical agents regulate GPx1 expression: docosahexaenoic acid [157] and Lfcin-B [158] suppress GPx1 expression, whereas other compounds, such as genistein [159] and resveratrol [160], upregulate GPx1 expression. Although the mechanisms by which these reagents modulate GPx1 expression are not clear, it has been shown that GPx1 can be upregulated by activation of the peroxisome proliferator-activated receptor-γ-coactivator-1a (PGC1α) pathway, possibly via the subsequent activation of Nrf2 [161]. Nrf2 is well known for mediating the expression of antioxidant and detoxifying proteins in response to oxidative and electrophilic stress (reviewed in [162, 163]). Normally, Nrf2 is sequestered in the cytoplasm by Keap1 (Kelch-like ECH-associated protein 1), a protein that also acts as an adaptor protein for the Cullin 3-dependent E3 ubiquitin ligase to mediate the ubiquitination and degradation of Nrf2. Oxidative inactivation of redox sensitive cysteine residues in Keap1, specific phosphorylation of Nrf2, or increased expression of p62, which competes with Nrf2 for Keap 1 binding, disrupts the Keap1/Nrf2 interactions to foster the stabilization and nuclear translocation of Nrf2 where it promotes transcription of a variety of protective antioxidant genes via the antioxidant response element or ARE in their promoter regions. There is some controversy regarding whether Nrf2 directly regulates the transcription of GPx1; however, the activation (or suppression) of Nrf2 appears to be tightly associated with the increased (or decreased) expression of GPx1 in many instances [164–167], possibly owing to the regulation of GSH biosynthesis by Nrf2 and/or the concurrent activation of additional antioxidant response pathways. Nevertheless, the regulation (direct or indirect) of GPx1 by Nrf2-linked pathways is intriguing as GPx1 can modulate oxidant stress to mitigate Nrf2 activation.
Two classes of drugs can alter selenocysteine-dependent translation to suppress GPx1 expression. We found that aminoglycosides, a class of antibiotics that can promote the read-through of stop codons, decreased selenium-dependent expression of GPx1 and facilitated the insertion of L-arginine for selenocysteine to decrease GPx1 specific activity [168]. Subsequent studies found that selenium deficiency enhances the aminoglycoside-mediated misincorporation of amino acids at the selenocysteine-specific UGA-codon [169]. Statins were also found to decrease the expression of some selenoproteins, including GPx1 [170, 171] due to their effects on the biosynthesis of isopentyl pyrophosphate. Statins inhibit the rate-limiting step of cholesterol biosynthesis, blocking the reduction of HMG-CoA reductase to mevalonate. Isopentyl pyrophosphate, a metabolite of mevalonate, is important for the isopentenylation of tRNASec, a modification that regulates the expression of selenoproteins [172]. It has been suggested that the statin-induced decrease of antioxidant selenoprotein expression in muscle may contribute to oxidant stress-induced myotoxicity, which is a known side-effect of statins [172]. It is not clear why this side-effect of statins occurs in some but not all patients taking this medication; however, one possibility is that individuals with low selenium may be more susceptible to statin-induced decreases in selenoproteins [173].
Overview
Studies in human subjects and animal models highlight the role of the selenoenzyme GPx1 in many physiological functions. By modulating cellular ROS, GPx1 protects against the development of many diseases. GPx1 has an important role both in mitochondria as well as in cytoplasm in removing hydrogen peroxide and prevented ROS-mediated ROS production. Interestingly, only a few studies have examined the role of GPx1 in modulating mitochondrial ROS production. At baseline, GPx1 deficient cardiomyocyte and liver mitochondria show increased mitochondrial ROS production [41, 174], with measurable alterations in respiration in liver mitochondria. In human Chang liver cells, overexpression of GPx1 was found to decrease ATP production and mitochondrial ROS-mediated functions such as disulfide bond formation and growth factor-mediated signaling [16]. GPx1 deficiency was found to promote mitochondrial dysfunction in response to stress, such as I/R and doxorubicin exposure in cardiomyocytes and in trauma models in brain, whereas GPx1 overexpression limited the mitochondrial respiratory stress [41, 43, 44, 114]. Mitochondrial bioenergetics is important for the function of the heart and brain, however, in most other model systems discussed in this review the role of mitochondria have not been examined.
Cardiovascular studies provide the most complete picture of the role of GPx1 in health and disease. In cardiomyocytes GPx1 preserves mitochondrial function and protects against cardiac dysfunction in response to many physiological stressors (such as in ischemia/reperfusion [41]), as well as with cardiotoxins [43, 44]. Cardiac function in response to other stressors such as AII [42] or viral agents [28] is also preserved by GPx1 overexpression. In humans, GPx1 is one (of many) selenoprotein(s) that may play a role in Keshan disease, a cardiomyopathy which is found in areas of China with low selenium levels [23]. A combination of low selenium and genetics may play a role in the susceptibility to Keshan disease as individuals carrying the Leu198 variant of GPX1, which is associated with lower expression, especially in the context of lower selenium levels, are at greater risk of developing Keshan disease [36]. Studies in primary endothelial cells grown in culture, as well as in animal models illustrate that a deficiency of GPx1 alters endothelial dependent vasodilation by regulating bioavailable nitric oxide [39, 60, 61, 70, 71]. Deficiency of GPx1 also promotes pro-inflammatory activation of endothelial and other cell types, and accelerates atherogenesis in susceptible animal models [6, 78, 79, 84, 85]. In human subjects, erythrocyte GPX1 activity is correlated with the risk of future cardiovascular events [86], and the Leu198 allele has been associated with increased risk of cardiovascular disease [89–92]. Furthermore, animal studies suggest that a deficiency of GPx1 promotes VSMC proliferation after stenting [93], consistent with findings in human subjects that found the Leu allele was associated with lower erythrocyte GPX1 activity and an increased risk of restenosis [94].
Additional evidence for a protective role of GPx1 can be in many other disease models, including stroke [106–109], and neurodegeneration caused by trauma [114, 115], neurotoxins [116], and AD [123, 124]; however, far less information exists about how GPx1 influences these outcomes in humans. For stroke, a trial of ebselen treatment and stroke showed some promising results of ebselen in improving outcomes [110], however this study has not been replicated and there is no clear association of stroke risk or outcomes with GPx1 polymorphisms [111, 112]. In AD, the genetic studies that have been reported show opposite results regarding the association of the Pro198Leu (rs1050450) polymorphism with AD [127, 128].
The complex findings regarding GPx1 in diabetes and cancer can be related in part to the heterogeneity of these disorders. Thus, with metabolic function, it appears that too much and too little GPx1 can alter the response of insulin target cells in liver and skeletal muscle to attenuate (GPx1 overexpression) [100] or enhance (GPx1 knockdown) [97] insulin-mediated signaling. GPx1 overexpression and knockdown also cause changes in the β-cell, altering the production of insulin. Thus, GPX1 overexpressing β-cells produce more insulin, whereas β-cell mass is reduced in GPx1 deficient mice, leading to hypoinsulinemia and decreased glucose-stimulated insulin secretion [96]. In humans, high selenium supplementation (which may increase the expression of GPx1, as well as that of other selenoproteins) was associated with increased risk of type 2 diabetes [103, 104].
In cancer, GPx1 is thought to protect against cancer initiation, but in tumor cells, the role of GPx1 is not always protective. Thus, excess GPx1 can promote cancer cells survival in a number of cancers by a number of different mechanisms, including promoting cell survival pathways, such as those mediated by Akt, and inhibiting apoptotic cell death [142–144, 148]. In breast cancer and prostate cancer there was no consensus finding of association of the Pro198Leu (rs1050450) polymorphism with these cancers among various studies [150–154]; however, in bladder cancer the Leu allele was associated with increased risk in meta-analyses [155, 156].
Normal expression of GPx1, however, is associated overwhelmingly with protection, in that normal expression of GPx1 results in less severe consequences than conditions with GPx1 deficiency in many diseases and, in diseases where ROS-mediated damage plays a crucial role in the pathogenesis, excess GPx1 is beneficial. Only in a limited context is a loss of GPx1 beneficial. GPx1 knockout was found to attenuate epileptic seizures in response to kainic acid due to an increased oxidation of redox-sensitive thiols in NMDA receptors that block their activity [117, 118]. However, brains of GPx1 deficient mice have increased oxidative stress at baseline, and increased production of ROS in response to kainic acid. GPx1 deficiency may also (partially) protect liver hepatocytes against acetaminophen-mediated toxicity by mechanisms that decrease protein nitration and cell death, whereas GPx1 overexpressing mice are more sensitive to acetaminophen-mediated hepatotoxicity, in part due to an exhaustion of GSH [175]. Mitochondria isolated from livers of GPx1 deficient mice, however, were found to have an increased release of hydrogen peroxide, with a decreased respiratory control ratio [174]. As mentioned above, increased sensitivity to insulin-mediated signaling in GPx1 knockout mice is offset by increased ROS in the pancreas and decreased plasma insulin in these mice. These findings show how difficult it is to predict the effects, even of a protective, antioxidant enzyme, in every situation (see also [175] and references therein). Our growing understanding of redox-mediated cellular events, however, suggest that some ROS is essential for normal cellular processes, such as disulfide bond formation and growth factor receptor-mediated signaling, which requires oxidative inactivation of certain phosphatases. Therefore, it is not surprising that excess GPx1 may adversely alter other (normal) cellular processes. Thus, excess GPx1 was found to attenuate hydrogen peroxide-mediated vasodilation responses [74] and attenuate ROS-mediated signaling to affect insulin sensitivity and promote type 2 diabetes in a mouse model system [100]. Reductive stress, caused in part by excess GPx1 and enhanced GPx1 activity is also associated with protein aggregation-induced myopathies [47, 50]. Furthermore, in some cancers, excess GPx1 may allow for cell survival and promote cell growth by decreasing apoptosis or by stabilizing the activity of oncogenic proteins [142–144, 148] (Figure 6). These negative effects of GPx1 overexpression, however, are associated with prolonged upregulation of GPx1 or its activity, as found in GPx1 transgenic models, or in models with sustained activation of Nrf2 to foster increased production and recycling of GSH. A similar dysregulation of GPx1 expression concurrent with other oncogenic changes may contribute to a role for other mechanisms in cancer cells.
Figure 6.
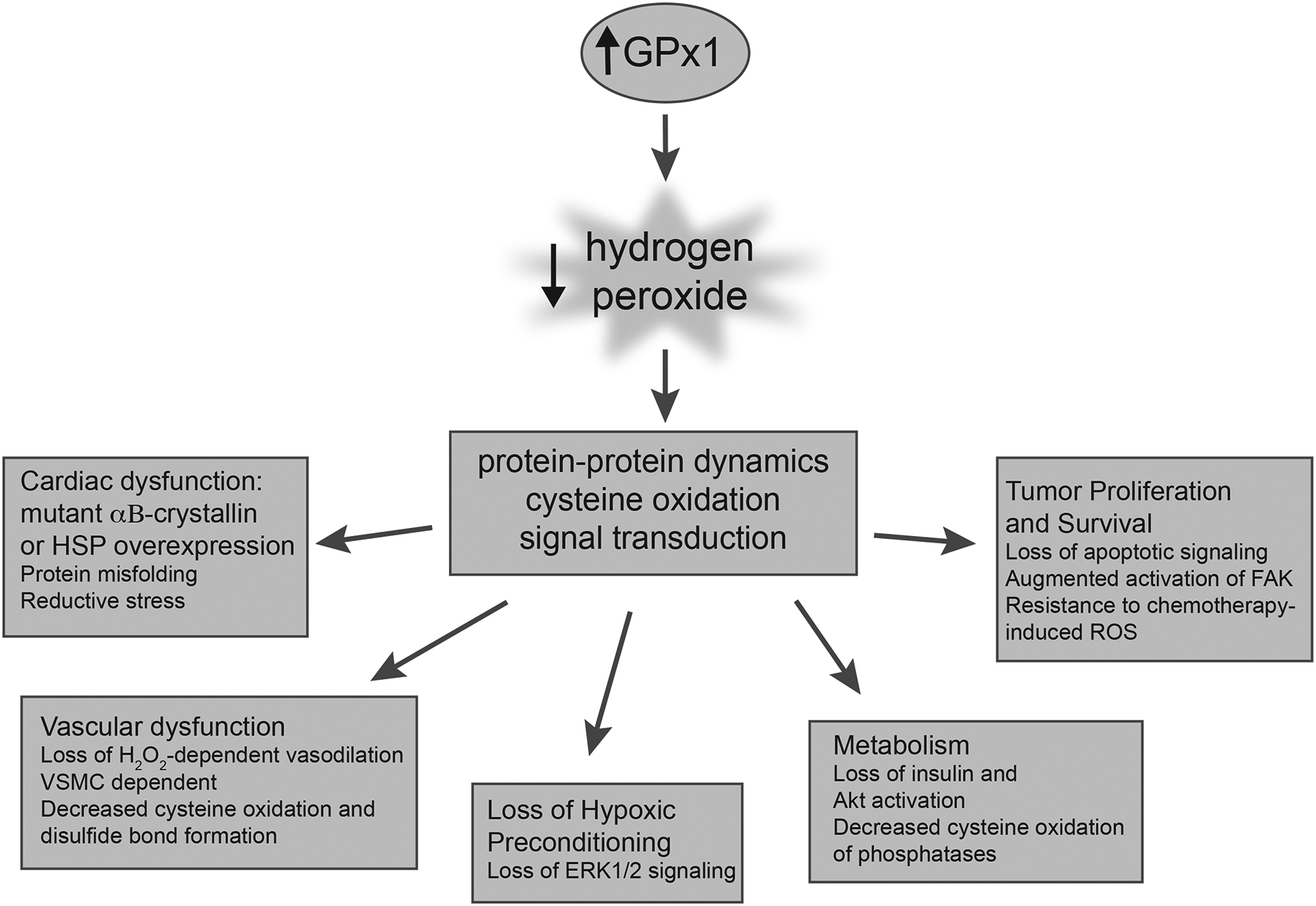
Excess GPx1 and dysfunction and disease. Although, GPx1 protects against dysfunction and disease in many biological systems, in some systems the effect of excess GPx1 is not beneficial. The effects of GPx1 are dependent on many factors including cellular redox state, signaling pathway, and biological or disease model. In some cases, excess GPx1 can eliminate essential ROS or create reductive stress to augment pathological effects.
Taken together, these findings suggest that there is an exquisite balance between necessary and harmful cellular oxidants and that GPx1 plays a crucial role in modulating this balance. As discussed in this review, in many pathophysiological conditions GPx1 plays a protective role and excess GPx1 can attenuate disease development and progression, however, evidence also suggests that the role of GPx1 can differ in other cellular contexts.
Highlights.
GPx1 is a selenoenzyme that reduces hydrogen peroxide and lipid hydroperoxides.
Selenium availability and translational mechanisms regulate the expression of GPx1.
GPx1 modulates the balance between necessary and harmful levels of H2O2.
Removal of essential reactive oxygen species can promote dysfunction and disease.
Overall, GPx1 protects against the progression and development of many diseases.
Acknowledgements
We thank Stephanie Tribuna for her assistance with manuscript preparation. This work was supported by the National Institutes of Health [grant numbers HG007690, HL061795, HL155107, GM107618].
Abbreviations used:
- Aβ
amyloid beta
- AD
Alzheimer disease
- Akt
protein kinase B
- AP-1
activator protein 1
- ApoE−/−
apolipoprotein E-deficient mice
- ARE
antioxidant response element
- ASK1
apoptosis signal-regulating kinase 1
- BH4
tetrahydrobiopterin
- CAD
coronary artery disease
- CBS+/−
mice heterozygous deficient for cystathionine beta-synthase
- ERK1/2
extracellular signal-regulated kinase 1/2
- eNOS
endothelial nitric oxide synthase
- FAK
focal adhesion kinase
- GPx1
glutathione peroxidase 1
- GPx1+/+
GPx1 wild type mice
- GPx1−/−
homozygous GPx1 knockout mice
- GPx1+/−
heterozygous GPx1 knockout mice
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- ICAM1
intercellular adhesion molecule 1
- I/R
ischemia reperfusion
- JNK
c-Jun N-terminal kinase
- Keap1
Kelch-like ECH-associated protein 1
- MAPK
mitogen-activated protein kinase
- MCAO
middle cerebral artery occlusion
- mcm5U
5-methoxy-carbonylmethyluridine
- mcm5Um
5-methoxy-carbonylmethyl-2’-O-methyluridine
- MCSF
macrophage colony stimulating factor
- NFκB
nuclear factor kappa B
- NMDA
N-methyl-D-aspartate
- NOX1
NADPH oxidase 1
- NOX2
NADPH oxidase 2
- NOX4
NADPH oxidase 4
- Nrf2
nuclear factor erythroid 2-related factor 2
- OTC
L-2-oxothiazolidine-4-carboxylic acid
- oxLDL
oxidized low-density lipoproteins
- PGC1α
peroxisome proliferator-activated receptor-γ-coactivator 1α
- PI3K
phosphatidylinositol 3-kinase
- PKC βII
protein kinase C beta II
- ROS
reactive oxygen species
- ROS1
ROS proto-oncogene 1, receptor tyrosine kinase
- SBP2
SECIS binding protein 2
- Sec
selenocysteine
- SECIS
selenocysteine incorporation sequence
- SHP-2
SH2 domain-containing protein tyrosine phosphatase-2
- TNFα
tumor necrosis factor alpha
- TRAF
tumor necrosis factor receptor-associated factor
- VCAM1
vascular cell adhesion molecule 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Flohé L, Günzler WA, Schock HH, Glutathione peroxidase: a selenoenzyme. FEBS Lett. 32:132–4; 1973. [DOI] [PubMed] [Google Scholar]
- [2].Lubos E, Loscalzo J, Handy DE, Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 15:1957–97; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fourquet S, Guerois R, Biard D, Toledano MB, Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem. 285:8463–71; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kabe Y, Ando K, Hirao S, Yoshida M, Handa H, Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 7:395–403; 2005. [DOI] [PubMed] [Google Scholar]
- [5].Yin Z, Machius M, Nestler EJ, Rudenko G, Activator Protein-1: redox switch controlling structure and DNA-binding. Nucleic Acids Res. 45:11425–11436; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lubos E, Kelly NJ, Oldebeken SR, Leopold JA, Zhang YY, Loscalzo J, Handy DE, Glutathione peroxidase-1 deficiency augments proinflammatory cytokine-induced redox signaling and human endothelial cell activation. J Biol Chem. 286:35407–17; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Senoner T, Dichtl W, Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients. 11: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Forman HJ, Zhang H, Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 20:689–709; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anrather J, Racchumi G, Iadecola C, NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 281:5657–67; 2006. [DOI] [PubMed] [Google Scholar]
- [10].Wu W, Li L, Su X, Zhu Z, Lin X, Zhang J, Zhuang Z, Cai H, Huang W, Nuclear factor-kappaB regulates the transcription of NADPH oxidase 1 in human alveolar epithelial cells. BMC Pulm Med. 21:98; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chalupsky K, Cai H, Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 102:9056–61; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG, Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 111:1201–9; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG, Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler Thromb Vasc Biol. 25:1623–8; 2005. [DOI] [PubMed] [Google Scholar]
- [14].Hauck AK, Bernlohr DA, Oxidative stress and lipotoxicity. J Lipid Res. 57:1976–1986; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zorov DB, Juhaszova M, Sollott SJ, Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 94:909–50; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Handy DE, Lubos E, Yang Y, Galbraith JD, Kelly N, Zhang YY, Leopold JA, Loscalzo J, Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 284:11913–21; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Handy DE, Loscalzo J, Responses to reductive stress in the cardiovascular system. Free Radic Biol Med. 109:114–124; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sunde RA, Raines AM, Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents. Adv Nutr. 2:138–50; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burk RF, Hill KE, Regulation of selenium metabolism and transport. Annu Rev Nutr. 35:109–34; 2015. [DOI] [PubMed] [Google Scholar]
- [20].Touat-Hamici Z, Bulteau AL, Bianga J, Jean-Jacques H, Szpunar J, Lobinski R, Chavatte L, Selenium-regulated hierarchy of human selenoproteome in cancerous and immortalized cells lines. Biochim Biophys Acta Gen Subj. 1862:2493–2505; 2018. [DOI] [PubMed] [Google Scholar]
- [21].Handy DE, Joseph J, Loscalzo J, Selenium, a micronutrient that modulates cardiovascular health via redox enzymology. Nutrients. 13:3238, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Joseph J, Selenium and cardiometabolic health: inconclusive yet intriguing evidence. Am J Med Sci. 346:216–20; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ge K, Xue A, Bai J, Wang S, Keshan disease-an endemic cardiomyopathy in China. Virchows Arch A Pathol Anat Histopathol. 401:1–15; 1983. [DOI] [PubMed] [Google Scholar]
- [24].Fuyu Y, Keshan disease and mitochondrial cardiomyopathy. Sci China C Life Sci. 49:513–8; 2006. [DOI] [PubMed] [Google Scholar]
- [25].Pei J, Fu W, Yang L, Zhang Z, Liu Y, Oxidative stress is involved in the pathogenesis of Keshan disease (an endemic dilated cardiomyopathy) in China. Oxid Med Cell Longev. 2013:474203; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ilback NG, Fohlman J, Friman G, Effects of selenium supplementation on virus-induced inflammatory heart disease. Biol Trace Elem Res. 63:51–66; 1998. [DOI] [PubMed] [Google Scholar]
- [27].Levander OA, Beck MA, Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol Trace Elem Res. 56:5–21; 1997. [DOI] [PubMed] [Google Scholar]
- [28].Beck MA, Esworthy RS, Ho YS, Chu FF, Glutathione peroxidase protects mice from viral-induced myocarditis. FASEB J. 12:1143–9; 1998. [DOI] [PubMed] [Google Scholar]
- [29].Yatmaz S, Seow HJ, Gualano RC, Wong ZX, Stambas J, Selemidis S, Crack PJ, Bozinovski S, Anderson GP, Vlahos R, Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation. Am J Respir Cell Mol Biol. 48:17–26; 2013. [DOI] [PubMed] [Google Scholar]
- [30].Zhou H, Wang T, Li Q, Li D, Prevention of Keshan disease by selenium supplementation: a systematic review and meta-analysis. Biol Trace Elem Res. 186:98–105; 2018. [DOI] [PubMed] [Google Scholar]
- [31].Zhu YH, Wang XF, Yang G, Wei J, Tan WH, Wang LX, Guo X, Lammi MJ, Xu JH, Efficacy of long-term selenium supplementation in the treatment of chronic Keshan disease with congestive heart failure. Curr Med Sci. 39:237–242; 2019. [DOI] [PubMed] [Google Scholar]
- [32].Alfthan G, Xu GL, Tan WH, Aro A, Wu J, Yang YX, Liang WS, Xue WL, Kong LH, Selenium supplementation of children in a selenium-deficient area in China: blood selenium levels and glutathione peroxidase activities. Biol Trace Elem Res. 73:113–25; 2000. [DOI] [PubMed] [Google Scholar]
- [33].Alehagen U, Aaseth J, Alexander J, Johansson P, Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS One. 13:e0193120; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kuria A, Tian H, Li M, Wang Y, Aaseth JO, Zang J, Cao Y, Selenium status in the body and cardiovascular disease: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 61:3616–25; 2021. [DOI] [PubMed] [Google Scholar]
- [35].Yan C, Luo R, Li F, Liu M, Li J, Hua W, Li X, The epidemiological status, environmental and genetic factors in the etiology of Keshan disease. Cardiovasc Endocrinol Metab. 10:14–21; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lei C, Niu X, Wei J, Zhu J, Zhu Y, Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin Chim Acta. 399:102–8; 2009. [DOI] [PubMed] [Google Scholar]
- [37].Hu YJ, Diamond AM, Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 63:3347–51; 2003. [PubMed] [Google Scholar]
- [38].Miller JC, Thomson CD, Williams SM, van Havre N, Wilkins GT, Morison IM, Ludgate JL, Skeaff CM, Influence of the glutathione peroxidase 1 Pro200Leu polymorphism on the response of glutathione peroxidase activity to selenium supplementation: a randomized controlled trial. Am J Clin Nutr. 96:923–31; 2012. [DOI] [PubMed] [Google Scholar]
- [39].Forgione MA, Cap A, Liao R, Moldovan NI, Eberhardt RT, Lim CC, Jones J, Goldschmidt-Clermont PJ, Loscalzo J, Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation. 106:1154–8; 2002. [DOI] [PubMed] [Google Scholar]
- [40].Lim CC, Bryan NS, Jain M, Garcia-Saura MF, Fernandez BO, Sawyer DB, Handy DE, Loscalzo J, Feelisch M, Liao R, Glutathione peroxidase deficiency exacerbates ischemia-reperfusion injury in male but not female myocardium: insights into antioxidant compensatory mechanisms. Am J Physiol Heart Circ Physiol. 297:H2144–53; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thu VT, Kim HK, Ha SH, Yoo JY, Park WS, Kim N, Oh GT, Han J, Glutathione peroxidase 1 protects mitochondria against hypoxia/reoxygenation damage in mouse hearts. Pflugers Arch. 460:55–68; 2010. [DOI] [PubMed] [Google Scholar]
- [42].Ardanaz N, Yang XP, Cifuentes ME, Haurani MJ, Jackson KW, Liao TD, Carretero OA, Pagano PJ, Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension. 55:116–23; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gao J, Xiong Y, Ho YS, Liu X, Chua CC, Xu X, Wang H, Hamdy R, Chua BH, Glutathione peroxidase 1-deficient mice are more susceptible to doxorubicin-induced cardiotoxicity. Biochim Biophys Acta. 1783:2020–9; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xiong Y, Liu X, Lee CP, Chua BH, Ho YS, Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free Radic Biol Med. 41:46–55; 2006. [DOI] [PubMed] [Google Scholar]
- [45].Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M, A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 20:92–5; 1998. [DOI] [PubMed] [Google Scholar]
- [46].Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ, Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 130:427–39; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, Hewett T, Robbins J, Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 89:84–91; 2001. [DOI] [PubMed] [Google Scholar]
- [48].Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C, Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal. 7:414–22; 2005. [DOI] [PubMed] [Google Scholar]
- [49].Baek SH, Min JN, Park EM, Han MY, Lee YS, Lee YJ, Park YM, Role of small heat shock protein HSP25 in radioresistance and glutathione-redox cycle. J Cell Physiol. 183:100–7; 2000. [DOI] [PubMed] [Google Scholar]
- [50].Zhang X, Min X, Li C, Benjamin IJ, Qian B, Zhang X, Ding Z, Gao X, Yao Y, Ma Y, Cheng Y, Liu L, Involvement of reductive stress in the cardiomyopathy in transgenic mice with cardiac-specific overexpression of heat shock protein 27. Hypertension. 55:1412–7; 2010. [DOI] [PubMed] [Google Scholar]
- [51].Kannan S, Muthusamy VR, Whitehead KJ, Wang L, Gomes AV, Litwin SE, Kensler TW, Abel ED, Hoidal JR, Rajasekaran NS, Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovasc Res. 100:63–73; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lincoln TM, Dey N, Sellak H, Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol (1985). 91:1421–30; 2001. [DOI] [PubMed] [Google Scholar]
- [53].Forgione MA, Leopold JA, Loscalzo J, Roles of endothelial dysfunction in coronary artery disease. Curr Opin Cardiol. 15:409–15; 2000. [DOI] [PubMed] [Google Scholar]
- [54].Jin RC, Loscalzo J, Vascular nitric oxide: formation and function. J Blood Med. 2010:147–162; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kietadisorn R, Juni RP, Moens AL, Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 302:E481–95; 2012. [DOI] [PubMed] [Google Scholar]
- [56].Lubos E, Loscalzo J, Handy DE, Homocysteine and glutathione peroxidase-1. Antioxid Redox Signal. 9:1923–40; 2007. [DOI] [PubMed] [Google Scholar]
- [57].Shi Y, Vanhoutte PM, Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 9:434–449; 2017. [DOI] [PubMed] [Google Scholar]
- [58].Upchurch GR Jr., Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF Jr., Loscalzo J, Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 272:17012–7; 1997. [DOI] [PubMed] [Google Scholar]
- [59].Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt-Clermont PJ, Farber HW, Cohen R, Loscalzo J, Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 106:483–91; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Weiss N, Zhang YY, Heydrick S, Bierl C, Loscalzo J, Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc Natl Acad Sci U S A. 98:12503–8; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Weiss N, Heydrick S, Zhang YY, Bierl C, Cap A, Loscalzo J, Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler Thromb Vasc Biol. 22:34–41; 2002. [DOI] [PubMed] [Google Scholar]
- [62].Chen M, Geng JG, P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp (Warsz). 54:75–84; 2006. [DOI] [PubMed] [Google Scholar]
- [63].Tvaroska I, Selvaraj C, Koca J, Selectins-The Two Dr. Jekyll and Mr. Hyde Faces of Adhesion Molecules-A Review. Molecules. 25:2835; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gonzalez-Flores JN, Shetty SP, Dubey A, Copeland PR, The molecular biology of selenocysteine. Biomol Concepts. 4:349–65; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bulteau AL, Chavatte L, Update on selenoprotein biosynthesis. Antioxid Redox Signal. 23:775–94; 2015. [DOI] [PubMed] [Google Scholar]
- [66].Xu XM, Carlson BA, Mix H, Zhang Y, Saira K, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL, Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 5:e4; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Carlson BA, Xu XM, Gladyshev VN, Hatfield DL, Selective rescue of selenoprotein expression in mice lacking a highly specialized methyl group in selenocysteine tRNA. J Biol Chem. 280:5542–8; 2005. [DOI] [PubMed] [Google Scholar]
- [68].Handy DE, Zhang Y, Loscalzo J, Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem. 280:15518–25; 2005. [DOI] [PubMed] [Google Scholar]
- [69].Barroso M, Florindo C, Kalwa H, Silva Z, Turanov AA, Carlson BA, de Almeida IT, Blom HJ, Gladyshev VN, Hatfield DL, Michel T, Castro R, Loscalzo J, Handy DE, Inhibition of cellular methyltransferases promotes endothelial cell activation by suppressing glutathione peroxidase 1 protein expression. J Biol Chem. 289:15350–62; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dayal S, Brown KL, Weydert CJ, Oberley LW, Arning E, Bottiglieri T, Faraci FM, Lentz SR, Deficiency of glutathione peroxidase-1 sensitizes hyperhomocysteinemic mice to endothelial dysfunction. Arterioscler Thromb Vasc Biol. 22:1996–2002; 2002. [DOI] [PubMed] [Google Scholar]
- [71].Forgione MA, Weiss N, Heydrick S, Cap A, Klings ES, Bierl C, Eberhardt RT, Farber HW, Loscalzo J, Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 282:H1255–61; 2002. [DOI] [PubMed] [Google Scholar]
- [72].Oelze M, Kroller-Schon S, Steven S, Lubos E, Doppler C, Hausding M, Tobias S, Brochhausen C, Li H, Torzewski M, Wenzel P, Bachschmid M, Lackner KJ, Schulz E, Munzel T, Daiber A, Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 63:390–6; 2014. [DOI] [PubMed] [Google Scholar]
- [73].Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM, Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension. 51:872–7; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Modrick ML, Didion SP, Lynch CM, Dayal S, Lentz SR, Faraci FM, Role of hydrogen peroxide and the impact of glutathione peroxidase-1 in regulation of cerebral vascular tone. J Cereb Blood Flow Metab. 29:1130–7; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Shimokawa H, Godo S, Nitric oxide and endothelium-dependent hyperpolarization mediated by hydrogen peroxide in health and disease. Basic Clin Pharmacol Toxicol. 127:92–101; 2020. [DOI] [PubMed] [Google Scholar]
- [76].Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H, Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch. 467:285–97; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K, Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 98:254–61; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Forstermann U, Munzel T, Lackner KJ, Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 27:850–7; 2007. [DOI] [PubMed] [Google Scholar]
- [79].Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME, de Haan JB, Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 115:2178–87; 2007. [DOI] [PubMed] [Google Scholar]
- [80].Cheng F, Torzewski M, Degreif A, Rossmann H, Canisius A, Lackner KJ, Impact of glutathione peroxidase-1 deficiency on macrophage foam cell formation and proliferation: implications for atherogenesis. PLoS One. 8:e72063; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chew P, Yuen DY, Koh P, Stefanovic N, Febbraio MA, Kola I, Cooper ME, de Haan JB, Site-specific antiatherogenic effect of the antioxidant ebselen in the diabetic apolipoprotein E-deficient mouse. Arterioscler Thromb Vasc Biol. 29:823–30; 2009. [DOI] [PubMed] [Google Scholar]
- [82].Beckman JA, Goldfine AB, Leopold JA, Creager MA, Ebselen does not improve oxidative stress and vascular function in patients with diabetes: a randomized, crossover trial. Am J Physiol Heart Circ Physiol. 311:H1431–H1436; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Montero D, Walther G, Stehouwer CD, Houben AJ, Beckman JA, Vinet A, Effect of antioxidant vitamin supplementation on endothelial function in type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 15:107–16; 2014. [DOI] [PubMed] [Google Scholar]
- [84].Lubos E, Mahoney CE, Leopold JA, Zhang YY, Loscalzo J, Handy DE, Glutathione peroxidase-1 modulates lipopolysaccharide-induced adhesion molecule expression in endothelial cells by altering CD14 expression. FASEB J. 24:2525–32; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sharma A, Yuen D, Huet O, Pickering R, Stefanovic N, Bernatchez P, de Haan JB, Lack of glutathione peroxidase-1 facilitates a pro-inflammatory and activated vascular endothelium. Vascul Pharmacol. 79:32–42; 2016. [DOI] [PubMed] [Google Scholar]
- [86].Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ, AtheroGene I, Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 349:1605–13; 2003. [DOI] [PubMed] [Google Scholar]
- [87].Schnabel R, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Torzewski M, Lubos E, Bickel C, Cambien F, Tiret L, Munzel T, Blankenberg S, Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene study. J Am Coll Cardiol. 45:1631–7; 2005. [DOI] [PubMed] [Google Scholar]
- [88].Ahmad A, Corban MT, Toya T, Sara JD, Lerman B, Park JY, Lerman LO, Lerman A, Coronary microvascular endothelial dysfunction in patients with angina and nonobstructive coronary artery disease Is associated with elevated serum homocysteine levels. J Am Heart Assoc. 9:e017746; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tang NP, Wang LS, Yang L, Gu HJ, Sun QM, Cong RH, Zhou B, Zhu HJ, Wang B, Genetic variant in glutathione peroxidase 1 gene is associated with an increased risk of coronary artery disease in a Chinese population. Clin Chim Acta. 395:89–93; 2008. [DOI] [PubMed] [Google Scholar]
- [90].Ye H, Li X, Wang L, Liao Q, Xu L, Huang Y, Xu L, Xu X, Chen C, Wu H, Le Y, Liu Q, Ye M, Dong C, Duan S, Genetic associations with coronary heart disease: meta-analyses of 12 candidate genetic variants. Gene. 531:71–7; 2013. [DOI] [PubMed] [Google Scholar]
- [91].Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, Sakagashira S, Nishi M, Sasaki H, Sanke T, Nanjo K, Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes. 53:2455–60; 2004. [DOI] [PubMed] [Google Scholar]
- [92].Wickremasinghe D, Peiris H, Chandrasena LG, Senaratne V, Perera R, Case control feasibility study assessing the association between severity of coronary artery disease with Glutathione Peroxidase-1 (GPX-1) and GPX-1 polymorphism (Pro198Leu). BMC Cardiovasc Disord. 16:111; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shuvalova YA, Kaminnyi AI, Meshkov AN, Shirokov RO, Samko AN, Association between polymorphisms of eNOS and GPx-1 genes, activity of free-radical processes and in-stent restenosis. Mol Cell Biochem. 370:241–9; 2012. [DOI] [PubMed] [Google Scholar]
- [94].Ali ZA, de Jesus Perez V, Yuan K, Orcholski M, Pan S, Qi W, Chopra G, Adams C, Kojima Y, Leeper NJ, Qu X, Zaleta-Rivera K, Kato K, Yamada Y, Oguri M, Kuchinsky A, Hazen SL, Jukema JW, Ganesh SK, Nabel EG, Channon K, Leon MB, Charest A, Quertermous T, Ashley EA, Oxido-reductive regulation of vascular remodeling by receptor tyrosine kinase ROS1. J Clin Invest. 124:5159–74; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].de Haan JB, Limiting reductive stress for treating in-stent stenosis: the heart of the matter? J Clin Invest. 124:5092–4; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Huang JQ, Zhou JC, Wu YY, Ren FZ, Lei XG, Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases. Free Radic Biol Med. 127:108–115; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wang X, Vatamaniuk MZ, Roneker CA, Pepper MP, Hu LG, Simmons RA, Lei XG, Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal. 14:391–401; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Merry TL, Tran M, Stathopoulos M, Wiede F, Fam BC, Dodd GT, Clarke I, Watt MJ, Andrikopoulos S, Tiganis T, High-fat-fed obese glutathione peroxidase 1-deficient mice exhibit defective insulin secretion but protection from hepatic steatosis and liver damage. Antioxid Redox Signal. 20:2114–29; 2014. [DOI] [PubMed] [Google Scholar]
- [99].Merry TL, Tran M, Dodd GT, Mangiafico SP, Wiede F, Kaur S, McLean CL, Andrikopoulos S, Tiganis T, Hepatocyte glutathione peroxidase-1 deficiency improves hepatic glucose metabolism and decreases steatohepatitis in mice. Diabetologia. 59:2632–44; 2016. [DOI] [PubMed] [Google Scholar]
- [100].McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG, Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 101:8852–7; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG, Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 51:1515–24; 2008. [DOI] [PubMed] [Google Scholar]
- [102].Harmon JS, Bogdani M, Parazzoli SD, Mak SS, Oseid EA, Berghmans M, Leboeuf RC, Robertson RP, beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 150:4855–62; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Vinceti M, Filippini T, Rothman KJ, Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Epidemiol. 33:789–810; 2018. [DOI] [PubMed] [Google Scholar]
- [104].Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E, Serum selenium concentrations and diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect. 117:1409–13; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN, Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 14:2327–36; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Crack PJ, Taylor JM, Flentjar NJ, de Haan J, Hertzog P, Iannello RC, Kola I, Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem. 78:1389–99; 2001. [DOI] [PubMed] [Google Scholar]
- [107].Ishibashi N, Prokopenko O, Weisbrot-Lefkowitz M, Reuhl KR, Mirochnitchenko O, Glutathione peroxidase inhibits cell death and glial activation following experimental stroke. Brain Res Mol Brain Res. 109:34–44; 2002. [DOI] [PubMed] [Google Scholar]
- [108].Crack PJ, Taylor JM, Ali U, Mansell A, Hertzog PJ, Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke. 37:1533–8; 2006. [DOI] [PubMed] [Google Scholar]
- [109].Wong CH, Bozinovski S, Hertzog PJ, Hickey MJ, Crack PJ, Absence of glutathione peroxidase-1 exacerbates cerebral ischemia-reperfusion injury by reducing post-ischemic microvascular perfusion. J Neurochem. 107:241–52; 2008. [DOI] [PubMed] [Google Scholar]
- [110].Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H, Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 29:12–7; 1998. [DOI] [PubMed] [Google Scholar]
- [111].Karahalil B, Elkama A, Orhan G, Oxidative stress gene polymorphisms may have an impact in the development of ischemic stroke. J Gene Med. 19:(3), DOI: 10.1002/jgm.2947; 2017. [DOI] [PubMed] [Google Scholar]
- [112].Forsberg L, de Faire U, Marklund SL, Andersson PM, Stegmayr B, Morgenstern R, Phenotype determination of a common Pro-Leu polymorphism in human glutathione peroxidase 1. Blood Cells Mol Dis. 26:423–6; 2000. [DOI] [PubMed] [Google Scholar]
- [113].Bhowmick S, D’Mello V, Caruso D, Abdul-Muneer PM, Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J Mol Med (Berl). 97:1627–41; 2019. [DOI] [PubMed] [Google Scholar]
- [114].Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS, The protective role of cellular glutathione peroxidase against trauma-induced mitochondrial dysfunction in the mouse brain. J Stroke Cerebrovasc Dis. 13:129–37; 2004. [DOI] [PubMed] [Google Scholar]
- [115].Flentjar NJ, Crack PJ, Boyd R, Malin M, de Haan JB, Hertzog P, Kola I, Iannello R, Mice lacking glutathione peroxidase-1 activity show increased TUNEL staining and an accelerated inflammatory response in brain following a cold-induced injury. Exp Neurol. 177:9–20; 2002. [DOI] [PubMed] [Google Scholar]
- [116].Lei XG, Cheng WH, McClung JP, Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr. 27:41–61; 2007. [DOI] [PubMed] [Google Scholar]
- [117].Boonplueang R, Akopian G, Stevenson FF, Kuhlenkamp JF, Lu SC, Walsh JP, Andersen JK, Increased susceptibility of glutathione peroxidase-1 transgenic mice to kainic acid-related seizure activity and hippocampal neuronal cell death. Exp Neurol. 192:203–14; 2005. [DOI] [PubMed] [Google Scholar]
- [118].Jiang D, Akopian G, Ho YS, Walsh JP, Andersen JK, Chronic brain oxidation in a glutathione peroxidase knockout mouse model results in increased resistance to induced epileptic seizures. Exp Neurol. 164:257–68; 2000. [DOI] [PubMed] [Google Scholar]
- [119].Berg M, Bruhn T, Johansen FF, Diemer NH, Kainic acid-induced seizures and brain damage in the rat: different effects of NMDA- and AMPA receptor antagonists. Pharmacol Toxicol. 73:262–8; 1993. [DOI] [PubMed] [Google Scholar]
- [120].Aizenman E, Hartnett KA, Reynolds IJ, Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron. 5:841–6; 1990. [DOI] [PubMed] [Google Scholar]
- [121].Sucher NJ, Lipton SA, Redox modulatory site of the NMDA receptor-channel complex: regulation by oxidized glutathione. J Neurosci Res. 30:582–91; 1991. [DOI] [PubMed] [Google Scholar]
- [122].Varga V, Jenei Z, Janaky R, Saransaari P, Oja SS, Glutathione is an endogenous ligand of rat brain N-methyl-D-aspartate (NMDA) and 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors. Neurochem Res. 22:1165–71; 1997. [DOI] [PubMed] [Google Scholar]
- [123].Crack PJ, Cimdins K, Ali U, Hertzog PJ, Iannello RC, Lack of glutathione peroxidase-1 exacerbates Abeta-mediated neurotoxicity in cortical neurons. J Neural Transm (Vienna). 113:645–57; 2006. [DOI] [PubMed] [Google Scholar]
- [124].Shin EJ, Chung YH, Sharma N, Nguyen BT, Lee SH, Kang SW, Nah SY, Wie MB, Nabeshima T, Jeong JH, Kim HC, Glutathione peroxidase-1 knockout facilitates memory impairment induced by beta-amyloid (1–42) in mice via inhibition of PKC betaII-mediated ERK signaling; application with glutathione peroxidase-1 gene-encoded adenovirus vector. Neurochem Res. 45:2991–3002; 2020. [DOI] [PubMed] [Google Scholar]
- [125].Medina JH, Viola H, ERK1/2: A key cellular component for the formation, retrieval, reconsolidation and persistence of memory. Front Mol Neurosci. 11:361; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Cosentino-Gomes D, Rocco-Machado N, Meyer-Fernandes JR, Cell signaling through protein kinase C oxidation and activation. Int J Mol Sci. 13:10697–721; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Paz-y-Mino C, Carrera C, Lopez-Cortes A, Munoz MJ, Cumbal N, Castro B, Cabrera A, Sanchez ME, Genetic polymorphisms in apolipoprotein E and glutathione peroxidase 1 genes in the Ecuadorian population affected with Alzheimer’s disease. Am J Med Sci. 340:373–7; 2010. [DOI] [PubMed] [Google Scholar]
- [128].da Rocha TJ, Silva Alves M, Guisso CC, de Andrade FM, Camozzato A, de Oliveira AA, Fiegenbaum M, Association of GPX1 and GPX4 polymorphisms with episodic memory and Alzheimer’s disease. Neurosci Lett. 666:32–37; 2018. [DOI] [PubMed] [Google Scholar]
- [129].Tran TV, Shin EJ, Nguyen LTT, Lee Y, Kim DJ, Jeong JH, Jang CG, Nah SY, Toriumi K, Nabeshima T, Yamada K, Kim HC, Protein kinase Cdelta gene depletion protects against methamphetamine-induced impairments in recognition memory and ERK1/2 signaling via upregulation of glutathione peroxidase-1 gene. Mol Neurobiol. 55:4136–4159; 2018. [DOI] [PubMed] [Google Scholar]
- [130].Martini F, Rosa SG, Klann IP, Fulco BCW, Carvalho FB, Rahmeier FL, Fernandes MC, Nogueira CW, A multifunctional compound ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer’s disease. J Psychiatr Res. 109:107–117; 2019. [DOI] [PubMed] [Google Scholar]
- [131].Autheman D, Sheldon RA, Chaudhuri N, von Arx S, Siegenthaler C, Ferriero DM, Christen S, Glutathione peroxidase overexpression causes aberrant ERK activation in neonatal mouse cortex after hypoxic preconditioning. Pediatr Res. 72:568–75; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Brigelius-Flohé R, Kipp A, Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 1790:1555–68; 2009. [DOI] [PubMed] [Google Scholar]
- [133].Liu J, Hinkhouse MM, Sun W, Weydert CJ, Ritchie JM, Oberley LW, Cullen JJ, Redox regulation of pancreatic cancer cell growth: role of glutathione peroxidase in the suppression of the malignant phenotype. Hum Gene Ther. 15:239–50; 2004. [DOI] [PubMed] [Google Scholar]
- [134].Meng Q, Shi S, Liang C, Liang D, Hua J, Zhang B, Xu J, Yu X, Abrogation of glutathione peroxidase-1 drives EMT and chemoresistance in pancreatic cancer by activating ROS-mediated Akt/GSK3beta/Snail signaling. Oncogene. 37:5843–57; 2018. [DOI] [PubMed] [Google Scholar]
- [135].Chen S, Su X, Mi H, Dai X, Li S, Chen S, Zhang S, Comprehensive analysis of glutathione peroxidase-1 (GPX1) expression and prognostic value in three different types of renal cell carcinoma. Transl Androl Urol. 9:2737–50; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Cheng Y, Xu T, Li S, Ruan H, GPX1, a biomarker for the diagnosis and prognosis of kidney cancer, promotes the progression of kidney cancer. Aging (Albany NY). 11:12165–12176; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhang Q, Xu H, You Y, Zhang J, Chen R, High Gpx1 expression predicts poor survival in laryngeal squamous cell carcinoma. Auris Nasus Larynx. 45:13–9; 2018. [DOI] [PubMed] [Google Scholar]
- [138].Lee JR, Roh JL, Lee SM, Park Y, Cho KJ, Choi SH, Nam SY, Kim SY, Overexpression of glutathione peroxidase 1 predicts poor prognosis in oral squamous cell carcinoma. J Cancer Res Clin Oncol. 143:2257–65; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wei J, Xie Q, Liu X, Wan C, Wu W, Fang K, Yao Y, Cheng P, Deng D, Liu Z, Identification the prognostic value of glutathione peroxidases expression levels in acute myeloid leukemia. Ann Transl Med. 8:678; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lv S, Luo H, Huang K, Zhu X, The prognostic role of glutathione peroxidase 1 and immune infiltrates in glioma investigated using public datasets. Med Sci Monit. 26:e926440; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Jardim BV, Moschetta MG, Leonel C, Gelaleti GB, Regiani VR, Ferreira LC, Lopes JR, Zuccari DA, Glutathione and glutathione peroxidase expression in breast cancer: an immunohistochemical and molecular study. Oncol Rep. 30:1119–28; 2013. [DOI] [PubMed] [Google Scholar]
- [142].Chen B, Shen Z, Wu D, Xie X, Xu X, Lv L, Dai H, Chen J, Gan X, Glutathione peroxidase 1 promotes NSCLC resistance to cisplatin via ROS-induced activation of PI3K/AKT pathway. Biomed Res Int. 2019:7640547; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Gong TT, Guo Q, Li X, Zhang TN, Liu FH, He XH, Lin B, Wu QJ, Isothiocyanate Iberin inhibits cell proliferation and induces cell apoptosis in the progression of ovarian cancer by mediating ROS accumulation and GPX1 expression. Biomed Pharmacother. 142:111533; 2021. [DOI] [PubMed] [Google Scholar]
- [144].Lee E, Choi A, Jun Y, Kim N, Yook JI, Kim SY, Lee S, Kang SW, Glutathione peroxidase-1 regulates adhesion and metastasis of triple-negative breast cancer cells via FAK signaling. Redox Biol. 29:101391; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Brigelius-Flohé R, Flohé L, Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid Redox Signal. 33:498–516; 2020. [DOI] [PubMed] [Google Scholar]
- [146].Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H, ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222–8; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H, ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 2:389–95; 1998. [DOI] [PubMed] [Google Scholar]
- [148].Lee S, Lee EK, Kang DH, Lee J, Hong SH, Jeong W, Kang SW, Glutathione peroxidase-1 regulates ASK1-dependent apoptosis via interaction with TRAF2 in RIPK3-negative cancer cells. Exp Mol Med. 53:1080–91; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Rayman MP, Selenoproteins and human health: insights from epidemiological data. Biochim Biophys Acta. 1790:1533–40; 2009. [DOI] [PubMed] [Google Scholar]
- [150].Hu J, Zhou GW, Wang N, Wang YJ, GPX1 Pro198Leu polymorphism and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 124:425–31; 2010. [DOI] [PubMed] [Google Scholar]
- [151].Meplan C, Dragsted LO, Ravn-Haren G, Tjonneland A, Vogel U, Hesketh J, Association between polymorphisms in glutathione peroxidase and selenoprotein P genes, glutathione peroxidase activity, HRT use and breast cancer risk. PLoS One. 8:e73316; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Jablonska E, Gromadzinska J, Peplonska B, Fendler W, Reszka E, Krol MB, Wieczorek E, Bukowska A, Gresner P, Galicki M, Zambrano Quispe O, Morawiec Z, Wasowicz W, Lipid peroxidation and glutathione peroxidase activity relationship in breast cancer depends on functional polymorphism of GPX1. BMC Cancer. 15:657; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Blein S, Berndt S, Joshi AD, Campa D, Ziegler RG, Riboli E, Cox DG, N.C.I. Breast, C. Prostate Cancer Cohort, Factors associated with oxidative stress and cancer risk in the Breast and Prostate Cancer Cohort Consortium. Free Radic Res. 48:380–6; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Liwei L, Wei Z, Ruifa H, Chunyu L, Association between genetic variants in glutathione peroxidase 1 gene and risk of prostate cancer: a meta-analysis. Mol Biol Rep. 39:8615–9; 2012. [DOI] [PubMed] [Google Scholar]
- [155].Men T, Zhang X, Yang J, Shen B, Li X, Chen D, Wang J, The rs1050450 C > T polymorphism of GPX1 is associated with the risk of bladder but not prostate cancer: evidence from a meta-analysis. Tumour Biol. 35:269–75; 2014. [DOI] [PubMed] [Google Scholar]
- [156].Cao M, Mu X, Jiang C, Yang G, Chen H, Xue W, Single-nucleotide polymorphisms of GPX1 and MnSOD and susceptibility to bladder cancer: a systematic review and meta-analysis. Tumour Biol. 35:759–64; 2014. [DOI] [PubMed] [Google Scholar]
- [157].Vibet S, Goupille C, Bougnoux P, Steghens JP, Gore J, Maheo K, Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radic Biol Med. 44:1483–91; 2008. [DOI] [PubMed] [Google Scholar]
- [158].Wang S, Tu J, Zhou C, Li J, Huang L, Tao L, Zhao L, The effect of Lfcin-B on non-small cell lung cancer H460 cells is mediated by inhibiting VEGF expression and inducing apoptosis. Arch Pharm Res. 38:261–71; 2015. [DOI] [PubMed] [Google Scholar]
- [159].Suzuki K, Koike H, Matsui H, Ono Y, Hasumi M, Nakazato H, Okugi H, Sekine Y, Oki K, Ito K, Yamamoto T, Fukabori Y, Kurokawa K, Yamanaka H, Genistein, a soy isoflavone, induces glutathione peroxidase in the human prostate cancer cell lines LNCaP and PC-3. Int J Cancer. 99:846–52; 2002. [DOI] [PubMed] [Google Scholar]
- [160].Hu Y, Rahlfs S, Mersch-Sundermann V, Becker K, Resveratrol modulates mRNA transcripts of genes related to redox metabolism and cell proliferation in non-small-cell lung carcinoma cells. Biol Chem. 388:207–19; 2007. [DOI] [PubMed] [Google Scholar]
- [161].Spiegelman BM, Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 287:60–3; discussion 63–9; 2007. [PubMed] [Google Scholar]
- [162].Baird L, Yamamoto M, The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 40: e00099–20; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J, Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 73:3221–47; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Arbab AAI, Lu X, Abdalla IM, Idris AA, Chen Z, Li M, Mao Y, Xu T, Yang Z, Metformin inhibits lipoteichoic acid-induced oxidative stress and inflammation through AMPK/NRF2/NF-kappaB signaling pathway in bovine mammary epithelial cells. Front Vet Sci. 8:661380; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Chen X, Xue H, Fang W, Chen K, Chen S, Yang W, Shen T, Chen X, Zhang P, Ling W, Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox Biol. 21:101068; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Liu Y, Lu F, Kang L, Wang Z, Wang Y, Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 17:63; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Roos NJ, Duthaler U, Bouitbir J, Krahenbuhl S, The uricosuric benzbromarone disturbs the mitochondrial redox homeostasis and activates the NRF2 signaling pathway in HepG2 cells. Free Radic Biol Med. 152:216–26; 2020. [DOI] [PubMed] [Google Scholar]
- [168].Handy DE, Hang G, Scolaro J, Metes N, Razaq N, Yang Y, Loscalzo J, Aminoglycosides decrease glutathione peroxidase-1 activity by interfering with selenocysteine incorporation. J Biol Chem. 281:3382–8; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Renko K, Martitz J, Hybsier S, Heynisch B, Voss L, Everley RA, Gygi SP, Stoedter M, Wisniewska M, Kohrle J, Gladyshev VN, Schomburg L, Aminoglycoside-driven biosynthesis of selenium-deficient Selenoprotein P. Sci Rep. 7:4391; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Diamond AM, Jaffe D, Murray JL, Safa AR, Samuels BL, Hatfield DL, Lovastatin effects on human breast carcinoma cells. Differential toxicity of an adriamycin-resistant derivative and influence on selenocysteine tRNAS. Biochem Mol Biol Int. 38:345–55; 1996. [PubMed] [Google Scholar]
- [171].Fuhrmeister J, Tews M, Kromer A, Moosmann B, Prooxidative toxicity and selenoprotein suppression by cerivastatin in muscle cells. Toxicol Lett. 215:219–27; 2012. [DOI] [PubMed] [Google Scholar]
- [172].Moosmann B, Behl C, Selenoproteins, cholesterol-lowering drugs, and the consequences: revisiting of the mevalonate pathway. Trends Cardiovasc Med. 14:273–81; 2004. [DOI] [PubMed] [Google Scholar]
- [173].Watanabe LM, Navarro AM, Seale LA, Intersection between obesity, dietary selenium, and statin therapy in Brazil. Nutrients. 13:2027; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Esposito LA, Kokoszka JE, Waymire KG, Cottrell B, MacGregor GR, Wallace DC, Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic Biol Med. 28:754–66; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Lei XG, Zhu JH, Cheng WH, Bao Y, Ho YS, Reddi AR, Holmgren A, Arner ES, Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol Rev. 96:307–64; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


