Abstract
Objective.
Continuous renal replacement therapy (CRRT) is widely utilized to support critically ill patients with AKI. Artificial intelligence (AI) has the potential to enhance CRRT delivery, but evidence is limited. We reviewed existing literature on the utilization of AI in CRRT with the objective of identifying current gaps in evidence and research considerations.
Methods.
We conducted a scoping review searching PubMed, OVID Embase, Web of Science, Cochrane, Scopus, and ProQuest for original papers published or translated in English (2012-2022). We included studies focusing on the development or use of AI-based tools in patients receiving CRRT.
Results.
Ten papers were identified, 6/10 (60%) published in 2021, and 6/10 (60%) focused on machine learning models to augment CRRT delivery. All innovations were in the design/early validation phase of development. Primary research interests focused on early indicators of CRRT need, clinical prognostication of mortality and kidney recovery, and identifying risk factors for mortality. Secondary research priorities included dynamic CRRT monitoring, predicting CRRT-related complications, and automated data pooling for point-of-care analysis. Identified literature gaps included prospective validation and implementation barriers, biases ascertainment, and quantifying social or AI-generated healthcare disparities.
Conclusion.
Research on AI applications to enhance CRRT delivery has grown exponentially in the last few years, but the field remains premature. There is a need to evaluate how these applications could enhance bedside decision-making capacity and assist structure and processes of CRRT delivery.
Keywords: continuous renal replacement therapy, CRRT, information technology, artificial intelligence, machine learning
1. INTRODUCTION
More than half of critically ill patients admitted to intensive care units (ICU) suffer from acute kidney injury (AKI), and about 10% of these patients require renal replacement therapy (RRT). Up to 75% of RRT in the ICU is delivered as continuous RRT (CRRT).1 CRRT is preferred for patients with hemodynamic instability and hypercatabolic states to better achieve fluid management goals and solute control.1,2 Despite advances in RRT technologies, the overall mortality rate for ICU patients on RRT is over 50%.1 Moreover, delivery of CRRT is costly, requires specific protocols, equipment, monitoring, and training, as well as multi-specialty collaborations.2,3 Importantly, demand for CRRT is increasing due to expansion of ICU capacity, in part exacerbated by the COVID-19 pandemic, but also due to the aging US population2 and the increased recognition of its utility in patients receiving other type of extracorporeal organ support.4 While expert consensus has identified candidate CRRT quality indicators,5–7 prospective validation of these performance indicators to assess structure, processes and outcomes of CRRT delivery is needed.8
Digital health is a broad term for the adoption of various digital technologies in healthcare, including wearable devices, telemedicine, and mobile health platforms.9 On the other hand, Artificial intelligence (AI) is a subset of data science, which refers to the replacement of the human intellect through a set of coding algorithms that enable computers to perceive, reason, and respond to a given dataset or stimuli.10–12 The term is often interchanged with a similar concept, augmented intelligence, which focuses on ‘augmenting’ human intellect rather than replacing it.10 Both digital health and AI/augmented intelligence have propelled virtually all aspects of medical practice, including medical diagnostics, risk-stratification, identification of therapeutic or procedural candidates, monitoring and anticipating treatment complications, dynamic data acquisition and point-of-care analytics for clinical decision support, remote delivery of health services, quality improvement, and healthcare resource optimization such as machine allocation and patient scheduling, and more.11,13,14
Whereas these advances are well documented in other fields such as radiology,15 molecular biology,11 and even nephrology,16 the state of AI and digital health in the CRRT space remains unknown and understudied.13 In this review, we summarize the available literature on the current state of AI and digital health research in the CRRT domain, provide insights on research trends, identify developmental gaps based on the available literature, and establish future research priorities for accelerated enhancement of the field.
2. POTENTIAL AREAS OF CRRT DELIVERY THAT CAN BE IMPROVED WITH AI
Figure 1 summarizes potential applications of AI to improve CRRT delivery and quality assurance.
Figure 1.
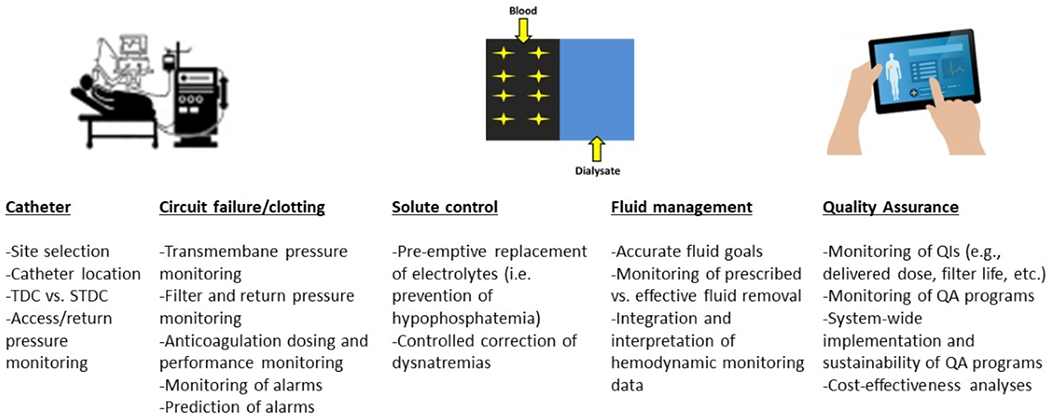
Potential applications of AI to improve CRRT delivery and quality assurance.
CRRT = Continuous Renal Replacement Therapy; TDC = Tunneled Dialysis Catheter; STDC = Short-Term Dialysis Catheter; QI = Quality Indicator; QA = Quality Assurance.
Access.
Access-related complications remain a common issue in establishing effective and sustained CRRT delivery. Short-term dialysis catheters (STDC’s) are the main catheters used for CRRT unless patients are expected to require CRRT for 3 weeks or more.17 Suboptimal positioning, bending and thrombosis not only result in catheter dysfunction and STDC failure necessitating replacement of the catheter,17 but is also the major cause of premature CRRT circuit failure.18 Moreover, the use of Tunneled Dialysis Catheters (TDCs) for CRRT has not been validated in clinical trials yet are postulated to have fewer complications than STDCs.17,19 Ruiz et al. reported 43% fewer access alarms with prioritization of right internal jugular vein for access placement, and 5-hour longer average filter life when they standardized protocols for CRRT delivery-including catheter access site-and enabled a quality assurance program.20 In this context, it is foreseeable that AI can assist with dynamic catheter pressure (i.e. access and return) and alarm monitoring.
Circuit clotting.
There is no standardization on CRRT anticoagulation. While use of pre-dilution Regional Citrate Anticoagulation (RCA) prolongs filter lifespan21–23 it requires a specialized protocol and could also increase risk of bleeding or other complications.23 Non-anticoagulation methods (i.e. saline flushes, minimization of filtration fraction, etc.) are proposed to reduce bleeding risk while maintaining filter patency with the use of new membrane biomaterials, although there are no solid data to prove this is indeed effective.24 Further, there is still debate on whether adjuvant systemic heparinization may add benefit in hypercoagulable states such as COVID-19. New generation CRRT machines are equipped with RCA dosing programs that allow for dynamic citrate and calcium dose adjustments. However, dose adjustments require continuous monitoring of the blood flow, effluent volume, and patient’s calcium levels and clinical status, thereby increasing nurse workload. AI-enabled remote monitoring and automated adjustment of RCA/calcium doses would reduce both nurse and clinician workload. Also, predicting the need for RCA dose adjustments could reduce the occurrence of citrate-related complications such as toxicity or bleeding.
Solute control.
Electrolyte disturbances such as hypokalemia and hypophosphatemia are common complications during CRRT.25,26 Further, customized rate of correction of dysnatremias could help preventing risk of overcorrection and neurological disturbances.27,28 AI-enhanced solute clearance monitoring and electrolyte replacement may positively impact CRRT delivery.
Quality assurance.
A multitude of reports highlight the importance of quality assurance in attaining efficient CRRT delivery.6,8,13,20,29–32 Lack of oversight and standardized protocols have been shown to cause treatment interruptions, poor solute clearance, and off-target fluid management or effluent dose delivery.33,34 Some of these outcomes were ameliorated by establishing multidisciplinary quality assurance programs20, although some may argue that the lack of field-wide standardization of quality metrics may be the leading cause of delayed improvement in CRRT delivery.31 Nonetheless, consensus has been reached around selection of CRRT quality metrics and interventional studies to establish the system-wide performance of these quality metrics in improving CRRT delivery are underway.8,35,36 For example, standardizing continuous monitoring of fluid management during CRRT could lead to timely adjustments and adherence to treatment goals. In this context, AI can assist with monitoring, incorporating and interpreting data from non-invasive hemodynamic devices to facilitate the workflow of clinicians evaluating fluid status and prescribing/monitoring fluid goals with CRRT.34,37
3. CURRENT LITERATURE ON THE USE OF AI APPLIED TO CRRT DELIVERY
We did a scoping review of current literature for studies on CRRT and digital health in patients with AKI using PubMed, OVID Embase, Web of Science, Cochrane, Scopus, and ProQuest dissertation databases. We selected studies in English, from database inception to present, except when the search returned over 5000 articles, for which we narrowed the timeline from 2012 to 2022. Literature reviews were scoured for additional studies not included in the original search. Full search strategy and list of included terms are reported in supplement 1. Collectively, there were 3460 studies included in the search results. After removal of duplicate studies, 3065 remained. Most studies were then excluded as they were not focused on CRRT, interventions were not based on AI-derived tools, or English translations for foreign studies were not found. After initial screening, we selected ten studies for reporting in this manuscript (7 full-length studies; 3 conference abstracts; see Table 1). We organized included studies by year and analyzed the innovations proposed, developmental stage of the proposed innovations (research development and/or validation; implementation and/or clinical utility), and the potential AI-based enhancement in CRRT delivery. After comprehensive review, the following insights emerged on the current state of AI research in CRRT, stratified in three critical domains of CRRT delivery such as structure, processes, and outcomes (Figure 2).
Table 1.
Summary of Selected Studies Reflecting the Design Stage of Various AI Tools for Enhancement of Structure, Processes or Outcomes of CRRT Delivery
| Study, Year & Country | Stage of Development | Population, Sample & Setting | Intervention | Study Outcomes & Results | Gaps Filled & Gaps Remaining |
|---|---|---|---|---|---|
|
Zhang et al. 2015 Australia |
Design | 1 AKI patient with sepsis on CVVH (UF 2L/hr. BFR 200ml/min) | E-monitoring of CRRT pressures and clotting prediction | Monitoring of progressive increases in TMP, filter pressure drop, and negative effluent pressure may predict clotting |
Dynamic monitoring and prevention of CRRT complications is possible with machine learning. Validation studies needed to reproduce these results across patient strata, and to test clinical effectiveness; characterization of the data management process needed to maintain accurate dynamic monitoring, as well as implementation protocols and performance metrics. |
|
Guru et al. 2016 United States |
Design, Validation | 488 ECMO patients, 213 of them on CRRT. | Automated EHR-based algorithm for prediction of CRRT initiation in ECMO patients. Performance of the algorithm was validated against manual searches. | Percent agreement between automated and manual search strategies: 89% or excellent, Kappa agreement statistic 0.99; training and validation cohorts’ agreement 90% or excellent, Kappa 1.0 |
EHR-based search algorithm can identify CRRT candidates in ECMO patients. External and cross-validation studies needed for reliability across patient strata; clinical validation needed for effectiveness; data management plans for ongoing data curation and monitoring; gap analysis, studies on performance metrics, implementation barriers and protocols. |
|
Keith et al. 2017 United States (Conference abstract) |
Design | CRRT patients 2012-2016 EHR data combined with operational data from CRRT machine (sample size and patient characteristics unknown) | Combining EHR flowsheets with CRRT machine data using automated scripts and dynamic data viewing | Multimodal database created successfully with 7,977,878 variables (205,910 EHR and CRRT flowsheet + 7,771,968 CRRT machine variables) |
Automation of data pooling and dynamic viewing is possible. Study details, including full methodology not available for review. Further validation needed for accuracy and reliability; characterization of the data management process needed, as well as implementation protocols and performance metrics. |
|
Kang et al. 2019 South Korea |
Design, Validation | 1571 ICU patients with AKI on CRRT (1094 test, 477 validation cohorts) | ML models (KNN, SVM, MARS, RF, XGB, ANN) for mortality prediction in CRRT, validated against disease severity scores (APACHE II, SOFA, MOSAIC). | Primary outcomes: probability of mortality during hospital/ICU admission. ML models outperformed disease severity scores. AUC values for all models (highest for RF model, 0.784 [0.744–0.825], followed by XGB 0.776 [0.735–0.818]) |
Machine learning outperforms disease severity scores in predicting mortality in CRRT patients. External validation studies needed for reliability, cross-validation for generalizability; clinical validation studies needed for clinical effectiveness; studies on data management and implementation barriers. |
|
Lee et al. 2021 United States (Conference abstract) |
Design | COVID-19 patients with AKI requiring CRRT (sample size and patient characteristics unknown). | Automated CRRT machine allocation scheduling for COVID-19 patients based on need (6-12 h vs. 24 h) | List of needs generated for the upcoming 24 hours on a shared server; machine-specific patient list generated within minutes of server updates, based on demand and machine location |
Automated scheduling improves workflow and optimizes use of CRRT resources. Full methodology, including feature selection and model development, needed; validation of the method for accuracy and reliability; data management plan and gap analysis; implementation barriers and performance metrics. |
|
Roy et al. 2021 United Kingdom |
Design, Validation | 36,498 patients (80% training, 10% validation, 10% test sets) at risk of developing 6 endpoints, including AKI and CRRT. | Comparing ML models (ST, SB multitask, SeqSNR) for CRRT initiation in the ICU using clinical data from the MIMIC-III dataset. Six endpoints were predicted, including AKI, CRRT, vasoactive medications, mortality, mechanical ventilation, and length of stay. | SeqSNR outperformed ST and SB multitask in predicting 4 endpoints (AKI, CRRT, vasoactive drugs, mortality). SeqSNR showed superior label efficiency when reducing training dataset (2.1%, 2.9%, 2.1% additional AUC for tasks using 1%, 5%, and 10% of labels, respectively). |
Deep learning SeqSNR has superior capacity to predict clinical endpoints, with less data, than traditional machine learning. Further research needed on clinical validation, identification of implementation barriers, and data curation and maintenance. |
|
Kang et al. 2021 South Korea |
Design, Validation | 2349 ICU patients with AKI on CRRT (70% training, 30% testing sets) | ML models (XGM, LGBM, DNN, SVM, LR) against disease severity scores (APACHE II, SOFA, MOSAIC) for hypotension prediction after CRRT initiation (MAP reduction ≥ 20 mmHg in 6 hours) | XGM model showed highest AUROC (0.828 [0.796–0.861]); all ML models outperformed disease severity scores |
Machine learning outperforms disease severity scores in predicting hypotension during CRRT. External validation studies needed for reliability, cross-validation for generalizability; clinical validation studies needed for clinical effectiveness; studies on data management and implementation barriers. |
|
Chen et al. 2021 China |
Design, Validation | Testing: All ICU patients 18+ years receiving RCA for CRRT, obtained from the institutional ICU database. 312 patients for database 1, 81 for database 2; Validation: 314 patients from the MIMIC III database. |
Comparing ML algorithms (Adaboost, XGBoost, SVM, SNN) to predict post-filter ionized calcium levels as a measure of citrate overdose. Clinical parameters used included patient age, gender, citrate dose, sodium bicarbonate solvent, replacement fluid solvent, body temperature, and replacement fluid pH. | SNN showed highest F1 score for classifying patients based on ionized calcium levels (90.8%, AUROC 0.86) in the training cohort, Adaboost in the validation cohort (80.5%, AUC 0.81) |
Variation In ML-based models’ performances in predicting post-filter ionized calcium. More validation studies needed to ascertain accuracy and reliability of various algorithms; direct comparison of more algorithms and potentially meta-analysis to identify those with consistently higher performance; characterization of the data management process needed to maintain data curation and processing; identification of implementation barriers and validation of quality improvement metrics for CRRT anticoagulation. |
|
Pattharanitima et al. 2021 United States |
Design, Validation | 684 ICU patients 18+ years with AKI requiring CRRT from MIMIC-III database | Comparing ML models (LR, SVM, AdaBoost, XGB, MLP, MLP+LSTM) for RRTFS prediction in CRRT (alive, not on RRT ≥7 days prior to discharge) | 30% had RRTFS; MLP+LSTM model showed the highest AUROC (0.70 [0.67–0.73]). |
Deep learning outperforms traditional machine learning for prognostication of RRT-free survival. Internal, external, and cross-validation studies needed for accuracy and reliability ascertainment; clinical validation studies needed to ascertain effectiveness; data curation and maintenance protocols, with gap analysis for performance optimization; studies on identifying implementation barriers. |
|
Yoo et al. 2021 South Korea (Conference abstract) |
Design, Validation | 784 AKI patients on CRRT (data source unknown) | ML (decision tree) for prediction of early (<60days) vs late (>60 days) mortality in patients with severe AKI undergoing CRRT based on changes in fluid balance. Body Composition Monitoring (BCM) was used as an indicator of sequential changes in total body water and validated against body weight at baseline. Other clinical parameters were included in the prediction models (not listed). | No difference in BCM vs body weight assessment of volume status at baseline; BCM showed marginal benefit from fluid balance in survivor group (p=0.074). In addition to BCM, decision tree model showed platelet count predicts mortality > 60 days; SOFA score, serum sodium, bilirubin, and target clearance for mortality <60 days (AUC = 0.957). |
Machine learning highlights the interplay of clinical variables in predicting mortality for CRRT patients. Internal, external, and cross-validation studies needed for accuracy and reliability ascertainment of the prediction model; clinical validation studies needed to ascertain effectiveness of the prediction model; further studies needed on the reliability and clinical utility of BCM. |
AKI = Acute Kidney Injury; ANN = artificial neural network; AUC = Area Under receiver operating characteristics Curve; CKD = Chronic Kidney Disease; CRRT = Continuous Renal Replacement Therapy; CVVHD = Continuous Veno-Venous Hemodialysis; DNN = Deep Neural Network; ECMO = Extra-Corporeal Membrane Oxygenation; EHR = Electronic Health Record; APACHE II = Acute Physiology and Chronic Health Evaluation II; ESRD = End Stage Renal Disease; ICU = Intensive Care Unit; KNN = K-nearest neighbor; SVM = support vector machine; LGBM = Light Gradient Boosting Machine; LR = Logistic Regression; LSTM = Long Short-Term Memory; MARS = multivariate adaptive regression splines; MIMIC III = Multiparameter Intelligent Monitoring in Intensive Care III; ML = Machine Learning; MLP = Multilayer Perception; MOSAIC = Mortality Scoring system for AKI with CRRT; MTL = Multitask Learning; NN = Neural Network; PUMCH = Peking Union Medical College Hospital; RCA = Regional Citrate Anticoagulation; RF = random forest; XGB = extreme gradient boost; RRTFS = RRT-Free Survival; SB = Shared Bottom (multitask model with shared parameters); SCUF = Slow Continuous Ultrafiltration; SeqSNR = Sequential Subnetwork Routing; SNN = Shallow Neural Network; SOFA = Sequential Organ Failure Assessment; ST = Single-Task (Recurrent Neural Network); UF = Ultrafiltrate; RBF = Renal Blood Flow.
Figure 2.
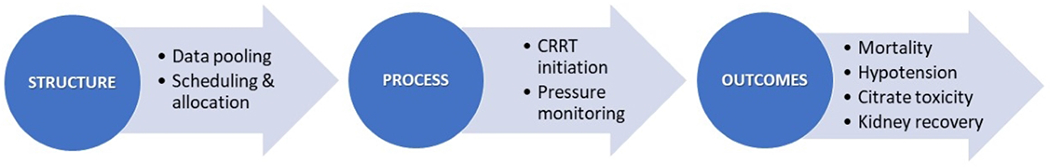
State of current evidence on the use of AI-based tools in CRRT delivery stratified by CRRT domains. Most of literature has been centered on clinical outcomes with little representation of structure and process of CRRT care.
CRRT = Continuous Renal Replacement Therapy
Structure:
Only two papers explored the implementation of AI in the structure of CRRT delivery.38,39 With the advancement of cloud-based systems and electronic task automation, Keith et al. programmed Python to download data from patient charts as well as the CRRT machine into a Structured Query Language (SQL) database, and to display the combined data for manipulation and analysis on novel digital platforms. More recently, Lee et al. examined the use of AI in the operational decision-making surrounding CRRT allocation for COVID-19 patients with AKI.39 A Python 3.6 code connected multidisciplinary teams on a shared server, pooling patient clinical data and prioritizing CRRT machine allocation according to specific patient needs and locations. The model successfully generated a list of patients for each machine within minutes of server updates.
Processes:
Guru et al. designed an automated algorithm to continuously scan patient charts for CRRT candidacy.40 The search algorithm achieved 100% agreement with standard manual chart reviews, with 90% of CRRT candidates identified at the same time and 10% within 15 minutes of manual reviews. Roy et al compared various ML algorithms for prognostication of CRRT initiation.41 For CRRT monitoring, efforts aimed at automating periodic data downloads from CRRT machines to be viewed in conjunction with clinical data. The earliest of those attempts was Zhang et al., who automated the recording and download of operational data from CRRT machines into a card reader at 1-minute intervals. The reader was then manually inserted into a computer card slot to view the files and identify various pressure patterns.42 Today, card readers and manual transfers have been replaced with cloud-based uploads and storage, online data downloads, and automated computer scripts deploying custom-based algorithms with countless functions.
Outcomes:
Predicting CRRT-related complications and patient prognosis are top research priorities, particularly with the advent of clinical decision support systems and point-of-care analytics. In 2021, Chen et al. compared 4 ML models to predict citrate overdose during CRRT.43,44 Neural networks scored the highest accuracy (F-1 score 90.8%) and performance (AUROC 0.86) in predicting early citrate overdose, allowing for timely adjustment of RCA. In the same year, Kang et al. validated 4 more ML models against disease-severity scores to predict hypotension during CRRT.45 All ML models outperformed standard clinical scores, with Extreme gradient boosting showing the highest AUROC scores (0.82) compared to other ML models. ML algorithms were also studied for applications in mortality risk-classification46,47 and dialysis-free prognostication.48 Most ML models reported in these studies significantly outperformed the predictive ability of standard clinical parameters, mortality scores and regression analyses. Nonetheless, large-scale ML studies are needed to rank ML models in terms of comparability and external validation, and clinical trials for comparing the impact of ML-enhanced vs. standard CRRT delivery on patient-relevant outcomes.
Overall, interest in AI-enhanced optimization of CRRT delivery has risen considerably. In 2021 alone, six original studies were published (60% of those reported in Table 1). Moreover, there is an uptrend in exploring the role of AI in all aspects of critical care nephrology, including patient risk-classification, optimization of CRRT prescriptions, prediction of CRRT-related complications, and prioritization of operational workflow. This rising trend reflects an increased awareness on the need for digital enhancement of CRRT delivery, and the potential implications of AI in the decision-making process surrounding its use, prescription, monitoring, and quality assurance. However, despite a comprehensive search strategy, only 10 original studies were available, all in adult patients without pediatric representation. Absence of detailed methods, lack of external validation to larger and diverse cohorts, and inherent database and/or algorithmic bias remain an issue and warrant further confirmatory studies before we can translate these innovations to the clinical setting. Therefore, the development of AI in the CRRT space remains premature overall, with persistent demand for more research to optimize CRRT structures, processes, and outcomes.
4-. IMPORTANT CONSIDERATIONS OF AI/ML APPLICATIONS FOR CRRT DELIVERY
There is implicit bias in ML algorithms, namely selection, lead-time, and algorithmic bias. In 2020, Wenlong et al. described the vicious cycle of feeding machines with biased information from unchecked and incomplete patient datasets, the biased predictions selecting a subset of the information to represent, the biased decision-making process based on these predictions, and the biased feedback that is fed back into the machine, etc.49 Researchers must be careful when using electronic data, as billing-derived diagnoses may not necessarily reflect the actual clinical context. Moreover, patients with normal or missing data may be counted in the control group, augmenting the selection and sampling biases of the study.50 Further, ascertainment bias can result from poor follow up and reporting.51 Therefore, it is essential to produce reliable metrics for detection and measurement of bias in ML algorithms52, and to understand their capacity as a decision aid and not a decision-making tool.53 In the context of CRRT, practice variations and resource access and allocation should be considered for implementation of AI-based tools. Development of multicenter datasets of patients undergoing CRRT such as CRRTnet could assist with contemporary assessment of epidemiology and variations in patient characteristics, prescription and delivery of therapy.54
Deployment of AI-based tools could highlight healthcare inequities.
AI could be a two-edge sword: if algorithms are developed and validated based on inherently inequitable databases, AI may bias the clinical decision-making, deepening current racial and economic health disparities. Alternatively, if compensatory algorithms were built into these databases and models, AI may help reduce inequity.55 The medical imaging field is an example on this struggle: Currie et al. reported persistent economic, geographic, and cultural inequities in the delivery of medical imaging services across the United States; 53 Waite et al. confirmed the relative scarcity and longer delivery time windows of advanced imaging services such as Computed Tomography and Magnetic Resonance Imaging in non-Caucasian predominant hospitals. 54 For AKI, health disparities are also of consideration: AKI occurs 28% more frequently in African American than Caucasian patients,56 and six times more frequently in low to middle-income countries than high-income countries.57 Used correctly, AI can help mitigate these issues with its ability to categorize and discern heterogenous subgroups of patients based on race, gender, socioeconomic status and more. Johnson et al. identified social determinants of health, including structural racism, as inherent causes of health service disparities among heart failure patients.58 The authors proposed that AI could help close the gap by diversifying data sources and investigators, increasing awareness on the inherent biases in AI and its impact on clinical decision-making, addressing heterogeneity in clinical studies with minority populations, and intentionally examining current and future research for structural racial or economic inequity. This applies to CRRT as this extracorporeal multiorgan support therapy is typically available in high and middle-income countries but not in low-income ones.
Deployment of tools to augment clinical decisions such as electronic alerts could also cause harm.
Several studies have identified alert fatigue as an implementation barrier for the use of electronic alert systems.59–61 A recent multicenter randomized clinical trial on the use of e-alerts for AKI patients found a 49% increase in adverse outcomes at the two smaller non-teaching hospitals, despite increased AKI care practices in the e-alert group.62 Overall, e-alerts did not reduce the risk of death, AKI progression, or RRT. Instead, the trial highlighted the role of alert fatigue on attention burnout and overtreatment. Another study by Baird et al. concluded e-alerts did not improve AKI severity or mortality, possibly due to alert fatigue.63 There is a need to examine implementation barriers to the use of AI-based tools, including clinician response and burnout, how and if those barriers can be mitigated, and the differential role of hospital logistics in tool utility. Therefore, AI-based tools to enhance CRRT delivery need to be adapted to the specific context of its deployment, taking into consideration the human and infrastructural planning component that is essential for CRRT delivery.
Monitoring of utility, implementation, and sustainability of AI-based tools is not standardized.
A new body of research will be needed to identify quality metrics and quality assurance processes that can assist, evaluate, and monitor AI-based tools for utility, implementation, and sustainability in CRRT applications. These metrics will need to be validated so healthcare professionals, patients/care partners, and stakeholders can trust and understand the integration of AI-based processes into current practice of CRRT delivery. Feng et al. suggested that hospitals should create specialized AI-based Quality Improvement units to continuously monitor and update AI algorithms, including statistical markers that track changes in model input/output variables, model behavior changes over time, and performance metrics.14 Importantly, standardization of policies to update AI models is also needed.
In Table 2, we summarize the advantages and challenges of using AI to enhance CRRT delivery.
Table 2.
Advantages and Challenges of AI Implementation to Enhance CRRT Delivery
| Advantages | Challenges |
|---|---|
| Automated learning from multimodal data (CRRT machine, monitors, EHRs, etc.) supersedes human accuracy in characterizing disease pathophysiology and predicting clinical outcomes | Inherent algorithmic bias may compound in a vicious cycle, misleading clinical decisions that ultimately result in worse outcomes. Standardized metrics and policy to evaluate utility, implementation, and sustainability of AI-based tools are needed |
| Inclusive databases and fair algorithmic models can identify marginalized populations, reduce health service disparity, and optimize treatment benefit based on complex sociodemographic, resource allocation and access, and clinical characteristics | Propensity to exacerbate healthcare inequities given that CRRT is mostly available in high and middle-income countries. Databases must be checked for diversity and algorithms for structural inequities and biases |
| Optimized allocation of resources such as CRRT machines based on specific patient needs and resource availability | Alert/monitoring fatigue nullifies or even reverses benefit of early risk identification/allocation, as true clinical deterioration could be ignored in a stream of low priority alerts |
| Automated clinical and quality assurance workflow streamlines effective CRRT service delivery across a health system | Implementation and deployment may become a burden in small size hospitals and/or low-income countries |
5-. CONCLUSIONS
AI-based tools have the potential to enhance and facilitate CRRT delivery. Current research has mostly focused on the development of ML-based models for early identification of patients requiring CRRT, and their risk-classification of mortality or kidney recovery. However, there is need to evaluate how the use of AI-based tools could improve bedside decision-making capacity, and –importantly– assist structure and processes of CRRT delivery. One should also note that major challenges in AI research are identifying implementation and deployments goals and barriers, measuring biases inherent to the use of big data and AI-based applications, and eliminating social and “machine-generated” disparities in health service utilization. Future studies examining the utility of AI in CRRT need to evaluate these implementation barriers, diverse and underrepresented populations including children, and effective methods and policy to mitigate AI-inherent biases to promote equity.
Supplementary Material
CLINICAL SUMMARY.
CRRT is a common organ support therapy for critically ill patients with AKI in the ICU
AI-based tools have the potential to enhance CRRT delivery
Current research has mostly focused on the development of ML-based models of clinical prognostication (i.e., mortality, kidney recovery), with little evaluation of enhancements in structure or processes of CRRT delivery
The evaluation of how AI-based tools could be successfully validated and implemented to enhance bedside decision-making capacity and assist structure and processes of CRRT delivery is needed
Future studies examining the utility of AI in CRRT need to evaluate implementation barriers and how to mitigate AI-inherent biases and promote equity
Acknowledgements
We would like to thank Ms. Emma O’Hagan for her feedback and assistance with building the search strategy of this review.
Funding
JAN is supported by grants from NIDDK (R01DK128208, R56 DK126930 and P30 DK079337) and NHLBI (R01 HL148448-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
JAN have received consulting honoraria from Baxter Healthcare Inc and Leadiant Biosciences Inc.
References
- 1.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez G, Chrusch C, Hulme T, Posadas-Calleja JG. Renal replacement therapy: a practical update. Can J Anaesth 2019;66:593–604. [DOI] [PubMed] [Google Scholar]
- 3.Lau D, Pannu N, James MT, et al. Costs and consequences of acute kidney injury after cardiac surgery: A cohort study. J Thorac Cardiovasc Surg 2021;162:880–7. [DOI] [PubMed] [Google Scholar]
- 4.Selewski DT, Wille KM. Continuous renal replacement therapy in patients treated with extracorporeal membrane oxygenation. Semin Dial 2021;34:537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mottes TA, Goldstein SL, Basu RK. Process based quality improvement using a continuous renal replacement therapy dashboard. BMC Nephrol 2019;20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordoza M, Rachinski K, Nathan K, et al. A Quality Improvement Initiative to Reduce the Frequency of Delays in Initiation and Restarts of Continuous Renal Replacement Therapy. J Nurs Care Qual 2021;36:308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rewa OG, Tolwani A, Mottes T, et al. Quality of care and safety measures of acute renal replacement therapy: Workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. J Crit Care 2019;54:52–7. [DOI] [PubMed] [Google Scholar]
- 8.Opgenorth D, Reil E, Lau V, et al. Improving the quality of the performance and delivery of continuous renal replacement therapy (CRRT) to critically ill patients across a healthcare system: QUALITY CRRT: a study protocol. BMJ Open 2022;12:e054583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.What is Digital Health? U.S Food and Drug Administration, 2020. at https://www.fda.gov/medical-devices/digital-health-center-excellence/what-digital-health.) [Google Scholar]
- 10.Niel O, Bastard P. Artificial Intelligence in Nephrology: Core Concepts, Clinical Applications, and Perspectives. Am J Kidney Dis 2019;74:803–10. [DOI] [PubMed] [Google Scholar]
- 11.Yi TW, Laing C, Kretzler M, et al. Digital health and artificial intelligence in kidney research: a report from the 2020 Kidney Disease Clinical Trialists (KDCT) meeting. Nephrol Dial Transplant 2022;37:620–7. [DOI] [PubMed] [Google Scholar]
- 12.Harrer S, Shah P, Antony B, Hu J. Artificial Intelligence for Clinical Trial Design. Trends Pharmacol Sci 2019;40:577–91. [DOI] [PubMed] [Google Scholar]
- 13.Neyra JA, Nadkarni GN. Continuous Kidney Replacement Therapy of the Future: Innovations in Information Technology, Data Analytics, and Quality Assurance Systems. Adv Chronic Kidney Dis 2021;28:13–9. [DOI] [PubMed] [Google Scholar]
- 14.Feng J, Phillips RV, Malenica I, et al. Clinical artificial intelligence quality improvement: towards continual monitoring and updating of AI algorithms in healthcare. NPJ Digit Med 2022;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer 2018;18:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CT, Liu KD. Exciting developments in the field of acute kidney injury. Nat Rev Nephrol 2020;16:69–70. [DOI] [PubMed] [Google Scholar]
- 17.Juncos LA, Chandrashekar K, Karakala N, Baldwin I. Vascular access, membranes and circuit for CRRT. Semin Dial 2021;34:406–15. [DOI] [PubMed] [Google Scholar]
- 18.Kim IB, Fealy N, Baldwin I, Bellomo R. Premature circuit clotting due to likely mechanical failure during continuous renal replacement therapy. Blood Purif 2010;30:79–83. [DOI] [PubMed] [Google Scholar]
- 19.Mendu ML, May MF, Kaze AD, et al. Non-tunneled versus tunneled dialysis catheters for acute kidney injury requiring renal replacement therapy: a prospective cohort study. BMC Nephrol 2017;18:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz EF, Ortiz-Soriano VM, Talbott M, et al. Development, implementation and outcomes of a quality assurance system for the provision of continuous renal replacement therapy in the intensive care unit. Sci Rep 2020;10:20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu MY, Hsu YH, Bai CH, Lin YF, Wu CH, Tam KW. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2012;59:810–8. [DOI] [PubMed] [Google Scholar]
- 22.Bai M, Zhou M, He L, et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med 2015;41:2098–110. [DOI] [PubMed] [Google Scholar]
- 23.Kośka A, Kirwan CJ, Kowalik MM, et al. Filter life span in postoperative cardiovascular surgery patients requiring continuous renal replacement therapy, using a postdilution regional citrate anticoagulation continuous hemofiltration circuit. Cardiol J 2022;29:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raina R, Sethi S, Khooblall A, et al. Non-anticoagulation pediatric continuous renal replacement therapy methods to increase circuit life. Hemodial Int 2022. [DOI] [PubMed] [Google Scholar]
- 25.Thompson Bastin ML, Adams PM, Nerusu S, Morris PE, Mayer KP, Neyra JA. Association of Phosphate Containing Solutions with Incident Hypophosphatemia in Critically III Patients Requiring Continuous Renal Replacement Therapy. Blood Purif 2022;51:122–9. [DOI] [PubMed] [Google Scholar]
- 26.Thompson Bastin ML, Stromberg AJ, Nerusu SN, et al. Association of Phosphate-Containing versus Phosphate-Free Solutions on Ventilator Days in Patients Requiring Continuous Kidney Replacement Therapy. Clin J Am Soc Nephrol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neyra JA, Ortiz-Soriano VM, Ali D, Morris PE, Johnston CM. A Multidisciplinary Approach for the Management of Severe Hyponatremia in Patients Requiring Continuous Renal Replacement Therapy. Kidney Int Rep 2019;4:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yessayan LT, Szamosfalvi B, Rosner MH. Management of dysnatremias with continuous renal replacement therapy. Semin Dial 2021;34:472–9. [DOI] [PubMed] [Google Scholar]
- 29.Vásquez Jiménez E, Anumudu SJ, Neyra JA. Dose of Continuous Renal Replacement Therapy in Critically III Patients: A Bona Fide Quality Indicator. Nephron 2021;145:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neyra JA, Tolwani AJ. A Quality Improvement Initiative Targeting CRRT Delivered Dose: The What, the How, and the Why. Am J Kidney Dis 2019;74:721–3. [DOI] [PubMed] [Google Scholar]
- 31.See E, Ronco C, Bellomo R. The future of continuous renal replacement therapy. Semin Dial 2021;34:576–85. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Ni H, Fan H, Li D, Xu X. Actually delivered dose of continuous renal replacement therapy is underestimated in hemofiltration. Asaio j 2013;59:622–6. [DOI] [PubMed] [Google Scholar]
- 33.Tseng MF, Wu CC. Cvvh yields better renal outcomes than intermittent hemodialysis among traumatic intracranial hemorrhage patients with AKI: A nationwide population-based retrospective study in Taiwan. Journal of the American Society of Nephrology 2018;29:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connor MJ Jr., Karakala N. Continuous Renal Replacement Therapy: Reviewing Current Best Practice to Provide High-Quality Extracorporeal Therapy to Critically III Patients. Adv Chronic Kidney Dis 2017;24:213–8. [DOI] [PubMed] [Google Scholar]
- 35.Rewa OG, Villeneuve PM, Lachance P, et al. Quality indicators of continuous renal replacement therapy (CRRT) care in critically ill patients: a systematic review. Intensive Care Med 2017;43:750–63. [DOI] [PubMed] [Google Scholar]
- 36.Rewa OG, Eurich DT, Noel Gibney RT, Bagshaw SM. A modified Delphi process to identify, rank and prioritize quality indicators for continuous renal replacement therapy (CRRT) care in critically ill patients. J Crit Care 2018;47:145–52. [DOI] [PubMed] [Google Scholar]
- 37.Afshinnia F, Belanger K, Palevsky PM, Young EW. Effect of ionized serum calcium on outcomes in acute kidney injury needing renal replacement therapy: secondary analysis of the acute renal failure trial network study. Ren Fail 2013;35:1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keith BH, Lusk J, Gist KM, Stidham T, Soranno DE. The CRRT dashboard: Utilizing software engineering to provide access to multimodal data. Pediatric Nephrology 2017;32:2187. [Google Scholar]
- 39.Lee H, Wong AK, Connor M. Using artificial intelligence to optimize RRT machine allocation during COVID-19-related RRT surge. Critical Care Medicine 2021;49:45. [Google Scholar]
- 40.Guru PK, Singh TD, Passe M, Kashani KB, Schears GJ, Kashyap R. Derivation and Validation of a Search Algorithm to Retrospectively Identify CRRT Initiation in the ECMO Patients. Applied clinical informatics 2016;7:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy S, Mincu D, Loreaux E, et al. Multitask prediction of organ dysfunction in the intensive care unit using sequential subnetwork routing. Journal of the American Medical Informatics Association 2021;28:1936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Baldwin I, Zhu G, Tanaka A, Bellomo R. Automated electronic monitoring of circuit pressures during continuous renal replacement therapy: a technical report. Crit Care Resusc 2015;17:51–4. [PubMed] [Google Scholar]
- 43.Chen H, Ma YY, Hong N, et al. Early warning of citric acid overdose and timely adjustment of regional citrate anticoagulation based on machine learning methods. Bmc Medical Informatics and Decision Making 2021;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Ma Y, Hong N, et al. Early warning of citric acid overdose and timely adjustment of regional citrate anticoagulation based on machine learning methods. BMC medical informatics and decision making 2021;21:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang MW, Kim S, Kim YC, et al. Machine learning model to predict hypotension after starting continuous renal replacement therapy. Sci Rep 2021;11:17169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang MW, Kim DK, Oh KH, Joo KW, Kim YS, Han SS. Machine learning algorithm to predict mortality in patients undergoing continuous renal replacement therapy. Journal of the American Society of Nephrology 2019;30:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo KD, Noh J, An JN, et al. Prediction of the clinical outcomes in patients with CRRT using body composition monitoring: A machine learning approach to a multicenter cohort study. Journal of the American Society of Nephrology 2021;32:129–30. [Google Scholar]
- 48.Pattharanitima P, Vaid A, Jaladanki SK, et al. Comparison of Approaches for Prediction of Renal Replacement Therapy-Free Survival in Patients with Acute Kidney Injury. Blood Purification 2021;50:621–7. [DOI] [PubMed] [Google Scholar]
- 49.Sun W, Nasraoui O, Shafto P. Evolution and impact of bias in human and machine learning algorithm interaction. PLoS One 2020;15:e0235502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang J Selection Bias in the Predictive Analytics With Machine-Learning Algorithm. Ann Emerg Med 2021;77:272–3. [DOI] [PubMed] [Google Scholar]
- 51.Flannery AH, Thompson Bastin ML. Oseltamivir Dosing in Critically III Patients With Severe Influenza. Ann Pharmacother 2014;48:1011–8. [DOI] [PubMed] [Google Scholar]
- 52.Thompson HM, Sharma B, Bhalla S, et al. Bias and fairness assessment of a natural language processing opioid misuse classifier: detection and mitigation of electronic health record data disadvantages across racial subgroups. J Am Med Inform Assoc 2021;28:2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan T, Gurupur V. Understanding the Difference Between Healthcare Informatics and Healthcare Data Analytics in the Present State of Health Care Management. Health Serv Res Manag Epidemiol 2020;7:2333392820952668-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heung M, Bagshaw SM, House AA, Juncos LA, Piazza R, Goldstein SL. CRRTnet: a prospective, multi-national, observational study of continuous renal replacement therapy practices. BMC Nephrol 2017;18:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Char DS, Shah NH, Magnus D. Implementing Machine Learning in Health Care - Addressing Ethical Challenges. The New England journal of medicine 2018;378:981–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grams ME, Matsushita K, Sang Y, et al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol 2014;25:1834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int 2013;84:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson AE, Brewer LC, Echols MR, Mazimba S, Shah RU, Breathett K. Utilizing Artificial Intelligence to Enhance Health Equity Among Patients with Heart Failure. Heart Fail Clin 2022;18:259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott J, Finch T, Bevan M, et al. Acute kidney injury electronic alerts: mixed methods Normalisation Process Theory evaluation of their implementation into secondary care in England. BMJ Open 2019;9:e032925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawhney S, Fluck N, Marks A, et al. Acute kidney injury-how does automated detection perform? Nephrol Dial Transplant 2015;30:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanagasundaram NS, Bevan MT, Sims AJ, Heed A, Price DA, Sheerin NS. Computerized clinical decision support for the early recognition and management of acute kidney injury: a qualitative evaluation of end-user experience. Clin Kidney J 2016;9:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson FP, Martin M, Yamamoto Y, et al. Electronic health record alerts for acute kidney injury: multicenter, randomized clinical trial. Bmj 2021;372:m4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baird D, De Souza N, Logan R, Walker H, Guthrie B, Bell S. Impact of electronic alerts for acute kidney injury on patient outcomes: interrupted time-series analysis of population cohort data. Clin Kidney J 2021;14:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


