Recently, it was published in Circulation that empagliflozin inhibits H2O2-induced cardiac late sodium current (late INa).1 Using computational modeling and point mutagenic approaches, Philippaert et al suggested a possible site of empagliflozin-binding within NaV1.5 similar to that of local anesthetics, supportive of direct drug binding to NaV1.5, although this remains to be determined conclusively and alternative mechanisms may exist.1 We have previously shown that CaMKII (Ca/calmodulin-dependent kinase II) binds to NaV1.5, stimulates late INa, and affects its H2O2-dependent regulation.2,3 We also demonstrated that empagliflozin inhibits CaMKII in failing human and murine cardiomyocytes.4
Here we show that inhibition of H2O2-induced late INa by empagliflozin cannot solely be mediated by direct drug binding but depends on CaMKII-dependent phosphorylation of NaV1.5 at serine 571. We demonstrate that empagliflozin inhibits late INa in patients with aortic stenosis (AS) and phenotypic features of heart failure (HF) with preserved ejection fraction.
Raw data/analytic methods can be made available for purposes of reproducing results or replicating procedures. Human tissue/proprietary antibodies cannot be made available because of legal constraints. Experiments conform to the Declaration of Helsinki. Human/murine studies were approved by institutional committee. Written informed consent was obtained from patients before tissue donation. Left ventricular samples were obtained from septal resections of 11 patients (8 male/3 female, age 69.3±2.6 years) with AS undergoing valve replacement. Patients had a HF with preserved ejection fraction–like phenotype with hypertrophy and preserved ejection fraction (59.4±1.7%). Murine models of CaMKIIδ knock-out (CaMKIIδ-/-),3 inhibition of CaMKII-dependent NaV1.5 phosphorylation at serine 571 (S571A), and with CaMKII phosphomimetic NaV1.5 S571E mutation were tested for involvement of CaMKII-NaV1.5 phosphorylation. Isolated ventricular myocytes were incubated (30 min) with empagliflozin (1 µmol/L) or control (dimethyl sulfoxide). Some cardiomyocytes were incubated with inhibitors of open-state Na channel inactivation (ATX-II or veratridine) or lidocaine (100 µmol/L, 30 min) for direct Na channel inhibition. H2O2 (100 µmol/L, 5 min) was used to induce reactive oxygen species, which stimulate late INa in HF via CaMKII3 (tested with CaMKII-inhibitor myristoylated-autocamtide-2-related inhibitory peptide (AiP); 2 µmol/L, 30 min). For some experiments, empagliflozin was washed in to ATX-II or H2O2 preincubated myocytes.
Late INa was measured as described previously.2,3 Resting membrane potential was held at –120 mV and INa elicited by depolarizing to –20 mV for 1000 ms, quantified by integrating from 100 to 500 ms of the start of depolarization (normalized to membrane capacitance). Western blots used human ventricular tissue exposed to empagliflozin/vehicle (30 min).4 Data were analyzed using mixed-effects analysis with Holm-Sidak, linear mixed model with random factor “individual” and Sidak correction, or paired t test (GraphPad Prism 9).
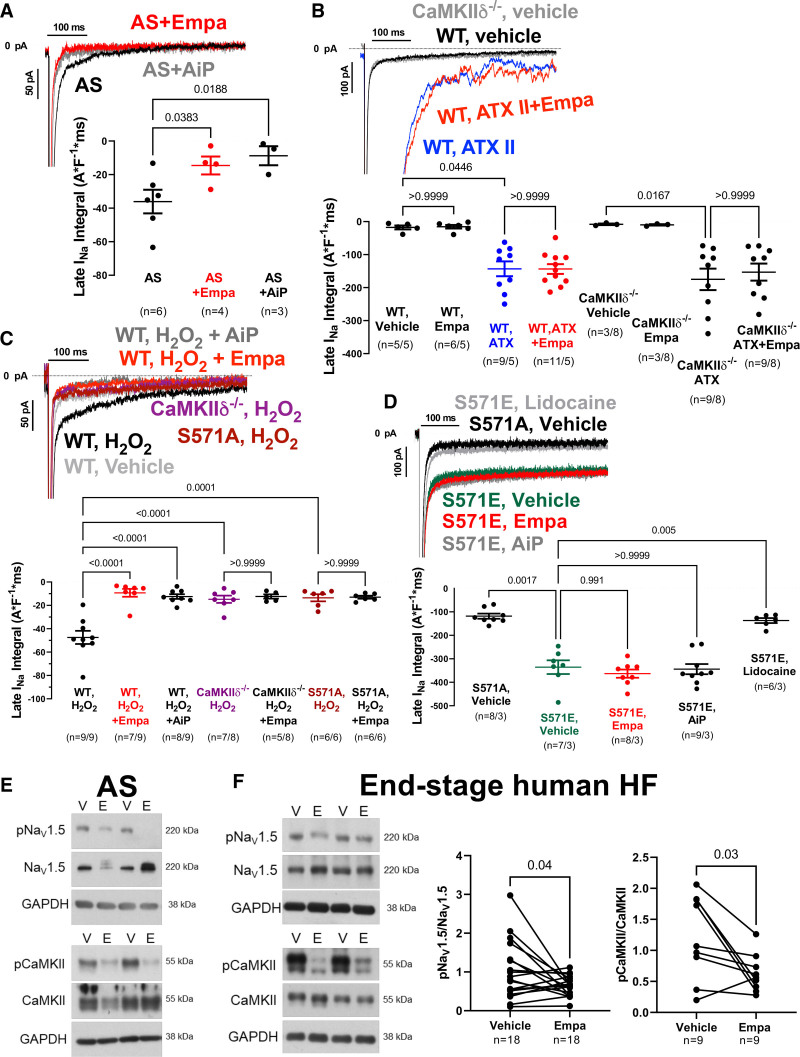
We demonstrate that late INa can be reduced by empagliflozin in ventricular myocytes from patients with AS similar to CaMKII-inhibitor AiP (Figure [A]). ATX-II–dependent (Figure [B]) enhancement of late INa in murine wild-type cardiomyocytes was not affected by empagliflozin (not even at 10 and 100 µmol/L), or after wash-in (at 1 µmol/L) to ATX-II preincubated myocytes, which would be expected if empagliflozin were a direct NaV1.5 inhibitor. Moreover, wash-in of empagliflozin (up to 10 µmol/L) also did not inhibit late INa in myocytes preincubated with a moderate concentration of veratridine (16 nmol/L, experimentally determined as EC50 by dose–response; data not shown). In sharp contrast, both veratridine and ATX-II–enhanced late INa were blocked by lidocaine (not shown). Empagliflozin robustly inhibited H2O2-induced late INa (Figure [C]), with maximal efficacy at 6 minutes but not at 2 minutes after onset of exposure (late INa integral during wash-in: 0 minutes, –50.8±4.3 A*F-1 [ampere*farad]*ms; 2 minutes, –39.9±4.6 A*F-1*ms, P=0.0934 versus 0 minutes; 4 minutes, –24.2±4.6 A*F-1*ms, P=0.0007 versus 0 minutes; 6 minutes, –17.2±4.0 A*F-1*ms, P<0.0001 versus 0 minutes, n=6). No additional effect of empagliflozin on late INa was observed with AiP (not shown) or in myocytes lacking either CaMKIIδ (CaMKIIδ−/−) or CaMKII-dependent NaV1.5 phosphorylation at serine 571 (S571A, Figure [C]). Accordingly, the enhanced late INa in mice with CaMKII phosphomimetic NaV1.5 S571E was blocked by neither empagliflozin nor AiP (Figure [D]). In contrast, lidocaine inhibited late INa in S571E cells, underscoring that empagliflozin primarily acts by CaMKII-NaV1.5 phosphorylation. Empagliflozin dose-response revealed an IC50 for inhibition of H2O2-dependent late INa of 0.086 µmol/L in murine myocytes (not shown). Empagliflozin inhibited CaMKII autophosphorylation and CaMKII-dependent phosphorylation of NaV1.5 in AS and HF (Figure [E and F]).
Figure.
Late INa inhibition by empagliflozin requires CaMKII. A, Original recordings and mean data of empagliflozin- or AiP-mediated inhibition of late INa in human ventricular cardiomyocytes from patients with AS (n=patients). B, Original recordings and mean data of late sodium current (late INa) in murine cardiomyocytes from wild-type (WT) or CaMKIIδ-/- mice (n=cells per mice). The ATX-dependent enhancement of late INa could not be blocked by empagliflozin. C, In contrast, the H2O2-dependent stimulation of late INa was blocked by CaMKII inhibition (AiP, CaMKII−/−), by transgenic inhibition of CaMKII-dependent NaV1.5 phosphorylation (S571A), or in the presence of empagliflozin. D, In contrast with local anesthetic lidocaine, neither empagliflozin nor AiP could block enhanced late INa in mice with phosphomimetic substitution of glutamic acid for serine at 571 (S571E). E and F, Western blots of cardiomyocytes on empagliflozin show reduced CaMKII-autophosphorylation (T287) and reduced CaMKII-dependent NaV1.5 phosphorylation. For comparison of multiple groups, mixed-effects analysis plus Holm-Sidak (A) or linear mixed model plus Sidak were performed. For comparison of 2 groups, paired t test was done (F). A indicates ampere; AIP, autocamtide-2-related inhibitory peptide; AS, aortic stenosis; ATX II or ATX, Anemonia viridis toxin 2; CaMKII, Ca/calmodulin-dependent kinase II; CaMKIIdelta-/-, CaMKII delta knock out δ; E, Empa; Empa, empagliflozin; F, farad; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; HF, heart failure; kDa= kilo Dalton; ms, miliseconds; p, phosphorylated; pA, picoampere; S571A and S571E: Nav1.5 with a phosphomimetic mutation at Ser571 (S571E), or Nav1.5 with the phosphorylation site ablated (S571A); V, vehicle; and WT, wildtype.
In conclusion, inhibition of late INa by empagliflozin is at least in part caused by inhibition of CaMKII-dependent regulation of NaV1.5.2,4 If cardiac Na channels were solely directly inhibited, empagliflozin, like local anesthetics, should have blocked ATX-II/veratridine–stimulated late INa, but it did not. Nevertheless, the target of empagliflozin in the heart remains unclear,5 and further research is needed to better understand direct versus indirect effects on late INa. We demonstrate that empagliflozin also inhibits late INa in patients with AS and features of HF with preserved ejection fraction, which may reduce the propensity for arrhythmias and contribute to the positive results of the EMPEROR-Preserved trial (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction).
Article Information
Acknowledgments
J.M., M.J.B., and S.W. designed experiments, interpreted data, wrote the article, and are responsible for the integrity of the article. J.M., M.J.B., T.S., M.T., Z.P., T.J.H., H.M., and S.P. acquired data and revised the article. S.S., P.J.M., and L.S.M. revised the article for critical intellectual content.
Sources of Funding
J.M. is funded by the German Cardiac Society Clinician Scientist program and by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) grant MU 4555/2-1. L.S.M. is funded by DFG grant MA1982/5-1. S.W. and L.S.M. are funded by SFB 1350 TPA6 and the University of Regensburg ReForM C program. S.W. is funded by DFG grants WA 2539/4-1, 5-1, 7-1, and 8-1. M.J.B. is supported by German Heart Foundation/German Foundation of Heart Research grant F/50/20. S.P., T.S., and M.T. are funded by the Else-Kröner-Fresenius Stiftung (EKFS, 2019_A84). S.S. is funded by DFG (SO 1223/4-1) and EKFS (2017_A137). P.J.M. is funded by National Institute of Health grant R35 HL135754 and by Leducq Foundation. T.J.H. is funded by National Institute of Health grants R01 HL156652 and R01 HL135096.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- AS
- aortic stenosis
- CaMKII
- Ca/calmodulin-dependent kinase II
- HF
- heart failure
- late /Na
- late sodium current
J. Mustroph and M.J. Baier contributed equally.
Circulation is available at www.ahajournals.org/journal/circ
For Sources of Funding and Disclosures, see page 1261.
Contributor Information
Maria J. Baier, Email: maria.baier@ukr.de.
Steffen Pabel, Email: steffen.pabel@ukr.de.
Thea Stehle, Email: Thea.Stehle@stud.uni-regensburg.de.
Maximilian Trum, Email: Maximilian.Trum@ukr.de.
Zdenek Provaznik, Email: zdenek.provaznik@ukr.de.
Peter J. Mohler, Email: peter.mohler@osumc.edu.
Hassan Musa, Email: Hassan.musa@osumc.edu.
Thomas J. Hund, Email: thomas.hund@osumc.edu.
Samuel Sossalla, Email: ssossalla@med.uni-goettingen.de.
Lars S. Maier, Email: lars.maier@ukr.de.
Stefan Wagner, Email: stefan.wagner@ukr.de.
References
- 1.Philippaert K, Kalyaanamoorthy S, Fatehi M, et al. The cardiac late sodium channel current is a molecular target for the sodium-glucose co-transporter 2 inhibitor empagliflozin. Circulation. 2021. Accessed May 21, 2021. 10.1161/CIRCULATIONAHA.121.053350 [DOI] [PMC free article] [PubMed]
- 2.Wagner S, Dybkova N, Rasenack E, Jacobshagen C, Fabritz L, Kirchhof P, Maier S, Zhang T, Hasenfuss G, Brown JH. Ca/calmodulin-dependent protein kinase II regulates cardiac Na channels. J Clin Investig. 2006;116:3127–3138. doi: 10.1172/JCI26620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J. Reactive oxygen species–activated Ca/calmodulin kinase IIδ is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Sag CM, Lebek S, Tarnowski D, Reinders J, Perbellini F. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Failure. 2018;5:642–648. doi: 10.1002/ehf2.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopaschuk GD, Subodh V. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors. JACC Basic Transl Sci. 2020;5:632–644. doi: 10.1016/j.jacbts.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]