Abstract
The “trophic downgrading of planet Earth” refers to the systematic decline of the world’s largest vertebrates. However, our understanding of why megafauna extinction risk varies through time and the importance of site- or species-specific factors remain unclear. Here, we unravel the unexpected variability in remaining terrestrial megafauna assemblages across 10 Southeast Asian tropical forests. Consistent with global trends, every landscape experienced Holocene and/or Anthropocene megafauna extirpations, and the four most disturbed landscapes experienced 2.5 times more extirpations than the six least disturbed landscapes. However, there were no consistent size- or guild-related trends, no two tropical forests had identical assemblages, and the abundance of four species showed positive relationships with forest degradation and humans. Our results suggest that the region’s megafauna assemblages are the product of a convoluted geoclimatic legacy interacting with modern disturbances and that some megafauna may persist in degraded tropical forests near settlements with sufficient poaching controls.
Large Asian mammals contradict global extinction trends and form bizarre assemblages.
INTRODUCTION
The loss of the world’s largest terrestrial vertebrates is a conspicuous portent of the Anthropocene mass extinction (1). Megafauna, defined here as large carnivores (Carnivora) with average adult body masses ≥15 kg (2) and terrestrial megaherbivores with average adult body masses ≥100 kg (3), are disproportionately targeted by hunters for both food and trade, which is often unsustainable because of their comparatively slow reproductive cycles (1). Megafauna are also acutely affected by habitat loss and fragmentation because of their large home ranges and dietary requirements (2, 3). More than 60% of all large carnivores and megaherbivores are now threatened with extinction (3, 4). Downgraded megafauna assemblages have important ramifications for conservation because these species are disproportionately important for maintaining diverse food webs and ecological processes, and their extinctions have often triggered unexpected cascading effects that degrade ecosystems (5, 6). Understanding and halting local megafauna abundance declines that rapidly reduce ecosystem functioning and foreshadow extinctions is urgently needed (7).
The drivers of megafauna extinctions have drastically shifted between the Pleistocene (between 2.6 million and 11,700 years before present), Holocene [11,700 to 100 years before present (8, 9)], and Anthropocene epochs [<70 years before present (1)]. During the Pleistocene, slowly operating geological, climatic, and biological processes, such as fluctuations in sea levels and the resulting suitable habitat, were key factors shaping extinction dynamics (10, 11). The Late Pleistocene and Early Holocene epochs saw a surge of megafauna extinctions coinciding with human colonization and settlement patterns (12) in combination with ongoing climatic factors that drove fluctuations in habitat availability (13, 14). Humans’ influence on biodiversity patterns has only intensified through time, and since the mid–20th century (i.e. ~1950 CE), a period now referred to as the Anthropocene epoch, the direct and indirect actions of humans are now the primary drivers of species extinctions, culminating in at least 322 terrestrial vertebrate extinctions since 1500 CE (1, 15). However, there is a tendency to assume that globally emergent patterns will manifest locally with less attention paid to the immense variations in species- and site-specific circumstances (16).
Southeast Asia is an ideal setting to study dynamic conservation threats because it retains high megafauna diversity yet suffers from extreme deforestation and poaching and has the highest percentage of threatened megafauna globally (4, 17). While it is often assumed or argued that extant megafauna remains largely confined to remote areas characterized by intact forests and minimal anthropogenic pressure (2, 3), there are numerous Southeast Asian examples of small protected areas near human settlements that retain high megafauna richness (18–20). The region also has a unique geological, climatic, and anthropogenic history that includes the Quaternary period’s largest volcanic super-eruption [Toba Caldera Complex ~75,000 years ago (21)], several dramatic changes in sea level that connected and separated Java, Sumatra, Borneo, and the Malay Peninsula as recently as ~10,000 years ago (8, 9), and more than 60,000 years of continued human existence, including the earliest pronounced human population expansion outside Africa (22). Even in contemporary Southeast Asian tropical forests, megafauna face unique challenges due to the forest’s irregular supra-annual mast fruiting phenology, where most fruiting plants in a forest release >90% of viable fruit in ~6 weeks followed by years without fruit (23). However, the degree to which Southeast Asia’s unique history and dynamic ecological forces have resulted in homogenization or variation in megafaunal assemblages remains unclear.
Here, we assess patterns in Holocene mammal extinctions, contemporary mammal abundances, and resulting Anthropocene assemblages for 14 megafauna species across 10 Southeast Asian tropical forest landscapes using a synthesis of occurrence records and 21 new camera trapping surveys (Tables 1 and 2 and table S1). We examine the role of key variables related to forest quality, anthropogenic, and abiotic influences to explain these patterns (table S2). We test three broadly held hypotheses to determine whether Southeast Asian megafauna assemblages follow globally emergent and well-documented patterns resulting in systematically downgraded assemblages (5), or if assemblages are more idiosyncratic than previously appreciated, suggesting the importance of site- and species-specific factors. First, we predicted that large carnivores would suffer more extirpations than megaherbivores because of their territoriality and reliance on healthy prey populations that put them at a higher risk than megaherbivores (4, 24). Second, we predicted that larger species within each guild would suffer more extirpations than smaller species (1). Last, we predicted that landscapes with more forest degradation and human pressure would suffer more extirpations and have lower abundances (5).
Table 1. Study site descriptions.
All landscape covariates were calculated in a 10-km radius area around the centroid of our camera trapping surveys in each landscape, except for forest size. FLII stands for Forest Landscape Integrity Index (26), and HFP stands for Human Footprint Index (38). NP = national park, WS = wildlife sanctuary, PF = production forest, and CA = conservation area. + refers to protected areas that are connected to larger expanses of forest.
| Landscape | Region | Forest size (km2) |
Intact forest cover
(%) |
FLII score |
Human density
(km−2) |
HFP score |
| 1) Khao Yai NP | Central Thailand | 2168 | 68.7 | 8.7 | 742 | 6.1 |
| 2) Khao Ban That WS | N. Malay Peninsula | 300 | 58.2 | 5.4 | 21,161 | 14.2 |
| 3) Ulu Muda PF | N. Malay Peninsula | 1152 | 76.9 | 9.0 | 34 | 4.3 |
| 4) Pasoh Forest Reserve |
S. Malay Peninsula | 130 | 49.4 | 3.3 | 10,595 | 11.0 |
| 5) Singapore | Singapore | 49 | 12.5 | 0.4 | 3,224,817 | 42.0 |
| 6) Gunung Leuser NP | North Sumatra | 8630+ | 69.6 | 7.9 | 7602 | 6.4 |
| 7) Kerinci Seblat NP | Central Sumatra | 13,300 | 64.9 | 7.7 | 1295 | 6.1 |
| 8) Bukit Barisan Selatan NP |
South Sumatra | 3568 | 80.0 | 8.8 | 0 | 5.6 |
| 9) Lambir Hills NP | Sarawak | 70 | 53.4 | 2.6 | 6349 | 8.4 |
| 10) Danum Valley CA | Sabah | 438+ | 72.0 | 9.6 | 0 | 6.9 |
Table 2. Southeast Asian large carnivores and megaherbivores at our study landscapes.
Table S3 provides additional details.
| Weight range (kg) |
IUCN Red List
status |
Detections (all
sites) |
Holocene
landscapes (no. occupied) |
Anthropocene
landscapes (no. occupied) |
Landscapes
detected in our surveys |
|
| Tiger (Panthera tigris) | 180–245 | Endangered | 72 | 10 | 5 | 3 |
| Sun bear (Helarctos
malayanus) |
27–63 | Vulnerable | 447 | 10 | 9 | 8 |
| Leopard (Panthera
pardus) |
45–65 | Vulnerable | 14 | 5 | 2 | 2 |
| Dhole (Cuon alpinus) | 15–21 | Endangered | 30 | 8 | 4 | 2 |
| Clouded leopard (Neofelis nebulosa and diardi) |
15–23 | Vulnerable | 120 | 10 | 8 | 7 |
| Asian elephant (Elephas maximus) |
3000–5000 | Endangered | 194 | 10 | 7 | 5 |
| Sumatran rhinoceros (Dicerorhinus sumatrensis) |
900–1000 | Critically Endangered |
0 | 10 | 3 | 0 |
| Gaur (Bos gaurus) | 650–900 | Vulnerable | 8 | 5 | 1 | 1 |
| Banteng (Bos
javanicus) |
600–800 | Endangered | 1 | 7 | 1 | 1 |
| Malay tapir (Tapirus
indicus) |
250–350 | Endangered | 396 | 9 | 5 | 5 |
| Sambar deer (Rusa
unicolor) |
180–260 | Vulnerable | 647 | 10 | 9 | 8 |
| Mainland serow (Capricornis sumatraensis) |
110–160 | Vulnerable | 46 | 7 | 7 | 7 |
| Wild boar (Sus scrofa) | 75–200 | Least Concern | 7656 | 8 | 8 | 8 |
| Bearded pig (Sus
barbatus) |
50–120 | Vulnerable | 2153 | 9 | 4 | 3 |
RESULTS
Species detections
We detected five large carnivore and nine megaherbivore species in 11,784 captures over a trapping effort of 63,423 days across 10 tropical forest landscapes in Thailand, Peninsular Malaysia, Singapore, Sumatra, and Borneo (Fig. 1, Tables 1 and 2, and tables S1 and S3). At the extremes of species detections, wild boar (Sus scrofa) represented 65% of all megafauna detections (7656 detections, present in all naturally occurring landscapes), while we failed to detect any Sumatran rhinoceros (Dicerorhinus sumatrensis) despite a trapping effort of 14,507 trap nights across three landscapes where the species is currently thought to persist (25). Accordingly, Anthropocene extirpations were defined as when a species went undetected in large camera trapping surveys at landscapes where they are currently recognized as extant by the IUCN Red List, noting that this definition includes species that persist at such low abundances, they can be considered functionally extinct, such as Sumatran rhinoceros (25). For brevity, we consider nondetections as either functional extinctions or real extirpations and refer to all nondetections as “extirpations” hereafter.
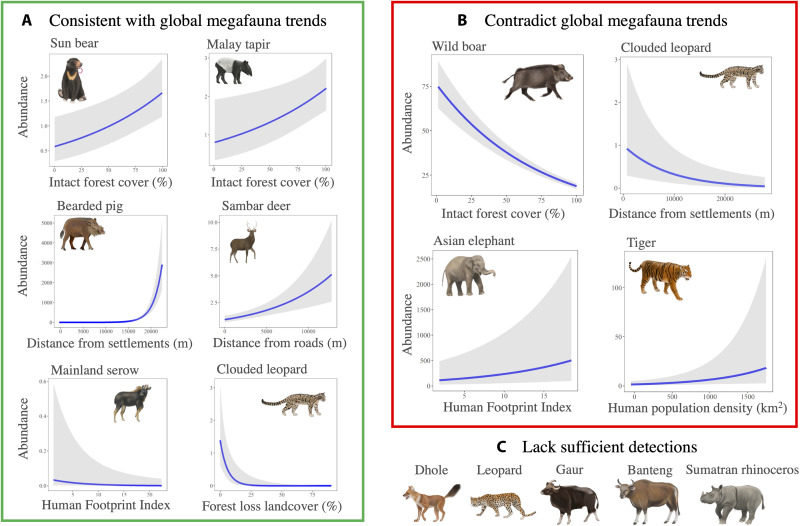
Fig. 1. Idiosyncratic megafauna assemblages are not systematically shaped by disturbances.
(A) Highly variable Anthropocene megafauna assemblages across our 10 study landscapes, and each circle represents the Forest Landscape Integrity Index (FLII) (26) in a 10-km radius around the center of each landscape. In (B), the base layer of the map depicts the contemporary habitat quality using the FLII, binned into high (FLII scores 9 to 10), medium (6 to 9), and poor (<6) integrity forest. The tan coloration shows land area that was exposed 50 m above current water levels at the onset of the Holocene [~12,000 years ago (68)]. See Table 1 for more information about each landscape and table S3 for all megafauna detected and abundance relative to each landscape. (C and D) Variation in FLII and HFP (38) for each study site. The study landscapes with new camera trapping on all panels are numbered as follows: 1, Khao Yai National Park; 2, Khao Ban That Wildlife Sanctuary; 3, Ulu Muda Forest Reserve; 4, Pasoh Forest Reserve; 5, Singapore; 6, Gunung Leuser National Park; 7, Kerinci Seblat National Park; 8, Bukit Barisan Selatan National Park; 9, Lambir Hills National Park; and 10, DVCA.
Megafauna extirpations
We documented 74 megafauna extirpations total (both the Holocene and Anthropocene) and recorded few significant predictors of extirpations (Fig. 2). The relative number of extirpations did not significantly differ between large carnivores and megaherbivores (Welch two-sample t test, tdf=11.2 = −0.06, P = 0.95 for the Holocene; t8.6 = −1.1, P = 0.30 for the Anthropocene; Fig. 3A) or between the largest and smallest species within each guild (Holocene carnivores: t2.7 = −0.23, P = 0.83; Anthropocene carnivores: t2 = −0.76, P = 0.53; Holocene herbivores: t6.1 = 2.1, P = 0.08; Anthropocene herbivores: t6.9 = −0.32, P = 0.76; Fig. 3B). Similarly, the proportion of species extirpated per landscape did not significantly vary with any of the 12 habitat covariates describing forest quality, anthropogenic conditions, or abiotic conditions [all coefficients in the univariate binomial generalized linear models (GLMs) had P > 0.05; table S4]. However, the four most disturbed landscapes—as measured by the Forest Landscape Integrity Index (FLII) (26) (Table 1)—experienced significantly more total extirpations than the six least disturbed landscapes (9 versus 3.6 total extirpations per landscape; t4.7 = −4.9, P = 0.005; Table 1 and Fig. 2C).
Fig. 2. Highly variable megafauna extirpation dynamics among species and sites in Southeast Asia.
(A) The Holocene and Anthropocene extirpations of 14 megafauna across 10 tropical forest landscapes (table S3). The relative abundance of extant species is shown by the size of points as determined from hierarchical abundance modeling. High abundance corresponds to abundance >75% quartile of each species’ relative abundance estimates, medium corresponds to relative abundance estimates between 25 and 75% quartiles, and low is <25% quartile. Bar chart (B) shows the number of landscapes each species was extirpated from, and bar chart (C) shows the number of species extirpated from each landscape. The 14 study species are grouped as large carnivores first followed by megaherbivores. Within each guild, species are listed from largest to smallest on the basis of average adult body size (Table 2). Landscapes are organized from left to right by increasing FLII scores (Table 1). Species listed in descending order are as follows: tiger, sun bear, leopard, dhole, clouded leopard, Asian elephant, Sumatran rhino, gaur, banteng, tapir, sambar, bearded pig, mainland serow, and wild boar (scientific names, descriptions, and relative abundance estimates in Table 2 and table S3).
Fig. 3. Species traits (guild and body size) do not explain extirpation patterns.
We conducted Welch two-sample t tests and found no significant differences between the relative number of extirpations between large carnivores and megaherbivores (A) or between different sized species within each guild (B). The maximum number of Holocene extirpations possible was 10 for species whose range covered the whole region and fewer for species with smaller distributions, including leopards, dhole, gaur, banteng, tapir, bearded pigs, mainland serow, and wild boar.
Holocene extirpations did not vary significantly among guilds or between subregions but did exhibit large variation among species. We documented 58 Holocene extirpations, which were defined as when the species’ IUCN Red List range no longer includes landscapes present in its Holocene range, as defined in prior work (table S3). There were 21 large carnivore extirpations out of 43 possible and there were 37 of 75 possible megaherbivore extirpations (Fig. 2, orange bars). Sumatran rhinoceros suffered the highest number of Holocene extirpations, being lost from 7 of 10 landscapes. This was followed by banteng (Bos javanicus), which were extirpated from six of seven landscapes, and bearded pigs (Sus barbatus), which were extirpated from five of nine landscapes. Meanwhile, there were no Holocene extirpations of wild boar or mainland serow (Capricornis sumatraensis; Fig. 2A). There were 6.2 Holocene extirpations per landscape in mainland Southeast Asia, 3 extirpations per landscape in Borneo, and 0.6 in Sumatra, noting that Kerinci Seblat National Park in central Sumatra did not experience any extirpations. The two most modern disturbed landscapes of Singapore [FLII = 0.4, with 10 being the most pristine, and Human Footprint Index (HFP) = 42, with 50 having the most human influence] and Lambir Hills National Park in Borneo (FLII = 2.6 and HFP = 8.4) lost 85 and 78% of their megafauna assemblages during the Holocene, respectively (Fig. 2C). However, the two least modern disturbed landscapes of Danum Valley Conservation Area (DVCA) in Borneo (FLII = 9.6 and HFP = 6.9) and Ulu Muda Forest Reserve in Peninsular Malaysia (FLII = 9.0 and HFP = 4.3) also lost 33 and 43% of their megafauna, respectively (Fig. 2A).
For the Anthropocene, there were 16 extirpations including 8 of 30 possible extirpations of large carnivores and 8 of 46 possible for megaherbivores (Fig. 2, gray bars). All landscapes experienced at least one Anthropocene megafauna extirpation except for the highly intact forest of DVCA in Borneo and Singapore, which had already lost most species before 1950, which counted toward the Holocene (Fig. 2C). Tigers (Panthera tigris) and Sumatran rhinoceros experienced the most Anthropocene extirpations (each extirpated from three landscapes), while six species did not experience any (Fig. 2B). By region, there were 1.6 Anthropocene extirpations per landscape in mainland Southeast Asia, 1 in Borneo, and 1.6 in Sumatra.
Megafauna abundance trends
We assessed relationships between local megafauna abundances using hierarchical N-mixture abundance modeling while accounting for imperfect detection. Elevation was the most important variable assessed using AICc model selection and appeared in eight species’ top models, including significant positive relationships for tigers, clouded leopards (Neofelis nebulosa and diardi), sun bears (Helarctos malayanus), Malay tapir (Tapirus indicus), and bearded pigs; a significant negative relationship for sambar deer (Rusa unicolor); and nonsignificant relationships with Asian elephants (Elephas maximus) and mainland serow (see table S5 for full competing model descriptions for all species).
Most of the top models explaining megafauna abundance included negative effects from habitat degradation or humans (Fig. 4A, green). The top models for sun bear and Malay tapir showed significant positive relationships with intact forest cover (βsun bear = 0.30, SE = 0.10, P = 0.002; βtapir = 0.29, SE = 0.12, P = 0.014). The top model for bearded pigs included a positive relationship with distance from human settlements (β = 3.42, SE = 0.20, P < 0.0001), sambar deer had a positive relationship with distance from roads (β = 0.25, SE = 0.06, P < 0.0001), and serow had a negative relationship with HFP (β = −0.79, SE = 0.35, P = 0.024). The top model for clouded leopard included a negative relationship with recent forest loss (β = −2.55, SE = 0.86, P = 0.003).
Fig. 4. Species-specific responses to habitat degradation and humans.
The response variable (y axis) is the predicted abundance from hierarchical abundance modeling in relation to covariates (x axis), with each panel showing an important variable from each species’ top model based on AICc model selection. (A) Six negative relationships between megafauna abundance and forest degradation or human pressure that are consistent with globally emergent trends. (B) Four relationships that contradict global trends and suggest positive relationships with forest degradation or human pressure. (C) Five species that had insufficient independent detections (≤30) to implement hierarchical abundance models. Table S5 provides the full model descriptions.
Contrary to our predictions, four species showed positive associations with habitat degradation or humans in their top models (Fig. 4B, red). Tigers showed a positive relationship with human population density (β = 0.35 SE = 0.14, P = 0.014), clouded leopards showed a significant negative relationship with distance from human settlements (β = −0.72, SE = 0.23, P = 0.002), wild boar showed a negative relationship with intact forest cover (β = −0.40, SE = 0.02, P < 0.001), and Asian elephants showed a positive relationship with HFP (β = 0.41, SE = 0.11, P < 0.001).
DISCUSSION
We documented remarkable variation in the extirpation patterns and abundances of Southeast Asia’s megafauna, suggesting that species responses to disturbances are more complex than previously appreciated. We expected to find “trophic downgrading” with the systematic loss of the largest species from landscapes experiencing the highest rates of forest degradation and human pressures. There was some general support for the role of forest degradation and human pressure driving megafauna losses because the four most disturbed landscapes experienced 2.5 times more extirpations than the six least disturbed landscapes. However, we did not identify a clear pattern of trophic downgrading, and there were no crucial landscape-level drivers of Holocene or Anthropocene extirpations. Instead, our most poignant observation was peculiar mammal assemblages in remaining protected areas with no two sites having identical assemblages, suggesting that the complex synergy between multiple threats and the unique responses of each species appear to be changing megafaunal assemblages in ways unique to each landscape (14, 27). We also found mixed support for the prediction that contemporary megafauna abundances would be positively associated with forest cover metrics and negatively associated with humans. Tigers, clouded leopards, Asian elephants, and wild boar showed positive relationships with forest degradation or humans, suggesting that these factors are not necessarily prescriptive. There was also little difference in the relative number of extirpations between guilds or body sizes, lending no support for the hypothesis that the largest carnivores are more threatened than other megafauna. The final outcomes are idiosyncratic assemblages in the Anthropocene that do not clearly reflect globally emergent trends in trophic downgrading.
While megafauna assemblages were more variable than expected as a whole, there were still some predictable trends. Eight of 14 megafauna species experienced extirpations during both the Holocene and Anthropocene, suggesting alarming ongoing range contractions that is consistent with other work regionally and globally (1, 28). The extirpation of Sumatran rhinoceros from all our study landscapes likely resulted from well-documented poaching pressure targeting rhino horn and the low reproductive performance among the few remaining females (29). On the other hand, wild boar experience very little poaching pressure due to the regionally widespread Halal dietary taboo on pork, and they have the highest reproductive capabilities of the region’s megafauna (30), likely explaining their long-term persistence across the region. Furthermore, crop-raiding provides important cross-boundary food subsidies allowing wild boar populations to increase 100-fold in forest fragments nearby agriculture (30, 31). Dietary breadth may also explain contrasting extirpation patterns in large carnivores. Obligate carnivores such as tigers, leopards, and clouded leopards are acutely threatened by the loss of prey from poaching (4), and these species experienced more extirpations than sun bears that are primarily insectivorous and frugivorous (32). Positive abundance relationships with human pressures may be explained by landscape-specific conservation and enforcement. Heightened protection appears successful in Khao Yai in central Thailand and in Bukit Barisan Selatan in southern Sumatra where there is intense patrolling to protect the few remaining Sumatran rhinoceros (33, 34). High mammal abundances of Khao Yai may also arise from being part of the Indo-Malayan biogeographic subregion, being more deciduous, and having managed grasslands, compared to all other study landscapes located in the Sundaic biogeographic subregion and which have evergreen rainforest and no grasslands (35). On the contrary, the lack of anti-poaching enforcement in some larger and more remote parks (36) may explain the recent extirpation of tigers from Ulu Muda, one of our largest and least disturbed landscapes. Overall, while there were some predictable outcomes, Southeast Asia’s remaining megafauna often showed divergent responses to the same threats, which has been partially noted by past work (37).
Of the major tropical forest zones globally (e.g., the Amazon and Congo), Southeast Asia suffers the most forest degradation (26) and human pressures (38). Often missing from this alarming state is the large variation among its four major landmasses that span several biodiversity hotspots (39). We found that mainland Southeast Asia and Borneo experienced more extirpations during the Holocene, while Sumatra experienced more extirpations during the Anthropocene, although this may be biased by Sumatra’s lack of paleontological research to establish Holocene ranges (35). Recent Sumatran extirpations have likely been driven by rapid deforestation for pulp, rubber, and oil palm production, and this deforestation has occurred at a rate faster than Borneo and mainland Southeast Asia (17). Forest fragmentation and landcover changes in the matrix between fragments across vast areas are strongly associated with extinction risk in terrestrial mammals (40). We fear that megafauna extirpations in Sumatra may increase through the Anthropocene because of its growing extinction debt (41), such as on Java where considerably more megafaunal extinctions have accumulated after its longer history of land clearing (42).
Southeast Asia’s idiosyncratic Anthropocene megafauna assemblages are partially explained by the region’s biogeographic history. The Quaternary period saw multiple megafauna extinction events in Southeast Asia (43), and while past researchers believed that early human hunting and extreme geological events shaped Pleistocene distributions, such as the Toba Caldera Complex super-eruption (44, 45), contemporary researchers find little support for this (21, 43). Instead, pre-Holocene and Holocene megafauna distributions were primarily shaped by the rapid loss of tropical grasslands and forest habitats due to climate changes and sea-level rise that isolated Sumatra, Borneo, Java, and Peninsular Malaysia (10). These habitat changes would have favored rainforest specialists over more open-adapted species (43). In turn, the more recent anthropogenic degradation of tropical forests may positively affect those species adapted to more open habitats, such as wild boar, Asian elephants, and tigers, and this has been argued for orangutans (14). Given the profound habitat changes before the Holocene and the long exposure to humans, species remaining in the Anthropocene may have passed through an extinction filter and are more disturbance-resistant than previously appreciated (11), potentially explaining how megafauna can persist in disturbed tropical forests near human settlements.
The rise and fall of megafauna species trigger ecological cascades that change ecosystems (5, 6). For example, apex predators are keystone species in some ecosystems and structure food webs via top-down regulation of herbivores (46). However, Southeast Asian apex predators may not play such important roles because of their naturally low densities and possible bottom-up control associated with the strict mast fruiting phenology in the region (23, 33, 47). Asian elephants are ecosystem engineers whose physical disturbances reshape the understory, and they disperse some of the largest seeded trees (48). In addition, the ability of smaller vertebrates to compensate for the loss of megafauna to perpetuate their vital ecological roles remains under doubt (48). For example, the Malay tapir is unlikely to fully substitute the ecological role of Asian elephants because it cannot disperse the region’s largest seeded plants (49). The rise of some megafauna in degraded forests is also a problem. High densities of wild boars in forest edges produce strong shifts in plant diversity and function (50, 51), and their collapse due to African swine fever could be equally disruptive (52). Ecological cascades research will be especially important for this region.
The peculiar distributions of Asian megafauna are an enduring puzzle for biogeographers, ecologists, and conservation biologists. While there is broad consensus that poaching and deforestation are the primary causes of dramatic declines across the region (26, 36), we also noted high diversity in tropical forests surrounded by agriculture and settlements, such as Bukit Barisan Selatan, Pasoh, and Khao Yai. This suggests that some megafauna can persist in disturbed landscapes where there is effective management. Last, the recent natural rewilding of wild boar and sambar deer in Singapore following a strict ban on poaching and substantial reforestation and conservation efforts (53) underscores the tenuous ability of humans to positively shape biodiversity patterns into the Anthropocene.
METHODS
Study landscapes
We conducted camera trapping surveys to document megafauna extirpations and measure megafauna local abundance in 10 lowland primary tropical forest landscapes in Sumatra (3), Borneo (2), Singapore (1), Peninsular Malaysia (2), and Thailand (2) (Fig. 1, map). We refer to our sampling areas as a “landscape,” typically a national park, production forest, or collection of forest patches. In Sumatra, Indonesia, we surveyed Gunung Leuser National Park (8630 km2), Kerinci Seblat National Park (13,753 km2), and Bukit Barisan Selatan National Park (3568 km2), which all comprise the UNESCO Tropical Rainforest Heritage of Sumatra (33). All Sumatran landscapes had a mixture of primary forest and some smaller forests with selective logging over 20 years ago and moderate levels of hunting (33). In Malaysian Borneo, we surveyed one primary forest fragment, Lambir Hills National Park (69.5 km2), which has experienced high historical hunting pressure (54) and one continuous primary forest site, DVCA (438 km2). In Singapore, we surveyed the Central Catchment Nature Reserve (37 km2) and a small offshore island Palau Ubin (10 km2) and treated them as a single landscape due to their proximity and similar historic hunting pressure. In Peninsular Malaysia, we surveyed Pasoh Forest Reserve (130 km2) and its adjacent selectively logged production forest and the Ulu Muda Forest Reserve (1152.6 km2), a largely intact primary forest on the border with Southern Thailand. In Thailand, we surveyed Khao Ban That Wildlife Sanctuary (1267 km2), which is a fragmented sliver of forest along a low mountain range near densely populated human settlements, and Khao Yai National Park (2168 km2), an intact primary forest connected to the larger UNESCO World Heritage Dong Phayayen-Khao Yai Forest Complex (34). All study landscapes fall within the Sundaic biogeographical subregion with the key exception of Khao Yai National Park, which sits in the Indo-Malaysian biogeographical subregion. We included Khao Yai because it retains a similar megafauna assemblage and has experienced similar Anthropocene disturbances to the study landscapes in the Sundaic biogeographical subregion. See Fig. 1 for a map of all study landscapes and Table 1 for a characterization of each landscape.
Study species
We collected data on five apex predator species [Carnivora with average adult body masses ≥15 kg (2)]: The largest were tigers (Panthera tigris), followed by sun bears (Helarctos malayanus), leopards (Panthera pardus), dholes (Cuon alpinus), and clouded leopards (Neofelis nebulosa and diardi, but analyzed as one species). We also collected data on nine megaherbivore species [terrestrial mammalian herbivores with average adult body masses ≥100 kg (3)]: The largest were Asian elephants (Elephas maximus), followed by Sumatran rhinoceros (Dicerorhinus sumatrensis), gaur (Bos gaurus), banteng (Bos javanicus), Malay tapir (Tapirus indicus), sambar deer (Rusa unicolor), bearded pig (Sus barbatus), mainland serow (Capricornis sumatraensis), and wild boar (Sus scrofa). Average adult body masses for all species was determined by the PanTHERIA database (55) and by Francis and Barrett (56) for species not included in the database (e.g., gaur). We limited our analysis to megafauna species currently recognized by the IUCN as extant in at least one of our 10 landscapes; thus, we did not include species historically distributed across our 10 landscapes [e.g., Javan rhino (Rhinoceros sondaicus) (57) or spotted hyena (Crocuta crocuta) (35)]. For species weights, IUCN status, and detection histories, see Table 2. For detailed accounts of each study species’ Holocene and Anthropocene distributions, see table S3.
Camera trapping
We collected information on megafauna populations using 21 systematic camera trapping sessions at our 10 landscapes. At each landscape, we deployed 22 to 112 passive infrared camera traps set across areas of 48 to 830 km2 (see table S1 for full deployment details). We standardized camera deployment between landscapes by attaching cameras to trees 0.2 to 0.3 m high and placing them along natural wildlife trails without baits. Cameras were deployed for approximately 60 to 90 days (mean = 40.4, SD = 31.6) at each site with 63,423 total trap nights across the entire study (table S1). We considered captures independent if they occurred at least 30 min apart. Cameras were systematically deployed in each landscape, but because of varying camera spacing between large forests (> 0.5 km) and small fragments (< 0.5 km), we spatially resampled our camera trapping locations by overlaying a grid of 7.79 km2 hexagonal cells (hereafter “sampling unit”), and when multiple cameras fell within the same sampling unit, we aggregated the detections on the basis of their date. This ensures comparability among landscapes, prevents spatial pseudo-replication, and, by using a sampling unit size larger than the species’ home range, ensures that abundance is being measured, as opposed to habitat use (58).
Generating covariates
We collected data on 12 forest quality, anthropogenic, and abiotic variables for each of our camera trapping deployments to examine how they affect megafauna extirpations and local abundance (table S2). Our covariates were derived from GIS layers of varying resolution and included the linear distance from the camera trap location to the covariate of interest (e.g., distance to forest edge, rivers, and settlements), and the percentage of a landcover type in a 1-km radius around each camera trap for various landcover classes common in Southeast Asia (e.g., oil palm plantations, intact forest cover, and degraded landcover) (59). We also calculated point values of several covariates at each camera trap location, including elevation, annual precipitation, FLII, and HFP. The FLII is a globally continuous measure of the world’s forests status that integrates both observed and inferred human pressures (e.g., deforestation and hunting pressure, respectively) with the loss of forest connectivity to represent landscape-level ecological integrity (26). The HFP is a globally continuous measure combining eight variables that measure the direct and indirect human pressures on the environment to represent landscape-level anthropogenic disturbances (38). When multiple camera traps fell into a single 7.79 km2 sampling unit (described above), we averaged the values of the covariates for each camera. Last, we calculated the same covariates in a 10-km radius around each study landscape to characterize each landscape, which allowed us to group the landscapes into binary categories following prior examples (26, 38) into “most disturbed” (i.e., intact forest cover <60%, FLII <7, human population density >6000, and HFP > 7) or “least disturbed” (i.e., intact forest cover >60%, FLII >7, human population density <6000, and HFP < 7). While we acknowledge an HFP score of 6+ would suggest a high anthropogenic pressure (38), the omnipresent grasp of humanity across our study region inflates the HFP values within each landscape above global mean values; hence, we have chosen a higher threshold to distinguish between least and most disturbed landscapes. See table S2 for a full list of covariates and sources.
Determining extirpation
Holocene ranges were defined as the most likely distribution across our 10 study landscapes ~11,700 years ago, at the beginning of the Holocene epoch (8, 9). Holocene ranges were extracted and fact-checked in the published literature, primarily using two main references (35, 57). We also used “snowball sampling,” where we examined the reference lists from the two main references (37, 53), and IUCN Red List reports for each species (table S3). Because of some incompleteness of paleontological records and the wide-ranging movements of megafauna plus fluctuations in available land area and suitable habitat, we intentionally treat our time definition as loosely covering the Late Pleistocene–Holocene boundary (10). We acknowledge that there were megafaunal range contractions occurring throughout the Early Pleistocene–Holocene (2.6 million to ~11,700 years ago) and excluded species from areas wherever there was strong evidence that they were lost before the Late Pleistocene–Holocene boundary (28).
To determine Anthropocene extirpations, we used distributions defined by the IUCN Red List (as of 2021; see table S3 for sources). A species was documented as present if their IUCN Red List range overlapped with our camera trap detections (plus 10-km buffer), assessed using the “sf” package (60) in R version 4.0.2 (61). We did not count Anthropocene extirpations in areas the IUCN distribution defined as “Extinct” or “Possibly Extinct,” and only counted Anthropocene extirpations in areas the IUCN defined as “Extant” and “Possibly Extant.” In the event a species was not detected in a camera trapping session, we assume that it was extirpated from the landscape or persists at such a low abundance that it is functionally extinct (hereafter “extirpated”). One limitation to this approach is that extirpations were treated as a binary variable despite some species potentially naturally occurring at extremely low densities or being so cryptic as to have no detections. Last, we determined contemporary extirpations from the small island nation of Singapore using the government’s official list of mammals (62) to exclude species within 10 km on the Malaysia-Singapore border (e.g., Asian elephants; table S3).
Examining extirpations as a function of guild, size, and environmental factors
To test our prediction that large carnivores would experience significantly more extirpations than megaherbivores, we conducted a Welch two-sample t test to examine whether the relative number of species extirpated between guilds was significantly different from each other, and we repeated this for both Anthropocene and Holocene extirpations. In addition, we used t tests to compare the relative number of species extirpated during the Holocene and Anthropocene within each guild by splitting species by the median average adult body weights as determined by the PanTHERIA database (55) and by Francis and Barrett (56) for species not included in the database (e.g., gaur). Last, we used a t test to compare total extirpations between our four least disturbed and six most disturbed landscapes. To examine whether forest degradation and anthropogenic pressures drive both Anthropocene and Holocene extirpations, we assessed the impact of our 12 covariates (table S2) by implementing univariate GLMs. Our response variable was the proportion of megafauna species extirpated relative to the number of potential species present per landscape and was treated with a binomial error distribution. We ran univariate GLMs for each covariate averaged across all cameras in a single landscape and scaled the covariate with a mean of 0 and SD of 1 to ensure comparable results. We evaluated which covariates were important by inspecting beta (β) coefficients, SE, and P values using the “glm” function in R. The β, SE, residual deviance, and P values from all univariate GLMs are included in table S4, and all statistics were implemented in R (61).
Hierarchal abundance modeling
We estimated species abundance at each landscape using hierarchal N-mixture models that account for imperfect detection (63). This detection-corrected estimated local abundance (hereafter just “abundance”) serves as an unbiased metric compared to traditional camera trapping measures (64). A key advantage of using N-mixture models is that it accurately quantifies the spatial variation in abundance as a function of covariates and is useful for elucidating key variables that drive abundance trends across species’ populations (65). Instead of interpreting the output as true densities, we consider that the variation in abundance across the range of observed covariates reflects true differences (65). For each species with more than 30 independent detections, we produced count history matrices from each landscape where the species was detected at least once and used the total number of individuals captured in a window of 3 days. All N-mixture models were implemented in the R package “unmarked” (66). To account for differing effort per sampling units, the number of trap nights per sampling unit was included as a detection probability covariate in all N-mixture models. To account for landscapes with repeated surveys and unmodeled variation between landscapes, a landscape fixed effect was included as an abundance covariate in all N-mixture models. Therefore, our “null” model to compare competing models contained a fixed effect for effort on the detection probability formula and a fixed effect of landscape on the abundance formula. We tested how the abundance of each species was affected by each forest quality, anthropogenic, and abiotic covariate (table S2) by implementing univariate models that built upon our null model. We compared models per species using AICc scores, with the best AICc being the lowest by at least 2 (67). If a species had multiple univariate models with a ∆AICc <2, and the variables were not strongly correlated (r < |0.6|), we implemented multivariate models to determine the best combination of covariates that explain the species abundance. Full model selection information with covariate effect sizes, P values, AIC scores, and R2 values are provided for all species assessed in table S5.
Acknowledgments
We thank Yayasan Sabah, the Sabah Forest Department, the Sabah Biodiversity Council, and the Danum Valley Management Committee, A. Hamid, G. Renolds, J. Brodie, K. Doehla, and R. Karolus for permission and help conducting fieldwork at Danum Valley. We thank P. Jansen, the Smithsonian Institute, and the Tropical Ecology Assessment and Monitoring (TEAM) network for help collecting data from Pasoh, as well as Y. L. Leong and the Forest Research Institute Malaysia (FRIM) for permissions to work at Pasoh. We thank M. B. Mohamad, J. Kulis, and the Sarawak Forestry Department for permission to conduct fieldwork at Lambir Hills. We thank A. Loo, M. Khoo, B. Lee, J. Chan, A. Goh, and NParks for permission and help with fieldwork in Singapore. We thank W. Chantorn, A. Nathalang, S. Bunyavejchewin, R. Sukmasuang, F. Gutierez, and C. Scanlon for permissions and help at Khao Yai and Khao Ban That. We thank W. R. Albert, M. Linkie, R. Dinata, H. Wibisono, E. D. Sembiring, Tarmizi and E. Ramadiyanta, Salpayanri, I. Tanjung, and C. Decky, Institution Conservation Society (ICS)—Solok Selatan Wahana Konservasi Masyarakat, the Leuser International Foundation, Fauna and Flora International, Wildlife Conservation Society, and HarimauKita for help in Sumatra. Original artwork was provided by T. Barber from Talking Animals and is copyrighted. We thank J. E. M. Watson, the members of the Ecological Cascades Lab at the University of Queensland, and two anonymous reviewers for providing invaluable feedback on earlier drafts.
Funding: The research was funded by the Smithsonian Institution’s ForestGEO program, Nanyang Technological University in Singapore, the National Geographic Society from Committee for the Research and Exploration no. 9384-13, and numerous small grants for fieldwork. M.S.L. was supported by an Australian Research Council Discovery Early Career Researcher Award no. DE210101440.
Author contributions: Conceptualization: Z.A. and M.S.L. Methodology: Z.A., J.H.M., P.J.N., and M.S.L. Investigation: Z.A., P.J.N., and M.S.L. Visualization: Z.A., J.H.M., and M.S.L. Supervision: M.S.L. Writing—original draft: Z.A. and M.S.L. Writing—review and editing: Z.A., J.H.M., P.J.N., and M.S.L.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Data and R code to implement our analysis can be found at the following DOI: https://doi.org/10.6084/m9.figshare.20489046.
Supplementary Materials
This PDF file includes:
Tables S1 to S5
References
REFERENCES AND NOTES
- 1.Dirzo R., Young H. S., Galetti M., Ceballos G., Isaac N. J., Collen B., Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Ripple W. J., Estes J. A., Beschta R. L., Wilmers C. C., Ritchie E. G., Hebblewhite M., Berger J., Elmhagen B., Letnic M., Nelson M. P., Schmitz O. J., Smith D. W., Wallach A. D., Wirsing A. J., Status and ecological effects of the world’s largest carnivores. Science 343, 1241484–1241484 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Ripple W. J., Newsome T. M., Wolf C., Dirzo R., Everatt K. T., Galetti M., Hayward M. W., Kerley G. I. H., Levi T., Lindsey P. A., Macdonald D. W., Malhi Y., Painter L. E., Sandom C. J., Terborgh J., van Valkenburgh B., Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf C., Ripple W. J., Prey depletion as a threat to the world’s large carnivores. R. Soc. Open Sci. 3, 160252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estes J. A., Terborgh J., Brashares J. S., Power M. E., Berger J., Bond W. J., Carpenter S. R., Essington T. E., Holt R. D., Jackson J. B., Marquis R. J., Oksanen L., Oksanen T., Paine R. T., Pikitch E. K., Ripple W. J., Sandin S. A., Scheffer M., Schoener T. W., Shurin J. B., Sinclair A. R., Soulé M. E., Virtanen R., Wardle D. A., Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Malhi Y., Doughty C. E., Galetti M., Smith F. A., Svenning J. C., Terborgh J. W., Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. U.S.A. 113, 838–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaston K. J., Fuller R. A., Commonness, population depletion and conservation biology. Trends Ecol. Evol. 23, 14–19 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Hanebuth T. J. J., Stattegger K., Bojanowski A., Termination of the last glacial maximum sea-level lowstand: The Sunda-Shelf data revisited. Global Planet. Change 66, 76–84 (2009). [Google Scholar]
- 9.Lambeck K., Rouby H., Purcell A., Sun Y., Sambridge M., Sea level and global ice volumes from the last glacial maximum to the Holocene. Proc. Natl. Acad. Sci. U.S.A. 111, 15296–15303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon C. H., Morley R. J., Bush A. B. G., The current refugial rainforests of Sundaland Are unrepresentative of their biogeographic past and highly vulnerable to disturbance. Proc. Natl. Acad. Sci. U.S.A. 106, 11188–11193 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betts M. G., Wolf C., Pfeifer M., Banks-Leite C., Arroyo-Rodríguez V., Ribeiro D. B., Barlow J., Eigenbrod F., Faria D., Fletcher R. J. Jr., Hadley A. S., Hawes J. E., Holt R. D., Klingbeil B., Kormann U., Lens L., Levi T., Medina-Rangel G. F., Melles S. L., Mezger D., Morante-Filho J. C., Orme C. D. L., Peres C. A., Phalan B. T., Pidgeon A., Possingham H., Ripple W. J., Slade E. M., Somarriba E., Tobias J. A., Tylianakis J. M., Urbina-Cardona J. N., Valente J. J., Watling J. I., Wells K., Wearn O. R., Wood E., Young R., Ewers R. M., Extinction filters mediate the global effects of habitat fragmentation on animals. Science 366, 1236–1239 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Andermann T., Faurby S., Turvey S. T., Antonelli A., Silvestro D., The past and future human impact on mammalian diversity. Sci. Adv. 6, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett L. J., Williams D. R., Prescott G. W., Balmford A., Green R. E., Eriksson A., Valdes P. J., Singarayer J. S., Manica A., Robustness despite uncertainty: Regional climate data reveal the dominant role of humans in explaining global extinctions of Late Quaternary megafauna. Ecography 39, 152–161 (2016). [Google Scholar]
- 14.Spehar S. N., Sheil D., Harrison T., Louys J., Ancrenaz M., Marshall A. J., Wich S. A., Bruford M. W., Meijaard E., Orangutans venture out of the rainforest and into the anthropocene. Sci. Adv. 4, e1701422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters C. N., Zalasiewicz J., Summerhayes C., Barnosky A. D., Poirier C., Gałuszka A., Cearreta A., Edgeworth M., Ellis E. C., Ellis M., Jeandel C., Leinfelder R., McNeill J. R., Richter D. B., Steffen W., Syvitski J., Vidas D., Wagreich M., Williams M., Zhisheng A., Grinevald J., Odada E., Oreskes N., Wolfe A. P., The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351, 137–137 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Banks-Leite C., Betts M. G., Ewers R. M., Orme C. D. L., Pigot A. L., The macroecology of landscape ecology. Trends Ecol. Evol. 37, 480–487 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Wilcove D. S., Giam X., Edwards D. P., Fisher B., Koh L. P., Navjot’s nightmare revisited: Logging, agriculture, and biodiversity in Southeast Asia. Trends Ecol. Evol. 28, 531–540 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Ardiantiono, Sugiyo, Johnson P. J., Lubis M. I., Amama F., Sukatmoko, Marthy W., Zimmermann A., Towards coexistence: Can people’s attitudes explain their willingness to live with Sumatran elephants in Indonesia? Conservation Science and Practice 3, (2021). [Google Scholar]
- 19.Wilson S. G., Biggs D., Kark S., Protecting an icon: Javan rhinoceros frontline management and conservation. Oryx , 1–7 (2021). [Google Scholar]
- 20.Estes J. G., Othman N., Ismail S., Ancrenaz M., Goossens B., Ambu L. N., Estes A. B., Palmiotto P. A., Quantity and configuration of available elephant habitat and related conservation concerns in the lower Kinabatangan Floodplain of Sabah, Malaysia. PLOS ONE 7, e44601 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louys J., Limited effect of the Quaternary’s largest super-eruption (Toba) on land mammals from Southeast Asia. Quat. Sci. Rev. 26, 3108–3117 (2007). [Google Scholar]
- 22.Atkinson Q. D., Gray R. D., Drummond A. J., mtDNA variation predicts population size in humans and reveals a major Southern Asian chapter in human prehistory. Mol. Biol. Evol. 25, 468–474 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Curran L. M., Leighton M., Vertebrate responses to spatiotemporal variation in seed production of mast-fruiting Dipterocarpaceae. Ecol. Monogr. 70, 101–128 (2000). [Google Scholar]
- 24.Carbone C., Gittleman J. L., A common rule for the scaling of carnivore density. Science 295, 2273–2276 (2002). [DOI] [PubMed] [Google Scholar]
- 25.S. Ellis, B. Talukdar, Dicerorhinus sumatrensis. The IUCN Red List of Threatened Species 2020: e.T6553A18493355 (2020).
- 26.Grantham H. S., Duncan A., Evans T. D., Jones K. R., Beyer H. L., Schuster R., Walston J., Ray J. C., Robinson J. G., Callow M., Clements T., Costa H. M., DeGemmis A., Elsen P. R., Ervin J., Franco P., Goldman E., Goetz S., Hansen A., Hofsvang E., Jantz P., Jupiter S., Kang A., Langhammer P., Laurance W. F., Lieberman S., Linkie M., Malhi Y., Maxwell S., Mendez M., Mittermeier R., Murray N. J., Possingham H., Radachowsky J., Saatchi S., Samper C., Silverman J., Shapiro A., Strassburg B., Stevens T., Stokes E., Taylor R., Tear T., Tizard R., Venter O., Visconti P., Wang S., Watson J. E. M., Anthropogenic modification of forests means only 40% of remaining forests have high ecosystem integrity. Nat. Commun. 11, 5978–5978 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symes W. S., Edwards D. P., Miettinen J., Rheindt F. E., Carrasco L. R., Combined impacts of deforestation and wildlife trade on tropical biodiversity are severely underestimated. Nat. Commun. 9, 4052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.J. Louys, in Paleontology in Ecology and Conservation (Springer, 2012), pp. 227–238. [Google Scholar]
- 29.Kretzschmar P., Kramer-Schadt S., Ambu L., Bender J., Bohm T., Ernsing M., Göritz F., Hermes R., Payne J., Schaffer N., Thayaparan S. T., Zainal Z. Z., Hildebrandt T. B., Hofer H., The catastrophic decline of the Sumatran rhino (Dicerorhinus sumatrensis harrissoni) in Sabah: Historic exploitation, reduced female reproductive performance and population viability. Glob. Ecol. Conserv. 6, 257–275 (2016). [Google Scholar]
- 30.Luskin M. S., Brashares J. S., Ickes K., Sun I. F., Fletcher C., Wright S. J., Potts M. D., Cross-boundary subsidy cascades from oil palm degrade distant tropical forests. Nat. Commun. 8, 2231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luskin M. S., Christina E. D., Kelley L. C., Potts M. D., Modern hunting practices and wild meat trade in the oil palm plantation-dominated landscapes of Sumatra, Indonesia. Hum. Ecol. 42, 35–45 (2014). [Google Scholar]
- 32.McConkey K., Galetti M., Seed dispersal by the sun bear Helarctos malayanus in Central Borneo. J. Trop. Ecol. 15, 237–241 (1999). [Google Scholar]
- 33.Luskin M. S., Albert W. R., Tobler M. W., Sumatran tiger survival threatened by deforestation despite increasing densities in parks. Nat. Commun. 8, 1783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pla-Ard M., Sukmasuang R., Srinopawan K., Population characteristics and habitat suitability of Asian elephants (Elephas maximus Linnaeus, 1758) in the Khao Yai National Park, Thailand. Eur. J. Ecol. 5, 62–71 (2019). [Google Scholar]
- 35.Louys J., Curnoe D., Tong H., Characteristics of Pleistocene megafauna extinctions in Southeast Asia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 243, 152–173 (2007). [Google Scholar]
- 36.Figel J. J., Hambal M., Krisna I., Putra R., Yansyah D., Malignant snare traps threaten an irreplaceable megafauna community. Trop. Conserv. Sci. 14, 194008292198918 (2021). [Google Scholar]
- 37.Tilker A., Abrams J. F., Mohamed A., Nguyen A., Wong S. T., Sollmann R., Niedballa J., Bhagwat T., Gray T. N. E., Rawson B. M., Guegan F., Kissing J., Wegmann M., Wilting A., Habitat degradation and indiscriminate hunting differentially impact faunal communities in the Southeast Asian tropical biodiversity hotspot. Commun. Biol. 2, 396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venter O., Sanderson E. W., Magrach A., Allan J. R., Beher J., Jones K. R., Possingham H. P., Laurance W. F., Wood P., Fekete B. M., Levy M. A., Watson J. E. M., Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7, 12558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers N., Mittermeier R. A., Mittermeier C. G., Da Fonseca G. A. B., Kent J., Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Ramírez-Delgado J. P., di Marco M., Watson J. E. M., Johnson C. J., Rondinini C., Corredor Llano X., Arias M., Venter O., Matrix condition mediates the effects of habitat fragmentation on species extinction risk. Nat. Commun. 13, 595 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Figueiredo L., Krauss J., Steffan-Dewenter I., Sarmento Cabral J., Understanding extinction debts: Spatio–temporal scales, mechanisms and a roadmap for future research. Ecography 42, 1973–1990 (2019). [Google Scholar]
- 42.Louys J., Braje T. J., Chang C. H., Cosgrove R., Fitzpatrick S. M., Fujita M., Hawkins S., Ingicco T., Kawamura A., MacPhee R. D. E., McDowell M. C., Meijer H. J. M., Piper P. J., Roberts P., Simmons A. H., van den Bergh G., van der Geer A., Kealy S., O’Connor S., No evidence for widespread island extinctions after Pleistocene hominin arrival. Proc. Natl. Acad. Sci. U.S.A. 118, e2023005118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louys J., Roberts P., Environmental drivers of megafauna and hominin extinction in Southeast Asia. Nature 586, 402–406 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Martin P. S., Africa and Pleistocene overkill. Nature 212, 339–342 (1966). [DOI] [PubMed] [Google Scholar]
- 45.Ambrose S. H., Late Pleistocene human population bottlenecks, volcanic winter, and differentiation of modern humans. J. Hum. Evol. 34, 623–651 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Terborgh J., Lopez L., Nuñez P., Rao M., Shahabuddin G., Orihuela G., Riveros M., Ascanio R., Adler G. H., Lambert T. D., Balbas L., Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Brodie J. F., Giordano A., Lack of trophic release with large mammal predators and prey in Borneo. Biol. Conserv. 163, 58–67 (2013). [Google Scholar]
- 48.Prugh L. R., Stoner C. J., Epps C. W., Bean W. T., Ripple W. J., Laliberte A. S., Brashares J. S., The rise of the Mesopredator. BioScience 59, 779–791 (2009). [Google Scholar]
- 49.Campos-Arceiz A., Traeholt C., Jaffar R., Santamaria L., Corlett R. T., Asian tapirs are no elephants when it comes to seed dispersal. Biotropica 44, 220–227 (2012). [Google Scholar]
- 50.Luskin M. S., Ickes K., Yao T. L., Davies S. J., Wildlife differentially affect tree and liana regeneration in a tropical forest: An 18-year study of experimental terrestrial defaunation versus artificially abundant herbivores. J. Appl. Ecol. 56, 1379–1388 (2019). [Google Scholar]
- 51.Luskin M. S., Johnson D. J., Ickes K., Davies S. J., Wildlife disturbances as a source of conspecific negative density-dependent mortality in tropical trees. Proc. R. Soc. B 288, 20210001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albrechtsen L., Macdonald D. W., Johnson P. J., Castelo R., Fa J. E., Faunal loss from bushmeat hunting: Empirical evidence and policy implications in Bioko Island. Environ. Sci. Policy 10, 654–667 (2007). [Google Scholar]
- 53.Lum S., Kang Min N., Lessons in ecology and conservation from a tropical forest fragment in Singapore. Biol. Conserv. 254, 108847 (2021). [Google Scholar]
- 54.Harrison R. D., Emptying the forest: Hunting and the extirpation of wildlife from tropical nature reserves. Bioscience 61, 919–924 (2011). [Google Scholar]
- 55.Jones K. E., Bielby J., Cardillo M., Fritz S. A., O’Dell J., Orme C. D. L., Safi K., Sechrest W., Boakes E. H., Carbone C., Connolly C., Cutts M. J., Foster J. K., Grenyer R., Habib M., Plaster C. A., Price S. A., Rigby E. A., Rist J., Teacher A., Bininda-Emonds O. R. P., Gittleman J. L., Mace G. M., Purvis A., PanTHERIA: A species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648–2648 (2009). [Google Scholar]
- 56.C. M. Francis, P. Barrett, A Field Guide to the Mammals of South-East Asia (New Holland, 2008). [Google Scholar]
- 57.Mahmood T., Vu T. T., Campos-Arceiz A., Akrim F., Andleeb S., Farooq M., Hamid A., Munawar N., Waseem M., Hussain A., Fatima H., Khan M. R., Mahmood S., Historical and current distribution ranges and loss of mega-herbivores and carnivores of Asia. PeerJ 9, e10738 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rayan D. M., Linkie M., Managing threatened ungulates in logged-primary forest mosaics in Malaysia. PLOS ONE 15, e0243932 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miettinen J., Shi C., Liew S. C., 2015 Land cover map of Southeast Asia at 250 m spatial resolution. Remote Sens. Lett. 7, 701–710 (2016). [Google Scholar]
- 60.Pebesma E., Simple features for R: Standardized support for spatial vector data. R J. 10, 439–446 (2018). [Google Scholar]
- 61.R Development Core Team. (R Foundation for Statistical Computing, Vienna, Austria, 2021).
- 62.Terrestrial and Marine Mammals Checklist (2021);https://nparks.gov.sg/biodiversity/wildlife-in-singapore/species-list/mammal.
- 63.Royle J. A., N-mixture models for estimating population size from spatially replicated counts. Biometrics 60, 108–115 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Sollmann R., Mohamed A., Samejima H., Wilting A., Risky business or simple solution – Relative abundance indices from camera-trapping. Biol. Conserv. 159, 405–412 (2013). [Google Scholar]
- 65.Gilbert N. A., Clare J. D. J., Stenglein J. L., Zuckerberg B., Abundance estimation of unmarked animals based on camera-trap data. Conserv. Biol. 35, 88–100 (2021). [DOI] [PubMed] [Google Scholar]
- 66.Fiske I., Chandler R., ‘unmarked’: An R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Soft. 43, 1–23 (2011). [Google Scholar]
- 67.K. P. Burnham, D. R. Anderson, Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, ed. 2, 2002). [Google Scholar]
- 68.Voris H. K., Maps of Pleistocene sea levels in Southeast Asia: Shorelines, river systems and time durations. J. Biogeogr. 27, 1153–1167 (2000). [Google Scholar]
- 69.J. Goodrich, A. Lynam, D. Miquelle, H. Wibisono, K. Kawanishi, A. Pattanavibool, S. Htun, T. Tempa, J. Karki, Y. Jhala, U. Karanth, Panthera tigris, The IUCN Red List of Threatened Species 2015: e.T15955A50659951 (2015).
- 70.Meijaard E., Biogeographic history of the Javan Leopard panthera pardus based on a craniometric analysis. J. Mammal. 85, 302–310 (2004). [Google Scholar]
- 71.Rostro-García S., Kamler J. F., Ash E., Clements G. R., Gibson L., Lynam A. J., McEwing R., Naing H., Paglia S., Endangered leopards: Range collapse of the Indochinese leopard (Panthera pardus delacouri) in Southeast Asia. Biol. Conserv. 201, 293–300 (2016). [Google Scholar]
- 72.A. B. Stein, V. Athreya, P. Gerngross, G. Balme, P. Henschel, U. Karanth, D. Miquelle, S. Rostro-Garcia, J. F. Kamler, A. Laguardia, I. Khorozyan, A. Ghoddousi, Panthera pardus, The IUCN Red List of Threatened Species 2020: e.T15954A163991139 (2020).
- 73.Chiang P.-J., Allen M. L., A review of our current knowledge of clouded leopards (Neofelis nebulosa). Int. J. Avian Wildlife Biol. 2, 00032 (2017). [Google Scholar]
- 74.D’Cruze N., Macdonald D. W., Clouded in mystery: The global trade in clouded leopards. Biodivers. Conserv. 24, 3505–3526 (2015). [Google Scholar]
- 75.A. Hearn, J. Ross, J. Brodie, S. Cheyne, I. A. Haidlir, B. Loken, J. Mathai. A. Wilting, J. McCarthy, Neofelis diardi, The IUCN Red List of Threatened Species 2015: e.T136603A97212874 (2015).
- 76.T. Gray, J. Borah, C. N. Z. Coudrat, Y. Ghimirey, A. Giordano, E. Greenspan, W. Petersen, S. Rostro-Garcia, M. Shariff, W. Wai-Ming, Neofelis nebulosa, The IUCN Red List of Threatened Species 2021: e.T14519A198843258 (2021).
- 77.J. F. Kamler, N. Songasen, K. Jenks, A. Srivathsa, L. Sheng, K. E. Kunkel, Cuon alpinus, The IUCN Red List of Threatened Species 2015: e.T5953A72477893 (2015).
- 78.Thinley P., Rajaratnam R., Kamler J. F., Wangmo C., Conserving an endangered canid: Assessing distribution, habitat protection, and connectivity for the dhole (Cuon alpinus) in Bhutan. Front. Conserv. Sci. 2, 654976 (2021). [Google Scholar]
- 79.Scotson L., Fredriksson G., Ngoprasert D., Wong W.-M., Fieberg J., Projecting range-wide sun bear population trends using tree cover and camera-trap bycatch data. PLOS ONE 12, e0185336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.L. Scotson, G. Fredriksson, D. Augeri, C. Cheah, D. Ngoprasert, W. Wai-Ming, Helarctos malayanus, The IUCN Red List of Threatened Species 2017: e.T9760A123798233 (2017).
- 81.C. Williams, S. K. Tiwari, V. R. Goswami, S. de Silva, A. Kumar, N. Baskaran, K. Yoganand, V. Menon, Elephas maximus, The IUCN Red List of Threatened Species 2020: e.T7140A45818198 (2020).
- 82.Fernando P., Vidya T. N. C., Payne J., Stuewe M., Davison G., Alfred R. J., Andau P., Bosi E., Kilbourn A., Melnick D. J., DNA analysis indicates that asian elephants are native to borneo and are therefore a high priority for conservation. PLOS Biol. 1, e6 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Havmøller R. G., Payne J., Ramono W., Ellis S., Yoganand K., Long B., Dinerstein E., Williams A. C., Putra R. H., Gawi J., Talukdar B. K., Burgess N., Will current conservation responses save the critically endangered Sumatran rhinoceros Dicerorhinus sumatrensis? Oryx 50, 355–359 (2016). [Google Scholar]
- 84.Ahmad Zafir A. W., Payne J., Mohamed A., Lau C. F., Sharma D. S. K., Alfred R., Williams A. C., Nathan S., Ramono W. S., Clements G. R., Now or never: What will it take to save the Sumatran rhinoceros Dicerorhinus sumatrensis from extinction? Oryx 45, 225–233 (2011). [Google Scholar]
- 85.J. W. Duckworth, K. Sankar, A. C. Williams, N. Samba Kumar, R. J. Timmins, Bos gaurus, The IUCN Red List of Threatened Species 2016: e.T2891A46363646 (2016).
- 86.Pedrono M., Tuan H. M., Chouteau P., Vallejo F., Status and distribution of the endangered banteng Bos javanicus birmanicus in Vietnam: A conservation tragedy. Oryx 43, 618–625 (2009). [Google Scholar]
- 87.P. Gardner, S. Hedges, S. Pudyatmoko, T. N. E. Gray, R. J. Timmins, Bos javanicus, The IUCN Red List of Threatened Species 2016: e.T2888A46362970 (2016).
- 88.Cranbrook E. O., Piper P. J., Borneo records of Malay tapir, Tapirus indicus Desmarest: A zooarchaeological and historical review. Int. J. Osteoarchaeol. 19, 491–507 (2009). [Google Scholar]
- 89.C. Traeholt, W. Novarino, S. bin Saaban, N. M. Shwe, A. Lynam, Z. Zainuddin, B. Simpson, S. bin Mohd, Tapirus indicus, The IUCN Red List of Threatened Species 2016: e.T21472A45173636 (2016).
- 90.R. Timmins, K. Kawanishi, B. Giman, A. Lynam, B. Chan, R. Steinmetz, H. Sagar Baral, N. Samba Kumar, Rusa unicolor, The IUCN Red List of Threatened Species 2015: e.T41790A85628124 (2015).
- 91.Kantapon S., Jaeger J.-J., Shoocongdej R., Chaimanee Y., Wattanapituksakul A., Bocherens H., Long-term isotope evidence on the diet and habitat breadth of pleistocene to holocene caprines in Thailand: Implications for the extirpation and conservation of himalayan gorals. Front. Ecol. Evol. 8, 67 (2020). [Google Scholar]
- 92.T. D. Phan, S. Nijhawan, S. Li, L. Xiao, Capricornis sumatraensis, The IUCN Red List of Threatened Species 2020: e.T162916735A162916910 (2020).
- 93.O. Keuling, K. Leus, Sus scrofa, The IUCN Red List of Threatened Species 2019: e.T41775A44141833 (2019).
- 94.Ke A., Luskin M. S., Integrating disparate occurrence reports to map data-poor species ranges and occupancy: A case study of the Vulnerable bearded pig Sus barbatus. Oryx 53, 377–387 (2019). [Google Scholar]
- 95.M. Luskin, A. Ke, E. Meijaard, M. Gumal, K. Kawanishi, Sus barbatus, The IUCN Red List of Threatened Species 2017: e.T41772A123793370 (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S5
References