Abstract
RORγt is known to instruct the differentiation of T helper 17 (TH17) cells that mediate the pathogenesis of autoimmune diseases. However, it remains unknown whether RORγt plays a distinct role in the differentiation and effector function of TH17 cells. Here, we show that mutation of RORγt lysine-256, a ubiquitination site, to arginine (K256R) separates the RORγt role in these two functions. Preventing ubiquitination at K256 via arginine substitution does not affect RORγt-dependent thymocyte development, and TH17 differentiation in vitro and in vivo, however, greatly impaired the pathogenesis of TH17 cell–mediated experimental autoimmune encephalomyelitis (EAE). Mechanistically, K256R mutation impairs RORγt to bind to and activate Runx1 expression critical for TH17-mediated EAE. Thus, RORγt regulates the effector function of TH17 cells in addition to TH17 differentiation. This work informs the development of RORγt-based therapies that specifically target the effector function of TH17 cells responsible for autoimmunity.
RORγt is required for both the effector function of TH17 cells and TH17 differentiation.
INTRODUCTION
Interleukin-17 (IL-17)–producing CD4+ T helper, or T helper 17, (TH17) cells participate in immune responses against pathogens and the pathogenesis of diverse immunological diseases such as autoimmune diseases and even autism (1–6). The transcription factor retinoid-related orphan receptor γt (RORγt), encoded by the gene Rorc, instructs the differentiation of TH17 cells (7–9). Mutations in Rorc affect IL-17 production and lead to severe immune deficiency in both mouse (7) and human (10). Thus, therapies that control the pathogenic TH17 responses are needed clinically (11, 12). Numerous pharmacological RORγt inhibitors have been developed for clinical application (5, 11–13). Those inhibitors are believed to prevent TH17-dependent autoimmunity by inhibiting the generation of TH17 cells due to the essential function of RORγt in TH17 differentiation.
The strength of the TH17 immune responses is determined by the overall number of TH17 cells and their effector function. TH17 cells are derived from naïve CD4+ T cells upon activation in the presence of an appropriate cytokine milieu including IL-6, transforming growth factor–β (TGF-β), and/or IL-23 (7, 14). Although the function of RORγt in TH17 differentiation has long been demonstrated, it remains unknown whether RORγt plays a role in the effector function of TH17 cells and whether RORγt has a distinct role in the differentiation versus effector function of TH17 cells. In addition to regulating TH17 cells, RORγt enhances thymocyte survival (15–18) and is required for lymph node development (8, 18–20). Previously, we have generated a mutation in RORγt that disrupts TH17 differentiation but not thymocyte development (8), indicating that RORγt uses different mechanisms to regulate the function of TH17 cells and thymocytes.
Ubiquitination is a posttranslational modification that regulates many aspects of cellular function (21). Ubiquitin is conjugated to the lysine residues of the proteins to modulate protein function by regulating protein stability and/or protein-protein interactions. Thus, cellular context-dependent ubiquitination of RORγt may be a mechanism to modulate the diverse RORγt functions. In vitro studies indicate the regulation of RORγt stability by ubiquitination (22–25). However, in vivo function of RORγt ubiquitination is difficult to prove, as it requires the generation of the mice expressing mutant RORγt incapable of being ubiquitinated. Previous studies used mice deficient in ubiquitin ligases or deubiquitinases to understand the role of ubiquitination in immune responses (26). Such an approach prevents the ubiquitination of all the substrates; thus, it is impossible to determine the function of a specific substrate and even less about the function of a specific ubiquitination site on the substrate in immunological function.
In this study, three in vitro assays were developed to dissect RORγt function in thymocyte development, TH17 differentiation, and effector function in experimental autoimmune encephalomyelitis (EAE) induction. A RORγt mutation at a ubiquitination site, lysine (K)–256 to arginine (RORγtK256R), was found to specifically impair the effector function of TH17 cells in inducing EAE without interfering with RORγt function in TH17 differentiation and thymocyte development. A strain of mice was established to express RORγtK256R (RORγtK256R/K256R), which cannot be ubiquitinated at this site. This strain of mice allows us to determine the in vivo function of ubiquitination of RORγt at a specific site. RORγtK256R/K256R mice have normal thymocyte development, lymph node development, and TH17 differentiation. However, RORγtK256R/K256R mice display greatly impaired TH17 immune responses leading to EAE. Further, RORγtK256R/K256R TH17 cells showed decreased RORγtK256R binding to the promoter region of the Runx1 gene and reduced expression of Runx1. Forced expression of Runx1 in RORγtK256R/K256R TH17 cells restored the ability to induce EAE. Therefore, RORγt regulates the effector function of TH17 cells in addition to TH17 differentiation.
RESULTS
Three assays are developed to dissect RORγt function
RORγt−/− mice display defects in thymic T cell development, TH17 differentiation, and development of TH17-dependent EAE (7, 18). To dissect the function of RORγt, we developed three assays to separate these three functions. To determine the function of RORγt in thymocytes, we used an in vitro thymocyte differentiation system (27). CD4−CD8− thymocytes from wild-type (WT) but not RORγt−/− mice could differentiate into CD4+CD8+ and CD4+ cells (Fig. 1A). Consistently, RORγt−/− CD4−CD8− thymocytes transduced with a retrovirus expressing a RORγt (RORγt) but not green fluorescent protein (GFP) alone [empty virus (EV)] rescued the development of CD4+CD8+ and CD4+ from RORγt−/− thymocyte (Fig. 1B and fig. S1A for gating strategy). We next used an in vitro assay to determine the function of RORγt in TH17 differentiation. Under TH17 polarization conditions, RORγt−/− naïve CD4+ T cells could not differentiate into TH17 cells unless exogenous RORγt was provided via retrovirus transduction (Fig. 1C and fig. S1B for gating strategy). Last, adoptive transfer EAE model enabled the testing of RORγt TH17 effector function by using 2D2 T cell receptor (TCR) transgenic mice (TgTcr2D2) that recognize myelin oligodendrocyte glycoprotein (MOG35–55) (28, 29). RORγt−/−/TgTcr2D2 CD4+ T cells were transduced with virus expressing RORγt, polarized under TH17 conditions and adoptively transferred into Rag1−/− recipients for inducing EAE (30). RORγt−/−/TgTcr2D2 CD4+ T cells expressing RORγt induced very severe EAE (RORγt−/−/TgTcr2D2 + RORγt); these mice had the highest disease score of 5 (the Institutional Animal Care and Use Committee protocol does not permit disease development beyond this point) equivalent to Rag1−/− recipients with WT TgTcr2D2 CD4+ T cells transduced with GFP alone (WT TgTcr2D2 + EV) (Fig. 1D). In contrast, RORγt−/−/TgTcr2D2 CD4+ T cells expressing GFP alone (RORγt−/−/TgTcr2D2 + EV) resulted in greatly delayed and impaired EAE with the highest disease score of 3. RORγt deficiency did not affect the proliferation and survival of TgTcr2D2 CD4+ T cells in vitro (fig. S1, C and D) and in vivo (fig. S1E), which is also indicated by an equivalent number CD4+ T cells recovered from the spleen after adoptive transfer (fig. S1F). The successful establishment of above assays allowed us to dissect three RORγt-regulated functions.
Fig. 1. Three assays are developed to dissect RORγt function.
(A) Representative flow cytometric analysis (left) of CD4+ and CD8+ thymocytes ex vivo developed from indicated genotypes of CD4−CD8− thymocytes placed on the OP9-DL4 stroma cells for 3 days (n = 3 per genotype). The number indicates the percentage of cells in the gated area throughout. Right: Percentage of CD4+CD8+ and CD4+ cells. (B) Representative flow cytometric analysis (left) of CD4+ and CD8+ thymocytes ex vivo developed from sorted RORγt−/−CD4−CD8− thymocytes transduced with retrovirus expressing GFP alone (EV) or with RORγt, and cultured on OP9-DL4 stroma cells for 3 days (n = 3 per genotype). Right: Percentage of CD4+CD8+ + CD4+ cells. (C) Representative flow cytometric analysis of IL-17A (left) and percentage of IL-17A+ cells (right) among indicated genotypes of CD4+ T cells transduced with retrovirus expressing GFP alone (EV) or with RORγt and polarized under TH17 conditions for 3 days (n = 3 per genotype). (D) Mean clinical EAE score of Rag1−/− mice adoptively transferred with same number of TgTcr2D2 or RORγt−/−/TgTcr2D2 CD4+ T cells transduced with retrovirus expressing GFP alone (EV) or with RORγt and polarized under TH17 conditions for 3 days. Bars are means ± SE. ***P < 0.001 (two-tailed Student’s t test).
RORγt-K256, a ubiquitination site, is critical for the effector function but not the differentiation of TH17 cells
RORγt has been shown to regulate TH17 differentiation; it, however, remains unknown whether and how RORγt regulates the effector function of TH17 cells. To address this question, we aimed to identify RORγt mutations that specifically disrupt RORγt function in effector TH17 cell–mediated EAE but not in TH17 differentiation and thymocyte development. Previously, we mutated lysine residues (K) on RORγt to arginine (R) to study the function of posttranslational modification of RORγt (8). Thus, we first compared the ability of WT and RORγt mutants to rescue RORγt−/− thymocyte development. Only RORγt-K31R mutation moderately affected thymocyte development (Fig. 2A). In terms of TH17 differentiation, K31R, K69R, and K313R impaired TH17 differentiation (Fig. 2, B to D), consistent with our published results (8, 9, 31). Further, most RORγt mutations either impaired or potentiated TH17 differentiation (Fig. 2B), suggesting that RORγt uses very different mechanisms to regulate the function of thymocytes and TH17 cells.
Fig. 2. RORγt-K256, a ubiquitination site, is critical for the effector function but not the differentiation of TH17 cells.
(A) The ratio of rescued thymocyte development of RORγt−/−CD4−CD8− by indicated RORγt K-R mutants relative to that by WT RORγt (1 = 100%). (B) Ratio of rescued TH17 differentiation of RORγt−/−CD4+ T cells by indicated RORγt K-R mutants relative to WT RORγt (1 = 100%). (C) Flow cytometric analysis of IL-17A+ cells among RORγt−/− CD4+ T cells transduced with retroviruses expressing GFP alone (EV) or indicated RORγt mutants and subsequently polarized for 3 days under TH17 conditions. (D) Percentages of IL-17A+ cells rescued by indicated RORγt mutants shown in (C) (n = 5 to 12). (E) Immunoblot (IB) of ubiquitinated (Ub) RORγtK256(K1) immunoprecipitated (IP) from human embryonic kidney (HEK) 293T cells expressing RORγtK256(K1) alone (none) or together with hemagglutinin (HA)–tagged WT or mutated ubiquitin (K0, all K mutated to R). WCL, whole-cell lysates. (F) Immunoblot of ubiquitinated WT RORγt or RORγtK256R (only K256 mutated to R) enriched by Tandem Ubiquitin Binding Entities 2 (TUBE2) from RORγt−/− TH17 cells expressing indicated RORγt type. (G) Mean clinical score of Rag1−/− mice adoptively transferred with sorted RORγt−/−/TgTcr2D2 TH17 cells expressing GFP alone (EV) or with indicated RORγt (n = 5 to 7 mice per group). (H) Total number and percentage of indicated immune cells recovered from the CNS of EAE-induced mice from (G). (I) Flow cytometric analysis of IL-17A+ cells among CD4+ T cells recovered from the CNS of EAE-induced mice shown in (G). (H and I) Box plots show median (central line), maximum, minimum (box ends), and outliers (extended lines). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant (P > 0.05); two-tailed Student’s t test.
To separate RORγt function in TH17 differentiation versus effector TH17 cells, we focused on mutants that did not perturb TH17 differentiation and thymocyte development, such as K99R, K256R, and K288R (Fig. 2, A to D). K256 was identified as a prominent ubiquitination site by mass spectrometry analysis of immunoprecipitated RORγt (fig. S2A). To validate the K256 ubiquitination site, we generated a RORγt mutant with all K mutated to R except K256 (RORγtK256(K1)) so that only K256 can be ubiquitinated. In the presence of WT ubiquitin, RORγtK256(K1) was ubiquitinated, whereas the ubiquitination signals were absent in the presence of a mutant ubiquitin that had all K mutated to R (K0) so that it cannot be attached to the substrates (Fig. 2E). We next used a RORγt mutant carrying a single K256 to R mutation (RORγtK256R) that cannot be ubiquitinated only at the K256 site. WT RORγt or RORγtK256R was then expressed in RORγt−/− T cells under TH17-polarizing conditions. Ubiquitinated RORγt was readily detected in TH17 cells (Fig. 2F). Compared to the WT RORγt, RORγtK256R had obviously reduced ubiquitination signals. RORγt-K256 is thus ubiquitinated in TH17 cells.
Next, we tested the effector function of TH17 cells in the induction of EAE. As described above (Fig. 1D), in vitro–differentiated RORγt−/−/TgTcr2D2 TH17 cells expressing exogenous RORγt, RORγtK99R, and RORγtK288R induced severe EAE after adoptive transfer into Rag1−/− recipients (Fig. 2G), suggesting that K99R and K288R do not affect the effector function of TH17 cells in the induction of EAE, whereas RORγtK256R-expressing RORγt−/−/TgTcr2D2 TH17 cells induced greatly impaired EAE. RORγtK69R also induced impaired EAE, as we previously observed because of reduced TH17 differentiation (8). There was no difference in the proliferation of the RORγt−/−/TgTcr2D2 cells expressing various RORγt (fig. S2B). The central nervous system (CNS) immune cell infiltrate was also analyzed (Fig. 2H and fig. S2C for gating strategy). Consistent with the impaired EAE, the total number of CD4+ T cells and monocytes/macrophages in the CNS was significantly reduced in recipients with RORγtK256R or RORγtK69R mutants, indicating reduced inflammation. In addition, recipients with RORγtK256R and RORγtK69R cells also showed reduced infiltrate IL-17A+ cells in the CNS (Fig. 2I and fig. S2D for gating strategy), whereas not obvious changes in interferon-γ–positive (IFN-γ+) TH1 cells from recipients with RORγtK256R compared to that with WT RORγt were observed (fig. S2, E and F). Therefore, the RORγt-K256 ubiquitination site, although dispensable for TH17 differentiation, is required for the effector function of TH17 cells in the pathogenesis of EAE.
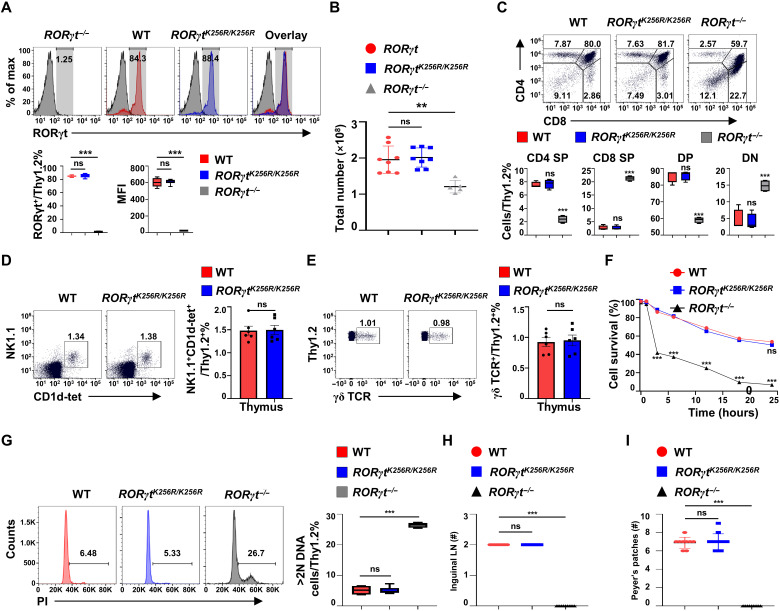
RORγt-K256R mutation does not affect RORγt-dependent development of thymocytes and lymph nodes
To investigate the function of RORγt-K256 ubiquitination in vivo, we generated homozygous mice for RORγtK256R (RORγtK256R/K256R) under the control of endogenous RORγt locus (fig. S3A for genetic engineering strategy and fig. S3B for confirming the K256R mutation by sequencing). We first examined RORγt-dependent thymocyte development (18–20, 32). WT RORγt and RORγtK256R had the same expression patterns in thymocytes (Fig. 3A); our gene-targeting strategy thus did not affect RORγtK256R expression. Furthermore, RORγtK256R was as stable as WT RORγt (fig. S3, C to D), suggesting that K256 ubiquitination site does not affect RORγt stability. Thymic cellularity (Fig. 3B) and distribution of different developmental stages of thymocytes, CD4−CD8− double-negative (DN), CD4+CD8+ double-positive (DP), and CD4+ or CD8+ single-positive thymocytes (Fig. 3C) were comparable between RORγtK256R/K256R and WT mice, which were different from RORγt−/− mice, suggesting normal thymocyte development in RORγtK256R/K256R mice. Furthermore, the percentage of natural killer T (NKT) and γδ T cells in the thymus (Fig. 3, D and E) and spleens (fig. S3, E and F) was equivalent between WT and RORγtK256R/K256R mice. The accelerated CD4+CD8+ thymocyte apoptosis (Fig. 3F and fig. S3G for analysis of apoptotic cells) accounted for the reduced percentage of CD4+CD8+ thymocytes (Fig. 3C) and decreased thymic cellularity (Fig. 3B) in RORγt−/− mice (18), whereas the apoptosis of CD4+CD8+ thymocytes from RORγtK256R/K256R mice was the same as that of the WT mice (Fig. 3F). In addition, thymocytes from RORγt−/− mice had a higher percentage of cells with >2N of DNA (Fig. 3G), indicating more cells in the DNA synthesis phase of the cell cycle (18), while thymocytes from RORγtK256R/K256R mice did not show an increased percentage of the cells in the DNA synthesis phase compared to the WT mice. Therefore, RORγt-K256R mutation does not affect RORγt function in thymocyte development. Furthermore, unlike RORγt −/− mice that lack all the peripheral lymph nodes (18), RORγtK256R/K256R mice developed lymph nodes including inguinal lymph nodes (Fig. 3H) and Peyer’s patches (Fig. 3I), same as the WT mice. Together, prevention of RORγt-K256 ubiquitination does not affect the development of thymocytes and lymph nodes.
Fig. 3. RORγt-K256R mutation does not affect RORγt-dependent development of thymocytes and lymph nodes.
(A) Flow cytometric analysis of RORγt in thymocytes obtained from indicated mice (n = 5 to 8). MFI, mean fluorescence intensity. (B) Total thymocyte numbers of the indicated mice (n = 5 to 8). The graph shows mean ± SD. (C) Flow cytometric analysis of CD4 and CD8 on thymocytes from indicated mice (n = 4 per genotype). (D) Representative flow cytometric analysis of NKT cells (n = 4) from the thymus of the indicated mice. (E) Representative flow cytometric analysis of γδ T cells (n = 4) from the thymus of the indicated mice. (F) Flow cytometric analysis of the survival of DP thymocytes cultured in vitro for the indicated time (n = 4 per genotype). (G) Representative flow cytometric analysis of DNA content of indicated thymocytes stained by propidium iodide (PI) (n = 4 to 6 per genotype). (H and I) Number of inguinal lymph nodes (LN) (H) and Peyer’s patches (I) in the indicated mice (n = 9 to 12 per genotype). **P < 0.01; ***P < 0.001 (two-tailed t test). (A, C, E, F, and G) Box plots or scatter plots show median (central line), maximum, minimum (box ends), and outliers (extended lines).
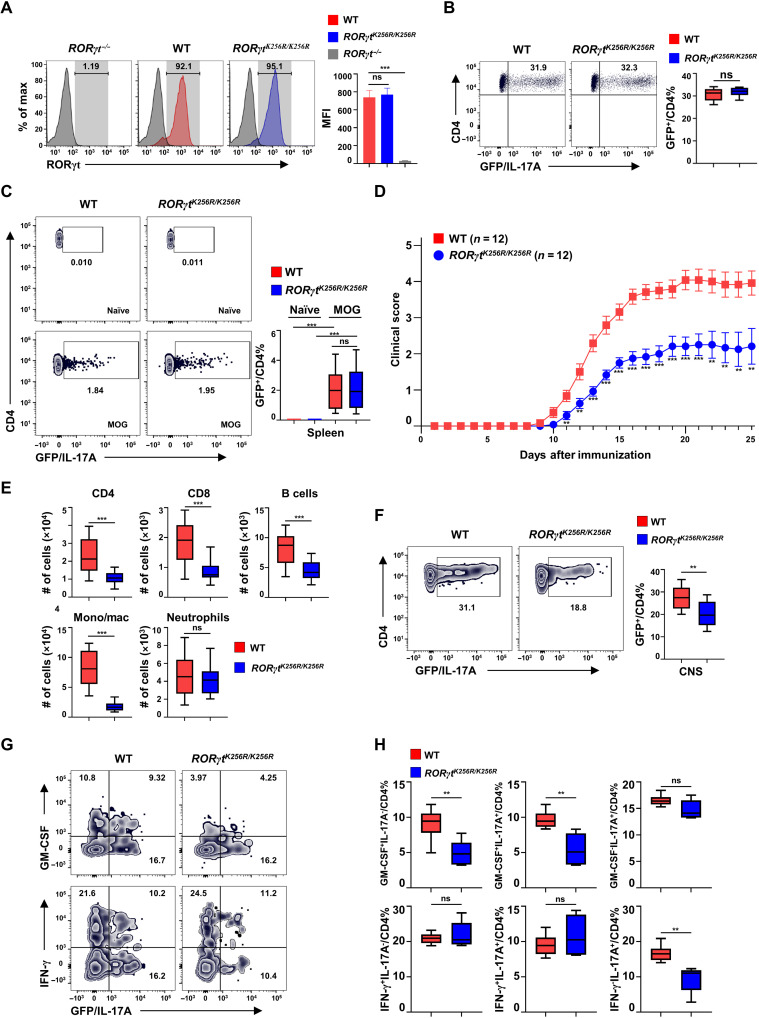
RORγtK256R/K256R mice have normal TH17 differentiation but develop impaired TH17-dependent EAE
Upon maturation in the thymus, T cells migrate to the periphery to mediate immune responses. RORγtK256R/K256R and WT mice had comparable splenocytes (fig. S4A), CD4+, CD8+ (fig. S4B), CD62LhiCD44lo naïve, and CD62LloCD44hi memory-like T cell counts (fig. S4, C to D). We next examined TH17 differentiation using a GFP reporter mouse line of IL-17 (Il17aGFP). We confirmed that in vitro–differentiated RORγtK256R/K256R TH17 cells showed comparable RORγt (Fig. 4A) and IL-17 (Fig. 4B) expression compared to WT cells, consistent with the notion that RORγt-K256R mutation does affect TH17 differentiation (Fig. 2, B and D). In addition, RORγtK256R/K256R mice showed a normal percentage of splenic regulatory T cells (Tregs) (fig. S4E) and normal differentiation of Tregs from naïve CD4+ T cells (fig. S4F). To test whether RORγt-K256R affects the effector function of TH17 cells responsible for the induction of EAE, we induced EAE by immunization with MOG35–55 peptide. Before immunization, there were almost no TH17 cells detected in the spleens from WT and RORγtK256R/K256R mice (Fig. 4C, top). Six days after immunization, equivalent TH17 (fig. S4, G and H) as well as TH1 (fig. S4, I to J) cells were induced in spleens (fig. S4, G and I) and lymph nodes (fig. S4, H and J) of WT and RORγtK256R/K256R mice. Again, on day 12 after immunization, when EAE symptoms started to develop, the percentage of TH17 cells still showed no obvious difference in the spleens of WT and RORγtK256R/K256R mice (Fig. 4C, bottom), confirming that RORγt-K256R does not affect the generation of TH17 cells in vivo. However, compared to WT mice, RORγtK256R/K256R mice developed greatly impaired EAE (Fig. 4D), supporting impaired RORγtK256R/K256R TH17 effector function. Consistently, adoptive transfer of in vitro–differentiated RORγtK256R/K256R TH17 cells also induced less severe EAE in Rag1−/− recipients compared to that induced by WT TH17 cells (fig. S4K). In addition, impaired EAE induction was associated with reduced immune cell infiltrate including CD4+, CD8+, B cells, and monocytes in the CNS of RORγtK256R/K256R mice, although no obvious changes were observed in neutrophil numbers (Fig. 4E and fig. S4L for gating strategy). Further analysis of CNS lymphocyte infiltrate in the RORγtK256R/K256R mice showed a decreased percentage of IL-17A+CD4+ cells (Fig. 4F), particularly pathogenic IL-17A+ granulocyte-macrophage colony-stimulating factor–positive (GM-CSF+) cells that play an important role in EAE development (Fig. 4, G and H, and fig. S4M for gating strategy) (2, 3). These results suggest that RORγt-K256R mutation, which prevents the ubiquitination, impairs the effector function but not the differentiation of TH17 cells responsible for the pathogenesis of EAE.
Fig. 4. RORγtK256R/K256R mice have normal TH17 differentiation but develop impaired TH17-dependent EAE.
(A) Flow cytometric analysis of RORγt in WT and RORγtK256R/K256R CD4+ T cells polarized for 3 days under TH17 conditions (n = 4 to 6 per genotype). Bars are means ± SEM. (B) Flow cytometric analysis of GFP+ (IL-17A+) cells among CD4+ T cells from indicated genotypes of cells differentiated under TH17 conditions as described in (A) (n = 4 per genotype). (C) Representative flow cytometric analysis of IL-17A+ cells among CD4+ T cells in the spleen of indicated genotype of mice either before (naïve) or 12 days after MOG35–55 immunization (n = 4). (D) Mean clinical score of indicated mice different days after MOG35–55 immunization. Bars are means ± SEM. (E) Number of indicated immune cells recovered from the CNS of indicated EAE-induced mice from (D). (F) Representative flow cytometric analysis of GFP+ (IL-17A+) among CD4+ T cell infiltrate to the CNS of indicated mice shown in (D) (n = 10 per genotype). (G) Representative flow cytometric analysis of GM-CSF or IFN-γ expression in the lymphocyte infiltrated to the CNS of indicated mice shown in (D). (H) Quantification of GM-CSF+/CD4+ % and IFN-γ+/CD4+ % cells in the CNS (n = 5 per genotype). **P < 0.01; ***P < 0.001 (two-tailed t test). (E, F, and H) Box plots: Median (central line), maximum, minimum (box ends), and outliers (extended lines).
RORγt-K256R impairs the pathways critical for the pathogenesis of EAE but not for the TH17 differentiation
To understand the mechanisms responsible for the RORγt-K256R mutation-disrupted effector function of TH17 cells responsible for EAE, GFP+ TH17 cells derived from WT and RORγtK256R/K256R/IL-17GFP CD4+ T cells were sorted to high purity (>98%) (fig. S5A for gating strategy) and subjected to RNA sequencing (RNA-seq) analysis. On the basis of the computational principal components analysis, the six RNA-seq samples were divided into two groups: WT and RORγtK256R/K256R TH17 cells (fig. S5B), indicating reproducible gene expression patterns within each group and thus the high quality of RNA-seq results. We identified 1375 differentially expressed genes (DEGs) [P < 0.05 and fold change (FC) > 1.5], with 943 up-regulated and 432 down-regulated genes, between WT and RORγtK256R/K256R TH17 cells (Fig. 5A, fig. S5C, and tables S2 and S3). Subjection of DEGs to pathway analysis did not find significant changes in the TH17 differentiation pathway (Fig. 5, B and C) between WT and RORγtK256R/K256R cells, confirming that RORγt-K256R mutation does not affect TH17 differentiation. Down-regulated pathways in RORγtK256R/K256R cells include IL-23 signaling (Fig. 5, B and D) and glycolysis pathways (Fig. 5, B and E); both play important roles in the pathogenesis of EAE (33, 34). In addition, inflammatory cytokines such as IL-9, IL-17, and IL-22 signaling pathways were also down-regulated, likely reflecting a reduced ability to induce inflammation responsible for the tissue damages. We next subjected DEGs to gene set enrichment analysis (GSEA) using the gene set specifically expressed in TH17 cells responsible for the development of pathogenic EAE (fig. S5D) (35) and found that RORγtK256R/K256R TH17 cells had significantly reduced enrichment of the genes important for the pathogenesis of EAE when compared to the WT TH17 cells (Fig. 5F). This result is consistent with the impaired EAE observed in RORγtK256R/K256R mice. To determine the DEGs that are directly regulated by RORγt, we performed chromatin immunoprecipitation sequencing (ChIP-seq) analysis to detect genome-wide RORγt occupancy. ChIP-seq analysis in TH17 cells revealed obvious RORγt-binding peaks at Il17a and Il17f loci (Fig. 5G), consistent with published results (8, 36). RORγtK256R-binding peaks at the Il17a and Il17f loci were comparable to that of WT RORγt, supporting that K256R did not affect RORγt binding to Il17 gene and thus its expression. Using our RNA-seq and ChIP-seq data, we cross-examined genes that were down-regulated in RORγtK256R/K256R TH17 cells with the genes that had reduced RORγtK256R-binding signals (see table S4 for the full list), identifying 31 genes (Fig. 5H). These 31 genes are considered directly regulated by RORγt, and their reduced expression in RORγtK256R/K256R TH17 cells is likely due to reduced RORγtK256R binding and activating their expression. The 31 genes were then cross-examined with the gene set specifically expressed in TH17 cells and responsible for the pathogenesis of EAE (fig. S5D) (35) and identified Runx1 (Fig. 5H). Furthermore, Runx1 was found to be a core regulator for the IL-23 signaling pathway that was down-regulated in RORγtK256R/K256R TH17 cells by a protein-protein interaction network analysis (Fig. 5I), indicating that down-regulated IL-23 signaling pathway is likely due to down-regulated Runx1 expression. Therefore, computational analysis of the transcriptome is consistent with the phenotypes observed in RORγtK256R/K256R mice that RORγt-K256R mutation does not affect TH17 differentiation but impairs the effector function of TH17 cells responsible for the development of EAE. Runx1 is thus a possible RORγt-regulated gene that is down-regulated in RORγtK256R/K256R TH17 cells and responsible for the observed defective effector function of RORγtK256R/K256R TH17 cells in the induction of EAE.
Fig. 5. RORγt-K256R impairs the pathways critical for the pathogenesis of EAE but not for the TH17 differentiation.
(A to F) Computation analysis of RNA-seq data obtained from GFP+/(IL-17A+) WT and RORγtK256R/K256R CD4+ T cells polarized under TH17 conditions. (A) The number of DEGs (black) including up-regulated (red) and down-regulated (blue) genes with a cutoff at P value of <0.05 and FC > 1.5. (B) Ingenuity pathway analysis (IPA) canonical signaling pathway analysis of down-regulated pathways (z-score < 0) in RORγtK256R/K256R versus WT TH17 cells. The dots denote P value, the vertical dotted line marks P value of 0.05 (log10P = 1.3). JAK, Janus kinase; STAT, signal transducer and activator of transcription. (C to F) GSEA plots showing the enrichment of genes critical for TH17 differentiation (C), IL-23 signaling (D), glycolysis (E), and pathogenesis of TH17-mediated EAE (F) between RORγtK256R/K256R versus WT TH17 cells. NES, normalized enrichment score. Gene sets were derived from the molecular signaling database (MSigDB) for (C) to (E) and the previous report for (F) (35). (G) ChIP-seq analysis of RORγt DNA-binding peaks at the Il17a and Il17f loci in RORγt−/− TH17 cells expressing indicated RORγt. (H) Venn diagram of gene overlapping among 432 down-regulated genes in RORγtK256R/K256R TH17 cells, 1277 genes with decreased RORγtK256R-binding activity, and 224 TH17 genes critical for the pathogenesis of EAE. (I) Protein-protein network analysis of differentially expressed regulators critical for the IL-23 signaling pathway between RORγtK256R/K256R versus WT TH17 cells. Genes critical for IL-23 signaling pathway were customized on the basis of IPA-curated pathways.
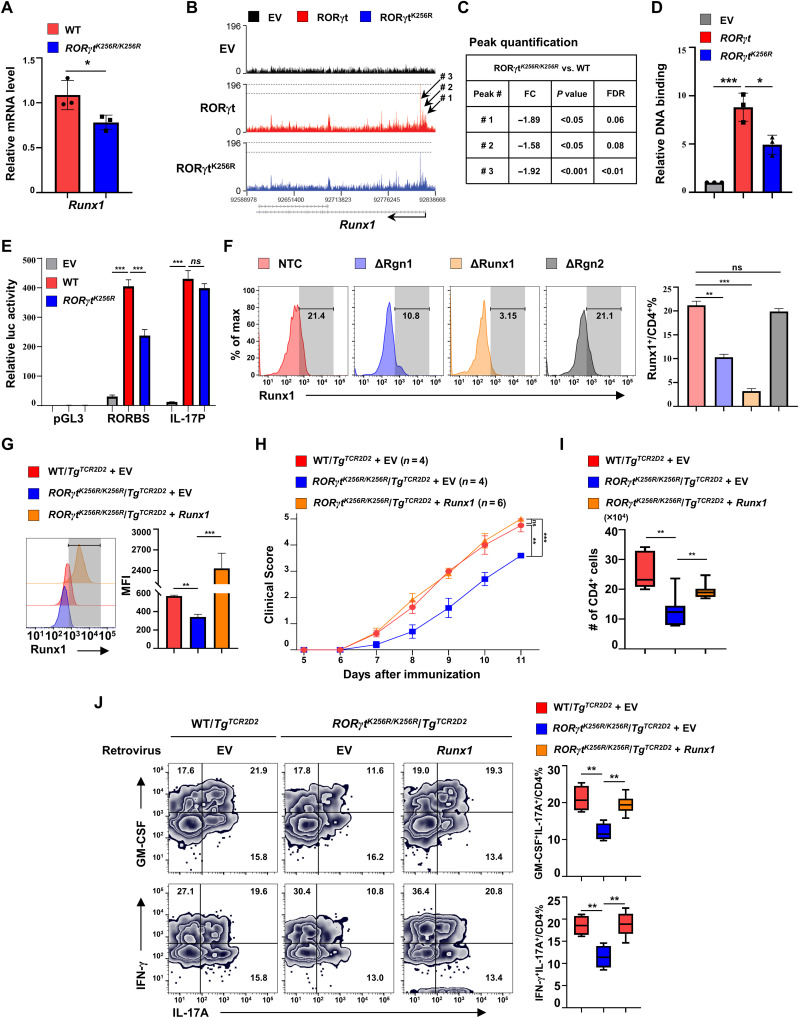
RORγt-K256R mutation impairs RORγt to bind and activate Runx1 gene critical for EAE development
Our computational analysis identified Runx1 as a potential gene responsible for impaired effector function of RORγtK256R/K256R TH17 cells, because (i) Runx1 was down-regulated in RORγtK256R/K256R/TgTcr2D2 TH17 cells, which was also confirmed by individual quantitative polymerase chain reaction (qPCR) analysis (Fig. 6A); (ii) three prominent RORγt-binding peaks close to the transcription start site of Runx1 gene were identified by ChIP-seq analysis (Fig. 6B), and RORγtK256R binding signals at those peaks were substantially decreased (Fig. 6, B and C). RORγt-binding sites were identified within the peak region by sequencing analysis (fig. S6A). Furthermore, individual ChIP assays also confirmed RORγt binding to the peak region, whereas RORγtK256R-binding signals to this region were greatly decreased (Fig. 6D), which correlates to the decreased levels of Runx1 mRNA in RORγtK256R/K256R TH17 cells (Fig. 6A); (iii) Runx1 was a gene expressed in TH17 cells and responsible for the pathogenesis of EAE, as deletion of Runx1 impairs the EAE development (35, 37). To determine the effects of decreased RORγtK256R-binding peak signals on Runx1 gene transcription, we cloned the DNA fragment covering the region with two potential RORγt-binding sites (fig. S6A) to a luciferase reporter gene (pGL3) driven by a basic thymidine kinase (TK) promoter. The reporter activity was greatly stimulated by WT RORγt but not as much by RORγtK256R (Fig. 6E). Therefore, reduced RORγtK256R binding to the Runx1 gene correlates well with the reduced ability of RORγtK256R to stimulate Runx1 gene expression. In contrast, WT RORγt and RORγtK256R equivalently stimulated IL-17 promoter-luciferase report activity, which correlates with the equivalent binding of RORγt and RORγtK256R to and activation of the Il17 gene (Figs. 4B and 5G). To further determine whether identified RORγt-binding peaks are important for the expression of endogenous Runx1 gene, the region containing the RORγt-binding peaks was deleted using CRISPR-Cas9 with two guiding RNAs (ΔRgn1) in CD4+ T from mice expressing Cas9 (fig. S6A). At the same time, we used a nontargeting control (NTC) and a deleted adjacent region (ΔRgn2) as negative controls, whereas deleted Runx1 gene itself as a positive control (ΔRunx1) (fig. S6B for gating strategy for detecting Runx1 expression after deletion). Deletion of the Runx1 gene prevented Runx1 expression [Fig. 6F and fig. S6C for mean fluorescence intensity (MFI) of Runx1], demonstrating a successful deletion strategy with CRISPR-Cas9. Furthermore, deletion of the RORγt-binding region, but not the adjacent region or NTC, greatly reduced expression of Runx1, strongly indicating the critical function of the RORγt-binding region in the stimulation of Runx1 expression. Together with the results that RORγtK256R had decreased binding signals to Runx1 (Fig. 6, B to D), these results suggest that reduced Runx1 expression in RORγtK256R/K256R TH17 cells is due to impaired RORγtK256R-binding and activating Runx1 gene expression. To determine whether the reduced level of Runx1 in RORγtK256R/K256R cells is responsible for the impaired development of EAE, we force-expressed Runx1 in RORγtK256R/K256R/TgTcr2D2 CD4+ T cells (Fig. 6G) and adoptively transferred them to Rag1−/− mice to induce EAE (Fig. 6H). Forced expression of Runx1 significantly enhanced RORγtK256R/K256R/TgTcr2D2 CD4+ T cell function in the induction of EAE comparable to that of WT TgTcr2D2 T cells, which was also confirmed by increased infiltration of CD4+ T cells to the CNS (Fig. 6I). Furthermore, the percentage of pathogenic IL-17+GM-CSF+ and IL-17+IFN-γ+ cells in CNS was reduced in Rag1−/− mice transferred with RORγtK256R/K256R/TgTcr2D2 CD4+ T cells compared to that transferred with WT TgTcr2D2 T cells but restored to WT levels in RORγtK256R/K256R/TgTcr2D2 CD4+ T cells expressing Runx1 (Fig. 6J), consistent with rescuing the effector function of RORγtK256R/K256R T cells by expressing Runx1. Collectively, our results demonstrated that the ubiquitination site of RORγt-K256, which is not essential for TH17 differentiation, regulates the effector function of TH17 cells required for inducing EAE via up-regulating Runx1. By decoupling the function of RORγt in the differentiation and effector function of TH17 cells, we demonstrated that RORγt also regulates the effector function of TH17 cells.
Fig. 6. RORγt-K256R mutation impairs RORγt to bind and activate Runx1 gene critical for EAE development.
(A) qPCR of Runx1 mRNA in indicated genotypes of TH17 cells (n = 3). Bars are means ± SEM. (B) ChIP-seq analysis of RORγt DNA-binding peaks at the Runx1 locus in RORγt−/− TH17 cells transduced with indicated vectors. (C) FC of RORγt-binding peaks indicated in (B). FDR, false discovery rate. (D) ChIP analysis of RORγt-binding signals at the Runx1 gene locus shown in (B) (n = 3). Bars are means ± SEM. (E) Luciferase (luc) activity from HEK 293T cells transfected with a pGL3 basic luciferase vector, a luciferase reporter with RORγt-binding sites (RORBS)–containing DNA fragment cloned from Runx1 locus and a basic TK promoter or IL-17 promoter (IL-17P) luciferase reporter under indicated conditions. (F) Flow cytometric analysis of Runx1 among GFP+(Cas9+) TH17 cells retrovirally transduced with indicated single-guide RNAs (n = 4). (G) Flow cytometric analysis of Runx1 among indicated genotypes of TH17 cells retrovirally transduced with GFP alone (EV) or together with Runx1 (n = 3). (H) Mean clinical score of Rag1−/− mice adoptively transferred with indicated genotypes of TH17 cells expressing GFP alone or with Runx1 shown in (G). (I) Number of infiltrating CD4+ T cells recovered from the CNS of the EAE-induced mice shown in (H). (J) Flow cytometric analysis of GM-CSF, IFN-γ, and IL-17A in CD4+ T cells recovered from the CNS of EAE-induced mice shown in (H). *P < 0.05; **P < 0.01; ***P < 0.001 (two-tailed t test). (I and J) Box plot: Median (central line), maximum, minimum (box ends), and outliers (extended lines).
DISCUSSION
The transcription factor RORγt, which is encoded by gene Rorc, is well known for instructing the differentiation of TH17 cells. Activation of naïve T cells in the presence of TGF-β, IL-6, and/or IL-23 is sufficient to up-regulate RORγt, which instructs the differentiation into TH17 cells (14, 38–41). The hallmark of TH17 differentiation is the activation and expression of IL-17. RORγt directly binds to Il17a and Il17f gene loci to stimulate their expression (8, 36), which explains the essential function of RORγt in the differentiation of IL-17–producing TH17 cells. Differentiated TH17 cells are able to induce tissue inflammation involved in the pathogenesis of autoimmune diseases, including psoriasis, inflammatory bowel disease, and multiple sclerosis (2, 3, 14). Previous studies using RORγt−/− mice demonstrated the essential function of RORγt in TH17 cell–mediated autoimmunity such as EAE (7, 9). RORγt−/− mice are resistant to EAE and other TH17-mediated autoimmunity, which is due to the lack of RORγt-dependent generation of TH17 cells. The question remains whether RORγt plays a role in the effector function of TH17 cells involved in autoimmunity. The mutation RORγtK256R we identified at the ubiquitination site does not affect the generation of IL-17–producing TH17 cells but impairs the effector function of TH17 cells in the induction of EAE. Further, RORγtK256R/K256R mice have normal TH17 differentiation both in vitro and in vivo but have greatly impaired TH17 immune responses that led to EAE. In addition, RORγtK256R/K256R mice have normal RORγt-dependent thymocyte development and lymph node genesis including Peyer’s patches. This study thus reveals a previously unidentified and essential RORγt function in effector TH17 cells in addition to TH17 differentiation. This study informs the development of RORγt-based therapies that specifically target the effector function of TH17 cells responsible for the pathogenesis of autoimmunity. This will have a significant impact on the clinical treatment of TH17-mediated autoimmunity, as usually medical treatment is performed after a diagnosis of autoimmune diseases resulting from the effector function of already developed TH17 cells.
Runx1 is a transcription factor known to regulate hematopoiesis (42–44) and oncogenesis (45). Runx1 was reported to play a role in TH17 cells. Particularly relevant to TH17 cells involved in the pathogenesis of EAE, Runx1 has been shown to be up-regulated in TH17 cells and work together with T-bet to stimulate IFN-γ expression that is believed to be responsible for the induction of EAE (37). However, it is not clear how Runx1 is up-regulated in TH17 cells. Our results demonstrate that Runx1 expression is stimulated by RORγt, as RORγt-binding peaks were detected by ChIP-seq on the promoter region of the Runx1 gene. The conserved RORγt-binding sequence was identified within the detected peaks, and the deletion of the DNA fragment containing the RORγt-binding site greatly reduced Runx1 expression. Moreover, RORγtK256R has reduced binding signals at the RORγt-binding region, which correlates with the impaired ability of RORγtK256R in stimulating a luciferase reporter gene driven by the RORγt-binding region identified in the Runx1 locus. These results demonstrate the mechanisms for how RORγt regulates the effector function of TH17 cells via up-regulating Runx1 expression and why RORγt-K256R mutation impairs the effector function of TH17 cells. An in vitro study showed that Runx1 can stimulate RORγt expression, which is, however, inhibited by T-bet (46). Because T-bet is required for IFN-γ expression, this seems to suggest that T-bet and RORγt inhibit each other. In addition, Runx1 has been shown to be required for forkhead box P3 (Foxp3) expression (47), and Runx1 is able to stimulate itself expression via an autoregulation mechanism (48). The function of Runx1 is thus complicated and dependent on the microenvironment. In our study, both in vitro and in vivo, we did not find obvious changes in the levels of RORγt expression in RORγtK256R/K256R TH17 cells that have lower levels of Runx1, thus not supporting the role of K256 ubiquitination in the regulation of RORγt and TH17 differentiation. Furthermore, even forced expression of Runx1 in RORγtK256R/K256R CD4+ T cells does not affect TH17 differentiation. Therefore, RORγt-regulated Runx1 expression does not affect TH17 differentiation but is required for the effector function of TH17 cells that mediate pathogenic EAE.
Dysregulated TH17 cells are often associated with autoimmune diseases such as EAE and psoriasis resulting from a reaction to self-antigens (49). In addition to IL-17, IL-23 also plays an important role in TH17 cell–dependent autoimmune diseases (1, 50) such as EAE and psoriasis (51, 52). Neutralizing antibodies for IL-23 and IL-17 or their receptors are used for the treatment of these autoimmune conditions (53–55). Therefore, inhibiting the TH17 pathway is effective for treating autoimmune conditions (52, 56). Our results show that the IL-23 signaling pathway is down-regulated in RORγtK256R/K256R TH17 cells. Further, network analysis supports that Runx1 is a core regulator for the IL-23 pathway and down-regulated IL-23 pathway thus likely resulting from down-regulated Runx1. Therefore, RORγt-regulated Runx1 seems to control the effector function of TH17 cells at least partially through regulating the IL-23 pathway known to be critical for TH17-mediated autoimmunity.
Considering the essential function of RORγt in TH17 cells, RORγt inhibitors are being developed to treat TH17-dependent autoimmunity (5, 11–13, 57). However, these RORγt inhibitors mostly target TH17 differentiation and RORγt-dependent thymocyte development. Inhibition of RORγt-dependent thymocyte development leads to a high frequency of thymic lymphoma (8, 58, 59). Our results demonstrate that a posttranslational ubiquitination event can dictate RORγt function in TH17-dependent responses involved in autoimmunity. However, this ubiquitination event is dispensable for thymocyte development and TH17 differentiation. Therefore, targeting this RORγt ubiquitination event is a potential treatment for TH17-dependent autoimmune disease without induction of thymocyte lymphoma. Currently, it remains unknown about the RORγt ubiquitination pathway including the enzymes involved in the ubiquitination of RORγt at K256. Illustrating the detailed mechanisms responsible for ubiquitination of RORγt will facilitate the development of novel treatments that target the RORγt-dependent effector function of TH17 cells responsible for autoimmunity while minimizing the other toxic effects such as lymphoma.
MATERIALS AND METHODS
Experimental design
The objective of this study was to determine whether RORγt plays a role in effector function of TH17 cells in addition to its known function in TH17 differentiation. To achieve this goal, we dissected the function of RORγt with K-R mutations in thymus development, TH17 differentiation, and induction of EAE. RORγt-K256R mutation did not affect TH17 differentiation but impaired the effector function of TH17 cells responsible for inducing EAE, which was also confirmed by the in vivo studies using RORγtK256R/K256R mice. RNA-seq and ChIP-seq assays identified Runx1 as a direct target of RORγt in the regulation of effector function of TH17 cells.
Mice
All male and female mice used for experiments were between 6 and 12 weeks old; age-matched littermates were used. The RORγt−/− (Rorctm1Litt, stock no. 007571) mouse strain was described previously (18). The RORγtK256R/K256R point-mutated mice were designed and generated by Biocytogen LLC. Rag1−/− (Rag1tm1Mom, stock no. 002216), TgTcr2D2 (Tcra2D2 and Tcrb2D2, stock no. 006912), IL-17A–GFP (Il17atm1Bcgen, stock no. 018472), CRISPR-Cas9– enhanced GFP (EGFP) [Gt(ROSA)26Sorem1.1(CAG-cas9*,-EGFP)Rsky, stock no. 028555], and C57BL/6J (stock no. 000664) mice were purchased from the Jackson Laboratory. For some assays, the mice were crossed to generate RORγt−/−/TgTcr2D2, RORγtK256R/K256R/TgTcr2D2, and RORγtK256R/K256R/IL-17A-IRES-GFP-KI mice. All mice were bred at the C57BL/6J background and maintained in a pathogen-free animal facility at City of Hope. All animal experiments were conducted per the protocols approved by the Institutional Animal Care and Use Committee at City of Hope. Statistical tests were not used to predetermine sample sizes. The sample sizes were chosen on the basis of previous studies of our own and by others in the field (8). The sample sizes are indicated in the figure legends or figures. Allocation of mice to experimental groups was random.
Induction and assessment of EAE
Active EAE was induced and assessed as previously described (8). Briefly, mice were immunized with 200 mg of MOG35–55 (Hooke Laboratories) in complete Freund’s adjuvant by subcutaneous injection at two dorsal sites at day 0, followed by two intraperitoneal injections of 80 ng of pertussis toxin at days 0 and 1. For passive EAE, Rag1−/− mice were adoptively transferred with 1 × 105 TCRMOG-expressing (TgTcr2D2) TH17 cells that were differentiated under TH17 polarization condition, followed by an immunization with MOG at 7 days after injection. In certain experiments, WT RORγt cells were virally transduced with retrovirus expressing GFP alone, while RORγtK256R/K256R cells were transduced with retrovirus expressing Runx1 and GFP before in vitro TH17 differentiation. In other experiments, RORγt−/− cells were transduced with an empty vector or vectors encoding WT RORγt, RORγtK87R, RORγtK99R, RORγtK256R, or RORγtK288R. All transduced cells were sorted for CD4 and GFP expression before adoptive transfer into mice. Severity of EAE was monitored, and a clinical score of 0 to 5 was assigned (30): 0 = no disease, 0.5 = partially limp tail, 1 = paralyzed tail, 2 = hindlimb weakness, 3 = hindlimb paralysis, 4 = hindlimb and forelimb paralysis, and 5 = moribundity and death.
Isolation of naïve CD4+ T cell isolation and in vitro TH17 differentiation
Murine CD4+ T cells were isolated from spleens by negative selection using the Naive CD4+ T Cell Isolation Kit (Miltenyi Biotec). Suspensions of 4 × 105 cells/ml in RPMI 1640 medium (Corning Inc.) containing 2 mM l-glutamine, 50 μM β-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% fetal bovine serum (FBS) were activated with hamster anti-CD3 (0.25 μg/ml; 145-2C11, BioLegend) and hamster anti-CD28 (1 μg/ml; 37.51, BioLegend) antibodies overnight in 24-well plates precoated with rabbit anti-hamster immunoglobulin G fraction (0.1 mg/ml; catalog no. 55398, MP Biomedicals). The following TH17 differentiation was carried out by supplementing to the culture medium mentioned above with TGF-β (2 ng/ml; Miltenyi Biotec), IL-6 (20 ng/ml; Miltenyi Biotec), anti–IL-4 (2 μg/ml; 11B11, BioLegend), and anti–IFN-γ (2 μg/ml; XMG 1.2, BioLegend), and additional IL-23 (20 ng/ml; Miltenyi Biotec) was also added for the induction of pathogenic TH17 cells.
Retroviral transduction
The retroviral vector murine stem cell virus (MSCV)–internal ribosomal entry site (IRES)–GFP (MIGR1, a gift from W.S. Pear, University of Pennsylvania) was used to clone WT or mutated RORγt. MSCV vector for expressing Runx1 was a gift from I. Taniuchi (RIKEN Center for Integrative Medical Sciences, Japan). Vectors were first transfected to Platinum-E (Cell Biolabs) retroviral packaging cells using BioT transfection reagent (Bioland Scientific), followed by a change of fresh medium at 24 hours. The virus-containing supernatant was collected at 48 and 72 hours, filtered with a 0.45-μm polyvinylidene difluoride (PVDF) syringe filter (Millipore), and used to transduce T cells or stored for future use at −80°C. Transduction of activated CD4+ T was performed by spin infection with viral supernatants (two, 500g, 30°C for 2 hours) in the presence of polybrene (8 μg/ml; Sigma-Aldrich). After spinning, the plates were incubated at 37°C for 3 hours. The viral supernatant was replaced with fresh culture medium with polarizing cytokines for in vitro differentiation.
CRISPR-Cas9–mediated genomic DNA deletion
Single-guide RNA (sgRNA) of Runx1, targeting the exon region (Addgene, library 67988), was cloned to pMSCV-U6sgRNA(Bbs I)-PGKpuro2ABFP (Addgene, 102796) with modification of Bbs I sites (table S1) for using universal primer design through this study. To generate plasmids for the deletion of large fragments of genomic DNA, PCR products of two U6 promoter–sgRNA cassettes and a phosphoglycerate kinase (PGK) promoter–TagBFP cassette were assembled using the Golden Gate assembly method and inserted into the MIGR1 vector with disrupted Bsp MI site. Bbs I sites and newly introduced Bsp MI sites were used for the insertion of gRNAs into each cassette. sgRNAs were delivered to the cells by retroviral transduction. The U6 promoter–driven transcription of sgRNAs in each cassette was confirmed by examining sgRORγt expression in TH17 cells together with a simultaneously expressed NTC sgRNA in another cassette. Three sgRNAs (sgRNA1, sgRNA2, and sgRNA3; fig. S6A) targeting the sequence of the Runx1 gene were designed by using an online tool (CRISPOR, http://crispor.tefor.net/). A simultaneous expression of sgRNA1 with sgRNA2 or sgRNA2 with sgRNA3 in Cas9-expressing cells was designed for deletion of the RORγt binding region (Rgn1) and the adjacent region (Rgn2) without RORγt biding site. sgRNA primers are listed in table S1.
In vitro T cell–development assay
Murine thymocytes from RORγt−/− mice were subjected to fluorescence-activated cell sorting for isolating DN (Thy1.2+CD4−CD8−) cells. Sorted cells at 5 × 105 cells/ml were cultured overnight on an 80% confluent bone marrow-derived stromal cell line (OP9) expressing the delta like canonical Notch ligand 4 (Dll4/DL4) (OP9-DL4) monolayer (a gift from E.V. Rothenberg) in 24-well culture plates with α-modified minimum essential medium (Invitrogen Life Technologies) supplemented with 20% FBS, penicillin-streptomycin (100 U/ml), 2 mM l-glutamine (Invitrogen Life Technologies), and recombinant mouse IL-7 (5 ng/ml; PeproTech). The cells were then transduced with RORγt carrying K/R mutations as described above. Cocultures were maintained for an additional 3 days in the fresh medium containing murine IL-7 (5 ng/ml). Cells were harvested for flow cytometry analysis.
Apoptosis assays
Murine thymocytes were collected by smashing the thymus in a 40-μm cell strainer. Cells were suspended in RPMI 1640 medium supplemented with 10% FBS, 1% penicillin-streptomycin, and 2 mM l-glutamine at 1 × 106 cells/ml and cultured for 0, 3, 6, 12, 18, or 24 hours. Thymocytes were then incubated with anti-Thy1.2 antibody and a fixable LIVE/DEAD near-infrared dye (Thermo Fisher Scientific). After two washes, the cells were stained with 5 μl of phycoerythrin–annexin V in 100 μl of binding buffer containing 0.01 M Hepes (pH 7.4), 0.14 M NaCl, and 2.5 mM CaCl2 for 15 min. An additional 400 μl of binding buffer was added to the suspension before analysis.
RNA-seq and analysis
CD4+ T cells isolated from RORγtWT/IL-17A-GFP+/− and RORγtK256R/K256R/IL-17A-GFP+/− mice were differentiated into pathogenic TH17 cells as described above. RNA was extracted from sorted ~1 × 106 GFP-expressing TH17 cells (CD4+GFP+) using an RNAeasy mini kit (QIAGEN). Each group contained three replicates from different mice. Quality control, library preparation, and sequencing were performed at Novogene. The analysis was performed through Partek Flow. Briefly, the sequence reads were aligned to the mouse whole genome (GRCm38) with validation of quality through prealignment and postalignment quality assurance/quality control (QA/QC). Aligned reads were further subjected to quantification using the Partek expectation/maximization (E/M) algorithm and normalization to counts per million with 0.001 added to each. The identification of differentially expressed features was performed through the Partek gene specific analysis (GSA) algorithm that applies multiple statistical models to each gene. Genes with total counts over 30 were considered to be statistically expressed in the cells. The expression values of pathogenic genes were extracted and subjected to ingenuity pathway analysis (IPA), gene set enrichment assay (GSEA), and network analysis.
Chromatin immunoprecipitation sequencing
In vitro–activated (see above) RORγt−/− CD4+ T cells that were transduced with retroviruses carrying GFP, RORγt-3xFlag/GFP, or RORγtK256R-3xFlag/GFP were used. After TH17 polarization, 2 × 107 cells were fixed in 1% formaldehyde at room temperature for 10 min to cross-link proteins with chromatin. The reaction was stopped with incubation in glycine for 5 min. Genomic DNA was fragmented with enzyme cocktail (ChIP-IT Express Enzymatic kit, Active Motif) for 10 min as directed. Cell lysates were centrifuged at 15,000 rpm for 10 min to remove debris, and the supernatant was used for immunoprecipitation. An equal amount of DNA was incubated with anti-FLAG (M2, Sigma-Aldrich) overnight, followed by precipitation with protein G agarose beads. Beads complexed with DNA fragments were extensively washed five times, and DNA was eluted, followed by reverse cross-linking. Recovered DNA was subjected to NovaSeq with 51–base pair (bp) paired-end sequencing length. Primers used in reverse-transcription quantitative PCR (RT-qPCR) are listed in table S1. Reads were analyzed using Partek Flow through alignment to the mm10 mouse genome using the Burrow-Wheeler aligner (BWA). Peaks were identified with the model-based analysis of ChIP-seq 2 (MACS2) tool (version 2.1.1) and quantified with a minimum region size of 50 bp.
Flow cytometry
For surface staining, cells isolated from mice or in vitro culture were directly stained with antibodies in phosphate-buffered saline (PBS) with 2% FBS and 1 mM EDTA at 4°C for 15 min. A blocking of Fc receptors with anti-CD16/32 antibody was carried out in case monocytes/macrophages were present. For staining transcription factors, cells were prestained for surface markers, fixed, and permeabilized in TF Fix/Perm buffer (BD Biosciences) at 4°C for 20 min and washed once with TF Perm/Wash buffer. Cells were stained for target proteins (see antibody list below) in the TF Perm/Wash buffer at 4°C for 15 min. For cytokine staining, cells were prestimulated with phorbol 12-myristate 13-acetate (50 ng/ml; Sigma-Aldrich) and ionomycin (750 ng/ml; Sigma-Aldrich) for 3 hours ahead of staining. Meanwhile, GolgiStop (BD Biosciences) was cotreated to block protein transport. In certain experiments, cells were stained with surface markers and/or fixable live/dead dye (Thermo Fisher Scientific). Cells were fixed and permeabilized with CytoFix/CytoPerm buffer (BD Biosciences), followed by staining for cytokines in the Perm/Wash buffer (BD Biosciences) after washing. To measure cell proliferation, either naïve CD4+ T cells or in vitro–differentiated TH17 cells for adoptive transfer were stained with CellTrace Violet dye (Thermo Fisher Scientific) in PBS (1:5000) at room temperature for 20 min. After washing, naïve CD4+ T cells were subjected to anti-CD3/anti-CD28 stimulation and TH17 differentiation for measuring in vitro proliferation, and TH17 cells were sorted out and injected to Rag1−/− mice for measuring hemostatic proliferation at day 3. Subsequent analysis was performed in the BD LSRFortessa flow cytometer.
The following antibodies were used for flow cytometric assay: anti-CD45 (BioLegend, clone 30-F11), anti-CD3 (BioLegend, 145-2C11), CD4 (BioLegend, RM4-5), anti-CD8 (BioLegend, 53-6.7), anti-CD19 (BioLegend, 1D3), anti-lymphocyte antigen 6 complex locus G6D (Ly6G) (BioLegend, 1A8), anti-CD62L (BioLegend, MEL-14), anti-CD44 (BioLegend, IM-7), anti–IFN-γ (BioLegend, XMG-1.2), anti–GM-CSF (BioLegend, MP1-22F9), killer cell lectin-like receptor subfamily B member 1C (Klrb1c/NK1.1) (BioLegend, PK136), anti-CD11b (eBioscience, M1/70), anti-Ly6C (eBioscience, HK1.4), anti-Thy1.2 (eBioscience, 53-1.2), anti–IL-17A (eBioscience, eBio17B7), anti-Runx1 (eBioscience, RXDMC), anti–IL-22 (eBioscience, 1H8PWSR), anti-Foxp3 (eBioscience, FJK-16 s), anti-RORγt (BD Biosicences, Q31-378), and CD1d-tetramer [National Institutes of Health (NIH), PBS-57].
Western blotting
A total 1.5 × 107 TH17 cells were lysed in radioimmunoprecipitation assay buffer containing 20 mM tris-HCl (pH 7.4), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and leupeptin (1 μg/ml). Ubiquitinated proteins were precipitated and enriched with 20 μl of equilibrated agarose-Tandem Ubiquitin Binding Entities 2 (TUBE2) (Lifesensors) at 4°C for 4 hours. Agarose-TUBE2-protein complex were washed with Tris buffered saline containing 0.1% Tween-20 (TBST) and subjected to heating in 2× Laemmli sample buffer (Bio-Rad) with β-mercaptoethanol at 90°C for 5 min. The supernatant containing precipitated proteins was subjected to SDS–polyacrylamide gel electrophoresis, and the protein was transferred to the PVDF membrane. Target proteins were sequentially immunoblotted with relevant primary antibodies and fluorescent secondary antibodies (LI-COR Biosciences), followed by measuring fluorescent intensity with a LI-COR Odyssey blot imager (LI-COR Biosciences). The eventual samples for Western blotting were pooled from three different experiments. Quantification of ubiquitination signals of blots showing in Fig. 2 (E and F) was performed to the area above 50 kDa.
Reverse-transcription quantitative PCR
Total RNA was extracted using the RNeasy mini kit (QIAGEN) as directed. A Tetro complementary DNA synthesis kit (Bioline) was used for reverse transcription. Subsequent qPCR was performed using PowerUp SYBR Green Master Mix (Applied Biosystems) in the QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific). The primers used for qPCR are listed in table S1. The amplification efficiency of all primers was tested and optimized. Gene expression was calculated with the delta-delta Ct (∆∆Ct) method normalized to the control gene encoding β-actin and glyceraldehyde-3-phosphate dehydrogenase, and all measurements were performed in triplicate.
Luciferase assay
Human embryonic kidney 293T cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 mg/ml). A total of 8 × 105 cells were seeded to each well of a six-well plate and transfected with the reporter vectors (400 ng), pSV40-Renilla luciferase vector (200 ng), and expression vectors (2 μg) using BioT transfection reagent (Bioland Scientific, Paramount, CA). The same amount of plasmid DNA was used by adjusting with an empty vector. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) per the manufacturer’s instruction and normalized against Renilla luciferase activities. “Relative luciferase activities” were plotted with further normalization of luciferase activities of each group to the pGL3-basic reporter vector plus the empty vector group. The generation of reporter plasmids was done by PCR amplification of Runx1 genomic DNA containing the RORγt-binding region and a subsequent insertion upstream of a mini TK promoter that was cloned to pGL3-basic vector (Promega) for a minimal expression of luciferase. Cloning primers and mini TK promoter sequence are listed in table S1.
Statistical analysis
The ratio of rescue for thymocyte development and TH17 differentiation in Fig. 2 (A and B) was calculated as relative ratio of the extent of RORγt−/− cells transduced with RORγt mutants to that of cells transduced with WT RORγt. The statistical parameters are indicated in the figure legends. The results were analyzed for statistical significance with unpaired Student’s t test. Bodyweights are presented as means ± SD, and other data are shown as means ± SEM. P values are calculated using GraphPad Prism and presented where the statistical significance (P < 0.05) was found.
Acknowledgments
We thank J. C. Zuniga-Pflucker (University of Toronto) for the DP9-DL4 stroma cell line, W.S. Pear (University of Pennsylvania) for the retroviral vector MIGR1, I. Taniuchi for the MSCV-Runx1 retroviral vector, Y.C. Yuan (Bioinformatics Core of City of Hope) for the guidance of Partek Flow, and Biocytogen for assisting with the design and generation of RORγtK256R/K256R mice. We also thank the following City of Hope core services: Animal Resource Center, Integrative Genomics Core, Flow Cytometer Core, Mass Spectrometry Core, and Bioinformatics Core. We thank C. S. Jayasena for reviewing and editing the manuscript.
Funding: This work was supported by grants from NIH R01-AI109644, R21-AI163256, institutional pilot funding, Jackie and Bruce Barrow Cancer Research Scholars’ Program, and Caltech-CoH Biomedical Initiative. The research reported in this publication included work performed in the animal, genomic, flow cytometry, and mass spectrometry cores supported under NIH grant P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions: Conceptualization: Z.S., Z.H., and X.Z. Methodology: X.Z., H.W., W.Z., Z.H., Y.G., N.I., and K.O.L. Investigation: X.Z., H.W., W.Z., Z.H., and K.O.L. Visualization: X.Z., H.W., W.Z., Z.H., and K.O.L. Funding acquisition: Z.S. Supervision: Z.S. Writing—original draft: Z.S., X.Z., and H.W. Writing—review and editing: Z.S., X.Z., H.W., Y.G., N.I., and Z.H.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. RNA-seq and ChIP-seq data is available on Gene Expression Omnibus (GEO) repository (accession #: GSE211414 and GSE211509).
Supplementary Materials
This PDF file includes:
Figs. S1 to S7
Tables S1 to S4
REFERENCES AND NOTES
- 1.Lee Y., Awasthi A., Yosef N., Quintana F. J., Xiao S., Peters A., Wu C., Kleinewietfeld M., Kunder S., Hafler D. A., Sobel R. A., Regev A., Kuchroo V. K., Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Codarri L., Gyulveszi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., Becher B., RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011). [DOI] [PubMed] [Google Scholar]
- 3.El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G. X., Dittel B. N., Rostami A., The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elloso M. M., Gomez-Angelats M., Fourie A. M., Targeting the Th17 pathway in psoriasis. J. Leukoc. Biol. 92, 1187–1197 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Skepner J., Ramesh R., Trocha M., Schmidt D., Baloglu E., Lobera M., Carlson T., Hill J., Orband-Miller L. A., Barnes A., Boudjelal M., Sundrud M., Ghosh S., Yang J., Pharmacologic inhibition of RORγt regulates Th17 signature gene expression and suppresses cutaneous inflammation in vivo. J. Immunol. 192, 2564–2575 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Choi G. B., Yim Y. S., Wong H., Kim S., Kim H., Kim S. V., Hoeffer C. A., Littman D. R., Huh J. R., The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R., The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006). [DOI] [PubMed] [Google Scholar]
- 8.He Z., Ma J., Wang R., Zhang J., Huang Z., Wang F., Sen S., Rothenberg E. V., Sun Z., A two-amino-acid substitution in the transcription factor RORγt disrupts its function in TH17 differentiation but not in thymocyte development. Nat. Immunol. 18, 1128–1138 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Z., Zhang J., Huang Z., Du Q., Li N., Zhang Q., Chen Y., Sun Z., Sumoylation of RORγt regulates TH17 differentiation and thymocyte development. Nat. Commun. 9, 4870 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada S., Markle J. G., Deenick E. K., Mele F., Averbuch D., Lagos M., Alzahrani M., Al-Muhsen S., Halwani R., Ma C. S., Wong N., Soudais C., Henderson L. A., Marzouqa H., Shamma J., Gonzalez M., Martinez-Barricarte R., Okada C., Avery D. T., Latorre D., Deswarte C., Jabot-Hanin F., Torrado E., Fountain J., Belkadi A., Itan Y., Boisson B., Migaud M., Arlehamn C. S., Sette A., Breton S., McCluskey J., Rossjohn J., de Villartay J. P., Moshous D., Hambleton S., Latour S., Arkwright P. D., Picard C., Lantz O., Engelhard D., Kobayashi M., Abel L., Cooper A. M., Notarangelo L. D., Boisson-Dupuis S., Puel A., Sallusto F., Bustamante J., Tangye S. G., Casanova J. L., Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349, 606–613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao S., Yosef N., Yang J., Wang Y., Zhou L., Zhu C., Wu C., Baloglu E., Schmidt D., Ramesh R., Lobera M., Sundrud M. S., Tsai P. Y., Xiang Z., Wang J., Xu Y., Lin X., Kretschmer K., Rahl P. B., Young R. A., Zhong Z., Hafler D. A., Regev A., Ghosh S., Marson A., Kuchroo V. K., Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 40, 477–489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z., Xie H., Wang R., Sun Z., Retinoid-related orphan receptor γt is a potential therapeutic target for controlling inflammatory autoimmunity. Expert Opin. Ther. Targets 11, 737–743 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Huh J. R., Littman D. R., Small molecule inhibitors of RORγt: Targeting Th17 cells and other applications. Eur. J. Immunol. 42, 2232–2237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaffen S. L., Jain R., Garg A. V., Cua D. J., The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bezbradica J. S., Hill T., Stanic A. K., Van Kaer L., Joyce S., Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc. Natl. Acad. Sci. U.S.A. 102, 5114–5119 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rachitskaya A. V., Hansen A. M., Horai R., Li Z., Villasmil R., Luger D., Nussenblatt R. B., Caspi R. R., Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 180, 5167–5171 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egawa T., Eberl G., Taniuchi I., Benlagha K., Geissmann F., Hennighausen L., Bendelac A., Littman D. R., Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity 22, 705–716 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Sun Z., Unutmaz D., Zou Y. R., Sunshine M. J., Pierani A., Brenner-Morton S., Mebius R. E., Littman D. R., Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Wang R., Xie H., Huang Z., Ma J., Fang X., Ding Y., Sun Z., Transcription factor network regulating CD+CD8+ thymocyte survival. Crit. Rev. Immunol. 31, 447–458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie H., Sadim M. S., Sun Z., RORγt recruits steroid receptor coactivators to ensure thymocyte survival. J. Immunol. 175, 3800–3809 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Komander D., Rape M., The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Rutz S., Kayagaki N., Phung Q. T., Eidenschenk C., Noubade R., Wang X., Lesch J., Lu R., Newton K., Huang O. W., Cochran A. G., Vasser M., Fauber B. P., DeVoss J., Webster J., Diehl L., Modrusan Z., Kirkpatrick D. S., Lill J. R., Ouyang W., Dixit V. M., Deubiquitinase DUBA is a post-translational brake on interleukin-17 production in T cells. Nature 518, 417–421 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Han L., Yang J., Wang X., Wu Q., Yin S., Li Z., Zhang J., Xing Y., Chen Z., Tsun A., Li D., Piccioni M., Zhang Y., Guo Q., Jiang L., Bao L., Lv L., Li B., The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor γt (RORγt) in Th17 cells. J. Biol. Chem. 289, 25546–25555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Yang J., Han L., Zhao K., Wu Q., Bao L., Li Z., Lv L., Li B., TRAF5-mediated Lys-63-linked polyubiquitination plays an essential role in positive regulation of RORγt in promoting IL-17A expression. J. Biol. Chem. 290, 29086–29094 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J., Xu P., Han L., Guo Z., Wang X., Chen Z., Nie J., Yin S., Piccioni M., Tsun A., Lv L., Ge S., Li B., Cutting edge: Ubiquitin-specific protease 4 promotes Th17 cell function under inflammation by deubiquitinating and stabilizing RORγt. J. Immunol. 194, 4094–4097 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Hu H., Sun S. C., Ubiquitin signaling in immune responses. Cell Res. 26, 457–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes R., Zúñiga-Pflücker J. C., The OP9-DL1 system: Generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb. Protoc. 2009, pdb.prot5156 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Bettelli E., Pagany M., Weiner H. L., Linington C., Sobel R. A., Kuchroo V. K., Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L., Hollinshead K. E. R., Hao Y., Au C., Kroehling L., Ng C., Lin W. Y., Li D., Silva H. M., Shin J., Lafaille J. J., Possemato R., Pacold M. E., Papagiannakopoulos T., Kimmelman A. C., Satija R., Littman D. R., Niche-selective inhibition of pathogenic Th17 cells by targeting metabolic redundancy. Cell 182, 641–654.e20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stromnes I. M., Goverman J. M., Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 1, 1810–1819 (2006). [DOI] [PubMed] [Google Scholar]
- 31.He Z., Zhang J., Du Q., Xu J., Gwack Y., Sun Z., SRC3 is a cofactor for RORγt in Th17 differentiation but not thymocyte development. J. Immunol. 202, 760–769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R., Xie H., Huang Z., Ma J., Fang X., Ding Y., Sun Z., T cell factor 1 regulates thymocyte survival via a RORγt-dependent pathway. J. Immunol. 187, 5964–5973 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cua D. J., Sherlock J., Chen Y., Murphy C. A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., Zurawski S., Wiekowski M., Lira S. A., Gorman D., Kastelein R. A., Sedgwick J. D., Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Xu K., Yin N., Peng M., Stamatiades E. G., Chhangawala S., Shyu A., Li P., Zhang X., Do M. H., Capistrano K. J., Chou C., Leslie C. S., Li M. O., Glycolytic ATP fuels phosphoinositide 3-kinase signaling to support effector T helper 17 cell responses. Immunity 54, 976–987.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaublomme J. T., Yosef N., Lee Y., Gertner R. S., Yang L. V., Wu C., Pandolfi P. P., Mak T., Satija R., Shalek A. K., Kuchroo V. K., Park H., Regev A., Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 163, 1400–1412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciofani M., Madar A., Galan C., Sellars M., Mace K., Pauli F., Agarwal A., Huang W., Parkurst C. N., Muratet M., Newberry K. M., Meadows S., Greenfield A., Yang Y., Jain P., Kirigin F. K., Birchmeier C., Wagner E. F., Murphy K. M., Myers R. M., Bonneau R., Littman D. R., A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Godec J., Ben-Aissa K., Cui K., Zhao K., Pucsek A. B., Lee Y. K., Weaver C. T., Yagi R., Lazarevic V., The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity 40, 355–366 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangan P. R., Harrington L. E., O’Quinn D. B., Helms W. S., Bullard D. C., Elson C. O., Hatton R. D., Wahl S. M., Schoeb T. R., Weaver C. T., Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Veldhoen M., Hocking R. J., Atkins C. J., Locksley R. M., Stockinger B., TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Miossec P., Korn T., Kuchroo V. K., Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361, 888–898 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Korn T., Bettelli E., Oukka M., Kuchroo V. K., IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Okuda T., Nishimura M., Nakao M., Fujita Y., RUNX1/AML1: A central player in hematopoiesis. Int. J. Hematol. 74, 252–257 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Egawa T., Tillman R. E., Naoe Y., Taniuchi I., Littman D. R., The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J. Exp. Med. 204, 1945–1957 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuda T., van Deursen J., Hiebert S. W., Grosveld G., Downing J. R., AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321–330 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Sood R., Kamikubo Y., Liu P., Role of RUNX1 in hematological malignancies. Blood 129, 2070–2082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarevic V., Chen X., Shim J. H., Hwang E. S., Jang E., Bolm A. N., Oukka M., Kuchroo V. K., Glimcher L. H., T-bet represses TH17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat. Immunol. 12, 96–104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L., Patsoukis N., Petkova V., Boussiotis V. A., Runx1 and Runx3 are involved in the generation and function of highly suppressive IL-17-producing T regulatory cells. PLOS ONE 7, e45115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez M., Hinojosa M., Trombly D., Morin V., Stein J., Stein G., Javed A., Gutierrez S. E., Transcriptional auto-regulation of RUNX1 P1 promoter. PLOS ONE 11, e0149119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasuda K., Takeuchi Y., Hirota K., The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 41, 283–297 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Ghoreschi K., Laurence A., Yang X. P., Tato C. M., McGeachy M. J., Konkel J. E., Ramos H. L., Wei L., Davidson T. S., Bouladoux N., Grainger J. R., Chen Q., Kanno Y., Watford W. T., Sun H. W., Eberl G., Shevach E. M., Belkaid Y., Cua D. J., Chen W., O’Shea J. J., Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langrish C. L., Chen Y., Blumenschein W. M., Mattson J., Basham B., Sedgwick J. D., McClanahan T., Kastelein R. A., Cua D. J., IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tonel G., Conrad C., Laggner U., Di Meglio P., Grys K., McClanahan T. K., Blumenschein W. M., Qin J. Z., Xin H., Oldham E., Kastelein R., Nickoloff B. J., Nestle F. O., Cutting edge: A critical functional role for IL-23 in psoriasis. J. Immunol. 185, 5688–5691 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fotiadou C., Lazaridou E., Sotiriou E., Ioannides D., Targeting IL-23 in psoriasis: Current perspectives. Psoriasis (Auckl) 8, 1–5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanzel J., D’Haens G. R., Anti-interleukin-23 agents for the treatment of ulcerative colitis. Expert Opin. Biol. Ther. 20, 399–406 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Moschen A. R., Tilg H., Raine T., IL-12, IL-23, and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 16, 185–196 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Segal B. M., Constantinescu C. S., Raychaudhuri A., Kim L., Fidelus-Gort R., Kasper L. H.; Ustekinumab MS Investigators , Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: A phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7, 796–804 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Sheridan C., Footrace to clinic heats up for T-cell nuclear receptor inhibitors. Nat. Biotechnol. 31, 370 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Ueda E., Kurebayashi S., Sakaue M., Backlund M., Koller B., Jetten A. M., High incidence of T-cell lymphomas in mice deficient in the retinoid-related orphan receptor RORγ. Cancer Res. 62, 901–909 (2002). [PubMed] [Google Scholar]
- 59.Liljevald M., Rehnberg M., Soderberg M., Ramnegard M., Borjesson J., Luciani D., Krutrok N., Branden L., Johansson C., Xu X., Bjursell M., Sjogren A. K., Hornberg J., Andersson U., Keeling D., Jirholt J., Retinoid-related orphan receptor γ (RORγ) adult induced knockout mice develop lymphoblastic lymphoma. Autoimmun. Rev. 15, 1062–1070 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S7
Tables S1 to S4