Abstract
Actinic keratosis (AK) is a chronic disease resulting from deleterious effects of long-term, cumulative, epidermal exposure to ultraviolet (UV) light. UV-induced mutations in p53, ras, and p16 genes lead to the emergence of abnormal epidermal actinic keratosis (AKs) cells, which proliferate while avoiding apoptosis and may lead to invasive squamous cell carcinoma. There are both lesion-targeted and field-directed topical treatments. This review is of new and emerging information on tirbanibulin and tirbanibulin 1% ointment, which is approved for topical field treatment of actinic keratosis on the face and scalp. Potent antiproliferative and proapoptotic activities result from tirbanibulin’s inhibition tubulin polymerization and disruption of microtubule formation and Src kinase signaling. Tirbanibulin 1% ointment is an effective treatment of facial and scalp AK after five consecutive once-daily applications, as measured by complete and partial clearance and percent reduction in the number of lesions. Localized skin reactions are usually mild to moderate, resolving within a month. The short and well-tolerated course of therapy results in very high patient adherence to the treatment regimen
Keywords: Tirbanibulin ointment, actinic keratosis, field therapy
Actinic keratosis (AK) is a commonly occurring skin disease that results from cumulative ultraviolet (UV) irradiation.1 Estimates approximate that 60 percent of people over 40 years of age with a history of UV exposure have at least one AK lesion2 and that AK affects 58 million people in the United States, leading to treatment costs (in 2004) in excess of $1,000,000,000.3 AK is a clinical diagnosis of scaly, rough, pink-to-red papules on sun-exposed skin. Histopathologic examination is warranted when there is a concern of squamous cell carcinoma (SCC).4,5
AK falls within a continuum from sun-damaged skin to AKs to in-situ SCC to invasive SCC. Intraepidermal AK cells exhibit histopathologic features detected in invasive SCC, including atypical keratinocytes, nuclear pleomorphism, and disordered keratinocyte maturation.4,6 Alterations at the molecular level in specific genes, such as p53,7-10, and changes in gene expression profiles11 confirm a similar genetic evolution of AK and SCC. Insights into the genomic landscape of actinic keratoses and their progression to cutaneous SCC (cSCC) were recently achieved with whole exome sequencing of 37 AKs. Forty-four significantly mutated AK driver genes were identified and confirmed to be similarly altered in cSCC. Integration with gene expression datasets confirmed that dysregulated of tumor growth factor-beta (TGF-β) signaling may represent an important event in AK to cSCC progression.7
The natural history of AK lesions encompasses regression, persistence, or progression to in-situ or invasive SCC. The overall risk of progression of an AK lesion is low; however, the disease is chronic with patients having multiple AK lesions at any point in time and developing new lesions over time, which increases a patient’s overall risk of developing invasive disease.12,13 In a study of nearly 500,000 people, half of which had AK and the other half of which were matched controls without AK, it was clearly detected that AK increased the risk of developing cSCC. The yearly risk of developing SCC was double in patients with AK (1.97% vs 0.835%) than in controls and was triple after 10 years of follow-up (17.1% vs 5.7%).14 Beyond the risk, modeled mathematically, of a patient with 7 to 8 AK lesions developing invasive SCC (6.1– 10.2% spanning 10 years),15 there are data that most SCCs arise from AK lesions, with an AK lesion being concomitant or contiguous with SCC in 65 to 97 percent of reviewed cases.13,16–18 The need to treat AK is supported by the histologic, molecular genetic, and epidemiologic relationship between AK and risk of developing SCC.5,19,20
Treatment of AK is usually classified as lesion-directed or field-directed therapy. Investigators who biopsied and examined clinically normal skin adjacent to biopsy-proven AK lesions found that in 75 percent of the “normal” skin there was histological evidence of AK. These “subclinical” lesions of AK indicate the presence of “field cancerization,” which requires field therapy, rather than solely targeted therapy, to treat not only the clinically visible but also the affected, albeit normal appearing, intervening areas between AK lesions.
Counter to the evidence that field therapy is needed to address both visible and subclinical AK lesions, targeted cryotherapy is by far the most common treatment of AK in the United States (US). Survey data representing approximately 57.9 million AK visits in the US indicated that cryotherapy was administered in 50.8 percent of AK visits and field therapies (e.g., 5-fluoruracil [5FU], imiquimod, ingenol mebutate, photodynamic therapy) in less than 3.2 percent of visits.22
For limited numbers of AK lesions, cryosurgery, most commonly utilizing liquid nitrogen as the cryogen, is effective and the most frequently used method in the US.23–25 Unfortunately, to be effective, the required duration of freezing at the extremely low temperature of liquid nitrogen is lethal to normal melanocytes in the epidermis and often results in loss of pigment in the treated areas.26 Relying on targeted treatment limited to visible lesions that avoids perilesional subclincal lesions results in significant recurrences, which may be due, in part, to subclinical AK lesions becoming visible AKs over time.27–29
United States Food and Drug Administration (FDA)-approved field therapies, which include 5FU, imiquimod, diclofenac, and tirbanibulin, address field cancerization in AK by eliminating transformed cells in the treated field. It is not surprising that during such field treatments new visible lesions that were not present at baseline arise and resolve, suggesting effectiveness for subclinical lesions.28,30,31 Patient adherence is challenging due to required large number of applications (as many as 180) and prolonged (up to 4 months) treatment and to the resulting localized erythema, scaling, edema, erosions, and discomfort (e.g., burning, itching). To achieve greater patient adherence and persistence, greater regimen simplicity and greater tolerability has been the goal of using modified dosing schedules, lower drug concentrations, and novel formulations,31–33 as well as sequential treatment employing both lesion- and field-directed methods, to achieve greater effectiveness.34–40
Shergil et al41 reported that the majority (63%) of patients being treated topically for AKs did not adhere to their treatment regimen, primarily due to local skin reactions and treatment being excessive and not simple. Experts have suggested that keeping AK field treatment short and simple could improve patient adherence.41,42 Of the available US Food and Drug Administration (FDA)-approved, topically applied AK field therapies and dosing regimens, the number of applications range from as few as two to as many as 180 and require up to four months duration of therapy. Following ingenol mebulate, with its once-daily application for 2 to 3 days, being removed from the market, tirbanibulin 1% ointment, approved for once-daily application for five consecutive days, is the shortest topical AK therapy available. Ninety-nine–percent patient adherence rates have been reported in large Phase III studies using this short, simple regimen of tirbanibulin 1% ointment. The mostly mild-to-moderate severity local skin reactions (LSRs) reported in these studies peak at three days following completion of all applications and quickly resolve.
This article reviews the mechanisms of action of tirbanibulin and highlights clinical studies and data supporting the efficacy, safety, tolerability, and patient acceptance of tirbanibulin 1% ointment for the treatment of AK.
MECHANISMS OF ACTION
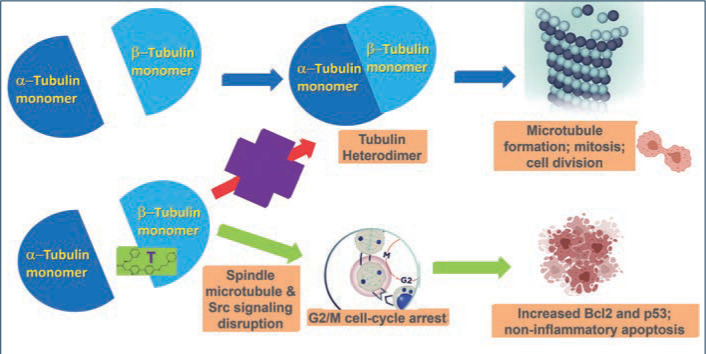
Tirbanibulin is a novel synthetic chemical entity (Figure 1) that has shown potent antiproliferative and antitumoral effects in vitro and in vivo by inducing cell cycle arrest and ultimately apoptotic cell death. These effects have been attributed to the ability of tirbanibulin to reversibly bind to the colchicine-binding site on b-tubulin43 and inhibit tubulin microfilament polymerization (Figure 2).44 Additional studies are needed to confirm whether tirbanibulin also binds to b-tubulin and/or a novel site on the a-b-tubulin heterodimer.44 Immunofluorescence staining has shown that tirbanibulin effectively disrupts the cellular MTs network immortalized keratinocyte CCD 1106 KERTr cells and HeLa cells. Tirbanibulin appears to induce complete cell cycle arrest at G2/M phase43 and trigger signals of programmed cell death by activating both intrinsic and extrinsic apoptotic pathways via Bcl-2, cleavage of caspase 8 and 9, and activation of caspase 3, in colon cancer HT-29 cells.
FIGURE 1.
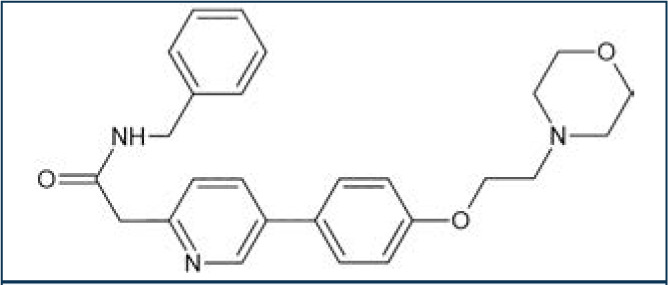
Molecular structure of tirbanibulin
FIGURE 2.
Src family kinases (SFKs) are a family of nine nonreceptor tyrosine kinases45 involved in vascular epithelial growth factor and angiogenesis.46,47 Elevated Src expression has been observed in AK and SCC, suggesting that increased signaling is necessary for keratinocyte migration and squamous carcinoma invasion.48 Tirbanibulin was shown to significantly down-regulate phospho-Src (p-Src) and Src signaling molecules and disrupt SFKs signaling in various cancer cell lines and tumor xenografts in mice.49 Whether the effects of tirbanibulin on Src signaling result from its direct binding to Src or secondary to its disruption of cellular microtubule networks is unclear.
CLINICAL DATA
Over 700 adult patients with 4 to 8 nonhypertrophic AKs in a contiguous area of 25cm2 on the face (68%) or scalp (32%) were randomized (1:1) in two identical Phase III trials to determine the tolerability and efficacy of tirbanibulin 1% ointment for AK compared to the ointment vehicle.50 The trials were carried out with the subjects and investigators/evaluators blinded to the randomized treatment. The average composite subject was a 70-year-old man with Fitzpatrick Skin Type I or II with facial AKs. Subjects received five consecutive days of once-daily treatment to the targeted 25cm2 field with either tirbanibulin (n=353) or vehicle control (n=349). Subjects with complete (100%) clearance of AK lesions in the treatment area at Day 57 were assessed for AK lesion recurrence every three months for a total of 12 months post-Day 57.
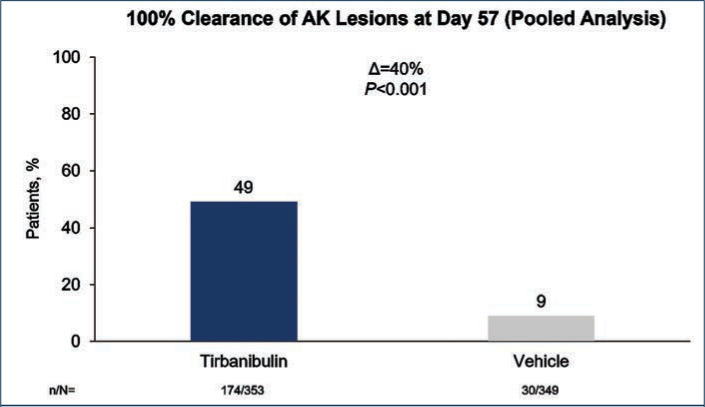
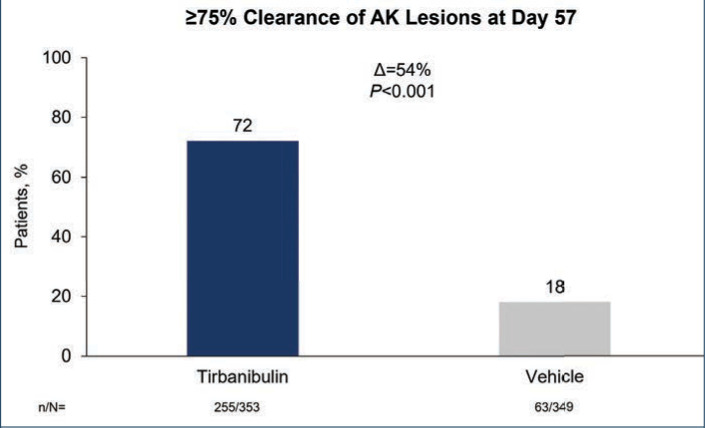
The primary efficacy endpoint was complete (100%) clearance of AK lesions in the treatment area, defined as the proportion of subjects at Day 57 with no clinically visible AK lesions in the treatment area, and the secondary endpoint was partial (≥75%) clearance of AK lesions in the treatment area.
Efficacy (pooled). Statistically significant higher Day 57 clinical endpoint complete and partial responses (Figures 3 and 4) were achieved in those receiving tirbanibulin treatment compared to those receiving vehicle. Median percent reduction from baseline, an important, unique endpoint being independent of the starting number of AK lesions, was higher (83%) in the active treatment arm compared with vehicle (20%).50
FIGURE 3.
Percent of patients with complete clearance of actinic keratosis (AK) lesions after treatment with tirbanibulin 1% ointment vs. vehicle control treatment, once daily for 5 days—Data are from the intent-to treat population and included both face and scalp treatment areas. Patients who discontinued before the Day 57 assessment were considered treatment failures. Significance determined by Cochran-Mantel-Haenszel method stratified by treatment group.50
D: difference between treatment groups; n/N: number of patients with 100% clearance/total number of patients
FIGURE 4.
Percent of patients with at least 75% clearance of actinic keratosis (AK) lesions after treatment with tirbanibulin 1% ointment vs. vehicle control treatment once daily for 5 days—Data are from the intent-to treat population and included both face and scalp treatment areas. Patients who discontinued before the Day 57 assessment were considered treatment failures. Significance determined by Cochran-Mantel-Haenszel method stratified by treatment group.50
D: difference between treatment groups; n/N: number of patients with ≥75% clearance/total number of patients
Consistent with the knowledge that AK lesions on the face have been found to be more responsive to all approved field therapies than AK lesions on the scalp, tirbanibulin treatment also exhibited significantly enhanced effectiveness rates on facial AK lesions than on scalp AK lesions, compared to vehicle (Table 1). Subgroup analyses revealed the efficacy of tirbanibulin to be consistent across sex and in those above or below 65 years of age.50
TABLE 1.
Efficacy of treatment of facial and scalp actinic keratoses with tirbanibulin 1% ointment versus vehicle control treatment once daily for 5 days
| EFFICACY AT DAY 57 | LOCATION | TIRBANIBULIN | VEHICLE | P-VALUE |
|---|---|---|---|---|
| Complete (100%) Clearance | Face | 55.9% | 9.6% | P<0.0001 |
| Scalp | 35.7% | 6.4% | P<0.0001 | |
| Partial (≥75%) Clearance | Face | 77.7% | 20.5% | P<0.0001 |
| Scalp | 60.9% | 12.7% | P<0.0001 | |
| Median % reduction in AK lesion count | Face | 100% | 25% | P<0.0001 |
| Scalp | 83.33% | 18.33% | P<0.0001 |
Blauvelt A, Kempers S, Lain E, et al. Phase III trials of tirbanibulin ointment for actinic keratosis. New Eng J Med. 2021;384(6):512–520.
Phototoxicity and contact sensitization. Clinical studies in healthy subjects have reported that tirbanibulin ointment did not cause contact sensitization (n=261), phototoxic skin reactions (n=31), or photoallergic skin reactions (n=64).51
Safety (pooled). Treatment-related adverse events (AEs). AEs were few and mostly mild transient application-site pruritus (tirbanibulin vs. vehicle: 9% vs. 6%) and pain (tirbanibulin vs. vehicle: 10% vs. 3%) (Table 2). No deaths, discontinuations, or serious AEs related to tirbanibulin occurred.50,51
TABLE 2.
Investigator assessment of maximal post-baseline local skin reactions greater than baseline in the treatment area (face or scalp): pooled data from 2 Phase III controlled clinical trials
| LOCAL SKIN REACTIONS | VEHICLE (N=349) | TIRBANIBULIN 1% OINTMENT (N=353) | ||||
|---|---|---|---|---|---|---|
| Mild n (%) |
Moderate n (%) |
Severe n (%) |
Mild n (%) |
Moderate n (%) |
Severe n (%) |
|
| Erythema | 98 (28%) | 20 (6%) | 0 | 76 (22%) | 223 (63%) | 22 (6%) |
| Flaking/Scaling | 86 (25%) | 33 (9%) | 1 (<1%) | 92 (26%) | 166 (47%) | 31 (9%) |
| Crusting | 31 (9%) | 8 (2%) | 0 | 107 (30%) | 50 (14%) | 7 (2%) |
| Swelling | 15 (4%) | 1 (<1%) | 0 | 102 (29%) | 32 (9%) | 2 (<1%) |
| Vesiculation /Pustulation | 3 (<1%) | 0 | 0 | 25 (7%) | 2 (<1%) | 2 (<1%) |
| Erosion /Ulceration | 10 (3%) | 0 | 0 | 32 (9%) | 9 (3%) | 0 |
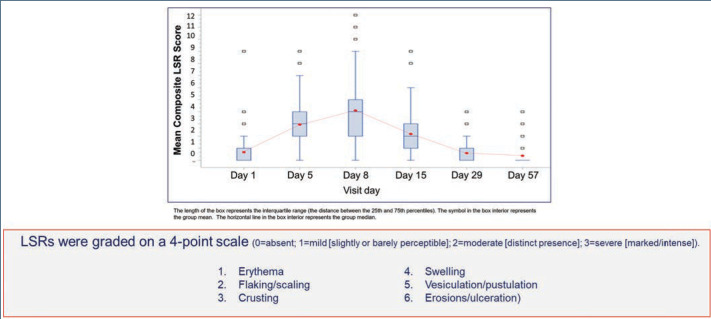
Local skin reactions were mostly mild to moderate, peaked on Day 8 and returned to baseline by Day 29. 0-3 LSR scale: 0=absent; 1=mild (slightly or barely perceptible); 2=moderate (distinct presence); 3=severe (marked, intense).
Blauvelt A, Kempers S, Lain E, et al. Phase III trials of tirbanibulin ointment for actinic keratosis. N Eng J Med. 2021;384(6):512-520.
LSRs. LSRs were collected independent of AEs and included erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosions/ulcerations. LSRs were assessed by the investigators using a four-point grading scale of 0=absent, 1=mild (slightly, barely perceptible), 2=moderate (distinct presence), and 3=severe (marked, intense). Composite score was the sum of all six LSR grades with a possible range of 0 to 18.50 Incidence and severity of LSRs greater than baseline was higher with tirbanibulin versus vehicle. LSRs were mostly mild to moderate, with severe reactions observed in less than 10 percent of subjects receiving tirbanibulin.51 LSRs peaked on Day 8 with tirbanibulin, decreased significantly by Day 15, and completely resolved by Days 29 to 57 (Figure 5). No significant difference was observed in LSR severity in patients younger or older than 65 years of age. No patients discontinued the study due to LSRs.50,52 Complete clearance of AKs using tirbanibulin 1% ointment was associated with mild-to-moderate LSRs, with 70.2 percent of patients showing a composite LSR score of 5 or less, highlighting that complete clearance was not correlated with the severity of LSRs.53
FIGURE 5.
Composite local skin reaction (LSR) scores to tirbanibulin 1% ointment once-daily treatment (Days 1–5) over time; composite score is the sum of all six LSR grades with a possible range of 0–18.; pooled results from 2 Phase III studies52
A post-hoc analysis of the two Phase III clinical trials, in which 353 participants having 4 to 8 clinically visible AK lesions (25cm2 area) were randomized to receive tirbanibulin (self-applied once daily, 5 consecutive days) was performed to assess the clearance rates of facial and scalp AK following treatment with tirbanibulin ointment 1% in different patient subgroups by body mass index (BMI), Fitzpatrick skin type, and previous AK treatment. There was no statistically significant relationship between the baseline variables of previous AK treatment (Table 3), Fitzpatrick skin type (Table 4), or BMI (29kg/m2) (Table 5) and complete (100%) and partial (>75%) clearance (CC, PC) at Day 57. In contrast, although found to be effective for both facial and scalp AK lesions, their location on the face did predict greater CC and PC success rates after two months.54
Additional post-hoc analyses of the two Phase III clinical trials evaluated CC and PC rates of AKs and severity of LSRs at time points (Day 8 earlier than end of study Day 57). At study visits (Days 8–15 to Days 29–57) CC, PC, and LSRs, including erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration, were assessed. Each LSR was scored between 0 and 3 (0=absent, 3=severe). Individual scores were added together, resulting in a LSR composite score between 0 and 18. The maximum LSR composite scores reached up to Day 57 were averaged for participants achieving CC at each visit.
A CC rate of 13.4 percent was achieved at Day 8, increasing during treatment to 24.7 percent (Day 15), 36.4 percent (Day 29), and 49.3 percent (Day 57). PC rate was 20.2 percent at Day 8 and gradually rose to 41.2 percent (Day 15), 62.8 percent (Day 29), and 72.2 percent (Day 57). Among patients reaching CC at each visit, baseline characteristics were similar except for a trend to higher percentage of face treatments in those achieving CC at Days 8 to 15 versus Days 29 to 57. The mean (±standard deviation) maximum LSR composite score reached during the follow-up was similarly low regardless of whether CC was obtained at Day 8 (4.7±1.8), Day 15 (4.8±2.2), Day 29 (4.9±2.1), or Day 57 (4.9±2.1). Although the highest CC rate with tirbanibulin was observed at Day 57, this analysis confirms that patients with AK can show much earlier responses (from Day 8). These were not accompanied by an increase in the severity of LSRs.55
Recurrence. For the one-year follow-up study, 174 subjects treated with tirbanibulin 1% ointment for once daily for five days who achieved CC at Day 57 were included. Post-hoc analysis of Kaplan-Meier estimates showed that at one year, 53 percent of patients maintained CC from recurrent lesions. The Kaplan-Meier estimate indicated that 27 percent remained free of any new or recurrent AK lesions in the 25cm2 treated area up to one year. Patients who had more than five baseline AK lesions [OR 2.1] or had prior AK treatments to the study treatment area [OR 3.0] correlated with recurrence of any AKs. Importantly, no skin cancers were detected in the treatment area throughout the one-year follow-up period.50
Based upon these studies, tirbanibulin was approved by the FDA as a field therapy for AK with the application of 2.5mg tirbanibulin in 250mg ointment to a 25cm2 contiguous area of skin on the face or scalp once daily for five consecutive days.51 The FDA accepts the defined 25cm2 area as a “field” of AK lesions, but in clinical practice, treatment requires a greater area. In a recent report, a single packet of 2.5mg tirbanibulin dissolved in 250mg ointment was sufficient to be evenly applied to a patient’s balding scalp, forehead, and two facial target AK lesions, for a total area of 317.82cm2 (digitally measured).56 As expected, LSRs peaked at Day 8 in the treatment areas and resolved by Day 39, similar to the findings in the Phase III trials. Presence of LSRs which peaked at Day 8 and resolved by Day 39 and clearance of the two target AK lesions (last to be treated at each of the 5 consecutive once-daily applications) suggest that the thin layer of ointment applied to this large area was sufficient to penetrate and be effective.56
ADDITIONAL CONSIDERATIONS
Recently, the American Academy of Dermatology (AAD) released a focused update of its guidelines of care for the management of AK.57 In this update, tirbanibulin is unique among all other listed topical treatments of AKs, in that it received the AAD AK guidelines committee’s strongest recommendation based on the highest certainty of evidence. The use of other field therapies, including imiquimod and ingenol mebuate, following cryotherapy of AK with liquid nitrogen, have resulted in additional benefits. We look forward to future studies to determine the effectiveness and safety of sequential treatment of AK with targeted cryotherapy followed by tirbanibulin 1% ointment.
An active comparator study of the efficacies and tolerabilities of tirbanibulin 1% ointment versus 5FU preparations would help direct the clinical choice of AK treatment. If such studies are undertaken, the following caveat regarding extemperaneous 5FU preparations should be noted. Investigators compared the efficacy and tolerability of 5% 5 fluorouracil cream + 0.005% calcipotriol ointment combination (FU+C) with 5% 5FU + petroleum jelly (Vaseline®, Unilever plc., London, England, United Kingdom) combination (FU+V) in the treatment of actinic keratoses.58 The FU+V control resulted in zero percent of participants having CC of facial AKs and an unexpectedly low four percent developing severe erythema, possibly due to the added petroleum jelly interfering with FU absorption. A recent study comparing the skin absorption and penetration of four different 5% 5FU formulations, including FU+V, found that combining 5FU cream with petroleum jelly interfered with the 5FU penetration through skin, resulting in a 20-fold reduced eight-hour penetration of 5FU when in the presence of petroleum jelly compared to 5% 5FU cream, and a 73-percent reduction in 5FU penetration compared to the 5-FU + calcipotriol ointment combination.59 These findings point to the inappropriateness of using 5FU in petroleum jelly as a control for clinical studies, which could lead to erroneous superiority claims.
Human papillomavirus 16 (HPV 16) has been detected in periungual SCCs and the HPV 16 E7 oncoprotein up-regulates Src family kinases, which tirbanibulin has been found to inhibit. Therefore, it is intriguing, albeit not surprising, that tirbanibulin 1% ointment has been reported to be effective in the treatment of in-situ cSCC.60 As previously discussed, SCC falls in the AK-to-SCC continuum, and therefore this possible indication for its treatment with tirbanibulin should be pursued further.
IN SUMMARY
Tirbanibulin 1% ointment is novel as a topical field therapy for AK, both in its mechanisms of action and short treatment duration. Its antiproliferative and proapoptotic activities result in an 83-percent median reduction in the number of AK lesions from baseline. It does so in a relatively noninflammatory fashion, with LSRs being rated as mostly mild to moderate. Importantly, the contents of a single packet of tirbanibulin 1% ointment were measured to cover more than the FDA-approved area of 25cm2, while maintaining effectiveness. At one year following once daily treatment for five days, 53 percent of the patients maintained 100-percent clearance, without recurrence of any of treated AK lesions. The findings outlined in this review suggest that tirbanibulin 1% ointment is a valuable, effective, and safe addition to our treatment armamentarium for AK.
Contributor Information
Brian Berman, Dr. B. Berman is with the Department of Dermatology and Cutaneous Surgery at the University of Miami Miller School of Medicine in Miami, Florida, and the Center for Clinical and Cosmetic Research in Aventura, Florida..
Ayman Grada, Dr. Grada is with the Department of Dermatology at Case Western Reserve University School of Medicine in Cleveland, Ohio..
Daniela K. Berman, Ms. D. Berman is with the University of California Berkeley Plant Gene Expression Center in Berkeley, California..
REFERENCES
- Uhlenhake EE. Optimal treatment of actinic keratoses. Clin Interv Aging. 2013;8:29. doi: 10.2147/CIA.S31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake LA, Ceilley RI, Cornelison RL et al. Guidelines of care for actinic keratoses. J Am Acad Dermatol. 1995;32(1):95–98. doi: 10.1016/0190-9622(95)90191-4. [DOI] [PubMed] [Google Scholar]
- https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf Society for Investigative Dermatology, American Academy of Dermatology Association. The burden of skin diseases, 2005. Accessed 2 Feb 2022. [DOI] [PubMed]
- Cockerell CJ, Wharton JR. New histopathological classification of actinic keratosis (incipient intraepidermal squamous cell carcinoma). J Drug Dermatol. 2005;4(4):462–467. [PubMed] [Google Scholar]
- Berman B, Bienstock L, Kuritzky L et al. Actinic keratosis: sequelae and treatments: recommendations from a consensus panel. J Fam Pract. 2006;55(5):S1–S1. [PubMed] [Google Scholar]
- Schwartz RA. The actinic keratosis: a perspective and update. Dermatolog Surg. 1997;23(11):1009–1019. doi: 10.1111/j.1524-4725.1997.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Thomson J, Bewicke-Copley F, Anene CA. et al. The genomic landscape of actinic keratosis. J Invest Dermatol. 2021. 141 7 1664 1674e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll A, Salgado R, Yebenes M et al. MYC gene numerical aberrations in actinic keratosis and cutaneous squamous cell carcinoma. Br J Dermatol. 2009;161(5):1112–1118. doi: 10.1111/j.1365-2133.2009.09351.x. [DOI] [PubMed] [Google Scholar]
- Toll A, Salgado R, Yébenes M et al. Epidermal growth factor receptor gene numerical aberrations are frequent events in actinic keratoses and invasive cutaneous squamous cell carcinomas. Exp dermatol. 2010;19(2):151–153. doi: 10.1111/j.1600-0625.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- Kanellou P, Zaravinos A, Zioga M et al. Genomic instability, mutations and expression analysis of the tumour suppressor genes p14ARF, p15INK4b, p16INK4a and p53 in actinic keratosis. Cancer Lett. 2008;264(1):145–161. doi: 10.1016/j.canlet.2008.01.042. [DOI] [PubMed] [Google Scholar]
- Padilla RS, Sebastian S, Jiang Z et al. Gene expression patterns of normal human skin, actinic keratosis, and squamous cell carcinoma: a spectrum of disease progression. Arch Dermatol. 2010;146(3):288–293. doi: 10.1001/archdermatol.2009.378. [DOI] [PubMed] [Google Scholar]
- Marks R, Foley P, Goodman G et al. Spontaneous remission of solar keratoses: the case for conservative management. Br J Dermatol. 1986;115(6):649–655. doi: 10.1111/j.1365-2133.1986.tb06644.x. [DOI] [PubMed] [Google Scholar]
- Criscione VD, Weinstock MA, Naylor MF et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention trial. Cancer. 2009;115(11):2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- Madani S, Marwaha S, Dusendang JR et al. Ten-year follow-up of persons with sun-damaged skin associated with subsequent development of cutaneous squamous cell carcinoma. JAMA Dermatol. 2021;157(5):559–565. doi: 10.1001/jamadermatol.2021.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson JM, DeSpain J, Hewett JE, Clark DP. Malignant potential of actinic keratoses and the controversy over treatment: a patient-oriented perspective. Arch Dermatol. 1991;127(7):1029–1031. [PubMed] [Google Scholar]
- Czarnecki D, Meehan C, Bruce F, Culjak G. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6(3):207–209. doi: 10.1007/s10227-001-0041-x. [DOI] [PubMed] [Google Scholar]
- Mittelbronn MA, Mullins DL, Ramos-Caro FA, Flowers FP. Frequency of pre-existing actinic keratosis in cutaneous squamous cell carcinoma. Int J Dermatol. 1998;37(9):677–681. doi: 10.1046/j.1365-4362.1998.00467.x. [DOI] [PubMed] [Google Scholar]
- Hurwitz RM, Monger LE. Solar keratosis: an evolving squamous cell carcinoma. benign or malignant? Dermatolog Surg. 1995;21(2):184. doi: 10.1111/j.1524-4725.1995.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Feldman SR, Fleischer Jr AB. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87(4):201–207. [PubMed] [Google Scholar]
- Stockfleth E, Ferrandiz C, Grob JJ et al. Development of a treatment algorithm for actinic keratoses: a European consensus. Eur J Dermatol. 2008;18(6):651–659. doi: 10.1684/ejd.2008.0514. [DOI] [PubMed] [Google Scholar]
- Markowitz O, Wang K, Levine A et al. Noninvasive long-term monitoring of actinic keratosis and field cancerization following treatment with ingenol mebutate gel 0.015%. J Clin Aesthet Dermatol. 2017;10(10):28. [PMC free article] [PubMed] [Google Scholar]
- Ranpariya VK, Muddasani S, Mahon AB, Feldman SR. Frequency of procedural and medical treatments of actinic keratosis. J Am Acad Dermatol. 2022;86(4):916–918. doi: 10.1016/j.jaad.2021.03.047. [DOI] [PubMed] [Google Scholar]
- Dinehart SM. The treatment of actinic keratoses. J Am Acad Dermatol. 2000;42(1):S25–S28. doi: 10.1067/mjd.2000.103338. [DOI] [PubMed] [Google Scholar]
- Halpern AC, Hanson LJ. Awareness of, knowledge of and attitudes to nonmelanoma skin cancer (NMSC) and actinic keratosis (AK) among physicians. Int J Dermatol. 2004;43(9):638–642. doi: 10.1111/j.1365-4632.2004.02124.x. [DOI] [PubMed] [Google Scholar]
- Balkrishnan R, Cayce KA, Kulkarni AS et al. Predictors of treatment choices and associated outcomes in actinic keratoses: results from a national physician survey study. J Dermatologic Treat. 2006;17(3):162–166. doi: 10.1080/09546630600765081. [DOI] [PubMed] [Google Scholar]
- Thai KE, Fergin P, Freeman M et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43(9):687–692. doi: 10.1111/j.1365-4632.2004.02056.x. [DOI] [PubMed] [Google Scholar]
- Krawtchenko N, Roewert-Huber J, Ulrich M et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157:34–40. doi: 10.1111/j.1365-2133.2007.08271.x. [DOI] [PubMed] [Google Scholar]
- Tanghetti E, Werschler P. Comparison of 5% 5-fluorouracil cream and 5% imiquimod cream in the management of actinic keratoses on the face and scalp. J Drugs in Dermatol. 2007;6(2):144–147. [PubMed] [Google Scholar]
- Ulrich M, Krueger-Corcoran D, Roewert-Huber J et al. Reflectance confocal microscopy for noninvasive monitoring of therapy and detection of subclinical actinic keratoses. Dermatology. 2010;220(1):15–24. doi: 10.1159/000254893. [DOI] [PubMed] [Google Scholar]
- Rivers J, Arlette J, Shear N et al. Topical treatment of actinic keratoses with 3· 0% diclofenac in 2· 5% hyaluronan gel. Br J Dermatol. 2002;146(1):94–100. doi: 10.1046/j.1365-2133.2002.04561.x. [DOI] [PubMed] [Google Scholar]
- Swanson N, Abramovits W, Berman B et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62(4):582–590. doi: 10.1016/j.jaad.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Loven K, Stein L, Furst K, Levy S. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clinic Therapeut. 2002;24(6):990–1000. doi: 10.1016/s0149-2918(02)80012-1. [DOI] [PubMed] [Google Scholar]
- Werschler WP. Considerations for use of fluorouracil cream 0.5% for the treatment of actinic keratosis in elderly patients. J Clin Aesthet Dermatol. 2008;1(2):22. [PMC free article] [PubMed] [Google Scholar]
- Lee AD, Jorizzo JL. Optimizing management of actinic keratosis and photodamaged skin: utilizing a stepwise approach. Cutis. 2009;84(3):169–175. [PubMed] [Google Scholar]
- Eisen DB, Asgari MM, Bennett DD et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85(4):e209–e233. doi: 10.1016/j.jaad.2021.02.082. [DOI] [PubMed] [Google Scholar]
- Serra-Guillén C, Nagore E, Hueso L et al. A randomized pilot comparative study of topical methyl aminolevulinate photodynamic therapy versus imiquimod 5% versus sequential application of both therapies in immunocompetent patients with actinic keratosis: clinical and histologic outcomes. J Am Acad Dermatol. 2012;66(4):e131–e137. doi: 10.1016/j.jaad.2011.11.933. [DOI] [PubMed] [Google Scholar]
- Berlin JM, Rigel DS. Diclofenac sodium 3% gel in the treatment of actinic keratoses postcryosurgery. J Drugs Dermatol. 2008;7(7):669–673. [PubMed] [Google Scholar]
- Tan JK, Thomas DR, Poulin Y et al. Efficacy of imiquimod as an adjunct to cryotherapy for actinic keratoses. J Cutan Med Surg. 2007;11(6):195–201. doi: 10.2310/7750.2007.00033. [DOI] [PubMed] [Google Scholar]
- Jorizzo JL, Markowitz O, Lebwohl MG et al. A randomized, double-blinded, placebo-controlled, multicenter, efficacy and safety study of 3.75% imiquimod cream following cryosurgery for the treatment of actinic keratoses. J Drug Dermatol. 2010;9(9):1101–1108. [PubMed] [Google Scholar]
- Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drug Dermatol. 2006;5(2):133–139. [PubMed] [Google Scholar]
- Shergill B, Zokaie S, Carr AJ. Non-adherence to topical treatments for actinic keratosis. Patient Preference Adherence. 2014;8:35. doi: 10.2147/PPA.S47126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grada A, Feldman SR, Bragazzi NL, Damiani G. Patient-reported outcomes of topical therapies in actinic keratosis: a systematic review. Dermatolog Ther. 2021;34(2):e14833. doi: 10.1111/dth.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L, Yang J, Yan W et al. Reversible binding of the anticancer drug KXO1 (tirbanibulin) to the colchicine-binding site of β-tubulin explains KXO1’s low clinical toxicity. J Biologic Chem. 2019;294(48):18099–18108. doi: 10.1074/jbc.RA119.010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolinski MP, Bu Y, Clements J et al. Discovery of novel dual mechanism of action Src signaling and tubulin polymerization inhibitors (KX2-391 and KX2-361. J Med Chem. 2018;61:4707–4719. doi: 10.1021/acs.jmedchem.8b00164. [DOI] [PubMed] [Google Scholar]
- Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochimica et Biophysica Acta (BBA) Review Cancer. 2002;1602(2):114–130. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19(49):5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- Munshi N, Groopman JE, Gill PS, Ganju RK. c-Src mediates mitogenic signals and associates with cytoskeletal proteins upon vascular endothelial growth factor stimulation in Kaposi’s sarcoma cells. J Immunol. 2000;164(3):1169–1174. doi: 10.4049/jimmunol.164.3.1169. [DOI] [PubMed] [Google Scholar]
- Ainger SA, Sturm RA. Src and SCC: getting to the FAK s. Exp Dermatol. 2015;24(7):487–488. doi: 10.1111/exd.12725. [DOI] [PubMed] [Google Scholar]
- Kim S, Min A, Lee K-H et al. Antitumor effect of KX-01 through inhibiting Src family kinases and mitosis. Cancer Res Treat. 2017;49(3):643. doi: 10.4143/crt.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt A, Kempers S, Lain E et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Eng J Med. 2021;384(6):512–520. doi: 10.1056/NEJMoa2024040. [DOI] [PubMed] [Google Scholar]
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213189s000lbl.pdf Klisyri ointment. Package insert. Almirall, LLC. Accessed 2 Feb 2022.
- Schlesinger T, Bhatia N, Berman B et al. Favorable safety profile of tirbanibulin ointment 1% for actinic keratosis: pooled results from two Phase III studies. SKIN J Cutan Med. 2020;4(6):s120–s120. [Google Scholar]
- Berman B, Schlesinger T, Bhatia N . Fall Clinical Dermatology Conference. Las Vegas; NV, USA: 2021. Complete clearance of actinic keratosis with tirbanibulin ointment 1% is not correlated with the severity of local skin reactions. Poster presentation. [PMC free article] [PubMed] [Google Scholar]
- Berman B, Gual A, Grada A . Scottsdale, AZ; USA: 2022. Efficacy of tirbanibulin ointment 1% across different patient populations: pooled results from two Phase 3 studies. Poster presentation. Fall Clinical Dermatology Conference. pp. 3–5. Jun. [Google Scholar]
- Berman B, Gupta G, Laura Padullés L . Portland, OR; USA: 2022. Complete clearance of actinic keratosis observed from Day 8 of tirbanibulin treatment, along with good tolerability: post-hoc analysis of two Phase 3 studies. Poster Presentation. Society for Investigative Dermatology Annual Meeting; pp. 18–21. May. [Google Scholar]
- Dunn A, Han H, Gade A, Berman B. Determination of the area of skin capable of being covered by the application of 250mg of tirbanibulin ointment, 1%. SKIN J Cutan Med. 2021;5(6):s82–s82. [Google Scholar]
- Eisen DB, Dellavalle RP, Frazer-Green L et al. Focused update: guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2022;87(2):373–374. doi: 10.1016/j.jaad.2022.04.013. [DOI] [PubMed] [Google Scholar]
- Cunningham TR, Tabacchi M, Eliane J-P et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106–116. doi: 10.1172/JCI89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman B, Tomondy P. Skin absorption and penetration study of four different topical formulations of 5-fluorouracil. 2022. SKIN J Cutan Med. in press.
- Moore A, Moore S. Topical tirbanibulin eradication of periungual squamous cell carcinoma. 2021. pp. 101–103. J Am Acad Dermatol Case Rep. [DOI] [PMC free article] [PubMed]