Abstract
Amyotrophic lateral sclerosis, when viewed as a biological entity rather than a clinical syndrome, probably evolves along a continuum, with the initial clinically silent phase eventually evolving into clinically manifest amyotrophic lateral sclerosis. Since motor neuron degeneration is incremental and cumulative over time, it stands to reason that the clinical syndrome of amyotrophic lateral sclerosis is probably preceded by a prodromal state characterized by minor motor abnormalities that are initially insufficient to permit a diagnosis of amyotrophic lateral sclerosis. This prodromal period, however, is usually missed, given the invariably long delays between symptom onset and diagnostic evaluation. The Pre-Symptomatic Familial ALS Study, a cohort study of pre-symptomatic gene mutation carriers, offers a unique opportunity to observe what is typically unseen. Here we describe the clinical characterization of 20 pre-symptomatic mutation carriers (in SOD1, FUS and C9orf72) whose phenoconversion to clinically manifest disease has been prospectively studied. In so doing, we observed a prodromal phase of mild motor impairment in 11 of 20 phenoconverters. Among the n = 12 SOD1 A4V mutation carriers, phenoconversion was characterized by abrupt onset of weakness, with a short (1–3.5 months) prodromal period observable in a small minority (n = 3); the observable prodrome invariably involved the lower motor neuron axis. By contrast, in all n = 3 SOD1 I113T mutation carriers, diffuse lower motor neuron and upper motor neuron signs evolved insidiously during a prodromal period that extended over a period of many years; prodromal manifestations eventually coalesced into a clinical syndrome that is recognizable as amyotrophic lateral sclerosis. Similarly, in all n = 3 C9orf72 hexanucleotide repeat expansion mutation carriers, focal or multifocal manifestations of disease evolved gradually over a prodromal period of 1–2 years. Clinically manifest ALS also emerged following a prodromal period of mild motor impairment, lasting >4 years and ∼9 months, respectively, in n = 2 with other gene mutations (SOD1 L106V and FUS c.521del6). On the basis of this empirical evidence, we conclude that mild motor impairment is an observable state that precedes clinically manifest disease in three of the most common genetic forms of amyotrophic lateral sclerosis (SOD1, FUS, C9orf72), and perhaps in all genetic amyotrophic lateral sclerosis; we also propose that this might be true of non-genetic amyotrophic lateral sclerosis. As a diagnostic label, mild motor impairment provides the language to describe the indeterminate (and sometimes intermediate) transition between the unaffected state and clinically manifest amyotrophic lateral sclerosis. Recognizing mild motor impairment as a distinct clinical entity should generate fresh urgency for developing biomarkers reflecting the earliest events in the degenerative cascade, with potential to reduce the diagnostic delay and to permit earlier therapeutic intervention.
Keywords: pre-symptomatic, phenoconversion, prodromal disease
Having observed ALS phenoconversion in 20 pre-symptomatic gene mutation carriers, Benatar et al. conclude that a prodromal period of mild motor impairment (MMI) precedes many (if not most) forms of ALS. They highlight the implications for reducing diagnostic delay and for early therapeutic intervention.
Introduction
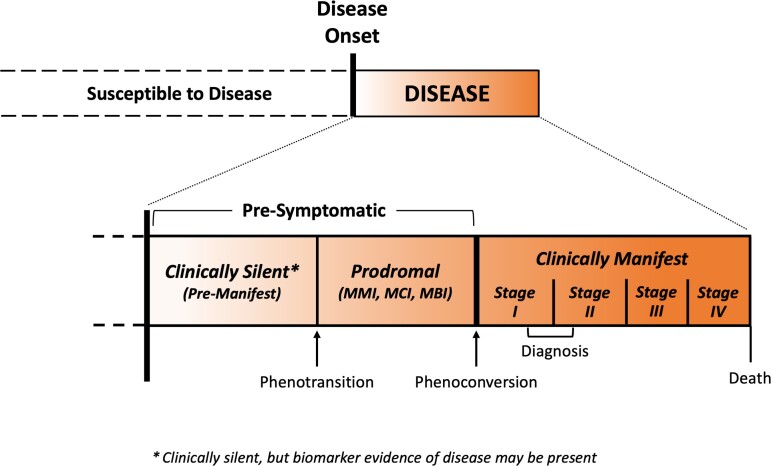
Amyotrophic lateral sclerosis (ALS) has traditionally been viewed as a clinical syndrome characterized by progressive weakness of the limbs, bulbar and respiratory muscles; and frequently also cognitive or behavioural abnormalities of the frontotemporal variety.1 The emergence of biomarker evidence of disease among clinically unaffected individuals at elevated genetic risk for ALS,2,3 however, has engendered the view that ALS, like other neurodegenerative diseases, should instead be regarded as a biological entity, with both pre-symptomatic and symptomatic (clinically manifest) phases of disease.4–6 Furthermore, it has recently been proposed that the clinical syndrome of ALS cannot be viewed simply as a dichotomy, with individuals categorized as either unaffected or as having clinically manifest ALS. Instead, ALS evolves along a continuum—passing from a clinically silent (pre-manifest) phase, through a prodromal period of mild motor impairment (MMI), before phenoconverting to the well-recognized and readily diagnosed syndrome of clinically manifest (or clinically overt) ALS (Fig. 1).4–7
Figure 1.
Clinically silent, prodromal and clinically manifest ALS. The natural history of ALS, as a biological entity, includes a clinically silent (also known as pre-manifest) stage that is typically not observable except when disease-related biomarker abnormalities are detected. These biomarker abnormalities, if present, serve as the first (and only) indication that the disease process has begun. The clinically silent stage is followed by a prodromal stage characterized by mild motor, cognitive or behavioural impairment (MMI, MCI or MBI, respectively); the prodromal stage is more likely to be observed in slower progressing disease. As motor deficits accumulate, the prodromal stage gives way to clinically manifest ALS. The term phenotransition describes the transition from the clinically silent to the prodromal stage, and the term phenoconversion describes the transition to clinically manifest ALS.7 The shaded gradient indicates that these periods exist along a continuum. (Note that the figure is not drawn to scale, as the relative duration of each period is largely unknown and may vary between individuals.)
Evidence for these paradigm shifts in conceptualizing ALS derives in large part from Pre-fALS (Pre-Symptomatic Familial ALS), a prospective natural history and biomarker cohort study, which was initiated in 2007 and focuses on characterizing the pre-symptomatic phase of ALS as well as identifying biomarkers of pre-symptomatic disease that might facilitate early intervention or disease prevention trials.8 Pre-fALS enrols unaffected individuals who are carriers of an ALS-associated gene mutation, the only population known to be at a significantly elevated risk for ALS and in whom a pre-symptomatic study is practicable. Participants are followed longitudinally. Should any of them develop symptoms or signs, follow-up continues through phenoconversion, into the early stages of clinical manifestation and for as long as participants are willing and able.
Through Pre-fALS, we have now observed the emergence of ALS in 20 previously unaffected individuals. In this report, we describe our experience with these phenoconverters and illuminate the early clinical evolution of ALS in a genetically and phenotypically heterogeneous population. On the basis of these new data, we conclude that there is an observable prodromal period of MMI in most, if not all, genetic ALS and propose that this might be true of non-genetic ALS as well. We also highlight the implications for efforts to prevent disease among those at high risk for ALS, as well as efforts to reduce the long diagnostic delay that is currently the norm for most patients with ALS.
Materials and methods
Study population
Details of the Pre-fALS study, initiated in 2007, have previously been described.2,3,8 Briefly, Pre-fALS recruits, from across North America, individuals who are carriers of any ALS-causing gene mutation [in SOD1, C9orf72 hexanucleotide repeat expansion (HRE), TARDBP, FUS, VCP, etc.], and who, at the time of enrolment, are clinically pre-symptomatic for ALS. Recruitment and follow-up have been ongoing since study initiation. As of the time this report was prepared, we have enrolled ∼210 pre-symptomatic gene mutation carriers, accrued ∼1110 person-years follow-up and there have been 20 phenoconverters. This study was approved by the University of Miami Institutional Review Board. All participants provided written informed consent. The study is registered on clinicaltrials.gov (NCT00317616).
Study procedures
At each in-person study visit, all participants undergo careful assessments—including detailed neuromuscular examination, EMG, cognitive/behavioural evaluation (a full neuropsychology battery7 that also includes the Edinburgh Cognitive and Behavioural ALS Screen), environmental exposure questionnaires and other biomarker procedures—at a single study centre every ∼12–24 months. Additional data and biological samples were also collected between in-person study visits. In the early years of Pre-fALS, EMG was only performed unilaterally (along with assessment of the bulbar region and thoracic paraspinal muscles); after the first few phenoconverters, the protocol was expanded to include bilateral EMG in the arms and legs, with systematic sampling of both proximal and distal limb muscles, as well as cranial innervated and thoracic paraspinal muscles. Those who develop motor or cognitive/behavioural symptoms or signs suggestive of disease at any point during follow-up are further evaluated at ad hoc visits to the study centre, and followed with more frequent visits as needed, depending on the degree of suspicion at the initial ad hoc assessment and the evolution of symptoms/signs over time. We thus acquire longitudinal data prior to the appearance of symptoms/signs; around the time that symptoms/signs begin to emerge and in the early (and, when possible, through the mid- or even late) stages of manifest disease.
Neurofilament light chain quantification
Neurofilament light chain (NfL) concentration was previously quantified in 50 serum samples from n = 17 of the phenoconverters using a Meso Scale Discovery (MSD) electrochemiluminescence immunoassay.3 In addition, NfL concentration has also been quantified in 97 plasma samples (collected in EDTA tubes) from all n = 20 phenoconverters using the Quanterix Simoa assay.3,9 A normative threshold of 30.4 pg/ml for serum MSD NfL, and 17.5 pg/ml for plasma Simoa NfL, was determined on the basis of the 95th percentile of, respectively, 59 serum samples from n = 34 healthy controls and 141 plasma samples from n = 80 healthy controls; controls and Pre-fALS participants were comparable in age and sex distribution. All samples were measured in duplicate at the same dilution, and all assays were performed blind to clinical information. Since we have plasma Simoa NfL data available for all phenoconverters, we rely primarily on these data to determine the timing of NfL rise relative to phenoconversion.
Data availability
Data are available from the corresponding author upon reasonable request.
Results
The 20 Pre-fALS phenoconverters include 12 SOD1 A4V (Human Genome Variation Society, A5V), three SOD1 I113T (I114T), one SOD1 L106V (L107V), one FUS c.521del6 (6 bp deletion at 521_IVS5+3) and three C9orf72 HRE mutation carriers. Their demographic, clinical and biomarker characteristics are summarized in Tables 1–3.
Table 1.
Demographic, clinical and biomarker characteristics in Pre-fALS phenoconverters
| SOD1 | FUS | C9orf72 | All (n = 20) | |||
|---|---|---|---|---|---|---|
| A4V (n = 12) | I113T (n = 3) | L106V (n = 1) | c.521del6 (n = 1) | HRE (n = 3) | ||
| Sex | ||||||
| Male | 7 | 1 | 0 | 0 | 2 | 10 |
| Female | 5 | 2 | 1 | 1 | 1 | 10 |
| Age at phenoconversion | ||||||
| Median (range) | 56 (33–76) | 55 (53–58) | 38 | 57 | 64 (44–69) | 56 (33–76) |
| Region of clinical onset (i.e. region of initial weakness or non-motor impairment) | ||||||
| Bulbar only | 2 | 0 | 0 | 0 | 0 | 2 |
| Limb(s) only | 8 | 2 | 1 | 1 | 1 | 13 |
| Axial only | 1 | 0 | 0 | 0 | 0 | 1 |
| Frontotemporal only | 0 | 0 | 0 | 0 | 1 | 1 |
| Mixeda | 1 | 1 | 0 | 0 | 1 | 3 |
| Months from phenoconversion b to diagnosis | ||||||
| Median (range) | 1.8 (0.5–5.3) | 0.0 (0.0–3.1) | 2.1 | 3.1 | 0.7 (0.0–3.0) | 1.8 (0.0–5.3) |
| Regional distribution at time of ALS diagnosis (or first examination after phenoconversion) | ||||||
| Focal | 12 | 0 | 1 | 1 | 2 | 16 |
| Multifocal | 0 | 3 | 0 | 0 | 1 | 4 |
| Neuronal axis | ||||||
| Any LMN involvement | 12 | 3 | 1 | 1 | 3 | 20 |
| Any UMN involvement | 8 | 3 | 1 | 1 | 2 | 15 |
| Any FT involvement | 0 | 0 | 0 | 0 | 1 | 1 |
| Clinical prodromal period | ||||||
| Observed | 3 | 3 | 1 | 1 | 3 | 11 |
| Plasma NfL elevation observed before or shortly after c phenoconversion | ||||||
| Before | 8 | 1 | 0 | 1 | 2 | 12 |
| Shortly afterc | 3 | 0 | 1 | 0 | 0 | 4 |
| Not elevated | 0 | 1 | 0 | 0 | 1 | 2 |
| N/A | 1 | 1 | 0 | 0 | 0 | 2 |
FT = frontotemporal; N/A = not available, due to insufficient data.
All were limb(s) and at least one other region.
Or first examination after phenoconversion. A value of 0.0 indicates that a diagnosis of ALS was made at the time that phenoconversion was confirmed to have occurred based on cumulative presence of UMN and LMN findings on examination.
For n = 4, no plasma sample was available in the 6–12 months before phenoconversion, but only shortly [i.e. median 5 (range 2–9) weeks] after phenoconversion. These NfL levels, from shortly after phenoconversion, were significantly elevated (1.6- to 16.1-fold above normative threshold). It is likely that, had samples been available before phenoconversion, those NfL levels would have also been elevated.
Table 2.
Phenoconversion and prodromal MMI in SOD1 ALS
| Case | Sex | Phenoconversion | Clinical prodrome (MMI) observed | Neuroanatomical distributionb at time when MMI first documented | Motor prodrome duration: from MMI onset to ALS phenoconversion | Timing of NfL↑ relative to ALS phenoconversion | |
|---|---|---|---|---|---|---|---|
| Agea | Site of initial weakness | ||||||
| SOD1 (A4V) | |||||||
| 1 | M | 44 | L arm | — | — | — | Likely beforem |
| 2 | F | 46 | L leg | Yes | Both legs (LMNc,d) | ∼1.9 months | ≥5.6 months beforek |
| 3 | F | 44 | R leg | — | — | — | Likely beforem |
| 4 | M | 54 | R leg | — | — | — | Likely beforem |
| 5 | F | 76 | L leg | — | — | — | ≥11.9 months beforek |
| 6 | F | 73 | L arm | — | — | — | ≥7.6 months beforek |
| 7 | M | 59 | Bulbar | Yes | Face, neck, and upper chest (LMNd) | 3.6 months | ≥7.1 months beforek,n |
| 8 | F | 57 | L arm | — | — | — | ≥1.6 months beforek |
| 9 | M | 46 | Bulbar | — | — | — | n/a |
| 10 | M | 68 | R arm + breathing | — | — | — | ≥9.7 months beforek |
| 11 | M | 33 | R leg | — | — | — | ≥7.4 months beforek |
| 12 | M | 63 | Trunk | Yes | Thoracic (LMNe), R leg (LMNe) | 1.2 months | ≥1.2 months beforek |
| SOD1 (I113T) | |||||||
| 13 | F | 55 | Bulbar + L arm + breathing | Yes | R arm (LMNe), both legs (LMNf) | ≥3.4 yearsk | ≥6.5 months beforek |
| 14 | F | 53 | Both arms + both legs | Yes | Both arms (UMNg,h), both legs (UMNg + LMNf) | ≥7.5 yearsk | n/ao |
| 15 | M | 58 | L leg | Yes | Both arms (UMNg,h + LMNi), R leg (UMNg,j + LMNd), L leg (UMNf,j + LMNd,e,f) |
≥1.6 yearsk,l | Pre-symptomatic NfL↑ not observed |
| SOD1 (L106V) | |||||||
| 16 | F | 38 | R arm | Yes | R arm (UMNg,h) | 4.4 years | Likely beforem |
F = female; M = male; NfL↑ = neurofilament light elevation (i.e. above normative threshold) based on plasma Simoa assay; L = left; R = right; n/a = insufficient data available.
In years.
Based on clinical and EMG findings.
Twitching, cramping and soreness of muscles after exercise.
Fasciculations.
Ongoing denervation changes.
Absent ankle reflex(es).
Pathologically brisk reflex(es).
Hoffman’s sign(s).
Atrophy and reduced muscle tone.
Extensor plantar response.
Greater than or equal symbol (≥) indicates that the actual duration (or length of time) may be longer than the observed duration (or length of time) reported here.
Prodrome duration could be at least 2.3 years based on external EMG, and potentially as much as ∼10 years based on participant self-report.
Blood samples not available in the 6–12 months before phenoconversion, but first sample collected immediately [i.e. median 5 (range 2–9) weeks] after phenoconversion showed such markedly elevated NfL that it is likely levels would have been elevated before phenoconversion had samples been available. Based on available data, however, we cannot determine the estimated timing of the pre-symptomatic rise in NfL.
Based on serum MSD but not plasma Simoa result.
The timing of this participant’s NfL elevation is as yet unknown, but it is definitely preceded by MMI onset.
Table 3.
Phenoconversion and prodromal MMI, MCI or MBI in FUS and C9orf72 ALS
| Case | Sex | Phenoconversion | Prodromal syndrome observed | Neuroanatomical distributionb at time when prodrome first documented | Prodrome duration: From onset of prodromal syndrome to |
Timing of NfL↑ relative to ALS or ALS-FTD phenoconversion | |||
|---|---|---|---|---|---|---|---|---|---|
| Agea | Site of initial weakness | ALS phenoconversion | bvFTD phenoconversion | ALS-FTD phenoconversion | |||||
| FUS (c.521del6) | |||||||||
| 17 | F | 57 | L leg | MMI | L leg (LMNc,d) | 8.8 months | — | — | ≥1.9 years beforej |
| C9orf72 (HRE) | |||||||||
| 18 | M | 64 | Bulbar + R leg | MMI | Bulbare, R leg (UMNf + LMNd) | 10.8 months | — | — | ≥4.7 years beforej |
| 19 | F | 44 | R arm | MMI | Both arms (UMNg,h), both legs (UMNg) | 12.1 months | — | — | Pre-symptomatic NfL↑ not observed |
| 20 | M | 69 | FT | MBIi + MMI | FT, L arm (LMNd), thoracic (LMNd) | — | ≥21 monthsi,j | ≥34 monthsi,j | ≥0.76 years before bvFTDj ≥1.8 years before ALS-FTDj |
F = female; M = male; FT = frontotemporal; NfL↑ = neurofilament light elevation (i.e. above normative threshold) based on plasma Simoa assay; n/a = insufficient data available.
In years.
Based on clinical and EMG findings and neuropsychological testing.
Focal atrophy of the extensor digitorum brevis muscle and toe extensor weakness.
Isolated denervation on EMG.
Excess saliva.
Extensor plantar response.
Pathologically brisk reflex(es).
Hoffman’s sign(s).
MBI reportedly started ∼1 year before the formal documentation of MBI and MMI at time of first assessment. Prodrome durations were estimated based on the report of initial MBI onset.
≥ indicates that the actual duration (or length of time) may be longer than the observed duration (or length of time) reported here.
SOD1 A4V mutation carriers (n = 12)
Age at phenoconversion varied from 33 to 76 years. The initial clinical manifestation of disease was most often focal weakness in a single limb (n = 8, 67%). One additional subject (Case 10) had upper limb onset concomitant with diaphragmatic weakness (Table 2). Three subjects (all male) had non-limb onset—two with bulbar weakness and one with axial (truncal) weakness (Table 2). At the first examination after phenoconversion (typically also when the diagnosis of ALS was made), lower motor neuron (LMN) axis was involved in all 12 subjects, with concurrent upper motor neuron (UMN) involvement in eight (67%). The initial appearance of weakness (i.e. phenoconversion) was often very abrupt, with some participants able to discern the particular day—sometimes even the time of day or the particular activity they were engaged in—when they first noticed weakness.
Importantly, we observed a short prodromal period of MMI before phenoconversion in three SOD1 A4V mutation carriers (Table 2). In one subject (Case 7) with bulbar onset, weakness was preceded by facial, neck and upper chest fasciculations 3.6 months before. In a second subject (Case 12) with initial truncal weakness, prescheduled evaluation 1.2 months before onset of weakness revealed ongoing denervation changes in the thoracic paraspinal muscles and the right tibialis anterior muscle. Finally, one subject (Case 2), weakness in the left leg was preceded by a 1.9-month period during which she reported muscle twitching, cramps and soreness after exercise in both legs. We did not observe prodromal manifestation of disease before the onset of weakness in the other nine SOD1 A4V mutation carriers.
We previously reported elevated serum NfL levels in SOD1 A4V mutation carriers as far back as 12 months before phenoconversion.2 In the current, expanded cohort of phenoconverters, blood samples within 12 months before phenoconversion were available in eight SOD1 A4V mutation carriers. Among them, n = 7 were found to have had elevated plasma NfL levels (first observed elevation, median = 24.1 pg/ml, range = 18.2–101.7) before phenoconversion; of them, n = 4 also had concurrent serum NfL data, which were also elevated (median = 69.7 pg/ml, range = 51.0–104.3). One other subject (Case 7) had serum NfL levels (33.6 pg/ml) above normative threshold and a borderline plasma NfL level (13.4 pg/ml), ∼7 months before phenoconversion. Three other SOD1 A4V mutation carriers (Cases 1, 3 and 4) did not have a blood sample available in the months preceding phenoconversion; however, their first samples collected shortly after phenoconversion (within 2–5 weeks of initial symptoms) showed such markedly elevated plasma and serum NfL (1.6- to 16.1-fold above normative threshold) that it is likely that levels would have been elevated before phenoconversion had samples been available. NfL results were uninformative for one other subject (Case 9) due to insufficient sample collection. Of note, in all three SOD1 A4V mutation carriers (Cases 2, 7 and 12) with a prodromal period of MMI, NfL was elevated at least as far back as, or even before, the emergence of prodromal manifestation of disease (Table 2).
SOD1 I113T mutation carriers (n = 3)
Age at phenoconversion ranged from 53–58 years. The initial prodromal manifestations of disease were invariably multifocal, and included symptoms other than weakness and/or subtle motor findings on examination or EMG; these were insufficient to declare phenoconversion to clinically manifest ALS (Table 2). These non-specific symptoms and signs evolved into overt weakness in one subject (Case 13); but in the other two subjects, it was the gradual accumulation of LMN and UMN findings over time, along with weakness on examination, that eventually led to confidence that phenoconversion had occurred. In all three subjects, therefore, phenoconversion was preceded by a years-long prodromal period of MMI. The exact duration of their respective prodrome is, however, unknown, as our data only went as far back as their initial evaluation (i.e. when they first joined the study), and all n = 3 already had MMI present by that time. We do know, however, that this duration (i.e. from MMI onset to phenoconversion) was at least 3.4 years for one subject (Case 13) (signs only); at least 7.5 years for another (Case 14) (signs only) and, for the third subject (Case 15), at least 1.6 years based on signs—and possibly 2.3 years (based on an external EMG) or as much as ∼10 years (based on self-reported symptoms) (Table 2).
Of the two subjects in whom we have blood samples available in the 12 months before phenoconversion, NfL levels (plasma and serum) were elevated above the normative threshold in only one (Case 13) (Table 2). This individual had more rapidly progressive symptomatic disease, and the motor prodrome appeared ∼2 years before NfL elevation.
SOD1 L106V mutation carrier (n = 1)
One SOD1 L106V subject (Case 16) developed ALS at age 38. Phenoconversion was preceded by a long (at least 4.4 years) prodromal period of MMI (Table 2). The initial MMI presentation was characterized by asymmetric UMN signs in one arm, followed ∼2 years later by contralateral UMN signs and a further ∼2 years later by weakness, atrophy and fasciculations in the arm where UMN signs were first observed. Plasma NfL levels were normal >2.5 years before phenoconversion and were significantly elevated (15-fold above normative threshold) within 2 months after phenoconversion (Table 2). With no data available in the 2.5 years immediately preceding phenoconversion, however, it is not possible to define the timing of initial NfL rise, except that it was at least 1.5 years after MMI onset.
FUS c.521del6 mutation carrier (n = 1)
One FUS c.521del6 mutation carrier (Case 17) developed ALS at age 57. Phenoconversion was preceded by 8.8 months of prodrome characterized by asymptomatic left sided findings of extensor digitorum brevis atrophy, mild toe extensor weakness and EMG evidence of isolated ongoing denervation changes in tibialis anterior and extensor hallucis longus (Table 3). Subsequently, phenoconversion was deemed to have occurred based on the emergence of a left foot drop, at which time examination showed diffuse weakness in both legs with brisk reflexes bilaterally, as well as weakness atrophy and brisk reflexes in the left arm. EMG showed ongoing denervation changes in both legs, the left arm and the thoracic paraspinal muscles. Plasma NfL levels were elevated 1.9 years before phenoconversion, and well before the emergence of MMI (Table 3).
C9orf72 HRE mutation carriers (n = 3)
Age at phenoconversion ranged from 44 to 69 years, with the youngest being a female (Case 19). Two subjects (Cases 18 and 19) had motor onset disease (one with focal weakness, and one with multifocal onset of weakness), with prodromal motor dysfunction preceding the onset of weakness by ∼11–12 months and neither developed cognitive/behaviour impairment (Table 3). One C9orf72 phenoconverter (Case 20) had behavioural symptoms (reported by a reliable informant) as the first known clinical manifestation of disease. These symptoms were reported by family members to have begun ∼12 months before his initial Pre-fALS assessment, at which time he was found to have mild behavioural (but not cognitive) impairment, based on our proposed criteria,7 as well as MMI. Within 9 months of this initial assessment (i.e. 21 months since onset of behavioural symptoms) he developed behavioural variant frontotemporal dementia (bvFTD), followed ∼13 months later by ALS, with initial weakness being focal in the left arm (Table 3). Plasma NfL levels were elevated in two (Cases 18 and 20) of the three C9orf72 HRE mutation carriers before symptom onset, one as far back as 4.7 years before (motor) phenoconversion. There were insufficient NfL data available for the third subject (Table 3).
Discussion
The Pre-fALS study, which follows unaffected gene mutation carriers longitudinally from the pre-symptomatic state through phenoconversion and clinically manifest ALS, provides a unique opportunity to prospectively observe, and in turn to shed light on, the earliest clinical and molecular features of neurodegeneration. On the basis of our empirical data, it is clear that, in at least a subset of individuals, the earliest symptoms/signs of disease are mild or non-specific, with some of these individuals reporting no symptoms but displaying subtle abnormal findings on examination or EMG. These earliest manifestations of motor impairment, while clearly abnormal, are of insufficient severity, extent or distribution, however, for one to conclude that the clinical syndrome of ALS has definitely emerged. Examples of early motor manifestations that constitute MMI include very mild focal weakness that is asymptomatic, hyper-reflexia (especially if new or asymmetric) and scattered ongoing denervation (e.g. fibrillations or positive sharp waves in muscles restricted to a single peripheral nerve or nerve root). These mild motor manifestations then evolve over varying lengths of time, with phenoconversion (to clinically manifest ALS) becoming apparent either because of the emergence of weakness, for example, or because of the accumulation of symptoms, signs and EMG findings. In these individuals, therefore, phenoconversion is preceded by a prodromal period of MMI. This is analogous to the prodromal period of mild cognitive impairment (MCI) that may precede Alzheimer’s disease. MCI, which is characterized by mild cognitive deficits but no dementia, may or may not portend future Alzheimer’s disease.10 Similarly, MMI also lacks specificity insofar as it may represent the first motor manifestation of ALS, but it may also be secondary to other disease processes. In addition, we recognize that a pre-paretic phase of ALS (with symptoms of stiffness, cramps, pain and paraesthesia) has been described in homozygous D90A SOD1 mutation carriers resembling Patrikios’ pseudopolyneuritic variant of ALS,11 and that periods of MCI and mild behavioural impairment (MBI) may precede ALS/FTD among gene mutation carriers who are at elevated risk for both ALS and FTD.7,12
While we have only observed a prodromal period of MMI in a subset of Pre-fALS phenoconverters, we speculate that a prodromal period may exist for all ALS (and ALS/FTD). It is, however, difficult to ‘catch’ the prodromal period when the onset and progression are abrupt, or if initial symptoms are neglected because the individual is not attuned to the possibility that these represent early ALS. For example, the prodrome duration for SOD1 A4V ALS may be so short (e.g. 1–3.5 months as our data indicate) that, by the time symptoms are reported and an electrodiagnostic study and detailed examination are scheduled and performed, the individual may have already phenoconverted. It is also possible that we missed prodromal manifestations of disease in some phenoconverters in the early years of Pre-fALS, when EMG was only performed unilaterally. It is for this reason that we have presented the data in terms of whether we were able to observe and document a prodrome period in each individual, rather than whether the participant had a prodromal period.
The temporal course of pre-symptomatic disease, and the likelihood that a prodromal period of MMI may be observed, appear to vary on the basis of the underlying genotype. For example, the prodrome for SOD1 A4V is short. By contrast, the SOD1 I113T mutation yields a prodromal period of MMI that may evolve gradually and insidiously over many years, consistent with previous observations.13 Interestingly, while there is some symmetry between the duration of the prodrome and duration of disease following phenoconversion for SOD1 A4V and I113T, our experience from other phenoconverters, however, suggests that this may not necessarily be the case for all genotypes. For instance, the motor prodrome of the single SOD1 L106V phenoconverter was long (∼4.5 years), but her clinical course of symptomatic disease was much more aggressive (onset of weakness to permanent assisted ventilation ∼15 months, consistent with published literature14,15). By contrast, we observed a relatively short motor prodrome (∼0.7 years) in our FUS mutation carrier, whose symptomatic course of disease extended over 2.4 years. The duration of symptomatic ALS in the three C9orf72 HRE phenoconverters is less clear as it is confounded, for example, by clinical trial participation.
Since the clinical phenotype of sporadic ALS more often resembles that of non-SOD1-A4V genetic forms of ALS, it is possible that many (if not all) patients with ALS of unknown aetiology also transition through a period of MMI. If true, this would have profound implications for ongoing efforts to reduce the long diagnostic delay that is so common in ALS.16–21 Notably, the symptoms, signs and EMG abnormalities that constitute MMI are not specific to ALS. As such, the clinical syndrome of MMI will invariably carry a broader differential diagnosis, with some cases of MMI reflecting early ALS and some attributable to other causes. Accordingly, patients with MMI will probably require additional investigative tests (and perhaps longitudinal follow-up) before the neurologist can make a definitive diagnosis. Diagnostic biomarkers for ALS have not been of great practical use in the past, given the relatively late stage in the disease course when patients are typically seen by a specialist, by which time the diagnosis is readily apparent based on clinical findings alone. With MMI becoming a clinically recognizable syndrome, however, early ALS-specific biomarkers will become important—and indeed necessary—as patients may be seen earlier in the disease course, when presenting with MMI rather than clinically manifest ALS. Such biomarkers would, therefore, add the most value if they could differentiate between MMI as a precursor to ALS versus MMI due to some other cause. For example, biomarker evidence of TDP-43 pathology might enable attribution of MMI to ALS, thereby permitting earlier diagnosis before emergence of the full-blown clinical syndrome. Moreover, even in the absence of such biomarkers, defining and recognizing MMI as a ‘grey zone’ between the unaffected state on the one hand and ALS on the other, permits diagnosis of an intermediate and indeterminate state. Recognition of MMI would also obviate the need for the neurologist to categorize patients as either unaffected or as having ALS, when such a dichotomization is an over-simplification and may be misleading. Individuals suspected of having MMI should be referred with high priority to a neuromuscular clinic for further evaluation as needed.
As illustrated by the sorites problem,22 and as is probably true of many diseases, the gradual accumulation of motor deficits in ALS makes it difficult to precisely define when phenoconversion occurs. While the emergence of ALS is a process that evolves over time, for practical reasons, it is necessary to demarcate (at roughly the right point in the evolution of disease) the transition between pre-symptomatic disease and clinically manifest ALS. Clear communication that phenoconversion has occurred (i.e. that the person now has ALS) enables the individual to access appropriate clinical care and potentially participate in treatment trials. In addition, an operational definition of phenoconversion enables its use as an outcome measure in early therapeutic intervention or disease prevention trials. In Pre-fALS6,7 and in the ATLAS trial,23 for example, we define phenoconversion based on the emergence of symptoms or objective motor signs that a trained evaluator would reasonably interpret as unequivocal evidence of clinically manifest ALS.
Consistent with our prior observations,2,3 NfL is increased before phenoconversion among SOD1 A4V mutation carriers; furthermore, for those in whom a prodrome was apparent, the elevation in NfL levels was evident at least as far back as, or even earlier than, the appearance of MMI. The same pattern was seen in our FUS and one C9ORF72 phenoconverter. For one other C9ORF72 and the non-A4V SOD1 phenoconverters, however, the opposite was true—for them, MMI appeared before NfL elevation. These observations underscore the complexity and nuances of using NfL as a biomarker of early disease. One hypothesis is that changes in NfL reflect the relative contribution of LMN versus UMN pathology to the prodromal syndrome. All subjects in whom the rise in NfL preceded (or occurred concurrently with) the emergence of MMI, had a prodrome dominated by LMN findings; by contrast, an increase in NfL was not observed among those in whom UMN signs were the dominant (or exclusive) abnormality. Alternatively, or in addition, the timing of the rise in NfL relative to the emergence of MMI might be a function of the aggressivity of neurodegeneration and the temporal dynamics of axonal loss. Early axonal injury in a less aggressive form of disease, for example, may be episodic with transient spikes in neurofilament that return to normal as clearance mechanisms remove NfL from the circulation. In this scenario, whether an increase in NfL is observed, would depend on when it is measured relative to the axonal injury event. In a more aggressive form of disease, on the other hand, axonal injury might be relentless from the outset, with neurofilament continuing to rise until reaching a plateau when production and clearance are in a steady state. In this scenario, NfL would be elevated when measured any time after the onset of axonal degeneration. In summary, therefore, the foregoing considerations suggest that NfL should be regarded as a marker of disease activity (i.e. axonal degeneration), whereas neurological deficits reflect the cumulative degree of previous axonal loss.
Strengths of this clinical report of 20 phenoconverters in the Pre-fALS study include: the systematic, long-term, longitudinal follow-up; the detailed and careful phenotyping of motor and cognitive/behavioural manifestation and progression; minimal inter-observer variability in assessment and interpretation of findings, given that the same neurologist (MB) evaluated all phenoconverters at almost every visit, and that this is, to date, the largest series reported of individuals who were prospectively observed to convert from the pre-symptomatic to clinically manifest phase of ALS. Moreover, the diverse genotypes among these phenoconverters, coupled with our having observed multiple phenoconverters with the same genetic mutation (e.g. SOD1 A4V, SOD1 I113T or C9orf72 HRE), has enabled us to identify similarities as well as differences among those with shared genotype, and between those with different genotypes. The phenotypic heterogeneity among these phenoconverters also suggests that our results may be more generalizable to patients with other forms of ALS. A limitation of our study is that the density of clinical observations and biological sample collection is not as high as we would like. This is, however, intrinsic to the practical limitations of how often study subjects are willing/able to be evaluated and the uncertainty of when MMI or clinically manifest ALS may emerge.
In conclusion, our observations offer rare insights into the earliest manifestations of ALS. They support the proposed working definition of phenoconversion, in which onset of the clinical syndrome is based on the emergence of symptoms, examination findings or EMG evidence that a trained neurologist would recognize as definitely indicative of ALS. They also provide the first evidence of a prodromal period of MMI that is characterized by non-specific symptoms, mild or non-specific signs on examination, or scattered ongoing denervation changes on EMG. The duration of this prodromal period may extend over many years or may be short—lasting only weeks or months, and possibly so short in some patients that it is missed. As the neurodegenerative field, and ALS specifically, increasingly embrace the prospect of early and pre-symptomatic therapeutic intervention, recognition of MMI as a clinical entity, represents a critical advance in our collective efforts to prevent ALS.
Acknowledgements
We extend thanks to our research staff (M. Catalina Fernandez, Danielle Dauphin, Dani Sheldon, Eliana Reyes, Sumaira Hussain, Alexa Gonzalez, Katja McBane, Jessica Stark, Yindi Li and other past and current members of the Pre-fALS team) at the University of Miami for participant recruitment, study coordination and data management; as well as Christine Stanislaw at Emory University for providing genetic counselling. Most importantly, we are grateful and indebted to all Pre-fALS study participants for their altruism and contribution to advancing ALS therapy development efforts. We dedicate this paper to the Pre-fALS phenoconverters whom we have lost to ALS/FTD, and to their families, who supported them throughout their courageous battle with this disease.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- (bv)FTD
(behavioural variant) frontotemporal dementia
- HRE
hexanucleotide repeat expansion
- LMN
lower motor neuron
- MBI
mild behavioural impairment
- MCI
mild cognitive impairment
- MMI
mild motor impairment
- NfL
neurofilament light chain
- Pre-fALS
Pre-Symptomatic Familial ALS Study
- UMN
upper motor neuron
Contributor Information
Michael Benatar, Department of Neurology, University of Miami, Miami, FL, USA.
Volkan Granit, Department of Neurology, University of Miami, Miami, FL, USA.
Peter M Andersen, Department of Clinical Science, Neurosciences, Umeå University, Umeå, Sweden.
Anne-Laure Grignon, Department of Neurology, University of Miami, Miami, FL, USA.
Caroline McHutchison, Department of Psychology, University of Edinburgh, Edinburgh, UK; Euan MacDonald Center for MND Research, University of Edinburgh, Edinburgh, UK.
Stephanie Cosentino, Department of Psychiatry, Columbia University, New York, NY, USA.
Andrea Malaspina, Department of Neurology, University College London, London, UK.
Joanne Wuu, Department of Neurology, University of Miami, Miami, FL, USA.
Funding
The Pre-fALS study has been supported by the Muscular Dystrophy Association (grant no. 4365 and #172123), the ALS Association (grant no. 2015), the National Institutes of Health (R01 NS105479), the ALS Recovery Fund, the Kimmelman Estate, the Swedish Brain Foundation (grant nos. 2016-0303, 2018-0310, 2020-0353), the Swedish Research Council (grant nos. 2012-3167, 2017-03100), the Knut and Alice Wallenberg Foundation (grant nos. 2012.0091, 2014.0305, 2020.0232), the Ulla-Carin Lindquist Foundation, and Neuroförbundet. C.M. is supported as a CReATe Scholar funded by the National Institutes of Health (U54 NS090291) and as a Milton Safenowitz Postdoctoral Fellow funded by the ALS Association.
Competing interests
M.B. reports grants from the National Institutes of Health and the Muscular Dystrophy Association; as well as consulting fees for Biogen, Denali, Alector, Novartis and Orphazyme A/S. The University of Miami has licensed intellectual property to Biogen to support design of the ATLAS study. V.G. reports being an employee of Biohaven (although this work was completed prior to employment at Biohaven). A.-L.G. reports no relevant disclosures. P.M.A. reports consultancies or advisory boards for Biogen, Roche, Avrion, Regeneron and Orphazyme; clinical trial site investigator for Biogen, Alexion, Sanofi, Lilly AL-S Pharma, Amylyx, Orion Pharma and Orphazyme. Since 1993, he has served as Director of the ALS-genetic laboratory at Umeå University Hospital that performs clinical and research genetic testing. He is also a member of the ClinGen ALS Gene Curation Expert panel. C.M. reports fellowship funding from the CReATe Consortium and the ALS Association. S.C. reports consulting fees from Sage Therapeutics and the Association for Frontotemporal Degeneration. J.W. reports grants from the National Institutes of Health.
References
- 1. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis—Frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A. Neurofilament light: A candidate biomarker of pre-symptomatic ALS and phenoconversion. Ann Neurol. 2018;84:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benatar M, Wuu J, Lombardi V, et al. Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swash M, Ingram D. Preclinical and subclinical events in motor neuron disease. J Neurol Neurosurg Psychiatry. 1988;51:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Carvalho M, Swash M. The onset of ALS? Clin Neurophysiol. 2010;121:1709–1710. [DOI] [PubMed] [Google Scholar]
- 6. Benatar M, Turner MR, Wuu J. Defining pre-symptomatic amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benatar M, Wuu J, McHutchison C, et al. Preventing amyotrophic lateral sclerosis: Insights from pre-symptomatic neurodegenerative diseases. Brain. 2022;145:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benatar M, Wuu J. Presymptomatic studies in ALS: Rationale, challenges, and approach. Neurology. 2012;79:1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benatar M, Zhang L, Wang L, et al. Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology. 2020;95:e59–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 11. Andersen P, Forsgren L, Binzer M, et al. Autosomal recessive adult-onset amyotrophic lateral sclerosis associated with homozygosity for Asp90Ala CuZn-superoxide dismutase mutations. Brain. 1996;119:1153–1172. [DOI] [PubMed] [Google Scholar]
- 12. Lule DE, Muller HP, Finsel J, et al. Deficits in verbal fluency in presymptomatic C9orf72 mutation gene carriers-a developmental disorder. J Neurol Neurosurg Psychiatry. 2020;91:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopate G, Baloh RH, Al-Lozi MT, et al. Familial ALS with extreme phenotypic variability due to the I113T SOD1 mutation. Amyotroph Lateral Scler. 2010;11:232–236. [DOI] [PubMed] [Google Scholar]
- 14. Juneja T, Pericak-Vance MA, Laing NG, Dave S, Siddique T. Prognosis in familial amyotrophic lateral sclerosis: Progression and survival in patients with glu100gly and ala4val mutations in Cu, Zn superoxide dismutase. Neurology. 1997;48:55–57. [DOI] [PubMed] [Google Scholar]
- 15. Cudkowicz ME, McKenna-Yasek D, Sapp PE, et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann Neurol. 1997;41:210–221. [DOI] [PubMed] [Google Scholar]
- 16. Benatar M, Wuu J. The challenge of early therapeutic intervention in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:5. [Google Scholar]
- 17. Cellura E, Spataro R, Taiello AC, La Bella V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin Neurol Neurosurg. 2012;114:550–554. [DOI] [PubMed] [Google Scholar]
- 18. Kraemer M, Buerger M, Berlit P. Diagnostic problems and delay of diagnosis in amyotrophic lateral sclerosis. Cli Neurol Neurosurg. 2010;112:103–105. [DOI] [PubMed] [Google Scholar]
- 19. Nzwalo H, de Abreu D, Swash M, Pinto S, de Carvalho M. Delayed diagnosis in ALS: The problem continues. J Neurol sci. 2014;343:173–175. [DOI] [PubMed] [Google Scholar]
- 20. Paganoni S, Macklin EA, Lee A, et al. Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zoccolella S, Beghi E, Palagano G, et al. Predictors of delay in the diagnosis and clinical trial entry of amyotrophic lateral sclerosis patients: A population-based study. J Neurol Sci. 2006;250:45–49. [DOI] [PubMed] [Google Scholar]
- 22. Britannica Encyclopaedia (Editors of) . ‘Sorites problem’. Encyclopedia Britannica 2011. Accessed 18 December 2021. https://www.britannica.com/topic/sorites-problem
- 23.Benatar M, Wuu J, Andersen PM, et al. Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Pre-Symptomatic SOD1 Variant Carriers: the ATLAS Study. Neurotherapeutics. Published online 18 May 2022. doi:10.1007/s1311-022-01237-4. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.