Abstract
Epilepsy is well-recognized as a disorder of brain networks. There is a growing body of research to identify critical nodes within dynamic epileptic networks with the aim to target therapies that halt the onset and propagation of seizures. In parallel, intracranial neuromodulation, including deep brain stimulation and responsive neurostimulation, are well-established and expanding as therapies to reduce seizures in adults with focal-onset epilepsy; and there is emerging evidence for their efficacy in children and generalized-onset seizure disorders. The convergence of these advancing fields is driving an era of ‘network-guided neuromodulation’ for epilepsy. In this review, we distil the current literature on network mechanisms underlying neurostimulation for epilepsy. We discuss the modulation of key ‘propagation points’ in the epileptogenic network, focusing primarily on thalamic nuclei targeted in current clinical practice. These include (i) the anterior nucleus of thalamus, now a clinically approved and targeted site for open loop stimulation, and increasingly targeted for responsive neurostimulation; and (ii) the centromedian nucleus of the thalamus, a target for both deep brain stimulation and responsive neurostimulation in generalized-onset epilepsies. We discuss briefly the networks associated with other emerging neuromodulation targets, such as the pulvinar of the thalamus, piriform cortex, septal area, subthalamic nucleus, cerebellum and others. We report synergistic findings garnered from multiple modalities of investigation that have revealed structural and functional networks associated with these propagation points — including scalp and invasive EEG, and diffusion and functional MRI. We also report on intracranial recordings from implanted devices which provide us data on the dynamic networks we are aiming to modulate. Finally, we review the continuing evolution of network-guided neuromodulation for epilepsy to accelerate progress towards two translational goals: (i) to use pre-surgical network analyses to determine patient candidacy for neurostimulation for epilepsy by providing network biomarkers that predict efficacy; and (ii) to deliver precise, personalized and effective antiepileptic stimulation to prevent and arrest seizure propagation through mapping and modulation of each patients’ individual epileptogenic networks.
Keywords: epilepsy, deep brain stimulation, responsive neurostimulation, networks, connectivity
Piper et al. describe the current state of network-guided neuromodulation for epilepsy and speculate on future directions. They review the mechanisms by which intracranial neurostimulation therapies reduce the frequency and severity of seizures and examine the neural networks underpinning current stimulation targets.
Glossary
Connectivity: The relationship that a node(s) has with another or others. In terms of brain connectivity, there is a common reference to ‘structural connectivity’ (e.g. integrity of white matter connections between regions) and ‘functional connectivity’ [e.g. correlation in brain activity (EEG or fMRI signal) between brain regions].
Network: A group of interconnected entities. Networks exist in different scales – for example an ‘epileptogenic network’.
Neuromodulation: ‘The alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body’ (The International Neuromodulation Society194).
Node: A specific entity within a network. Connections (or ‘edges’) may be measured between nodes.
Seizure-onset zone: A brain region or network (of multiple regions) that are responsible for the development of seizures.
Synchrony: A measure of functional connectivity that may be determined as the similarity or correlation between the signal timeseries (e.g. those dervied from EEG or fMRI) of two or more nodes.
Introduction
We now understand that epilepsy is a disorder that alters the normal connectivity of the brain.1–6 Advances in brain connectivity (‘network neuroscience’ or ‘connectomics’) research over the last few decades7,8 have driven the study of epilepsy as a network disorder. The propagation of abnormal brain activity both during (ictal) and between (inter-ictal) seizures alters widespread networks in generalized-onset epilepsy, but we also now understand that focal epilepsy subtypes have a wider implicated network than previously considered,9,10 i.e. a persistent group of around 30% of patients with epilepsy will develop drug-resistant epilepsy (DRE) and require alternative forms of therapy.11 Not all patients with DRE, however, are eligible for surgical resection of the seizure-onset zone (SOZ). Whilst traditional epilepsy surgical options—including resections and disconnections—have the potential to decouple the epileptogenic network from the normal networks of the brain,12,13 they are limited by their morbidity and irreversibility. Stimulation therapies provide a greater degree of control and reversibility while being minimally-invasive, potentially offering a more accurate and controllable treatment option. Intracranial neurostimulation interventions, such as deep brain stimulation (DBS) and responsive neurostimulation (RNS), have become effective and available treatment options to reduce seizure burden for selected patients with DRE.14–16 Although not further discussed in this review, vagus nerve (extracranial) stimulation also delivers neuromodulation in order to reduce seizure frequency by altering brain networks via the afferent innervation of the vagus nerve.17
Advances in brain network analyses and the fortuitous availability of data gathered from long-term implants in the human epileptic brain has enabled a cascade of research in the field of ‘network-guided neuromodulation’.18–21 Our understanding of how neurostimulation works on a network level has been made possible by studying and combining multiple complimentary methods such as diffusion and functional MRI (fMRI),22–25 scalp EEG and intracranial EEG.26
Whilst there have been recent reviews that have summarized the current availability and efficacy of intracranial neurostimulation therapies for epilepsy,14–16,27,28 we here approach these therapies from a network neuroscience perspective. A network-guided neuromodulation framework for epilepsy allows us to ask questions that may advance the treatment options and efficacy that we can offer to patients. These include but are not limited to:
What are the mechanisms through which current neurostimulation therapies inhibit epileptic activity in brain networks?
What are the network properties of the thalamic regions currently implanted with antiepileptic devices that make them useful targets for neurostimulation?
Are there other potential stimulation targets and what are the network properties associated with these?
Are there properties of pre-operative epileptic networks (biomarkers) that are predictive of clinical response to neurostimulation therapies?
How can we use networks to optimize and personalize neurostimulation to maximize its efficacy?
This review of intracranial neuromodulation approaches these questions, draws on the latest studies and suggests future research that may help us to advance in this field.
The mechanistic role of network modulation in neurostimulation for epilepsy
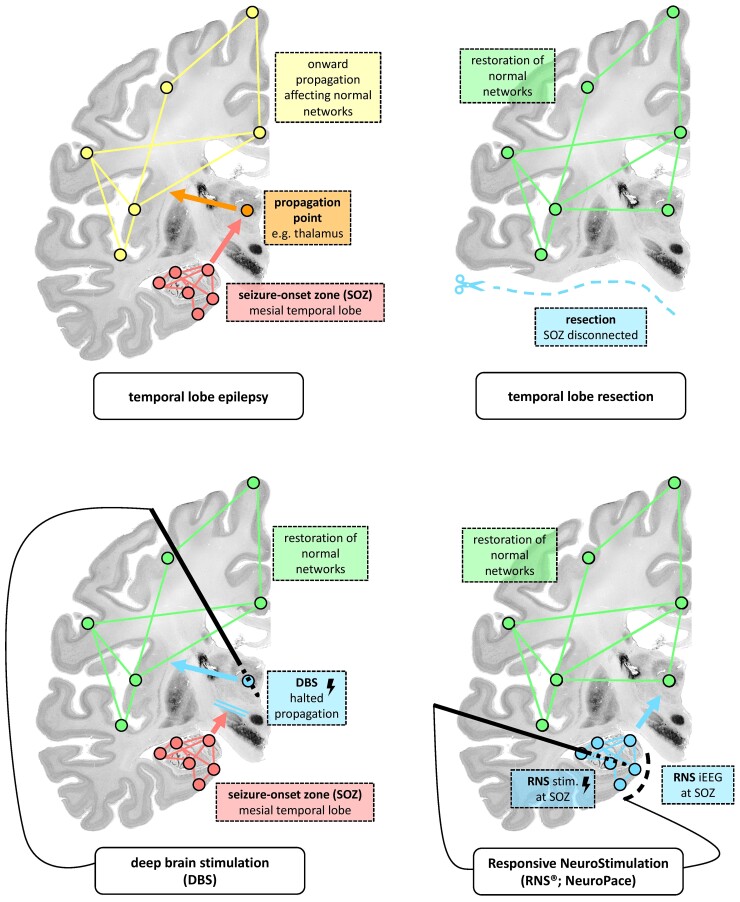
From a connectivity perspective, all epilepsy surgery interventions are an attempt to sufficiently disrupt the epileptogenic network to prevent seizures and to prevent the alteration of ‘healthy’ brain networks.29 Surgical resection and thermal ablation directly target and destroy the putative SOZ and hemispherotomy or corpus callosotomy surgeries structurally ‘disconnect’ the white matter bridging the epileptogenic and normal networks. DBS instead targets the most influential downstream ‘propagation points’ within the epileptogenic network and aims to prevent onward spread of seizure activity. RNS aims to suppress seizure generation by stimulating the SOZ in ‘response’ to epileptiform activity recorded at the SOZ. These concepts are illustrated in Fig. 1.
Figure 1.
Potential network modulation mechanisms of resective surgery, DBS and RNS using mesial temporal lobe epilepsy as an example of a focal epileptogenic network. This annotation uses coronal sections of an ex vivo brain from the BigBrain Project (open-source; https://bigbrainproject.org/).30
This review does not attempt to describe all of the hypotheses that have been postulated to explain the efficacy of DBS and RNS. Other articles have specifically set out to summarize these approaches across multiple scales and modes.31–33 These include, but are not limited to, mechanisms of DBS at the local/target level; for example, high-frequency stimulation has been suggested to prevent onward propagation of seizures by either direct inhibition (‘functional lesioning’) of the target or activation disrupting pathological activity in circuits (‘jamming theory’).34 In addition, studies have suggested that DBS disrupts pathological oscillations as a therapeutic mechanism.35 At the cellular level, for example, it has been suggested that thalamic stimulation causes glutamate35,36 and adenosine36 release that may reduce thalamic oscillations. Since these mechanisms have already been reviewed in detail, our review summarizes and questions the main contributions that intracranial neurostimulation may offer in terms of wider ‘network’ modulation.
Neurostimulation desynchronizes the epileptogenic network
Desynchronization of epileptogenic neural networks has been shown in multiple investigations as a responsible mechanism for the efficacy of neurostimulation.37 In 1954, Penfield and Jasper commented on the hyper-synchronization of brain activity occurring during epileptiform activity,38 and this observation remains corroborated in the literature.39–44 For example, the study in sheep by Stypulkowski et al.45 compared the network alterations in the Papez circuit between anterior nucleus of the thalamus (ANT; ‘indirect’) and hippocampal (‘direct’) stimulation. Both indirect and direct high-frequency stimulation suppressed theta-band power of local field potentials (LFPs) in the hippocampus, but only direct stimulation caused a ‘post-ictal suppression’ (defined as a higher threshold to produce further discharges from the hippocampus following stimulation). A more recent in-human study by Yu et al.43 investigated nine patients with temporal lobe epilepsy (TLE) undergoing intracranial EEG through inclusion of an electrode in the ANT. They showed that high-frequency stimulation of the ANT caused the broadband LFPs measured in the ANT to become desynchronized with LFPs in the ipsilateral hippocampus and neocortex. A subsequent study by Scherer et al.42 supported these findings of desynchronization in 14 patients with TLE with intermittent ANT DBS investigated with scalp EEG. They found that stimulation caused desynchronization of scalp-recorded theta and alpha band activity in responders, but not in non-responders, which supports this finding as an important therapeutic mechanism.
DBS therefore uses high-frequency stimulation to prevent so-called ‘propagation points’ (stimulation targets, e.g. the thalamus) from allowing seizure activity from the SOZ to propagate to and synchronize with the unaffected networks of the brain (Fig. 1). DBS may also prevent seizure propagation by suppressing the local generation of seizure activity through common projections, for example along the Circuit of Papez in the case of ANT stimulation (Fig. 2). In comparison, RNS offers a closed-loop system in which the receiving and delivering electrodes are both located in the SOZ, and RNS suppresses synchronization locally or regionally during the occurrence of ictal and inter-ictal epileptiform activity.
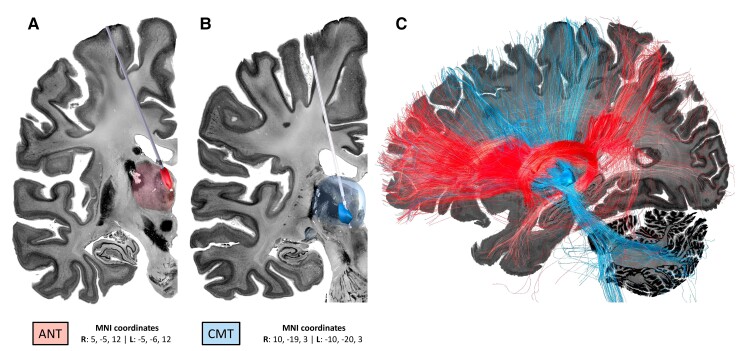
Figure 2.
Demonstration of the anatomical locations of the current propagation points/stimulation targets. (A) The ANT and (B) CMT. The images were created using LeadDBS46,47 with simulated trajectories within the BigBrain backdrop.30 The ANT (anteroventral) and CMT regions of interest and respective MNI coordinates were taken from the THOMAS atlas.48,49 The MNI coordinates are derived according to the centre of the regions of interest of the THOMAS atlas: anteroventral (right, 5, −5, 12; left, −5, −6, 12) and CMT (right, 10, −19,3; left, −10, −20,3). (C) Unconstrained fibres were seeded from each target using the normative fibre-tracking dataset of 32 adult participants from the Human Connectome Project.50,51
The concept of desynchronization is relatively straightforward to apply to focal-onset epilepsies (Fig. 1) but more challenging to understand in generalized-onset or multifocal-onset epilepsies. Bilateral thalamic stimulation may desynchronize cortically driven epileptiform activity from the subcortical networks, as suggested by studies that have shown that Lennox-Gastaut syndrome is a cortically-driven network disorder.52
Can neurostimulation normalize brain networks?
We question if electrically disrupting the epileptogenic network allows restoration of normal cortical network functioning. This concept of ‘neural hijacking’ has been postulated before by Cheney et al.53, who suggest that ‘high-frequency stimulation eliminates and replaces natural activity’. However, whilst functional network normalization has been shown in resective epilepsy surgery,54 antiseizure medication therapy55,56 and in DBS for other conditions such as Parkinson’s disease,57 there remains a lack of evidence and need for investigation for this effect in neurostimulation for DRE.
There is an understandable focus within the current neurostimulation literature on seizure frequency reduction as the primary outcome measure for neurostimulation strategies. There have been a number of studies, however, that have investigated the neuropsychological improvement associated with neurostimulation for epilepsy, most comprehensively covered by Chan et al.58 in their review. Longer-term data at five59 and nine years60 following the SANTE trial of DBS for DRE showed that patients gained neuropsychological improvement—including improvement in attention, executive function, mood (including depression, tension and anxiety) and subjective cognitive function. Similarly, a study that examined cognitive outcomes 2 years following the RNS trial identified a small yet significant improvement in cognition.61 Of significance, there has recently been a prospective clinical trial examining the cognitive effects of DBS of the anterior nucleus of the thalamus for epilepsy. Heminghyt et al.62 randomized eight adults to active stimulation and 10 adults to no stimulation for 6 months following implantation but did not show any cognitive differences between the groups at the 6-month endpoint. However, at 1 year (the open-label phase), there was a reduction in the proportion of patients experiencing executive dysfunction.
It may be, however, that a mechanism that allows these neuropsychological improvements is the relief from seizures or weaning of antiseizure medications, but some have argued that this may not be the only mechanism.63 In addition, neurostimulation may allow neuropsychological improvements by normalizing brain networks. Regardless, neurostimulation may be of particular therapeutic value in the developing brains of children—in whom the comorbidities of epilepsy may be equally or more concerning than seizures. Data to shed light on paediatric outcomes is likely to soon become available owing to a recent increase in DBS and RNS studies in children.64–66
Lastly, neurostimulation approaches must consider the risk of iatrogenic and negative implications to the normal brain network(s). Adverse events are not uncommon in neurostimulation therapies—for example, the SANTÉ trial of DBS reported that 18.2% of participants had a paresthesia and 16.4% withdrew due to adverse effects. Studies of diffusion MRI in patients undergoing DBS for movement disorders have demonstrated that it is possible to model optimal electrode positioning that allows for targeting efficacious white matter connections (tracts) but avoiding those associated with adverse effects.20 Other adverse effects that remain uninvestigated from a network perspective include the potential effects of neurostimulation on cognition, mood and sleep.67
Neurostimulation alters temporally dynamic brain networks
As well as considering stationary (single time point) brain networks, there is a need to consider that brain networks are dynamic over time. Patients with epilepsy demonstrate temporally organized seizure occurrences that respect either circadian (in the course of a day), multidien (over multiple days) or circannual (over years) patterns.68,69 The opportunity to study data recorded from intracranial devices, such as patients undergoing intracranial EEG for pre-surgical assessment, has demonstrated changing ‘seizure pathways’ within the epileptogenic network of individual patients across time.70 Whilst RNS is responsive to seizure activity in real-time, both DBS and RNS therapy may be refined by further ‘adaptive’ stimulation regimes that account for cyclical seizure patterns.
The ability of RNS and more recent DBS technologies to detect LFPs within the epileptogenic network over time allows for further investigation of the neurophysiological impact of stimulation on the patient’s epileptic network. There is a growing consensus that the efficacy of RNS is likely due to an long-term neuromodulatory effect on the epileptogenic network, rather than only arresting seizures.71 Sisterson et al.72 proposed this temporal effect to result from progressive disruption of epileptogenic network connectivity and reduction of the core synchronized population, rendering the clinical manifestation of seizures less severe, rather than RNS just being a ‘defibrillator for the brain’. In a recent in-human study, Khambhati et al.73 used the long-term data from 51 patients with DRE (with either a mesial temporal or neocortical SOZ) treated with RNS to examine the dynamics in interictal epileptiform connectivity over time by constructing a network using device-recorded LFPs. In patients with RNS electrodes in two SOZs the inter-electrode network was temporally plastic, meaning that they were able to detect alterations in functional connectivity (measured as ‘phase locking values’) between these electrodes over time and particularly within the first year post-implantation. For patients with neocortical electrodes, they found that functional connectivity was decreased in alpha and beta bands but increased in gamma bands between SOZs in ‘super’ responders (>90% reduction in seizures) compared to poor responders (<50% reduction in seizures). This led the authors to propose a ‘spark-on-kindling’ hypothesis, suggesting that RNS desynchronizes the epileptogenic network (‘kindling’) and reduces the risk of a seizure generation caused by inter-ictal epileptiform discharges (‘spark’). This may provide a mechanistic explanation for the observations from both RNS and DBS long-term clinical studies showing that seizure frequency often gradually decreases over time.60,74 This concept of stimulation-induced plasticity agrees with the observations from the literature on dystonia showing gradual improvement in symptoms with DBS over a number of months.31
It is conceptually plausible that closed-loop RNS, with both sensing and stimulation electrodes in the SOZ, may induce plastic change in the SOZ and reduce the number of focal-onset seizures. However, it is intriguing to consider how this mechanism might occur during DBS to reduce the frequency of focal-onset seizures. This raises the question as to whether DBS also has a ‘plastic’ influence on the SOZ as well as working to isolate/desynchronize the SOZ from the rest of the brain’s network. However, the data from the aforementioned study by Yu et al.43 showed that ANT stimulation decreased the rate of inter-ictal epileptiform discharges and high-frequency oscillations, supporting the plasticity concept. A provisional longer-term study has been reported in one patient who first had RNS with receiving and stimulating leads in the seizure onset and who then went on to have ANT DBS.75 In this patient, over 1.5 years, ANT DBS progressively suppressed hippocampal epileptiform activity. Overall, however, further research is required into the effects of stimulation on brain networks and their dynamics.
Propagation points within the epileptogenic network
In this section, we focus on network studies concerning the intracranial targets of neuromodulation therapies for epilepsy. We have focused primarily on the regions of the thalamus—the ANT and centromedian nucleus of the thalamus (CMT) that are DBS and RNS targets in current clinical practice. We also highlight hypothetical targets that either have previously been targeted or may bring future opportunities, including the pulvinar of the thalamus, piriform cortex (PC), septal area (SA), subthalamic nucleus (STN) and cerebellum.
Thalamus
The notion that the thalamus is a critical hub in the propagation of seizures is not a new concept. Following the dawn of Penfield’s ‘Montreal Procedure’ for epilepsy in 1930s, during which patients with epilepsy undergoing awake craniotomy would have cortical stimulation followed by ablation, attention was turned to deeper structures.76 The thalamus became a target of therapeutic neurostimulation in the animal studies by Penfield and Jasper as early as 1949.38,77,78 The thalamus is responsible for the mediation of reciprocal cortical to subcortical connections and thus defined as an ‘integrative hub’ for functional brain networks.79
The thalamus has classically been implicated as a propagation point in generalized-onset seizures80,81 and focal-onset seizures with secondary generalization on account of the onward bilateral cortical spread of epileptiform activity and connection to the brainstem. However, more recent evidence suggests that the thalamus also has a significant network role in focal-onset epilepsies without secondary generalization. From a clinical perspective, in the landmark SANTÉ trial of DBS for adult DRE there was also significant improvement in seizure frequency seen in those patients with focal-onset epilepsy.82
The role of the thalamus has been a particular focus in studying the epileptogenic network of TLE,41 partly due to the relative amenability to group studies owing to the homogeneity of the epileptic network in this condition. He et al.83 demonstrated that patients who were not rendered seizure-free following temporal lobe resection surgery for TLE were more likely to have a higher functional connectivity of the thalami on preoperative resting-state fMRI, suggesting that thalamic ‘hubness’ within the epileptogenic network could be used as a biomarker of postoperative seizure recurrence.
The thalamus is a complex structure with nuclei that each have distinct connectivity profiles. The two common targets of DBS are the ANT and CMT (shown in Fig. 2). Of note, ANT stimulation remains the only Food and Drug Administration (USA) and National Institute for Health and Care Excellence (UK) approved stimulation target for adults with DRE. The pulvinar is another nucleus of the thalamus that has been shown to be a component in the epileptogenic network84,85 but has been less well studied.
Anterior nucleus of the thalamus
The ANT has been a stimulation target for epilepsy for several decades. Early studies in humans include a study by Mullan et al. of nine patients who had lesioning of the ANT in the 1960s86 and a small cohort reported by Upton and Cooper87 of six adults who underwent ANT stimulation in the 1980s. The SANTÉ trial validated the efficacy of ANT stimulation, in which adults with focal-onset (TLE or extra-TLE) DRE underwent bilateral ANT DBS.82 Despite this early success of clinical translation, subsequent and ongoing research continues in order to further understand the network mechanisms that explain the efficacy of this therapy and to refine neurostimulation strategies.
The ANT has been described as a component of the ‘extended hippocampal system’,88 as it receives inputs from the mamillary body (via the mammillothalamic tract), subicular and retrosplenial cortex, whilst it has outputs to the medial prefrontal cortex (detailed in Fig. 3). These brain regions connected to the ANT are components of the so-called ‘Papez circuit’.89 This network of cortical and subcortical structures gives a route of seizure propagation between the hippocampus and thalamus by connections through the mammillothalamic tract and the fornix. The ANT feeds back to the hippocampus via the cingulum to the parahippocampal gyrus and entorhinal cortex.90 Neurostimulation that targets nodes (including the ANT) within the Papez circuit may desynchronize, or even recalibrate, this network.
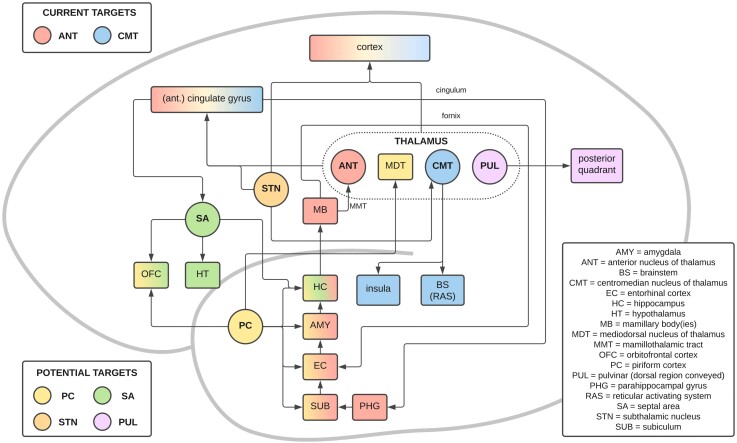
Figure 3.
A simplified schematic of the connections of the current and potential propagation points/stimulation targets. This figure demonstrates the common connections between these current and potential stimulation targets, including the ‘Circuit of Papez’. Current targets: ANT (red), CMT (blue). Potential targets: PC (yellow), septal area (SA; green), pulvinar (PUL; purple) and STN (orange). Connections with multiple colours show common connections with the respective stimulation targets.
The ANT is composed the anteroventral, anterodorsal and anteromedial subnuclei. There are ANT subnucleus-specific differences in connectivity,88,91 and studies have investigated the differences in lead placement between patients who have and have not responded to ANT DBS. Multiple retrospective studies have shown that stimulation of the anterior-ventral and anterior-medial ANT is more associated with responder status,92–95 but the wider network substrates that underpins this more efficacious target have not yet been demonstrated. A recent connectivity study by Schaper et al.96 looked at 20 patients undergoing ANT DBS and found that responders (>50% seizure reduction) had a shorter distance of the contacts to the junction of the ANT and mammillothalamic tract.
Stereo-EEG (SEEG) has offered an opportunity to investigate the connectivity profile of the ANT in the epileptogenic network, as demonstrated in the aforementioned study by Yu et al.43 Another interesting SEEG study by Chaitanya et al.97 has examined 26 seizures in seven patients with drug-resistant TLE and investigated the dynamic changes in synchronization between the SOZ and ANT. They showed that there was an increase in coupling between the amplitude of high gamma band in the SOZ and the phases of low-frequency oscillations (alpha, delta and theta) in the ANT. They also showed, however, that the synchronization between the ANT and the epileptic network preceded seizure-onset, suggesting that the ANT has a key role in the ictogenesis as well as seizure propagation. A further study by Toth et al.98 used an epileptogenicity index based on SEEG data and found that seizures that had an onset in the mesial temporal lobe (compared with other SOZs) had a higher and faster rate of ANT recruitment and that ANT recruitment preceded clinical onset. They also found that seizures that recruited the ANT lasted longer. The authors suggest that the ANT has a key role in the early organization and maintenance of seizure activity. Also, data from LFPs captured from DBS devices with sensing capabilities (e.g. the ‘Medtronic Percept’) are beginning to emerge that may provide further information on long-term network effects of ANT stimulation in DRE.99
The abnormal synchronization of thalamo-cortical seizure activity has been best studied in the TLE paradigm. The results from the SANTÉ trial suggest that patients with TLE are more likely to respond to DBS than extratemporal epilepsy or generalized epilepsy cases.82 The SANTÉ trial showed that although patients with TLE had a median of 44% seizure frequency reduction from baseline, there was no significant difference in seizure frequency reduction in patients with seizures with onset in the frontal, parietal or occipital lobes. That said, the SANTÉ trial was not statistically powered to compare rates of efficacy according to different SOZs, and other studies have identified connectivity alterations that are suggestive of a role of the ANT in a wider cortical network. For example, a study of five patients with epilepsy undergoing ANT DBS measured a transient reduction in intracortical inhibition within the motor cortex, determined by increases in motor thresholds during transcranial magnetic stimulation.100 Additionally, in a study of five patients with either multifocal or generalized epilepsy undergoing ANT stimulation, time-locked cortical responses (estimated using scalp EEG and source modelling) during ANT stimulation were increased in ipsilateral cingulate gyrus, insular cortex and lateral temporal cortices.44 Lastly, a study of 10 patients with idiopathic generalized epilepsy were studied with paired EEG-fMRI which showed that the ANT (as well as CMT) was activated during spike-and-wave discharges,101 suggesting ANT synchrony with the generalized epileptogenic network and thus a potential propagation point.
RNS has been used to treat generalized epilepsy, with a receiving detector on the cortex and the stimulation contacts in the ANT.102 Further studies are required to understand the network mechanisms by which extratemporal epileptogenic networks may benefit from ANT stimulation. Lastly, further studies are also required in order to determine the potential for ANT stimulation to be of benefit in children with epilepsy.64,65,103
Centromedian nucleus of the thalamus
The CMT (shown in Fig. 2) is an intralaminar nucleus sited at the lateral wall of the third ventricle.104 The CMT has been a neurostimulation target for 30 years, particularly for the treatment of generalized-onset epilepsies, namely Lennox-Gastaut syndrome. Studies from as early as the 1990s105–111 identified the CMT as a potentially efficacious stimulation target, but CMT DBS has only recently been supported by prospective clinical trials112 including a randomized controlled trial of CMT DBS for Lennox-Gastaut syndrome (the ‘ESTEL trial’).113 Furthermore, corresponding in vivo human studies investigating the network substrates of the CMT are only beginning to emerge.
A study by Torres Diaz et al.114 used both diffusion and functional MRI acquired in 10 adults with generalized epilepsy undergoing CMT DBS. Although the cohort was small, the clinical effect was striking—with 8/10 achieving at least 50% seizure frequency reduction. The structural (diffusion tractography) and functional (fMRI) networks of the CMT were determined using the lead contacts as seeds—‘volume of tissue activated’. Improved outcomes were associated with increased connectivity between the volume of tissue activated and the reticular system, supporting the hypothesis that the brainstem is an important component of CMT network.52 The functional connections of the CMT, derived from resting-state fMRI, involved the anterior cingulate, pre-frontal, pre-central, post-central, insular, medial temporal and occipital cortices. Similarly, results from simultaneous EEG-fMRI in young adults with Lennox-Gastaut syndrome in the ESTEL trial identified significant connectivity with the basal ganglia, brainstem, cerebellum, sensorimotor cortex, premotor cortex and limbic cortex.115 The same group have published two other paired EEG-fMRI studies showing that, in both adults and children with Lennox-Gastaut syndrome, generalized paroxysmal fast activity starts in the cortex and involves the CMT only after a delay,116 perhaps propagating to the brainstem via cortico-reticular pathways first before involving the CMT afterwards.115 A study by Kim et al.,117 using both diffusion MRI and EEG and in a cohort of 10 patients undergoing DBS for either generalized or multifocal epilepsy, also showed that the anterior cingulate gyrus and frontotemporal regions had significant connections with the CMT.
A recently published study, also from the ESTEL trial,113 used probabilistic mapping, structural connectivity (tractography) and functional connectivity (simultaneous EEG-fMRI) to refine the ‘sweet spot’ (the target of area of maximal efficacy) for CMT DBS in 20 young adults with Lennox-Gastaut syndrome.118 The study identified that DBS lead localization in the anterior-inferior-lateral CMT border (the parvocellular region) was associated with patients with higher seizure frequency reduction. Structural connectivity profiles associated with greater seizure frequency reduction showed higher connectivity with the premotor cortex, frontal operculum, putamen, globus pallidus, hippocampus, cerebellum and brainstem. Posterior (parietal, occipital and temporal) cortical connectivity was, in contrast, associated with lesser seizure frequency reduction.
Lastly, so far, only a few reports exist of efficacy of RNS for the treatment of drug-resistant idiopathic generalized epilepsy. For example, a recent retrospective cohort of four patients with idiopathic generalized epilepsy treated with CMT RNS demonstrated seizure frequency reductions ranging between 75–99%, seizure duration reduction and quality of life improvements. Another group have reported that bilateral RNS was used to detect seizure activity from and deliver neurostimulation to the CMT in a 16-year-old boy with primary generalized epilepsy.119 It will be intriguing to review the results from the upcoming RNS trial for patients with Lennox-Gastaut syndrome that will detect seizure activity at the cortex and will use the CMT as the stimulation target.120
Alternate and prospective stimulation targets
Whilst we will not discuss these in such detail as currently targeted propagation points, there are several other stimulation targets that have been or could be explored for the treatment of epilepsy. We have chosen to discuss also the pulvinar of the thalamus, piriform cortex (PC), septal area (SA), subthalamic nucleus (STN) and cerebellum, which may be emerging as potential therapeutic stimulation targets.
Pulvinar of the thalamus
The pulvinar of the thalamus has received less attention than ANT and CMT. The pulvinar is a large region of the thalamus that has distinct zones with differing connectivity profiles. The inferior and lateral subregions are considered the ‘visual pulvinar’ with strong connectivity to the occipital lobe121 and has been a suggested stimulation target for patients with posterior quadrant SOZs.85
The medial pulvinar has connections with the frontal and medial temporal lobes.122,123 A study of eight patients with TLE undergoing SEEG showed that seizures triggered by hippocampal stimulation were rendered less severe with high-frequency medial pulvinar stimulation than those without.122 This study noted that reduction in seizure severity was noted to occur with an improvement of awareness during seizures. A follow-on study of the same data measured functional connectivity (correlation in broad band SEEG) between temporal and extratemporal regions and compared connectivity differences between (i) stimulation on and stimulation off; and (ii) responders and non-responders.124 ‘Synchrony’ (i.e. connectivity) was found to be lower during stimulation in responders. The authors hypothesized that medial pulvinar stimulation may ‘reduce global synchrony’ and relate to improved awareness during TLE seizures.
Piriform cortex
The PC is a region of paleocortex that bridges the medial temporal and inferior frontal lobes superficial to the limen insulae (Fig. 4). Whilst in health the PC is a primary olfactory cortex, the PC has been implicated as a key zone of seizure propagation and kindling for several decades now.125–127 An early study in rats identified the ‘deep prepiriform’ cortex as a potent seizure zone,128 leading to a deep zone of the PC named as ‘area tempestas’ (Latin for ‘storm area’).129 There has been a recent renewal of interest in the PC’s role in epilepsy, which has been made possible by the availability of non-invasive investigations (e.g. scalp EEG, MRI and PET) to study the functional network of the human PC in vivo.130 The PC has been demonstrated as an important node within the epileptogenic network in independent cohort studies showing that extent of PC resection was associated with a higher rate of seizure freedom following anterior temporal lobe resection.131,132 This raises the question as to whether the PC is not only a seizure propagation zone, but, as previously thought, a site of epileptogenesis in TLE.
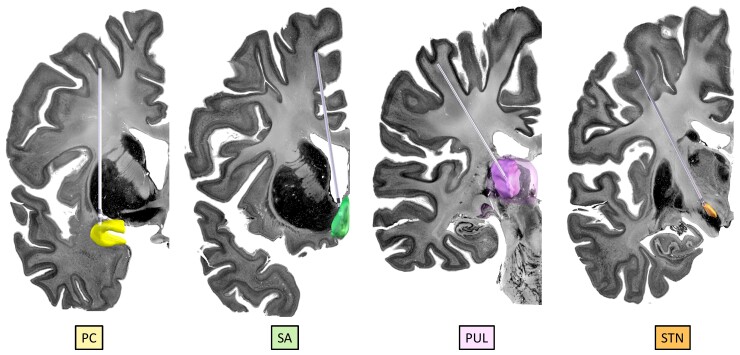
Figure 4.
Demonstration of the anatomical locations of some of the potential propagation points/stimulation targets: The PC (yellow), septal area (SA; green), pulvinar of thalamus (PUL; purple) and STN (orange). The images were created using LeadDBS46,47 with simulated trajectories within the BigBrain backdrop.30 The PC was manually segmented according to the Mai et al. atlas138; the SA was manually segmented; the PUL is a reconstruction from the THOMAS atlas48,49 within LeadDBS and the STN is a reconstruction from the DISTAL atlas139 within LeadDBS.
Laufs et al.133 used simultaneous EEG-fMRI in adults with focal-onset seizures to demonstrate increased activity of the frontal component of the PC ipsilateral to the putative SOZ was associated with interictal epileptiform discharges. Another EEG-fMRI study in 27 patients with either TLE or extratemporal epilepsy showed that the PC was a common hyperconnected node,134 supported also by a resting-state fMRI study in extratemporal epilepsy by Pedersen.135 The PC has also been implicated in generalized-onset epilepsies, but this has been less studied.125
The PC may, therefore, be implicated as a propagation point within the epileptogenic network of focal epilepsies and could thus serve as a stimulation target.125 The PC is a structural and functional connection between the temporal lobe and the limbic system.125 As such, the PC is connected to the medial temporal lobe and its associated network—including the hippocampus, amygdala, entorhinal and perirhinal cortices,136 orbitofrontal cortex,137 and the circuit of Papez. Studies of olfaction using fMRI have shown functional connectivity between the PC and the mediodorsal thalamus.137
Focus now turns to how the PC may be modulated for the treatment of DRE, particularly within TLE which seems to be the most related epilepsy type thus far. Further studies to refine our understanding of the network of the PC are required,130 and movement towards ultra-high-field imaging (7-T MRI) may facilitate studies of small structures such as the PC. As it stands, there is currently a shortage of network-based analyses of the PC analogous to those described above for other stimulation targets.
Septal area
The SA (also termed the ‘medial septum’ or ‘medial frontal zone’) is a small region of the cortex at the most posterior and deep portion of the frontal lobe (Fig. 4). Although less well explored, there has been an interest in the septal area as a neurostimulation zone for epilepsy.140
The SA has been an area of particular interest in the context of TLE considering the septo-hippocampal structural and functional connectivity. There is coupling of epileptiform activity between the septal area and hippocampus,141 and septal area stimulation inhibits hippocampal neuronal activity.142 An MRI study showed that patients with TLE (but without mesial temporal sclerosis) have higher volumes of the septal area nuclei compared to patients with extratemporal epilepsy and controls.143 The authors stated that this finding was ‘evidence of neuroplasticity/augmentation of the septal-hippocampal system in TLE’.
As it stands, studies performing neurostimulation of the septal area to treat epilepsy have been limited to animal models. A study by Takeuchi et al.144 demonstrated that closed-loop stimulation of the medial septum was able to terminate seizures in Long-Evans rats with TLE. A study by Izadi et al.145 showed that continuous stimulation of the medial septum in Sprague-Dawley rats with pilocarpine-induced TLE was able to raise the seizure threshold and improve cognitive performance measured using the Barnes maze. Further studies are required in order to determine the role of the SA in the epileptogenic network of both TLE and extratemporal epilepsy and its potential as a propagation point and stimulation target.
Subthalamic nucleus
The STN, more typically a target for DBS in Parkinson’s disease, has also been proposed as a stimulation target in epilepsy.146–148 The STN has connections with the cortex, both directly and via the thalamus.149 Following reports in animal models,150 Chabardès, Benabid and colleagues151,152 first performed STN DBS in a child with focal cortical dysplasia followed by four other patients. They hypothesized that stimulation of the STN acts on a ‘cortico-subcortical network’ by anti-dromic neuromodulation of the cortex,152 but data available in the study could not corroborate this and the network mechanism of STN stimulation in epilepsy remains unknown.
We found one study that used SEEG to investigate the role of the STN in seven patients with epilepsy undergoing presurgical evaluation and who had SOZs in the motor area.147 The investigators reported a downstream propagation of epileptiform activity from the motor cortex to the ipsilateral STN. Furthermore, the study used trials of high-frequency stimulation to the STN to show reductions in interictal spiking and high-frequency oscillations, leading to their conclusion that the STN is a key node/propagation point in the network for these patients and thus a potential stimulation target.
Cerebellum
In 1976, Cooper and colleagues published their results on using stimulation at the cerebellar cortex to inhibit seizures in 10 of 15 human subjects.153 Whilst the results suggested that anterior cerebellar lobe stimulation was more efficacious than posterior cerebellar lobe stimulation, there was no further data to refine our network understanding of this clinical effect. A small number of further human studies109,154,155 have not convincingly replicated the finding of seizure reduction with cerebellar stimulation156 and subsequently the cerebellum has not been further explored like other targets have.
Others
Alternate targets include the central lateral thalamus,157,158 pontine nucleus oralis,158 hypothalamus159 and caudate nucleus,160 as well as others.15,161 Further pre-clinical (including network analyses) and clinical evidence are required to investigate these potential seizure propagation points.
Towards personalized, network-guided neurostimulation
This review has so far discussed the mechanisms by which network augmentation delivers therapeutic effect to patients with epilepsy, the network properties of particular propagation points within the epileptogenic network and how network differences are related to varying degrees of therapeutic benefit of neuromodulation (seizure frequency reduction). This section discusses how we may be able to employ pre-implantation network metrics to guide our clinical decision making in neurostimulation and personalize therapies to maximize the delivered clinical impact to our patients.
The next translational step in network-guided neuromodulation for epilepsy is to apply the patient’s epileptogenic network to a candidacy algorithm—i.e. can we use preoperative network data to predict which patients will benefit from neurostimulation? Studies have predicted postoperative seizure outcomes based on preoperative multi-modal network data in patients undergoing resective surgery162–167 and vagus nerve stimulator implantation168 for DRE. For example, a study by Li et al.169 developed the network-based concept of ‘neural fragility’ to predict surgical failure in 43 of 47 patients undergoing resective surgery for epilepsy. Only recently, however, a small number of published studies have reported the ability of pre-implantation networks to predict response to intracranial neurostimulation for epilepsy.
Whilst we await prospective studies of network-predicted DBS or RNS efficacy in epilepsy, a number of retrospective studies have been performed that speak to the ability of preoperative data to be associated with response to neurostimulation. For example, a study by Middlebrooks et al.170 showed that, in six patients undergoing ANT DBS for DRE, the volume of tissue activated by stimulation in responders was hyperconnected to the default mode network (derived within a normative dataset from resting-state fMRI data) when compared to non-responders. A recent study by Charlebois et al.171 concluded that higher structural connectivity of the volume of tissue activated was correlated with greater seizure reduction in patients treated with hippocampal RNS. These studies raise the possibility that preoperative network measures may provide biomarkers to determine stimulation candidacy and tailor targeting to the individual patient’s network. Furthermore, Scheid et al.26 used pre-RNS functional network data derived from 30 patients undergoing intracranial EEG. They tested the hypothesis that wide-scale networks (i.e. those that incorporate nodes beyond the SOZ) can be identified as a predictive marker of RNS responder rate. They found that, compared with non-responders, responders to RNS had a smaller decrease in the functional connectivity [high-γ band (95–105 Hz)] measured between EEG contacts. Intracranial EEG could therefore be used as a pre-neurostimulation investigation, but there is still a need to determine whether predictive network signatures can be identified non-invasively.
As well as predicting patient responsiveness to neurostimulation and determining candidacy, a future objective of this field is to use preoperative measures of brain connectivity to deliver personalized and network-guided neurostimulation114,172,173 (Fig. 5). As one would expect, it has been clearly demonstrated that brain connectivity is to some degree individual in health,174 as well as in disease paradigms such as epilepsy. Stimulation targeting, therefore, must be individualized. There has recently been a significant drive towards these ‘precision’ DBS approaches within the context of adult movement disorders such as Parkinson’s disease,20 but epilepsy remains a step behind in terms of available evidence. There are moves to provide ‘adaptive’ neurostimulation, such as alteration of stimulation paradigms in response to temporally-variant neurophysiological (e.g. LFPs in RNS) or manual programming based on clinical feedback (seizure frequency).
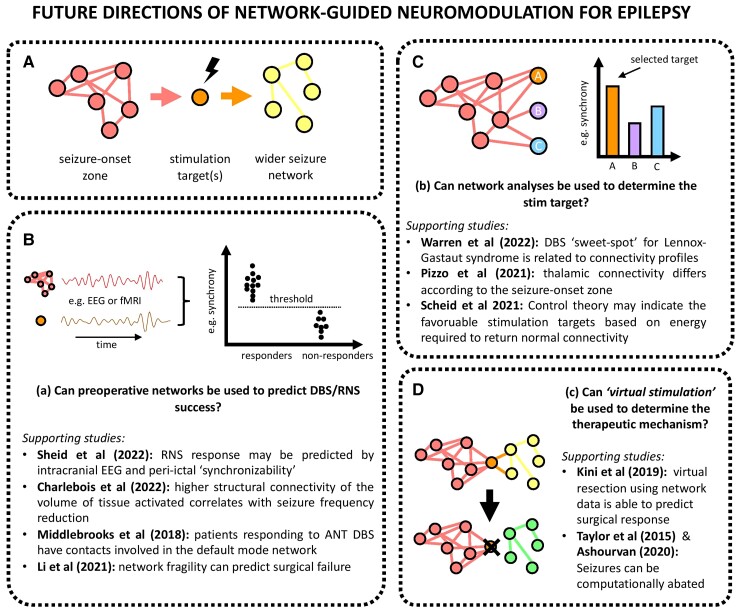
Figure 5.
Future directions of network-guided neuromodulation for epilepsy. (A) Seizures begin in the SOZ and propagate to a wider seizure network. Neurostimulation at stimulation targets prevent or limit the spread of seizure activity from the SOZ to the wider network. Panels B, C and D ask forward-thinking questions in network-guided neuromodulation epilepsy and draw on recent key studies.26,84,118,163,169–171,175–177
Although invasive, SEEG offers a clinically-viable opportunity for network-guided and individualized neuromodulation planning and has already begun transitioning in routine clinical practice.18,43,178,179 Richardson’s18 review of paradigm shifts in closed-loop neuromodulation suggests that by changing the current ‘(seizure) focus-guided’ decision-making framework to a ‘network-orientated’ framework, SEEG implantation that includes potential propagation points may identify both sites for seizure detection in RNS and sites for the delivery of neuromodulation (DBS or RNS). Similarly, the latest ‘DBS Think Tank’ report describes ‘reassessing the purpose of SEEG’ by moving away from a ‘node based philosophy’ towards a ‘network based philosophy’.180 The authors challenge the notion that ‘one size fits all’ in thalamic stimulation and suggest that SEEG may allow quantitative determination of the optimal stimulation target per patient. A retrospective study of 74 patients undergoing thalamic SEEG supports an individualized and data-driven approach to thalamic connectivity—they revealed that thalamic epileptogenicity was different according to epilepsy localization and was correlated with the extent of the epileptogenic network.84 Further retrospective studies claim that SEEG can be used to optimally place the receiving RNS lead178 and that graph theory metrics can identify the most ‘controllable’ node(s) within the epileptogenic network.175 However, further prospective evidence for the utility of SEEG-guided neuromodulation is required—including proof of concept for the network-guided placement of the stimulating lead(s). Stimulation during SEEG investigation may also provide further inferences to the optimal stimulation target(s).181
The intent of network-guided neuromodulation for epilepsy is to isolate an individual’s unique epileptogenic network and to identify key locations responsible for generating seizures, perhaps the SOZ, and an optimal propagation point in order to normalize brain connectivity. As stated in the ‘DBS Think Tank’ report,173 this would require integrating neuroimaging and network data to deliver ‘precision DBS’. Whilst many of the network-based neurostimulation studies in adult disorders outside of epilepsy predominantly use structural data, such as diffusion tractography, epilepsy would more than likely require a more advanced and multi-modality approach that incorporates functional connectivity (including EEG and fMRI) in order to incorporate data describing the dynamic and temporal network properties. Seizure occurrences in epilepsy are not random, but hold a chronotype—a temporal variation that respects cyclical patterns and may eventually allow for seizure forecasting in some patients.68,182,183
We suggest that existing data could be used to, at first, retrospectively test the idea that the preoperative and individual network can identify the patient’s optimal stimulation target. Simulated lesioning (a.k.a. ‘virtual resection’) has been used in neuroimaging studies of patients with DRE in attempts to manipulate the preoperative epileptogenic network.163,184,185 A small number of studies have similarly attempted ‘virtual stimulation’ experiments that computationally abate seizures,176,177,186 but further clinical validation and prospective studies are required. As mentioned, the availability of network data following implantation and during stimulation would be a powerful addition to allow validation of these models’ predictions in terms of network modulation and outcome. For example, the recording of LFPs simultaneous to whole-brain connectivity measures, for example fMRI or scalp EEG, could further explain the network effects of neurostimulation at different targets or stimulation regimes.187,188
The availability of normative datasets—for example structural normative networks in the Human Connectome Project189 or epilepsy-specific data such as stereo-EEG datasets—may allow for the identification of key propagation points in the individual.190 A recent example of applying normative data is in the study by Vetkas et al.,161 who used the normative functional (fMRI) dataset from 1000 adults to derive the nodes that are common to the networks of three clinically-used neurostimulation targets—the ANT, CMT and hippocampus. They used graph theory to show that the anterior cingulate and other regions of the default mode and salience networks were common nodes connected with these stimulation targets. The ultimate goal is to use the pre-operative and non-invasively derived network to identify the particular propagation point (stimulation target) where DBS would produce the greatest effect for an individual patient.
Lastly, unlike movement disorders, where the effects of altering stimulation parameters can be measured quickly, the clinical effects of such augmentations on seizure patterns can take days to weeks to become apparent. Whilst we have so far discussed pre-implantation investigations that could inform of DBS or RNS efficacy, another potential application of network analyses is to determine how alterations in stimulation regimes will affect seizure control. A catalyst in this regard could be the capability to perform neuroimaging studies with these implanted stimulation devices in situ, to measure how stimulation alters network dynamics.187,188,191 For example, Middlebrooks et al.187,188 used fMRI during active ANT DBS to demonstrate the network differences of patients with high (145 Hz) versus low (35 Hz) stimulation frequency regimes. Provided safety risks can be managed,192,193 observations of acute and chronic effects of DBS modulation can substantially improve our understanding of their mechanism of action and ultimately clinical efficacy.
Conclusions
The convergence of the fields of network neuroscience and neurostimulation are leading towards an exciting opportunity for personalized, network-guided approaches to neuromodulation for patients with epilepsy. The opportunity to combine data derived from implanted neuromodulation devices and studies of whole-brain networks gives us the opportunity to work towards this goal. Further studies are required to (i) determine the mechanistic role of network modulation; (ii) define the critical nodes within the epileptogenic network (at disease paradigm, syndrome and individual levels); (iii) to use preoperative network data to deliver precision neurostimulation to individual patients; and (iv) validate markers and models with post-operative data. As always, we need prospective clinical trials of these technologies and philosophies in order to demonstrate their clinical utility. This will require a multi-site, international and coordinated effort.
Funding
This work is supported by the NIHR GOSH BRC. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. R.J.P. is funded by the Great Ormond Street Hospital Children’s Charity Lewis Spitz Surgeon-Scientist PhD programme. B.L. is funded by National Institutes of Health grants 1DP1 OD029758-01, R56 NS 099348-05A1, The Pennsylvania Health Research Formula Fund, and the Mirowski Family Foundation. D.W.C. is supported by the Wellcome Centre for Medical Engineering. R.M.R. is funded by National Institutes of Health grant R01 NS110424. G.W. is supported by National Institutes of Health (UH2/UH3 NS95495 and R01 NS09288203).
Competing interests
D.W.C. provides consultancy on device safety in MRI. R.M.R. provides consultancy to NeuroPace, Inc. T.D. has provided consulting services for Cortec Neuro, Inspire and Synchron, and device-related intellectual property licensed with Medtronic and Bioinduction. G.W. declares intellectual property licensed to Cadence Neuroscience Inc. and NeuroOne, Inc. G.W. is an investigator for the Medtronic Deep Brain Stimulation Therapy for Epilepsy Post-Approval Study (EPAS). The other authors report no competing interests.
Contributor Information
Rory J Piper, Department of Neurosurgery, Great Ormond Street Hospital, London, UK; Developmental Neurosciences, UCL Great Ormond Street Institute of Child Health, University College London, London, UK.
R Mark Richardson, Department of Neurosurgery, Massachusetts General Hospital and Harvard Medical School, Boston, USA.
Gregory Worrell, Department of Neurology, Mayo Clinic, Rochester, USA.
David W Carmichael, Department of Biomedical Engineering, King’s College London, London, UK.
Torsten Baldeweg, Developmental Neurosciences, UCL Great Ormond Street Institute of Child Health, University College London, London, UK.
Brian Litt, Department of Neurology and Bioengineering, University of Pennsylvania, Philadelphia, USA.
Timothy Denison, MRC Brain Networks Unit, University of Oxford, Oxford, UK.
Martin M Tisdall, Department of Neurosurgery, Great Ormond Street Hospital, London, UK; Developmental Neurosciences, UCL Great Ormond Street Institute of Child Health, University College London, London, UK.
References
- 1. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the international league against epilepsy: Position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
- 2. Richardson MP. Large scale brain models of epilepsy: Dynamics meets connectomics. J Neurol Neurosurg Psychiatry. 2012;83:1238–1248. [DOI] [PubMed] [Google Scholar]
- 3. Spencer SS. Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia. 2002;43:219–227. [DOI] [PubMed] [Google Scholar]
- 4. Van Diessen E, Diederen SJHH, Braun KPJJ, Jansen FE, Stam CJ. Functional and structural brain networks in epilepsy: What have we learned? Epilepsia. 2013;54:1855–1865. [DOI] [PubMed] [Google Scholar]
- 5. Centeno M, Carmichael DW. Network connectivity in epilepsy: Resting state fMRI and EEG-fMRI contributions. Front Neurol. 2014;5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Royer J, Bernhardt BC, Larivière S, et al. Epilepsy and brain network hubs. Epilepsia. 2022;63:537–550. [DOI] [PubMed] [Google Scholar]
- 7. Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. [DOI] [PubMed] [Google Scholar]
- 8. Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larivière S, Bernasconi A, Bernasconi N, Bernhardt BC. Connectome biomarkers of drug-resistant epilepsy. Epilepsia. 2021;62:6–24. [DOI] [PubMed] [Google Scholar]
- 10. Laufs H. Functional imaging of seizures and epilepsy: Evolution from zones to networks. Curr Opin Neurol. 2012;25:194–200. [DOI] [PubMed] [Google Scholar]
- 11. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. [DOI] [PubMed] [Google Scholar]
- 12. Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377:1639–1647. [DOI] [PubMed] [Google Scholar]
- 13. Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group . A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 14. Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol. 2014;10:261–270. [DOI] [PubMed] [Google Scholar]
- 15. Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017:CD008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy: State-of-the-art approved therapies. Lancet Neurol. 2021;20:1038–1047. [DOI] [PubMed] [Google Scholar]
- 17. Hachem LD, Wong SM, Ibrahim GM. The vagus afferent network: Emerging role in translational connectomics. Neurosurg Focus. 2018;45:E2. [DOI] [PubMed] [Google Scholar]
- 18. Richardson RM. Closed-loop brain stimulation and paradigm shifts in epilepsy surgery. Neurol Clin. 2022;40:355–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horn A, Fox MD. Opportunities of connectomic neuromodulation. Neuroimage. 2020;221:117180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hollunder B, Rajamani N, Siddiqi SH, et al. Toward personalized medicine in connectomic deep brain stimulation. Prog Neurobiol. 2022;210:102211. [DOI] [PubMed] [Google Scholar]
- 21. Denison T, Morrell MJ. Neuromodulation in 2035. Neurology. 2022;98:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tavakol S, Royer J, Lowe AJ, et al. Neuroimaging and connectomics of drug-resistant epilepsy at multiple scales: From focal lesions to macroscale networks. Epilepsia. 2019;60:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Middlebrooks EH, Domingo RA, Vivas-Buitrago T, et al. Neuroimaging advances in deep brain stimulation: review of indications, anatomy, and brain connectomics. Am J Neuroradiol. 2020;41:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foit NA, Bernasconi A, Ladbon-Bernasconi N. Contributions of imaging to neuromodulatory treatment of drug-refractory epilepsy. Brain Sci. 2020;10:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu C, Ferreira F, Fox M, et al. Clinical applications of magnetic resonance imaging based functional and structural connectivity. Neuroimage. 2021;244:118649. [DOI] [PubMed] [Google Scholar]
- 26. Scheid BH, Bernabei JM, Khambhati AN, et al. Intracranial electroencephalographic biomarker predicts effective responsive neurostimulation for epilepsy prior to treatment. Epilepsia. 2022;63:652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Starnes K, Miller K, Wong-Kisiel L, Lundstrom BN. A review of neurostimulation for epilepsy in pediatrics. Brain Sci. 2019;9:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Touma L, Dansereau B, Chan AY, et al. Neurostimulation in people with drug-resistant epilepsy: Systematic review and meta-analysis from the ILAE surgical therapies commission. Epilepsia. 2022;63:-1314–1329. [DOI] [PubMed] [Google Scholar]
- 29. Chari A, Thornton RC, Tisdall MM, Scott RC. Microelectrode recordings in human epilepsy: A case for clinical translation. Brain Commun. 2020;2:fcaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amunts K, Lepage C, Borgeat L, et al. Bigbrain: An ultrahigh-resolution 3D human brain model. Science. 2013;340:1472–1475. [DOI] [PubMed] [Google Scholar]
- 31. Ashkan K, Rogers P, Bergman H, Ughratdar I. Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol. 2017;13:548–554. [DOI] [PubMed] [Google Scholar]
- 32. Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: Circuits, targets, and trials. Neurotherapeutics. 2014;11:508–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: Current challenges and future directions. Nat Rev Neurol. 2019;15:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carlson JD, Cleary DR, Cetas JS, Heinricher MM, Burchiel KJ. Deep brain stimulation does not silence neurons in subthalamic nucleus in Parkinson’s patients. J Neurophysiol. 2010;103:962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee KH, Hitti FL, Chang S-Y, et al. High frequency stimulation abolishes thalamic network oscillations: An electrophysiological and computational analysis. J Neural Eng. 2011;8:046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tawfik VL, Chang S-Y, Hitti FL, et al. Deep brain stimulation results in local glutamate and adenosine release. Neurosurgery. 2010;67:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Medeiros D de C, Moraes MFD. Focus on desynchronization rather than excitability: A new strategy for intraencephalic electrical stimulation. Epilepsy Behav. 2014;38:32–36. [DOI] [PubMed] [Google Scholar]
- 38. Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain; Little, Brown & Co;1954. [Google Scholar]
- 39. Kramer MA, Cash SS. Epilepsy as a disorder of cortical network organization. Neurosci. 2012;18:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chaitanya G, Toth E, Pizarro D, et al. Anterior nucleus of thalamus gates progression of mesial temporal seizures by modulating thalamocortical corresponding. bioRxiv. [Preprint] doi.org/ 10.1101/2020.09.17.301812 [DOI] [Google Scholar]
- 41. Guye M, Régis J, Tamura M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129:1917–1928. [DOI] [PubMed] [Google Scholar]
- 42. Scherer M, Milosevic L, Guggenberger R, et al. Desynchronization of temporal lobe theta-band activity during effective anterior thalamus deep brain stimulation in epilepsy. Neuroimage. 2020;218:116967. [DOI] [PubMed] [Google Scholar]
- 43. Yu T, Wang X, Li Y, et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain. 2018;141:2631–2643. [DOI] [PubMed] [Google Scholar]
- 44. Zumsteg D, Lozano AM, Wieser HG, Wennberg RA. Cortical activation with deep brain stimulation of the anterior thalamus for epilepsy. Clin Neurophysiol. 2006;117:192–207. [DOI] [PubMed] [Google Scholar]
- 45. Stypulkowski PH, Stanslaski SR, Jensen RM, Denison TJ, Giftakis JE. Brain stimulation for epilepsy – local and remote modulation of network excitability. Brain Stimul. 2014;7:350–358. [DOI] [PubMed] [Google Scholar]
- 46. Horn A, Kühn AA. Lead-DBS: A toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–135. [DOI] [PubMed] [Google Scholar]
- 47. Horn A, Li N, Dembek TA, et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage. 2019;184:293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Su JH, Thomas FT, Kasoff WS, et al. Thalamus optimized multi atlas segmentation (THOMAS): Fast, fully automated segmentation of thalamic nuclei from structural MRI. Neuroimage. 2019;194:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saranathan M, Iglehart C, Monti M, Tourdias T, Rutt B. In vivo high-resolution structural MRI-based atlas of human thalamic nuclei. Sci Data. 2021;8:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horn A, Kühn AA, Merkl A, Shih L, Alterman R, Fox M. Probabilistic conversion of neurosurgical DBS electrode coordinates into MNI space. Neuroimage. 2017;150:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Setsompop K, Kimmlingen R, Eberlein E, et al. Pushing the limits of in vivo diffusion MRI for the human connectome project. Neuroimage. 2013;80:220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Warren AEL, Harvey AS, Vogrin SJ, et al. The epileptic network of Lennox-Gastaut syndrome: cortically driven and reproducible across age. Neurology. 2019;93:E215–E226. [DOI] [PubMed] [Google Scholar]
- 53. Cheney PD, Griffin DM, Van Acker GM. Neural hijacking: action of high-frequency electrical stimulation on cortical circuits. Neuroscientist 2013;19:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boerwinkle VL, Cediel EG, Mirea L, et al. Network-targeted approach and postoperative resting-state functional magnetic resonance imaging are associated with seizure outcome. Ann Neurol. 2019;86:344–356. [DOI] [PubMed] [Google Scholar]
- 55. Xiao F, Koepp MJ, Zhou D. Pharmaco-fMRI: a tool to predict the response to antiepileptic drugs in epilepsy. Front Neurol. 2019;10:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wandschneider B, Stretton J, Sidhu M, et al. Levetiracetam reduces abnormal network activations in temporal lobe epilepsy. Neurology. 2014;83:1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Horn A, Wenzel G, Irmen F, et al. Deep brain stimulation induced normalization of the human functional connectome in Parkinson’s disease. Brain. 2019;142:3129–3143. [DOI] [PubMed] [Google Scholar]
- 58. Chan AY, Rolston JD, Rao VR, Chang EF. Effect of neurostimulation on cognition and mood in refractory epilepsy. Epilepsia Open. 2018;3:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Salanova V, Witt T, Worth R, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nair DR, Laxer KD, Weber PB, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95:e1244–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Loring DW, Kapur R, Meador KJ, Morrell MJ. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia. 2015;56:1836–1844. [DOI] [PubMed] [Google Scholar]
- 62. Heminghyt E, Herrman H, Skogan AH, et al. Cognitive change after DBS in refractory epilepsy: a randomized-controlled trial. Acta Neurol Scand. 2022;145:111–118. [DOI] [PubMed] [Google Scholar]
- 63. Spencer D. Responsive neurostimulation and cognition. Epilepsy Curr. 2016;16:98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khan M, Paktiawal J, Piper RJ, Chari A, Tisdall MM. Intracranial neuromodulation with deep brain stimulation and responsive neurostimulation in children with drug-resistant epilepsy: a systematic review. J Neurosurg Pediatr. 2022;29:208–217. [DOI] [PubMed] [Google Scholar]
- 65. Nagahama Y, Zervos TM, Murata KK, et al. Real-world preliminary experience with responsive neurostimulation in pediatric epilepsy: a multicenter retrospective observational study. Neurosurgery. 2021;89:-997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kerezoudis P, Gyftopoulos A, Alexander AY, et al. Safety and efficacy of responsive neurostimulation in the pediatric population: evidence from institutional review and patient-level meta-analysis. Epilepsy Behav. 2022;129:108646. [DOI] [PubMed] [Google Scholar]
- 67. Voges BR, Schmitt FC, Hamel W, et al. Deep brain stimulation of anterior nucleus thalami disrupts sleep in epilepsy patients. Epilepsia. 2015;56:e99–e103. [DOI] [PubMed] [Google Scholar]
- 68. Karoly PJ, Rao VR, Gregg NM, et al. Cycles in epilepsy. Nat Rev Neurol. 2021;17:267–284. [DOI] [PubMed] [Google Scholar]
- 69. Baud MO, Kleen JK, Mirro EA, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schroeder GM, Diehl B, Chowdhury FA, et al. Seizure pathways change on circadian and slower timescales in individual patients with focal epilepsy. Proc Natl Acad Sci U S A. 2020;117:11048–11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kokkinos V, Sisterson ND, Wozny TA, Richardson RM. Association of closed-loop brain stimulation neurophysiological features with seizure control among patients with focal epilepsy. JAMA Neurol. 2019;76:800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sisterson ND, Wozny TA, Kokkinos V, Constantino A, Richardson RM. Closed-loop brain stimulation for drug-resistant epilepsy: Towards an evidence-based approach to personalized medicine. Neurotherapeutics. 2019;16:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Khambhati AN, Shafi A, Rao VR, Chang EF. Long-term brain network reorganization predicts responsive neurostimulation outcomes for focal epilepsy. Sci Transl Med. 2021;13:eabf6588. [DOI] [PubMed] [Google Scholar]
- 74. Salanova V, Sperling MR, Gross RE, et al. The SANTÉ study at 10 years of follow-up: effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 2021;62:1306–1317. [DOI] [PubMed] [Google Scholar]
- 75. Silva AB, Khambhati AN, Speidel BA, Chang EF, Rao VR. Effects of anterior thalamic nuclei stimulation on hippocampal activity: chronic recording in a patient with drug-resistant focal epilepsy. Epilepsy Behav Reports. 2021;16:100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gardner J. A history of deep brain stimulation: technological innovation and the role of clinical assessment tools. Soc Stud Sci. 2013;43:707–728. [Google Scholar]
- 77. Jasper H, Naquet R, King EE. Thalamocortical recruiting responses in sensory receiving areas in the cat. Electroencephalogr Clin Neurophysiol. 1955;7:99–114. [DOI] [PubMed] [Google Scholar]
- 78. Hunter J, Jasper HH. Effects of thalamic stimulation in unanaesthetised animals. Electroencephalogr Clin Neurophysiol. 1949;1:305–324. [DOI] [PubMed] [Google Scholar]
- 79. Hwang K, Bertolero MA, Liu WB, D’Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci. 2017;37:5594–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Blumenfeld H. The thalamus and seizures. Arch Neurol. 2002;59:135–137. [DOI] [PubMed] [Google Scholar]
- 81. Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. [DOI] [PubMed] [Google Scholar]
- 82. Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. [DOI] [PubMed] [Google Scholar]
- 83. He X, Doucet GE, Pustina D, Sperling MR, Sharan AD, Tracy JI. Presurgical thalamic “hubness” predicts surgical outcome in temporal lobe epilepsy. Neurology. 2017;88:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pizzo F, Roehri N, Giusiano B, et al. The ictal signature of thalamus and basal ganglia in focal epilepsy: A SEEG study. Neurology. 2021;96:e280–e293. [DOI] [PubMed] [Google Scholar]
- 85. Burdette D, Mirro EA, Lawrence M, Patra SE. Brain-responsive corticothalamic stimulation in the pulvinar nucleus for the treatment of regional neocortical epilepsy: a case series. Epilepsia Open. 2021;6:611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mullan S. Thalamic lesions for the control of epilepsy. Arch Neurol. 1967;16:277. [DOI] [PubMed] [Google Scholar]
- 87. Upton ARM, Amin I, Garnett S, Springman M, Nahmias C, Cooper IS. Evoked metabolic responses in the Limbic Striate system produced by stimulation of anterior thalamic nucleus in man. Pacing Clin Electrophysiol. 1987;10:217–225. [DOI] [PubMed] [Google Scholar]
- 88. Child ND, Benarroch EE. Anterior nucleus of the thalamus: Functional organization and clinical implications. Neurology. 2013;81:1869–1876. [DOI] [PubMed] [Google Scholar]
- 89. Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725. [Google Scholar]
- 90. Li DH, Yang XF. Remote modulation of network excitability during deep brain stimulation for epilepsy. Seizure. 2017;47:42–50. [DOI] [PubMed] [Google Scholar]
- 91. Aggleton JP, O’Mara SM. The anterior thalamic nuclei: core components of a tripartite episodic memory system. Nat Rev Neurosci. 2022;23:505–516. [DOI] [PubMed] [Google Scholar]
- 92. Krishna V, King NKK, Sammartino F, et al. Anterior nucleus deep brain stimulation for refractory epilepsy: insights into patterns of seizure control and efficacious target. Neurosurgery. 2016;78:802–811. [DOI] [PubMed] [Google Scholar]
- 93. Lehtimäki K, Möttönen T, Järventausta K, et al. Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimul. 2016;9:268–275. [DOI] [PubMed] [Google Scholar]
- 94. Koeppen JA, Nahravani F, Kramer M, et al. Electrical stimulation of the anterior thalamus for epilepsy: clinical outcome and analysis of efficient target. Neuromodulation. 2019;22:465–471. [DOI] [PubMed] [Google Scholar]
- 95. Gross RE, Fisher RS, Sperling MR, Giftakis JE, Stypulkowski PH. Analysis of deep brain stimulation lead targeting in the stimulation of anterior nucleus of the thalamus for epilepsy clinical trial. Neurosurgery. 2021;89:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schaper FLWVJ, Plantinga BR, Colon AJ, et al. Deep brain stimulation in epilepsy: a role for modulation of the mammillothalamic tract in seizure control? Neurosurgery. 2020;87:602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chaitanya G, Toth E, Pizarro D, Irannejad A, Riley K, Pati S. Precision mapping of the epileptogenic network with low- and high-frequency stimulation of anterior nucleus of thalamus. Clin Neurophysiol. 2020;131:2158–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Toth E, Chaitanya G, Pizarro D, et al. Ictal recruitment of anterior nucleus of thalamus in human focal epilepsy. [Preprint] bioRxiv doi:10.1101/788422.
- 99. Gregg NM, Marks VS, Sladky V, et al. Anterior nucleus of the thalamus seizure detection in ambulatory humans. Epilepsia. 2021;62:e158–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Molnar GF, Sailer A, Gunraj CA, et al. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology. 2006;66:566–571. [DOI] [PubMed] [Google Scholar]
- 101. Tyvaert L, Chassagnon S, Sadikot A, LeVan P, Dubeau F, Gotman J. Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology. 2009;73:2018–2022. [DOI] [PubMed] [Google Scholar]
- 102. Herlopian A, Cash SS, Eskandar EM, Jennings T, Cole AJ. Responsive neurostimulation targeting anterior thalamic nucleus in generalized epilepsy. Ann Clin Transl Neurol. 2019;6:2104–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Piper RJ, Tangwiriyasakul C, Shamshiri EA, et al. Functional connectivity of the anterior nucleus of the thalamus in pediatric focal epilepsy. Front Neurol. 2021;12:670881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Warsi NM, Yan H, Suresh H, et al. The anterior and centromedian thalamus: anatomy, function, and dysfunction in epilepsy. Epilepsy Res. 2022;182:106913. [DOI] [PubMed] [Google Scholar]
- 105. Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841–851. [DOI] [PubMed] [Google Scholar]
- 106. Velasco F, Velasco M, Jiménez F, et al. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. 2000;47:295–305. [DOI] [PubMed] [Google Scholar]
- 107. Velasco AL, Velasco F, Jimenez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox–Gastaut syndrome. Epilepsia. 2006;47:1203–1212. [DOI] [PubMed] [Google Scholar]
- 108. Velasco M, Velasco F, Velasco AL, Jiménez F, Brito F, Márquez I. Acute and chronic electrical stimulation of the centromedian thalamic nucleus. Arch Med Res. 2000;31:304–315. [DOI] [PubMed] [Google Scholar]
- 109. Velasco F, Carrillo-Ruiz JD, Brito F, et al. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia. 2005;46:1071–1081. [DOI] [PubMed] [Google Scholar]
- 110. Velasco F, Velasco M, Velasco AL, Jimenez F, Marquez I, Rise M. Electrical stimulation of the centromedian thalamic nucleus in control of seizures: Long-term studies. Epilepsia. 1995;36:63–71. [DOI] [PubMed] [Google Scholar]
- 111. Velasco AL, Velasco F, Velasco M, Trejo D, Castro G, Carrillo-Ruiz JD. Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia. 2007;48:1895–1903. [DOI] [PubMed] [Google Scholar]
- 112. Valentín A, García Navarrete E, Chelvarajah R, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54:1823–1833. [DOI] [PubMed] [Google Scholar]
- 113. Dalic LJ, Warren AEL, Bulluss KJ, et al. DBS Of thalamic centromedian nucleus for Lennox-Gastaut syndrome (ESTEL trial). Ann Neurol. 2022;91:253–267. [DOI] [PubMed] [Google Scholar]
- 114. Diaz C V. T, González-Escamilla G, Ciolac D, et al. Network substrates of centromedian nucleus deep brain stimulation in generalized pharmacoresistant epilepsy. Neurotherapeutics. 2021;18:1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Warren AEL, Dalic LJ, Thevathasan W, Roten A, Bulluss KJ, Archer J. Targeting the centromedian thalamic nucleus for deep brain stimulation. J Neurol Neurosurg Psychiatry. 2020;91:339–349. [DOI] [PubMed] [Google Scholar]
- 116. Dalic LJ, Warren AEL, Young JC, et al. Cortex leads the thalamic centromedian nucleus in generalized epileptic discharges in Lennox-Gastaut syndrome. Epilepsia. 2020;61:2214–2223. [DOI] [PubMed] [Google Scholar]
- 117. Kim SH, Lim SC, Yang DW, et al. Thalamo-cortical network underlying deep brain stimulation of centromedian thalamic nuclei in intractable epilepsy: a multimodal imaging analysis. Neuropsychiatr Dis Treat. 2017;13:2607–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Warren AEL, Dalic LJ, Bulluss KJ, Roten A, Thevathasan W, Archer JS. The optimal target and connectivity for DBS in Lennox-Gastaut syndrome. Ann Neurol. 2022;92:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Welch WP, Hect JL, Abel TJ. Case report: Responsive neurostimulation of the centromedian thalamic nucleus for the detection and treatment of seizures in pediatric primary generalized epilepsy. Front Neurol. 2021;12:656585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. NeuroPace . NeuroPace Awarded Five-Year NIH Grant Funding of More than $9 M to Study RNS System in Patients with Lennox-Gastaut Syndrome. Accessed December 8, 2021. https://www.neuropace.com/neuropace-awarded-five-year-nih-grant-funding/
- 121. Bridge H, Leopold DA, Bourne JA. Adaptive pulvinar circuitry supports visual cognition. Trends Cogn Sci. 2016;20:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Filipescu C, Lagarde S, Lambert I, et al. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia. 2019;60:e25–e30. [DOI] [PubMed] [Google Scholar]
- 123. Homman-Ludiye J, Bourne JA. The medial pulvinar: Function, origin and association with neurodevelopmental disorders. J Anat. 2019;235:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Deutschová B, Pizzo F, Giusiano B, et al. Ictal connectivity changes induced by pulvinar stimulation correlate with improvement of awareness. Brain Stimul. 2021;14:344–346. [DOI] [PubMed] [Google Scholar]
- 125. Young JC, Vaughan DN, Paolini AG, Jackson GD. Electrical stimulation of the piriform cortex for the treatment of epilepsy: A review of the supporting evidence. Epilepsy Behav. 2018;88:152–161. [DOI] [PubMed] [Google Scholar]
- 126. Vaughan DN, Jackson GD. The piriform cortex and human focal epilepsy. Front Neurol. 2014;5:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Löscher W, Ebert U. The role of the piriform cortex in kindling. Prog Neurobiol. 1996;50:427–481. [DOI] [PubMed] [Google Scholar]
- 128. Piredda S, Gale K. A crucial epileptogenic site in the deep prepiriform cortex. Nature. 1985;317:623–625. [DOI] [PubMed] [Google Scholar]
- 129. Gale K. Progression and generalization of seizure discharge: Anatomical and neurochemical substrates. Epilepsia. 1988;29(Suppl 2):S15–S34 [DOI] [PubMed] [Google Scholar]
- 130. Koepp M, Galovic M. Functional imaging of the piriform cortex in focal epilepsy. Exp Neurol. 2020;330:113305. [DOI] [PubMed] [Google Scholar]
- 131. Borger V, Schneider M, Taube J, et al. Resection of piriform cortex predicts seizure freedom in temporal lobe epilepsy. Ann Clin Transl Neurol. 2021;8:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Galovic M, Baudracco I, Wright-Goff E, et al. Association of piriform cortex resection with surgical outcomes in patients with temporal lobe epilepsy. JAMA Neurol. 2019;76:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Laufs H, Richardson MP, Salek-Haddadi A, et al. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology. 2011;77:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Flanagan D, Badawy RAB, Jackson GD. EEG-fMRI in focal epilepsy: local activation and regional networks. Clin Neurophysiol. 2014;125:21–31. [DOI] [PubMed] [Google Scholar]
- 135. Pedersen M, Curwood EK, Vaughan DN, Omidvarnia AH, Jackson GD. Abnormal brain areas common to the focal epilepsies: multivariate pattern analysis of fMRI. Brain Connect. 2016;6:208–215. [DOI] [PubMed] [Google Scholar]
- 136. Vismer MS, Forcelli PA, Skopin MD, Gale K, Koubeissi MZ. The piriform, perirhinal, and entorhinal cortex in seizure generation. Front Neural Circuits. 2015;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Plailly J, Howard JD, Gitelman DR, Gottfried JA. Attention to odor modulates thalamocortical connectivity in the human brain. J Neurosci. 2008;28:5257–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mai JK, Majtanik M, Paxinos G. Atlas of the Human Brain. 3rd ed.Academic Press; 2015. [Google Scholar]
- 139. Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage. 2018;170:271–282. [DOI] [PubMed] [Google Scholar]
- 140. Fisher RS. Stimulation of the medial septum should benefit patients with temporal lobe epilepsy. Med Hypotheses. 2015;84:543–550. [DOI] [PubMed] [Google Scholar]
- 141. Sinel’nikova VV, Popova IY, Kichigina VF. Correlational relationships between the hippocampus and medial septal area and their changes during epileptogenesis. Neurosci Behav Physiol. 2009;39:619–623. [DOI] [PubMed] [Google Scholar]
- 142. Vinogradova OS, Brazhnik ES, Kitchigina VF, Stafekhina VS. Acetylcholine, theta-rhythm and activity of hippocampal neurons in the rabbit—IV. Sensory stimulation. Neuroscience. 1993;53:993–1007. [DOI] [PubMed] [Google Scholar]
- 143. Butler T, Zaborszky L, Wang X, et al. Septal nuclei enlargement in human temporal lobe epilepsy without mesial temporal sclerosis. Neurology. 2013;80:487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Takeuchi Y, Harangozó M, Pedraza L, et al. Closed-loop stimulation of the medial septum terminates epileptic seizures. Brain. 2021;144:885–908. [DOI] [PubMed] [Google Scholar]
- 145. Izadi A, Pevzner A, Lee DJ, Ekstrom AD, Shahlaie K, Gurkoff GG. Medial septal stimulation increases seizure threshold and improves cognition in epileptic rats. Brain Stimul. 2019;12:735–742. [DOI] [PubMed] [Google Scholar]
- 146. Benabid AL, Koudsié A, Benazzouz A, et al. Deep brain stimulation of the corpus luysi (subthalamic nucleus) and other targets in Parkinson’s disease. Extension to new indications such as dystonia and epilepsy. J Neurol. 2001;248:37–47. [DOI] [PubMed] [Google Scholar]
- 147. Ren L, Yu T, Wang D, et al. Subthalamic nucleus stimulation modulates motor epileptic activity in humans. Ann Neurol. 2020;88:283–296. [DOI] [PubMed] [Google Scholar]
- 148. Handforth A, DeSalles AAF, Krahl SE. Deep brain stimulation of the subthalamic nucleus as adjunct treatment for refractory epilepsy. Epilepsia. 2006;47:1239–1241. [DOI] [PubMed] [Google Scholar]
- 149. Benarroch EE. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology. 2008;70:1991–1995. [DOI] [PubMed] [Google Scholar]
- 150. Dybdal D, Gale K. Postural and anticonvulsant effects of inhibition of the rat subthalamic nucleus. J Neurosci. 2000;20:6728–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Benabid AL, Minotti L, Koudsié A, de Saint Martin A, Hirsch E. Antiepileptic effect of high-frequency stimulation of the subthalamic nucleus (corpus luysi) in a case of medically intractable epilepsy caused by focal dysplasia: a 30-month follow-up: technical case report. Neurosurgery. 2002;50:1385–1392. [DOI] [PubMed] [Google Scholar]
- 152. Chabardès S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid A-L. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4(Suppl 3):S83–S93. [PubMed] [Google Scholar]
- 153. Cooper IS. Chronic cerebellar stimulation in epilepsy. Arch Neurol. 1976;33:559. [DOI] [PubMed] [Google Scholar]
- 154. Van Buren JM, Wood JH, Oakley J, Hambrecht F. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg. 1978;48:407–416. [DOI] [PubMed] [Google Scholar]
- 155. Wright GD, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984;47:769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kellinghaus C, Loddenkemper T. Double-blind, randomized controlled study of bilateral cerebellar stimulation. Epilepsia. 2006;47:1247–1247. [DOI] [PubMed] [Google Scholar]
- 157. Redinbaugh MJ, Phillips JM, Kambi NA, et al. Thalamus modulates consciousness via layer-specific control of cortex. Neuron. 2020;106:66–75.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb Cortex. 2017;27:1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Benedetti-Isaac JC, Torres-Zambrano M, Vargas-Toscano A, et al. Seizure frequency reduction after posteromedial hypothalamus deep brain stimulation in drug-resistant epilepsy associated with intractable aggressive behavior. Epilepsia. 2015;56:1152–1161. [DOI] [PubMed] [Google Scholar]
- 160. Šramka M, Chkhenkeli SA. Clinical experience in intraoperational determination of brain inhibitory structures and application of implanted neurostimulators in epilepsy. Stereotact Funct Neurosurg. 1990;54:56–59. [DOI] [PubMed] [Google Scholar]
- 161. Vetkas A, Germann J, Elias G, et al. Identifying the neural network for neuromodulation in epilepsy through connectomics and graphs. Brain Commun. 2022;4:fcac092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gleichgerrcht E, Munsell B, Bhatia S, et al. Deep learning applied to whole-brain connectome to determine seizure control after epilepsy surgery. Epilepsia. 2018;59:1643–1654. [DOI] [PubMed] [Google Scholar]
- 163. Kini LG, Bernabei JM, Mikhail F, et al. Virtual resection predicts surgical outcome for drug-resistant epilepsy. Brain. 2019;142:3892–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Taylor PN, Sinha N, Wang Y, et al. The impact of epilepsy surgery on the structural connectome and its relation to outcome. NeuroImage Clin. 2018;18:202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hutchings F, Han CE, Keller SS, Weber B, Taylor PN, Kaiser M. Predicting surgery targets in temporal lobe epilepsy through structural connectome based simulations. PLOS Comput Biol. 2015;11:e1004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Johnson GW, Cai LY, Narasimhan S, et al. Temporal lobe epilepsy lateralisation and surgical outcome prediction using diffusion imaging. J Neurol Neurosurg Psychiatry. 2022;93:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Morgan VL, Sainburg LE, Johnson GW, et al. Presurgical temporal lobe epilepsy connectome fingerprint for seizure outcome prediction. Brain Commun. 2022;4:fcac128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Workewych AM, Arski ON, Mithani K, Ibrahim GM. Biomarkers of seizure response to vagus nerve stimulation: A scoping review. Epilepsia. 2020;61:2069–2085. [DOI] [PubMed] [Google Scholar]
- 169. Li A, Huynh C, Fitzgerald Z, et al. Neural fragility as an EEG marker of the seizure onset zone. Nat Neurosci. 2021;24:1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Middlebrooks EH, Grewal SS, Stead M, Lundstrom BN, Worrell GA, Van Gompel JJ. Differences in functional connectivity profiles as a predictor of response to anterior thalamic nucleus deep brain stimulation for epilepsy: a hypothesis for the mechanism of action and a potential biomarker for outcomes. Neurosurg Focus. 2018;45:E7. [DOI] [PubMed] [Google Scholar]
- 171. Charlebois CM, Anderson DN, Johnson KA, et al. Patient-specific structural connectivity informs outcomes of responsive neurostimulation for temporal lobe epilepsy. Epilepsia. 2022;63:2037–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Denison T, Koubeissi M, Krook-Magnuson E, Mogul D, Worrell G, Schevon C. Stimulating solutions for intractable epilepsy. Epilepsy Curr. 2021;21:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Vedam-Mai V, Deisseroth K, Giordano J, et al. Proceedings of the eighth annual deep brain stimulation think tank: advances in optogenetics, ethical issues affecting DBS research, neuromodulatory approaches for depression, adaptive neurostimulation, and emerging DBS technologies. Front Hum Neurosci. 2021;15:644593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Scheid BH, Ashourvan A, Stiso J, et al. Time-evolving controllability of effective connectivity networks during seizure progression. Proc Natl Acad Sci U S A. 2021;118:e2006436118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Taylor PN, Thomas J, Sinha N, et al. Optimal control based seizure abatement using patient derived connectivity. Front Neurosci. 2015;9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ashourvan A, Pequito S, Khambhati AN, et al. Model-based design for seizure control by stimulation. J Neural Eng. 2020;17:026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Feldman L, Krishnan B, Alexopoulos A, Mackow M, Taylor K, Bulacio J. Brain-Responsive Neurostimulation Guided by Stereo-EEG. American Epilepsy Society; 2018. [Google Scholar]
- 179. Gadot R, Korst G, Shofty B, Gavvala JR, Sheth SA. Thalamic stereoelectroencephalography in epilepsy surgery: a scoping literature review. J Neurosurg. 2022:1–16. [DOI] [PubMed] [Google Scholar]
- 180. Wong JK, Deuschl G, Wolke R, et al. Proceedings of the ninth annual deep brain stimulation think tank: advances in cutting edge technologies, artificial intelligence, neuromodulation, neuroethics, pain, interventional psychiatry, epilepsy, and traumatic brain injury. Front Hum Neurosci. 2022;16:813387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. George DD, Ojemann SG, Drees C, Thompson JA. Stimulation mapping using stereoelectroencephalography: current and future directions. Front Neurol. 2020;11:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Gregg NM, Nasseri M, Kremen V, et al. Circadian and multiday seizure periodicities, and seizure clusters in canine epilepsy. Brain Commun. 2020;2:fcaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Toth R, Zamora M, Ottaway J, et al. Dyneumo mk-2: an investigational circadian-locked neuromodulator with responsive stimulation for applied chronobiology. Conf Proceedings IEEE Int Conf Syst Man, Cybern. 2020; 2020:3433–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Chari A, Seunarine KK, He X, et al. Drug-resistant focal epilepsy in children is associated with increased modal controllability of the whole brain and epileptogenic regions. Commun Biol. 2022;5:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Khambhati AN, Davis KA, Lucas TH, Litt B, Bassett DS. Virtual cortical resection reveals push-pull network control preceding seizure evolution. Neuron. 2016;91:1170–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kramer MA, Lopour BA, Kirsch HE, Szeri AJ. Bifurcation control of a seizing human cortex. Phys Rev E. 2006;73:041928. [DOI] [PubMed] [Google Scholar]
- 187. Middlebrooks EH, Jain A, Okromelidze L, et al. Acute brain activation patterns of high-versus low-frequency stimulation of the anterior nucleus of the thalamus during deep brain stimulation for epilepsy. Neurosurgery. 2021;89:901–908. [DOI] [PubMed] [Google Scholar]
- 188. Middlebrooks EH, Lin C, Okromelidze L, et al. Functional activation patterns of deep brain stimulation of the anterior nucleus of the thalamus. World Neurosurg. 2020;136:357–363.e2. [DOI] [PubMed] [Google Scholar]
- 189. Toga AW, Clark KA, Thompson PM, Shattuck DW, Van Horn JD. Mapping the human connectome. Neurosurgery. 2012;71:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Taylor PN, Papasavvas CA, Owen TW, et al. Normative brain mapping of interictal intracranial EEG to localize epileptogenic tissue. Brain. 2022;145:939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Saenger VM, Kahan J, Foltynie T, et al. Uncovering the underlying mechanisms and whole-brain dynamics of deep brain stimulation for Parkinson’s disease. Sci Rep. 2017;7:9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Carmichael DW, Pinto S, Limousin-Dowsey P, et al. Functional MRI with active, fully implanted, deep brain stimulation systems: Safety and experimental confounds. Neuroimage. 2007;37:508–517. [DOI] [PubMed] [Google Scholar]
- 193. Carmichael DW, Thornton JS, Rodionov R, et al. Feasibility of simultaneous intracranial EEG-fMRI in humans: A safety study. Neuroimage. 2010;49:379–390. [DOI] [PubMed] [Google Scholar]
- 194. International Modulation Society . How has neuromodulation been developed and used? Accessed 2021. https://www.neuromodulation.com/learn-more [Google Scholar]