Abstract
At the frontline of the host defence response, neutrophil antimicrobial functions have adapted to combat infections and injuries of different origins and magnitude. The release of web-like DNA structures named neutrophil extracellular traps (NETs) constitutes an important mechanism by which neutrophils prevent pathogen dissemination or deal with microorganisms of a bigger size. At the same time, nuclear and granule proteins with microbicidal activity bind to these DNA structures promoting the elimination of entrapped pathogens. However, these toxic properties may produce unwanted effects in the host, when neutrophils uncontrollably release NETs upon persistent inflammation. As a consequence, NET accumulation can produce vessel occlusion, tissue damage, and prolonged inflammation associated with the progression and exacerbation of multiple pathologic conditions. This review outlines recent advances in understanding the mechanisms of NET release and functions in sterile disease. We also discuss mechanisms of physiological regulation and the importance of neutrophil heterogeneity in NET formation and composition.
Keywords: Neutrophil, NETosis, Sterile inflammation
1. Introduction
Neutrophils constitute our first line of defence against microbial pathogens but can also mediate tissue injury and sterile inflammation. Among the variety of antimicrobial weapons with which neutrophils are armed, neutrophil extracellular traps (NETs) are released to limit pathogen dissemination and kill microbes. NETs are DNA structures decorated with cytosolic, granule, and nuclear proteins,1 and can entrap microorganisms including bacteria, viruses, or fungi.2 Importantly, unbalanced immune responses might result in the dysregulated release of NETs which accounts for exacerbated inflammation and host tissue damage beyond their antimicrobial functions, and thus contribute to multiple diseases. NET-associated material stems predominantly from the nucleus, and is therefore highly enriched in core histones but also includes high levels of granule proteins [neutrophil elastase (NE), cathepsin G, and proteinase-3], myeloperoxidase (MPO), or cytosolic proteins such as S100 proteins.3 Although the NET proteome composition is rather stable, the relative abundance of its constituent proteins and also its composition may vary depending on the stimulus.4 Notably, upon certain stimuli NETs may originate from the mitochondria,5 ultimately altering NET composition and function, as these organelles lack histones.
This review outlines mechanisms underlying the molecular control of NET formation and describes the pathogenic contribution of NETs to sterile acute and chronic diseases. We also contemplate the contribution of circadian rhythms, the microbiome, or tissue location to the regulation of NET release and consider the impact of neutrophil heterogeneity on NETosis susceptibility and NET composition.
2. Molecular mechanism and triggers of NETosis
NETs arise predominately via a cell death program termed lytic or suicidal NETosis.1 The process starts with the activation of surface receptors that trigger a program that is completed over several hours and performs four critical tasks: the permeabilization of the plasma membrane, the disassembly of the cytoskeleton and nuclear envelope, the decondensation of chromatin, and the assembly of antimicrobial proteins onto the chromatin scaffold. NETosis externalizes both nuclear and mitochondrial DNA. In addition to the lytic program, an alternative rapid NET release mechanism known as ‘vital NETosis’ extrudes nuclear or mitochondria DNA from live cells (reviewed in ref.6) Here, we focus on lytic NETosis as it is the predominant mechanism implicated in inflammatory disease.
2.1 The lytic NETosis ‘machinery’
Reactive oxygen species (ROS) play a central role as signalling mediators linking the upstream regulatory pathways with the machinery driving NETosis (Figure 1).7 ROS can be generated either by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or by mitochondrial respiration.5,8 The assembly of active NADPH oxidase 2 on phagocytic or plasma membranes is induced by the GTPase Rac, and by p47phox phosphorylation by the protein kinase C (PKC) and Raf–MAPK/ERK kinase (MEK)–extracellular signal-regulated kinase (ERK) pathway.9–11 NADPH oxidase reduces molecular oxygen by transferring electrons from NADPH across membranes to generate superoxide—a powerful but short-lived oxidant that is rapidly converted to hydrogen peroxide—which serves as a central ROS mediator in NETosis. Mitochondrial respiration can also produce superoxide by electron leakage and is powered by pyruvate generated during glycolysis. The link between ROS generation and neutrophil metabolism renders NETosis sensitive to metabolic alterations. Adaptations in neutrophil metabolism can differ in neutrophil subsets associated with a number of conditions and may sustain pathogenesis by favouring NET release.12,13 Diabetic patients furnish a classic example in which elevated glucose levels augment NETosis. This phenomenon may contribute to the impaired wound healing characteristic of these patients.14,15 Another example relates to the regulation of intracellular cholesterol levels by ATP binding cassette subfamily A member 1 (ABCA1) and subfamily G member 1 (ABCG1) transporters, whose function suppresses inflammasome activation, limits NETosis, and alleviates atherosclerosis in mice.16 The redundancy and specific requirements for these different ROS generators likely depend on the upstream inducers. For example, while mitochondrial ROS promote NETosis, in NADPH oxidase-deficient neutrophils stimulated with immune complexes in a PKC and Raf–MEK–ERK-independent manner, the NADPH oxidase is required for NET release induced by phorbol 12-myristate 13-acetate (PMA) or fungi, suggesting that mitochondrial respiration remains low or is insufficient with these stimuli.5,17,18
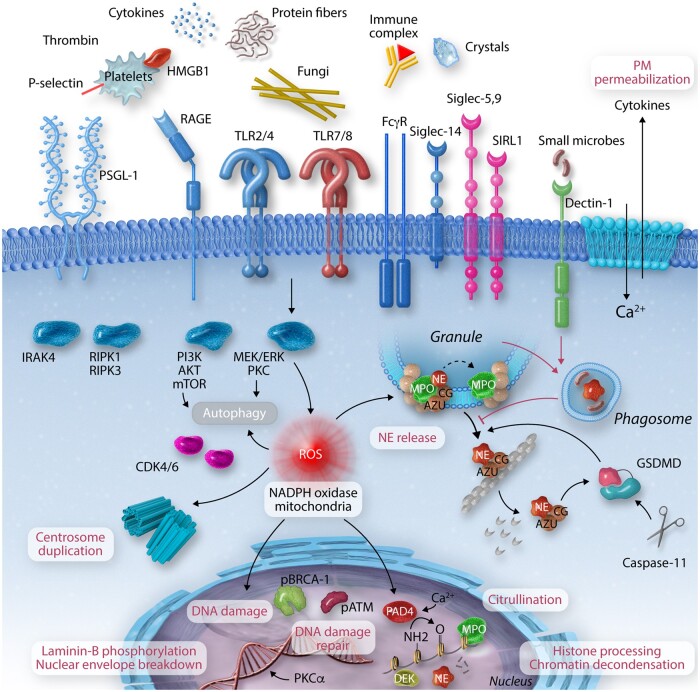
Figure 1.
Pathways and mechanisms regulating lytic NETosis. NETosis is triggered by microbial and endogenous stimuli via several activating molecules such as receptor for advanced glycation end products (RAGE), P-selectin–P-selectin glycoprotein ligand 1 (PSGL1), toll-like receptors (TLR), low-affinity immunoglobulin gamma receptor (FcγR), or sialic acid-binding immunoglobulin-type lectins (Siglec), among others. Activation of MAP kinase signalling induces reactive oxygen species (ROS) generation by the NADPH oxidase 2 (Nox2). Alternative ROS can be generated by mitochondria. ROS plays a central role in NETosis triggering NE release from the azurosome complex, a process aided by gasdermin D (GSDMD) which is activated by caspase-11 upon exposure to intracellular cytosolic bacteria. NE degrades F-actin and translocates to the nucleus where it will partially cleave histones promoting chromatin decondensation. Chromatin decondensation is also enhanced by the binding of cationic proteins like MPO or DEK and by protein-arginine deiminase type 4 (PAD4)-mediated histone citrullination. Phosphorylation of the lamin network drives its disassembly and the breakdowns of the nuclear envelope. High levels of ROS promote DNA damage triggering DNA repair via ataxia-telangiectasia mutated (ATM) and BReast CAncer gene (BRCA)-1. NETosis also depends on cell cycle cyclin-dependent kinase 4/6 (CDK4/6) and the duplication of centrosomes and autophagy. Inhibitory receptors such as sialic acid-binding immunoglobulin-type lectin-5 and 9 (Siglec-5,9) or signal inhibitory receptor on leukocytes 1 (SIRL1) block NEtosis. Phagocytic receptors like Dectin-1 inhibit NETosis in response to small microorganisms by sequestering NE to phagosomes. ATG7, autophagy-related protein 7; AZU, azurophilic granule; CG, cathepsin G; CR3, complement receptor 3; IRAK, IL-1 receptor-associated kinase; MEK, MAPK/ERK kinase; mTOR, mechanistic target of rapamycin; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; RIPK1/3, receptor-interacting serine/threonine-protein kinase 1/3.
ROS promote the activation of a number of downstream effectors. First, neutrophil activation requires actin cytoskeleton dynamics, and inhibition of actin polymerization within the firsts 30 min post-stimulation reduces the efficiency of NET formation.19 Cytoskeletal dynamics depend on ROS-mediated cysteine glutathionylation of actin and tubulin.20 At later stages, ROS orchestrate the degradation of the actin cytoskeleton by activating the protease NE. In resting neutrophils, NE resides in phagocytic granules within a fraction that is localized in the lumen and a fraction bound to MPO and associated with granule membranes. ROS trigger the activation and release of NE from the MPO-containing azurosome complex into the cytosol where NE binds to F-actin and degrades actin filaments.18 Subsequently, NE translocates to the nucleus, likely via passive diffusion since NE is a 28.5 kDa highly basic protein. Upon entering the nucleus, NE partially cleaves histones to promote chromatin decondensation.21 NE cleaves lysine and arginine-rich C-terminal histone H3 tails that are critical for inter-nucleosomal interactions.22,23 Histones are cleaved in response to diverse stimuli such as the mitogen PMA, Candida albicans, bacterial toxin nigericin, and Group B streptococcus.3,17,21 Chromatin decondensation is further enhanced by the binding of cationic proteins such as MPO and the nuclear protein DEK.21,24,25 MPO also promotes protein carbamylation of NET histones in human neutrophils that drive tissue damage in rheumatoid arthritis (RA).26
Another factor implicated in NETosis is protein-arginine deiminase type 4 (PAD4). PAD4 reduces the positive charge of histones and their electrostatic interactions with DNA by converting arginine to citrulline.27 The enzyme requires the binding of five calcium ions to adopt a catalytically active conformation.28,29 Hence, most studies investigating the role of PAD4 in NETosis employ stimulation with calcium ionophores. ROS also contribute to PAD4 activation. PAD4-mediated citrullination can be triggered by hydrogen peroxide and inhibition of NADPH oxidase reduces citrullination, providing a link between PAD4 and ROS production.30,31 In contrast, PAD4-mediated citrullination of p67phox and p47phox promotes their dissociation from the NADPH oxidase to suppress ROS production.32 How this negative feedback mechanism affects ROS-mediated mechanisms and how PAD4 synergizes with other chromatin decondensation-promoting factors remains unclear.
Recent studies suggest that NETosis requires PAD4 primarily in response to calcium ionophores and immune complexes. However, PAD4 is dispensable for NET formation induced by PMA, fungi, or cholesterol crystals as demonstrated in human neutrophils.17,30,33 These findings helped uncover a role for chromatin citrullination in NET-mediated inflammation which may account for some of the pathologic effects of PAD4 in vivo. For instance, blocking citrullination decreases the pro-inflammatory capacity of histones and atherosclerotic plaque formation in mice without inhibiting NET formation.34 Conversely, granule proteases in mouse neutrophils may be dispensable for NETosis in response to Ca2+ ionophores.35 Hence, PAD4-mediated citrullination and NE-dependent proteolytic histone cleavage are common features of NETosis but may be critical under distinct circumstances.
Another important step is the activation of the cell cycle and DNA repair signalling. The cell cycle regulator cyclin-dependent kinase (CDK) 4/6 is activated during NETosis and is required for the duplication of centrosomes which are later dismantled along with the nuclear envelope. Inhibition of CDK4/6 in human neutrophils also decreases NE translocation to the nucleus.36 Moreover, phosphorylated PKCα phosphorylates lamin B to facilitate nuclear envelope breakdown.37 Furthermore, human neutrophils employ DNA repair mechanisms to cope with ROS-mediated DNA damage by activating ataxia-telangiectasia mutated (ATM) and BReast CAncer gene (BRCA)-1.38 Additionally, DNA repair facilitates chromatin decondensation39 and together with the disassembly of lamins generate physical pressure that promotes nuclear envelope expansion and breakdown.19
The disassembly of the cytoskeleton and chromatin decondensation reduces plasma membrane stability. However, cell death and membrane permeabilization are primarily accelerated by the inflammasome40 and the assembly of gasdermin D (GSDMD) pores on the plasma membrane.41 GSDMD activation in human neutrophils can follow activation with PMA, cytosolic lipopolysaccharide (LPS), or virulent Gram-negative bacteria that trigger caspase-11 mediated GSDMD activation and NETosis.42 GSDMD is in a feedback loop with NE, with NE promoting its activation and GSDMD promoting NE release from azurophilic granules. Moreover, by assembling pores on the plasma membrane GSDMD alters cellular ion gradients, a process that might facilitate PAD4 activation.43 Caspase-b- and gasdermin Eb-mediated pyroptosis is also required for NETosis in response to bacterial infection in zebrafish.44 Finally, conflicting evidence exists over the role of necroptosis and its dedicated receptor-interacting serine/threonine-protein kinase (RIPK)-1 and -3 kinases, and several reports have implicated autophagy in NETosis via interrogation of PI3K, autophagy-related gene 7 (ATG7) and 5 (ATG5), but their mechanistic contribution in either mouse or human neutrophils is unclear.45–48 Therefore, neutrophils incorporate various elements with features from other processes such as the cell cycle, DNA repair, and pyroptosis to orchestrate a unique antimicrobial strategy.
2.2. Endogenous inducers of NETosis in disease
Several host factors trigger NET formation in pathological sterile inflammatory conditions (Figure 1). Endogenous crystals act as danger signals that promote inflammatory diseases such as atherosclerosis, gout, and pancreatitis. Cholesterol and monosodium urate crystals implicated in these conditions potently trigger NETosis via RIPK1–RIPK3-mixed lineage kinase domain-like protein (MLKL) signalling in an ROS and NE-dependent manner.33,48,49 Calcium carbonate crystals found in pancreatic secretions can induce PAD4-dependent NETosis that promotes pancreatic duct occlusion and pancreatitis in mice.50 As large extracellular crystals promote NETosis, these cells may interact with crystals via surface receptors that have yet to be identified. Moreover, NETs are induced by amyloid-β deposits in a mouse model of Alzheimer’s disease, and depletion of neutrophils or inhibition of neutrophil extravasation delays disease progression.51 Other amyloid fibrils such as α-synuclein, Sup35, and transthyretin trigger NADPH oxidase-dependent NET formation in human neutrophils as well.52
Another class of NET inducers is immune complexes which places NET release under the control of the adaptive immune system in autoimmune conditions such as systemic lupus erythematosus (SLE) and anti-neutrophil cytoplasmic antigens (ANCA) vasculitis.53,54 Ribonucleoprotein immune complexes drive NET formation via FcγRIIIb-mediated receptor signalling in neutrophils primed with type I interferon (IFN).53,55–57 Moreover, in lupus patients NETs activate components of the complement system leading to the deposition of C1q which impairs NET clearance.58 The ability of type I IFNs to exacerbate NET formation may also occur in chronic infections such as tuberculosis and human genetic disorders such as ataxia telangiectasia and Artemis deficiency.59,60 Furthermore, several pro-inflammatory cytokines such as interleukin-17A (IL-17A), tumour necrosis factor-α, IL-1β, and midkine induce NETs in an NADPH oxidase-dependent manner.55,61–63 Dysregulated NETosis induced by these cytokines has been implicated in multiple conditions such as RA and systemic inflammatory syndrome in humans and mice, or in interfering with cytotoxic CD8 T cells’ anti-tumour functions in mice. Moreover, chemokines that activate C-X-C motif chemokine receptor 1 (CXCR1) and CXCR2 trigger NET formation via the Src–p38–ERK signalling pathway to promote disease (discussed below) in mouse models.64,65
The importance of neutrophil adhesion and integrin activation in the formation of NETs still engenders debate. Studies in mice and human neutrophils have shown opposite results with regard to the role of integrins in NET release.66–69 Genetic ablation or pharmacological blockade of β2 integrins impede NETosis in experimental hantavirus infection67 or sepsis,69 and in human neutrophils incubated with Bacterium Acinetobacter baumannii, however, this effect might be independent on cell adhesion.66 It is hence unclear whether neutrophils require physical interaction with other cells (i.e. endothelial cells or platelets) to release NETs, or if this process can occurin the absence of juxtacrine contact. In vitro studies in human neutrophils have shown that NET release requires neutrophil adhesion and surface stiffness,70 however, this requirement might be stimulus-dependent as PMA can induce NETosis in absence of cell adhesion. These in vitro studies await in vivo validation.
Several signals have also been implicated in NET induction in cancer. Platelet-activating factor drives NET-mediated thrombosis during cancer.71 Moreover, cancer-associated fibroblast-derived amyloid-β triggers NADPH oxidase-dependent pro-tumorigenic NET release via CD11b.72 In addition, tumours induce NETosis in human and mouse neutrophils by releasing cathepsin C, high mobility group protein B1 (HMGB1), and other alarmins73,74 and exacerbate NETosis by upregulating the generation, recruitment, and polarization of immature neutrophils via granulocyte colony-stimulating factor, transforming growth factor-β, and chemokines in mice.71,75–77
Direct cell-to-cell interactions can also regulate NETosis. Activated platelets can relay signals such as LPS to neutrophils, triggering NETosis in mouse models of thrombosis with NETs serving as a binding scaffold for Von Willebrand factor and NET proteases degrading coagulation inhibitors such as tissue factor (TF) pathway inhibitor.78–81 Platelet-derived alarmins such as HMGB1 can mediate intercellular signalling via the receptor for advanced glycation endproducts82,83 triggering MLKL-mediated NET release that contributes to venous thrombosis.84 In heparin-induced thrombocytopenia/thrombosis, IgG binding to heparin/platelet factor 4 complexes can trigger NETosis via FcγRIIa binding.85 Similarly, pro-thrombotic autoantibodies targeting phospholipids and phospholipid-binding proteins in SARS-CoV-2 patient sera can induce NETosis.86 These pathways increase the risk for venous thromboembolism during sepsis and sterile disease.78,87–89
A number of mechanisms can suppress NETosis to limit immune pathologenesis, but may also be exploited by microorganisms to evade capture. Phagocytosis is a basic mechanism that disrupts NETosis by sequestering NE to phagosomes.90 Hence, phagocytic receptors such as Dectin-1, suppress chromatin decondensation upon ingestion of small particles. Other receptors that suppress NETosis are signal inhibitory receptor on leukocytes 1 and sialic acid-binding immunoglobulin-type lectin-5 (Siglec-5) and Siglec-9.91–93 The exploitation of these pathways may have important therapeutic potential.94,95
Despite these advances, our understanding of the endogenous signals and mechanisms regulating NETosis remains limited and will be critical for the development of therapeutic interventions that suppress pathological NETosis without interfering with immune protection.
3. Physiological regulation of NET release
It has become increasingly clear that not all neutrophils are equally prone to release NETs, as variability exists across different species, or among tissues and physiological states of the organism. It is also likely that the quality of the released NETs varies between different neutrophil types or neutrophils at different stages of maturation. Both changes in the quantity and quality of released NETs likely have a major impact on the magnitude and type of inflammatory response, however, the underlying causes for this variability remain poorly defined. Variations in NET release may intertwine with regulators of the neutrophil life cycle, including circadian oscillations, spatial distribution, the microbiome, and even the age of the organism.
3.1. Circadian regulation
During normal granulopoiesis, neutrophils synthesize granules store antimicrobial peptides, adhesion molecules, and proteolytic enzymes.96 As mentioned in the previous section, NET formation depends on the granule content of neutrophils. This concept has particularly relevance as recent observations have shown that degranulation occurs not only during acute activation, such as seen during infections and exposure to pathogen-associated molecular patterns (PAMPs), but can also takes place in the circulation under steady-state conditions. Mechanistic studies demonstrated that autocrine signals delivered via CXCR2 can elicit this progressive degranulation such that neutrophils, which are mobilized into the blood at night (in mice), display marked reductions in their content in primary granules by daytime.97 This temporal degranulation, which is subject to the core circadian machinery and follows strict circadian patterns, in turn, predicted that NET formation depends critically on the time of day. Indeed, analysis of neutrophils isolated at different times (noon vs. evening) evidenced substantial differences in NET formation ex vivo both in mice and humans, and this variability could be recapitulated in living tissues subjected to ischaemia–reperfusion injury or acute lung injury.97 The genetic determinism of NET formation by the circadian clock was additionally evidenced by using not only time of day as a variable but also use of mice bearing a neutrophil-specific deletion of the core circadian gene Arntl (encoding Bmal1) which feature night-type neutrophils, or the gene encoding CXCR4 which harbours day-type neutrophils.97,98 Notably, elimination of these time-sensing molecules exclusively in neutrophils rendered NET formation and severity of the pulmonary disease independent of diurnal time in mice.97 Studies in a small cohort of pneumonia patients undergoing pulmonary distress suggested that similar principles of circadian regulation apply in humans.97
3.2. Microbiome
Signals derived from the commensal flora of the intestine have been associated with intimate regulation of immune development and competence against pathogen invasion.99 Bacteria, particularly segmented filamentous bacteria, have been shown to provide signals locally responsible for neutrophil priming through a Th17-dependent mechanism and increased barrier permeability that mediates expansion, dissemination of PAMPs released by these groups of bacteria, and sensing through toll-like receptor (TLR)/MyD88 signalling.100 This neutrophil priming, referred to as ageing despite the lack of evidence for circadian regulation,98 predispose neutrophils for activation (loss of CD62L and gain of CD11b) and NET release and associated with vascular occlusion in mouse models of sickle cell disease and psychological stress-induced inflammation.100,101 In humans, the number of NETs in pulmonary sputum of patients with chronic obstructive pulmonary disease correlated with the dominance of Haemophilus sp. in the sputum.102 Consequently, depletion of the intestinal microbiome by antibiotics in mice reduces NET production and confers protection during chronic inflammatory disease. Although these approaches might alleviate NET-driven disease in the clinic, depleting strategies of the microbiome might result in opposing effects. Indeed, the absence of commensal bacteria result in elevated NET release in the context of mouse mesenteric ischaemia/reperfusion injury,103 suggesting an inhibitory or tolerogenic action of the microbiome. These results should, however, be taken with caution as the divergence in the techniques employed in these studies to detect NETs or the antibiotic cocktail and regime used to deplete the microbiome might account for these differences.
Contrasting with the NET-promoting effects of segmented filamentous bacteria, some bacteria used in probiotic regimes (Lactobacillus rhamnosus strain GG) can inhibit NET formation induced by different stimuli in vitro in human neutrophils, possibly by blunting ROS formation.104 While the in vivo benefits (or risk) of strains impairing NET formation remain to be evaluated, it is now increasingly evident that microbial-derived metabolites can influence NET formation in variable, and even opposed ways. Interestingly, this regulation of NET release might also depend on the diurnal oscillations in the microbiome location and function and its control of intestinal permeability.105 As TLR expression in neutrophils follows a circadian pattern,98 the coordinated expression of these receptors and the daily influx of microbial products may result in neutrophil priming and susceptibility to NETosis in a circadian fashion.
3.3. Age
Of particular interest for our discussion is the varying capacity of neutrophils to release NETs with age, as this may account for enhanced susceptibility of older individuals to infections106 and may represent a contributor to inflamm-ageing. Various studies have reported a marked loss of NET-forming capacity in older individuals (both human and mice, averaging ∼70 years or 18 months, respectively), in response to a variety of stimuli including endotoxins, chemokines (IL-8), or TLR2 ligands.46,107 It is interesting that these studies implicated both reduced production of ROS (an obligate signal for NET formation) and defective autophagy (common in older organisms) as potential culprits causing defective NET release by neutrophils. These, and possibly other features of neutrophils from older individuals, may stem from accelerated and defective maturation of neutrophils in the BM with age, and in turn, be associated with the aforementioned dependence of efficient formation of NETs on intact granule content.97 The observation, however, that potent downstream activation with phorbol esters induces comparable levels of NETs as in young individuals further suggests that signalling leading to NET formation may also be compromised in neutrophils from older individuals,107 and these possibilities merit future exploration. Following the same principle, one must consider that even for NETs that form in neutrophils of aged organisms, their composition is likely to vary due to different granule composition, in turn affecting their immunomodulatory (e.g. by degrading inflammatory cytokines49) or thrombo-inflammatory properties.
3.4. Location
Although formally unexplored, the distribution of neutrophils in specific niches or different tissues deserves special consideration, as there are hints that it can influence NET formation in ways that may have considerable disease relevance. For example, baseline release of NETs in the circulation is inferred from their marked accumulation in the absence of plasma host DNAses, at least under conditions of neutrophilia.88 In this context, the enhanced capacity of relatively immature neutrophils (which have not yet been cleared from the circulation) to release NETs97 may underlie the enhanced susceptibility to thrombo-inflammatory injury of organs in which neutrophils remain preferentially intravascular, such as the lung and liver.108 In the lumen of mouse atherosclerotic arteries, released NETs on the activated endothelium serve as a platform for inflammatory monocytes to adhere and transmigrate into the inflamed tissue helping to overcome the elevated blood flow.109 In scenarios of chronic inflammation, activation of neutrophils in certain tissues may incite exaggerated production of NETs. For example, it may be driven by factors present in the plasma of patients that cause systemic NET release, as seen in autoimmune disease,5,53 locally in lungs of patients with acute lung injury (ALI) (including SARS-CoV-2 patients110) or driven by specific cytokines produced in the vascular wall, as demonstrated in mouse atherosclerosis.111
4. Neutrophil diversity and NETs
Neutrophils are considered no longer a homogeneous population but rather plastic cells that can adapt to the environment and modulate their phenotype for different functional needs.112 Although evidence is still limited, this functional diversification of neutrophils can also be epitomized in a differential capacity to release NETs. In line with our previous discussion, physiological but also pathological (discussed below) insults regulate NETosis but also the appearance of neutrophil subpopulations.112 Thus, such environmental signals, and also intrinsic characteristics or the combination of both, may alter the propensity of certain neutrophil subpopulations to release NETs. Congruent with this idea, activation of mouse or human neutrophils results in only 30% or 60% of NETosis, respectively.21,113 These results illustrate that under the same stimulation not all neutrophils can undergo NETosis, however, the underlying causes for these differences are unclear. One hypothesis is that as neutrophils exit the BM and circulate throughout different tissues acquire a primed or aged state required to permit NETosis, as induced by microbiome-elicited signals.100 Accordingly, BM-derived mouse neutrophils—considered to be in a more immature stage—exhibit diminished NETosis capacity upon IFN priming and C5 stimulation, a function that increases as they mature (circulating neutrophils), and it is concomitant with the acquisition of an IFN signature.114 Once in the circulation—although still able to release NETs—neutrophils with an immature phenotype have reduced NETting capability.115 Interestingly, circulating immature neutrophils are more prevalent in males and in pregnant but not non-pregnant females. In females, a major proportion of neutrophils exhibit the expression of type I interferon-stimulated genes (ISGs), a mature signature, and elevated NETosis ability.56 The similarities of this subset and isolated low-density granulocytes (LDGs) from autoimmune patients are obvious,116 and may explain the higher predisposition of females to develop such diseases. Likewise, it is reasonable to think that the expansion and activation of this ISG-expressing subset results in the origination of LDGs in autoimmunity, however, this connection requires further investigation. Another unexplored question concerns the role of this IFN signalling as a specific signalling pathway determining the susceptibility of neutrophils to form NETs. On the contrary, the importance of neutrophil maturation as a determining factor for NET release seems to be incomplete. For instance, although these NEtting LDGs in autoimmune disease exhibit a mature phenotype,116 blood immature LDGs isolated from tumour-bearing mice have increased NET release as compared to normal-density counterparts.13 This observation suggests that the degree of maturation is not a unique property that influences NETosis capacity. Finally, it merits mention that the existence of a subpopulation of olfactomedin-4-expressing human neutrophils that constitutes ∼10–30% of all circulating neutrophils and possess an increased capacity to undergo NETosis,117 numbers that fit the aforementioned observations on partial NET formation by mouse neutrophils.
5. Pathogenic functions of NETs
NETs are important mediators of the neutrophil antimicrobial response in body surfaces and vessels.2 However, the NET release also comes with detrimental actions associated with occlusion, tissue damage, or amplification of the immune response. As consequence, NETs have increasingly been associated with multiple human pathologies by exerting a variety of pathogenic functions (Figure 2). Here, we will focus our discussion on the contributions of NETs during sterile immunopathology. The use of NETs as biomarkers for disease severity and outcome and the current developing NET-directed therapies are listed in Tables 1 and 2, respectively.
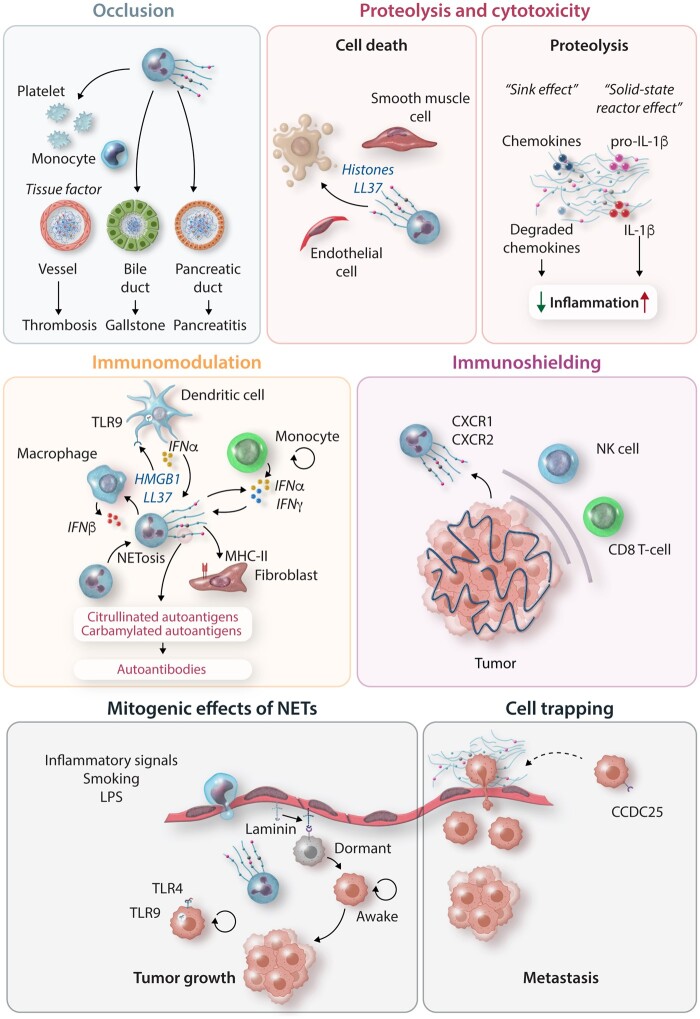
Figure 2.
Pathomechanisms of NETosis: excessive release of NETs drives disease through multiple mechanisms involving vessel occlusion, tissue injury, modulation of immune cell function, and pro-tumorigenic and pro-metastatic functions. NETs promote thrombosis through a coagulant activity by the induction of tissue factor release by activated platelets and monocytes and by providing a physical scaffold for platelets and thrombotic molecules (fibrin) to deposit and aggregate to form the thrombus. NET aggregation can also occlude other tube-shaped structures such as the bile and pancreatic ducts provoking alterations of organ function and inflammation. NET components cause different effects depending on their nature, amount, and targeted cell. The toxic cargo (histones or granule proteins such as LL37) of NETs induces cell apoptosis and lysis causing death and promoting tissue injury and inflammation. However, NETs also contain multiple proteases that degrade (‘sink effect’) or activate (‘solid-state reactor’) entrapped cytokines and chemokines through a proteolytic activity, hence modulating the inflammatory response. The immunomodulatory function of NETs occurs upon interaction with phagocytes such as macrophages and dendritic cells resulting in activation cell activation, and the release of inflammasome-dependent IL-1β or TLR9-mediated signalling release of IFNα. NETs can also activate T-cell to release IFNα and IFNγ and serve as autoantigens to synovial fibroblasts to initiate autoimmune responses. Among the NET-driven tumorigenic activities, NETs promote tumour growth through direct induction of cell proliferation or the awakening of dormant tumour cells after the remodelling of the surrounding extracellular matrix. The metastatic function of NETs involves their ability to attract and trap circulating tumour cells, providing a physical niche for the development of the metastasis. Finally, NETs can be released by recruited neutrophils around the primary tumour, thus preventing the entrance and function of anti-tumoural cytotoxic T cells and natural killer cells. LPS, lipopolysaccharide; NET, neutrophil extracellular trap.
Table 1.
NET-associated biomarkers in sterile inflammatory diseases
| Biomarker | Disease | Detection method | Correlation | Reference | |
|---|---|---|---|---|---|
| Blood plasma/serum | Cit-H3 | Advanced cancer | ELISA | Predictive of poor clinical outcome | 118 |
| Advanced cancer | ELISA | Predictive of poor clinical outcome independent of coagulation | 119 | ||
| Acute ischaemic stroke | ELISA | Positive association with atrial fibrillation and all-cause mortality | 120 | ||
| MPO–DNA complexes | Advanced oesophageal, gastric, and lung cancer | ELISA | Positive association with advanced cancer stage | 121 | |
| Metastatic colorectal cancer | ELISA | Positive association with risk of cancer recurrence after resection | 122 | ||
| SLE | ELISA | Positive association with risk of nephritis and cardiovascular events | 123 | ||
| RA | ELISA | Positive association with inflammatory markers and the appearance of extra-articular nodules | 124 | ||
| CAD | ELISA | Positive association with disease severity and thrombosis | 125 | ||
| NE–DNA | Breast cancer | ELISA | Positive association with advanced cancer stage | 126 | |
| Nucleosome | Lung cancer | ELISA | Positive association with cancer-related stroke | 127 | |
| Tissue | Tumour | Pancreatic ductal adenocarcinoma | Cit-H3+CD15+ | Positive association with poor survival and cancer recurrence | 128 |
| Thrombi | Acute ischaemic stroke, CAD | Cit-H3+ extDNA+ | Positive association with systemic inflammation | 129 | |
| Thrombi | Acute coronary syndrome | Cit-H3+ extDNA+ | Positive association with infarct size | 130 | |
| Thrombi | Acute ischaemic stroke | Cit-H4+MPO+ extDNA+ | Positive association with reperfusion resistance | 131 | |
| Ex vivo NET formation | Critically ill patients | Propidium iodide staining | Positive association with disease severity and predicts the development of disseminated intravascular coagulation and mortality. | 132 | |
CAD, coronary artery disease; Cit-H3, citrullinated histone H3; MPO, myeloperoxidase; NE, neutrophil elastase; NET, neutrophil extracellular trap; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Table 2.
NET-directed therapeutic strategies
| Biological target | Compound | Targeting disease | Study | Reference, Identifier |
|---|---|---|---|---|
| DNAse | Dornase Alfa | SARS-CoV-2 | COVIDORNASE, Phase III | NCT04355364 |
| COVASE, Phase II | NCT04359654 | |||
| DORNASESARS2, Phase III | NCT04402970 | |||
| Respiratory failure after trauma | TRAUMADORNASE, Phase III | NCT03368092 | ||
| Ischaemic stroke | NETs-target, Phase II | NCT04785066 | ||
| Histones | Heparin | Sepsis | Preclinical | 133 |
| Non-coagulant heparin | Sepsis | Preclinical | 134 | |
| Chondroitin sulphate E | Endotoxaemia | Preclinical | 135 | |
| Anti-histone antibodies | Sepsis, kidney injury, atherosclerosis | Preclinical | 111,136,137 | |
| Anti-citrullinated antibodies | RA | Preclinical | 138 | |
| PAD4 | GSK199 | Thrombosis, sepsis-induced coagulation | Preclinical | 83,139 |
| Microtubular function | Colchicine | Myocardial infarction | COLCOT | 140 |
| Gasdermin D | Disulfiram | SARS-CoV-2 | DISCO, Phase II | NCT04485130 |
| DEK | DEK aptamers | RA | Preclinical | 25 |
NET, neutrophil extracellular trap; PAD4, protein-arginine deiminase type 4; RA, rheumatoid arthritis.
5.1. Occlusive NETs
Aggregated NETs frequently localize in intravascular thrombi and occluded ducts (i.e. biliopancreatic ducts) blocking blood and other fluid circulation and secretion. Deposited NETs on the vasculature can actively contribute to thrombus formation by exerting pro-coagulant and pro-thrombotic activities resulting in venous and arterial thrombosis, adverse consequences observed in infection,141 cardiovascular diseases142 (atherosclerosis, stroke), or cancer.71 Mechanistically, NETs provides a scaffold for platelets and erythrocytes to adhere, inducing platelet aggregation and permitting fibrin accumulation.79 This process can be prevented by DNA degradation after DNAse I treatment in mice and depends on PAD4 activity.143 At the same time, the polyanionic DNA backbone of NETs can interact and retain factor XII to initiate the intrinsic coagulation pathway in a mouse model of venous thrombosis.144 TF—the main initiator of the coagulation cascade—is expressed in activated human neutrophils and it is externalized through NETs to promote thrombosis.145 In turn, NETs or NET-derived histones can also stimulate endothelial cells146 or monocytes147in vitro to produce more TF. Whether these processes contribute to thrombosis in vivo requires further investigation. Interestingly, although NET inhibition or degradation reduces thrombosis in animal models, the thrombotic effects of intact NETs are in doubt148 and seem to depend on the action of their individualized components such as DNA or histones.147
Vascular NET release and subsequent thrombosis involve a cascade of events that rely on neutrophil adhesion with the endothelium through PSGL-1 and CXCR2 receptors149 and their interaction with activated platelets.79 Indeed, blockade of NET release, degradation of NETs, or interfering with neutrophil–endothelium or neutrophil–platelet interactions blunts thrombus formation and reduces vascular tissue damage in mice.79,143,144,149 However, in mice with induced neutrophilia inhibition of the coagulation cascade (anti-thrombin treatment) or platelet depletion, is insufficient to prevent NETs to obstruct vessels in absence of host DNases.88 These results illustrate the importance of host DNAses to tolerate neutrophil-derived toxic responses and explains patient susceptibility to develop thrombosis and autoimmune responses in individuals with impaired DNAse activities.
5.2. NETs damage tissues
Neutrophil tissue infiltration frequently comes at the expense of host cell damage. Indeed, excessive neutrophil infiltration associated with tissue damage in a large variety of clinical settings including acute lung injury, myocardial infarction, stroke, as well as kidney and liver failure.150–152 Such collateral damage can result from the production of ROS, the release of cytotoxic granule proteins, and the release of NETs. The latter also integrates the former two, as ROS is important for NET release, and NETs contain granule proteins. Previous observations regarding the importance of ROS and granule proteins in the context of tissue damage and employing gene targeting or therapeutic neutralization revealed the importance of NET-driven cell death. Thus, it is not surprising that NETs reportedly trigger cytotoxicity in various tissues and diseases including sepsis, kidney injury, acute lung injury, and atherosclerosis, and delay wound healing.14,111,136,153
The negatively charged nucleic acid of NETs associated with cationic proteins of nuclear, cytosolic, and granule origin. These proteins include cationic antimicrobial peptides or cell-penetrating peptides including histones, cathelicidins, and α-defensins. These peptides exert antimicrobial activity by generating pores in the membranes of prokaryotes,154 but the selectivity of candidate proteins such as histones, cathelicidin, and α-defensins is only moderate. Consequently, they also attack host cells at concentrations in the range of those needed for their antimicrobial activity. The membrane of eukaryotic cells is very different in its composition, overall charge, and transmembrane potential to that of prokaryotes as its outer leaflet is composed mainly of zwitterionic phosphatidylcholine and sphingomyelin phospholipids thus rendering the net charge less anionic. Hence, the question arises of how these peptides gain access to mammalian cell membranes to form pores. Cathelicidin, for example, interacts less with membranes composed of neutral lipids, but after forming oligo-homomers, cathelicidin can efficiently interact also with neutral membranes and penetrate these, leading to lytic cell death.155 While such mechanisms may offer therapeutic value when designing novel anti-tumour strategies,156 in the context of inflammation this process will lead to tissue damage. In fact, many NET-resident proteins can exert cytotoxicity in host cells111,155,157 and in connection with NET histones, which account for ∼70% of NET-associated proteins,3 hold a prominent position in causing host cell death.111,134,136,137,153 Similar to cathelicidins, the interaction of histones with cell membranes heavily relies on charge. Hence, histones preferably bind to anionic phospholipids including cardiolipin and phosphatidylserine, but not zwitterionic phospholipids such as phosphatidylcholine.158 One possible means for histones to gain access to neutral cell membranes is the induction of phosphatidylserine exposure hence increasing negative surface charge.159 In small unilamellar vesicles addition of cholesterol to the lipid mixture increased the ability of histone H4 to increase membrane bending and eventually pore formation.111 However, while bactericidal properties of histone fragments depend on their ability to form amphipathic α‐helices160 with potential membrane-spanning domains, understanding the precise mechanism of histone-driven membrane permeation will require additional structural analyses. Given that electrostatic interactions are key to the toxic effects of NETs, several strategies have emerged to mitigate these toxicity. These include plasma proteins (including albumin or apolipoprotein AI), (poly-)peptides, and polysaccharides (including modified heparins).134,161,162 In addition, specific interference using antibodies is conceivable but requires accessibility of the epitope in the presence of DNA or other histone binding partners.
Yet, while we consider the cytotoxic effect of NETs in general harmful, the net effect of such activity may be context-dependent. As an example, the efficient removal of senescent cells is key during embryogenic development and in ageing. Recently, NETs were found to be critically involved in the removal of senescent vascular cells during remodelling in a mouse model of ischaemic retinopathy.163 Mechanistically, the secretome of senescent vascular units attracts neutrophils and promotes the release of NETs. These NETs in turn induce cell death of the senescent cells thereby promoting their clearance. In yet another scenario, mouse and human NETs can dampen inflammation by acting as a sink for inflammatory cytokines and chemokines.49 Serine proteases in NETs can cleave cytokines hence rendering them inactive.
5.3. Atherogenic role of NETs
Many of the properties of NETs may wreak havoc in tissues, for example, within the atherosclerotic plaque.142 In experimental atherosclerosis in mice, NETs can contribute to the initiation and progression of this disease involving a variety of mechanisms depending on disease stage and tissue location. At the core of the lesion, NETs amplify the inflammatory response by priming macrophages as the first hit for inflammasome activation.33,164 Similarly, TLR9-dependent sensing of NET structures can render interferogenic responses by plasmacytoid dendritic cells (pDCs) that help to perpetuate vascular inflammation.165 Interestingly, when released at the fibrous cap region, NET-containing histone H4 causes smooth muscle cell lysis and death provoking fibrous cap thinning and potentially plaque destabilization.111 These contrasting effects suggest that NETs’ pathogenic effects strongly depend on dose but also on the susceptibility of the targeted cell to their toxic effects. In the vasculature proper, NETs may play also a pernicious pathophysiologic role.142 Strategically situated at the intimal surface, the interface between the lumen and the arterial wall or the microvasculature and tissue parenchyma, NETs are poised to contribute to sterile inflammation. The tendrils of extended DNA, derived from disintegrating nucleosomes, become decorated with numerous proteins capable of amplifying and extending local inflammation. These filamentous structures carry a cargo that includes constituents of the neutrophil itself, and proteins recruited from the bloodstream. Tethered to the intimal surface, NETs can act as a ‘solid-state reactor’ that constrains enzymes and other biologically active constituents to the crucial interface of the bloodstream with tissues. Rather than depending on stochastic interactions in the fluid face of blood, this localization can facilitate the encounter of enzymes and substrates thus facilitating their reactions. Interestingly, whether these NETs generate a ‘solid-state reactor’ or ‘sink’ effect on the accumulated constituents will depend on their location, time of release (e.g. acute or resolution phase of inflammation), and composition of the aggregated components, ultimately determining their impact on the outcome of the inflammatory process.
The intrinsic proteins derived from granulocytic granules include a number of enzymes that can amplify local inflammatory responses through their catalytic functions.158 Localized on the fibres of NETs just at the intimal surface, MPO-derived hypochlorous acid can provoke endothelial cell apoptosis and TF gene expression in vitro.166 Thus, NET formation can propagate endothelial injury and provoke local thrombosis. MPO in blood associated with first-ever and recurrent cardiovascular events and serves as a biomarker.167 Indeed, DNA-associated MPO serves as a commonly used, albeit imperfect, a marker of NET formation in clinical studies. Among the serine proteinases found in granulocytes, proteinase 3 joins MPO as one of the key antigens that comprise ANCAs implicated in ANCA-positive vasculitides. Perhaps presentation on NETs provides a source of antigen for mononuclear phagocytes involved in the afferent limb of the immune response that contributes to ANCA vasculitis. Thus, in addition to contributing to acute forms of sterile inflammation and thrombosis in the vasculature, NETs can contribute to instigating chronic conditions that affect blood vessels as well.
NETs also can bind cytokines of neutrophil origin. These include IL-1 alpha, IL-33, and members of the IL-36 family. Another NET-associated serine proteinase, cathepsin G, can process these cytokines to more active forms.146,168,169 IL-1 beta also associated with NETs, but cathepsin G degrades it to inactive fragments. In regard to endothelial activation, NET-associated IL-1 alpha appears to exert the strongest action. IL-1 alpha mediates induction of vascular cell adhesion molecule-1 expression and TF gene expression in human endothelial cells in vitro.146 In addition to endothelial cell expression of TF induced by NET components or products such as hypochlorous acid, NETs can bind TF from the blood. Thrombi retrieved from culprit lesions from patients with acute coronary syndromes (ACS) contain NETs bearing TF, providing a source of this potent procoagulant at a site of clinically important thrombosis.145,170
Indeed, NETs may have a particular role in superficial erosion, a form of atherosclerotic plaque disruption that currently accounts for about a third of ACS.171 Human plaques with the morphology associated with erosion contain more markers of NET formation than plaques with thin fibrous caps and large lipid cores more associated with plaque rupture.172 As endothelial cells slough and uncover the basement membrane, adherent neutrophils can undergo NET formation at the site of an intimal breach. NET functions can then extend endothelial injury and amplify thrombus formation by the mechanisms described above. ACS due to erosion associate with higher MPO levels than those due to fibrous cap rupture.173,174
In an experimental mouse preparation that recapitulates certain features of superficial erosion, myeloid deficiency of PAD4 preserves endothelial integrity.175 Delivery of a small molecule PAD4 inhibitor via nanoparticles that target collagen IV, a protein abundant in the basement membrane exposed by endothelial cell desquamation, can likewise preserve endothelial structure and function.176 Thus, NET formation may be a therapeutic target in ACS precipitated by several forms of plaque disruption. Administration of DNAse can mitigate reperfusion injury after experimental myocardial infarction.177 This action likely relates to the preservation of microvascular perfusion.
5.4. NETs break immune tolerance
NETs can contribute to the loss of immunological tolerance characteristic of autoimmune diseases. Patients with SLE,178 RA,55 or AAV179 accumulate NETs in the circulation and tissues, and their concentrations associate with the severity of the disease and poor clinical outcome. This accumulation is explained by aberrant activation of neutrophils and elevated release of NETs together with the defective clearance of NETs seen in these patients.173 This augmented NET release may derive from a particular neutrophil subpopulation of LDGs.116 LDGs—which are overrepresented in SLE and RA patients—and may represent an example of neutrophil functional diversity defined by their capacity to release NETs. LDGs are dysfunctional neutrophils characterized by a highly activated phenotype, enhanced ability to release pro-inflammatory cytokines, and propensity for spontaneous NETosis. Human LDGs exhibit a strong signature of ISGs which accounts for increased responsiveness to the higher levels of systemic type I IFNs.53,116 As LDGs isolated from healthy individuals are not prone to release NETs,180 the buoyancy properties of this neutrophil subset cannot explain their increased NETosis capacity in SLE and RA patients. Instead, their particular sensitivity to IFN priming may facilitate the predisposition to do NETosis,53 however, additional work is required to provide a mechanistic link between these two processes.
Neutrophils respond early during the preclinical stages of autoimmune diseases, and the release of NET-derived autoantigens may initiate loss of immune tolerance and generation of autoantibodies.181 As the disease progresses, released NETs exacerbate the inflammatory response through their interaction with different innate and adaptive immune cells. Finally, excessive NETosis also accounts for host tissue damage and organ dysfunction, supporting contributions of NETs at all stages of autoimmune diseases.
NETs as a source of self-antigens: The presence of autoantibodies against proteinase 3 or MPO is a hallmark of AAV.181 Other autoantibodies targeting double-stranded DNA or citrullinated proteins can also be detected in SLE or RA patients. Due to the nature of the protein cargo, neutrophils are enriched in these proteins that, when released during NETosis, serve as antigens for the generation of autoantibodies. In particular, protein citrullination by PAD enzymes is highly active during NETosis and is responsible for the generation of the citrullinated antigens observed in RA55 and SLE182 patients. At the same time, generated ANCAs and anti-citrullinated protein antibodies can potently induce NETosis in human neutrophils, thus completing an inflammatory loop that contributes to the progression of the disease.54,55 In addition to their antigenicity, protein citrullination increases their immune reactivity, amplifying the inflammatory responses, and contributing to RA pathogenesis.55 Similarly, carbamylated proteins were recently identified as an furnishing antigenic epitopes for anti-carbamylated protein antibodies, whose accumulation associated with excessive inflammation and exacerbated bone destruction in RA patients.26
Immunomodulatory effects of NETs: The immunogenic properties of NETs manifest at different stages of autoimmune diseases and involve multiple cell types. In human synovial fluid, NETs can be internalized by fibroblast-like synoviocytes inducing antigen presentation of citrullinated peptides to T cells mediating Th1 responses.183 In an in vitro setting, T-cell recognition of NET components via T-cell receptor have priming effects, reducing their activation threshold and increasing their response to antibodies.184 An important property of NET-derived structures is their capacity to induce potent production of interferons, a hallmark of many autoimmune diseases. pDCs are main producers of IFN through TLR9-dependent recognition of DNA-bound HMGB1 protein and LL37 complexes.53,185 In macrophages, phagocyted NETs are sensed through the cytosolic sensor cyclic GMP–AMP synthase triggering IFN I production and amplifying autoimmune responses in mice.186 The interferogenic properties of NETs can partly be explained by their partial mitochondrial origin. In response to ribonucleoprotein immune complexes, neutrophils extrude NETs depending on mitochondrial ROS,53 and this oxidized DNA material induces a strong production of IFNs that can promote autoimmunity.5 Mechanistically, the release of mitochondrial DNA fragments in stressed cells and during NETosis seems to depend on the formation of pores in the mitochondrial outer membrane by the oligomerization of the voltage-dependent anion channel, whose pharmacological inhibition prevents NETosis and IFN responses in a mouse model of lupus.187 Interestingly, another stress signalling pathway, the endoplasmic reticulum stress, was recently associated with mitochondrial ROS-induced NETosis and the development of experimental lupus.188 It remains to be clarified whether stress in hyperactive neutrophils (e.g. SLE or RA LDGs) is then a common cellular state preceding NAPDH-independent and mitochondrial ROS-dependent NETosis, and that results in the release of immunogenic oxidized DNA.
5.5. Tumorigenic and metastatic NETs
Neutrophils are increasingly being recognized as contributors to cancer initiation, development, and progression, ultimately impacting on patient survival or resistance to immunotherapy.189 Tumour-elicited signals have a profound impact on neutrophil production, mobilization, and function over the course of the disease. Among these functional alterations, cancer-induced NETosis has gained attention as it has been implicated in multiple tumorigenic and pro-metastatic processes, and tumour-associated thrombosis as well. NETs accumulate in advanced human cancer190 and liver121 metastases associated with poor prognosis and reduced survival. Similarly, NET remnants in plasma such as NE-DNA complexes191 or citrunillated histone H3118 potently predict survival in cancer patients.
Experimental blockade of NETosis or NET degradation strategies highlights a causal role of NETs in cancer.192 However, the mechanisms underpinning these pro-tumoural functions are just beginning to be understood. Indeed, the variety of pro-tumoural and pro-metastatic functions ascribed to NETs has exceeded initially expectations.
NETs feed the tumour: NETs can act as inflammatory signals to stimulate cancer cell proliferation. In mice with metastatic colorectal cancer, NETs induce mitochondrial biogenesis, increased ATP production and, oxygen consumption in cancer cells.193 NET-borne NE signals through TLR4 receptor to induce in vitro metabolic reprogramming and foster cell proliferation. NET-mediated TLR9 signalling can also activate cancer cell proliferation, and consequently, genetic disruption of TLR9 reduces tumour growth and prevents dissemination.64 Although robust evidence of the in vivo relevance of these mechanisms is still needed, NETs might act as initiators of tumour growth particularly in patients with pre-existing low-grade inflammation or chronic inflammation after non-resolved infection. In agreement with this idea, early NET-driven inflammation in experimental non-alcoholic steatohepatitis promotes subsequent development of hepatocellular carcinoma.194 A recent pioneer study also supports this concept by suggesting that NETs mediate the awakening of dormant cancer cells to form lung metastasis.195 Inflammatory stimulation of NET release after LPS instillation or exposure to tobacco smoke in mice results in the exposure of extracellular matrix-degrading enzymes NE and MMP9. Here, matrix remodelling through sequential cleavage of laminin-111 by these NET-derived proteases generates an altered form of laminin that activates integrin α3β1 signalling to induce cancer cell proliferation. Interestingly, remodelling of the tumour extracellular matrix by proteases can exert alternative effects beyond acceleration of cancer cell proliferation, such as angiogenesis or tumour dissemination. Indeed, neutrophils in the tumour furnish MMP9 which is a potent inductor of angiogenesis independent of NETs,196 however, whether NET-bound or NET-free MMP9 exerts distinct pro-tumoural processes is still unknown.
Nesting metastasis: NETs released in the tumour-free environment might also serve as physical scaffolds of the metastatic niche. Deposited on the microvasculature of the liver and lung after septic inflammation, NETs can efficiently trap circulating cancer cells, hence facilitating their adhesion to the tissue stroma and the subsequent metastasis.197 Notably, the NET-bound protein carcinoembryonic antigen-related cell adhesion molecule 1 mediates the interaction between NETs and cancer cells in mice.198 Before the formation of metastases, neutrophil activation and NETosis in the vasculature are often induced remotely by soluble factors originated in the primary tumour. This process—observed in human ovarian and breast cancer—links neutrophil infiltration and NET release in the omentum199 and lung75 with experimental metastasis. In the pre-metastatic niche, NETs can also function as a potent chemoattractant for disseminated cancer cells.190 Human and mouse NETs are sensed by cancer cells through the transmembrane receptor coiled-coil domain-containing protein 25 (CCDC25), whose activation induces cell motility. Beyond the apparent therapeutic opportunities based on CCDC25 targeting, a comprehensive analysis of the function of this receptor in other pathologic conditions and homeostasis is of the utmost interest to better understand NET-associated biology.
Shielding from anti-tumoural immunity: Tumours can hijack anti-tumoural immunity by using NETs as physical shields. Induced by CXCR1 and CXCR2 ligands secreted by the tumour, NETs are ejected around the tumour stroma in humans and mice.65 Interestingly, tumours surrounded by NETs exhibit an augmented resistance towards the immune response elicited by effector CD8+ T cells and natural killer cells. This mechanism might mediate tumour resistance to immune checkpoint blockade strategies. Indeed, experimental NET inhibition can restore responsiveness to checkpoint inhibitors.65 Similar observations pertain to pancreatic cancer, where IL-17 recruits neutrophils and promotes NETosis to induce immunosuppression.61 Blockade of IL-17 or inhibition of NET release in combination with anti-PD1 consequently improved treatment efficacy by reducing tumour growth and metastasis in a mouse model of pancreatic cancer.
6. Conclusion
Since the discovery of NETosis, our understanding of the biology of NET formation and its implications for disease have expanded enormously. Yet, the variety of ascribed functions described to date—aside from their antimicrobial activity—suggest that we are only at the beginning of understanding its biology. Although many of these actions have pathological consequences, the importance of NETs in host defence and as regulators of the inflammatory response cannot be ignored. Evidence from disease studies suggests that the immunomodulatory properties of NETs might be required to permit a proper inflammatory response (priming effect) or to limit inflammation to ensure resolution. These immunoregulatory properties also likely have important implications under homeostatic conditions. Another important factor influencing NET function relies on variations in their composition and structure. Such changes might originate from differences in the proteomic and transcriptomic landscape among neutrophil subpopulations and upon activation with distinct stimuli. Although plausible, this concept will require future investigation. Furthermore, and as it occurs with neutrophils, how much, when, and where NETs are released, critically shifts the balance between a harmful or beneficial outcome. Here, evidence of temporal (circadian) and spatial regulation of NETosis is emerging, and pinpoint the importance of the physiological regulation of this process to ensure maximal efficacy in situations of host defence while preventing tissue injury or vascular occlusion. All these aspects merit consideration when understanding NET biology and identifying physiological factors controlling NET release or clearance. Therapeutic strategies that intervene on these physiological processes entail an alternative to treatments based on inhibition of NETosis or NET degradation, preventing their pathogenic dysregulation without altering their function in host defence.
7. Perspective
The concept of NETs as relevant pathological drivers of human disease has gained increasing acceptance in the scientific community. The identification of NETs and their remnants in multiple human diseases (as recently exemplified by the number of studies implicating NETs in the aetiology of SARS-Cov-2 infection), or as markers of treatment efficiency, together with mechanistic evidence in animal models have sustained this idea. This increasing interest will stimulate the development of sensitive, specific, and standardized techniques for NET detection, a requirement for their utility as biomarkers in clinical practice. Similarly, the clinical translation of NETs also requires overcoming other obstacles such as the design of novel therapeutic strategies that ensure targeting NETosis with high specificity. In this sense, a better understating of the molecular and physiological mechanisms preceding and driving NET release will enable such possibilities. We propose that the introduction of single-cell genomic technologies in the study of NET biology is necessary to decipher at single-cell resolution the epigenetic, transcriptomic, and molecular changes occurring in specific neutrophil subpopulations prior to NET release, thereby permitting to specifically counter their toxic consequences. The application of newly developed spatial ‘omic’ techniques combining transcriptomic and immunostaining analyses will also help to solve the current technical limitations when detecting NETs in tissue, and should expand our understating on NET regulation and function in the native tissue.
Authors’ contributions
The authors contributed equally to all aspects of the article.
Funding
C.S.-R. receives funding from the Deutsche Forschungsgemeinschaft (SFB1123 TP A6). O.S. receives funding from the Deutsche Forschungsgemeinschaft (SFB914 TP B8, SFB1123 TP A6, TP B5, SFB1009 TP A13), the Vetenskapsrådet (2017-01762), the Else-Kröner-Fresenius Stiftung (2017_A13), the Swedish Heart–Lung Foundation (20190317), and the Leducq foundation (TNE-18CVD04). P.L. receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund. V.P. receives core funding from the Francis Crick institute funded by UK Medical Research Council, Cancer Research UK and the Wellcome Trust (FC0010129, FC001134). I.V.A was funded by an EMBO LTF (ALTF 113-2019). A.H. is funded by Ministerio de Ciencia e Innovacion (RTI2018-095497-B-I00), La Caixa Foundation (HR17_00527), and the European Commision (FET-OPEN 861878).
Contributor Information
Andres Hidalgo, Area of Cell and Developmental Biology, Fundación Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Melchor Fernández Almagro 3, 28029, Madrid, Spain.
Peter Libby, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Oliver Soehnlein, Institute for Experimental Pathology (ExPat), Center for Molecular Biology of Inflammation (ZMBE), University of Münster, Von-Esmarch-Straße 56, 48149, Münster, Germany; Department of Physiology and Pharmacology (FyFa), Karolinska Institute, Solnavägen 1, 171 77, Stockholm, Sweden.
Iker Valle Aramburu, Laboratory of Antimicrobial Defence, The Francis Crick Institute, London NW1 1AT, UK.
Venizelos Papayannopoulos, Laboratory of Antimicrobial Defence, The Francis Crick Institute, London NW1 1AT, UK.
Carlos Silvestre-Roig, Institute for Experimental Pathology (ExPat), Center for Molecular Biology of Inflammation (ZMBE), University of Münster, Von-Esmarch-Straße 56, 48149, Münster, Germany.
References
- 1. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A.. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 2. Burgener SS, Schroder K.. Neutrophil extracellular traps in host defense. Cold Spring Harb Perspect Biol 2020;12:a037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A.. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 2009;5:e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X, Zhao J, Cai C, Tang X, Fu L, Zhang A, Han L.. A label-free quantitative proteomic analysis of mouse neutrophil extracellular trap formation induced by Streptococcus suis or phorbol myristate acetate (PMA). Front Immunol 2018;9:2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, Ravin SS. D, Smith CK, Malech HL, Ledbetter JA, Elkon KB, Kaplan MJ.. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016;22:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castanheira FVS, Kubes P.. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019;133:2178–2185. [DOI] [PubMed] [Google Scholar]
- 7. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A.. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007;176:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J.. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 2009;114:2619–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benna JE, Han J, Park JW, Schmid E, Ulevitch RJ, Babior BM.. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys 1996;334:395–400. [DOI] [PubMed] [Google Scholar]
- 10. Wolfson M, McPhail LC, Nasrallah VN, Snyderman R.. Phorbol myristate acetate mediates redistribution of protein kinase C in human neutrophils: potential role in the activation of the respiratory burst enzyme. J Immunol 1985;135:2057–2062. [PubMed] [Google Scholar]
- 11. Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H.. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol 2011;7:75–77. [DOI] [PubMed] [Google Scholar]
- 12. Sadiku P, Willson JA, Ryan EM, Sammut D, Coelho P, Watts ER, Grecian R, Young JM, Bewley M, Arienti S, Mirchandani AS, Sanchez Garcia MA, Morrison T, Zhang A, Reyes L, Griessler T, Jheeta P, Paterson GG, Graham CJ, Thomson JP, Baillie K, Thompson AAR, Morgan J-M, Acosta-Sanchez A, Dardé VM, Duran J, Guinovart JJ, Rodriguez-Blanco G, Von Kriegsheim A, Meehan RR, Mazzone M, Dockrell DH, Ghesquiere B, Carmeliet P, Whyte MKB, Walmsley SR.. Neutrophils fuel effective immune responses through gluconeogenesis and glycogenesis. Cell Metab 2021;33:411–423.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsu BE, Tabaries S, Johnson RM, Andrzejewski S, Senecal J, Lehuede C, Annis MG, Ma EH, Vols S, Ramsay L, Froment R, Monast A, Watson IR, Granot Z, Jones RG, St-Pierre J, Siegel PM.. Immature low-density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis. Cell Rep 2019;27:3902–3915.e6. [DOI] [PubMed] [Google Scholar]
- 14. Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD.. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015;21:815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Zhou X, Yin Y, Mai Y, Wang D, Zhang X.. Hyperglycemia induces neutrophil extracellular traps formation through an NADPH oxidase-dependent pathway in diabetic retinopathy. Front Immunol 2018;9:3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM, Wang W, Abramowicz S, Bastide-van Gemert S. L, Wang N, Welch CL, Reilly MP, Stroes ES, Moore KJ, Tall AR.. Cholesterol efflux pathways suppress inflammasome activation, NETosis, and atherogenesis. Circulation 2018;138:898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Bernuth H. V, Zychlinsky A.. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 2017;6:e24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V.. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep 2014;8:883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neubert E, Meyer D, Rocca F, Günay G, Kwaczala-Tessmann A, Grandke J, Senger-Sander S, Geisler C, Egner A, Schön MP, Erpenbeck L, Kruss S.. Chromatin swelling drives neutrophil extracellular trap release. Nat Commun 2018;9:3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stojkov D, Amini P, Oberson K, Sokollik C, Duppenthaler A, Simon HU, Yousefi S.. ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J Cell Biol 2017;216:4073–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A.. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 2010;191:677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tilley DO, Abuabed U, Zimny Arndt U, Schmid M, Florian S, Jungblut R, Brinkmann V, Herzig A, Zychlinsky A. Histone H3 clipping is a novel signature of human neutrophil extracellular traps. bioRxiv 2021. [DOI] [PMC free article] [PubMed]
- 23. Krajewski WA. On the role of inter-nucleosomal interactions and intrinsic nucleosome dynamics in chromatin function. Biochem Biophys Rep 2016;5:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A.. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 2011;117:953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mor-Vaknin N, Saha A, Legendre M, Carmona-Rivera C, Amin MA, Rabquer BJ, Gonzales-Hernandez MJ, Jorns J, Mohan S, Yalavarthi S, Pai DA, Angevine K, Almburg SJ, Knight JS, Adams BS, Koch AE, Fox DA, Engelke DR, Kaplan MJ, Markovitz DM.. DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis. Nat Commun 2017;8:14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Neil LJ, Barrera-Vargas A, Sandoval-Heglund D, Merayo-Chalico J, Aguirre-Aguilar E, Aponte AM, Ruiz-Perdomo Y, Gucek M, El-Gabalawy H, Fox DA, Katz JD, Kaplan MJ, Carmona-Rivera C.. Neutrophil-mediated carbamylation promotes articular damage in rheumatoid arthritis. Sci Adv 2020;6:eabd2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S, Stallcup MR, Allis CD, Coonrod SA.. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004;306:279–283. [DOI] [PubMed] [Google Scholar]
- 28. Kearney PL, Bhatia M, Jones NG, Yuan L, Glascock MC, Catchings KL, Yamada M, Thompson PR.. Kinetic characterization of protein arginine deiminase 4: a transcriptional corepressor implicated in the onset and progression of rheumatoid arthritis. Biochemistry 2005;44:10570–10582. [DOI] [PubMed] [Google Scholar]
- 29. Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M.. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol 2004;11:777–783. [DOI] [PubMed] [Google Scholar]
- 30. Neeli I, Radic M.. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol 2013;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neeli I, Khan SN, Radic M.. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 2008;180:1895–1902. [DOI] [PubMed] [Google Scholar]
- 32. Zhou Y, An LL, Chaerkady R, Mittereder N, Clarke L, Cohen TS, Chen B, Hess S, Sims GP, Mustelin T.. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci Rep 2018;8:15228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V.. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015;349:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsourouktsoglou TD, Warnatsch A, Ioannou M, Hoving D, Wang Q, Papayannopoulos V.. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep 2020;31:107602. [DOI] [PubMed] [Google Scholar]
- 35. Martinod K, Witsch T, Farley K, Gallant M, Remold-O'Donnell E, Wagner DD.. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J Thromb Haemost 2016;14:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amulic B, Knackstedt SL, Abu Abed U, Deigendesch N, Harbort CJ, Caffrey BE, Brinkmann V, Heppner FL, Hinds PW, Zychlinsky A.. Cell-cycle proteins control production of neutrophil extracellular traps. Dev Cell 2017;43:449–462.e5. [DOI] [PubMed] [Google Scholar]
- 37. Li Y, Li M, Weigel B, Mall M, Werth VP, Liu ML.. Nuclear envelope rupture and NET formation is driven by PKCalpha-mediated lamin B disassembly. EMBO Rep 2020;21:e48779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harbort CJ, Soeiro-Pereira P. V, BH, von Kaindl AM, Costa-Carvalho BT, Condino-Neto A, Reichenbach J, Roesler J, Zychlinsky A, Amulic B.. Neutrophil oxidative burst activates ATM to regulate cytokine production and apoptosis. Blood 2015;126:2842–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azzouz D, Khan MA, Palaniyar N.. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov 2021;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Münzer P, Negro R, Fukui S, Meglio L. D, Aymonnier K, Chu L, Cherpokova D, Gutch S, Sorvillo N, Shi L, Magupalli VG, Weber ANR, Scharf RE, Waterman CM, Wu H, Wagner DD.. NLRP3 inflammasome assembly in neutrophils is supported by PAD4 and promotes NETosis under sterile conditions. Front Immunol 2021;12:683803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sollberger G, Choidas A, Burn GL, Habenberger P, Lucrezia R D, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Kruger R, Herzig A, Zychlinsky A.. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 2018;3:eaar6689. [DOI] [PubMed] [Google Scholar]
- 42. Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, Pein J. V, Broz P, Sweet MJ, Schroder K.. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 2018;3:eaar6676. [DOI] [PubMed] [Google Scholar]
- 43. Chen X, He WT, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, Han J.. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res 2016;26:1007–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen W, Zhao J, Mu D, Wang Z, Liu Q, Zhang Y, Yang D.. Pyroptosis mediates neutrophil extracellular trap formation during bacterial infection in zebrafish. J Immunol 2021;206:1913–1922. [DOI] [PubMed] [Google Scholar]
- 45. Ma R, Li T, Cao M, Si Y, Wu X, Zhao L, Yao Z, Zhang Y, Fang S, Deng R, Novakovic VA, Bi Y, Kou J, Yu B, Yang S, Wang J, Zhou J, Shi J.. Extracellular DNA traps released by acute promyelocytic leukemia cells through autophagy. Cell Death Dis 2016;7:e2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu F, Zhang C, Zou Z, Fan EKY, Chen L, Li Y, Billiar TR, Wilson MA, Shi X, Fan J.. Aging-related Atg5 defect impairs neutrophil extracellular traps formation. Immunology 2017;151:417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Germic N, Stojkov D, Oberson K, Yousefi S, Simon HU.. Neither eosinophils nor neutrophils require ATG5-dependent autophagy for extracellular DNA trap formation. Immunology 2017;152:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Desai J, Kumar SV, Mulay SR, Konrad L, Romoli S, Schauer C, Herrmann M, Bilyy R, Müller S, Popper B, Nakazawa D, Weidenbusch M, Thomasova D, Krautwald S, Linkermann A, Anders H-J.. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur J Immunol 2016;46:223–229. [DOI] [PubMed] [Google Scholar]
- 49. Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, Holmdahl R, Krenn V, Harrer T, Jeremic I, Bilyy R, Schett G, Hoffmann M, Herrmann M.. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 2014;20:511–517. [DOI] [PubMed] [Google Scholar]
- 50. Leppkes M, Maueroder C, Hirth S, Nowecki S, Gunther C, Billmeier U, Paulus S, Biermann M, Munoz LE, Hoffmann M, Wildner D, Croxford AL, Waisman A, Mowen K, Jenne DE, Krenn V, Mayerle J, Lerch MM, Schett G, Wirtz S, Neurath MF, Herrmann M, Becker C.. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat Commun 2016;7:10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zenaro E, Pietronigro E, Bianca V. D, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nani S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G.. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 2015;21:880–886. [DOI] [PubMed] [Google Scholar]
- 52. Azevedo EP, Guimaraes-Costa AB, Torezani GS, Braga CA, Palhano FL, Kelly JW, Saraiva EM, Foguel D.. Amyloid fibrils trigger the release of neutrophil extracellular traps (NETs), causing fibril fragmentation by NET-associated elastase. J Biol Chem 2012;287:37206–37218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V.. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011;3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE.. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009;15:623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ.. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013;5:178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gupta S, Nakabo S, Blanco LP, O'Neil LJ, Wigerblad G, Goel RR, Mistry P, Jiang K, Carmona-Rivera C, Chan DW, Wang X, Pedersen HL, Gadkari M, Howe KN, Naz F, Dell'Orso S, Hasni SA, Dempsey C, Buscetta A, Frischmeyer-Guerrerio PA, Kruszka P, Muenke M, Franco LM, Sun H-W, Kaplan MJ.. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci USA 2020;117:16481–16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Behnen M, Leschczyk C, Moller S, Batel T, Klinger M, Solbach W, Laskay T.. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J Immunol 2014;193:1954–1965. [DOI] [PubMed] [Google Scholar]
- 58. Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, Bengtsson AA, Blom AM.. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 2012;188:3522–3531. [DOI] [PubMed] [Google Scholar]
- 59. Moreira-Teixeira L, Stimpson PJ, Stavropoulos E, Hadebe S, Chakravarty P, Ioannou M, Aramburu IV, Herbert E, Priestnall SL, Suarez-Bonnet A, Sousa J, Fonseca KL, Wang Q, Vashakidze S, Rodríguez-Martínez P, Vilaplana C, Saraiva M, Papayannopoulos V, O’Garra A.. Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis. Nat Commun 2020;11:5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gul E, Sayar EH, Gungor B, Eroglu FK, Surucu N, Keles S, Guner SN, Findik S, Alpdundar E, Ayanoglu IC, Kayaoglu B, Geckin BN, Sanli HA, Kahraman T, Yakicier C, Muftuoglu M, Oguz B, Cagdas Ayvaz DN, Gursel I, Ozen S, Reisli I, Gursel M.. Type I IFN-related NETosis in ataxia telangiectasia and Artemis deficiency. J Allergy Clin Immunol 2018;142:246–257. [DOI] [PubMed] [Google Scholar]
- 61. Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, Zoltan M, Arora N, Baydogan S, Horne W, Burks J, Xu H, Hussain P, Wang H, Gupta S, Maitra A, Bailey JM, Moghaddam SJ, Banerjee S, Sahin I, Bhattacharya P, McAllister F.. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med 2020;217:e20190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J, Barthwal MK, Dikshit M.. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One 2012;7:e48111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weckbach LT, Grabmaier U, Uhl A, Gess S, Boehm F, Zehrer A, Pick R, Salvermoser M, Czermak T, Pircher J, Sorrelle N, Migliorini M, Strickland DK, Klingel K, Brinkmann V, Abu Abed U, Eriksson U, Massberg S, Brunner S, Walzog B.. Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J Exp Med 2019;216:350–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, Liu P, Li Z, Xia Y, Jiang W.. Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res 2019;25:1867–1879. [DOI] [PubMed] [Google Scholar]
- 65. Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, Andrea C. D, Ochoa MC, Otano I, Etxeberria I, Andueza MP, Nieto CP, Resano L, Azpilikueta A, Allegretti M, Pizzol M, de Ponz-Sarvise M, Rouzaut A, Sanmamed MF, Schalper K, Carleton M, Mellado M, Rodriguez-Ruiz ME, Berraondo P, Perez-Gracia JL, Melero I.. CXCR1 and CXCR2 Chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 2020;52:856–871.e8. [DOI] [PubMed] [Google Scholar]
- 66. Xu Z, Cai J, Gao J, White GC, Chen F, Ma Y-Q.. Interaction of kindlin-3 and β2-integrins differentially regulates neutrophil recruitment and NET release in mice. Blood 2015;126:373–377. [DOI] [PubMed] [Google Scholar]
- 67. Raftery MJ, Lalwani P, Krautkrämer E, Peters T, Scharffetter-Kochanek K, Krüger R, Hofmann J, Seeger K, Krüger DH, Schönrich G.. β2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J Exp Med 2014;211:1485–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kamoshida G, Kikuchi-Ueda T, Nishida S, Tansho-Nagakawa S, Ubagai T, Ono Y.. Pathogenic bacterium Acinetobacter baumannii inhibits the formation of neutrophil extracellular traps by suppressing neutrophil adhesion. Front Immunol 2018;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P.. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012;12:324–333. [DOI] [PubMed] [Google Scholar]
- 70. Erpenbeck L, Gruhn AL, Kudryasheva G, Günay G, Meyer D, Busse J, Neubert E, Schön MP, Rehfeldt F, Kruss S.. Effect of adhesion and substrate elasticity on neutrophil extracellular trap formation. Front Immunol 2019;10:2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD.. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA 2012;109:13076–13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Munir H, Jones JO, Janowitz T, Hoffmann M, Euler M, Martins CP, Welsh SJ, Shields JD.. Stromal-driven and Amyloid beta-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat Commun 2021;12:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, Wang Y, Yang S, Liang C, Liang Y, Wen J, Liu Y, Luo W, Lv X, He Y, Cheng DD, Zhou T, Zhao W, Zhang P, Zhang X, Xiao Y, Qian Y, Wang H, Gao Q, Yang QC, Yang Q, Hu G.. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell 2021;39:423–437.e7. [DOI] [PubMed] [Google Scholar]
- 74. Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, Singhi AD, Kang R, Tang D, Lotze MT, Zeh HJ.. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther 2015;22:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, Nakasone ES, Hearn SA, Kuttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg MS, Egeblad M.. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 2016;8:361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ.. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett 2010;584:3193–3197. [DOI] [PubMed] [Google Scholar]
- 77. Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S.. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol 2005;66:1146–1154. [DOI] [PubMed] [Google Scholar]
- 78. Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P.. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007;13:463–469. [DOI] [PubMed] [Google Scholar]
- 79. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD.. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 2010;107:15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Andrews RK, Arthur JF, Gardiner E.. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb Haemost 2014;112:659–665. [DOI] [PubMed] [Google Scholar]
- 81. Massberg S, Grahl L, Bruehl M. V, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B.. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010;16:887–896. [DOI] [PubMed] [Google Scholar]
- 82. Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, Maiuri L, Maseri A, D'Angelo A, Bianchi ME, Rovere-Querini P, Manfredi AA.. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost 2014;12:2074–2088. [DOI] [PubMed] [Google Scholar]
- 83. Dyer MR, Chen Q, Haldeman S, Yazdani H, Hoffman R, Loughran P, Tsung A, Zuckerbraun BS, Simmons RL, Neal MD.. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci Rep 2018;8:2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nakazawa D, Desai J, Steiger S, Muller S, Devarapu SK, Mulay SR, Iwakura T, Anders HJ.. Activated platelets induce MLKL-driven neutrophil necroptosis and release of neutrophil extracellular traps in venous thrombosis. Cell Death Discov 2018;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Perdomo J, Leung HHL, Ahmadi Z, Yan F, Chong JJH, Passam FH, Chong BH.. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun 2019;10:1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, Sule G, Gockman K, Madison JA, Zuo M, Yadav V, Wang J, Woodard W, Lezak SP, Lugogo NL, Smith SA, Morrissey JH, Kanthi Y, Knight JS.. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med 2020;12:3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jiménez-Alcázar M, Limacher A, Panda R, Méan M, Bitterling J, Peine S, Renné T, Beer JH, Aujesky D, Lämmle B, Fuchs TA.. Circulating extracellular DNA is an independent predictor of mortality in elderly patients with venous thromboembolism. PLoS One 2018;13:e0191150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renné C, Renné T, Kluge S, Panzer U, Mizuta R, Mannherz HG, Kitamura D, Herrmann M, Napirei M, Fuchs TA.. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017;358:1202–1206. [DOI] [PubMed] [Google Scholar]
- 89. Kambas K, Mitroulis I, Apostolidou E, Girod A, Chrysanthopoulou A, Pneumatikos I, Skendros P, Kourtzelis I, Koffa M, Kotsianidis I, Ritis K.. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS One 2012;7:e45427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V.. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 2014;15:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Secundino I, Lizcano A, Roupe KM, Wang X, Cole JN, Olson J, Ali SR, Dahesh S, Amayreh LK, Henningham A, Varki A, Nizet V.. Host and pathogen hyaluronan signal through human siglec-9 to suppress neutrophil activation. J Mol Med (Berl) 2016;94:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Khatua B, Bhattacharya K, Mandal C.. Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J Leukoc Biol 2012;91:641–655. [DOI] [PubMed] [Google Scholar]
- 93. Steevels TAM, van Avondt K, Westerlaken GHA, Stalpers F, Walk J, Bont L, Coffer PJ, Meyaard L.. Signal inhibitory receptor on leukocytes-1 (SIRL-1) negatively regulates the oxidative burst in human phagocytes. Eur J Immunol 2013;43:1297–1308. [DOI] [PubMed] [Google Scholar]
- 94. Delaveris CS, Wilk AJ, Riley NM, Stark JC, Yang SS, Rogers AJ, Ranganath T, Nadeau KC, Stanford C-B, Blish CA, Bertozzi CR.. Synthetic Siglec-9 agonists inhibit neutrophil activation associated with COVID-19. ACS Cent Sci 2021;7:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bornhofft KF, Viergutz T, Kuhnle A, Galuska SP.. Nanoparticles equipped with alpha2,8-linked sialic acid chains inhibit the release of neutrophil extracellular traps. Nanomaterials (Basel) 2019;9:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Borregaard N. Neutrophils, from marrow to microbes. Immunity 2010;33:657–670. [DOI] [PubMed] [Google Scholar]
- 97. Adrover JM, Aroca-Crevillén A, Crainiciuc G, Ostos F, Rojas-Vega Y, Rubio-Ponce A, Cilloniz C, Bonzón-Kulichenko E, Calvo E, Rico D, Moro MA, Weber C, Lizasoaín I, Torres A, Ruiz-Cabello J, Vázquez J, Hidalgo A.. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat Immunol 2020;21:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, Huerga-Encabo H, Silvestre-Roig C, Rossaint J, Cossío I, Lechuga-Vieco AV, García-Prieto J, Gómez-Parrizas M, Quintana JA, Ballesteros I, Martin-Salamanca S, Aroca-Crevillen A, Chong SZ, Evrard M, Balabanian K, López J, Bidzhekov K, Bachelerie F, Abad-Santos F, Muñoz-Calleja C, Zarbock A, Soehnlein O, Weber C, Ng LG, Lopez-Rodriguez C, Sancho D, Moro MA, Ibáñez B, Hidalgo A.. A neutrophil timer coordinates immune defense and vascular protection. Immunity 2019;50:390–402.e10. [DOI] [PubMed] [Google Scholar]
- 99. Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK.. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 2014;15:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, Merad M, Frenette PS.. Neutrophil ageing is regulated by the microbiome. Nature 2015;525:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xu C, Lee SK, Zhang D, Frenette PS.. The gut microbiome regulates psychological-stress-induced inflammation. Immunity 2020;53:417–428.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dicker AJ, Crichton ML, Pumphrey EG, Cassidy AJ, Suarez-Cuartin G, Sibila O, Furrie E, Fong CJ, Ibrahim W, Brady G, Einarsson GG, Elborn JS, Schembri S, Marshall SE, Palmer CNA, Chalmers JD.. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2018;141:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ascher S, Wilms E, Pontarollo G, Formes H, Bayer F, Müller M, Malinarich F, Grill A, Bosmann M, Saffarzadeh M, Brandão I, Groß K, Kiouptsi K, Kittner JM, Lackner KJ, Jurk K, Reinhardt C.. Gut microbiota restricts NETosis in acute mesenteric ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 2020;40:2279–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vong L, Lorentz RJ, Assa A, Glogauer M, Sherman PM.. Probiotic lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J Immunol 2014;192:1870–1877. [DOI] [PubMed] [Google Scholar]
- 105. Tuganbaev T, Mor U, Bashiardes S, Liwinski T, Nobs SP, Leshem A, Dori-Bachash M, Thaiss CA, Pinker EY, Ratiner K, Adlung L, Federici S, Kleimeyer C, Moresi C, Yamada T, Cohen Y, Zhang X, Massalha H, Massasa E, Kuperman Y, Koni PA, Harmelin A, Gao N, Itzkovitz S, Honda K, Shapiro H, Elinav E.. Diet diurnally regulates small intestinal microbiome-epithelial-immune homeostasis and enteritis. Cell 2020;182:1441–1459.e21. [DOI] [PubMed] [Google Scholar]
- 106. Kauffman CA. Fungal infections in older adults. Clin Infect Dis 2001;33:550–555. [DOI] [PubMed] [Google Scholar]
- 107. Hazeldine J, Harris P, Chapple IL, Grant M, Greenwood H, Livesey A, Sapey E, Lord JM.. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell 2014;13:690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Casanova-Acebes M, Nicolás-Ávila JA, Yao Li JL, García-Silva S, Balachander A, Rubio-Ponce A, Weiss LA, Adrover JM, Burrows K, A-González N, Ballesteros I, Devi S, Quintana JA, Crainiciuc G, Leiva M, Gunzer M, Weber C, Nagasawa T, Soehnlein O, Merad M, Mortha A, Ng LG, Peinado H, Hidalgo A.. Neutrophils instruct homeostatic and pathological states in naive tissues. J Exp Med 2018;215:2778–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schumski A, Ortega-Gómez A, Wichapong K, Winter C, Lemnitzer P, Viola JR, Pinilla-Vera M, Folco E, Solis-Mezarino V, Völker-Albert M, Maas SL, Pan C, Perez Olivares L, Winter J, Hackeng T, Karlsson MCI, Zeller T, Imhof A, Baron RM, Nicolaes GAF, Libby P, Maegdefessel L, Kamp F, Benoit M, Döring Y, Soehnlein O.. Endotoxinemia accelerates atherosclerosis through electrostatic charge-mediated monocyte adhesion. Circulation 2021;143:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, Looney MR, McAllister F, Rayes R, Renaud S, Rousseau S, Salvatore S, Schwartz RE, Spicer JD, Yost CC, Weber A, Zuo Y, Egeblad M.. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med 2020;217:e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, Winter J, Adrover JM, Santos GS, Froese A, Lemnitzer P, Ortega-Gómez A, Chevre R, Marschner J, Schumski A, Winter C, Perez-Olivares L, Pan C, Paulin N, Schoufour T, Hartwig H, González-Ramos S, Kamp F, Megens RTA, Mowen KA, Gunzer M, Maegdefessel L, Hackeng T, Lutgens E, Daemen M, von Blume J, Anders H-J, Nikolaev VO, Pellequer J-L, Weber C, Hidalgo A, Nicolaes GAF, Wong GCL, Soehnlein O.. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019;569:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P.. Neutrophil diversity in health and disease. Trends Immunol 2019;40:565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V.. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun 2009;1:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Martinelli S, Urosevic M, Daryadel A, Oberholzer PA, Baumann C, Fey MF, Dummer R, Simon H-U, Yousefi S.. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem 2004;279:44123–44132. [DOI] [PubMed] [Google Scholar]
- 115. Blazkova J, Gupta S, Liu Y, Gaudilliere B, Ganio EA, Bolen CR, Saar-Dover R, Fragiadakis GK, Angst MS, Hasni S, Aghaeepour N, Stevenson D, Baldwin N, Anguiano E, Chaussabel D, Altman MC, Kaplan MJ, Davis MM, Furman D.. Multicenter systems analysis of human blood reveals immature neutrophils in males and during pregnancy. J Immunol 2017;198:2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mistry P, Nakabo S, O'Neil L, Goel RR, Jiang K, Carmona-Rivera C, Gupta S, Chan DW, Carlucci PM, Wang X, Naz F, Manna Z, Dey A, Mehta NN, Hasni S, Dell'Orso S, Gutierrez-Cruz G, Sun H-W, Kaplan MJ.. Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci USA 2019;116:25222–25228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Clemmensen SN, Bohr CT, Rorvig S, Glenthoj A, Mora-Jensen H, Cramer EP, Jacobsen LC, Larsen MT, Cowland JB, Tanassi JT, Heegaard NHH, Wren JD, Silahtaroglu A. N, Borregaard N.. Olfactomedin 4 defines a subset of human neutrophils. J Leukoc Biol 2012;91:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thålin C, Lundström S, Seignez C, Daleskog M, Lundström A, Henriksson P, Helleday T, Phillipson M, Wallén H, Demers M.. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One 2018;13:e0191231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rosell A, Aguilera K, Hisada Y, Schmedes C, Mackman N, Wallén H, Lundström S, Thålin C.. Prognostic value of circulating markers of neutrophil activation, neutrophil extracellular traps, coagulation and fibrinolysis in patients with terminal cancer. Sci Rep 2021;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Vallés J, Lago A, Santos MT, Latorre AM, Tembl JI, Salom JB, Nieves C, Moscardó A.. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb Haemost 2017;117:1919–1929. [DOI] [PubMed] [Google Scholar]
- 121. Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V, Bertos N, Cools-Lartigue J, Ferri LE, Spicer JD.. Primary tumors induce neutrophil extracellular traps with targetable metastasis-promoting effects. JCI Insight 2019;5:e128008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A.. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res 2016;76:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Moore S, Juo HH, Nielsen CT, Tyden H, Bengtsson AA, Lood C.. Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J Rheumatol 2020;47:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bach M, Moon J, Moore R, Pan T, Nelson JL, Lood C.. A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol 2020;72:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Cate H T, Hofstra L, Crijns HJ, Wagner DD, Kietselaer B.. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 2013;33:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rivera-Franco MM, Leon-Rodriguez E, Torres-Ruiz JJ, Gómez-Martín D, Angles-Cano E, la Luz Sevilla-González M. D.. Neutrophil extracellular traps associate with clinical stages in breast cancer. Pathol Oncol Res 2020;26:1781–1785. [DOI] [PubMed] [Google Scholar]
- 127. Bang OY, Chung JW, Cho YH, Oh MJ, Seo WK, Kim GM, Ahn MJ.. Circulating DNAs, a marker of neutrophil extracellular traposis and cancer-related stroke the Oasis-cancer study. Stroke 2019;50:2944–2947. [DOI] [PubMed] [Google Scholar]
- 128. Jin W, Xu H-X, Zhang S-R, Li H, Wang W-Q, Gao H-L, Wu C-T, Xu J-Z, Qi Z-H, Li S, Ni Q-X, Liu L, Yu X-J.. Tumor-infiltrating NETs predict postsurgical survival in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol 2019;26:635–643. [DOI] [PubMed] [Google Scholar]
- 129. Farkas ÁZ, Farkas VJ, Gubucz I, Szabó L, Bálint K, Tenekedjiev K, Nagy AI, Sótonyi P, Hidi L, Nagy Z, Szikora I, Merkely B, Kolev K.. Neutrophil extracellular traps in thrombi retrieved during interventional treatment of ischemic arterial diseases. Thromb Res 2019;175:46–52. [DOI] [PubMed] [Google Scholar]
- 130. Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, Simon D, Laimer D, Bangert C, Kammerlander A, Mascherbauer J, Winter MP, Distelmaier K, Adlbrecht C, Preissner KT, Lang IM.. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 2015;116:1182–1192. [DOI] [PubMed] [Google Scholar]
- 131. Ducroux C, Meglio L. D, Loyau S, Delbosc S, Boisseau W, Deschildre C, Maacha M. B, Blanc R, Redjem H, Ciccio G, Smajda S, Fahed R, Michel JB, Piotin M, Salomon L, Mazighi M, Ho-Tin-Noe B, Desilles JP.. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018;49:754–757. [DOI] [PubMed] [Google Scholar]
- 132. Abrams ST, Morton B, Alhamdi Y, Alsabani M, Lane S, Welters ID, Wang G, Toh C-H.. A novel assay for neutrophil extracellular trap formation independently predicts disseminated intravascular coagulation and mortality in critically ill patients. Am J Respir Crit Care Med 2019;200:869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang Z, Wang L, Cao C, Jin H, Zhang Y, Liu Y, Gao Y, Liang X, Li G, Shou S.. Heparin attenuates histone-mediated cytotoxicity in septic acute kidney injury. Front Med 2020;7:586652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wildhagen KCAA, Frutos P. D, Reutelingsperger CP, Schrijver R, Aresté C, Ortega-Gómez A, Deckers NM, Hemker HC, Soehnlein O, Nicolaes GAF.. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 2014;123:1098–1101. [DOI] [PubMed] [Google Scholar]
- 135. Li J, Sparkenbaugh EM, Su G, Zhang F, Xu Y, Xia K, He P, Baytas S, Pechauer S, Padmanabhan A, Linhardt RJ, Pawlinski R, Liu J.. Enzymatic synthesis of chondroitin sulfate E to attenuate bacteria lipopolysaccharide-induced organ damage. ACS Cent Sci 2020;6:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT.. Extracellular histones are major mediators of death in sepsis. Nat Med 2009;15:1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nakazawa D, Kumar S. V, Marschner J, Desai J, Holderied A, Rath L, Kraft F, Lei Y, Fukasawa Y, Moeckel GW, Angelotti ML, Liapis H, Anders HJ.. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol 2017;28:1753–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chirivi RGS, van Rosmalen JWG, van der Linden M, Euler M, Schmets G, Bogatkevich G, Kambas K, Hahn J, Braster Q, Soehnlein O, Hoffmann MH, Van Es HHG, Raats JMH.. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol Immunol 2021;18:1528–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. McDonald B, Davis R, Lewis H, Kubes P, Jenne C.. In vivo imaging of disseminated intravascular coagulation in sepsis reveals a pathological role for neutrophil extracellular traps (NETs). Chest 2015;148:188A. [Google Scholar]
- 140. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L'Allier PL, Guertin M-C, Roubille F.. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 141. Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, Vanwinge C, Cataldo D, Oury C, Delvenne P, Marichal T.. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med 2020;217:e20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Döring Y, Libby P, Soehnlein O.. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res 2020;126:1228–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD.. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA 2013;110:8674–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. von Brühl M-L, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Köllnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S.. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012;209:819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, Tsironidou V, Giatromanolaki A, Skendros P, Konstantinides S, Ritis K.. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J 2015;36:1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P.. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscl Thromb Vasc Biol 2018;38:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gould TJ, Lysov Z, Swystun LL, Dwivedi DJ, Zarychanski R, Fox-Robichaud AE, Liaw PC; Canadian Critical Care Translational Biology Group . Extracellular histones increase tissue factor activity and enhance thrombin generation by human blood monocytes. Shock 2016;46:655–662. [DOI] [PubMed] [Google Scholar]
- 148. Noubouossie DF, Whelihan MF, Yu Y. B, Sparkenbaugh E, Pawlinski R, Monroe DM, Key NS.. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood 2017;129:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yago T, Liu Z, Ahamed J, McEver RP.. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood 2018;132:1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Akopov SE, Simonian NA, Grigorian GS.. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke 1996;27:1739–1743. [DOI] [PubMed] [Google Scholar]
- 151. Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR.. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 1983;67:1016–1023. [DOI] [PubMed] [Google Scholar]
- 152. Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT.. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 2011;179:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT.. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 2012;7:e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Matsuzaki K. Membrane permeabilization mechanisms. Advances in Experimental Medicine and Biology. 2019;1117: p9–16. [DOI] [PubMed] [Google Scholar]
- 155. Xhindoli D, Pacor S, Benincasa M, Scocchi M, Gennaro R, Tossi A.. The human cathelicidin LL-37—a pore-forming antibacterial peptide and host-cell modulator. Biochim Biophys Acta 2016;1858:546–566. [DOI] [PubMed] [Google Scholar]
- 156. Baxter AA, Lay FT, Poon IKH, Kvansakul M, Hulett MD.. Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects. Cellular and Molecular Life Sciences. 2017; 74 p3809–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lichtenstein A, Ganz T, Selsted ME, Lehrer RI.. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood 1986;68:1407–1410. [PubMed] [Google Scholar]
- 158. Pereira LF, Marco FM, Boimorto R, Caturla A, Bustos A, Concha E. L, Subiza JL.. Histones interact with anionic phospholipids with high avidity; its relevance for the binding of histone-antihistone immune complexes. Clin Exp Immunol 1994;97:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Semeraro F, Ammollo CT, Esmon NL, Esmon CT.. Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J Thromb Haemost 2014;12:1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Koo YS, Kim JM, Park IY, Yu BJ, Jang SA, Kim KS, Park CB, Cho JH, Kim SC.. Structure-activity relations of parasin I, a histone H2A-derived antimicrobial peptide. Peptides 2008;29:1102–1108. [DOI] [PubMed] [Google Scholar]
- 161. Wichapong K, Silvestre-Roig C, Braster Q, Schumski A, Soehnlein O, Nicolaes GAF.. Structure-based peptide design targeting intrinsically disordered proteins: novel histone H4 and H2A peptidic inhibitors. Comput Struct Biotechnol J 2021;19:934–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Meara CHO, Coupland LA, Kordbacheh F, Quah BJC, Chang CW, Simon Davis DA, Bezos A, Browne AM, Freeman C, Hammill DJ, Chopra P, Pipa G, Madge PD, Gallant E, Segovis C, Dulhunty AF, Arnolda LF, Mitchell I, Khachigian LM, Stephens RW, Itzstein M. V, Parish CR.. Neutralizing the pathological effects of extracellular histones with small polyanions. Nat Commun 2020;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Binet F, Cagnone G, Crespo-Garcia S, Hata M, Neault M, Dejda A, Wilson AM, Buscarlet M, Mawambo GT, Howard JP, Diaz-Marin R, Parinot C, Guber V, Pilon F, Juneau R, Laflamme R, Sawchyn C, Boulay K, Leclerc S, Abu-Thuraia A, Côté J-F, Andelfinger G, Rezende FA, Sennlaub F, Joyal J-S, Mallette FA, Sapieha P.. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 2020;369:eaay5356. [DOI] [PubMed] [Google Scholar]
- 164. Paulin N, Viola JR, Maas SL, Jong R D, Fernandes-Alnemri T, Weber C, Drechsler M, Döring Y, Soehnlein O.. Double-strand DNA sensing Aim2 inflammasome regulates atherosclerotic plaque vulnerability. Circulation 2018;138:321–323. [DOI] [PubMed] [Google Scholar]
- 165. Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, Busch M, Manca M, Koenen RR, Pelisek J, Daemen MJ, Lutgens E, Zenke M, Binder CJ, Weber C, Zernecke A.. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012;125:1673–1683. [DOI] [PubMed] [Google Scholar]
- 166. Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P.. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol 2004;24:1309–1314. [DOI] [PubMed] [Google Scholar]
- 167. Morrow DA, Sabatine MS, Brennan ML, Lemos J. D, Murphy SA, Ruff CT, Rifai N, Cannon CP, Hazen SL.. Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur Heart J 2008;29:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Liew FY, Girard JP, Turnquist HR.. Interleukin-33 in health and disease. Nat Rev Immunol 2016;16:676–689. [DOI] [PubMed] [Google Scholar]
- 169. Clancy DM, Henry CM, Sullivan GP, Martin SJ.. Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines. FEBS J 2017;284:1712–1725. [DOI] [PubMed] [Google Scholar]
- 170. Badimon L, Vilahur G.. Neutrophil extracellular traps: a new source of tissue factor in atherothrombosis. Eur Heart J 2015;36:1364–1366. [DOI] [PubMed] [Google Scholar]
- 171. Partida RA, Libby P, Crea F, Jang IK.. Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur Heart J 2018;39:2070–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P.. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J 2015;36:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ferrante G, Nakano M, Prati F, Niccoli G, Mallus MT, Ramazzotti V, Montone RA, Kolodgie FD, Virmani R, Crea F.. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation 2010;122:2505–2513. [DOI] [PubMed] [Google Scholar]
- 174. Tan Y, Yang S, Chen R, Sheng Z, Zhou P, Liu C, Zhao H, Song L, Li J, Zhou J, Chen Y, Yan H.. High plasma myeloperoxidase is associated with plaque erosion in patients with ST-segment elevation myocardial infarction. J Cardiovasc Transl Res 2020;13:908–915. [DOI] [PubMed] [Google Scholar]
- 175. Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, Liu X, Tesmenitsky Y, Shvartz E, Sukhova GK, Michel JB, Nicoletti A, Lichtman A, Wagner D, Croce KJ, Libby P.. Roles of PAD4 and netosis in experimental atherosclerosis and arterial injury implications for superfcial erosion. Circ Res 2018;123:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Molinaro R, Yu M, Sausen G, Bichsel CA, Corbo C, Folco EJ, Lee GY, Liu Y, Tesmenitsky Y, Shvartz E, Sukhova GK, Kloss F, Croce KJ, Farokhzad OC, Shi J, Libby P.. Targeted delivery of protein arginine deiminase-4 inhibitors to limit arterial intimal NETosis and preserve endothelial integrity. Cardiovasc Res 2021;117:2652–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Savchenko AS, Borissoff JI, Martinod K, Meyer S. D, Gallant M, Erpenbeck L, Brill A, Wang Y, Wagner DD.. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 2014;123:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Bruschi M, Bonanni A, Petretto A, Vaglio A, Pratesi F, Santucci L, Migliorini P, Bertelli R, Galetti M, Belletti S, Cavagna L, Moroni G, Franceschini F, Fredi M, Pazzola G, Allegri L, Sinico RA, Pesce G, Bagnasco M, Manfredi A, Ramirez GA, Ramoino P, Bianchini P, Puppo F, Pupo F, Negrini S, Mattana F, Emmi G, Garibotto G, Santoro D, Scolari F, Ravelli A, Tincani A, Cravedi P, Volpi S, Candiano G, Ghiggeri GM.. Neutrophil extracellular traps profiles in patients with incident systemic lupus erythematosus and lupus nephritis. J Rheumatol 2020;47:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Söderberg D, Kurz T, Motamedi A, Hellmark T, Eriksson P, Segelmark M.. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology 2015;54:2085–2094. [DOI] [PubMed] [Google Scholar]
- 180. Hardisty GR, LLanwarne F, Minns D, Gillan JL, Davidson DJ, Findlay EG, Gray RD. Ultra-pure isolation of low density neutrophils casts doubt on their exceptionality in health and disease. bioRxiv2020;2020.06.17.156588. [DOI] [PMC free article] [PubMed]
- 181. Liu Y, Kaplan MJ.. Neutrophils in the pathogenesis of rheumatic diseases: fueling the fire. Clin Rev Allergy Immunol 2021;60:1–16. [DOI] [PubMed] [Google Scholar]
- 182. Yaniv G, Twig G, Shor DBA, Furer A, Sherer Y, Mozes O, Komisar O, Slonimsky E, Klang E, Lotan E, Welt M, Marai I, Shina A, Amital H, Shoenfeld Y.. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev 2015;14:75–79. [DOI] [PubMed] [Google Scholar]
- 183. Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, Liu Y, Bicker KL, Wahamaa H, Hoffmann V, Catrina AI, Thompson PR, Buckner JH, Robinson WH, Fox DA, Kaplan MJ.. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol 2017;2:eaag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tillack K, Breiden P, Martin R, Sospedra M.. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol 2012;188:3150–3159. [DOI] [PubMed] [Google Scholar]
- 185. Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M.. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011;3:73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Apel F, Andreeva L, Knackstedt LS, Streeck R, Frese CK, Goosmann C, Hopfner K-P, Zychlinsky A.. The cytosolic DNA sensor cGAS recognizes neutrophil extracellular traps. Sci Signal 2021;14:eaax7942. [DOI] [PubMed] [Google Scholar]
- 187. Kim J, Gupta R, Blanco LP, Yang S, Shteinfer-Kuzmine A, Wang K, Zhu J, Yoon HE, Wang X, Kerkhofs M, Kang H, Brown AL, Park SJ, Xu X, Rilland E. V, Kim MK, Cohen JI, Kaplan MJ, Shoshan-Barmatz V, Chung JH.. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 2019;366:1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Sule G, Abuaita BH, Steffes PA, Fernandes AT, Estes SK, Dobry CJ, Pandian D, Gudjonsson JE, Kahlenberg JM, O’Riordan MX, Knight JS.. Endoplasmic reticulum stress sensor IRE1α propels neutrophil hyperactivity in lupus. J Clin Investig 2021;131:e137866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Shaul ME, Fridlender ZG.. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol 2019;16:601–620. [DOI] [PubMed] [Google Scholar]
- 190. Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F, Liu J, Xing Y, Chen X, Su S, Song E.. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020;583:133–138. [DOI] [PubMed] [Google Scholar]
- 191. Wan X, Guo G, Wang H, Gu K, Zhang Y, Hu Y, Ma C, Sun H, Wei X, Li M, Wei W, Zhang F, Yang F.. Diagnostic, therapeutic predictive, and prognostic value of neutrophil extracellular traps in patients with gastric adenocarcinoma. Front Oncol 2020;1:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Masucci MT, Minopoli M, Vecchio S. D, Carriero MV.. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol 2020;11:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Yazdani HO, Roy E, Comerci AJ, Windt D V D, Zhang H, Huang H, Loughran P, Shiva S, Geller DA, Bartlett DL, Tsung A, Sheng T, Simmons RL, Tohme S.. Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res 2019;79:5626–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Windt D V D, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O’Doherty RM, Minervini MI, Huang H, Simmons RL, Tsung A.. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018;68:1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, Bružas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JTH, Egeblad M.. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018;361:6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Nozawa H, Chiu C, Hanahan D.. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA 2006;103:12493–12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L.. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Investig 2013;123:3446–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Rayes RF, Vourtzoumis P, Bou Rjeily M, Seth R, Bourdeau F, Giannias B, Berube J, Huang Y-H, Rousseau S, Camilleri-Broet S, Blumberg RS, Beauchemin N, Najmeh S, Cools-Lartigue J, Spicer JD, Ferri LE.. Neutrophil extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J Immunol 2020;204:2285–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Lee WJ, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H.. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med 2019;216:176–194. [DOI] [PMC free article] [PubMed] [Google Scholar]