ABSTRACT
Background: The situation in the world today, encompassing multiple armed conflicts, notably in Ukraine, the Coronavirus pandemic and the effects of climate change, increases the likelihood of childhood exposure to physical injury and pain. Other effects of these worldwide hardships include poverty, malnutrition and starvation, also bringing with them other forms of trauma, including emotional harm, neglect and deliberate maltreatment.
Objective: To review the neurobiology of the systems in the developing brain that are most affected by physical and emotional trauma and neglect.
Method: The review begins with those that mature first, such as the somatosensory system, progressing to structures that have a more protracted development, including those involved in cognition and emotional regulation. Explored next are developing stress response systems, especially the hypothalamic–pituitary–adrenal axis and its central regulator, corticotropin-releasing hormone. Also examined are reward and anti-reward systems and genetic versus environmental influences. The behavioural consequences of interpersonal childhood trauma, focusing on self-harm and suicide, are also surveyed briefly. Finally, pointers to effective treatment are proffered.
Results: The low-threshold nature of circuitry in the developing brain and lack of inhibitory connections therein result in heightened excitability, making the consequences of both physical and emotional trauma more intense. Sensitive and critical periods in the development of structures such as the amygdala render the nervous system more vulnerable to insults occurring at those points, increasing the likelihood of psychiatric disorders, culminating in self-harm and even suicide.
Conclusion: In view of the greater excitability of the developing nervous system, and its vulnerability to physical and psychological injuries, the review ends with an exhortation to consider the long-term consequences of childhood trauma, often underestimated or missed altogether when faced with adults suffering mental health problems.
KEYWORDS: Development, childhood, neurobiology, pain, trauma, neglect
HIGHLIGHTS
Circumstances in the world today, including armed conflicts, the Coronavirus pandemic, climate change, famine and the worsening economic situation, increase the likelihood that children of all ages are exposed to multiple traumas, including physical as well as emotional pain.
Trauma of whatever kind in childhood has a greater impact than that occurring in adulthood, due to greater excitability and a lack of inhibition in the developing brain and nervous system.
If the trauma occurs during ‘sensitive’ or ‘critical’ periods of certain structures and systems, the potential for long-term damage and its behavioural consequences, including self-harm and even suicide, is heightened.
Abstract
Antecedentes: La situación en el mundo actual, que abarca múltiples conflictos armados, mayormente en Ucrania, la pandemia del Coronavirus y los efectos del cambio climático, aumenta la probabilidad de exposición infantil a lesiones físicas y dolor. Otros efectos de estas dificultades mundiales incluyen pobreza, desnutrición y hambruna, también traen consigo otras formas de trauma, incluyendo daño emocional, negligencia y maltrato deliberado.
Objetivo: Revisar la neurobiología de los sistemas en el cerebro en desarrollo que se ven más afectados por el trauma físico y emocional y la negligencia.
Método: La revisión comienza con aquellos que maduran primero, como el sistema somatosensorial, progresando hacia estructuras que tienen un desarrollo más prolongado, incluidas las involucradas en la cognición y regulación emocional. A continuación se exploraron los sistemas de respuesta al estrés en desarrollo, especialmente el eje hipotálamo-hipófisis-adrenal y su regulador central, la hormona liberadora de corticotrofina. También se examinaron los sistemas de recompensa y anti-recompensa e influencias genéticas versus ambientales. Tambien se investigaron brevemente, las consecuencias conductuales del trauma infantil interpersonal, centrándose en las autolesiones y suicidio. Finalmente, se ofrecieron indicaciones para un tratamiento eficaz.
Resultados: La naturaleza de bajo umbral de los circuitos en el cerebro en desarrollo y la falta de conexiones inhibitorias en ellos dan como resultado una mayor excitabilidad, lo que hace que las consecuencias del trauma físico y emocional sean más intensas. Los periodos críticos y sensibles en el desarrollo de estructuras como la amígdala hacen que el sistema nervioso sea más vulnerable a las agresiones que ocurren en esos puntos, lo que aumenta la probabilidad de trastornos psiquiátricos, culminando en autolesiones e incluso suicidio.
Conclusión: En vista de la mayor excitabilidad del sistema nervioso en desarrollo y su vulnerabilidad a las lesiones físicas y psicológicas, la revisión termina con una exhortación a considerar las consecuencias a largo plazo del trauma infantil, con frecuencia subestimado o ignorado totalmente cuando nos enfrentamos con adultos que sufren de problemas de salud mental.
PALABRAS CLAVE: Desarrollo, infancia, neurobiología, dolor, trauma, negligencia
Abstract
背景:当今世界的局势,包括多种武装冲突,尤其在乌克兰,冠状病毒疫情和气候改变的影响,增加了童年期身体伤害和疼痛暴露的可能性。这些全球性困难的其他影响包括贫困、营养不良和饥饿,还带来其他形式的创伤,包括情感伤害、忽视和蓄意虐待。
目的:综述受身体和情感创伤和忽视影响最大的发育中大脑系统神经生物学。
方法:综述从先成熟的结构开始,例如体感系统,然后发展到发育更持久的结构,包括涉及认知和情绪调节的结构。接下来考查应激反应发育系统,尤其是下丘脑-垂体-肾上腺轴及其中枢调节剂促肾上腺皮质激素释放激素。还考查了奖赏和反奖赏系统以及遗传与环境的影响。还简单调查了童年期人际创伤的行为后果,重点是自伤和自杀。最后,提出了有效治疗的建议。
结果:发育中的大脑环路的低阈值性质和缺乏抑制性连接导致兴奋性增强,使身体和情感创伤的后果更加强烈。杏仁核等结构发育的敏感和关键阶段使神经系统对于这些部位的损伤更易感,从而增加了患上精神障碍的可能性,最终导致自残甚至自杀。
结论:鉴于发育中的神经系统具有更大的兴奋性,以及其对身体和心理伤害的易感性,本综述最终建议考虑童年期创伤的长期后果,当面对遭受心理健康问题的成年人时,这些创伤常常被低估或完全被忽略。
关键词: 发育, 童年, 神经生物学, 疼痛, 创伤, 忽视
Introduction
By the middle of July 2022, almost 5 months since the Russian invasion of Ukraine on 24 February, 6.1 million people had fled the country, around half of them children, with 6.2 million internally displaced (UNICEF, n.d.), and hundreds injured and killed. The war has had a severely traumatising effect on children of all ages, involving both physical pain and emotional harm. The knock-on effects, not only of conflicts but also of the Coronavirus pandemic and worldwide economic downturn have widened already deepening inequalities. Increasing incidence of socioeconomic deprivation, including destitution, is impacting the lives of children around the world, rendering them and their families susceptible to trauma-related conditions including intense anxiety, depression and post-traumatic stress disorder (PTSD). In the UK, open referrals to NHS child and adolescent mental health services reached their highest ever monthly total, around 400,000, in February 2022 (NHS Digital, Mental Health Services Monthly Statistics, n.d.).
Potent factors in childhood vulnerability are the differing properties of the nervous system as it is developing, intensifying the impact of adverse and stressful events and increasing susceptibility to mental and physical health problems later in life. For example, the immature sensory system behaves in a different way to that of the older child and adult. It is a much more excitable, low-threshold system and stimuli, whether painful or not, have a much greater effect than they would at later stages of maturation (Andrews et al., 2002; Andrews & Fitzgerald, 1999). The continued proliferation of neuronal cells and development of neural pathways during the neonatal period makes it a time of particular vulnerability (Graham et al., 1999).
The developing nervous system: long-term effects of untreated pain and trauma
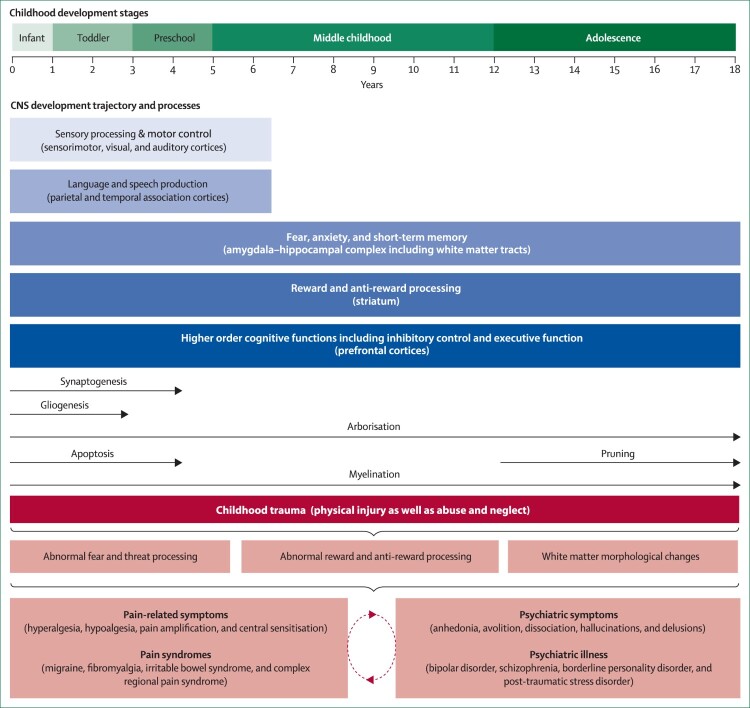
Scrutiny of the literature on developmental progression across different brain structures reveals that this is a process that is not uniformly linear (Shaw et al., 2008). Areas involved in sensation and some aspects of motor control mature earlier in development, since these are vital for immediate survival, whereas the neural substrates for cognition and emotional regulation, more complex functions, take longer to mature (Greenough et al., 1987). Nevertheless, common themes emerge during normal maturation, such as the pruning of exuberant synapses and development of inhibitory connections (Huttenlocher et al., 1982; LaMantia & Rakic, 1994; Petanjek et al., 2008) (see Figure 1).
Figure 1.
Childhood trauma and its effects on biobehavioural systems implicated in pain and psychiatric disorders During development, distinct neurological systems mature at different rates, accompanied by changes in neurobiological mechanisms at the molecular or cellular level. Throughout the neurodevelopmental trajectory, and as a result of ongoing CNS maturation processes, children are arguably more vulnerable to the unfortunate and negative impact of childhood trauma, maltreatment in particular. In the developing CNS, maltreatment is hypothesised to interfere especially with systems that regulate threat detection, fear processing, reward and anti-reward mechanisms, and other fundamental neurological properties (e.g. white matter integrity). Over time, the impediment of normal neurodevelopmental process stemming from childhood trauma might commonly facilitate abnormal pain and somatosensory processing, psychiatric symptoms and illnesses, and complex comorbid states. (Adapted from Cay et al., 2022; Govindan et al., 2010 with kind permission of the authors).
Although considerable plasticity occurs during normal brain development (Stiles, 2017), a wide range of neuropathological events can affect developmental trajectories. The immature brain adapts to the effects of the neural insult, but adaptation is not always benign and can produce long-term adverse consequences. For example, untreated pain in infancy, from whatever origin, is one type of stressful event that can affect the developing brain, potentially causing long-lasting damage. Although cortical responses to pain are measurable in the youngest preterm infant (Slater et al., 2006), and the neural circuits in the brain necessary for discrimination between touch and pain are formed near the end of the gestation period in humans (Fabrizi et al., 2011), inhibitory connections that dampen down the response to pain take longer to develop (Fitzgerald & Koltzenburg, 1986; Fitzgerald & Walker, 2009), meaning that repeated procedural interventions, such as on preterm infants during neonatal intensive care, surgery, or complications such as infection, can all contribute to the development of persistent hyperalgesia or chronic pain (Fitzgerald et al., 1989). Women from deprived areas are more likely to give birth to a very preterm baby than those from more affluent areas, compounding the risk of childhood trauma through social and economic disadvantage (Smith et al., 2009).
In adults, noxious or tissue-damaging stimuli and the consequent release of inflammatory molecules activate and sensitise nerve endings responsive to pain (termed ‘nociceptors’) in the damaged area. These send a barrage of signals into the central nervous system (CNS) (Hucho & Levine, 2007; Woolf & Ma, 2007), which in turn excites central nociceptive circuits in the spinal cord, brainstem, thalamus and other brain areas including the somatosensory cortex, cingulate cortex and the amygdala (Price, 2002; Tracey & Mantyh, 2007). Evidence suggests that there is substantial overlap between brain circuits that mediate the emotional distress seen in PTSD and those involving physiological threat, including pain. The resulting increased sensitivity, termed ‘central sensitisation’, is a common feature of both conditions (McFarlane, 2010). Neurobiological activators and modulators of these circuits are contributors both to PTSD symptoms and chronic pain. For example, reduced levels of neurotransmitters that have anti-stress and anti-nociceptive properties, including neuropeptide Y, allopregnanolone and pregnanolone, have been implicated in the pathophysiology of both PTSD, including that from childhood abuse (Seedat et al., 2003), and chronic pain (Scioli-Salter et al., 2015).
Although many of the brain regions and the connections between them that are affected by central sensitisation are working even in newborns, they undergo great structural and functional changes in the postnatal period and early years. This means that noxious stimulation does not produce the same pattern of activity as it does in the adult CNS (Baccei & Fitzgerald, 2006; Fitzgerald, 2005). Immaturity both of synaptic connections and neuronal circuitry in infancy produce a much more diffuse and less spatially localised experience of pain than in adults (Jones et al., 2022), but it may also be more powerful due to the lack of endogenous inhibitory control from higher CNS centres (Fitzgerald & Koltzenburg, 1986). The fine balance between excitation and inhibition, seen in structures such as the amygdala in adulthood (Marek et al., 2013), likely doesn't exist to the same degree in childhood meaning that insults such as tissue damage produce long-term changes (Abdulkader et al., 2008; Hermann et al., 2006).
This has been illustrated in studies examining the effects in later childhood (9-16 years) of burn injuries sustained during infancy (6-24 months). Compared to age-matched controls, both moderately and severely burned children show altered sensory and pain sensitivity, including greater sensitisation to repetitive mechanical stimuli, indicating that early traumatic and painful injuries such as burns can induce whole body, long-term changes in sensory and pain processing (Wollgarten-Hadamek et al., 2009). Furthermore, children who suffer pain and stress exposure due to severe burns in infancy demonstrate impaired functioning of descending pain inhibition, possibly heralding increased susceptibility to chronic pain problems in later life (Wollgarten-Hadamek et al., 2011). This is also true of other types of childhood trauma, causing an attentional bias towards physical rather than emotional pain and potentially prompting self-injurious behaviours that might relieve affective pain in conditions such as borderline personality disorder (Lane et al., 2018).
Early life stress (ELS), whether physical or emotional, not only increases predisposition to chronic pain in later life, but also to inflammation. Indeed, even in maltreated children who developed depression by 12 years, levels of inflammation were already elevated compared to controls (Danese et al., 2011). This has a knock-on effect, increasing the risk for depression and psychosis in young adulthood (Khandaker et al., 2014), and for other psychiatric disorders later, including depression, bipolar disorder, schizophrenia and PTSD (Danese & Lewis, 2017). One possible mechanism by which this occurs is that early-life immune activation can also cross-sensitise the neuroendocrine response to stress. Experimental studies have shown that, like psychosocial stress exposure, administration of interleukin-1 (IL-1) and to a lesser extent IL-6 and TNF-α can trigger hypothalamic–pituitary–adrenal (HPA) axis and central catecholamine activation (Besedovsky et al., 1986; Dunn, 2000).
Quantitative sensory testing studies in adult patients indicate that childhood trauma is also predictive of chronicity of low back pain and enhanced pressure pain sensitivity (Tesarz et al., 2015). Furthermore, early life stressors such as emotional and sexual abuse are associated with enhanced temporal summation of pain and heightened touch sensitivity (Tesarz et al., 2016). These phenomena are indicative of somatosensory hyperreactivity or hyperalgesia, which may result from hypervigilance and anxiety arising from childhood maltreatment. However, childhood trauma can also produce diminished pain sensitivity or hypoalgesia, commonly seen in schizophrenia (Stubbs et al., 2015), borderline personality disorder (Defrin et al., 2020) and PTSD (Defrin et al., 2015). This may occur because of avoidance, dissociative or deficit symptoms consequent to childhood maltreatment, especially neglect (Cay et al., 2022).
Thus, it isn't only what is done to children that has a lasting effect, but also what is not done to nurture them. Childhood neglect may be more damaging to the developing brain and stress systems than adversity in adulthood (De Bellis, 2010; Nelson et al., 2019). Compromised maternal care, including neglect, inconsistency and lack of sensitivity, is a significant contributor to ELS (Nelson et al., 2011; Sheridan & McLaughlin, 2014), resulting in increased numbers and function of excitatory synapses upon stress-sensitive neurons in the hypothalamus (Gunn et al., 2013), a critical structure in coordinating the autonomic response to stress as part of the HPA. This increased excitatory input may sensitise the central components of the neuroendocrine stress system to subsequent stress (Chen & Baram, 2016). Smaller intracranial volume, reduced hemispheric integration and a smaller corpus callosum constitute other neural correlates of deprivation and neglect (De Bellis et al., 1999; Noble et al., 2005; Teicher et al., 2004). Structural changes include stunting, atrophy or hypertrophy of dendritic arborisation and altered synapse number and function (Ivy et al., 2010). The resulting neural abnormalities observed may underpin the difficulties with emotional regulation seen in neglected children. Regardless of whether they have PTSD, these children have been found to score significantly lower on IQ than non-traumatised controls. Disruptions are observed also in neural systems involved in language, visuospatial processing and attention (Bick & Nelson, 2016).
Derailment of brain circuits mediating inhibitory control underlies numerous psychiatric and behavioural disorders, many of which are manifested during adolescence. Such disruption has been shown to be predictive of future substance abuse problems (Romer Thomsen et al., 2018). Structures involved in inhibitory control, the frontal lobes and striatum, including the caudate nuclei, areas whose function is impaired in PTSD, are not yet well developed by late adolescence and under normal circumstances demonstrate significant changes from that time to young adulthood, including increased functional connectivity between them (Wang et al., 2021).
However, this process can be disrupted by abuses that are relatively common. For example, chronic peer victimisation during adolescence has a detrimental effect on striatal structures such as the left caudate nucleus and the putamen, which have been shown to decrease in volume as a result, this being associated with generalised anxiety (Quinlan et al., 2020). A 2019 study published in Lancet Psychiatry, using a population-representative birth cohort of 2,232 children born in England and Wales in 1994-1995, revealed a prevalence of trauma exposure of 31.1% in 2,064 participants. The prevalence of PTSD in the same sample was 7.8% by age 18 years. They also reported that the risk of developing PTSD was greatest after ‘direct interpersonal index traumas’, including bullying by peers (Lewis et al., 2019). Furthermore, verbal bullying has long been associated with major depressive disorder (MDD) and suicidal ideation (Van der Wal et al., 2003), especially in females aged 14 years exposed to peer emotional abuse (Khan et al., 2015).
Studies of the effects of ELS on brain development have found that higher-order, complex cognitive and affective functions associated with brain regions that undergo protracted postnatal development are especially vulnerable to the deleterious effects of ELS. Several deficits, particularly those in the affective domain, persist for years after ELS has ceased and may increase the risk for future psychopathology (Pechtel & Pizzagalli, 2011). The 2010 US National Comorbidity Survey disclosed that adverse childhood experiences (ACEs) explained nearly 32% of adult psychiatric disorders and 44% of disorders with childhood onset, as well as 67% of the population-attributable risk for suicide (Green et al., 2010).
The importance of ‘sensitive periods’ in development
Studies on sensitive periods have revealed that the adverse effects of ELS are greatest in those brain regions undergoing growth spurts at the time (Teicher, Samson, et al., 2006; Teicher, Tomoda, et al., 2006). For example, the amygdala is particularly sensitive to early ELS. Its basic cytoarchitecture and function are present at birth and it develops rapidly until 4 years of age, after which it grows more slowly, attaining its maximum volume between 9 and 11 years of age (Uematsu et al., 2012). This early development renders it more reactive in childhood than in adulthood, and due to its central role in learning and responding to social and emotional stimuli, including facial expressions, disruptions in its developmental trajectory may sensitise it to emotional threat and contribute to psychopathology, especially anxiety (Juranek et al., 2006). A recent review of functional magnetic resonance imaging studies indicates that ELS is related to heightened bilateral amygdala reactivity to sad faces but not to other facial emotions. The same review also examined behavioural studies and found faster reaction times to angry and sad faces and lower accuracy rates to fearful and happy faces, especially in those who had been exposed to early adversity prior to the age of 3 years (Saarinen et al., 2021).
Studies involving institutionalised but later adopted children have revealed greater right amygdala volume in these children compared with controls (Mehta et al., 2009). A study of adults recruited from a 30-year longitudinal sample who had been followed from infancy and who had experienced significant levels of childhood adversity found a dose–response relationship between severity of exposure at 10–11 years of age and right amygdala volume in adulthood. The types of harm that were associated with larger volume of this structure included parental verbal abuse, physical maltreatment, non-verbal emotional abuse, witnessing sibling assault and peer verbal abuse (Pechtel et al., 2014). The amygdala also seems more resistant to changes in environment, such as removal from the adverse situation (Lupien et al., 2009), than other brain regions, which may contribute to the persistence of emotional problems in institutionalised children despite improvements in their circumstances (Tottenham et al., 2010).
Sexual abuse in females between the ages of 3 and 5 years (and to some extent between 11 and 13) is related to smaller hippocampal volume, whereas sexual abuse occurring between the ages of 9–10 and 11–14 years is linked to dysfunctions in the corpus callosum and prefrontal cortex respectively, all areas implicated in psychiatric disorders (Andersen et al., 2008). The cerebellum is another region with a prolonged developmental trajectory (Anderson et al., 2002; Gogtay & Thompson, 2010), leaving it vulnerable to long-term stress exposure and altered cytoarchitecture, thus interfering with later developing cognitive functions such as planning (Bauer et al., 2009), learning and working memory (Tiemeier et al., 2010).
Among the last areas to develop are higher-order structures containing complex association sites, such as those of the frontal lobe (e.g. dorsolateral prefrontal cortex) involved in executive functioning, attention and motor coordination, as well as the orbitofrontal cortex, temporal lobes and superior parietal lobes, which are linked to complex skills such as decision-making, executive functioning and inhibition (Gogtay et al., 2004; Molnar et al., 2019). Development of executive functions is thought to coincide with growth spurts in the maturation of the frontal cortex, the critical periods being between birth and 2 years, 7–9 years, during adolescence and continuing into the third decade of life (Jurado & Rosselli, 2007; Marsh et al., 2008).
As might be expected, ELS onset at older ages of youth (8–17 years) is associated with volumetric reductions in different brain regions, notably the anterior cingulate cortex (ACC) and insula, which were not seen in younger children. This may reflect the advent of more fully developed emotional processing of ELS in older children, implicating these areas in neuropsychiatric disorders and emotional regulation (Baker et al., 2013).
A meta-analysis of several studies investigated the impact of childhood trauma on the integrity of white matter tracts in children with PTSD as compared to healthy controls (Daniels et al., 2013). The majority of these studies focused on the largest fibre bundle, the corpus callosum. An increase in its growth occurs from 6–13 years as language and associative memory functions increase in importance, but it continues to increase in size until 29 years of age. Significant reductions in area and volume were found in those children with PTSD, with boys showing greater reductions than girls. The authors also analysed studies that examined white matter integrity in adults with a history of childhood trauma, finding that compromised connections were carried into adulthood and were correlated with depression, anxiety, anger, dissociation, somatisation and what they termed raised ‘limbic irritability’ scores. Similar reductions in white matter volume have also been noted in the cingulum bundle, the most prominent white matter tract in the limbic system (Choi et al., 2008), in a sample of young adults exposed to childhood trauma, including parental verbal abuse.
The integrity of important white matter tracts that provide connection between limbic structures such as the amygdala and frontal lobe regions including the ACC, including the left uncinate fasciculus (Eluvathingal et al., 2006), is clearly disrupted in childhood trauma exposed individuals (Govindan et al., 2010). This may contribute to impairments in threat and fear processing observed in PTSD, linked to dysfunction of prefrontal inhibition of amygdala fear-conditioning provided by structures such as the ACC (O’Doherty et al., 2018).
The effects of childhood poverty and the chronic stress thus engendered have been shown to affect adult neural activity, but here again, the stage and duration of the hardship experienced is important. In a longitudinal study, adults with lower family income aged 9 exhibited reduced ventrolateral and dorsolateral prefrontal cortex activity and failure to suppress amygdala activation during effortful regulation of negative emotion at age 24. The relationship between family income at 9 years and prefrontal cortex activity at 24 years was mediated by chronic stressor exposure across childhood (measured at ages 9, 13 and 17) (Kim et al., 2013). Low socioeconomic status in childhood has also been associated with impaired cognitive functioning, including deficits in sustained attention and executive functioning (Evans & Schamberg, 2009; Noble et al., 2007).
Stress response systems
Stress involves the activation of the HPA axis, and the resulting cascade of neurotransmitters causes the release of glucocorticoids throughout the brain and body. Brain regions with a high density of glucocorticoid receptors, such as the prefrontal cortex and hippocampus, are particularly susceptible to such disturbances, which can impair neural plasticity (Gunnar & Quevedo, 2007), thus sabotaging the potential for recovery.
Like the amygdala, the hippocampus is a heterogeneous structure, sub-regions being responsible for different functions and developing at varying rates (Gogtay et al., 2006). In adults who have experienced ELS, a common although not universal finding is a reduction in overall volume of the hippocampus (Logue et al., 2018). Since this structure is particularly rich in glucocorticoid receptors, increased receptor binding caused by elevated levels of corticotropin-releasing hormone (CRH) and glucocorticoids such as cortisol likely has a negative impact on cell growth, causing delayed or reduced dendritic branching and cell loss (Brunson et al., 2001; Sapolsky, 1996). Increased CRH expression has been found in both the hippocampus (Zhou & Fang, 2018), and amygdala (Dubé et al., 2015), after ELS.
Furthermore, persistent changes in CRH neurotransmission and alterations in other neurotransmitter systems implicated in the regulation of stress responses may mediate the relationship between ELS and the development of major depression and anxiety disorders (McCrory et al., 2011). Elevated levels of CRH in cerebrospinal fluid (CSF) are also found in individuals who have experienced intense ELS (Carpenter et al., 2004), and in those with PTSD (Bremner et al., 1997), although surprisingly, they tend to have lower glucocorticoid levels than people without PTSD (Mason et al., 1986). Low levels of glucocorticoids are especially prevalent among those with PTSD who have also experienced ELS (Raglan et al., 2017).
Reward and anti-reward systems
Reward and its opposite, anti-reward, are mediated by the mesocorticolimbic and nigrostriatal dopamine systems. These overlap substantially with the opioid system and have been implicated in acute as well as chronic pain states and analgesia (Navratilova & Porreca, 2014; Upadhyay et al., 2018). They are also integrated with brain areas regulating both emotional condition via the serotonergic system and attention via noradrenaline circuitry (see ref. Govindan et al., 2010 for review).
Individuals exposed to ELS have demonstrated hyporesponsiveness within dopaminergic systems that lasts well into adulthood (Boecker et al., 2014). Conversely, other investigators have found elevated striatal dopamine function in young adults who had experienced severe physical or sexual abuse in childhood compared to those who had not, which may also increase the risk for later psychotic illness, including schizotypy and schizophrenia (Egerton et al., 2016). Prior to that, in adolescence, dysregulation of reward networks caused by emotional neglect predicts the emergence of depressive symptoms (Hanson et al., 2015). Adults with MDD display increased levels of anhedonia (inability to feel pleasure) compared with adults with little or no childhood trauma (Fan et al., 2021). Impairments in reward and anti-reward systems in those with schizophrenia as well as MDD may also produce apathy, avolition and dissociation alongside anhedonia, the latter also possibly being a mechanism by which psychiatric illnesses and maybe chronic pain states increase in severity (Borsini et al., 2020). Furthermore, PTSD has been shown to be a risk factor for complex regional pain syndrome in both children and adults (Speck et al., 2017), indicative of maladaptive mechanisms affecting reward or anti-reward systems accompanying threat and fear processing.
Along with its central role in nociception and analgesia, the µ-opioid system plays a key role in modulating mood and well-being, as well as addictive behaviours, and is an essential element in the reward network (Le Merrer et al., 2009). Unsurprisingly perhaps, its disruption by ELS renders it a risk factor for opioid dependence in adulthood. In a case–control study of a US community-based sample, opioid-dependent males had a higher prevalence of physical and emotional abuse, whereas female subjects had a higher prevalence and greater severity of sexual abuse. Early parental separation was also a factor among female cases. The prevalence of childhood neglect was similar for both cases and controls (Conroy et al., 2009). The authors of this study postulate that the mechanism by which ELS and substance dependence may be related is through disruption of self-system processes leading to problems in impulse control, affect regulation and threat appraisal.
Genetic versus environmental factors relating to childhood trauma
Adverse early experiences can exert their effects at an even more fundamental level, inducing changes in gene expression through epigenetic mechanisms, thus altering stress reactivity, brain function and behaviour, and predisposing to later health problems (Turecki et al., 2014).
Much of the research on genetic effects has been investigated in animal studies, particularly those conducted on rats. These have shown that differences in maternal care, as measured by licking and grooming, produce stable expression changes in genes vital for key behavioural and stress responses. Offspring of mothers who provided low levels of licking and grooming, as opposed to those of mothers who provided high levels of these behaviours, were found to have lifelong increased stress reactivity and anxiety-like behaviours (Liu et al., 1997). Cross-fostering studies have verified that these phenomena are due to variations in maternal care, not to inherited characteristics (Weaver, Cervoni, et al., 2004).
Interestingly, postnatal handling of neonatal rats, whereby they are separated from their mothers for a short (3–15 min) daily period in the first few weeks of life results in decreased stress reactivity in adulthood. This and other environmental circumstances in early life may alter development of glucocorticoid receptor gene expression in the hippocampus and HPA responses to acute or chronic stress. These involve an activation of ascending serotonergic pathways and subsequent changes in the expression of transcription factors that might influence hippocampal glucocorticoid receptor expression (Weaver, Diorio, et al., 2004).
The complex relationship between genetic polymorphisms and environmental influences has been examined in rhesus monkey neonates, in which a length variation in the serotonin (5-HT) transporter gene regulatory region (5-HTTLPR) has been identified that is homologous to a human polymorphism. Animals that possessed this variation demonstrated increased struggling, were less easy to console and manifested greater emotional distress than those that did not, whether mother-reared or nursery-reared. But nursery-reared, not mother-reared, infants with this polymorphism exhibited lower orientation scores (ability to interact with their environment), compared to those without it. It is possible that mothers exerted protective or compensatory effects for their offspring, thus mitigating the effects of genotypic characteristics (Champoux et al., 2002).
The intergenerational effects of maternal abuse have also been investigated in rhesus monkey infants. Those who were exposed to higher levels of maternal rejection in their first six months of life exhibited significantly lower CSF concentrations of serotonin and dopamine metabolites in the first three years of life compared to those who were exposed to lower levels of maternal rejection. This phenomenon was also observed in highly rejected infants who were cross-fostered and reared by unrelated mothers. Females who were abused by their mothers and became abusive mothers themselves also had lower CSF concentrations of a serotonin metabolite than did abused females who did not exhibit abusive parenting. The authors of this study postulate that these findings result from early experience, not from genetic similarities between mothers and offspring (Maestripieri et al., 2006). Related to this, it has been suggested that preconception trauma in parents may produce epigenetic changes affecting the germline and impacting fetoplacental interactions, resulting in intergenerational transmission of trauma consequences. A comprehensive review indicates that this process is influenced by several factors, including gender-specific epigenetic effects following trauma and parental developmental stage at the time of exposure (Yehuda & Lehrner, 2018).
Childhood abuse has also been shown to induce epigenetic changes in hippocampal neurons in humans. Using postmortem brain from suicide victims, investigators observed a methylation pattern involving glucocorticoid receptors in those with documented histories of childhood abuse that was not observed in suicide victims who had not undergone childhood abuse. Such methylation results in decreased glucocorticoid receptor expression and therefore a potentially increased stress response (McGowan et al., 2009). Epigenetic processes are implicated in the regulation of several other biological pathways and may mediate the interaction between environmental exposure and genetic polymorphisms on the risk of developing stress related psychiatric disorders (Klengel et al., 2013).
Risk of abnormal behaviour including self-harm and suicide
Childhood trauma dramatically increases the risk for later suicide attempts (Heim et al., 2010). A landmark Center for Disease Control study back in 2001 reported an increased risk of suicide attempts throughout the life span among primary care patients with histories of childhood abuse. ACEs in any category increased the risk of attempted suicide two- to fivefold (Dube et al., 2001). A 2015 investigation found that sexual abuse at 18 years in females was the strongest predictor of suicidal ideation in the study sample (see ref. Khandaker et al., 2014). The 2019 Lancet Psychiatry study reported a prevalence of 25% for self-harm and 8.3% for suicide attempt since age 12 years. A more recent large Australian study of men recently transitioned from regular military service demonstrated clearly that childhood interpersonal trauma (including sexual assault, rape, physical or emotional abuse, stalking or kidnap) and childhood-onset anxiety had ‘a direct and significant association with past-year suicidality.’ (Syed Sherriff et al., 2020)
The conclusion from numerous studies is that adolescents and adults who have experienced ELS demonstrate increased tendency to maladaptive behaviours such as eating disorders, substance abuse, alcohol dependence, high risk sexual behaviours or disorders, dissociation and self-harm or suicide (Teicher et al., 2010).
Considerations for treatment
What follows is not a comprehensive review of treatment options but merely pointers to consider when embarking on therapeutic interventions. A significant problem is that any therapy for individuals who suffer severe sequelae from early-life trauma has the potential to re-traumatise them through resensitisation both to the trauma events and to the traumatic stimuli that act as triggers. Therefore the treatment should progress at a rate with which the survivor can cope, particularly any exposure element, and be tailored to the person's specific experiences and clinical states (Craighead & Craighead, 2003). This means that longer-term rather than acute treatment is what is needed (Craighead & Nemeroff, 2005).
In this context, the priority in establishing a therapeutic relationship with someone who has been exposed to childhood trauma is to build an alliance around trust and safety. An effective tool for building on such a base is Schema Focused Therapy, which allows the sufferer to cultivate or reinstate fundamental beliefs that others can be trusted, that the world can be safe and that social and interpersonal relationships can be maintained (Young & Klosko, 2003). To modify a person's underlying schema in this way is a process that usually takes several months.
Therapy programmes that address behavioural, emotional, cognitive and neurobiological dysfunction concurrently offer the greatest opportunities for success (Stevenson, 1999). Incorporating an awareness of the developmental stage at which any trauma occurred and therefore the brain regions and functions likely to be affected by it may also offer greater possibility of resolution.
Another essential element of helping an individual to deal with the emotional dysregulation resulting from early life trauma is to help that person to learn better emotional regulation. Interventions such as Emotion Focused Therapy, which helps survivors to access and modify trauma memories to address fear, shame, anger and sadness (Greenberg & Paivio, 1997), and those that utilise mindfulness, all help to improve emotional regulation, but they also require the development of a strong therapeutic alliance and time for the internalisation of emotional control to develop (Segal et al., 2002).
A different therapeutic approach is to help survivors to ‘tune in’ to the somatic sensations that accompanied the trauma and its aftermath, since words may be inadequate to fully describe emotions indicative of their inner state, especially if the trauma occurred when they were pre – or barely verbal. Once again though, this should be done in a very subtle and sometimes indirect way in order not to precipitate distress through recollection of the traumatic event(s), so that words can be used to describe bodily sensations rather emotional states, aiming to increase understanding and gain control over the emotional perturbations engendered by the trauma (Pain et al., 2010).
Conclusion
The far-reaching effects of trauma of all kinds and throughout all stages of childhood and adolescence continue to have vital contemporary relevance, whether considering the plight of children caught up in armed conflicts or the recruitment of minors into military service, especially those from disadvantaged areas, as happens in the UK (Abu-Hayyeh & Singh, 2019). Investigators have noted that: ‘The original traumatic pathophysiological insults may be ‘silent’ until much later in life, when they are likely to be overlooked by investigators and clinicians who are understandably prone to focus on proximate determinants of human well-being’ (Anda et al., 2006). The seriousness of this cannot be overstated: these changes are not short-term or easily corrected, and their effects may well be lifelong unless effective strategies for recovery are offered and implemented for those affected.
Acknowledgements
Some of the information included in this paper was submitted to the Australian Royal Commission into Defence and Veteran Suicide, in which the author was invited to participate and in which she is still involved. Grateful thanks for reading and commenting on the manuscript at various stages are due to Sandy McFarlane, Professor Emeritus of Psychiatry and former Director, Centre for Traumatic Stress Studies, University of Adelaide, and to Walter Busuttil, Professor Institute of Psychiatry, Psychology and Neurosciences Kings College London KCMHR, Consultant Psychiatrist and Director of Research and Training Combat Stress.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data included in this manuscript is cited fully in the references section at the end.
References
- Abdulkader, H. M., Freer, Y., Garry, E. M., Fleetwood-Walker, S. M., & McIntosh, N. (2008). Prematurity and neonatal noxious events exert lasting effects on infant pain behaviour. Early Human Development, 84(6), 351–355. 10.1016/j.earlhumdev.2007.09.018 [DOI] [PubMed] [Google Scholar]
- Abu-Hayyeh, R., & Singh, G. (2019). Adverse health effects of recruiting child soldiers. BMJ Paediatrics Open, 3(1), e000325. 10.1136/bmjpo-2018-000325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda, R. F., Felitti, V. J., Bremner, J. D., Walker, J. D., Whitfield, C., Perry, B. D., Dube, S. R., & Giles, W. H. (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. 10.1007/s00406-005-0624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, S. L., Tomada, A., Vincow, E. S., Valente, E., Polcari, A., & Teicher, M. H. (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences, 20(3), 292–301. 10.1176/jnp.2008.20.3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, C. M., Teicher, M. H., Polcari, A., & Renshaw, P. F. (2002). Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: Potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology, 27(1-2), 231–244. 10.1016/s0306-4530(01)00047-6 [DOI] [PubMed] [Google Scholar]
- Andrews, K. A., Desai, D., Dhillon, H. K., Wilcox, D. T., & Fitzgerald, M. (2002). Abdominal sensitivity in the first year of life: Comparison of infants with and without unilateral hydronephrosis. Pain, 100(1-2), 35–46. 10.1016/s0304-3959(02)00288-9 [DOI] [PubMed] [Google Scholar]
- Andrews, K. A., & Fitzgerald, M. (1999). The cutaneous flexion reflex in human neonates: A quantitative study of threshold and stimulus/response characteristics, following single and repeated stimuli. Developmental Medicine & Child Neurology, 41(10), 696–703. 10.1017/s0012162299001425 [DOI] [PubMed] [Google Scholar]
- Baccei, M., & Fitzgerald, M. (2006). Development of pain pathways and mechanisms. In McMahon S. B., & Koltzenburg M. (Eds.), The Textbook of Pain (pp. 143–148). Churchill Livingstone. [Google Scholar]
- Baker, L. M., Williams, L. M., Korgaonkar, M. S., Cohen, R. A., Heaps, J. M., & Paul, R. H. (2013). Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging and Behavior, 7(2), 196–203. 10.1007/s11682-012-9215-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, P. M., Hanson, J. L., Pierson, R. K., Davidson, R. J., & Pollak, S. D. (2009). Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry, 66(12), 1100–1106. 10.1016/j.biopsych.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky, H., del Rey, A., Sorkin, E., & Dinarello, C. A. (1986). Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science, 233(4764), 652–654. 10.1126/science.3014662 [DOI] [PubMed] [Google Scholar]
- Bick, J., & Nelson, C. J. (2016). Early adverse experiences and the developing brain. Neuropsychopharmacology, 41(1), 177–196. 10.1038/npp.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker, R., Holz, N. E., Buchmann, A. F., Blomeyer, D., Plichta, M. M., Wolf, I., Baumeister, S., Meyer-Lindenberg, A., Banaschewski, T., Brandeis, D., & Laucht, M. (2014). Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS One, 9(8), e104185. 10.1371/journal.pone.0104185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini, A., Wallis, A. S. J., Zunszain, P., Pariante, C. M., & Kempton, M. J. (2020). Characterising anhedonia: A systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cognitive, Affective, & Behavioral Neuroscience, 20(4), 816–841. 10.3758/s13415-020-00804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner, J. D., Licinio, J., Darnell, A., Krystal, J. H., Owens, M. J., Southwick, S. M., Nemeroff, C. B. & Charney, D. S. (1997). Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. American Journal of Psychiatry, 154(5), 624–629. 10.1176/ajp.154.5.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson, K. L., Eghbal-Ahmadi, M., Bender, R., Chen, Y., & Baram, T. Z. (2001). Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences, 98(15), 8856–8861. 10.1073/pnas.151224898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, L. L., Tyrka, A. R., McDougle, C. J., Malison, R. T., Owens, M. J., Nemeroff, C. B., & Price, L. H. (2004). Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology, 29(4), 777–784. 10.1038/sj.npp.1300375 [DOI] [PubMed] [Google Scholar]
- Cay, M., Gonzalez-Heydrich, J., Teicher, M. H., van der Heijden, H., Ongur, D., Shinn, A. K., & Upadhyay, J. (2022). Childhood maltreatment and its role in the development of pain and psychopathology. The Lancet Child & Adolescent Health, 6(3), 195–206. 10.1016/S2352-4642(21)00339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux, M., Bennett, A., Shannon, C., Higley, J. D., Lesch, K. P., & Suomi, S. J. (2002). Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry, 7(10), 1058–1063. 10.1038/sj.mp.4001157 [DOI] [PubMed] [Google Scholar]
- Chen, Y., & Baram, T. Z. (2016). Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology Reviews, 41(1), 197–206. 10.1038/npp.2015.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J., Jeong, B., Rohan, M. L., Polcari, A. M., & Teicher, M. H. (2008). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry, 65(3), 227–234. 10.1016/j.biopsych.2008.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy, E., Degenhardt, L., Mattick, R. P., & Nelson, E. C. (2009). Child maltreatment as a risk factor for opioid dependence: Comparison of family characteristics and type and severity of child maltreatment with a matched control group. Child Abuse & Neglect, 33(6), 343–352. 10.1016/j.chiabu.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead, W. E., & Craighead, LW (2003): Behavioral and cognitive-behavioral psychotherapy. In: I. B. Weiner (series Ed.); Stricker G & Widiger TA (vol. Eds.), Handbook of Psychology: vol. 8. Clinical Psychology, New York, Wiley, pp. 279-299.
- Craighead, W. E., & Nemeroff, C. B. (2005). The impact of early trauma on response to psychotherapy. Clinical Neuroscience Research, 4(5-6), 405–411. 10.1016/j.cnr.2005.03.004 [DOI] [Google Scholar]
- Danese, A., Caspi, A., Williams, B., Ambler, A., Sugden, K., Milka, J., Mika, J., Werts, H., Freeman, J., Pariante, C. M., Moffitt, T. E., & Arseneault, L. (2011). Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry, 16(3), 244–246. 10.1038/mp.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, A., & Lewis, S. J. (2017). Psychoneuroimmunology of early-life stress: The hidden wounds of childhood trauma? Neuropsychopharmacology, 42(1), 99–114. 10.1038/npp.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, J. K., Lamke, J.-P., Gaebler, M., Walter, H., & Scheel, M. (2013). White matter integrity and its relationship to PTSD and childhood trauma – a systematic review and meta-analysis. Depression and Anxiety, 30(3), 207–216. 10.1002/da.22044 [DOI] [PubMed] [Google Scholar]
- De Bellis, M. D. (2010). The neurobiology of child neglect. In Lanius R. A., Vermetten E., & Pain C. (Eds.), The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic (pp. 123–132). Cambridge University Press. [Google Scholar]
- De Bellis, M. D., Keshavan, M. S., Clark, D. B., Casey, B. J., Giedd, J. N., Boring, A. M., Frustaci, K., & Ryan, N. D. (1999). Developmental traumatology part II: Brain development. Biological Psychiatry, 45(10), 1271–1284. 10.1016/s0006-3223(99)00045-1 [DOI] [PubMed] [Google Scholar]
- Defrin, R., Cohen Sagy, N., Biran, I., Goor-Aryeh, I., Shai, R., & Ginzburg, K. (2020). Enhanced pain modulation capacity among individuals with borderline personality disorder: A possible mechanism underlying their hypoalgesia. European Journal of Pain, 24(3), 544–554. 10.1002/ejp.1504 [DOI] [PubMed] [Google Scholar]
- Defrin, R., Schreiber, S., & Ginzburg, K. (2015). Paradoxical pain perception in posttraumatic stress disorder: The unique role of anxiety and dissociation. The Journal of Pain, 16(10), 961–970. 10.1016/j.jpain.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Dube, S. R., Anda, R. F., Felitti, V. J., Chapman, D. P., Williamson, D. F., & Giles, W. H. (2001). Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: Findings from the Adverse Childhood Experiences Study. JAMA, 286(24), 3089–3096. 10.1001/jama.286.24.3089 [DOI] [PubMed] [Google Scholar]
- Dubé, C. M., Molet, J., Singh-Taylor, A., Ivy, A., Maras, P. M., & Baram, T. Z. (2015). Hyperexcitability and epilepsy generated by chronic early-life stress. Neurobiology of Stress, 2, 10–19. 10.1016/j.ynstr.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, A. J. (2000). Cytokine activation of the HPA axis. Annals of the New York Academy of Sciences, 917(1), 608–617. 10.1111/j.1749-6632.2000.tb05426.x [DOI] [PubMed] [Google Scholar]
- Egerton, A., Valmaggia, L. R., Howes, O. D., Day, F., Chaddock, C. A., Allen, P., Winton-Brown, T. T., Bloomfield, M. A. P., Bhattacharyya, S., Chilcott, J., Lappin, J. M., Murray, R. M., & McGuire, P. (2016). Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophrenia Research, 176(2-3), 171–176. 10.1016/j.schres.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal, T. J., Chugani, H. T., Behen, M. E., Juhász, C., Muzik, O., Maqbool, M., Chugani, D. C., & Makki, M. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics, 117(6), 2093–2100. 10.1542/peds.2005-1727 [DOI] [PubMed] [Google Scholar]
- Evans, G. W., & Schamberg, M. A. (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences, 106(16), 6545–6549. 10.1073/pnas.0811910106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi, L., Slater, R., Worley, A., Meek, J., Boyd, S., Olhede, S., & Fitzgerald, M. (2011). A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Current Biology, 21(18), 1552–1558. 10.1016/j.cub.2011.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J., Liu, W., Xia, J., Li, S., Gao, F., Zhu, J., Han, Y., Zhou, H., Liao, H., Yi, J., Tan, C., & Zhu, X. (2021). Childhood trauma is associated with elevated anhedonia and altered core reward circuitry in major depression patients and controls. Human Brain Mapping, 42(2), 286–297. 10.1002/hbm.25222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, M. (2005). The development of nociceptive circuits. Nature Reviews Neuroscience, 6(7), 507–520. 10.1038/nrn1701 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M., & Koltzenburg, M. (1986). The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Developmental Brain Research, 24(1-2), 261–270. 10.1016/0165-3806(86)90194-x [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M., Millard, C., & McIntosh, N. (1989). Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain, 39(1), 31–36. 10.1016/0304-3959(89)90172-3 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M., & Walker, S. (2009). Infant pain management: A developmental neurobiological approach. Nature Clinical Practice Neurology, 5(1), 35–50. 10.1038/ncpneuro0984 [DOI] [PubMed] [Google Scholar]
- Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., Nugent, T. F., Herman, D. H., Clasen, L. S., Toga, A. W., Rapoport, J. L., & Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay, N., Nugent, T. F., Herman, D. H., Ordonez, A., Greenstein, D., Hayashi, K. M., Clasen, L., Toga, A. W., Giedd, J. N., Rapoport, J. L., & Thompson, P. M. (2006). Dynamic mapping of Normal human hippocampal development. Hippocampus, 16(8), 664–672. 10.1002/hipo.20193 [DOI] [PubMed] [Google Scholar]
- Gogtay, N., & Thompson, P. M. (2010). Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain and Cognition, 72(1), 6–15. 10.1016/j.bandc.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan, R. M., Behen, M. E., Helder, E., Makki, M. I., & Chugani, H. T. (2010). Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS). Cerebral Cortex, 20(3), 561–569. 10.1093/cercor/bhp122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, Y. P., Heim, C., Goodman, S. H., Miller, A. H., & Nemeroff, C. B. (1999). The effects of neonatal stress on brain development: Implications for psychopathology. Development and Psychopathology, 11(3), 545–565. 10.1017/s0954579499002205 [DOI] [PubMed] [Google Scholar]
- Green, J. G., McLaughlin, K. A., Berglund, P. A., Gruber, M. J., Sampson, N. A., Zaslavsky, A. M., & Kessler, R. C. (2010). Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication 1: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, L. S., & Paivio, S. C. (1997). Working with the Emotions in Psychotherapy. Guilford. [Google Scholar]
- Greenough, W. T., Black, J. E., & Wallace, C. S. (1987). Experience and brain development. Child Development, 58(3), 539–559. 10.2307/1130197 [DOI] [PubMed] [Google Scholar]
- Gunn, B. G., Cunningham, L., Cooper, M. A., Corteen, N. L., Seifi, M., Swinny, J. D., Lambert, J. J., & Belelli, D. (2013). Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: Relevance to neurosteroids and programming of the stress response. Journal of Neuroscience, 33(50), 19534–19554. 10.1523/JNEUROSCI.1337-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar, M., & Quevedo, K. (2007). The neurobiology of stress and development. Annual Review of Psychology, 58(1), 145–173. 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Hanson, J. L., Hariri, A. R., & Williamson, D. E. (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78(9), 598–605. 10.1016/j.biopsych.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim, C., Shugart, M., Craighead, W. E., & Nemeroff, C. B. (2010). Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology, 52(7), 671–690. 10.1002/dev.20494 [DOI] [PubMed] [Google Scholar]
- Hermann, C., Hohmeister, J., Demirakça, S., Zohsel, K., & Flor, H. (2006). Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain, 125(3), 278–285. 10.1016/j.pain.2006.08.026 [DOI] [PubMed] [Google Scholar]
- Hucho, T., & Levine, J. D. (2007). Signalling pathways in sensitisation: Towards a nociceptor cell biology. Neuron, 55(3), 365–376. 10.1016/j.neuron.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Huttenlocher, P. R., de Courten, C., Garey, L. J., & Van der Loos, H. (1982). Synaptogenesis in human visual cortex–evidence for synapse elimination during Normal development. Neuroscience Letters, 33(3), 247–252. 10.1016/0304-3940(82)90379-2 [DOI] [PubMed] [Google Scholar]
- Ivy, A. S., Rex, C. S., Chen, Y., Dubé, C., Maras, P. M., Grigoriadis, D. E., Dube, C., Gall, C. M., Lynch, G., & Baram, T. Z. (2010). Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. Journal of Neuroscience, 30(39), 13005–13015. 10.1523/JNEUROSCI.1784-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, L., Verriotis, M., Cooper, R. J., Laudiano-Dray, M. P., Rupawala, M., Meek, J., Fabrizi, L., & Fitzgerald, M. (2022). Widespread nociceptive maps in the human neonatal somatosensory cortex. Elife, 11, e71655. 10.7554/eLife.71655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado, M., & Rosselli, M. (2007). The elusive nature of executive functions: A review of our current understanding. Neuropsychology Review, 17(3), 213–233. 10.1007/s11065-007-9040-z [DOI] [PubMed] [Google Scholar]
- Juranek, J., Filipek, P. A., Berenji, G. R., Modahl, C., Osann, K., & Spence, M. A. (2006). Association between amygdala volume and anxiety level: Magnetic resonance imaging (MRI) study in autistic children. Journal of Child Neurology, 21(12), 1051–1058. 10.1177/7010.2006.00237 [DOI] [PubMed] [Google Scholar]
- Khan, A., McCormack, H. C., Bolger, E. A., McGreenery, C. E., Vitaliano, G., Polcari, A., & Teicher, M. H. (2015). Childhood maltreatment, depression, and suicidal ideation: Critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Frontiers in Psychiatry, 6, 42. 10.3389/fpsyt.2015.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker, G. M., Pearson, R. M., Zammit, S., Lewis, G., & Jones, P. B. (2014). Association of serum interleukin 6 and C-Reactive protein in childhood with depression and psychosis in young adult life. JAMA Psychiatry, 71(10), 1121–1128. 10.1001/jamapsychiatry.2014.1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, P., Evans, G. W., Angstadt, M., Ho, S. S., Sripada, C. S., Swain, J. E., Liberzon, I., & Phan, K. L. (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences, 110(46), 18442–18447. 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel, T., Mehta, D., Anacker, C., Rex-Haffner, M., Pruessner, J. C., Pariante, C. M., Pace, T. W. W., Mercer, K. B., Mayberg, H. S., Bradley, B., Nemeroff, C. B., Holsboer, F., Heim, C. M., Ressler, K. J., Rein, T., & Binder, E. B. (2013). Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience, 16(1), 33–41. 10.1038/nn.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia, A. S., & Rakic, P. (1994). Axon overproduction and elimination in the anterior commissure of the developing rhesus monkey. The Journal of Comparative Neurology, 340(3), 328–336. 10.1002/cne.903400304 [DOI] [PubMed] [Google Scholar]
- Lane, R. D., Anderson, F. S., & Smith, R. (2018). Biased competition favoring physical over emotional pain: A possible explanation for the link between early adversity and chronic pain. Psychosomatic Medicine, 80(9), 880–890. 10.1097/PSY.0000000000000640 [DOI] [PubMed] [Google Scholar]
- Le Merrer, J., Becker, J. A., Befort, K., & Kieffer, B. L. (2009). Reward processing by the opioid system in the brain. Physiological Reviews, 89(4), 1379–1412. 10.1152/physrev.00005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. J., Arseneault, L., Caspi, A., Fisher, H. L., Matthews, T., Moffitt, T. E., Quinlan, E. B., Barker, E. D., Luo, Q., Banaschewski, T., Bokde, A. L. W., Bromberg, U., Büchel, C., Desrivières, S., Flor, H., Frouin, V., Garavan, H., Chaarani, B., Gowland, P., … Schumann, G. (2019). The epidemiology of trauma and post-traumatic stress disorder in a representative cohort of young people in England and Wales. Molecular Psychiatry, 6(3), 247–256. 10.1038/s41380-018-0297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D., Diorio, J., Tannenbaum, B., Caldji, C., Francis, S., Freedman, A., Sharma, S., Pearson, D., Plotsky, P. M., & Meaney, M. J. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277(5332), 1659–1662. 10.1126/science.277.5332.1659 [DOI] [PubMed] [Google Scholar]
- Logue, M. W., van Rooij, S. J. H., Dennis, E. L., Davis, S. L., Hayes, J. P., Stevens, J. S., Densmore, M., Haswell, C. C., Ipser, J., Koch, S. B. J., Korgaonkar, M., Lebois, L. A. M., Peverill, M., Baker, J. T., Boedhoe, P. S. W., Frijling, J. L., Gruber, S. A., Harpaz-Rotem, I., Jahanshad, N., … Morey, R. A. (2018). Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biological Psychiatry, 83(3), 244–253. 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Maestripieri, D., Higley, J. D., Lindell, S. G., Newman, T. K., McCormack, K. M., & Sanchez, M. M. (2006). Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta). Behavioral Neuroscience, 120(5), 1017–1024. 10.1037/0735-7044.120.5.1017 [DOI] [PubMed] [Google Scholar]
- Marek, R., Strobel, C., Bredy, T. W., & Sah, P. (2013). The amygdala and medial prefrontal cortex: Partners in the fear circuit. The Journal of Physiology, 591(10), 2381–2391. 10.1113/jphysiol.2012.248575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, R., Gerber, A. J., & Peterson, B. S. (2008). Neuroimaging studies of Normal brain development and their relevance for understanding childhood neuropsychiatric disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 47(11), 1233–1251. 10.1097/CHI.0b013e318185e703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, J. W., Giller, E. L., Kosten, T. R., Ostroff, R. B., & Podd, L. (1986). Urinary free-cortisol levels in posttraumatic stress disorder patients. The Journal of Nervous and Mental Disease, 174(3), 145–149. 10.1097/00005053-198603000-00003 [DOI] [PubMed] [Google Scholar]
- McCrory, E., De Brito, S. A., & Viding, E. (2011). The impact of childhood maltreatment: A review of neurobiological and genetic factors. Frontiers in Psychiatry, 2, 48. 10.3389/fpsyt.2011.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane, A. C. (2010). The long-term costs of traumatic stress: Intertwined physical and psychological consequences. World Psychiatry, 9(1), 3–10. 10.1002/j.2051-5545.2010.tb00254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan, P. O., Sasaki, A., D’Alessio, A. C., Dymov, S., Labonté, B., Szyf, M., Turecki, G., & Meaney, M. J. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348. 10.1038/nn.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, M. A., Golembo, N. I., Nosarti, C., Colvert, E., Mota, A., Williams, S. C. R., Rutter, M., & Sonuga-Barke, E. J. S. (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees Study Pilot. Journal of Child Psychology and Psychiatry, 50(8), 943–951. 10.1111/j.1469-7610.2009.02084.x [DOI] [PubMed] [Google Scholar]
- Molnar, Z., Clowry, G. J., Sestan, N., Alzu’bi, A., Bakken, T., Hevner, R. F., Molnár, Z., Šestan, N., Alzu'bi, A., Hüppi, P. S., Kostović, I., Rakic, P., Anton, E. S., Edwards, D., Garcez, P., Hoerder-Suabedissen, A., & Kriegstein, A. (2019). New insights into the development of the human cerebral cortex. Journal of Anatomy, 235(3), 432–451. 10.1111/joa.13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova, E., & Porreca, F. (2014). Reward and motivation in pain and pain relief. Nature Neuroscience, 17(10), 1304–1312. 10.1038/nn.3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, C. A. 3rd, Bos, K., Gunnar, M. R., & Sonuga-Barke, E. J. S. (2011). The neurobiological toll of early human deprivation. Monographs of the Society for Research in Child Development, 76(4), 127–146. 10.1111/j.1540-5834.2011.00630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, C. A. 3rd, Zeanah, C. H., & Fox, N. A. (2019). How early experience shapes human development: The case of psychosocial deprivation. Neural Plasticity, 1676285. 10.1155/2019/1676285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Digital, Mental Health Services Monthly Statistics . Performance February, Provisional March 2022 https://digital.nhs.uk/data-and-information/publications/statistical/mental-health-services-monthly-statistics.
- Noble, K. G., McCandliss, B. D., & Farah, M. J. (2007). Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science, 10(4), 464–480. 10.1111/j.1467-7687.2007.00600.x [DOI] [PubMed] [Google Scholar]
- Noble, K. G., Tottenham, N., & Casey, B. J. (2005). Neuroscience perspectives on disparities in school readiness and cognitive achievement. The Future of Children, 15(1), 71–89. 10.1353/foc.2005.0006 [DOI] [PubMed] [Google Scholar]
- O’Doherty, D. C. M., Ryder, W., Paquola, C., Tickell, A., Chan, C., Hermens, D. F., Bennett, M. R., & Lagopoulos, J. (2018). White matter integrity alterations in post-traumatic stress disorder. Human Brain Mapping, 39(3), 1327–1338. 10.1002/hbm.23920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain, C., Lanius, R. A., Ogden, P., & Vermetten, E. (2010). Psychodynamic psychotherapy: Adaptations for the treatment of patients with chronic complex post-traumatic stress disorder. In Lanius R. A., Vermetten E., & Pain C. (Eds.), The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic (pp. 286–294). Cambridge University Press. [Google Scholar]
- Pechtel, P., Lyons-Ruth, K., Anderson, C. M., & Teicher, M. H. (2014). Sensitive periods of amygdala development: The role of maltreatment in preadolescence. Neuroimage, 97, 236–244. 10.1016/j.neuroimage.2014.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel, P., & Pizzagalli, D. A. (2011). Effects of early life stress on cognitive and affective function: An integrated review of the human literature. Psychopharmacology, 214(1), 55–70. 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek, Z., Judas, M., Kostovic, I., & Uylings, H. B. (2008). Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer- specific pattern. Cerebral Cortex, 18(4), 915–929. 10.1093/cercor/bhm124 [DOI] [PubMed] [Google Scholar]
- Price, D. D. (2002). Central neural mechanisms that interrelate sensory and affective dimensions of pain. Molecular Interventions, 2(392-403), 339. 10.1124/mi.2.6.392 [DOI] [PubMed] [Google Scholar]
- Quinlan, E. B., Barker, E. D., Luo, Q., Banaschewski, T., Bokde, A. L. W., Bromberg, U., Büchel, C., Desrivières, S., Flor, H., Frouin, V., Garavan, H., Chaarani, B., Gowland, P., Heinz, A., Brühl, R., Martinot, J.-L., Martinot, M.-L. P., Nees, F., Orfanos, D. P., … Schumann, G. (2020). Peer victimization and its impact on adolescent brain development and psychopathology. Molecular Psychiatry, 25(1), 3066–3076. 10.1038/s41380-018-0297-9 [DOI] [PubMed] [Google Scholar]
- Raglan, G. B., Schmidt, L. A., & Schulkin, J. (2017). The role of glucocorticoids and corticotropin-releasing hormone regulation on anxiety symptoms and response to treatment. Endocrine Connections, 6(2), R1–R7. 10.1530/EC-16-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer Thomsen K, Blom Osterland, B., Hesse, M., & Feldstein Ewing, S. W. (2018). The intersection between response inhibition and substance use among adolescents. Addictive Behaviors, 78, 228–230. 10.1016/j.addbeh.2017.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarinen, A., Keltikangas-Järvinen, L., Jääskeläinen, E., Huhtaniska, S., Pudas, J., Tovar-Perdomo, S., Penttilä, M., Miettunen, J., & Lieslehto, J. (2021). Early adversity and emotion processing from faces: A meta-analysis on behavioral and neurophysiological responses. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6(7), 692–705. 10.1016/j.bpsc.2021.01.002 [DOI] [PubMed] [Google Scholar]
- Sapolsky, R. M. (1996). Stress, glucocorticoids, and damage to the nervous system: The current state of confusion. Stress, 1(1), 1–19. 10.3109/10253899609001092 [DOI] [PubMed] [Google Scholar]
- Scioli-Salter, E. R., Forman, D. E., Otis, J. D., Gregor, K., Valovski, I., & Rasmusson, A. M. (2015). The shared neuroanatomy and neurobiology of comorbid chronic pain & PTSD: Therapeutic implications. The Clinical Journal of Pain, 31(4), 363–374. 10.1097/AJP.0000000000000115 [DOI] [PubMed] [Google Scholar]
- Seedat, S., Stein, M. B., Kennedy, C. M., & Hauger, R. L. (2003). Plasma cortisol and neuropeptide Y in female victims of intimate partner violence. Psychoneuroendocrinology, 28(6), 796–808. 10.1016/s0306-4530(02)00086-0 [DOI] [PubMed] [Google Scholar]
- Segal, Z. V., Williams, J. M. G., & Teasdale, J. D. (2002). Mindfulness-based Cognitive Therapy for Depression – a new Approach for Preventing Relapse. Guilford. [Google Scholar]
- Shaw, P., Kabani, N. J., Lerch, J. P., Eckstrand, K., Lenroot, R., Gogtay, N., Greenstein, D., Clasen, L., Evans, A., Rapoport, J. L., Giedd, J. N., & Wise, S. P. (2008). Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience, 28(14), 3586–3594. 10.1523/JNEUROSCI.5309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, M. A., & McLaughlin, K. A. (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18(11), 580–585. 10.1016/j.tics.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater, R., Cantarella, A., Gallella, S., Worley, A., Boyd, S., Meek, J., & Fitzgerald, M. (2006). Cortical pain responses in human infants. Journal of Neuroscience, 26(14), 3662–3666. 10.1523/JNEUROSCI.0348-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, L. K., Draper, E. S., Manktelow, B. N., & Field, D. J. (2009). Socioeconomic inequalities in survival and provision of neonatal care: Population based study of very preterm infants. BMJ, 339(dec01 1), b4702. 10.1136/bmj.b4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck, V., Schlereth, T., Birklein, F., & Maihöfner, C. (2017). Increased prevalence of posttraumatic stress disorder in CRPS. European Journal of Pain, 21(3), 466–473. 10.1002/ejp.940 [DOI] [PubMed] [Google Scholar]
- Stevenson, J. (1999). The treatment of long-term sequelae of child abuse. Journal of Child Psychology and Psychiatry, 40(1), 89–111. 10.1111/1469-7610.00425 [DOI] [PubMed] [Google Scholar]
- Stiles, J. (2017). Principles of brain development. Wiley Interdisciplinary Reviews: Cognitive Science, 8(1-2), 10.1002/wcs.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs, B., Thompson, T., Acaster, S., Vancampfort, D., Gaughran, F., & Correll, C. U. (2015). Decreased pain sensitivity among people with schizophrenia: A meta-analysis of experimental pain induction studies. Pain, 156(11), 2121–2131. 10.1097/j.pain.0000000000000304 [DOI] [PubMed] [Google Scholar]
- Syed Sherriff, R., Van Hooff, M., Malhi, G. S., Grace, B., & McFarlane, A. (2020). Childhood determinants of suicidality in men recently transitioned from regular military service. Australian & New Zealand Journal of Psychiatry, 54(7), 743–754. 10.1177/0004867420924742 [DOI] [PubMed] [Google Scholar]
- Teicher, M. H., Dumont, N. L., Ito, Y., Vaituzis, C., Giedd, J. N., & Andersen, S. L. (2004). Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry, 56(2), 80–85. 10.1016/j.biopsych.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Teicher, M. H., Rabi, K., Sheu, Y.-S., Seraphin, S. B., Andersen, S. L., Anderson, C. M., Choi J., & Tomoda, A. (2010). Neurobiology of childhood trauma and adversity. In Lanius R. A., Vermetten E., & Pain C. (Eds.), The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic (pp. 112–122). Cambridge University Press. [Google Scholar]
- Teicher, M., Samson, J. A., Polcari, A., & McGreenery, C. E. (2006). Sticks, stones and hurtful words: Relative effects of various forms of childhood maltreatment. American Journal of Psychiatry, 163(6), 993–1000. 10.1176/ajp.2006.163.6.993 [DOI] [PubMed] [Google Scholar]
- Teicher, M., Tomoda, A., & Andersen, S. (2006). Neurobiological consequences of early stress and childhood maltreatment: Are results from human and animal studies comparable? Annals of the New York Academy of Sciences, 1071(1), 313–323. 10.1196/annals.1364.024 [DOI] [PubMed] [Google Scholar]
- Tesarz, J., Eich, W., Treede, R. D., & Gerhardt, A. (2016). Altered pressure pain thresholds and increased wind-up in adult patients with chronic back pain with a history of childhood maltreatment: A quantitative sensory testing study. Pain, 157(8), 1799–1809. 10.1097/j.pain.0000000000000586 [DOI] [PubMed] [Google Scholar]
- Tesarz, J., Gerhardt, A., Leisner, S., Janke, S., Treede, R. D., & Eich, W. (2015). Distinct quantitative sensory testing profiles in nonspecific chronic back pain subjects with and without psychological trauma. Pain, 156(4), 577–586. 10.1097/01.j.pain.0000460350.30707.8d [DOI] [PubMed] [Google Scholar]
- Tiemeier, H., Lenroot, R. K., Greenstein, D. K., Tran, L., Pierson, R., & Giedd, J. N. (2010). Cerebellum development during childhood and adolescence: A longitudinal morphometric MRI study. Neuroimage, 49(1), 63–70. 10.1016/j.neuroimage.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham, N., Hare, T. A., Quinn, B. T., McCarry, T. W., Nurse, M., Gilhooly, T., Millner, A., Galvan, A., Davidson, M. C., Eigsti, I.-M., Thomas, K. M., Freed, P. J., Booma, E. S., Gunnar, M. R., Altemus, M., Aronson, J., & Casey, B. J. (2010). Prolonged institutional rearing is associated with atypically larger amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey, I., & Mantyh, P. W. (2007). The cerebral signature for pain perception and its modulation. Neuron, 55(3), 377–391. 10.1016/j.neuron.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Turecki, G., Ota, V. K., Belangero, S. I., Jackowski, A., & Kaufman, J. (2014). Early life adversity, genomic plasticity, and psychopathology. The Lancet Psychiatry, 1(6), 461–466. 10.1016/s2215-0366(14)00022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu, A., Matsui, M., Tanaka, C., Takahashi, T., Noguchi, K., Suzuki, M., & Nishijo, H. (2012). Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PloS One, 7(10), e46970. 10.1371/journal.pone.0046970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF report on war in Ukraine . (n.d.). https://www.unicef.org/emergencies/war-ukraine-pose-immediate-threat-children. July 2022.
- Upadhyay, J., Geber, C., Hargreaves, R., Birklein, F., & Borsook, D. (2018). A critical evaluation of validity and utility of translational imaging in pain and analgesia: Utilising functional imaging to enhance the process. Neuroscience & Biobehavioral Reviews, 84, 407–423. 10.1016/j.neubiorev.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wal, M. F., de Wit, C. A., & Hirasing, R. A. (2003). Psychosocial health among young victims and offenders of direct and indirect bullying. Pediatrics, 111(6), 1312–1317. 10.1542/peds.111.6.1312 [DOI] [PubMed] [Google Scholar]
- Wang, H., Fan, L., Song, M., Liu, B., Wu, D., Jiang, R., Li, J., Li, A., Banaschewski, T., Bokde, A. L. W., Quinlan, E. B., Desrivières, S., Flor, H., Grigis, A., Garavan, H., Chaarani, B., Gowland, P., Heinz, A., Ittermann, B., … Schumann, G. (2021). Functional connectivity predicts individual development of inhibitory control during adolescence. Cerebral Cortex, 31(5), 2686–2700. 10.1093/cercor/bhaa383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, I. C., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., D'Alessio, A. C., Dymov, S., Szyf, M., & Meaney, M. J. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847–854. 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Weaver, I. C., Diorio, J., Seckl, J. R., Szyf, M., & Meaney, M. J. (2004). Early environmental regulation of hippocampal glucocorticoid receptor gene expression: Characterization of intracellular mediators and potential genomic target sites. Annals of the New York Academy of Sciences, 1024(1), 182–212. 10.1196/annals.1321.099 [DOI] [PubMed] [Google Scholar]
- Wollgarten-Hadamek, I., Hohmeister, J., Demirakça, S., Zohsel, K., Flor, H., & Hermann, C. (2009). Do burn injuries during infancy affect pain and sensory sensitivity in later childhood? Pain, 141(1-2), 165–172. 10.1016/j.pain.2008.11.008 [DOI] [PubMed] [Google Scholar]
- Wollgarten-Hadamek, I., Hohmeister, J., Zohsel, K., Flor, H., & Hermann, C. (2011). Do school-aged children with burn injuries during infancy show stress-induced activation of pain inhibitory mechanisms? European Journal of Pain, 15(4), 423. e1-10. 10.1016/j.ejpain.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Woolf, C. J., & Ma, Q. (2007). Nociceptors - noxious stimulus detectors. Neuron, 55(3), 353–364. 10.1016/j.neuron.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Yehuda, R., & Lehrner, A. (2018). Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry, 17(3), 243–257. 10.1002/wps.20568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J. E., & Klosko, J. S. (2003). Schema Therapy: A Practitioner’s Guide. Guilford. [Google Scholar]
- Zhou, J. N., & Fang, H. (2018). Transcriptional regulation of corticotropin-releasing hormone gene in stress response. IBRO Reports, 5, 137–146. 10.1016/j.ibror.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this manuscript is cited fully in the references section at the end.