ABSTRACT
Astragalus membranaceus is a traditional Chinese medicine and has been widely used in treating cardiovascular diseases (CVDs), such as asthma, edema, and chest tightness. Astragaloside IV (AS-IV), one of the major active components extracted from Astragalus membranaceus, has a series of pharmacological effects, including inhibiting inflammation, regulating energy metabolism, reducing oxidative stress and apoptosis. However, the effect of AS-IV on myocardial infarction (MI) and the underlying molecular mechanism remains unclear. The purpose of our study is to investigate the effects of AS-IV on MI-induced myocardial fibrosis and cardiac remodeling and to elucidate its underlying mechanisms. MI was induced by ligation of the left anterior descending (LAD) coronary artery. Echocardiography was used to evaluate cardiac function in mice. Pathological changes in cardiac tissues were analyzed with hematoxylin and eosin (H&E) staining, Masson staining, and wheat germ agglutinin (WGA) staining. Immunohistochemistry was used to detect the expression of fibrosis and inflammation-related proteins. Immunofluorescence and flow cytometry were used to detect ROS level. The expressions of α-SMA, Collagen I, NLRP3, cleaved cas-1, cleaved IL-18, cleaved IL-β, GSDMD-N, and cleaved caspase-1 were examined using western blot. The results of cardiac ultrasound showed that AS-IV could improve poor ventricular remodeling, myocardial pathological staining showed that AS-IV could significantly reduce the myocardial fibrosis and myocardial hypertrophy, ROS levels were also significantly reduced, and the protein expression of NLRP3/Caspase-1/GSDMD signaling pathway was remarkably decreased in the AS-IV group. Furthermore, immunohistochemical staining results showed that the expression of myocardial macrophages and neutrophils in AS-IV group decreased significantly, to further investigate whether the reduction of myocardial pyroptosis by AS-IV is related to the regulation of macrophages, in vitro, AS-IV was selected to stimulate bone marrow-derived macrophages (BMDMs). Our findings indicated that AS-IV protective effect of the heart might be related to the reduction of macrophage pyroptosis. These results demonstrate that AS-IV alleviated MI-induced myocardial fibrosis and cardiac remodeling by suppressing ROS/Caspase-1/GSDMD signaling pathway, AS-IV should be further studied in the future.
KEYWORDS: Astragaloside IV, pyroptosis, cardiac remolding
Introduction
MI is one of the major causes of death and disability worldwide [1]. Clinical treatments for MI mainly include anticoagulation, antiplatelet, thrombolysis, and myocardial reperfusion; on the one hand, these treatments can reduce the infarct area, but on the other hand, they will aggravate myocardial injury to varying degrees, and the treatment effect is not ideal [2]. Therefore, how to reduce the death of cardiomyocyte in ischemic area and protect impaired cardiac function is very crucial.
Pyroptosis is a programmed cell death mediated by gasdermin protein [3]. It is mainly mediated by caspase-1 and accompanied by the release of large number of pro-inflammatory cytokines, causing the body to produce inflammatory response [4]. Growing evidence indicated that pyroptosis is related to a variety of CVDs, such as ischemic heart disease, diabetic cardiomyopathy, and HF [5]. Lei Q et al. found that antioxidants can reduce ROS level and significantly inhibit the activation of NLRP3 inflammasome in the model of myocardial pyroptosis induced by oxygen and glucose deprivation [6]. The activated NLRP3 inflammasome triggers the cleavage of pro-caspase-1 to active caspase-1, and then the cytokine precursor pro-IL-1β and pro-IL-18 were transformed into bioactive IL-1β and IL-18, which exacerbated the inflammatory response after MI [7]. On the contrary, inhibition of NLRP3 inflammasome can protect cardiac function and reduce myocardial pyroptosis [6,8,9]. Therefore, target pyroptosis provides potential therapeutic strategies for MI.
AS-IV is a cycloartane triterpene saponin, pharmacological studies have confirmed that AS-IV can prevent HF by regulating sarcoplasmic reticulum Ca2+ pump, promoting angiogenesis, improving myocardial energy metabolism, and inhibiting myocardial hypertrophy [10–13]. In this study, we provided more evidence about whether ROS/Caspase-1/GSDMD signaling pathway was activated in MI mice and the cardioprotective mechanism of AS-IV on myocardial fibrosis and cardiac remodeling.
Here, we report that pyroptosis is a key event during MI injury and identify a previously unrecognized role of AS-IV after MI.
Methods and materials
Animal experimental protocols
Male C57BL/6 J (SPF level, body weight 25 ± 3 g) mice aged 6 to 8 weeks were purchased from the Shanghai Laboratory Animal Center (n = 20 for each group). All mice were maintained under a regular 12-h light/dark cycle, under a temperature-controlled (20 ± 3°C) and a humidity-controlled (40 ± 5%) environment. The mice were placed in the anesthesia box, rapid anesthesia with 5% isoflurane, after mice were placed on the experimental board in supine position, and maintained anesthesia with 1% isoflurane. After removing the chest hair and disinfecting the skin, break the skin from the 4th and 5th intercostals, passively separate the subcutaneous fascia and muscle, breaking through the 3rd and 4th intercostal space, and quickly squeeze out the heart. LAD was ligated by using a 6–0 polyester suture, and the needle inlet and outlet are about 2 mm. Return to the heart after ligation, squeeze out the chest gas and suture.
Experimental drugs and preparations
AS-IV was purchased from Selleck Medical Technology Co., Ltd. (China) and dissolved in 0.5% carboxymethyl cellulose (CMC). The sham group and the MI group received only 0.5% CMC (20 mL/kg) once a day. The AS-IV group was receiving MI surgery and were orally AS-IV (40 mg/kg) daily. All mice were intragastric gavage for 4 weeks.
Echocardiographic assessment
After 4-week treatment, all surviving mice underwent echocardiography measurement (Netherlands Philips IE33). All the mice were anesthetized with 2% isoflurane and fixed on a heating plate to maintain body temperature and supine position, after depilation, the heart was imaged in 2-D mode at 2 cm depth of the parasternal short axis. The results of echocardiography were an average of three independent cardiac cycles.
Heart weights
Both the heart weight index (heart weight/body weight ratio, HWI = HW/BW) and left ventricular weight index (left ventricular weight/body weight ratio, LVWI = LVW/BW) were calculated from the results of the associated measurements.
Heart histology and immunohistochemical analyses
The heart was fixed in 10% neutral formaldehyde and embedded in paraffin to make myocardial histopathological sections (5 μm). After dewaxing, H&E and Masson trichrome staining were performed respective, immunohistochemistry (IHC) staining for Collagen I (ab270993, Abcam, USA), Collagen III (ab7778, Abcam, USA), α-SMA (19245S, CST, USA), Fibronectin (ab2413, Abcam, USA), F4/80 (28, 463-1-AP, Proteintech, USA), Myeloperoxidase (MPO,012319, Biocare medical, USA), GSDMD (ab219800, Abcam, USA) were performed in paraffin sections according to the manufacturer’s instruction.
Analysis of ROS levels
Analysis of ROS levels was carried out according to the relevant literature [14]. Ventricular tissue near the apex of the heart was separated, then put it into tin foil paper and embedded with OCT, and quickly put it into liquid nitrogen, freeze for 15 s. After embedding, 5-μm-thick sections were generated with a tissue-processing equipment for ROS staining. After drying the frozen sections, 50 uL DHE working solution was added to each slice, incubated at 37°C for 30 min, rinse with PBS three times for 5 min. The slices were sealed with an anti-fluorescence quenching agent. Fluorescence microscope was used to take photos in a darkroom.
Immunofluorescence
Immunofluorescence staining of WGA was performed as described previously [15]. Paraffin slices were dewaxed, the slices were washed with double distilled water for 5 min and PBS for three times, 20 μg/mL protease K was incubated for 0.5 h and washed with PBS for three times, WGA (GTX01502, GeneTex, USA) was diluted with HBSS to 20 μg/mL, then added sufficient staining solution to completely cover the slices and incubated at 37°C in the dark for 1 h. PBS was rinsed three times again. Finally, 30 μL DAPI solution was added to each slice. After sealing the slide with cover glass, the images were observed and collected by fluorescence microscope in the dark room.
Enzyme-linked immunosorbent assay (ELISA)
The serum levels of IL-1β and IL-18 were measured using commercial ELISA kits according to the manufacturer’s instructions (70-EK201B/3-96, 70-EK118-48, Multi science, China).
Cell culture and treatment
For bone marrow-derived macrophages (BMDMs) experiment, the femur and tibia were isolated from 10 C57BL/6 J mice of 6-week-old, and then the bone marrow was then washed out with a 1-mL syringe containing 1640 medium (31,870,082, Gibco), repeated the above steps until a sufficient amount of bone marrow is collected, the cells are gently dispersed, centrifuged at 250 × g for 5 min, resuspend the cell precipitation with 1640 medium, and seed it in the well plate, and added 40 ng/mL macrophage colony-stimulating factor (M-CSF) (0518245, Peprotech, USA). After incubating for 6 days, then stimulated BMDMs with 1 μg/mL lipopolysaccharide (LPS) (L4130, Sigma-Aldrich, USA) for 4 h, and then treated with 20 uM nigericin (S6653, Selleck, USA) for 30 min, AS-IV is added to BMDMs in conjunction with LPS.
CCK-8 experiment
We configure 100 uL of BMDMs suspension in 96-well plate, and different concentrations of AS-IV were added to 96-well plate and cultured for 24 h. Then, 10 μL of CCK-8 solution was added to each hole, then put the plate in the incubator for 2 h. Absorbance at 450 nm was measured using a microplate reader.
Western blotting
The total protein of the left ventricle and the collected BMDMs were extracted according to the instructions, and the protein concentration was calculated by BCA protein kit (23,235, Thermo Fisher, USA). Proteins were transferred to the nitrocellulose membrane by SDS-polyacrylamide gel electrophoresis. The milk was sealed for 1 h at room temperature, and the TBST was washed 3 times for 10 minutes each time. The membranes were incubated at 4°C overnight with primary antibodies of NLRP3 (15101S, CST, USA), Caspase-1 (ab179515, Abcam, USA), IL-1β (ab9722, Abcam, USA), IL-18 (ab207323, Abcam, USA), GSDMD-N (96458S, CST, USA). After washing with TBST, the membranes were incubated with secondary antibody for 1 h at room temperature. The absorbance of the target protein band was scanned by a gel image analysis system (Tanon Science & Technology Co., Shanghai, China). GAPDH (ab8245, Abcam) served as an internal control.
Flow cytometry detection of ROS
After BMDMs were collected, they were suspended in 10 μmol/L DCFH-DA (S0033S, Beyotime, China) working solution, incubated for 20 minutes in a cell incubator, and then washed with a serum-free medium for 3 times, finally detected by flow cytometry (BD Biosciences). FlowJo software was used for the analysis of data [16].
Statistical analysis
All results are expressed as mean ± standard deviation (SD). GraphPad 9.0 was used for statistical analysis and drawing. Statistical analysis was tested by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests. Quantitative assessment of western blotting, Masson’s trichrome, and WGA staining were performed by Image J (Image J 1.48 v, NIH). P < 0.05 showed that the difference was statistically significant.
Results
AS-IV treatment preserves cardiac function after MI
To determine whether AS-IV can improve cardiac function after MI, we performed a survival analysis, gross morphology of whole hearts, echocardiography, body weights, and heart weights. The survival rate of mice after 4 weeks of treatment is shown in Figure 1(a). On the 28th day after MI, the AS-IV group have 17 mice survived, compared with 13 mice in the MI group, the survival rate of the AS-IV group was higher. Figure 1(b) shows a representative gross morphology of whole hearts, compared with sham group, the MI group heart size was significantly increased, whereas the AS-IV group heart size was smaller than MI group. Figure 1(c-e) shows representative echocardiograms of the three groups of mice. At 4-weeks post MI, LVEF and LVFS were significantly restored in mice treated with AS-IV compared to the MI groups. Furthermore, compared with the sham group, the HWI and LVWI (Figure 1(f, g)) were significantly increased in the MI group; however, after treatment with AS-IV, the HWI and LVWI in the AS-IV mice were decreased significantly. These results suggest that AS-IV can effectively protect cardiac function.
Figure 1.
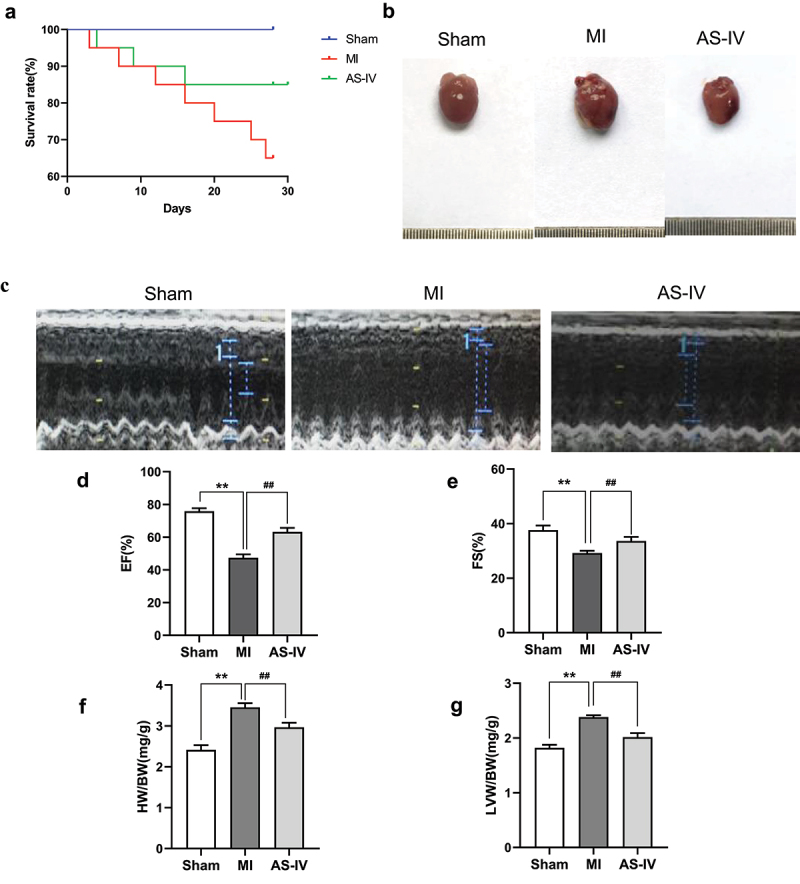
AS-IV helps preserve cardiac function after MI. (a) Kaplan–Meier survival analysis at different time points post-MI. (b) Representative gross morphology of whole hearts. (c) Representative echocardiograms of the three groups: sham group, MI group, AS-IV group (n = 20 of each group). (d)LVEF (n = 20 of Sham group, n = 13 of MI group, n = 17 of AS-IV group). (e)LVFS (n = 20 of Sham group, n = 13 of MI group, n = 17 of AS-IV group). (f) HWI = HW/BW. (g) LVWI = LVW/BW. **P < 0.01 vs the sham group, ##P < 0.01 vs the MI group.
AS-IV relieves myocardial injury and cardiomyocyte hypertrophy
To evaluate the effect of AS-IV on myocardial injury and hypertrophy in MI mice, H&E, Masson, and WGA staining were performed on myocardial tissue. As shown in Figure 2(a-b), the myocardial injury and fibrosis were observed by H&E and Masson staining. In the sham group, the shape of cardiomyocytes was clear and arranged orderly, while in the MI group, the morphology of cardiomyocytes was disordered and a large number of inflammatory cells infiltrate. A lot of cardiomyocytes were replaced by collagen fibers, and the myocardial fibers were disordered; however, after treatment with AS-IV, the cardiomyocytes morphology and fiber arrangement were obviously improved. WGA staining indicated that compared with sham group, myocyte cross-sectional area in MI group was significantly increased, after treatment with AS-IV, cardiomyocyte hypertrophy in AS-IV mice was significantly reduced (Figure 2(c)). The above results demonstrated that AS-IV can effectively reduce myocardial injury and hypertrophy after MI.
Figure 2.
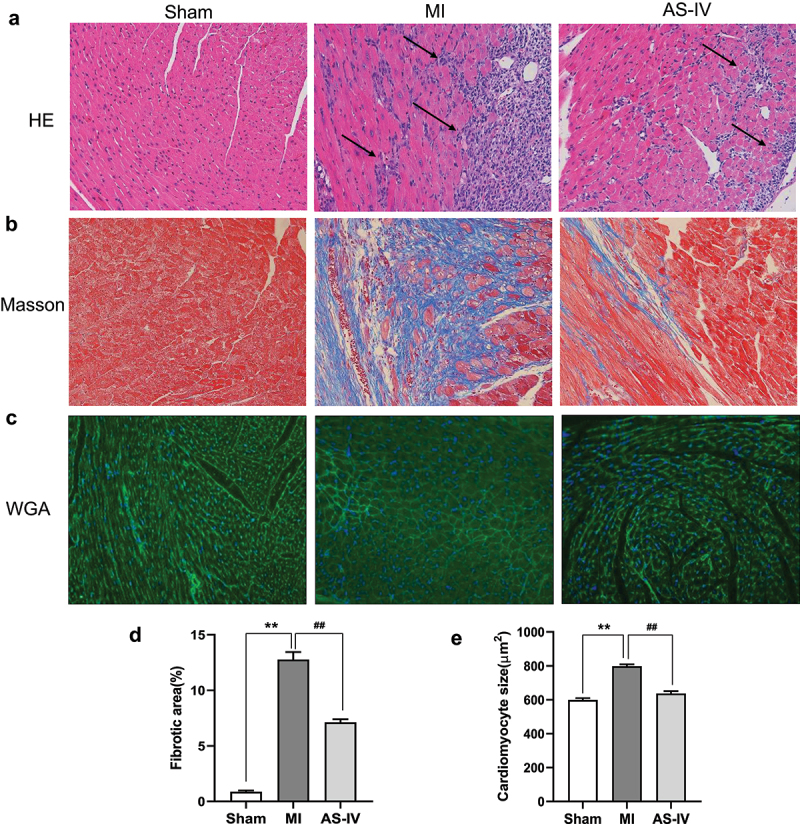
AS-IV relieves the myocardial injury and myocardial hypertrophy. (a) Representative H&E staining of the heart sections(magnification×200). (b) Representative Masson’s trichrome staining of the heart sections(magnification×200). (c) Representative WGA staining from each group(magnification×200). (d)The percentage of fibrotic area of each group (n = 3 of each group). (e) Quantitative analysis of the cardiomyocyte size (n = 3 of each group). **P < 0.01 vs the sham group, ##P < 0.01 vs the MI group.
AS-IV treatment attenuates myocardial fibrosis
Next, we further verified the effect of AS-IV on the expression of fibrosis markers by western blot and immunohistochemistry. Consistent with the results of cardiac histology analyses, the western blot results showed that the expression of α-SMA and Collagen I in MI mice was up-regulated; however, AS-IV significantly reduced the expression of these proteins (Figure 3(a-c)). In addition, immunohistochemical staining further showed that Collagen I, Collagen III, α-SMA, and Fibronectin expressions were significantly increased in MI mice, whereas AS-IV reversed these changes, which indicated that AS-IV had a good effect on myocardial fibrosis (Figure 3(d)).
Figure 3.
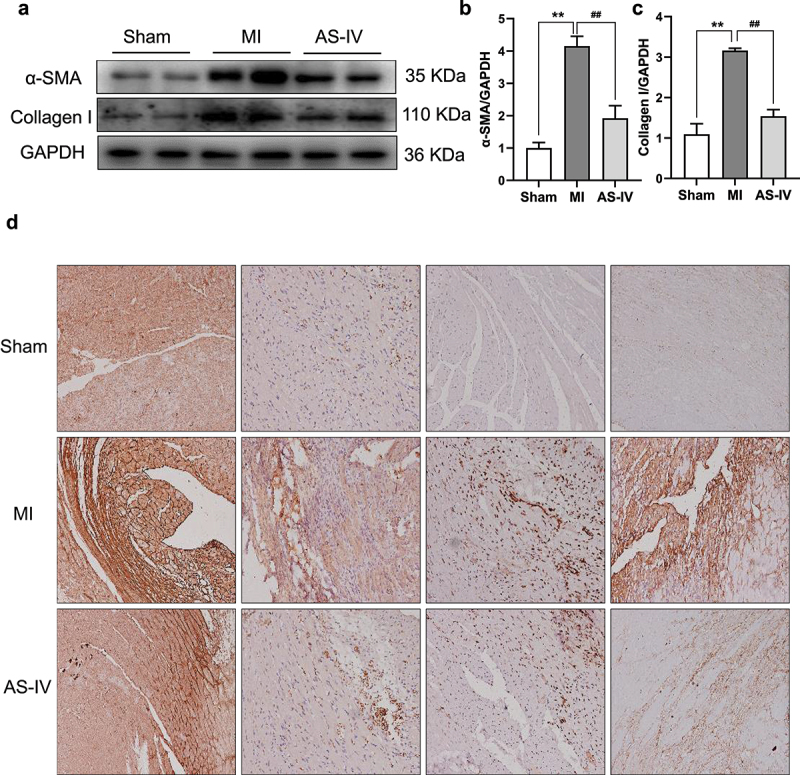
AS-IV treatment attenuates myocardial fibrosis. (a) The protein levels of α-SMA, Collagen I were determined by western blot in the heart. (b-c) Each band was quantified. Data represent four independent experiments, values are expressed as mean ± SD (n = 6 of each group, **P < 0.01 vs the sham group, ##P < 0.01 vs the MI group. (d) Representative Collagen I, Collagen III, α-SMA and Fibronectin immunohistochemical staining (magnification×200).
AS-IV alleviates inflammatory infiltration after MI
Studies have shown that after MI, necrotic myocardial fragments can be recognized by innate immunity as damage-related molecular patterns (DAMPs) and induce acute and severe inflammatory responses [17]. Neutrophils are the first recruited immune cell to enter the ischemic myocardium, and then monocyte macrophages are recruited to the ischemic myocardium to induce acute inflammatory response [18]. Therefore, we further detected the expression of neutrophils and macrophages in myocardial tissue. Immunohistochemical results showed that F4/80 and MPO expression were increased in MI group, however AS-IV alleviated F4/80 and MPO infiltration after MI (Figure 4(a-b)). In addition, ELISA data indicated that, compared with the MI group, IL-18 and IL-1β expression was considerably lower in the AS-IV group (Figure 4(c-d)). Taken together, these results showed that AS-IV could alleviate inflammatory infiltration after MI.
Figure 4.
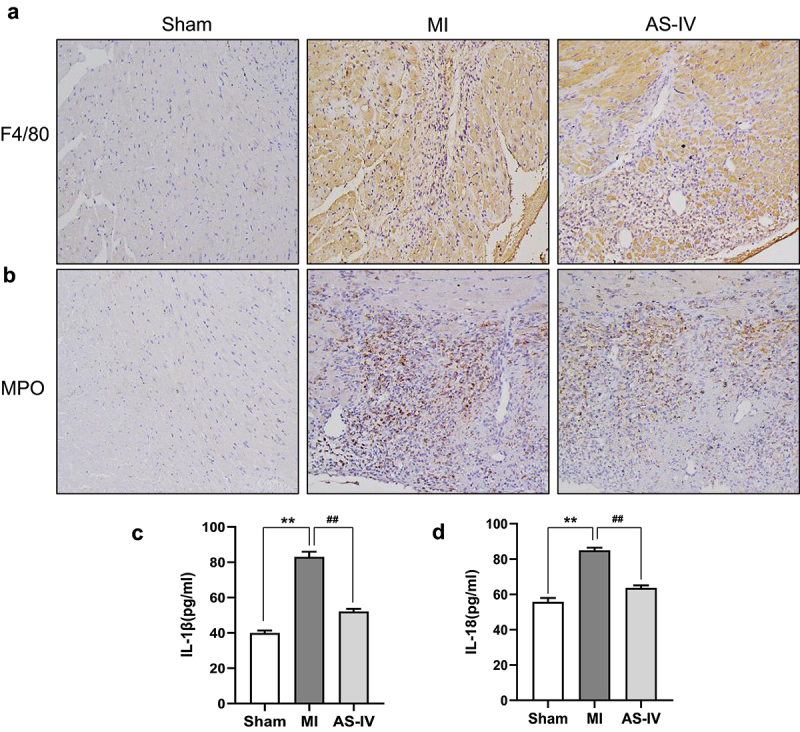
AS-IV alleviated inflammatory infiltration after MI.(a-b) Representative F4/80 and MPO immunohistochemical staining(magnification×200). (c-d) ELISA to evaluate the level of serum IL-1β and IL-18 in mice (n = 6 of each group). **P < 0.01 vs the sham group, ##P < 0.01 vs the MI group.
Effect of AS-IV on the ROS/NLRP3/GSDMD signaling pathway activation
A large number of studies have confirmed that oxidation stress is involved in the occurrence and development of MI, ROS plays a key role in the process. To clarify the impact of AS-IV on antioxidant stress, we detected the expression of ROS in myocardial tissue. As shown in Figure 5(a), ROS staining results showed that, compared with the sham group, the level of ROS in the MI group increased significantly, while AS-IV effectively reduced the release of ROS. These results suggested that AS-IV can reduce the release of ROS induced by MI. To further clarify the interaction between ROS production caused by MI and cardiomyocyte pyroptosis, we further detected the expression of pyroptosis-related protein, GSDMD-N, which is a key protein in the process of pyroptosis. As shown in Figure 5(b-g), the protein levels of NLRP3, cleaved cas-1, cleaved IL-1β, cleaved IL-18, and GSDMD-N were markedly elevated in MI group, and these effects were significantly reversed by AS-IV. In addition, immunohistochemical staining further showed that AS-IV could inhibit the expression of GSDMD after MI (Figure 5(h)). This illustrated that the AS-IV could reduce the release of ROS, thereby inhibiting the activation of NLRP3 inflammasome and cardiomyocyte pyroptosis.
Figure 5.
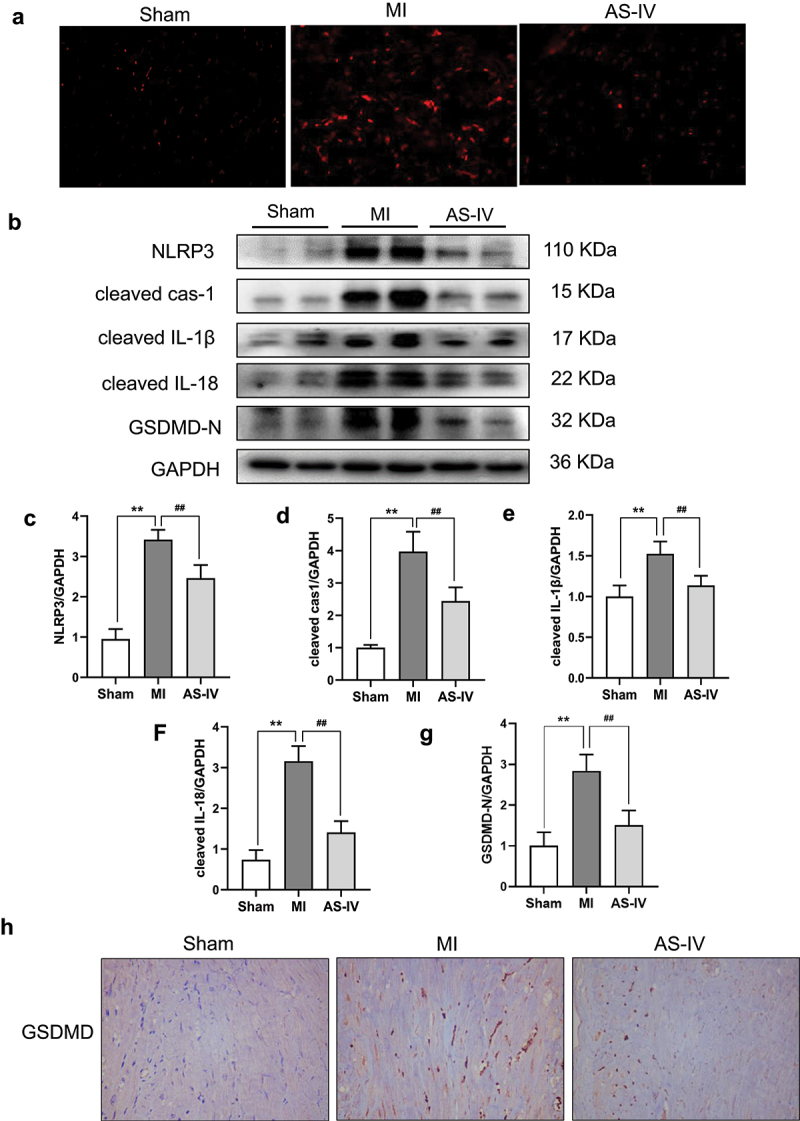
Effect of AS-IV on the ROS/NLRP3/GSDMD signaling pathway activation. (a) Representative images of ROS staining (magnification 200×) (b) The protein levels of NLRP3, cleaved cas-1, cleaved IL-1β, cleaved IL-18 and GSDMD-N were determined by western blot in the heart. (c-g) Each band was quantified. Data represent three independent experiments. Values are expressed as mean ± SD (n = 6 of each group). **P < 0.01 vs the sham group, ##P < 0.01 vs the MI group. (h) Representative GSDMD immunohistochemical staining (magnification×200).
Effect of AS-IV on pyroptosis of BMDMs
In vivo experiments showed that AS-IV can effectively improve cardiac function and reduce cardiomyocyte pyroptosis, to verify whether the reduction of cardiomyocyte pyroptosis by AS-IV is related to the regulation of macrophages; hence, we established the pyroptosis model of BMDMs in vitro, and further observed the effect of AS-IV on BMDMs. According to cell viability assay, 100 μM AS-IV had the best cell viability for BMDMs (Supplemental Fig.1); therefore, a concentration of 100 μM was chosen for the cell experiment. Based on the results reported in previous studies [19], we stimulated BMDMs with 1 μg/mL LPS for 4 h, and then treated with 20 uM nigericin for 30 min, AS-IV is added to BMDMs in conjunction with LPS. As shown in Figure 6(a), after treating with LPS and nigericin, the ROS level in model group has significantly increased compared with the control group; however, the level of ROS in AS-IV group was remarkably lower than control group. To further clarify the mechanism of AS-IV on BMDMs, the NLRP3/Caspase-1/GSDMD signaling pathway was detected by using western blot. The results showed that 100 μM AS-IV could significantly inhibit the expression of NLRP3, cleaved cas-1, cleaved IL-1β, cleaved IL-18, and GSDMD-N (Figure 6(b-g)). The results indicated that AS-IV may play a crucial effect in the anti-inflammatory process by reducing macrophage pyroptosis.
Figure 6.
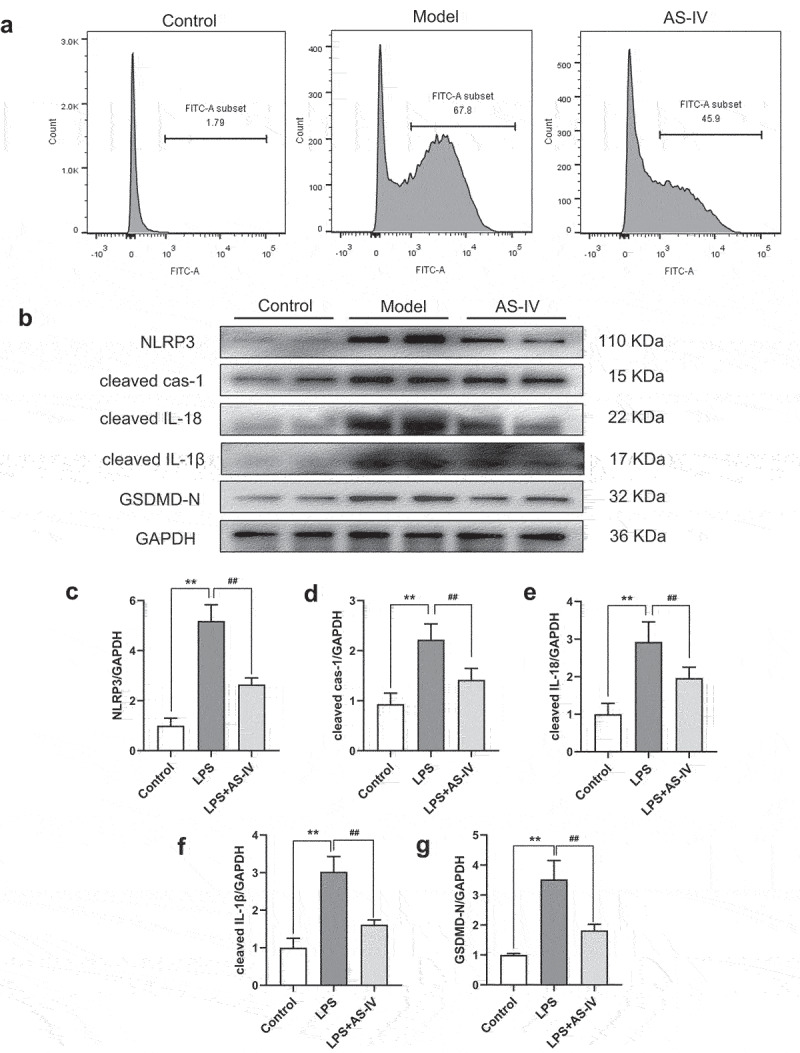
Effect of AS-IV on pyroptosis of BMDMs. (a) ROS was detected by flow cytometry. (b) The protein levels of NLRP3, cleaved cas-1, cleaved IL-1β, cleaved IL-18 and GSDMD-N were determined by western blot in the BMDMs. (c-g) each band was quantified. data represent three independent experiments. values are expressed as mean ± SD (n = 6 of each group). **P < 0.01 vs the sham group, ##P < 0.01 vs the MI group, #P < 0.05 vs the MI group.
Discussion
Multiple evidence supports the efficacy of AS-IV in different models, Zhao et al. have reported that AS-IV can improve cardiac function, inhibit compensatory hypertrophy of cardiomyocytes, and reduce the number of apoptotic cardiomyocytes in the LAD-induced CHF model [13]. Cheng et al. found that AS-IV by inhibiting TLR4/NF-κB pathway, effectively improved the survival rate and cardiac function, which indicated that AS-IV has a strong anti-inflammatory effect in AMI-induced HF rats [20]. Lu et al. also found that AS-IV can attenuate LPS-induced cardiac dysfunction and inflammatory response by inhibiting the NF-кB signaling and activate PI3K/AKT pathway [21]. Although previous studies have shown that AS-IV can reduce NLRP3 and IL-1β expression after MI, however, the effect of AS-IV on cardiomyocyte pyroptosis has not been reported. This study proves for the first time that AS-IV exerts myocardial protection by inhibiting pyroptosis in MI hearts. In our study, we confirmed that AS-IV can effectively reduce mortality, improve cardiac function, and reduce myocardial hypertrophy and fibrosis in MI-induced mice. These results suggest that AS-IV might develop into a promising drug for patients with myocardial injury.
Acute inflammatory response in the early stage of AMI is triggered by innate immune system against ischemic cardiomyocyte necrosis, necrotic myocardial fragments are recognized by the innate immune system as DAMPs, triggering an acute and intense inflammatory response [22,23]. At the same time, DAMPs induce the expression of pro-inflammatory chemokines and cytokines, which leads to the recruitment of immune cells and a series of inflammatory events. DAMPs can also activate the inflammasome of NLRP3, which is a multi-protein complex, NLRP3 can induce the production of active pro-inflammatory cytokines IL-1β and IL-18 [24]. Il-1β and IL-18 can induce myocardial inflammation, promote myocardial fibrosis, aggravate MI, and reduce myocardial systolic function [25–27]. Therefore, reducing the inflammatory response after MI is very important to alleviate myocardial fibrosis and cardiac remodeling [28,29]. Murphy et al. found that inhibition of inflammatory response can reduce the production of cytokines, thereby reducing the number of cardiac cell death and improving cardiac function in patients with HF [30]. The results of myocardial pathological staining showed that AS-IV could effectively reduce the infiltration of inflammatory cells in myocardium, Immunohistochemistry analysis further showed that AS-IV treatment could reduce the expression of F4/80 and MPO, what is more, the levels of serum IL-1β and IL-18 cytokines in AS-IV treatment group were decreased significantly.
A large amount of ROS can be released during myocardial ischemia, which plays a crucial role in oxidative stress [31,32]. On the one hand, ROS can directly damage myocardial function, on the other hand, it induces the activation of inflammatory signals [33]. Anti-oxidative stress therapy in preclinical environment has also been widely studied and showed very promising results. Qian Lie et al. demonstrated that suppression of ROS by NAC can reduced NF-κB and NLRP3 inflammasome activation [34]. In recent years, ROS has been considered as a key regulator of NLRP3 inflammasome activation [35], inhibit ROS can reverse the activation of NLRP3 inflammasome [36]. As a result, we demonstrated that AS-IV can reduce the release of ROS in myocardial mitochondria and decrease the expression of NLRP3, caspase-1, IL-18, and IL-1β proteins. Furthermore, our results also indicated the relationship between ROS release and NLRP3 inflammasome activation.
When cells are stimulated in different ways, they are induced by the classical inflammatory pathway dependent on caspase-1 and the non-classical pathway dependent on caspase-11. In the classical inflammasome pathways of pyroptosis, the signal is initiated by pathogen-related molecular patterns (PAMPs) or DAMPs, such as ischemia and hypoxia, uric acid crystals, ROS, and ATP, which cause inflammasomes to assemble and activate caspase-1 [37]. Activated caspase-1 cleaved GSDMD releases the active N-terminal domain [38] and promotes the maturation of pro-inflammatory precursor cytokines, which are released into the extracellular and cause the inflammatory response [4]. NLRP3 inflammasomes are activated by cardiovascular risk factors, such as bacteria, viruses, cholesterol crystals, and uric acid, which in turn activate downstream caspase-1, IL-18 and IL-1β activation, triggering cell pyroptosis [39,40]. Recently, it was reported that the inhibition of caspase-1 could attenuate cardiomyocyte hypertrophy [41]. GSDMD is the executor of pyroptosis, where inhibition of GSDMD pore formation also plays a protective role in myocardium. Small-molecule inhibitor necrosulfonamide (NSA) binds directly to GSDMD and inhibits the oligomerization of P30-GSDMD, blocking the pyroptosis in monocytes or macrophages secretion [42]. Our previous studies have showed that NLRP3 and IL-1β were up-regulated after MI [15]. Therefore, we selected mice after MI to observe the expression of pyroptosis-related proteins. Our study showed that AS-IV significantly suppresses the expression of GSDMD-N, which is the main functional domain. The immunohistochemical results also further verified that AS-IV could reduce the pyroptosis of cardiomyocytes. Consequently, our results indicated that AS-IV can effectively reduce pyroptosis induced by MI.
In the acute inflammatory stage, neutrophils are the earliest immune cell to enter the ischemic myocardium, and then mononuclear macrophages are recruited to the ischemic myocardium to induce acute inflammatory response, phagocytosis of necrotic myocardium, and repair the damaged myocardium [43]. Previous studies confirmed that LPS and nigericin can cause BMDMs pyroptosis [44]. Our results showed that the expressions of NLRP3, cleaved cas-1, IL-1β, IL-18, and GSDMD-N proteins were reduced in BMDMs treated with AS-IV, and we validated that the cardioprotective effect of AS-IV after MI is related to reduction of macrophage pyroptosis.
In summary, our study firstly demonstrated that AS-IV significantly attenuates MI-induced cardiac dysfunction and inflammatory response by down-regulating ROS/Caspase-1/GSDMD signaling pathways, and the underlying mechanism may be relevant to reduce pyroptosis of macrophages (Figure 7). Our results confirm the effectiveness of AS-IV in the treatment of MI. In the future, AS-IV may be a potential candidate for treatment of MI patients.
Figure 7.
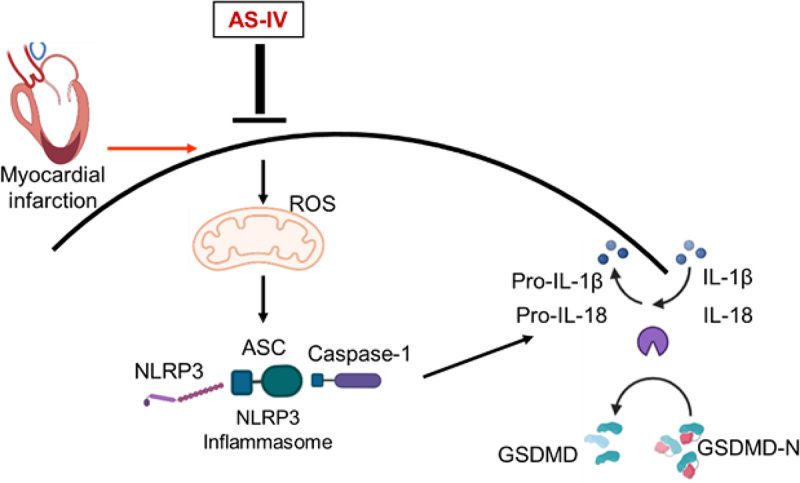
Mechanism underlying the effects of AS-IV after MI. MI induced mitochondria ROS production, activated the ROS/NLRP3/GSDMD pathway, AS-IV intervention inhibiting the production of ROS and the expression of NLRP3, cleaved cas-1, cleaved IL-1β, cleaved IL-18 and GSDMD-N, thus ameliorating myocardial fibrosis and cardiac remodeling.
Limitations
In the present study, we have just demonstrated that AS-IV can downregulate ROS/Caspase-1/GSDMD signaling pathways, and attenuate MI-induced myocardial fibrosis and cardiac remodeling, but the effect of AS-IV on cardiac fibroblasts needs further study.
Supplementary Material
Funding Statement
This study was supported by the Three-year Action Plan of Shanghai Shenkang Medical Development Center [grant no.SHDC2020CR1053B]; the Graduate Science and Technology Innovation Program of Shanghai University of Traditional Chinese Medicine [grant no.Y2020014].
Ethical approval
All animal experimental protocols were approved by the Shanghai University of Chinese Medicine (Safety Certificate NO: SYXK-HU-2020-0009, and Animal Ethics Code PZSHUTCM200807012).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
Xiaoqing Zhang wrote the manuscript. Hua Zhou and Huiyan Qu designed the study, and Qian Liu and Tao Yang revised this article. All authors read and approved the final manuscript.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384101.2022.2093598
References
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017 Mar 7;135(10):e146–e603. PubMed PMID: 28122885; PubMed Central PMCID: PMCPMC5408160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Task Force on the management of STseamiotESoC, Steg PG, James SK, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012. Oct;33(20):2569–2619. PubMed PMID: 22922416. [DOI] [PubMed] [Google Scholar]
- [3].Shi J, Gao W, Shao F.. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017Apr;42(4):245–254. PubMed PMID: 27932073. [DOI] [PubMed] [Google Scholar]
- [4].Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016 Jul 7;535(7610):153–158. PubMed PMID: 27383986; PubMed Central PMCID: PMCPMC5539988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang Y, Liu X, Shi H, et al. NLRP3 inflammasome, an immune‐inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin Transl Med. 2020;10(1):91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lei Q, Yi T, Chen C. NF-κB-gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. 2018;24:6044–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J. 2014;55(2):101–105. PubMed PMID: 24632952. [DOI] [PubMed] [Google Scholar]
- [8].Audia JP, Yang XM, Crockett ES, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018 Jul 10;113(5):32. PubMed PMID: 29992382; PubMed Central PMCID: PMCPMC6396295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen A, Chen Z, Xia Y, et al. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem Biophys Res Commun. 2018 May 5;499(2):267–272. PubMed PMID: 29571736. [DOI] [PubMed] [Google Scholar]
- [10].Xu XL, Ji H, Gu SY, et al. Modification of alterations in cardiac function and sarcoplasmic reticulum by astragaloside IV in myocardial injury in vivo. Eur J Pharmacol. 2007 Jul 30;568(1–3):203–212. PubMed PMID: 17509559. [DOI] [PubMed] [Google Scholar]
- [11].Wang SG, Xu Y, Chen JD, et al. Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules. 2013 Oct 16;18(10):12809–12819. PubMed PMID: 24135938; PubMed Central PMCID: PMCPMC6270590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dong Z, Zhao P, Xu M, et al. Astragaloside IV alleviates heart failure via activating PPARalpha to switch glycolysis to fatty acid beta-oxidation. Sci Rep. 2017 Jun 2;7(1):2691. PubMed PMID: 28578382; PubMed Central PMCID: PMCPMC5457407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhao Z, Wang W, Wang F, et al. Effects of astragaloside IV on heart failure in rats. Chin Med. 2009 Apr 2;4(1):6. PubMed PMID: 19338675; PubMed Central PMCID: PMCPMC2674594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang Y, Xie Z, Jiang N, et al. Hispidulin attenuates cardiac hypertrophy by improving mitochondrial dysfunction. Front Cardiovasc Med. 2020;7:582890. PubMed PMID: 33324687; PubMed Central PMCID: PMCPMC7726192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang X, Zhao D, Feng J, et al. LuQi formula regulates NLRP3 inflammasome to relieve myocardial-infarction-induced cardiac remodeling in mice. Evid Based Complement Alternat Med. 2021;2021:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu L, Wang H, Huang L, et al. The protective roles of ROS-mediated mitophagy on (125)I seeds radiation induced cell death in HCT116 cells. Oxid Med Cell Longev. 2016;2016:9460462. PubMed PMID: 28119765; PubMed Central PMCID: PMCPMC5227180 people or organizations that could inappropriately influence this work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016 Jun 24;119(1):91–112. PubMed PMID: 27340270; PubMed Central PMCID: PMCPMC4922528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yan X, Anzai A, Katsumata Y, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013. Sep;62:24–35. PubMed PMID: 23644221. [DOI] [PubMed] [Google Scholar]
- [19].Ye X, Liu X, Wei W, et al. Volume-activated chloride channels contribute to lipopolysaccharide plus nigericin-induced pyroptosis in bone marrow-derived macrophages. Biochem Pharmacol. 2021. Nov;193:114791. PubMed PMID: 34582774. [DOI] [PubMed] [Google Scholar]
- [20].Cheng S, Yu P, Yang L, et al. Astragaloside IV enhances cardioprotection of remote ischemic conditioning after acute myocardial infarction in rats. Am J Transl Res. 2016;8(11):4657–4669. PubMed PMID: 27904669; PubMed Central PMCID: PMCPMC5126311. [PMC free article] [PubMed] [Google Scholar]
- [21].Zhao P, Wang Y, Zeng S, et al. Protective effect of astragaloside IV on lipopolysaccharide-induced cardiac dysfunction via downregulation of inflammatory signaling in mice. Immunopharmacol Immunotoxicol. 2015;37(5):428–433. PubMed PMID: 26376109. [DOI] [PubMed] [Google Scholar]
- [22].Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018. Jun;186:73–87. PubMed PMID: 29330085; PubMed Central PMCID: PMCPMC5981007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu J, Wang H, Li J. Inflammation and Inflammatory cells in myocardial infarction and reperfusion injury: a double-edged sword. Clin Med Insights Cardiol. 2016;10:79–84. PubMed PMID: 27279755; PubMed Central PMCID: PMCPMC4892199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014 Dec 19;289(51):35237–35245. PubMed PMID: 25391648; PubMed Central PMCID: PMCPMC4271212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gross O, Thomas CJ, Guarda G, et al. The inflammasome: an integrated view. Immunol Rev. 2011Sep;243(1):136–151. PubMed PMID: 21884173. [DOI] [PubMed] [Google Scholar]
- [26].Toldo S, Mezzaroma E, O’Brien L, et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014 Apr 1;306(7):H1025–31. PubMed PMID: 24531812; PubMed Central PMCID: PMCPMC3962640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abbate A, Van Tassell BW, Biondi-Zoccai GG. Blocking interleukin-1 as a novel therapeutic strategy for secondary prevention of cardiovascular events. Biodrugs. 2012 Aug 1;26(4):217–233. PubMed PMID: 22571369. [DOI] [PubMed] [Google Scholar]
- [28].Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995 Mar 15;91(6):1872–1885. PubMed PMID: 7882499. [DOI] [PubMed] [Google Scholar]
- [29].Zouridakis EG, Garcia-Moll X, Kaski JC. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am J Cardiol. 2000;86(4):449–451. [DOI] [PubMed] [Google Scholar]
- [30].Murphy SP, Kakkar R, McCarthy CP, et al. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020 Mar 24;75(11):1324–1340. PubMed PMID: 32192660. [DOI] [PubMed] [Google Scholar]
- [31].Cai H, Liu Y, Men H, et al. Protective mechanism of humanin against oxidative stress in aging-related cardiovascular diseases. Front Endocrinol (Lausanne). 2021;12:683151. PubMed PMID: 34177809; PubMed Central PMCID: PMCPMC8222669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun Y, Rawish E, Nording HM, et al. Inflammation in metabolic and cardiovascular disorders-role of oxidative stress. Life (Basel). 2021 Jul 9;11(7). DOI: 10.3390/life11070672. PubMed PMID: 34357044; PubMed Central PMCID: PMCPMC8308054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tian X, He W, Yang R, et al. Dl-3-n-butylphthalide protects the heart against ischemic injury and H9c2 cardiomyoblasts against oxidative stress: involvement of mitochondrial function and biogenesis. J Biomed Sci. 2017 Jun 15;24(1):38. PubMed PMID: 28619102; PubMed Central PMCID: PMCPMC5471652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lei Q, Yi T, Chen C. NF-kappaB-gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. 2018 Aug 30;24:6044–6052. PubMed PMID: 30161099; PubMed Central PMCID: PMCPMC6128186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014 May 22;157(5):1013–1022. PubMed PMID: 24855941. [DOI] [PubMed] [Google Scholar]
- [36].Qiu Z, He Y, Ming H, et al. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-Induced injury through activating ROS-Dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J Diabetes Res. 2019;2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mezzaroma E, Toldo S, Farkas D, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19725–19730. PubMed PMID: 22106299; PubMed Central PMCID: PMCPMC3241791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016 Jul 7;535(7610):111–116. PubMed PMID: 27281216. [DOI] [PubMed] [Google Scholar]
- [39].Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008Aug;9(8):847–856. PubMed PMID: 18604214; PubMed Central PMCID: PMCPMC2834784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Orlowski GM, Colbert JD, Sharma S, et al. Multiple cathepsins promote Pro-IL-1beta synthesis and NLRP3-mediated IL-1beta activation. J Immunol. 2015 Aug 15;195(4):1685–1697. PubMed PMID: 26195813; PubMed Central PMCID: PMCPMC4530060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bai Y, Sun X, Chu Q, et al. Caspase-1 regulate AngII-induced cardiomyocyte hypertrophy via upregulation of IL-1beta. Biosci Rep. 2018 Feb 12. DOI: 10.1042/BSR20171438. PubMed PMID: 29440460; PubMed Central PMCID: PMCPMC5857903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rathkey JK, Zhao J, Liu Z, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018 Aug 24;3(26). DOI: 10.1126/sciimmunol.aat2738. PubMed PMID: 30143556; PubMed Central PMCID: PMCPMC6462819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Andreadou I, Cabrera-Fuentes HA, Devaux Y, et al. Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities. Cardiovasc Res. 2019 Jun 1;115(7):1117–1130. PubMed PMID: 30825305; PubMed Central PMCID: PMCPMC6529904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xiao J, Wang C, Yao JC, et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 2018Nov;16(11):e3000047. PubMed PMID: 30388107; PubMed Central PMCID: PMCPMC6235378 following competing interests: GM is a consultant for Aclaris Therapeutics, Inc., and holds stocks of this company. RC receives research support from Amgen, and holds stock of Amgen, Eli- Lilly, and Merck & Co. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


