Abstract
Objective
To explore the clinical value on combined detection of serum carbohydrate antigen 724 (CA724), secreted protein dickkopf-1 (DKK1), and thymidine kinase 1 (TK1) in the diagnosis of gastric cancer (GC).
Methods
The clinical data of 63 GC patients (GC group) and 54 patients with benign gastric lesions (control group) admitted to Zhu Xianyi Memorial Hospital of Tianjin Medical University from June 2020 to June 2021 were retrospectively analyzed. The levels of serum CA724, DKK1, and TK1 in the two groups were detected by electrochemiluminescence instrument, enzyme-linked immunosorbent assay, and enhanced chemiluminescence. The diagnostic efficacy of single detection and combined detection was analyzed by drawing the receiver operating characteristic (ROC) curve.
Results
Compared with the control group, the serological indexes of patients in GC group were markedly higher (P < 0.001). ROC curve analysis showed that the areas under the curve of serum CA724, DKK1, TK1, and combined detection in the diagnosis of GC were 0.849, 0.754, 0.685, and 0.923, respectively; and the sensitivity and specificity of their combined detection were higher than those of separate detection.
Conclusion
The levels of serum CA724, DKK1, and TK1 were highly expressed in GC patients, with a higher diagnostic value for GC in their combined detection, which can effectively screen and assist the diagnosis of GC.
1. Introduction
Gastric cancer (GC) is a common tumor disease in digestive tract and one of the most common cancers in the world. In 2018, there were more than 1 million new cases and about 783,000 dead cases worldwide. Southeast Asia is a high-incidence area of GC in the world, and the prevention and control of GC in China are also severe [1]. Patients had no specificity in early clinical symptoms of patients, so it is often confused with gastritis or gastric ulcer in clinical diagnostic process [2]. Although there are many examination methods for this tumor disease, such as gastroscopy, X-ray examination, and CT scanning, the detection rate of early GC is still low, and most patients are already in the middle and late stages when the diagnosis seriously affects the clinical therapeutic effect [3], so that the accurate diagnosis of early GC has become the focus of attention in clinic. With the continuous progress of molecular biology technique, serum tumor markers have been gradually applied to the prediction of benign and malignant tumors. Studies have found that [4] the abnormal expression of human serum tumor markers occurs earlier than clinical symptoms. Some studies have also found [5] that CA724 in the diagnosis of positive rate in GC is higher than that of other tumor markers, which can be used as an important serum factor to judge the process of GC and clinical efficacy. DKK can regulate intracellular signal transduction by binding to its corresponding receptors, thereby determining the characteristics such as cellular differentiation, survival, and apoptosis, with an important effect in tumorigenesis [6, 7]. TK1 is a kinase related to cell proliferation, which plays a positive role in evaluation of cellular proliferating activity. The literature report has pointed out that [8] serum CA724 has an abnormal expression in early GC. Based on these, this study detected the serological indexes of GC and patients with benign gastric diseases and analyzed the diagnostic value of indexes and combined detection, so as to provide more references for choosing the treatment plan of GC.
2. Materials and Methods
2.1. General Information
63 GC patients (GC group) and 54 patients with benign gastric lesions (control group) admitted in Zhu Xianyi Memorial Hospital of Tianjin Medical University from June 2020 to June 2021 were selected as the study subjects for the retrospective analysis. This study has been approved by the Ethics Committee of Zhu Xianyi Memorial Hospital of Tianjin Medical University (approval no. 20200413) and was in line with the Declaration of Helsinki (2013) [9].
2.2. Inclusion and Exclusion Criteria
Inclusion criteria. (1) All patients in GC group were confirmed by surgical pathology, with the first onset. (2) Patients did not receive the chemotherapy or immunotherapy before admission. (3) Patients with benign gastric lesions were diagnosed by gastroscope and pathological examination.
Exclusion criteria. (1) Patients with other malignant tumors; (2) female patients in pregnancy or lactation period; and (3) patients with immune system disease or hematological disease.
2.3. Detection Method
Fasting elbow vein blood (3 ml) of all study subjects was obtained in the morning of admission, and the physiological saline was added to dilute the samples. After coagulation, the blood was centrifuged at 3000 rev/min for 5 min to separate the serum, and then, the clear supernatant was stored in a refrigerator at -80°C for examination. The CA724 level (reference range of 0-6.9 U/ml) in serum sample was detected using an electrochemiluminescence instrument (manufacturer: Shanghai Mojin Medical Instrument Co., Ltd.; model: Roche cobas e411), the serum DKK1 level (reference range of 0-3.3 ng/ml) was detected by enzyme-linked immunosorbent assay (kit purchased from Shanghai Fusheng Industry Co., Ltd.), and the enhanced chemiluminescence (kit purchased from Shanghai Toscience Biotechnology Co., Ltd.) was used to detect the TK1 level (reference range of 0-75.0 pmol/ml). All operations were strictly in accordance with the kit instruction.
2.4. Statistical Method
The SPSS 26.0 was used for the data analysis, the enumeration data such as gender and measurement data such as age and BMI value were indicated by (n (%)) and (−x ± s), and the comparisons between the two groups were tested by x2 and independent sample t test, respectively. The efficacy of separate detection of serum CA724, DKK, and TK1 and combined detection under the parallel structure model in the diagnosis of GC was analyzed using the ROC curve to obtain the sensitivity and specificity of diagnosis. P < 0.05 indicated a statistic significance.
3. Results
3.1. Comparison of Clinical Data between the Two Groups
There was no significant difference in clinical data such as age, WBC values, MCV values, and educational level (P > 0.05), see details in Table 1.
Table 1.
Comparison of clinical data between the two groups.
| Projects | GC group (n = 63) | Control group (n = 54) | X 2/t | P |
|---|---|---|---|---|
| Gender | ||||
| Male/female | 35/28 | 30/24 | 0.000 | 1.000 |
| Average age (mean ± SD, years) | 48.92 ± 8.94 | 47.41 ± 8.18 | 0.947 | 0.346 |
| BMI (mean ± SD, kg/m2) | 21.36 ± 1.35 | 21.61 ± 1.33 | 1.005 | 0.317 |
| Types of GC | / | / | / | |
| Diffuse pattern | 29 (46.03) | / | ||
| Intestinal type | 34 (53.97) | / | ||
| TNM staging | / | / | ||
| Stage I | 32 (50.79) | / | ||
| Stage II | 17 (26.98) | / | ||
| Stage III | 8 (12.70) | / | ||
| Stage IV | 6 (9.52) | / | ||
| WBC (mean ± SD, ×109/L) | 6.01 ± 1.55 | 6.31 ± 1.53 | 1.258 | 0.223 |
| HCT (mean ± SD, %) | 0.39 ± 0.04 | 0.40 ± 0.06 | 1.537 | 0.097 |
| MCV (mean ± SD, fl) | 87.94 ± 5.21 | 86.57 ± 5.26 | 1.644 | 0.108 |
| Educational level | ||||
| College and above | 5 (7.94) | 4 (7.41) | 0.012 | 0.915 |
| Senior high school | 17 (26.98) | 13 (24.07) | 0.129 | 0.719 |
| Junior high school | 20 (31.75) | 15 (27.78) | 0.218 | 0.640 |
| Primary school | 12 (19.05) | 18 (33.33) | 3.112 | 0.078 |
| Illiteracy | 9 (14.29) | 4 (7.41) | 1.393 | 0.238 |
| Place of residence (n (%)) | 0.030 | 0.863 | ||
| Town | 27 (42.86) | 24 (44.44) | ||
| Countryside | 36 (57.14) | 30 (55.56) |
3.2. Comparison of Serological Indexes between the Two Groups
Compared with control group, the serological indexes in GC group were markedly higher (P < 0.001), see details in Table 2.
Table 2.
Comparison of serological indexes between the two groups (−X ± S).
| Groups | n | CA724 (U/ml) | DKK1 (ng/ml) | TK1 (pmol/ml) |
|---|---|---|---|---|
| GC group | 63 | 24.91 ± 2.01 | 4.56 ± 0.36 | 104.64 ± 11.48 |
| Control group | 54 | 4.05 ± 1.20 | 3.03 ± 0.40 | 66.36 ± 4.65 |
| t | 66.726 | 21.771 | 22.933 | |
| P | <0.001 | <0.001 | <0.001 |
3.3. Diagnostic Efficacy of Serum CA724, DKK1, TK1, and Combined Detection in GC
See Table 3 and Figure 1 for details.
Table 3.
Diagnostic efficacy of serum CA724, DKK1, TK1, and combined detection in GC.
| Diagnosis methods | Specificity | Sensitivity | Area under curve | Positive predictive value | Negative prediction value | Asymptotic 95% confidence interval |
|---|---|---|---|---|---|---|
| CA724 | 80.00% | 89.47% | 0.849 | 80.95% | 88.89% | 0.774-0.924 |
| DKK1 | 71.19% | 79.31% | 0.754 | 73.02% | 77.78% | 0.663-0.845 |
| TK1 | 64.41% | 72.41% | 0.685 | 66.67% | 70.37% | 0.587-0.783 |
| Combined detection | 90.91% | 93.55% | 0.923 | 92.06% | 92.59% | 0.867-0.979 |
Figure 1.
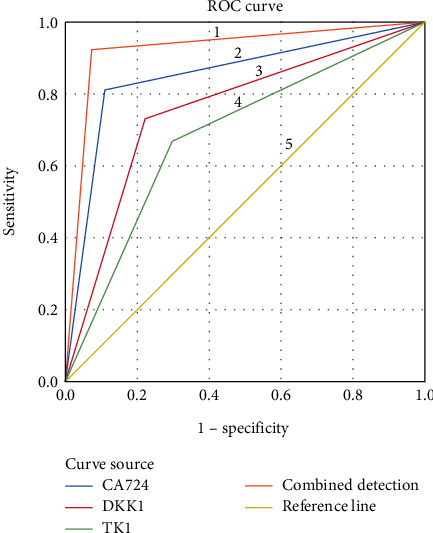
ROC curve of serum CA724, DKK1, TK1, and combined detection in the diagnosis of GC. Notes. 1 represented the ROC curve of combined detection for the diagnosis of GC, 2 represented the ROC curve of CA724 for the diagnosis of GC, 3 represented the ROC curve of DKK1 for the diagnosis of GC, 4 represented the ROC curve of TK1 for the diagnosis of GC, and 5 represented the reference line.
4. Discussion
GC, as a malignant tumor originating from gastric epithelium, is a common tumor disease in digestive system, with a higher incidence [10], and it is classified as human group 1 carcinogen by the International Agency for Research on Cancer in the World Health Organization (WHO) [11]. There are many incidence positions of GC, such as curvatura ventriculi minor and gastric antrum, which often has no obvious symptom in the early stage of this disease and is easy to be ignored. Therefore, the disease has entered the middle and late stages when patients are diagnosed, which is not conducive to the treatment of disease. Although pathological biopsy under gastroscopy is recognized as the gold standard for the diagnosis of GC, it is not widely used in clinic due to the invasive operation [12, 13], so that the diagnosis of this disease has attracted the attention of many physicians in oncology department and patients.
With the progress of medical diagnosis technology, the advantages of serum tumor markers with high specificity and sensitivity and convenient detection in the differential diagnosis of GC have become the focus of clinical study [8]. Some studies [14] have confirmed that the combined detection of serum CA724 and CA19-9 has high specificity and sensitivity in the diagnosis of gastric stromal tumor, and studies have also reported [15] that serum DKK1 has an obviously high expression in patients with primary liver cancer. However, the sensitivity and specificity of detection with single indicator are low, which cannot meet the clinical needs due to the diversity and complexity of biological characteristics in tumor cells [16, 17]. Therefore, it is very important to combine different serum tumor markers for early diagnosis and treatment of GC patients to perform the combined detection of multiple targets. However, the value on combined detection of serum CA724, DKK1, and TK1 in the diagnosis of GC is not clear. In this study, it was found that the levels of serum CA724, DKK1, and TK1 in GC group were higher than those in control group by detecting the serological indexes of GC group and control group (P < 0.001). The putative reasons are as follows. CA724 is mucinoid glycoprotein polymer recognized by two monoclonal antibodies of cc49 and b72.3, with an abnormal increase in various gastrointestinal tumors and ovarian cancer [18]. DKK1 has been proved to be a new therapeutic target for lung cancer and esophageal cancer and plays an important role in the pathogenesis and development of gastric cancer, cervical cancer, and other malignant tumors [19]. The Wnt/β-catenin signaling pathway is inhibited due to the changes of β-catenin in GC patients, resulting in a high expression of DKK1. DKK1 is a secreted protein that can be secreted into the blood, and the serum DKK1 level will be significantly elevated with its increased expression in gastric cancerous tissues [20]. As a marker of abnormal cell proliferation, the content of TK1 is positively correlated with the situation of DNA synthesis in the body. Clinical studies have found [21] that the content of TK1 in nonproliferative cells is extremely low, which is involved in cell proliferation and plays an important role in the pyrimidine salvage pathway. Therefore, TK1 concentration will increase with the lesion growth, disease progression, and distant metastasis.
Although most clinical studies have suggested that patients with malignant tumors have an increase of tumor markers, there are still some factors that can affect the diagnosis and judgment. As previously reported [22], CA724 was significantly increased in 3.5% of healthy adults and 6.7% of patients with gastrointestinal diseases, and the secretion of DKK1 can also be affected by other factors and liver metabolism after entering the blood, which are the reasons for missed diagnosis and misdiagnosis of tumor markers. In addition, a single test for each serological marker was conducted in this study, which can easily lead to inaccurate detecting results, so that multiple measurements can be performed when conditions permit to obtain more accurate detecting results.
The ROC curve for the diagnosis of GC was constructed in order to accurately and comprehensively evaluate the value of each serological index in the diagnosis of GC. The results showed that the areas under the curve of CA724, DKK1, and TK1 for the diagnosis of GC were 0.849, 0.754, and 0.685, respectively, suggesting that each serological index has certain diagnostic value for GC, with a high missed diagnosis and misdiagnosis. At present, the combined diagnosis with multiple indicators is often used in clinic, which can improve the diagnostic efficiency of diseases to a certain extent [23, 24]. In this study, the diagnosis status of GC by single and combined diagnostic methods was analyzed, and the results showed that compared with single diagnosis, the area under the curve of the combined diagnosis was the highest, suggesting that the combined detection with multiple indicators could improve the diagnostic efficiency of GC. Therefore, the combined detection has the highest diagnostic value for GC, which can make up for the shortcomings of insufficient sensitivity or specificity of single index, so as to provide more accurate information for clinical practice. Compared with the previous serological detection tests in clinic, the results of this study confirmed that this scheme had higher diagnostic efficiency, and the detection method was convenient and fast, so as to better make up for the shortcomings of previous diagnosis, which is the promotion and optimization of previous studies.
5. Conclusion
The detection of tumor markers has an important significance for the diagnosis of GC in clinic, while the detection of single indicator has limitations in specificity and sensitivity, and the combined detection with different indicators can improve the diagnostic efficiency through complementary advantages, which can provide more evidence-based proofs for the treatment protocol of subsequent disease. However, the detection of serum tumor markers has its unique advantages, but also has some defects, so that it is still the focus of future studies to find ideal tumor markers related to GC and explore new detection methods.
Acknowledgments
This research was funded by (1) The Scientific Research Project of Integrated Traditional Chinese and Western Medicine of Tianjin Health Commission Administration of Traditional Chinese Medicine Subject number: 2021010, (Study on the mechanism of Nobiletin regulated CD36/AKT/β-catenin signaling pathway inhibited peritoneal metastasis in gastric cancer). (2) Chen Xiao-ping Foundation for the Development of Science and Technology of Hubei Province Subject number: CXPJJH122002-073, (Study on the mechanism of Huaier granule inhibiting peritoneal metastasis of gastric cancer with oxaliplatin and fluorouracil). (3) Tianjin Key Medical Discipline (Specialty) Construction Project, Project number: TJYXZDXK-032A.
Contributor Information
Honglei Wang, Email: wanghonglei@tjnkh.org.cn.
Yu Wu, Email: wuyu@tjnkh.org.cn.
Data Availability
Data to support the findings of this study is available on reasonable request from the corresponding author.
Conflicts of Interest
The authors do not have conflicts of interest to declare.
Authors' Contributions
Yu Wang and Dongqing Cui contributed equally to this article.
References
- 1.Gullo I., Grillo F., Mastracci L., et al. Precancerous lesions of the stomach, gastric cancer and hereditary gastric cancer syndromes. Pathologica . 2020;112(3):166–185. doi: 10.32074/1591-951X-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito R., Kadota T., Murano T., et al. Clinical features and risk factors of gastric cancer detected by esophagogastroduodenoscopy in esophageal cancer patients. Esophagus . 2021;18(3):621–628. doi: 10.1007/s10388-021-00822-4. [DOI] [PubMed] [Google Scholar]
- 3.Chiang T. H., Chang W. J., Chen S. L. S., et al. mass eradication of <i>Helicobacter pylori</i> to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut . 2021;70(2):243–250. doi: 10.1136/gutjnl-2020-322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold M., Park J. Y., Camargo M. C., Lunet N., Forman D., Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut . 2020;69(5):823–829. doi: 10.1136/gutjnl-2019-320234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki S., Takahashi A., Ishikawa T., et al. Surgically treated gastric cancer in Japan: 2011 annual report of the national clinical database gastric cancer registry. Gastric Cancer . 2021;24(3):545–566. doi: 10.1007/s10120-021-01178-5. [DOI] [PubMed] [Google Scholar]
- 6.Collatuzzo G., Pelucchi C., Negri E., et al. Exploring the interactions between helicobacter pylori (Hp) infection and other risk factors of gastric cancer: a pooled analysis in the stomach cancer pooling (StoP) project. International Journal of Cancer . 2021;149(6):1228–1238. doi: 10.1002/ijc.33678. [DOI] [PubMed] [Google Scholar]
- 7.Gai Q.-Z., Li X.-L., Li N., Li L., Meng Z., Chen A. . F. Clinical significance of multi-slice spiral CT, MRI combined with gastric contrast-enhanced ultrasonography in the diagnosis of T staging of gastric cancer. Clinical & Translational Oncology . 2021;23(10):2036–2045. doi: 10.1007/s12094-021-02606-9. [DOI] [PubMed] [Google Scholar]
- 8.Kuang J., Gong Y., Xie H., et al. The prognostic value of preoperative serum CA724 for CEA-normal colorectal cancer patients. PeerJ . 2020;8:p. e8936. doi: 10.7717/peerj.8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association. World medical association Declaration of Helsinki. Journal of the American Medical Association . 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 10.Liang L., Fang J., Han X., et al. Prognostic value of CEA, CA19-9, CA125, CA724, and CA242 in serum and ascites in pseudomyxoma peritonei. Front Oncologia . 2021;11:p. 594763. doi: 10.3389/fonc.2021.594763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y., Joo J., Lee Y. J., et al. Randomized phase II study of platinum-based chemotherapy plus controlled diet with or without metformin in patients with advanced non-small cell lung cancer. Lung Cancer . 2021;151:8–15. doi: 10.1016/j.lungcan.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Wang S., Zhao M., Mu R., Zhang X., Yun K., Shang H. Biological variation of serum neuron-specific enolase and carbohydrate antigen 724 tumor markers. Journal of Clinical Laboratory Analysis . 2018;32(4):p. e22327. doi: 10.1002/jcla.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xuyang G., Heng Z. Diagnostic and prognostic values of anti-helicobacter pylori antibody combined with serum CA724, CA19-9, and CEA for young patients with early gastric cancer. Journal of Clinical Laboratory Analysis . 2020;34(7):p. e23268. doi: 10.1002/jcla.23268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lian M., Zhang C., Zhang D., et al. The association of five preoperative serum tumor markers and pathological features in patients with breast cancer. Journal of Clinical Laboratory Analysis . 2019;33(5):p. e22875. doi: 10.1002/jcla.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eldeeb M., Magour G., Bedair R., Shamseya M., Hammouda M. Study of Dickkopf-1 (DKK-1) in patients with chronic viral hepatitis C-related liver cirrhosis with and without hepatocellular carcinoma. Clinical and Experimental Hepatology . 2020;6(2):85–91. doi: 10.5114/ceh.2020.95831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fareez R. F., Kok-Yong C. A review of the potential application of osteocyte-related biomarkers, fibroblast growth factor-23, sclerostin, and Dickkopf-1 in predicting osteoporosis and fractures. Diagnostics (Basel) . 2020;10(3):p. 145. doi: 10.3390/diagnostics10030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinlin S., Xudong C., Yansen W. Comparison of the diagnostic value of CEA combined with OPN or DKK1 in non-small cell lung cancer. Oncology Letters . 2020;20(3):3046–3052. doi: 10.3892/ol.2020.11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabel L., Rosenblum D., Lerebours F., et al. Plasma thymidine kinase 1 activity and outcome of ER+ HER2- metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Research . 2020;22(1):1–9. doi: 10.1186/s13058-020-01334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernhard T. Early prediction of pathologic response to neoadjuvant treatment of breast cancer: use of a cell-loss metric based on serum thymidine kinase 1 and tumour volume. BMC Cancer . 2020;20(1):p. 440. doi: 10.1186/s12885-020-06925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velazquez E. J., Brindley T. D., Shrestha G., et al. Novel monoclonal antibodies against thymidine kinase 1 and their potential use for the immunotargeting of lung, breast and colon cancer cells. Cancer Cell International . 2020;20(1):p. 127. doi: 10.1186/s12935-020-01198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishino T., Oyama T., Tomori A., Takahashi A., Shinohara T. Usefulness and limitations of a serum screening system to predict the risk of gastric cancer. Internal Medicine . 2020;59(12):1473–1480. doi: 10.2169/internalmedicine.3521-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pih G. Y., Gong E. J., Choi J. Y., et al. Associations of serum lipid level with gastric cancer risk, pathology, and prognosis. Cancer Research and Treatment: Official Journal of Korean Cancer Association . 2021;53(2):445–456. doi: 10.4143/crt.2020.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han B. L., Wang Y. M., Xue Y. W. Effect of preoperative serum alanine aminotransferase and asparagine aminotransferase ratio on prognosis of patients with gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi . 2020;23(1):65–70. doi: 10.3760/cma.j.issn.1671-0274.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Kanda M., Suh Y. S., Park D. J., et al. Serum levels of ANOS1 serve as a diagnostic biomarker of gastric cancer: a prospective multicenter observational study. Gastric Cancer . 2020;23(2):203–211. doi: 10.1007/s10120-019-00995-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data to support the findings of this study is available on reasonable request from the corresponding author.


