Background:
There is paucity of data regarding the effects of delayed reperfusion (DR) on clinical outcomes in patients with incomplete reperfusion following mechanical thrombectomy. We hypothesized that DR has a strong association with clinical outcome in patients with incomplete reperfusion after mechanical thrombectomy (expanded Thrombolysis in Cerebral Infarction, 2a–2c).
Methods:
Single-institution’s stroke registry retrospective analysis of patients admitted from February 2015 to December 2020. DR was defined as the absence of any perfusion delay on ≈24-hour contrast-enhanced follow-up perfusion imaging, whereas persistent perfusion deficit denotes a perfusion delay corresponding to the catheter angiographic deficit directly after the intervention. The association of perfusion outcome (DR versus persistent perfusion deficit) with the occurrence of new infarcts and 90-day functional independence (modified Rankin Scale score 0–2) was evaluated using logistic regression analyses. Comparison of predictive accuracy was evaluated by calculating area under the curve for models with and without perfusion outcome.
Results:
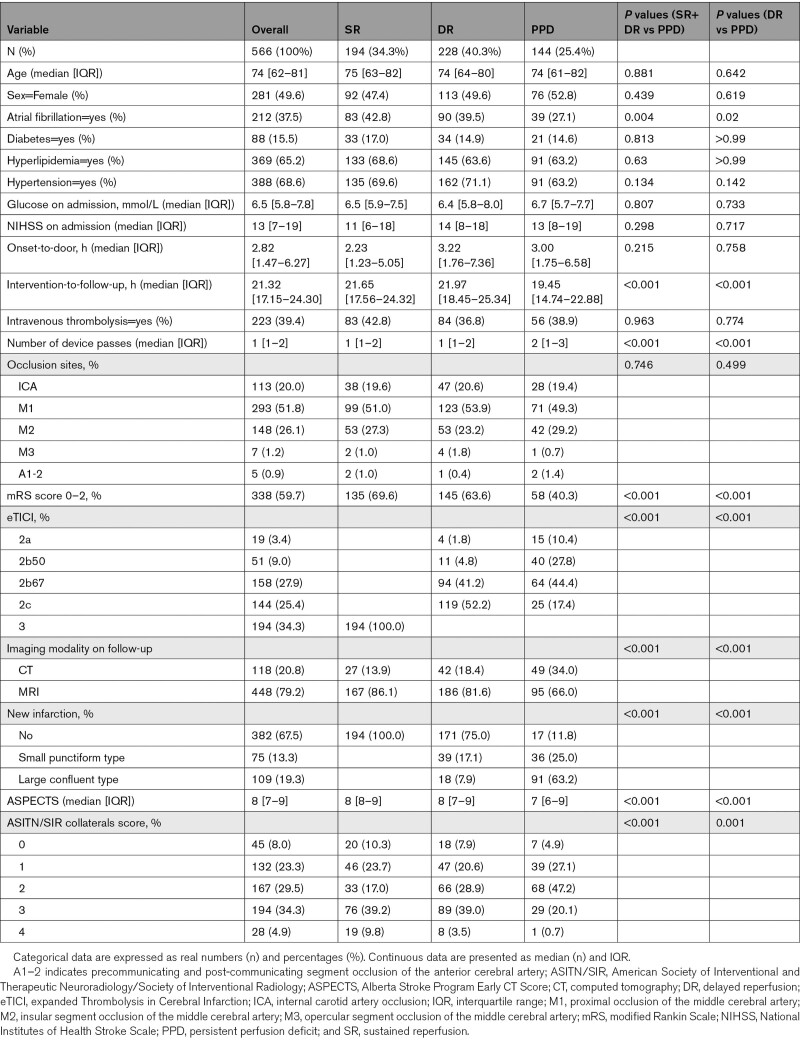
In 566 patients (mean age 74, 49.6% female), new infarcts in the incomplete reperfusion areas were less common in DR versus persistent perfusion deficit patients (small punctiform: 17.1% versus 25%, large confluent: 7.9% versus 63.2%; P=0.001). After adjustment for confounders, DR was a strong predictor of functional independence (adjusted odds ratio, 2.37 [95% CI 1.34–4.23]). There was a significant improvement in predictive accuracy of functional independence when perfusion outcome was added to expanded Thrombolysis in Cerebral Infarction alone (area under the curve 0.57 versus 0.62, P=0.01).
Conclusions:
Occurrence of DR is closely associated with tissue outcome and functional independence. DR may be an independent prognostic parameter, suggesting it as a potential outcome surrogate for medical rescue therapies.
Keywords: ischemic stroke, magnetic resonance imaging, reperfusion, thrombectomy, tomography
Achieving early and, at best, complete reperfusion, and salvaging tissue at risk is the current aim of all large vessel occlusion acute ischemic stroke treatment strategies.1–3 However, in a considerable proportion of patients, reperfusion is only achieved partially due to residual occlusions persisting after the intervention.4,5
As the effect of reperfusion is profoundly time-dependent, it remains unknown if—and to what extent—delayed reperfusion (DR) favors good clinical outcomes in patients undergoing mechanical thrombectomy (MT).6 Although there seems to be a good correlation between angiographically assessed reperfusion and post-interventional degree of tissue perfusion, detailed analyses suggest heterogeneity of post-interventional perfusion in patients with incomplete reperfusion.7–10
We hypothesized that patients with DR after incomplete MT have better tissue and clinical outcomes, and are more likely to resemble the clinical course of patients with immediate complete reperfusion during the intervention.
Methods
Study Design
Single-institution’s stroke registry retrospective observational analysis for all patients admitted between February 2015, and December 2020 with acute ischemic stroke. The study has been conducted in accordance with the 1964 Helsinki Declaration and its subsequent amendments,11 and was approved by the local ethics committee (Kantonale Ethikkomission Bern, reference ID 231/14) The data supporting the findings of this study are available from the corresponding author upon reasonable request and clearance by the ethics committee. Patient data has been processed in the registry according to the Swiss Federal Data Protection Act.12 This manuscript follows the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guideline (Supplemental Material).13
Patient Selection and Eligibility Criteria
All potential MT candidates were initially evaluated for eligibility. Patients who actively refused usage of their data for research purposes and who did not undergo MT were excluded. Patients who ultimately did not undergo MT (eg, spontaneous reperfusion after alteplase), or presented with an isolated extracranial, or posterior vessel occlusion, were excluded. Patients with final expanded Thrombolysis in Cerebral Infarction (eTICI) grade 2a to 3, and who had follow-up perfusion imaging at 24±12 hours after MT have been further analyzed. Flow chart details are shown in Figure S1. To address potential bias of inclusion, sensitivity analyses were performed between patients who did and did not undergo perfusion imaging on follow-up.
Clinical Variables
We screened for patients` baseline characteristics, interventional, neuroimaging, and outcome variables. Mortality and functional outcome were assessed with the modified Rankin Scale score at 90 days, where disability was categorized in one of the following categories: 0 (no symptoms), 1 (no significant disability), 2 (slight disability), 3 (moderate disability), 4 (moderately severe disability), 5 (severe disability), 6 (fatal outcome). The modified Rankin Scale score was assessed by an independent research nurse at a 90-day follow-up visit, or via a structured telephone interview, where modified Rankin Scale score of 0 to 2 was defined as functional independence. Long-term mortality data extraction methods have been previously described.14
Neuroimaging
Patients have received preinterventional and follow-up imaging with either a computed tomography (CT) or magnetic resonance imaging (MRI). CT (SOMATOM Definition Edge, Siemens, Erlangen, Germany) included following imaging modalities: noncontrast CT (slice thickness: 1 mm and/or 3 mm), CT angiography in early arterial (slice thickness: 0.6 mm), late venous (slice thickness: 1 mm) phase, and perfusion imaging. MRI (1.5/3T MRI Avanto, Avanto fit, Verio, Aera, Skyra fit and Vida, Siemens, Erlangen, Germany) included: fluid-attenuated inversion recovery, intracranial time-of-flight angiography, diffusion-weighted imaging, susceptibility-weighted imaging, contrast-enhanced cervical, and intracranial angiography, gradient-echo dynamic susceptibility contrast perfusion, and optional post-contrast T1-weighted imaging. Postprocessing software syngo.via (Siemens) was used to process CT perfusion data, as was Olea Sphere Software (Olea Sphere v2.3; Olea Medical, La Ciotat, France) to generate MRI perfusion maps. Following perfusion maps were generated: relative cerebral blood volume, time to maximum (Tmax), mean transit time, relative cerebral blood flow, time to peak, and temporal maximum intensity projection map. First, follow-up imaging was performed ≈24 hours after the intervention, where DR was evaluated on time to peak and Tmax maps due to their high sensitivity.15,16 Diffusion-weighted imaging and CT were used to screen for new infarcts within the hypoperfused territory after incomplete MT.17,18
Neuroimaging Variables
Based on initial imaging, sites of arterial occlusions were categorized into one of the following: intracranial carotid artery, proximal segment of the middle cerebral artery (M1), insular segment of the middle cerebral artery (M2), opercular segment of the middle cerebral artery (M3), precommunicating and post-communicating segment of the anterior cerebral artery (A1–2). eTICI scale was used for reperfusion success grading, where eTICI 2a corresponds to the 1% to 49%; 2b to 50% to 50% to 66%; 2b67 to 67% to 89%; 2c to 90% to 99%; and 3% to 100% reperfusion of the affected territory. eTICI grading was performed by a core lab of 4 neuroradiologists on final angiography runs, blinded to clinical data, where eTICI≥2b50 was categorized as successful reperfusion.19
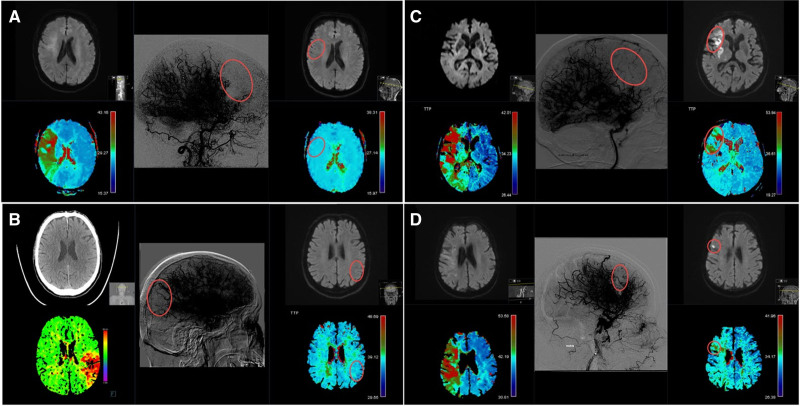
DR was defined as the absence of a focal perfusion delay with values of Tmax≥4 seconds on contrast-enhanced follow-up perfusion imaging within the territory of the initially occluded artery (Figure 1A and 1B). Conversely, a persistent perfusion deficit (PPD) was defined as a persisting perfusion imaging deficit on follow-up perfusion imaging with focally increased delays with values of Tmax≥4 seconds within the territory of the initially occluded large artery, corresponding to the location of the antegrade capillary phase deficit on the final angiography image area after incomplete MT (Figure 1C and 1D). To streamline perfusion outcome evaluation, there was a central adjudicated charter (see Methods S1). Perfusion outcome was rated by 4 independent neuroradiologists who were not involved in the treatment of these patients (Krippendorf alpha, 0.82 [95% CI, 0.80–0.84] for a random sample of 50 patients).
Figure 1.
Delayed reperfusion and persistent perfusion deficit on perfusion follow-up imaging. Time to maximum (Tmax) and diffusion-weighted imaging (DWI) imaging maps were evaluated on admission and follow-up examinations. Final angiography runs are displayed with high contrast to emphasize the capillary phase deficits. A, Patient with a right side M1 occlusion with delayed reperfusion on magnetic resonance imaging (MRI) follow-up after expanded Thrombolysis in Cerebral Infarction (eTICI) 2b50 reperfusion, together with corresponding admission DWI (top-left), admission perfusion imaging (bottom-left), final angiography imaging (middle), follow-up DWI (top-right), follow-up perfusion imaging (bottom-right). B, Patient with a left side M2 occlusion with delayed reperfusion on MRI follow-up after eTICI 2b67 reperfusion, together with corresponding admission CT imaging (top-left), admission perfusion imaging (bottom-left), final angiography imaging (middle), follow-up DWI (top-right), follow-up perfusion imaging (bottom-right). C, Patient with a right side M1 occlusion with persistent perfusion deficit and confluent infarct on MRI follow-up after eTICI 2b50 reperfusion together with corresponding admission DWI (top-left), admission perfusion imaging (bottom-left), final angiography imaging (middle), follow-up DWI (top-right), follow-up perfusion imaging (bottom-right). D, Patient with a right side M1 occlusion with persistent perfusion deficit and punctiform-infarct on MRI follow-up after eTICI 2b67 reperfusion together with corresponding admission DWI (top-left), admission perfusion imaging (bottom-left), final angiography imaging (middle), follow-up DWI (top-right), and follow-up perfusion imaging (bottom-right).
Presented definitions for DR and PPD are valid only for patients with incomplete reperfusion (eTICI 2a–2c), whereas for eTICI 3 patients, perfusion outcome was rated as either sustained reperfusion, meaning absence of focal perfusion delay after complete reperfusion, or a new focal perfusion deficit within the territory of the initially occluded artery. For the purposes of excluding false positive ratings of PPD caused by susceptibility artifacts/hemorrhagic transformations, other sequences, such as susceptibility-weighted imaging, were used (Figure S2).
For tissue outcome, new infarct was defined as demarcated infarcted area on follow-up imaging, which equates to the same area of brain parenchyma where there was capillary phase hypoperfusion due to incomplete reperfusion at the end of thrombectomy but no early demarcation on initial preinterventional imaging. This variable was further semi-quantitatively graded into either small punctiform infarction with each single hypodensity or diffusion-weighted imaging-lesion <2 mm) or large confluent infarct type (single lesion ≥2 mm; Figure 1C and 1D).
ASPECTS (Alberta Stroke Program Early CT Score) was rated on either noncontrast CT or diffusion-weighted imaging sequences to quantitatively estimate the extent of early ischemic changes. Collateral status was graded with the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology scale on preinterventional angiography imaging.20
Statistical Analysis
Results are reported as median (interquartile range) and n (%) unless specified otherwise. Fisher exact test has been used for categorical and Mann-Whitney U for continuous variables. Logistic regression was used with functional independence as a dependent variable. Results of the logistic regression analysis are displayed as adjusted odds ratios (aOR), with corresponding 95% CI, where aORs of the independent variables were plotted as forest plots. Regression was adjusted for baseline confounders and potentially pathophysiological covariates which could influence patient outcome: age (continuous variable), sex (binary variable), National Institutes of Health Stroke Scale on admission (continuous variable, aOR referring to one point increase), onset-to-door time (continuous variable, aOR referring to one hour delay), intravenous thrombolysis (binary variable), collateral status (ordinal variable with a step-wise increase), ASPECTS (ordinal variable with a step-wise increase), occlusion site (categorical variable), perfusion outcome (binary variable), and eTICI score (ordinal variable with a step-wise increase). Additionally, a new variable—eTICI—was computed as a combination of final incomplete reperfusion eTICI scores (2a, 2b50, 2b67, and 2c) and perfusion outcome (DR and PPD), amounting to a total of 8 categories (4x2), with the final, ninth, category encompassing only eTICI 3 patients. eTICI variable coding is presented in the Table S1. This variable was subsequently used as one of the covariates in the analyses in 2 different settings, first begin defined as a categorical, and later as ordinal variable with step-wise increase. To compare predictive accuracy of models, receiver operating characteristics were constructed and areas under the receiver operating characteristics curve (area under the curve [AUC]) were calculated. DeLong test was used for comparison between 2 bootstrapped receiver operating characteristics curves. Bar plots have been used to present tissue and perfusion outcomes stratified across the incomplete reperfusion scale (eTICI 2a–2c). Uniformly, clinical outcome is represented with modified Rankin Scale plots stratified by perfusion outcome across the eTICI spectrum. All statistical analyses were conducted using R v4.0.0.21 For a complete list of used statistical packages, refer to Table S2.
Results
Study Population and Inclusion Bias
The final study population constituted 566 patients, with a median age of 74 (interquartile range, 62.3–81.5), among which 281 (49.6%) were female. Better baseline and outcome characteristics have been observed in patients who underwent perfusion imaging on follow-up when compared with those who did not, as denoted in Table S3.
Follow-Up Perfusion Patterns
Overall, DR was present in 40.3% (n=228) patients with incomplete reperfusion following MT, whereas all eTICI 3 patients (n=194) showed sustained reperfusion without focal perfusion delays on follow-up imaging, as shown in the Table. The majority of patients underwent MRI imaging on follow-up, as opposed to CT (79.2% versus 20.8%). DR and PPD rates among patients with incomplete reperfusions (eTICI 2a–2c) varied across the eTICI spectrum, with higher rates of DR observed in better reperfusion scores (eTICI 2a versus 2c: 21.1% versus 82.6%, P<0.001). There was no significant difference between different occlusion sites and rates of DR (P=0.49). DR patients had higher ASPECTS (DR versus PPD: 8 [7–9] versus 7 [6–9]; P<0.001), and longer intervention-to-follow-up time window (DR versus PPD: 21.9 hours [18.5–25.3] versus 19.5 hours [14.7–22.9]; P<0.001).
Table.
Comparison of Perfusion Follow-Up Patients Stratified by Perfusion Outcome
Relevance of Delayed Reperfusion for Tissue Outcome
Increasing rates of DR strongly correlate with preserved tissue where there were no newly infarcted demarcations (DR versus PPD: 75% versus 11.8%, P<0.001). Inversely, with increasing rates of PPD more small punctiform- and, specifically, large confluent-type of new infarcts were observed on follow-up imaging (DR punctiform-type: 17.1% and confluent-type: 7.9% versus PPD punctiform-type: 25% and confluent-type: 63.2%; P=0.001). In patients with incomplete reperfusion, there was a close correlation between increasing eTICI grades, higher chances of delayed reperfusion, and lower chances of new infarct development despite incomplete reperfusion (see Figure S3).
Clinical Impact of Delayed Reperfusion
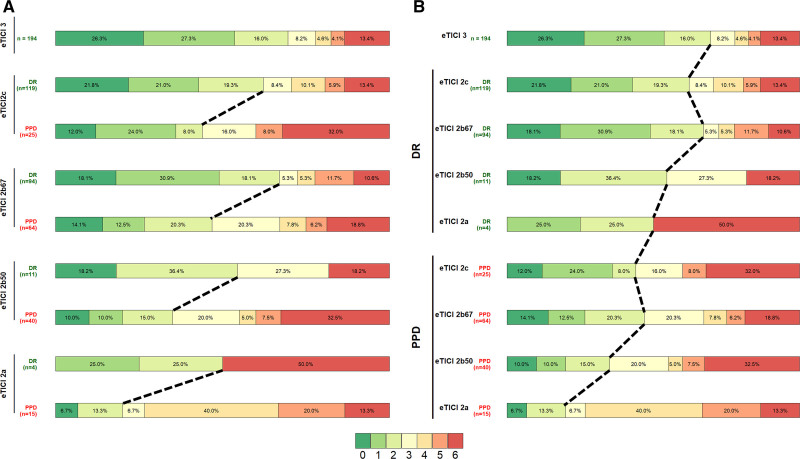
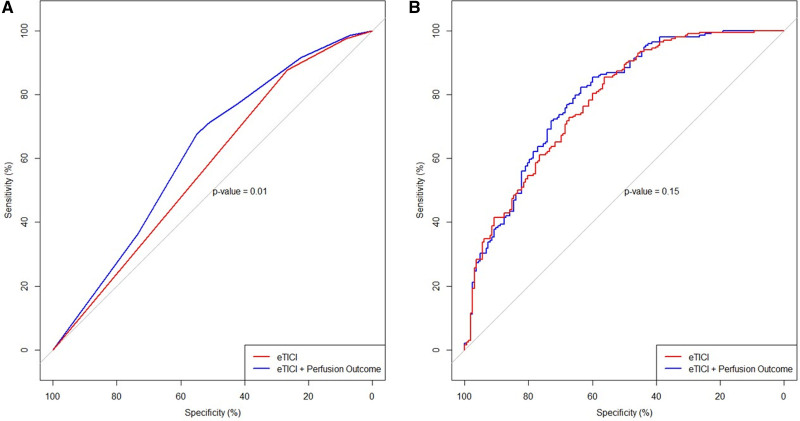
Better reperfusion rates across the eTICI spectrum were associated with better functional independence, as shown in Figure 2A. However, in each eTICI stratum denoting different levels of reperfusion (eTICI 2a–2c), there was a profound effect of DR on clinical outcomes. Vice versa, there was better outcome among patients with DR of any eTICI grade as opposed to patients with PPD of any eTICI grade (eg, better outcome of eTICI 2b50 with DR than eTICI 2c with PPD, Figure 2B). In patients with incomplete postprocedural reperfusion, DR showed independent association with functional independence (aOR, 2.37 [95% CI, 1.34–4.23]; Table S4) and lower mortality (adjusted hazard ratio, 0.60 [95% CI, 0.39–0.91]; Figure S4). There was a significant increase in predictive accuracy when eTICI was compared with a model which aside from eTICI also included perfusion outcome (AUC, 0.57 [95% CI, 0.52–0.63] versus AUC 0.62 [95% CI, 0.56–0.68]; P=0.01; Figure 3A). This increased accuracy did not reach significant levels in a model adjusted for other clinical cofactors (AUC, 0.77 [95% CI, 0.73–0. 82] versus AUC 0.79 [95% CI, 0.74–0.83]; P=0.15; Figure 3B).
Figure 2.
Clinical impact of delayed reperfusion. A, Better reperfusion rates across the expanded Thrombolysis in Cerebral Infarction (eTICI) spectrum were associated with better functional independence. B, There was better outcome among patients with delayed reperfusion (DR) of any eTICI grade as opposed to patients with persistent perfusion deficit (PPD) of any eTICI grade, eg, better outcome of eTICI 2b50 with DR than eTICI 2c with PPD.
Figure 3.
Predictor accuracy for favorable clinical outcome. A, Unadjusted regression model for only expanded Thrombolysis in Cerebral Infarction (eTICI; red) and eTICI with perfusion outcome (blue) showed significant effect of perfusion outcome on clinical outcome (area under the curve [AUC], 0.57 [95% CI, 0.52–0.63] vs AUC 0.62 [95% CI, 0.56–0.68], respectively; P=0.01). B, Multivariable logistic regression models adjusted to age, sex, National Institutes of Health Stroke Scale on admission, onset-to-door time, intravenous thrombolysis, collateral status, occlusion site, Alberta Stroke Program Early CT Score, and eTICI (colored in red, see Methods). Aside from the aforementioned, perfusion outcome was added as an additional variable to the comparator model (blue). There was no statistical significance between the 2 models (AUC, 0.77 [95% CI‚ 0.73–0. 82] vs AUC, 0.79 [95% CI‚ 0.74–0.83], respectively; P=0.15).
Expanded Thrombolysis in Cerebral Infarction
With few exceptions, there was a consistent increase in functional independence with increasing eTICI levels (see close to linear decrease in aOR across the eTICI spectrum denoted in Figure S5). Notably, all eTICI with DR showed higher aOR than all eTICI scores with PPD. Patients with eTICI 2b67 and eTICI 2c with DR closely resembled the clinical course of patients with eTICI 3 (eTICI 2b67 with DR aOR‚ 1.07 [95% CI, 0.57–2.03]; P=0.84; reference category eTICI 3, Figure S5). The averaged aOR was 1.21 (95% CI, 1.12–1.32) per eTICI step increase (Figure S5 last row).
Discussion
This study has the following main findings: (1) DR occurs in 20% to 80% of patients depending on the post-interventional eTICI score; (2) independent of the eTICI score, DR is strongly associated with preserved tissue status and favorable functional outcome; (3) patients with incomplete thrombectomy, but DR, closely resemble the clinical course of patients with complete reperfusion after thrombectomy; (4) incorporating DR significantly increases the chances for predicting functional independence when compared with the final eTICI score alone.
Delayed reperfusion appears to be common, occurring in ≈40% of patients with incomplete reperfusion after MT in our study. Similar DR rates have been observed by others, with higher chances of DR occurrence among patients with higher final TICI score.7,9,10,22 In the DEFUSE II trial (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution II), 58.46% of patients showed DR, with higher rates observed among patients with TICI 2B as opposed to patients with, for example, TICI 0 to 2A (72.7% versus 51%).7 Tan et al,22 shared the same observation of increasing TICI rates correlating well with increasing DR rates among 41.6% of their cohort who achieved DR (modified Thrombolysis in Cerebral Infarction [mTICI] score 0–2a versus mTICI 2b–2c: 0% versus 57.6%). Even when perfusion imaging is performed ≤30 minutes after MT, DR may be observed in approximately every fourth patient (mTICI 2A-2B).10 There have been observations of hypoperfusion patterns in patients with complete reperfusion (mTICI 3) when observed in short enough time window.10 Similarly to other studies,7,9,22 we have evaluated perfusion outcome in a follow-up period of 24±12 hours and were not able to delineate hypoperfusion patterns of Tmax>4 s delay in completely reperfused patients (eTICI 3). Potential explanation for this discrepancy to other studies23,24 could be that perfusion abnormalities are a strongly time-dependent phenomenon, but also due to different definitions of hypoperfusion in each study (ie, definition of no-reflow versus PPD, suggesting persistent vessel occlusions).23,24 It would also be reasonable to assume the presence of unrecognized distal occlusions on final angiography images, which could only be detected on time-sensitive follow-up perfusion imaging.15,16 This may occur more often when interventionalists have to grade their own interventions.25 To avoid potentially biased rating, we have used an independent core lab blinded to clinical data (see Methods).
Strong effect of DR on clinical outcome has been shown, with DR patients of any eTICI grade having a better clinical outcome than PPD patients of any eTICI grade. Other studies showcased similar results of perfusion outcome exhibiting a strong effect on clinical outcome across the TICI spectrum,7,10 although some studies included only patients with successful reperfusion.22 Although the exact time point of delayed reperfusion is unknown,7,9,10,22,26 the clinical magnitude of this association was considerable across all eTICI scores and notably independent of the patients' collateral status. In our large series of patients with follow-up perfusion imaging, this effect was so pronounced that patients who had poor reperfusion grade on their last catheter angiographic control run, but presented with DR on follow-up, had better clinical outcomes than patients with higher final eTICI scores, but PPD on follow-up. DR patients had also higher ASPECTS, which is not surprising considering the association between DR and collateral status we have observed. Even after including ASPECTS in all statistical models, significance and effect size of the association between DR with clinical outcome remained unchanged.
Current guidelines recommend to achieve TICI 2c/3 whenever possible.27 However, not all patients may benefit from rescue treatments aimed at improving incomplete reperfusion to a complete or near complete reperfusion.28 For more distal occlusions, intraarterial fibrinolytics may constitute a reasonable option,29,30 and is currently performed by some interventionalists.31,32 Identification of patients for medical rescue treatments is difficult as many unknowns are present at the time when a potential decision is being made after the endovascular procedure. The present study highlights that not all patients may benefit from such rescue strategies, as a considerable proportion of patients experience spontaneous DR. Patients with DR tend to resemble the clinical course and outcome of eTICI 3 patients, making it less likely for them to expect any benefit from pursuing further rescue medical treatments. Vice versa, patients with PPD have a high risk for infarct expansion and poor clinical outcomes, making them ideal candidates to pursue rescue treatments. Predicting perfusion outcome at 24 hours at the time point when the intervention ends with incomplete thrombectomy (eTICI 2a–2c), if achievable with high accuracy, would be useful in clinical practice. An accurate prediction could help steer the patient selection process for rescue treatments. Perfusion imaging could also be considered as a surrogate-imaging end point for clinical trials evaluating medical rescue treatment strategies (eg, additional administration of intravenous or intraarterial thrombolytics) after incomplete thrombectomy.
Currently, findings are sparse regarding factors associated with DR. We have found that higher post-interventional eTICI score, lower number of device passes, better collateral status, and history of atrial fibrillation are all factors which seem to favor DR. Accordingly, these factors might be taken into considerations in decisions regarding rescue treatments and their anticipated risks and benefits.
There are many predictors of favorable functional outcome such as early clinical improvement,33 periventricular white matter disease,34 speed of infarct progression,35 or age and early motor function.36 Although eTICI obtained at the end of the endovascular procedure is regarded as one of the strongest predictors of favorable 90-day outcome,37–39 we have found that DR is much stronger in predicting favorable outcome than is the final eTICI score alone. Bivariate comparison has shown an accuracy benefit of implementing DR rates into the prediction model, providing evidence that the predictive value of DR for clinical outcomes is independent of the achieved eTICI score. This difference was attenuated and nonsignificant when models were additionally adjusted for other confounders known to influence clinical outcomes. However, the study population may not be powered to detect increments in predictive accuracy in fully adjusted models.
Limitations
This study has several limitations. Generalizability of our results is limited by a single-center retrospective nature of our study. Selection bias was present in a sense that patients with follow-up perfusion imaging had better presenting clinical profile at baseline. Therefore, the absolute prevalence of patients with DR should also be interpreted carefully as DR rates may not be the same in the nonperfusion imaging group. Core volume is a known predictor of good outcome, but data on quantitative measurements of ischemic core were not available for the present cohort.40 Lastly, this study was not powered to show superiority of DR over eTICI as a predictor of 90-day outcome when adjusted for known factors influencing clinical outcomes.
Conclusions
In patients with incomplete reperfusion following MT, occurrence of DR is clinically relevant for functional recovery and preserving postprocedural tissue status. This association was stable across all incomplete eTICI scores, independent of collateral status, and hence, proposes DR as a useful surrogate-imaging marker for potential rescue strategies. Patients with DR closely resemble the clinical course of patients with complete reperfusion at the end of thrombectomy‚ making them less likely to benefit from rescue treatments.
Article Information
Presented in part at the joint annual meeting of the Austrian Stroke Society and the Swiss Stroke Society, Innsbruck, Austria, June 14–15, 2022.
Sources of Funding
None.
Disclosures
Dr Meinel reports research support from the Bangerter Rhyner Foundation, Swiss National Science Foundation (SNSF), and the Swiss Heart Foundation. Dr Heldner reports research support from Swiss Institute for Translational and Entrepreneurial Medicine (SITEM) Research Funds‚ Swiss Heart Foundation, and SNSF. Dr Mordasini reports research support from Siemens, Cerenovus, iSchemaview, Medtronic, and Stryker, and is receipt of honoraria and consultation fees from Medtronic, Cerenovus, Phenox, and Microvention. Dr Arnold reports personal fees from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Covidien, Daiichi Sankyo, Novartis, Sanofi, Pfizer, Medtronic. Dr Gralla is a global principal investigator of the SWIFT DIRECT study (Solitaire With the Intention for Thrombectomy Plus Intravenous Tissue Plasminogen Activator Versus DIRECT Solitaire Stent-Retriever Thrombectomy in Acute Anterior Circulation Stroke; Medtronic), therefore consultant for Medtronic, and receives SNSF grants for magnetic resonance imaging in stroke. Dr Fischer reports grants during the conduct of the study from Medtronic, Stryker, and CSL Behring. Dr Kaesmacher reports grants from the Swiss Academy of Medical Sciences/Bangerter Foundation, Swiss Stroke Society, Clinical Trials Unit Bern/Inselspital Bern, and the SNSF during the conduct of the study. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Figures S1–S5
Tables S1–S4
STROBE Statement
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ASPECTS
- Alberta Stroke Program Early CT Score
- CT
- computed tomography
- DR
- delayed reperfusion
- eTICI
- expanded Thrombolysis in Cerebral Infarction
- MRI
- magnetic resonance imaging
- MT
- mechanical thrombectomy
- PPD
- persistent perfusion deficit
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.122.040063.
For Sources of Funding and Disclosures, see page 3357.
Contributor Information
Adnan Mujanovic, Email: adnan.mujanovic@insel.ch.
Noel Jungi, Email: noel.jungi@hispeed.ch.
Christoph C. Kurmann, Email: christoph.kurmann@insel.ch.
Tomas Dobrocky, Email: tomas.klail@insel.ch.
Thomas R. Meinel, Email: thomas.meinel@insel.ch.
William Almiri, Email: william.almiri@insel.ch.
Lorenz Grunder, Email: lorenz.grunder@insel.ch.
Morin Beyeler, Email: morin.beyeler@insel.ch.
Matthias F. Lang, Email: matthias.lang@insel.ch.
Simon Jung, Email: simon.jung@insel.ch.
Tomas Klail, Email: tomas.klail@insel.ch.
Angelika Hoffmann, Email: angelika.hoffmann@insel.ch.
David J. Seiffge, Email: david.seiffge@insel.ch.
Mirjam R. Heldner, Email: mirjam.heldner@insel.ch.
Sara Pilgram-Pastor, Email: sara.pilgram-pastor@insel.ch.
Pasquale Mordasini, Email: pasquale.mordasini@insel.ch.
Marcel Arnold, Email: marcel.arnold@insel.ch.
Eike I. Piechowiak, Email: eike.piechowiak@insel.ch.
Jan Gralla, Email: jan.gralla@insel.ch.
References
- 1.Tachibana M, Ago T, Wakisaka Y, Kuroda J, Shijo M, Yoshikawa Y, Komori M, Nishimura A, Makihara N, Nakamura K, et al. Early reperfusion after brain ischemia has beneficial effects beyond rescuing neurons. Stroke. 2017;48:2222–2230. doi: 10.1161/STROKEAHA.117.016689 [DOI] [PubMed] [Google Scholar]
- 2.Yoo AJ, Soomro J, Andersson T, Saver JL, Ribo M, Bozorgchami H, Dabus G, Liebeskind DS, Jadhav A, Mattle H, et al. Benchmarking the extent and speed of reperfusion: first pass TICI 2c-3 is a preferred endovascular reperfusion endpoint. Front Neurol. 2021;12:669934. doi: 10.3389/fneur.2021.669934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning NW, Warne CD, Meyers PM. Reperfusion and clinical outcomes in acute ischemic stroke: systematic review and meta-analysis of the stent-retriever-based, early window endovascular stroke trials. Front Neurol. 2018;9:301. doi: 10.3389/fneur.2018.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, Poppe AY, Buck BH, Field TS, Dowlatshahi D, et al. ; ESCAPE-NA1 Investigators. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887. doi: 10.1016/S0140-6736(20)30258-0 [DOI] [PubMed] [Google Scholar]
- 5.Leischner H, Flottmann F, Hanning U, Broocks G, Faizy TD, Deb-Chatterji M, Bernhardt M, Brekenfeld C, Buhk JH, Gellissen S, et al. Reasons for failed endovascular recanalization attempts in stroke patients. J Neurointerv Surg. 2019;11:439–442. doi: 10.1136/neurintsurg-2018-014060 [DOI] [PubMed] [Google Scholar]
- 6.Shin J, Kim YS, Jang HS, Kim KH, Jeon P, Chung JW, Seo WK, Bang OY, Kim GM. Perfusion recovery on TTP maps after endovascular stroke treatment might predict favorable neurological outcomes. Eur Radiol. 2020;30:6421–6431. doi: 10.1007/s00330-020-07066-3 [DOI] [PubMed] [Google Scholar]
- 7.Marks MP, Lansberg MG, Mlynash M, Kemp S, McTaggart RA, Zaharchuk G, Bammer R, Albers GW; DEFUSE Investigators. Angiographic outcome of endovascular stroke therapy correlated with MR findings, infarct growth, and clinical outcome in the DEFUSE 2 trial. Int J Stroke. 2014;9:860–865. doi: 10.1111/ijs.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durán-Laforet V, Fernández-López D, García-Culebras A, González-Hijón J, Moraga A, Palma-Tortosa S, García-Yébenes I, Vega-Pérez A, Lizasoain I, Moro MÁ. Delayed effects of acute reperfusion on vascular remodeling and late-phase functional recovery after stroke. Front Neurosci. 2019;13:1–12. doi: 10.3389/fnins.2019.00767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brugnara G, Herweh C, Neuberger U, Bo Hansen M, Ulfert C, Mahmutoglu MA, Foltyn M, Nagel S, Schönenberger S, Heiland S, et al. Dynamics of cerebral perfusion and oxygenation parameters following endovascular treatment of acute ischemic stroke. J Neurointerv Surg. 2022;14:neurintsurg–neurint2020. doi: 10.1136/neurintsurg-2020-017163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubiera M, Garcia-Tornel A, Olivé-Gadea M, Campos D, Requena M, Vert C, Pagola J, Rodriguez-Luna D, Muchada M, Boned S, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke. 2020;51:1736–1742. doi: 10.1161/STROKEAHA.120.029212 [DOI] [PubMed] [Google Scholar]
- 11.Medical W, Wma A. Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc. 2009;107:403–405. doi: 10.1515/9783110208856.233 [PubMed] [Google Scholar]
- 12.Beranek Zanon N. Switzerland: Revision of Federal Data Protection Act (FDPA). Comput Law Rev Int. 2020;21:125–128. doi: 10.9785/cri-2020-210406 [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Beyeler M, Weber L, Kurmann CC, Piechowiak EII, Mosimann PJ, Zibold F, Meinel TR, Branca M, Goeldlin M, Pilgram-Pastor SM, et al. Association of reperfusion success and emboli in new territories with long term mortality after mechanical thrombectomy. J Neurointerv Surg. 2022;14:326–332. doi: 10.1136/neurintsurg-2021-017422 [DOI] [PubMed] [Google Scholar]
- 15.Wouters A, Christensen S, Straka M, Mlynash M, Liggins J, Bammer R, Thijs V, Lemmens R, Albers GW, Lansberg MG. A comparison of relative time to peak and tmax for mismatch-based patient selection. Front Neurol. 2017;8:539. doi: 10.3389/fneur.2017.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaro-Weber O, Moeller-Hartmann W, Siegmund D, Kandziora A, Schuster A, Heiss WD, Sobesky J. MRI-based mismatch detection in acute ischemic stroke: Optimal PWI maps and thresholds validated with PET. J Cereb Blood Flow Metab. 2017;37:3176–3183. doi: 10.1177/0271678X16685574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagaraja N. Diffusion weighted imaging in acute ischemic stroke: a review of its interpretation pitfalls and advanced diffusion imaging application. J Neurol Sci. 2021;425:117435. doi: 10.1016/j.jns.2021.117435 [DOI] [PubMed] [Google Scholar]
- 18.Schaefer PW, Copen WA, Lev MH, Gonzalez RG. Diffusion-weighted imaging in acute stroke. Magn Reson Imaging Clin N Am. 2006;14:141–168. doi: 10.1016/j.mric.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 19.Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, Mitchell PJ, van der Lugt A, Menon BK, San Román L, et al. ; HERMES Collaborators. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11:433–438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 20.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2020. [Google Scholar]
- 22.Tan Z, Bivard A, Sharma G. Optimal tissue reperfusion estimation by thrombectomy in acute ischemic stroke. Stroke. 2021;52:e760–e763. doi: 10.1161/STROKEAHA.121.034581 [DOI] [PubMed] [Google Scholar]
- 23.ter Schiphorst A, Charron S, Ben Hassen W, Provost C, Naggara O, Benzakoun J, Seners P, Turc G, Baron JC, Oppenheim C. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab. 2021;41:253–266. doi: 10.1177/0271678X20954929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng FC, Churilov L, Yassi N, Kleinig TJ, Thijs V, Wu TY, Shah DG, Dewey HM, Sharma G, Desmond PM, et al. ; EXTEND-IA TNK Part 1 and 2 Investigators. Microvascular dysfunction in blood-brain barrier disruption and hypoperfusion within the infarct posttreatment are associated with cerebral edema. Stroke. 2022;53:1597–1605. doi: 10.1161/STROKEAHA.121.036104 [DOI] [PubMed] [Google Scholar]
- 25.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO; SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1 [DOI] [PubMed] [Google Scholar]
- 26.Ng F, Churilov L, Yassi N, Kleinig T, Thijs V, Wu TY, Shah D, Dewey H, Sharma G, Desmond P, et al. Prevalence, characteristics and clinical significance of impaired tissue reperfusion despite complete recanalization in ischemic stroke (no-reflow): a pooled analysis of 3 randomized clinical trials. SSRN Electron J. 2021;12:23–54. doi: 10.2139/ssrn.3786059 [Google Scholar]
- 27.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, De Vries J, White P, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019;11:1–30. doi: 10.1136/neurintsurg-2018-014569 [DOI] [PubMed] [Google Scholar]
- 28.Kaesmacher J, Ospel JM, Meinel TR, Boulouis G, Goyal M, Campbell BCV, Fiehler J, Gralla J, Fischer U. Thrombolysis in cerebral infarction 2b reperfusions: to treat or to stop? Stroke. 2020;51:3461–3471. doi: 10.1161/STROKEAHA.120.030157 [DOI] [PubMed] [Google Scholar]
- 29.Diprose WK, Wang MTM, Ghate K, Brew S, Caldwell JR, McGuinness B, Barber PA. Adjunctive intra-arterial thrombolysis in endovascular thrombectomy. Neurology. 2021;96:1135–1143. doi: 10.1212/WNL.0000000000012112 [DOI] [PubMed] [Google Scholar]
- 30.Kaesmacher J, Abdullayev N, Maamari B, Dobrocky T, Vynckier J, Piechowiak EI, Pop R, Behme D, Sporns PB, Styczen H, et al. Safety and angiographic efficacy of intra-arterial fibrinolytics as adjunct to mechanical thrombectomy: results from the infinity registry. J Stroke. 2021;23:91–102. doi: 10.5853/jos.2020.01788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nolte CH, Fiehler J, Ringleb PA. Thrombolysis management in thrombectomy patients: Real-life data from German stroke centres. Eur Stroke J. 2017;2:356–360. doi: 10.1177/2396987317727229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaesmacher J, Meinel TR, Kurmann C, Zaidat OO, Castonguay AC, Zaidi SF, Mueller-Kronast N, Kappelhof M, Dippel DWJ, Soudant M, et al. Safety and efficacy of intra-arterial fibrinolytics as adjunct to mechanical thrombectomy: a systematic review and meta-analysis of observational data. J of Neurointerv Surg. 2021;13:1073–1080. doi: 10.1136/neurintsurg-2020-016680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudilosso S, Urra X, Amaro S, Llull L, Renú A, Laredo C, Obach V, Chamorro Á. Timing and Relevance of Clinical Improvement After Mechanical Thrombectomy in Patients With Acute Ischemic Stroke. Stroke. 2019;50:1467–1472. doi: 10.1161/STROKEAHA.118.024067 [DOI] [PubMed] [Google Scholar]
- 34.Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Flaherty ML, Air E, Broderick J, Tsevat J. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke. 2009;40:530–536. doi: 10.1161/STROKEAHA.108.521906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo WK, Liebeskind DS, Yoo B, Sharma L, Jahan R, Duckwiler G, Tateshima S, Nour M, Szeder V, Colby G, et al. ; UCLA Penumbra Imaging Investigators. Predictors and functional outcomes of fast, intermediate, and slow progression among patients with acute ischemic stroke. Stroke. 2020;51:2553–2557. doi: 10.1161/STROKEAHA.120.030010 [DOI] [PubMed] [Google Scholar]
- 36.Harvey RL. Predictors of functional outcome following stroke. Phys Med Rehabil Clin N Am. 2015;26:583–598. doi: 10.1016/j.pmr.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 37.Jang KM, Nam TK, Ko MJ, Choi HH, Kwon JT, Park SW, Byun JS. Thrombolysis in cerebral infarction grade 2C or 3 represents a better outcome than 2B for endovascular thrombectomy in acute ischemic stroke: a network meta-analysis. World Neurosurg. 2020;136:e419–e439. doi: 10.1016/j.wneu.2020.01.020 [DOI] [PubMed] [Google Scholar]
- 38.Kaesmacher J, Dobrocky T, Heldner MR, Bellwald S, Mosimann PJ, Mordasini P, Bigi S, Arnold M, Gralla J, Fischer U. Systematic review and meta-analysis on outcome differences among patients with TICI2b versus TICI3 reperfusions: success revisited. J Neurol Neurosurg Psychiatry. 2018;89:910–917. doi: 10.1136/jnnp-2017-317602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flottmann F, van Horn N, Maros ME, McDonough R, Deb-Chatterji M, Alegiani A, Thomalla G, Hanning U, Fiehler J, Brekenfeld C. Early TICI 2b or Late TICI 3—is perfect the enemy of good? Clin Neuroradiol. 2021;32:353–360. doi: 10.1007/s00062-021-01048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panni P, Gory B, Xie Y, Consoli A, Desilles JP, Mazighi M, Labreuche J, Piotin M, Turjman F, Eker OF, et al. ; ETIS (Endovascular Treatment in Ischemic Stroke) Investigators. Acute stroke with large ischemic core treated by thrombectomy. Stroke. 2019;50:1164–1171. doi: 10.1161/STROKEAHA.118.024295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.