Abstract
Major mental diseases such as autism, bipolar disorder, schizophrenia, and major depressive disorder are debilitating illnesses with complex etiologies. Recent findings show that the onset and development of these illnesses cannot be well described by the one-gene; one-disease approach. Instead, their clinical presentation is thought to result from the regulative interplay of a large number of genes. Even though the involvement of many genes are likely, up regulating and activation or down regulation and silencing of these genes by the environmental factors play a crucial role in contributing to their pathogenesis. Much of this interplay may be moderated by epigenetic changes. Similar to genetic mutations, epigenetic modifications such as DNA methylation, histone modifications, and RNA interference can influence gene expression and therefore may cause behavioral and neuronal changes observed in mental disorders. Environmental factors such as diet, gut microbiota, and infections have significant role in these epigenetic modifications. Studies show that bioactive nutrients and gut microbiota can alter either DNA methylation and histone signatures through a variety of mechanisms. Indeed, microbes within the human gut may play a significant role in the regulation of various elements of “gut-brain axis,” via their influence on inflammatory cytokines and production of antimicrobial peptides that affect the epigenome through their involvement in generating short chain fatty acids, vitamin synthesis, and nutrient absorption. In addition, they may participate in-gut production of many common neurotransmitters. In this review we will consider the potential interactions of diet, gastrointestinal microbiome, inflammation, and epigenetic alterations in psychiatric disorders.
Keywords: epigenetics, inflammation, microbiome, nutrition, psychiatric disorders
1 ∣. INTRODUCTION
A complex ecosystem consisting of viruses, bacteria, fungi, protozoa and archaea resides in human digestive track, respiratory system, and skin surface. This ecosystem has been estimated to have over 1,000 species and 8,000 strains, but a definition of what exactly is considered a healthy flora is still undefined (Dinan & Cryan, 2017). Recent findings indicate that the endogenous microbial flora, in particular gastrointestinal (GI) microbiota (or microbiome), play a fundamentally important role in health and disease (Paul et al., 2015). Most relevantly, alterations in intestinal microbial ecosystem and inflammation have been associated with autism, schizophrenia (SCZ) and bipolar disorder (Bloomfield et al., 2016; Meyer, Feldon, & Dammann, 2011).
Two of the more questions are; “how intestinal microbes impact brain function and how necessary are these microbes for normal brain function?” Several lines of evidence indicate that interplay between dietary components and microbiota may also play a key role in moderating disease pathogenesis through more classic mechanisms such as inflammation (Fond et al., 2015). Interestingly, as it was reviewed by Sampson and Mazmanian (2015), a number of studies have shown that in the absence of gut microbiome, brain fails to develop normally. For example, lower levels of BDNF, serotonin, and 5HT1A receptors were reported in the amygdala and hippocampus of germ free mice which were not recovered after re-colonization of normal microbiome in adult mice (Sampson & Mazmanian, 2015).
The conversation between the “brain and the gut” may be moderated by at least four different pathways such as (i) the parasympathetic nervous system (most notably the vagus nerve); (ii) the immune system; (iii) the gut neuroendocrine system; or (iv) via circulatory system delivery of neuroactive metabolites and neurotransmitters directly produced in the gut (Berger, Gray, & Roth, 2009). The extent of this activity is surprisingly large. For example, 90% of all serotonin is produced by enterochromaffin cells of the GI tract (Berger et al., 2009). This serotonin synthesis is influenced by gut microbiome (O’Mahony, Clarke, Borre, Dinan, & Cryan, 2015). Even though circulating serotonin is largely metabolized by liver, its normal range of blood levels remains significant (101–283 ng/ml, i.e., 1 mg/total blood) (https://medlineplus.gov). In fact, relative blood levels of serotonin is ~10,100 times more than dopamine and epinephrine, which are active circulatory catecholamines stimulating sympathetic nerves (note that; blood levels for dopamine, epinephrine, and norepinephrine are 10 ng/ml, 900 pg/ml, and 600 pg/ml, respectively). Despite the fact that serotonin cannot cross the blood brain barrier (BBB), it affects vagus nerve activity and BBB permeability, thus indirectly influences brain functions (Li, Wu, Zhu, & Owyang, 2001; Sharma, Olsson, & Dey, 1990; Sharma, Westman, Navarro, Dey, & Nyberg, 1995). Therefore, these pathways are not always distinct. For instance, instead of arriving via the circulatory system, some bacterial toxins can be transported to CNS by the GI vagus nerve (Bolte, 1998).
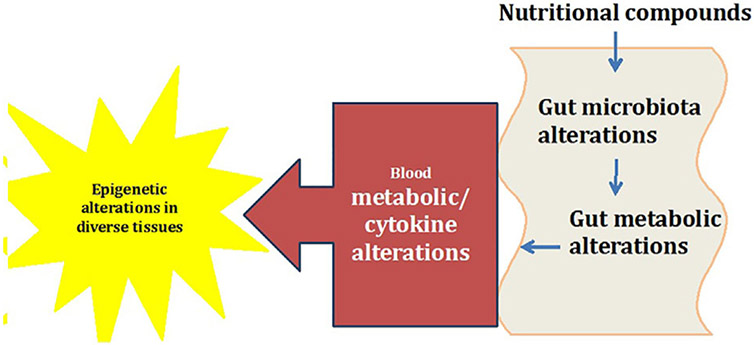
This relationship between gut microbiome and brain development is now sometimes referred to as the “microbiome-gut-brain axis,” which is composed of gut microbiome, enteric nervous system, vagus nerve, neuroendocrine system [that includes Hypothalamic-pituitary-adrenal axis (HPA axis)] and of course, the central nervous system (CNS) (Cong et al., 2016; Stilling, Dinan, & Cryan, 2014). Figure 1 depicts mechanisms through which gut microbiota may connect to immune system and affect brain functions. Here, we review the influence of these pathways in regards to epigenetic alterations associated with neuro-developmental and psychiatric illnesses such as autism, SCZ, bipolar disorder, and major depressive disorder.
FIGURE 1.
Interplay of nutrition, microbiome, and epigenome. This is a simplified depiction of what happens in the GI tract, and how the outcome in the form of metabolites and byproducts can ultimately affect the epigenetics in other tissues of the body. This change can be the result of direct interaction between these metabolites or secondary to activation of an immune response
2 ∣. MATERNAL NUTRITION AND THE ESTABLISHMENT OF GUT MICROBIOTA BEFORE AND AFTER BIRTH
It was long believed that neonates are born with sterile gastrointestinal tract (GIT) and the first microbial flora that colonizes humans’ GIT are those from maternal vaginal canal, skin and large intestine (Rook, Lowry, & Raison, 2014). Recent studies using culture-independent methods showed that humans start to acquire gut bacteria while they are in utero. Thus, like viruses, bacterial elements may pass through the maternal digestive tract or other internal mucosa to colonize the embryo’s digestive tract. The acquisition of GI flora is influenced by maternal diet and lifestyle and may be linked to developmental disorders in neonates (Collado, Rautava, Aakko, Isolauri, & Salminen, 2016; Gosalbes et al., 2013, 2016; Koleva, Kim, Scott, & Kozyrskyj, 2015). For example, one study that used non-human primates demonstrated that, mothers with fat rich diet (36%) during gestation and lactation deliver offspring with microbial dysbiosis, even after their diet was changed to an isocaloric (13% fat) diet (Ma et al., 2014). This earlier finding has been extended by a more recent study indicating that offspring of mothers with high-fat diet during pregnancy exhibit depletion of Bacteroides in meconium at birth that sustain at least for weeks (Chu et al., 2016).
When the baby is born, he or she is exposed to billions of microorganisms. Some of these new microbes eventually get introduced to the gut flora and “fight” for survival. This fight is influenced by many environmental factors. Some of the most significant determinants of gut microbiome composition are the type of delivery, infant feeding, duration of postpartum hospitalization, and the use of antibiotics. For example, infants born by vaginal delivery at term who were breastfed exclusively, have more beneficial microbiota and less number of C. difficile and Escherichia coli (Penders et al., 2006). Other major microbial components of baby’s gut flora include Bifidobacterium, Streptococcus, Lactococcus, and Lactobacillus (Yatsunenko et al., 2012). This flora changes over time. In comparison, adults GIT is mostly dominated by Firmicutes (38.8%), Bacteroidetes (27.8%), Actinobacteria (8.2%), Proteobacteria (2.1%) (D’Argenio & Salvatore, 2015).
It appears that after the first 2 weeks, the established microbial flora is relatively stable (Yu, Gadkari, & Zhou, 2015). However, introducing formula milk to neonate’s diet can rapidly shift the composition of microbiome. Also, the environment provided by child’s family has a crucial role in formation of this new microbial ecosystem. Several studies using real time PCR analysis, fluorescent in situ hybridization (FISH), 16S RNA cloning, and sequencing have shown a close similarity between neonate and the parent’s microbiome (Morelli, 2008). The next notable change in GI microbiome comes around the time of transitioning to solid food. However, it is clear from other studies that these transitions are fairly common with steady state not being complete until age 5 (Odamaki et al., 2016).
While maternal milk nutrients and bioactive components are crucial in early establishment and maintenance of the gut microbiome, a functioning GIT, and healthy intestinal epithelial cells establish a barrier against invading organisms and their toxins. GIT illnesses are ranked among the top causes of neonatal morbidity and mortality in humans. Therefore, the importance of GI maturation and development in newborns health has become more closely examined over the past several years. The scrutiny of complex interactions between host and gut microbiota has gained more attention with the realization that the number of microbes which colonize different segments of human’s gut are almost 10 times more than the number of host cells, and that the number of microbiome genes are estimated to be 100 times more than the number of human genes (Munyaka, Khafipour, & Ghia, 2014).
3 ∣. MICROBIOME, INFLAMMATION, AND BRAIN DEVELOPMENT
Several lines of evidence suggest that the GI microbial flora and inflammation are key players in brain development and function. One animal study examining the effects of microbiota in mouse showed that a germ-free mouse would have an increased myelin gene expression producing a thicker myelin sheet specifically in prefrontal cortex, which is involved in control of anxiety and social behavior. The same study showed that if a microbiome is introduced to germ free mouse, the affected neurons are capable of reforming and regressing to their normal state (Hoban et al., 2016). In addition, microbes, including gut microbiota directly shape the immune system by alteration of circulating levels of inflammatory and anti-inflammatory cytokines that in turn affect the development of oligodendrocytes (Andrews, Zhang, & Bhat, 1998; Hakansson & Molin, 2011). Studies using either monkeys or rodents both show that a nonspecific inflammation during pregnancy could also cause abnormalities in brain development. These abnormalities are associated with behavioral alterations that mimic autism and SCZ (Köler et al., 2014; Smith, Li, Garbett, Mirnics, & Patterson, 2007; Willette et al., 2011).
As the immune-inflammatory concept of psychosis has been explored in the past two decades, the relation between infections, the gut microbiota landscape, inflammatory markers, and brain development has become better understood. For example, some Studies of human subjects have shown that several perinatal infections and maternal immune disorders are risk factors for SCZ in offspring (Müller, Weidinger, Leitner, & Schwarz, 2015). Moreover, both animal and human studies showed an increased risk for SCZ with pre-or perinatal viral infections. In particular respiratory, genital and reproductive tract infections have been linked to an increased risk for SCZ in offspring (Buka, Cannon, Torrey, & Yolken, 2008). Some researchers have even taken a step further and investigated the impacts of specific infections and their exclusive effects on individual psychiatric illnesses. Yolken and Torrey (1995) showed a direct association between a history of Herpes Simplex 1 (HSV1) infection and decline in cognitive function in schizophrenic patients. They proposed that HSV1 could modulate the neurocognitive function of CNS due to its ability to establish a latent infection and chronic inflammation in neurons (Yolken & Torrey, 1995). Like any other tissue, inflammation in CNS can cause both protective and toxic effects. However, as described in following section, an excessive inflammatory response by pro inflammatory cytokines, glial cells, astrocytes, and T or B cells can be a source of injury to this organ.
4 ∣. MECHANISMS THROUGH WHICH INFECTION AND INFLAMMATION MAY INDUCE MENTAL DISORDERS
Genetic studies have shown that many genes that are primarily or in part responsible for immune functions and inflammation are affected in mental diseases. For example, several studies addressed the link between MHC genes on chromosome 6 and SCZ (Andreassen et al., 2014). In one longitudinal study, the relation between C-reactive protein (CRP) levels in adolescents was monitored and measured against their risk for SCZ in adulthood (Fernandes et al., 2016). The results showed a direct, and a linear relation between blood CRP levels in adolescents and later risk for SCZ. Others have shown that higher levels of CRP are associated with earlier age of disease onset (Metcalf et al., 2016). Still other studies have shown the effects of immune modulation on amyloidosis, a hallmark of Alzheimer’s disease (Philippens et al., 2017). A study of cytokine profiles in bipolar affective disorder also showed elevated pro-inflammatory cytokines both in the manic and depressed phase of bipolar disorder compared to the healthy subjects. A significant increased expression of inflammatory cytokines was also shown in the brain of those patients who attempted suicide (Raison & Miller, 2013). Additionally, several studies indicate that depression is linked to dysregulation of many inflammatory genes. A contributory roles of inflammatory markers in depression is further supported by observations that alpha interferon, a commonly prescribed medication for patients with multiple sclerosis, causes depression in almost 90% of patients to whom it is prescribed (Goeb et al., 2006; Haussleiter, Brüne, & Juckel, 2009). Hence, it is not surprising that anti-inflammatory drugs are amongst novel therapeutics for depressed patients, particularly those with increased inflammatory markers (Capuron et al., 2002).
Many other clinical trials have demonstrated anti-depressant effects from anti-inflammatory medications, in particular, NSAIDs and cytokine inhibiting medications. One recent study showed a direct relation between total white blood count and depression. In this study, Beydoun et al. (2016) proposed that women with increased WBC are more prone to depression whereas the converse was true for men. The authors attributed this differential effect to hormonal effects of stress and cortisol in women. While more recent studies have proposed an increased levels of cytokines such as IL-6, IL-8, and TNFα as biomarkers for prediction of psychotic disorders (Föcking et al., 2016), the reverse or the anti-inflammatory effects of antipsychotic medication have been known for a long time (Müller et al., 2015). Additionally, elevated levels of inflammatory markers such as IL-6, soluble IL-6 receptor (sIL-6R), sIL-2R, and transferrin receptor (TfR) have been found in the blood of patients with bipolar disorder and major depression (Maes et al., 1995). Further exploration of underlying mechanisms of infection in psychiatric symptoms in animal studies showed that chronic gastrointestinal inflammation induces anxiety-like behavior and altered central nervous system biochemistry in mice (Bercik et al., 2010). In this study, mice infected with parasite Trichuris muris showed increased anxiety-like behavior that was associated with decreased hippocampal BDNF messenger RNA (mRNA). Etanercept (a TNF inhibitor) was able to reduce the inflammatory marker and anxiety like behavior but could not normalize BNDF level. However, BNDF was normalized when the infected mice was treated with probiotic Bifidobacterium longum. More details of diverse effects of gut microbiome on various circulatory cytokines are described by Hakansson and Molin (2011). Given the potential roles of pro inflammatory cytokines in psychiatric illnesses combined with imaging and therapeutic studies, these and other findings strongly support the need for more extensive research in psychoneuroimmunology. Moreover, there is much to learn about the mechanisms mediating the influence of microbiome, infection and inflammation on psychiatric diseases. Some studies suggest that epigenetic alterations are that missing link.
5 ∣. EPIGENETIC ALTERATIONS AND INFECTIOUS ELEMENTS
Epigenetic (“on top of” genetic) refers to those modifications of the genome that do not change DNA sequences, and are potentially heritable and reversible. These alterations allow the single genome to adapt its transcriptional repertoire to the ever changing environmental conditions and/or to create different cell/tissue types in multi-cellular organisms (Abdolmaleky, Zhou, & Thiagalingam, 2015; Abdolmaleky, Zhou, Thiagalingam, & Smith, 2008). In fact, contrary to genetic codes which are fixed (with the exception of random mutations) and non adaptive to the external conditions, epigenetic codes are flexible and responsive to the environmental cues for fine-tuning of gene expression levels. Regulation of gene expression by DNA methylation, histone modifications, non-coding RNAs, and RNA editing are among the best known epigenetic mechanisms. In general, DNA methylation that consists of methylation of cytosine residues followed by guanine or adenine, suppresses gene expression. However, hydroxymethylation of the same residues can stimulate gene expression. These changes are mediated via a group of enzymes that promote DNA methylation (e.g., DNMT1, DNMT3A, and DNMT3B) or hydroxymethylation (e.g., TET1-3 and IDH1-3).
In contrast with DNA methylation, histone modifications are more diverse and complex. Different amino acids of histone tails could be methylated, acetylated, phosphorylated, etc. These modifications may increase or decrease gene expression depending on the type of change and its position. For example, acetylation of histone residues leads to increased accessibility of nucleosomal DNA to transcription factors, thus increasing the expression levels of corresponding genes. Histone acetylation is catalyzed by lysine acetyltransferases. Conversely, histone deacetylases (HDACs) remove the acetyl group from lysine residue (Paul et al., 2015). The nature of histone methylation is much more complex. Amino acids of histone residues can be mono, bi, or tri methylated. These modifications may inhibit or promote genes’ expression dependent on their positions and the affected amino acids (Abdolmaleky & Thiagalingam, 2014). This complex combination of DNA methylation and histone modifications interact with more than 1,000 microRNAs. In turn, each microRNA can target the transcript of hundreds of genes in a tissue specific manner thus increasing the complexity of the transcriptional response of an organism without increasing the number of genes.
In the last decade, studies have shown hundreds of epigenetic alterations in blood or brain of psychiatric subjects as compared to corresponding tissues of normal controls (reviewed in Abdolmaleky et al., 2015). For example, samples from those with autism have increased DNMT1, 3A, and 3B expression in their cerebellum, which supports the findings of global increase in DNA methylation and hydroxymethylation in these individuals (Keil & Lein, 2016). Another study using human fetal cortex samples ranging from 23 to 184 days post-conception showed altered DNA methylation in key neurodevelopmental gene suggesting that epigenetic mechanisms mediate some of the effects observed in SCZ (Pidsley et al., 2014). Similar findings with respect to other mental disorders are reviewed elsewhere (Abdolmaleky et al., 2015).
Conceivably, DNA methylation and other epigenetic marks can be influenced by either extrinsic environmental factors (e.g., alcohol) or intrinsic factors such as hormones. However, the exact list of environmental factors that may be linked to the epigenetic aberrations observed in mental disease is not well defined. In recent years, technologically advances and comprehensive multidirectional studies have provided tools and large data sets to understand the relations between diet, gut microbiome, chronic inflammation, and mental health. It has also become clear that all of these players (Jacobi & Odle, 2012), as well as epigenetic modifications have key roles in development of all human organs, especially CNS during the embryo-fetal, perinatal, and later stages of life. Epigenetic regulation itself is influenced by diet, infections, and inflammation as well as gut microbiome. Hullar and Fu (2014) showed that gut microbiome alterations via different means can induce epigenetic aberrations associated with human diseases and developmental maladies. For example, gut microbiome-derived metabolites can directly interact with the mammalian epigenome. The intestinal microbiome generates a variety of short chain fatty acids (SCFA) for energy and ATP production. Bacteria from Clostridium, Eubacterium, and Butyrivibrio genera are able to synthesis butyrate from non-digestive fibers in the GI lumen which has inhibitory effects on HDACs (Bourassa, Alim, Bultman, & Ratan, 2016). Butyrate and other SCFA byproducts (e.g., acetate, propionate) of GI microbiome are further metabolized by the colonocytes and mostly cleared in the liver and then enter systemic circulation, where butyrate consists ~20% of the total SCFA (Cummings, Pomare, Branch, Naylor, & Macfarlane, 1987). At least two G-protein coupled receptors (FFAR2 and FFAR2) are characterized for SCFA in diverse tissues which affect cell metabolism, inflammation and oxidative stress (Puddu, Sanguineti, Montecucco, & Viviani, 2014). The GI microbiome also contributes to the absorption and secretion of minerals such as iodine, zinc, selenium, cobalt and other cofactors that participate in epigenetic processes. Additionally, some other key metabolites of gut microbiota including S-adenosylmethionine (SAM), acetyl-CoA, NAD, alpha-KG, and ATP serve as essential cofactors for epigenetic enzymes that regulate DNA methylation and histone modifications (Paul et al., 2015).
Beside the general effects of gut bacterial flora which indirectly affects epigenetic landscape, infection with some bacteria such as Helicobacter pylori are specifically linked to DNA methylation and may decrease expression of O6-methylguanine DNA methyltransferase (Sepulveda et al., 2010) resulting in changes of local epigenetic signatures. Here, the epigenetic alteration is due to the induction of inflammation by H. pylori infection (Niwa et al., 2010). Therefore, not all of these effects are mediated by metabolic by-products. As another example, Mycobacterium tuberculosis produces a protein (Rv3423.1), which exhibits histone acetyltransferase activity in host cells and acetylates histone H3 at K9/K14 positions (Jose et al., 2016). In addition, Rv1988, another secreted mycobacterial protein that interacts with chromatin, has methyltransferase activity and methylates histone H3 at H3R42 position repressing the expression of affected genes (Yaseen, Kaur, Nandicoori, & Khosla, 2015).
Viral activity may also moderate certain epigenetic changes. For example, EBV and human papillomavirus infections are known to induce epigenetic modifications of several genes via altering H3K27me3 (histone H3 trimethylated at lysine 27), and DNA methylation of their promoter regions involving DNMTs expression or activation. The later is observed in Hepatitis B virus infection as well (Paschos & Allday, 2010). HIV infection also induces DNA methylation alterations in drug naïve patients which correspond to 10 years older age compared to age-matched control subjects (Nelson et al., 2017). Hence, based on these lines of evidence it is likely that other chronic bacterial, parasitic or viral infections which are linked to psychiatric diseases (e.g., brucellosis, toxoplasmosis, and herpes) may induce similar epigenetic alterations. Finally, although a tantalizing relationship between certain chronic infections (e.g., brucellosis) and major mental diseases (such as psychosis and depression) has been recognized for a long time (Sadock, Sadock, & Ruiz, 2009), it is difficult to establish whether these infections are coincidental or causal in the pathogenesis of behavioral illness. Extensive, well designed investigations will be needed before the potential impacts of specific infections to particular epigenetic alterations observed in mental diseases can be clarified.
6 ∣. CHRONIC INFLAMMATION AND EPIGENETIC ALTERATIONS
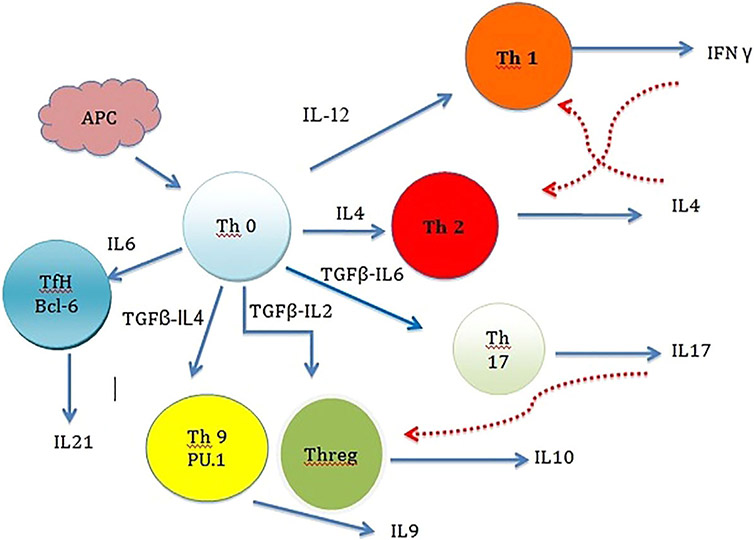
There are many chronic inflammatory diseases in humans that are considered as psychosomatic diseases. A short review of their nature may help to better understand the relation between inflammation and mental diseases. Inflammatory bowel diseases (IBD), ulcerative colitis (UC), and Crohns disease (CD) all follow similar pathways of inflammation. These diseases processes are complex and involve both environmental and genetic factors. Genetic studies have shown that polymorphisms in the Toll-like receptor (TLR), IL-23/IL-17, interferon gamma (IFNG), and apoptosis pathways are associated with susceptibility to CD and UC (Bank et al., 2015). In the IL-23/IL-17 pathway, IL-23 increases the excretion of the pro-inflammatory cytokine IL-17, which in turn enhances the production of proinflammatory mediators such as IL-1β, IL-6, IL-8, and TNF-α (Andrews et al., 1998). These molecules are produced by immune cells under chronic inflammatory conditions (Figure 2), which consequently lead to the development of many diseases such as UC, CD, and autoimmune disorders (Ciechomska & O’Reilly, 2016). Furthermore, recent studies have provided evidence that the imbalance of specific bacterial species in human gut contributes to IBD, CD, and UC pathogenesis through inflammatory processes which in part could be remedied by antibiotic therapy (Lopez-Siles et al., 2014; Mirsepasi-Lauridsen et al., 2016; Zhou et al., 2016).
FIGURE 2.
Cell-cell interactions in an immune response. When Antigen Presenting Cell (APC) comes in contact with Th0 (naïve T cell) the faith of immune response is decided. Th0 have the potential to differentiate to inflammatory Th1 cells or Th2 helper cells. This is done by cytokines in the environment, which are influenced by the antigen being presented via APC. Antigen induced IL4 production favors Th2 generation while IL12 favors Th1 cells. Once the faith of immune pathway is decided other Interleukins will inhibit the alternative pathways. IFN produced by Th1 will inhibit the differentiation of Th2 and in return IL4 produced by Th2 will inhibit Th1. Th17 is another subset of helper T cells. Th17 is a part of adoptive immune response and also play a significant role in maintaining mucosal barriers and contribute to pathogen clearance at mucosal surfaces. TGFβ, IL6, IL21, and IL23 contribute to Th17 differentiation. Another subpopulation of T cells is regulatory T cells (Th-reg). These actively suppress activation of the immune system and prevent pathological self-reactivity, that is, autoimmune disease. In the presence of TGFβ and IL4 naïve T cells can transform to T9 helper cells. The main product of Th9 is IL9, which is one of the major interleukins in mast cell production and T cell growth. It also stimulates mast cell accumulation in tissues, promotes ILC survival, enhances class-switching to IgE in B cells and alters haematopoietic progenitor cell activity.T Follicular Helper (Tfh) Cells are the T cells responsible for antigen specific B-Cell immunity. Tfh helps B-cells to produce antigen specific antibodies and form long term memory cells. Solid arrows indicate stimulatory and dashed arrows indicate inhibitory effects
Though the epigenome itself is affected by inflammation, epigenetic mechanisms are key mediators in the development of chronic inflammation by the expression of proinflammatory cytokines including IL1, IL2, IL6, TNFα, and induction of COX2 and transcription factor NF-κB (Wilson, Symons, McDowell, McDevitt, & Duff, 1997). As an example, while dysregulation of TNFα is linked to IBD, in a zebrafish model the loss of function of uhrf1 (ubiquitin-like protein containing PHD and RING finger domains 1), a gene which is an epigenetic regulator, results in TNFα promoter DNA hypomethylation. This in turn is associated with a microbe-dependent increase in TNFα expression leading to immune cell recruitment, chronic inflammation, apoptosis, and barrier dysfunction of intestinal epithelial cells (Marjoram et al., 2015). Other examples include, demethylation of a small promoter region of the IL2 gene in T cells soon after activation, resulting in IL2 production. Also the maturation of naive CD4 T cells to the T helper 2 cells is characterized by the rapid acquisition of H3 acetylation at the IL4/13 gene clusters (Wilson et al., 1997). Hence, demethylating agents and histone deacetylase inhibitors are amongst the currently proposed drugs for epigenetic therapy, which target chromatin in rapidly dividing cells and restore normal cell functions (Karberg, 2009).
Data also demonstrates that viral infections induce demethylation of inflammatory genes, increasing the expressions of IL6, IL13, and IL17 in infected individuals (Tang et al., 2011). Epigenetic modifications of these cytokine genes are known to regulate the differentiation of naïve CD4 cells to Th1, Th2 cells altering immune response. Additionally, demethylation of Ifng locus activates CD8 killer T cells (Frydecka, Karpiński, & Misiak, 2014).
7 ∣. THE IMPACTS OF MODERN LIFE ON HABITUAL OR NATURAL HUMAN MICROBIOME
While various infections and chronic inflammations are emerging as important risk factors in the pathogenesis of mental diseases, reduction in ancient and habitual human infections, disruption of the commensal microbes and diminished contact with environmental microbes may also increase the vulnerability to inflammatory as well as mental diseases (Meyer et al., 2011). Although it is best recognized as the causative agent for peptic ulcer disease, H. pylori has co-evolved with humans for thousands of years. Interestingly, in some cases, exposure has been associated with a protective effect with respect to chronic inflammatory disorders and allergies (Blaser & Atherton, 2004). Other organisms that are now regarded as “old friends” of GI, such as hepatitis A viruses, salmonella, mycobacterium tuberculosis have similar bivalent effects on the regulation of our immune system. These microbes often bind to dendritic cells, C-type lectin receptors and modulate an immune response, which can be chronic and last a lifetime. Other environmental organisms also induce an immune response following their ingestion or inhalation. Some soil-derived bacteria such as mycobacterium genus may influence the immune regulatory circuits and prevent inflammation (Bah, Dickinson, Forster, Kampmann, & Ghazal, 2017).
In modern life, antibiotics have been a mainstay of treatment for bacterial infections worldwide. However, despite their benefits, their use may have a disruptive effect on the GI microbiome. An experiment involving 12 healthy individuals taking broad-spectrum antibiotic (metronidazole, ciprofloxacin, vancomycin) showed significant decrease in microbiome diversity just 1 day after the start of treatment. Blood studies showed notable decrease in TNFα response to lipopolysaccharides (LPS) stimulation (Strawbridge et al., 2015). Additional studies in the same individuals showed reduced capacity to release IL1β and IL6 in antibiotic treated subjects (Lankelma et al., 2016).
Broad-spectrum antibiotics can affect the abundances of almost 30% of bacteria in the gut bacterial community, causing rapid and significant drops in microbial taxonomic richness, diversity, and consistency (Dethlefsen, Huse, Sogin, & Relman, 2008; Dethlefsen & Relman, 2011). Once the treatment is stopped the gut microbiome tries to recover and depending on individual microorganism’s resilience, the original composition may be restored. But in some cases, the initial state is often not totally recovered. The microbiome alterations secondary to the use of antibiotics, beyond increasing the risk for opportunistic infections, would also affect basic immune homeostasis. These effects will be even more significant if they occur early in life, which is the critical period for maturation of the immune system and establishment of immunological tolerance (Francino, 2014). Beside antibiotics, dietary elements are among other factors that affect microbial profiles in modern civilization. For example, a meat rich diet can increase “abundance and activity of Bilophilawadsworthia” which can trigger IBD (David et al., 2014).
8 ∣. MICROBIAL THERAPY IN MENTAL DISEASES
Having observed various interesting links between the microbiome, immunity, inflammation, epigenetic alterations and mental health, many investigators have considered whether microbiome manipulation may be a tool to fight mental illnesses in modern life. However, the challenge is to find a safe and healthy microbiota. This goal might not be as simple as it appears, given that the human microbiome is a changeable entity reacting to local stress, food intake, sleep, and baseline conditions.
Probiotics are considered as the oldest tools to normalize the GI microbiome. The Food and Agriculture Organization of the United Nations defined probiotics as “live microorganisms which when administered in adequate amounts, confer a health benefit on the host”. Probiotic experiments in animals have shown to be beneficial for inflammation and colitis (Sheil, Shanahan, & O’Mahony, 2007). Probiotics for humans, almost exclusively belong to the genera Lactobacillus and Bifidobacterium as they have a long history of safe use (Linares, Ross, & Stanton, 2016). Clinical trials have shown that probiotics favorably prevent or improve the symptoms of various disorders, including IBD and irritable bowel syndrome. Additionally, it has been shown that the gut microbial samples of healthy individuals can improve the disease symptoms in individuals suffering from UC (Suskind et al., 2016). These trials lead the experts in the field to conclude that; the use of probiotics will alternate the dominant pathologic GI microbial flora and may influence the health through three major pathways: (i) direct antimicrobial effects; (ii) enhancement of mucosal barrier integrity; and (iii) immune modulation (Patel & DuPont, 2015).
Newborn rats exposed to early life stress (ELS) showed that the use of proper probiotics could be an effective treatment to restore normal developmental course of the emotion-related behaviors (Cowan, Callaghan, & Richardson, 2016). More specifically, administration of Lactobacillus Rhamnosous for 10 days was shown to normalize anxiety-like behavior in rats (Foster, Lyte, Meyer, & Cryan, 2016). In humans, a randomized, placebo-controlled trial showed that probiotics could reduce the level of vWF (von Willebrand factor) and increase BDNF and MCP-1 (monocyte chemotactic protein-1) in SCZ patients (Tomasik, Yolken, Bahn, & Dickerson, 2015). Another randomized, placebo-controlled study reported a trend toward the improvement of positive psychiatric symptoms following 14 weeks probiotic treatment in male SCZ patients who were seronegative for Candida albicans (Severance et al., 2017). Although one prior study showed no improvement in psychiatric symptoms in those with SCZ (Dickerson et al., 2014), a recent meta-analysis of seven studies using healthy participants showed that probiotics supplementation improve psychological symptoms compared with placebo (McKean, Naug, Nikbakht, Amiet, & Colson, 2017). These findings may be more applicable to depression. A recent meta-analysis of randomized controlled trials in major depression showed that probiotics improve depressive symptoms providing supporting evidence that probiotic supplementation could reduce anxiety and depression symptoms (Huang, Wang, & Hu, 2016). A literature review on 10 randomized controlled trials also provided supporting evidence that probiotic supplementation could reduce anxiety and depression symptoms (Pirbaglou et al., 2016).
9 ∣. CONCLUSION
Epigenetic aberrations may have key roles in the pathogenesis of many major psychiatric diseases. However, the underlying causes of these regulatory changes are not well defined. New lines of evidence along with the recently introduced model of “microbiome-gut-brain axis” have opened a new window for our understanding of the pathogenesis of neuropsychiatric syndromes, in particular depression. Future studies to identify therapeutic diets, protective microbiome constituents, harmful bacterial metabolites, or epigenetic signatures predictive of future illnesses could have a major impact on the prevention or treatment of behavioral disorders.
REFERENCES
- Abdolmaleky HM, & Thiagalingam S (2014). Pathogenic histone modifications in schizophrenia are targets for therapy. In Grayson DR & Peedicayil J (Eds.), Epigenetics in psychiatry, dimitrios avramopoulos, Chapter 11. New York: Elsevier and Academic Press. [Google Scholar]
- Abdolmaleky HM, Zhou JR, & Thiagalingam S (2015). An update on the epigenetics of psychotic diseases and autism. Epigenomics, 7(3), 427–449. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Zhou JR, Thiagalingam S, & Smith CL (2008). Epigenetic and pharmacoepigenomic studies of major psychoses and potentials for therapeutics. Pharmacogenomics, 9(12), 1809–1823. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Harbo HF, Wang Y, Thompson WK, Schork AJ, Mattingsdal M, … Dale AM (2014). Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: Differential involvement of immune-related gene loci. Journal of Molecular Psychiatry, 20, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T, Zhang P, & Bhat NR (1998). TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyteprogenitors. Journal of Neuroscience Research, 54(5), 574–583. [DOI] [PubMed] [Google Scholar]
- Bah SY, Dickinson P, Forster T, Kampmann B, & Ghazal P (2017). Immune oxysterols: Role in mycobacterial infection and inflammation. The Journal of Steroid Biochemistry and Molecular Biology, 169, 152–163. [DOI] [PubMed] [Google Scholar]
- Bank S, Andersen PS, Burisch J, Pedersen N, Roug S, Galsgaard J, … Andersen V (2015). Polymorphisms in the toll-like receptor and the IL-23/IL-17 pathways were associated with susceptibility to inflammatory bowel disease in a danish cohort. PLoS ONE, 10(12), e0145302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, … Collins SM (2010). Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology, 139(6), 2102–2112. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, & Roth BL (2009). The expanded biology of serotonin. Annual Review of Medicine, 60, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Dore GA, Canas JA, Fanelli-Kuczmarski MT, Evans MK, & Zonderman AB (2016). White blood cell inflammatory markers are associated with depressive symptoms in a longitudinal study of urban adults. Translational Psychiatry, 6(9), e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ, & Atherton JC (2004). Helicobacter pylori persistence: Biology and disease. Journal of Clinical Investigation, 113(3), 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, … Howes OD (2016). Microglial activity in people at ultra high risk of psychosis and in schizophrenia: An [(11)C]PBR28 PET brain imaging study. The American Journal of Psychiatry, 173, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte ER (1998). Autism and clostridium tetani. Medical Hypotheses, 51(2), 133–144. [DOI] [PubMed] [Google Scholar]
- Bourassa M, Alim I, Bultman S, & Ratan R (2016). Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neuroscience Letters, 625, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Cannon TD, Torrey EF, & Yolken RH (2008). Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biological Psychiatry, 63(8), 809–815. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, & Miller AH (2002). Neurobehavioral effects ofinterferon-alpha in cancer patients: Phenomenology and paroxetineresponsiveness of symptomdimensions. Neuropsychopharmacology, 26, 643–652. [DOI] [PubMed] [Google Scholar]
- Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, & Aagaard KM (2016). The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Medicine, 8(1), 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechomska M, & O’Reilly S (2016). Epigenetic modulation as a therapeutic prospect for treatment of autoimmune rheumatic diseases. Mediators of Inflammation, 2016, Article ID 9607946, 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MC, Rautava S, Aakko J, Isolauri E, & Salminen S (2016). Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Scientific Reports, 6, 23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X, Xu W, Romisher R, Poveda S, Forte S, Starkweather A, & Henderson WA (2016). Gut microbiome and infant health: Brain-Gut-Microbiota axis and host genetic factors. The Yale Journal of Biology and Medicine, 89(3), 299–308. [PMC free article] [PubMed] [Google Scholar]
- Cowan CS, Callaghan BL, & Richardson R (2016). The effects of a probiotic formulation (Lactobacillus rhamnosus and L. helveticus) on developmental trajectories of emotional learning in stressed infant rats. Translational Psychiatry, 6(5), e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Pomare E, Branch W, Naylor C, & Macfarlane G (1987). Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut, 28(10), 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argenio V, & Salvatore F (2015). The role of the gut microbiome in the healthy adult status. Clinica Chimica Acta, 451(Pt A), 97–102. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, … Turnbaugh PJ (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Huse S, Sogin ML, & Relman DA (2008). The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biology, 6(11), e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, & Relman DA (2011). Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proceedings of the National Academy of Sciences of the United States America, 108(Suppl 1), 4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, … Yolken RH (2014). Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. The Primary Care Companion for CNS Disorders, 16(1). 10.4088/PCC.13m01579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, & Cryan JF (2017). Brain-gut-microbiota axis—Mood, metabolism and behaviour. Nature Reviews Gastroenterology & Hepatology, 14(2), 69–70. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, & Dean OM (2016). C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: Meta-analysis and implications. Molecular Psychiatry, 21, 554–564. [DOI] [PubMed] [Google Scholar]
- Föcking M, Dicker P, Lopez LM, Cannon M, Schäfer MR, McGorry PD, … Amminger GP (2016). Differential expression of the inflammation marker IL12p40 in the at-risk mental state for psychosis: A predictor of transition to psychotic disorder? BMC Psychiatry, 16(1), 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N, … Leboyer M (2015). The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathologie Biologie (Paris), 63(1), 35–42. [DOI] [PubMed] [Google Scholar]
- Foster JA, Lyte M, Meyer E, & Cryan JF (2016). Gut microbiota and brain function: An evolving field in neuroscience. International Journal of Neuropsychopharmacology, 19(5), pyv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francino MP (2014). Early development of the gut microbiota and immune health. Pathogens, 3(3), 769–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydecka D, Karpiński P, & Misiak B (2014). Unravelling immune alterations in schizophrenia: Can DNA methylation provide clues? Epigenomics, 6(3), 245–247. [DOI] [PubMed] [Google Scholar]
- Goeb JL, Even C, Nicolas G, Gohier B, Dubas F, & Garré JB (2006). Psychiatric side effects of interferon-beta in multiple sclerosis. European Psychiatry, 21(3), 186–193. [DOI] [PubMed] [Google Scholar]
- Gosalbes MJ, Llop S, Vallès Y, Moya A, Ballester F, & Francino MP (2013). Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clinical and Experimental Allergy, 43(2), 198–211. [DOI] [PubMed] [Google Scholar]
- Gosalbes MJ, Vallès Y, Jiménez-Hernández N, Balle C, Riva P, Miravet-Verde S, … Francino MP (2016). High frequencies of antibiotic resistance genes in infants’ meconium and early fecal samples. Journal of Developmental Origins of Health and Disease, 7(1), 35–44. [DOI] [PubMed] [Google Scholar]
- Hakansson A, & Molin G (2011). Gut microbiota and inflammation. Nutrients, 3(6), 637–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussleiter IS, Brüne M, & Juckel G (2009). Psychopathology in multiple sclerosis diagnosis, prevalence and treatment. Therapeutic Advances in Neurological Disorders, 2(1), 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, … Cryan JF (2016). Regulation of prefrontal cortex myelination by the microbiota. Translational Psychiatry, 6(4), e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Wang K, & Hu J (2016). Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients, 8(8), E483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar MA, & Fu BC (2014). Diet, the gut microbiome, and epigenetics. The Cancer Journal, 20(3), 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi SK, & Odle J (2012). Nutritional factors influencing intestinal health of the neonate. Advances in Nutrition, 3(5), 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose L, Ramachandran R, Bhagavat R, Gomez RL, Chandran A, Raghunandanan S, … Kumar RA (2016). Hypothetical protein Rv3423.1 of Mycobacterium tuberculosis is a histone acetyltransferase. FEBS Journal, 283(2), 265–281. [DOI] [PubMed] [Google Scholar]
- Karberg S (2009). Switching on epigenetic therapy. Cell, 139(6), 1029–1031. [DOI] [PubMed] [Google Scholar]
- Keil KP, & Lein PJ (2016). DNA methylation: A mechanism linking environmental chemical exposures to risk of autism spectrum disorders. Environmental Epigenetics, 2(1), dvv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, & Krogh J (2014). Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic reviewand meta-analysis of randomized clinical trials. JAMA Psychiatry, 71, 1381–1391. [DOI] [PubMed] [Google Scholar]
- Koleva PT, Kim JS, Scott JA, & Kozyrskyj AL (2015). Microbial programming of health and disease starts during fetal life. Birth Defects Research Part C: Embryo Today, 4, 265–277. [DOI] [PubMed] [Google Scholar]
- Lankelma JM, Belzer C, Hoogendijk AJ, de Vos AF, de Vos WM, van der Poll T, & Wiersinga WJ (2016). Antibiotic-induced gut microbiota disruption decreases TNF-α release by mononuclear cells in healthy adults. Clinical and Translational Gastroenterology, 7, e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu XY, Zhu JX, & Owyang C (2001). Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. The American Journal of Physiology-Gastrointestinal and Liver Physiology, 281(4), G916–G923. [DOI] [PubMed] [Google Scholar]
- Linares DM, Ross P, & Stanton C (2016). Beneficial Microbes: The pharmacy in the gut. Bioengineered, 7(1), 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Siles M, Martinez-Medina M, Busquets D, Sabat-Mir M, Duncan SH, Flint HJ, … Garcia-Gil LJ (2014). Mucosa-associated faecalibacteriumprausnitzii and Escherichia coli co-abundance can distinguish irritable bowel syndrome and inflammatory bowel disease phenotypes. International Journal of Medical Microbiology, 304(3–4), 464–475. [DOI] [PubMed] [Google Scholar]
- Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, … Aagaard KM (2014). High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nature Communications, 5, 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, & Desnyder R (1995). Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. Journal of Affective Disorders, 34(4), 301–309. [DOI] [PubMed] [Google Scholar]
- Marjoram L, Alvers A, Deerhake ME, Bagwell J, Mankiewicz J, Cocchiaro JL, … Bagnat M (2015). Epigenetic control of intestinal barrier function and inflammation in zebrafish. Proceedings of the National Academy of Sciences of the United States America, 112(9), 2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean J, Naug H, Nikbakht E, Amiet B, & Colson N(2017). Probiotics and subclinical psychological symptoms in healthy participants: A systematic review and meta-Analysis. Journal of Alternative and Complementary Medicine, 23(4), 249–258. [DOI] [PubMed] [Google Scholar]
- Metcalf SA, Jones PB, Nordstrom T, Timonen M, Mäki P, Miettunen J, … Khandaker GM (2016). Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: A prospective birth cohort study. Brain, Behavior, and Immunity, 59, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, & Dammann O (2011). Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric Research, 69, 26R––33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsepasi-Lauridsen HC, Halkjaer SI, Mortensen EM, Lydolph MC, Nordgaard-Lassen I, Krogfelt KA, & Petersen AM (2016). Extraintestinal pathogenic Escherichia coli are associated with intestinal inflammation in patients with ulcerative colitis. Scientific Reports, 6, 31152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli L (2008). Postnatal development of intestinal microflora as influenced by infant nutrition. The Journal of Nutrition, 138(9), 1791S–1795S. [DOI] [PubMed] [Google Scholar]
- Müller N, Weidinger E, Leitner B, & Schwarz MJ (2015). The role of inflammation in schizophrenia. Frontiers in Neuroscienc, 9, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyaka PM, Khafipour E, & Ghia JE (2014). External influence of early childhood establishment of gut microbiota and subsequent health implications. Frontiers in Pediatrics, 2, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KN, Hui Q, Rimland D, Xu K, Freiberg MS, Justice AC, … Sun YV (2017). Identification of HIV infection-related DNA methylation sites and advanced epigenetic aging in HIV+, treatment-naïve U.S. veterans. AIDS, 31(4), 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa T, Tsukamoto T, Toyoda T, Mori A, Tanaka H, Maekita T, … Ushijima T (2010). Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial-cells. Cancer Research, 70(4), 1430–1440. [DOI] [PubMed] [Google Scholar]
- Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, … Osawa R (2016). Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiology, 16, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, & Cryan JF (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research, 15(277), 32–48. [DOI] [PubMed] [Google Scholar]
- Patel R, & DuPont HL (2015). New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clinical Infectious Diseases, 60(Suppl 2), S108–S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos K, & Allday MJ (2010). Epigenetic reprogramming of host genes in viral and microbial pathogenesis. Trends Microbiology, 18(10), 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B, Barnes S, Demark-Wahnefried W, Morrow C, Salvador C, Skibola C, & Tollefsbol TO (2015). Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clinical Epigenetics, 7, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, … Stobberingh EE (2006). Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics, 118(2), 511–521. [DOI] [PubMed] [Google Scholar]
- Philippens IH, Ormel PR, Baarends G, Johansson M, Remarque EJ, & Doverskog M (2017). Acceleration of amyloidosis by inflammation in the amyloid-beta marmoset monkey model of alzheimer’s disease. Journal of Alzheimers Disease, 55(1), 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidsley R, Viana J, Hannon E, Spiers H, Troakes C, Al-Saraj S, … Mill J (2014). Methylomic profiling of human brain tissue supports a neurodevelopmental origin for schizophrenia. Genome Biology, 15(10), 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, & Ritvo P (2016). Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutrition Research, 36(9), 889–898. [DOI] [PubMed] [Google Scholar]
- Puddu A, Sanguineti R, Montecucco F, & Viviani GL (2014). Evidence for the gut microbiota short chain fatty acids as key pathophysiological moleculesimproving diabetes. Mediators Inflammation, 2014, Article ID 162021, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, & Miller AH (2013). The evolutionary significance of depressionin pathogen host defense (PATHOS-D). Molecular Psychiatry, 18, 15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Lowry CA, & Raison CL (2014). Hygiene and other early childhood influences on the subsequent function of the immune system. Brain Research, 1617, 47–62. [DOI] [PubMed] [Google Scholar]
- Sadock BJ, Sadock VA, & Ruiz P (2009). Kaplan and Sadock’s comprehensive textbook of psychiatry, vol. I. 9th ed. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Sampson TR, & Mazmanian SK (2015). Control of brain development, function, and behavior by the microbiome. Cell Host & Microbe, 17(5), 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda AR, Yao Y, Yan W, Park DI, Kim JJ, Gooding W, … Graham DY (2010). CpG methylation and reduced expression of O6-methylguanine DNA methyltransferase is associated with Helicobacter pylori infection. Gastroenterology, 138(5), 1836–1844. [DOI] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, … Yolken RH (2017). Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain, Behavior, and Immunity, 62, 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HS, Olsson Y, & Dey PK (1990). Changes in blood-brain barrier and cerebral blood flow following elevation of circulating serotonin level in anesthetized rats. Brain Research, 517(1–2), 215–223. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Westman J, Navarro JC, Dey PK, & Nyberg F (1995). Probable involvement of serotonin in the increased permeability of the blood-brain barrier by forced swimming. An experimental study using Evans blue and 131I-sodium tracers in the rat. Behavioural Brain Research, 72(1–2), 189–196. [DOI] [PubMed] [Google Scholar]
- Sheil B, Shanahan F, & O’Mahony L (2007). Probiotic effects on inflammatory bowel disease. Journal of Nutrition, 137(3 Suppl 2), 819S–824S. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, & Patterson PH (2007). Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience, 27, 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, Dinan TG, & Cryan JF (2014). Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes Brain Behavior, 13(1), 69–86. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane VA, & Cleare AJ (2015). Inflammation and clinical response to treatment indepression: A meta-analysis. European Neuropsychopharmacology, 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Suskind DL, Cohen SA, Brittnacher MJ, Wahbeh G, Lee D, Shaffer ML, … Miller SI (2016). Clinical and fecal microbial changes with diet therapy in active inflammatory bowel disease. Journal of Clinical Gastroenterology, 32(4), 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Zhao R, Sun Y, Zhu Y, Zhong J, Zhao G, & Zhu N (2011). Interleukin-6 expression was regulated by epigenetic mechanisms in response to influenza virus infection or dsRNA treatment. Molecular Immunology, 48(8), 1001–1008. [DOI] [PubMed] [Google Scholar]
- Tomasik J, Yolken RH, Bahn S, & Dickerson FB (2015). Immunomodulatory effects of probiotic supplementation in schizophrenia patients: A randomized, placebo-controlled trial. Biomarker Insights, 10, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, Gilmore JH, & Coe CL (2011). Brain enlargement and increased behavioral andcytokine reactivity in infant monkeys following acute prenatalendotoxemia. Behavioural Brain Research, 219, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AG, Symons JA, McDowell TL, McDevitt HO, & Duff GW (1997). Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proceedings of the National Academy of Sciences of the United States America, 94(7), 3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, … Gordon JI (2012). Human gut microbiome viewed across age and geography. Nature, 486(7402), 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen I, Kaur P, Nandicoori VK, & Khosla S (2015). Mycobacteria modulate host epigenetic machinery by Rv1988 methylation of a non-tail arginine of histone H3. Nature Communications, 6, 8922. [DOI] [PubMed] [Google Scholar]
- Yolken RH, & Torrey EF (1995). Viruses, schizophrenia, and bipolar disorder. Clinical Microbiology Reviews, 8(1), 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DH, Gadkari M, & Zhou Q (2015). Postnatal epigenetic regulation of intestinal stem cells requires DNA methylation and is guided by the microbiome. Genome Biology, 16, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Chen H, He H, Du Y, Hu J, Li Y, … Nie Y (2016). Increased Enterococcus faecalis infection is associated with clinically active Crohn disease. Medicine (Baltimore), 95(39), e5019. [DOI] [PMC free article] [PubMed] [Google Scholar]