Abstract
Objective:
To determine the prevalence of resistance to rifampicin alone; rifampicin and isoniazid, and second-line anti-TB drugs among sputum smear-positive tuberculosis patients in Zimbabwe.
Design:
A health facility-based cross-sectional survey.
Results:
In total, 1114 (87.6%) new and 158 (12.4%) retreatment TB patients were enrolled. MTB was confirmed by Xpert MTB/RIF among 1184 (93%) smear-positive sputum samples. There were 64 samples with Xpert MTB/RIF-determined rifampicin resistance. However, two were rifampicin susceptible on phenotypic drug susceptibility testing. The prevalence of RR-TB was [4.0% (95% CI, 2.9, 5.4%), n = 42/1043) and 14.2% (95% CI, 8.9, 21.1%; n = 20/141) among new and retreatment patients, respectively. The prevalence of MDR-TB was 2.0% (95% CI, 1.3, 3.1%) and 6.4% (95% CI, 2.4, 10.3%) among new and retreatment TB patients, respectively. Risk factors for RR-TB included prior TB treatment, self-reported HIV infection, travel outside Zimbabwe for ≥one month (univariate), and age <15 years. Having at least a secondary education was protective against RR-TB.
Conclusion:
The prevalence of MDR-TB in Zimbabwe has remained stable since the 1994 subnational survey. However, the prevalence of rifampicin mono-resistance was double that of MDR-TB.
Keywords: Drug resistant TB, Previously treated TB, Zimbabwe, Rifampicin resistant TB, MDR, Gene Xpert
Introduction
In the modern era, Mycobacterium tuberculosis (MTB) drug resistance is among the key challenges in ending TB (Mariandyshev and Eliseev 2017). In 2016, there were 600 000 new cases globally of multi-drug resistant tuberculosis (MDR-TB), defined as resistance to at least isoniazid and rifampicin (RIF), resulting in an estimated quarter-million annual deaths (World Health Organisation 2016). An estimated 92 629 MDR-TB cases (approximately 16% of the global burden) occurred on the African continent. However, 70% of these were not notified to health authorities, and only one-half of the countries have completed a formal drug resistance survey (DRS) (World Health Organisation 2017).
Although neighboring South Africa reports the second highest absolute number of notified rifampicin-resistant cases in the world (second only to India) (World Health Organisation 2015b), and studies from the north of Zimbabwe have indicated a possible increase in MDR-TB prevalence among retreatment cases (Metcalfe et al. 2014), no nationally representative DRS has been performed in Zimbabwe. In 1994 a sub-national DRS was conducted in Zimbabwe and the prevalence of MDR-TB was 1.9% (95% CI, 1.1, 3.2) and 8.3% (95% CI, 2.9, 21.8) among new and retreatment TB patients, respectively (Mwinga 2006). At this time in the mid-1990s, HIV was rapidly becoming hyperendemic in Zimbabwe (Harries et al. 2001), and standard “short-course” 6-month regimens including RIF were yet to be adopted (this was done in 1994), antiretroviral drugs (ARVs) were unavailable, and it was questioned whether the societal costs of treating MDR-TB was worth control of the relatively small risk it presented (Schaaf et al. 1996). Since that time, there have been dramatic changes to the TB diagnostic and MDR-TB treatment landscapes; substantial increases in movement of economic and political migrants across borders in the Southern African region; and rapid and sustained scale-up of ARVs.
The best estimates of the burden of drug resistant TB in Africa require well-performed, population-based survey findings (Ismail et al. 2018). We undertook a cross-sectional survey in 2015–2016 to determine the prevalence among new and retreatment TB cases of Rifampicin-mono-resistance, MDR-TB, and resistance to second-line agents among those with MDR-TB. We also sought to assess the risk factors for rifampicin-resistant TB and to compare the MDR-TB estimate to that obtained in 1994 as a measure of the burden of drug-resistant TB.
Methods
Study design
A population-based cross-sectional study. Initially, patients in sampled health facilities were screened and diagnosed for TB using smear microscopy. Those who were smear-positive were asked to enroll in the survey as per WHO guidelines (World Health Organisation 2015a). The survey was conducted from August 2015-September 2016 on sputum-positive new and retreatment TB patients, regardless of age or HIV status, and not already on anti-TB therapy.
Since rifampicin resistance has conventionally been considered a proxy for MDR, and due to resource constraints, only Xpert MTB/RIF-determined rifampicin-resistant (RR) specimens proceeded to solid culture and first- and second-line DST. All patients whose samples had RR-TB strains on Xpert were re-interviewed to verify history of TB treatment.
Survey procedures
A survey questionnaire eliciting socio-demographic and clinical information (e.g. self-reported HIV status and history of TB) was administered to all consenting participants at enrolment. Two spot-sputum specimens were collected from consenting patients within two days of a smear-positive TB diagnosis. Sputum collection was done under the supervision of trained nurses. About 5 mL of spot-sputum specimens were collected in two 50 mL screw-capped falcon tubes, each containing 5 mL of Cetyl-Pyridium-Chloride. This was done to maintain the integrity of the sample in case of delays (of up to 30 days) in sample transportation to the National Reference Laboratory. Each tube was labelled with a unique patient identification number (PIN). The specimens were triple-packaged in zip-lock bags to minimize spillage and contamination and were stored at room temperature. A private courier transported the specimens to the National TB Reference Laboratory (NTBRL).
At the NTBRL, both specimens were vortexed for 15 s, pooled, and then split again. One specimen was tested using the Xpert MTB/RIF assay and the other was archived. A barcode reader was used to minimize transcription errors when inputting PIN numbers. In case of errors, assays were repeated using the remaining specimens from first specimens. Subsequent procedures were based upon Xpert MTB/RIF results: if RR-TB was not detected or MTB was not detected, no further procedures were performed. If RR-TB was detected, archived specimens were retrieved, decontaminated and the resultant sputum deposits were inoculated on LJ and pyruvate agar media according to standard operating procedures (Stop TB Partnership 2014). The media were incubated at 37 °C and growth of MTB was observed weekly for up to 6 weeks. Part of the deposit was inoculated on LJ agar slants in 5 mL cryo-vials for shipment to a Supranational TB Reference Laboratory (SRL) in Antwerp, Belgium for external quality assurance. At the NTBRL, phenotypic culture and drug susceptibility testing (CDST) was done on LJ on all MTB positive isolates using the proportion method (Stop TB Partnership 2014). First-line DST was done for the drugs streptomycin, isoniazid, rifampicin and ethambutol (SIRE), and second-line DST for kanamycin, amikacin, ofloxacin, moxifloxacin and capreomycin. All the isolates were stored at −20 °C in cryo-vials with 10% glycerol. Hain Line Probe assay (LPA) (Hain LifeSciences, Nehren, Germany) was carried out on all cultures that failed to grow. Discordances between Xpert MTB/RIF RR-TB results and first-line phenotypic DST were resolved by conventional Sanger DNA sequencing of rpoB at the SRL. There was a 100% concordance in sensitivity and specificity between NTBRL and the SRL on the drugs kanamycin, capreomycin, ofloxacin and rifampicin. Sensitivity and specificity of isoniazid were 90% and 89%, respectively.
Sampling
Sampling was done as per WHO guidelines (World Health Organisation 2015a). First, probability proportional to size sampling was used to select 63 of 146 national TB diagnostic sites that were functional in 2012, and 20 of 56 national TB diagnostic sites that became functional between 2012 and 2014. Within each selected diagnostic site, consecutive eligible patients were enrolled until the required number of new cases for that site was reached, or the end of the survey period was reached.
As per WHO recommendations, sample size was calculated based on new patients only; retreatment patients were sampled on convenience. For new patients, a sample size of 677 was based on the following assumptions: (i) a total national notification of 12,405, based on 2012 programme data; (ii) an absolute precision of 1% at 95% confidence interval (CI); (iii) a priori estimated prevalence of MDR-TB of 1.9%, based on the 1994 sub-national survey. After factoring in a design effect of two and accounting for possible losses of up to 20%, a minimum sample of 1625 new smear-positive patients was estimated.
Survey and data management
A survey management team and a steering committee were established to ensure smooth implementation of the survey. A pilot survey was conducted in 10% of the sites. Three teams from the national office were trained and they later provided on-site trainings to survey teams (TB nurses and laboratory staff) in different provinces starting with low-volume sites.
Each recruiting facility maintained a survey register which captured patient demographic and clinical data. Each patient had a PIN which was linked to all the survey tools (survey register, laboratory request form and NTBRL laboratory register). Xpert MTB/RIF and CDST results were reported to facilities to inform clinical management of patients. Quality of data was ensured through training of survey teams, cross-checking original forms during support visits by local teams and during data monitoring missions supported by staff from WHO and KNCV.
De-anonymised data were sent to the central level by a courier for double-data entry into the Census and Surveys Processing System (CSPro) database by Zimbabwe National Statistics Agency staff. Electronic data were stored in a password-protected computer and backed-up on CDs stored in a locked-file cabinet. Source documents were stored in locked-file cabinets (Table 1).
Table 1.
Number and (proportion) of participants who were enrolled in the Zimbabwe Drug Resistant survey by province, 2015–16.
| Province | Total number notified during the survey period | Number of new patients | (%) | Expected number of new patients |
|---|---|---|---|---|
| Total | 5279 | 1114 | (65.5) | 1700 |
| Manicaland | 298 | 135 | (79.4) | 170 |
| Mashonaland Central | 250 | 138 | (73.8) | 187 |
| Mashonaland East | 304 | 78 | (65.5) | 119 |
| Mashonaland West | 282 | 133 | (65.2) | 204 |
| Matabeleland North | 152 | 71 | (54.9) | 153 |
| Matabeleland South | 328 | 83 | (46.4) | 119 |
| Midlands | 300 | 136 | (88.9) | 153 |
| Masvingo | 258 | 93 | (60.8) | 153 |
| Harare | 2879 | 166 | (46.5) | 357 |
| Bulawayo | 228 | 81 | (95.3) | 85 |
Data analysis
Data were exported to SPSS version 20 (Chicago, Illinois, USA) for analysis. Categorical variables were summarized using frequencies. Continuous variables were summarized using means and medians as appropriate. Weighted analysis of prevalence of RR-TB and MDR-TB were done using exact sampling probabilities to adjust for sampling error due to combining two sampling methods and the capping of patient recruitment at 12 months. Odds ratios and their 95% CI for factors associated with RR-TB were calculated using the stepwise logistic regression. Level of significance was set at p < 0.05.
Ethics
This survey was approved by the Medical Research Council of Zimbabwe and the Research Council of Zimbabwe. All the participants provided written informed consent/assent prior to enrolment and collection of sputum specimens.
Results
A total of 5279 sputum smear-positive patients were notified during the survey period. Of these, 1301 (24.6%) were initially enrolled and tested using Xpert MTB/RIF (Figure 1). Twenty-nine patients (2%) were excluded due to lack of survey forms and/or barcoding. The analysis population was 1272 patients: 1114 (87.6%) new and 158 (12.4%) retreatment (Figure 1). Of these, 766 (60.2%) were male, the median age was 34 years [(interquartile range (IQR), 27–42 years)], 699 (55.0%) self-reported a history of HIV infection, and 765 (60.1%) were recruited from urban clusters (Table 2). A total of 293 (23%) participants had a history of travel outside Zimbabwe of ≥one month’s duration.
Figure 1.
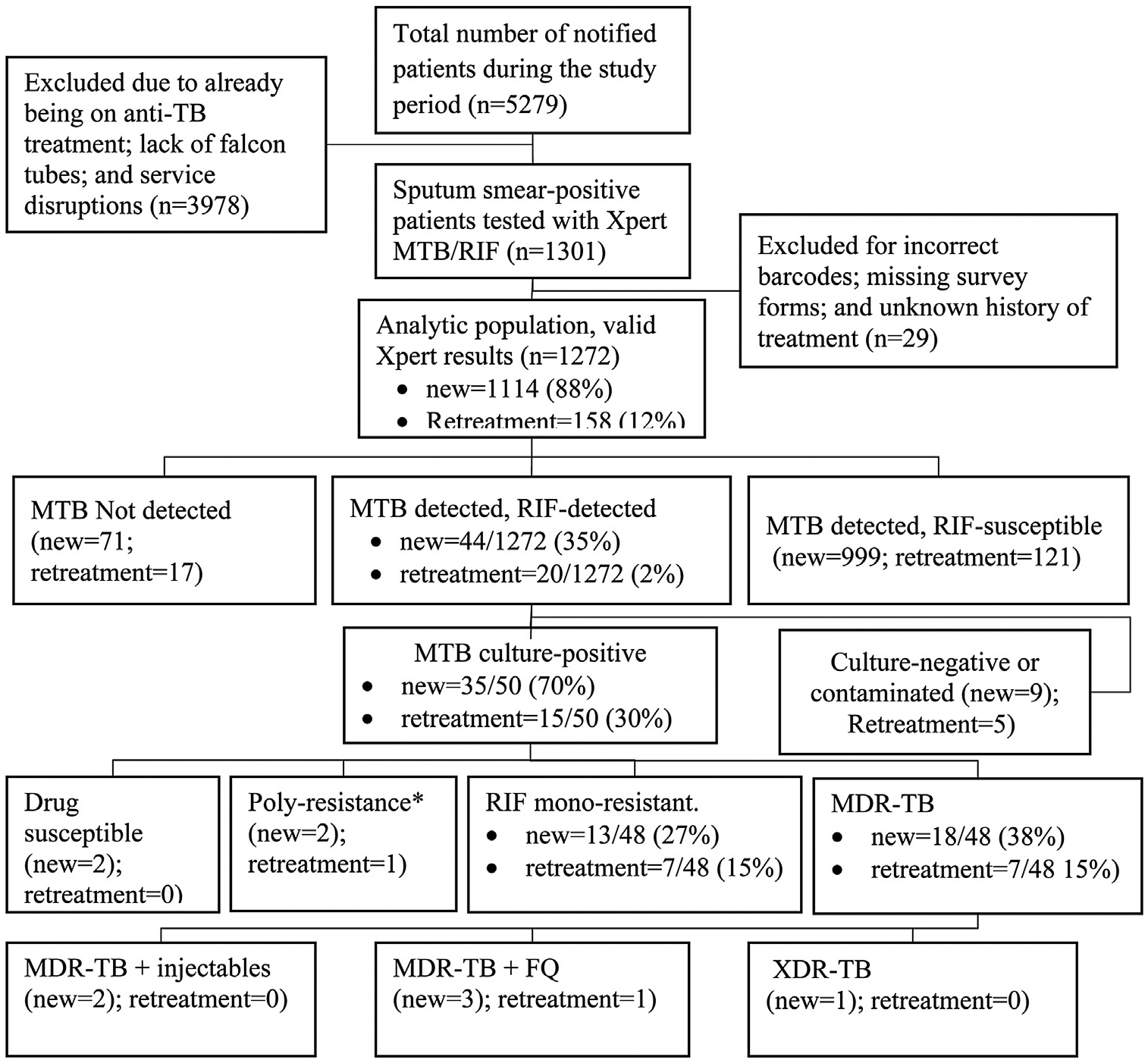
Flow of participants who were enrolled in the Zimbabwe DRS 2015–2016.
MDR-TB = multi-drug resistant TB; FQ = Fluoroquinolone; XDR-TB = extensively drug resistant TB; RMR = rifampicin mono-resistant TB; Poly-resistant = resistance to >one first-line anti-TB drug, other than both isoniazid and rifampicin.
Table 2.
Socio-demographic and clinical characteristics of patients enrolled in the Zimbabwe TB drug resistant survey, 2015–2016.
| Demographic characteristics | TB patients | Total | ||||
|---|---|---|---|---|---|---|
| New | Retreatment | |||||
| n | (%) | n | (%) | n | (%)a | |
| Total | 1114 | (87.6) | 158 | (12.4) | 1272 | |
| Sex | ||||||
| Male | 668 | (60.0) | 98 | (62.0) | 766 | (60.2) |
| Female | 446 | (40.0) | 60 | (38.0) | 506 | (39.8) |
| Age group | ||||||
| <15 | 18 | (1.6) | 1 | (0.6) | 19 | (1.5) |
| 15–24 | 171 | (15.4) | 13 | (8.2) | 184 | (14.5) |
| 25–34 | 415 | (37.3) | 43 | (27.2) | 458 | (36.0) |
| 35–44 | 315 | (28.3) | 50 | (31.6) | 365 | (28.7) |
| 45–54 | 116 | (10.4) | 33 | (20.9) | 149 | (11.7) |
| 55–64 | 46 | (4.1) | 7 | (4.4) | 53 | (4.2) |
| ≥65 | 31 | (2.8) | 11 | (7.0) | 42 | (3.3) |
| Unknown | 2 | (0.2) | 0 | (0) | 2 | (0.2) |
| HIV status | ||||||
| Positive | 580 | (52.1) | 119 | (75.3) | 699 | (55.0) |
| Negative | 492 | (44.2) | 34 | (21.5) | 526 | (41.4) |
| Unknown | 42 | (3.7) | 5 | (3.2) | 47 | (3.6) |
| History of any travel outside Zimbabwe | ||||||
| For ≥1 month | 243 | (21.8) | 50 | (31.6) | 293 | (23.0) |
| To South Africa | 166 | (14.9) | 32 | (20.3) | 198 | (15.6) |
| Other SADC countries | 62 | (5.6) | 17 | (10.8) | 79 | (6.2) |
| To other SADC countries | 8 | (0.7) | 1 | (0.6) | 9 | (0.7) |
| Unknown | 7 | (0.6) | 0 | (0.0) | 7 | (0.6) |
| Marital status | ||||||
| Never married | 229 | (20.6) | 22 | (13.9) | 251 | (19.7) |
| Married | 600 | (53.9) | 75 | (47.5) | 675 | (53.1) |
| Divorced | 177 | (15.9) | 33 | (20.9) | 210 | (16.5) |
| Widowed | 89 | (8.0) | 22 | (13.9) | 111 | (8.7) |
| Unknown | 19 | (1.7) | 6 | (3.8) | 25 | (2.0) |
| Level of education | ||||||
| None | 39 | (3.5) | 4 | (2.5) | 43 | (3.4) |
| Primary | 312 | (28.0) | 46 | (29.1) | 358 | (28.1) |
| Secondary | 700 | (62.8) | 94 | (59.5) | 794 | (62.4) |
| Tertiary | 55 | (4.9) | 13 | (8.2) | 68 | (5.3) |
| Missing | 8 | (0.7) | 1 | (0.6) | 9 | (0.7) |
| Cluster location | ||||||
| Urban | 671 | (60.2) | 94 | (59.5) | 765 | (60.1) |
| Rural | 443 | (39.8) | 64 | (40.5) | 507 | (39.9) |
SADC = Southern Africa Development Community; TB = tuberculosis.
Column percentages.
Bacteriologic results
Of the 1272 valid Xpert MTB/RIF assays, 1184 (93.1%) detected MTB. There were 44 (3.5%) new and 20 (1.6%) retreatment TB patients who had Xpert-determined RR-TB. Of these 64, 50 (78.1%) successfully grew on culture at the NTBRL. First and second-line phenotypic DST confirmed RR-TB in 48 (96%), while two cultures (4%) were susceptible to all the first-line drugs (SIRE) according to phenotypic CDST, Hain LPA (at the NTBRL), and rpoB gene sequencing at the SRL. Twenty-five cultures [(52.1%) (95% CI, 38.3, 65.5)] had MDR-TB; 20 demonstrated rifampicin mono-resistance (RMR); three had poly-resistance and two were rifampicin susceptible. Of the 25 MDR-TB cultures, one (4.0%) demonstrated fluoroquinolone and aminoglycoside resistance in addition to MDR (XDR-TB).
The crude prevalence of RR-TB was [4.0% (95% CI, 2.9, 5.4%), n = 42/1043] and [14.2% (95% CI, 8.9, 21.1%), n = 20/141] among new and retreatment patients, respectively. The crude prevalence of MDR-TB was 2.0% [(95% CI, 1.3, 3.1%)] and [6.4% (95% CI, 2.4, 10.3%)] among new and retreatment TB patients, respectively. Among new patients, the weighted prevalence of RR-TB and MDR-TB were [4.6% (95% CI, 3.0, 6.2)] and [1.8% (95% CI, 1.0, 2.5)] respectively.
Risk factors for RR-TB
In univariate analysis, a history of travel outside Zimbabwe for ≥one month [(odds ratio [(OR = 1.74, 95% CI, 1.02, 2.97)] had increased odds of RR-TB. In multivariate analysis, HIV-positivity [adjusted odds ratio (aOR) = 2.12 (95% CI, 1.09, 4.05)], age <15 years [aOR = 6.37 (95% CI, 1.51, 26.87)], and a previous history of TB treatment [aOR = 3.53 (95% CI,1.86, 6.25)] were associated with RRTB, while having at least a secondary education was protective [(aOR = 0.52; 95% CI, 0.29, 0.97)] (Table 3). After stratifying by type of TB patient, a positive HIV status [aOR = 2.19; 95% CI, 1.07, 4.46)] and history of travel outside Zimbabwe [aOR = 2.05; 95% CI, 1.05, 4.03)] were significantly associated with RR-TB among new patients (Table 4).
Table 3.
Risk factors for rifampicin resistance among patients diagnosed with smear-positive sputum during the TB drug resistant survey, Zimbabwe (2015–2016).
| Variable | Total | RR-TB detected | OR (95% CI) | aOR 95% CI | |
|---|---|---|---|---|---|
| n | (%)a | ||||
| 1184 | 62 | (5.2) | |||
| Sex | |||||
| Female | 466 | 20 | (4.3) | Ref | Ref |
| Male | 718 | 42 | (5.8) | 1.38 (0.78, 2.52) | 1.43 (0.69, 2.46) |
| Age group | |||||
| <15 | 18 | 4 | (22.2) | 6.90 (1.80, 26.45)b | 6.37 (1.51, 26.87)b |
| 15–24 | 176 | 7 | (4.0) | Ref | Ref |
| 25–34 | 431 | 19 | (4.4) | 1.11 (0.46, 2.70) | 0.96 (0.38, 2.42) |
| 35–44 | 337 | 22 | (6.5) | 1.68 (0.68, 4.77) | 1.25 (0.46, 3.27) |
| 45–54 | 128 | 5 | (3.6) | 0.91 (0.28, 2.92) | 0.52 (0.16, 1.75) |
| 55–64 | 48 | 3 | (6.2) | 1.61 (0.40, 6.47) | 1.04 (0.24, 4.42) |
| ≥65 | 34 | 2 | (5.9) | 0.51 (0.30, 7.60) | 0.90 (0.16, 4.98) |
| Level of education | |||||
| Primary and less | 363 | 27 | (7.4) | Ref | Ref |
| Secondary and above | 813 | 34 | (4.2) | 0.54 (0.31, 0.95)b | 0.52 (0.29, 0.97)b |
| Unknown | 8 | 1 | (12.5) | 1.78 (0.21, 14.99) | 2.83 (0.30, 27.08) |
| Cluster location | |||||
| Urban | 714 | 37 | (5.2) | Ref | |
| Rural | 470 | 25 | (5.3) | 1.03 (0.58, 1.78) | 0.90 (0.54, 1.71) |
| HIV status | |||||
| Negative | 508 | 14 | (2.8) | Ref | Ref |
| Positive | 632 | 46 | (7.3) | 2.77 (1.46, 5.52)b | 2.12 (1.09, 4.05)b |
| Unknown | 44 | 2 | (4.5) | 1.68 (0.18, 7.70) | 1.34 (0.29, 6.24) |
| History of any travel outside Zimbabwe | |||||
| for ≥1 month | 281 | 22 | (7.8) | 1.74 (1.02, 2.97)b | 1.69 (0.95, 2.99) |
| To South Africa | 190 | 17 | (8.9) | 1.55 (0.55, 4.36) | 1.49 (0.57, 4.39) |
| To other SADC countries | 270 | 21 | (7.8) | 0.84 (0.10, 6.91) | 0.87 (0.15, 6.42) |
| Treatment history | |||||
| New | 1043 | 42 | (4.0) | Ref | Ref |
| Retreatment | 141 | 20 | (14.2) | 3.94 (2.11, 7.11)b | 3.53 (1.86, 6.25)b |
OR = odds ratio; HIV = human immune-deficiency virus; aOR = adjusted odds ratio; SADC = Southern Africa Development Community; Ref = reference.
Row percentages.
Significant.
Table 4.
Factors associated with rifampicin resistance, among patients diagnosed with smear-positive sputum during the TB drug resistant survey, Zimbabwe (2015–2016), disaggregated by type of TB patient.
| Risk factors | Type of TB case | |||||
|---|---|---|---|---|---|---|
| New (n = 1043) | Retreatment (n = 141) | Total (n = 1184) | ||||
| aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | |
| Sex | ||||||
| Female | 1.08 | 0.55, 2.12 | 2.16 | 0.68, 6.82 | 1.43 | 0.69, 2.46 |
| Male | Reference | Reference | Reference | |||
| Age group | ||||||
| <15 | 8.59 | 1.47, 50.04a | – | 6.37 | 1.51, 26.87a | |
| 15–24 | Reference | Reference | Reference | |||
| 25–34 | 1.62 | 0.45, 5.84 | 0.28 | 0.05, 1.48 | 0.96 | 0.38, 2.42 |
| 35–44 | 2.44 | 0.68, 8.77 | 0.29 | 0.06, 1.40 | 1.25 | 0.46, 3.27 |
| 45–54 | 0.64 | 0.10, 4.06 | 0.19 | 0.03, 1.11 | 0.52 | 0.16, 1.75 |
| 55–64 | 2.43 | 0.45,13.27 | N/A | 1.04 | 0.24, 4.42 | |
| ≥65 | 1.91 | 0.19,19.80 | 0.20 | 0.02, 2.56 | 0.90 | 0.16, 4.98 |
| Level of education | ||||||
| ≤Primary | Reference | Reference | Reference | |||
| ≥Secondary | 0.52 | 0.27, 1.02 | 0.75 | 0.21, 2.67 | 0.52 | 0.29, 0.97a |
| Unknown | 5.11 | 0.51, 51.25 | – | 2.83 | 0.30, 27.08 | |
| HIV status | ||||||
| Negative | Reference | Reference | Reference | |||
| Positive | 2.19 | 1.07,4.46a | 1.76 | 0.44, 7.09 | 2.12 | 1.09, 4.05a |
| Unknown | 0.89 | 0.11, 7.25 | 2.72 | 0.21,34.71 | 1.34 | 0.29, 6.24 |
| History of travel outside Zimbabwe | ||||||
| for ≥ 1 month | 2.05 | 1.05,4.03a | 1.09 | 0.37, 3.16 | 1.69 | 0.95, 2.99 |
| Treatment history | ||||||
| New | Reference | |||||
| Re-treatment | 3.53 | 1.86, 6.25a | ||||
OR = odds ratio; HIV = human immune-deficiency virus; aOR = adjusted odds ratio. – = undefined.
Significant.
Discussion
This first nationally representative TB-DRS survey for Zimbabwe was conducted following significant socio-political and epidemiological changes in the country. We demonstrated that the prevalence of MDR-TB has remained stable since 1994 though the prevalence of RR-TB is now double that of MDR-TB. We also observed that the factors associated with RR-TB were a previous history of TB, HIV positivity, age <15 years, lower than secondary education and a stay outside Zimbabwe for ≥a month.
The observed prevalence of MDR-TB was consistent with prevalence reported from South Africa [(2.1% (95% CI, 1.5, 2.7) and 4.6% (CI, 95%: 3.2, 6.0) among new and previously treated patients, respectively in the 2012–2014 survey] and Botswana [(2.5%, 95% CI, 1.6, 3.7) and 6.6%, 95% CI, 3.3, 11.7) among new and previously treated patients during 2007–2008)] (National Institute for Communicable Diseases 2014; Menzies et al. 2014), but was lower than prevalence recorded in both Lesotho [(3.1% (95% CI, 2.1, 4.3) and (12.8% (95% CI, 8.8, 18.2)] among previously treated patients and Namibia [(3.8% (95% CI, 2.8, 5.1) and (16.4% (95% CI, 12.9, 20.6)] among previously treated patients for the survey carried out in 2008–2009 (Maama-Maime et al. 2015; Ministry of Health and Social Services: National Tuberculosis and Leprosy Programme 2012). The increase in the prevalence of RR-TB could be attributed to a high proportion of RMR observed in this study. This may be due to acquired resistance to rifampicin. Studies have shown associations between RMR and factors like a positive HIV status, previous histories of TB, use of antifungals and use of rifabutin (Meyssonnier et al. 2014; Ridzon et al. 2013; Villegas et al. 2016). The association between a positive HIV-status and RR-TB could be attributed to acquired drug resistance resulting from “preferential adherence” to antiretroviral drugs at the expense of anti-TB drugs among TB co-infected patients as reported in a previous qualitative study (Daftary et al., 2014).
The findings that the previous histories of TB and stay outside Zimbabwe for ≥1 month (bivariate analysis) were associated with RR-TB were not surprising. The latter may stem from the fact that most Zimbabweans go to neighbouring, high TB-burden countries as economic emigrants and living conditions there may foster acquisition of RR-TB. Neither DRS surveys in southern Africa nor studies, including systematic reviews, found any association between MDR-TB and HIV infection (Lukoye et al. 2015; National Institute for Communicable Diseases 2014; Maama-Maime et al. 2015; Ministry of Health and Social Services: National Tuberculosis and Leprosy Programme 2012; Menzies et al. 2014).
We do not know the reasons why attainment of secondary education was protective against RR-TB. Perhaps, attainment of secondary education is associated with better socio-economic status and positive health behavioral traits. By contrast, a study done in China showed that attainment of high school was a risk factor for RR-TB (Yang et al. 2015). Drug resistant surveys from southern Africa did not assess the relationship between education and risk of RR-TB. Studies from high-income countries failed to show the relationship between education and risk of RR-TB. Thus more research is needed to investigate this relationship.
This study enrolled more patients from urban than from rural areas, a consistent finding with program data in which high notifications are recorded in urban centers. This exposes geo-graphical health inequities despite a similar burden of MTB between rural and urban centers as evidenced in this study and the 2014 Zimbabwe National TB prevalence survey (Ministry of Health and Child Care 2014).
This survey had some limitations. First, in adopting sputum smear-positivity as our starting point, we invariably underreported RR-TB in this high HIV-burden setting given that children and HIV-positive patients often produce pauci-bacillary specimens. Second, all sputum specimens in which RR-TB was not detected did not undergo CDST. Thus, the prevalence of isoniazid mono-resistance is unknown, though it is known to be rising elsewhere in the southern Africa Development Community (SADC) (Variava and Martinson 2018; National Institute for Communicable Diseases 2014). Future DRS surveys should determine the prevalence of isoniazid-mono resistance. Third, 6% of new and 11% of retreatment sputum smear-positive specimens tested Xpert MTB/RIF-negative, raising the possibility of non-tuberculosis mycobacteria (NTM). Indeed the previn adopting sputum smear-positivity as our starting point, we invariably underreported RR-TB in this high HIV-burden setting given that children and HIV-positive patients often produce pauci-bacillary specimens. Second, all sputum specimens in which RR-TB was not detected did not undergo CDST. Thus, the prevalence of isoniazid mono-resistance is unknown, though it is known to be rising elsewhere in the southern Africa Development Community (SADC) (Variava and Martinson 2018; National Institute for Communicable Diseases 2014). Future DRS surveys should determine the prevalence of isoniazid-mono resistance. Third, 6% of new and 11% of retreatment sputum smear-positive specimens tested Xpert MTB/RIF-negative, raising the possibility of non-tuberculosis mycobacteria (NTM). Indeed the prevalence of NTM is very high in Zimbabwe. During the 2014 TB prevalence survey the prevalence of NTM was estimated to be 16.9% (964/5705) of all the survey presumptive TB cases. Of the NTM isolates obtained in a convenient sample of specimens collected during the 2014 TB prevalence survey, the prevalence of clinically significant NTM such as Mycobacteria Avium complex (MAC) was 51.5% (41/81) (Chin’ombe et al. 2016; Ministry of Health and Child Care 2014). Fourth, HIV status was obtained by self-report. Incorporation of HIV testing in TB-DRS surveys could have provided crucial information for the NTP on the relationship between HIV and drug-resistant TB (World Health Organization 2015). Lastly, we did not do multiple imputation to control for bias on the results for 14 samples that did not have DST results (no culture growths or contaminated cultures) since our data were robust. However, it would have been a useful exercise to compare potential differences in results between our models and the imputation models.
Several programmatic implications arise from this study. First, there is need to improve early and universal access to DST (in Zimbabwe and elsewhere in SADC) for at least rifampicin, in line with the WHO End TB strategy (STOP TB Partnership 2015). Second, since isoniazid prophylactic therapy (IPT) has been scaled up in Zimbabwe with ≥20,000 PLHIV having been started on IPT by December 2015 and IPT completion rates of ≥89% have been attained (Takarinda et al. 2017, 2019), and within the context of South African isoniazid-mono resistance of [4.9%, 95% CI: 4.1%–5.8%)],(National Institute for Communicable Diseases 2014) estimating the prevalence of and continued monitoring of isoniazid mono-resistance should be prioritized in Zimbabwe. Third, although sample size was small and should be interpreted as hypothesis-generating, we noted an increased risk of RR-TB among older children and adolescents, and warrants additional studies examining the determinants of childhood RR-TB in Zimbabwe. Lastly, we could not follow up on the treatment outcomes of this group.
In conclusion, the prevalence of MDR-TB in Zimbabwe has remained stable since the 1994 subnational survey.
Acknowledgements
A special thank you goes to the participants who volunteered to take part in the drug resistant survey. Thanks are also due to all staff who worked tirelessly to make this survey a success.
Funding
This survey was funded by the USAID’s Challenge TB through the World Health Organisation. The funders had no role in the design and interpretation of the findings.
Footnotes
Conflict of interest statement
None declared.
References
- Chin’ombe Nyasha, Muzividzi Boniface, Munemo Ellen, Nziramasanga Pasipanodya. Molecular identification of nontuberculous mycobacteria in humans in Zimbabwe Using 16S ribosequencing. Open Microbiol J 2016;10:113–23, doi: 10.2174/1874285801610010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftary Amrita, Padayatchib Nesri, Max O’Donnellb C. Preferential adherence to antiretroviral therapy over tuberculosis (TB) treatment: a qualitative study of drug–resistant TB/HIV co-infected patients in South Africa. Glob Public Health 2014;9(9):1107–16, doi: 10.1080/17441692.2014.934266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries Anthony D, Hargreaves James, Kemp JR, Jindani Amina, Enarson DA, Maher Dermot, et al. Deaths from tuberculosis in Sub-Saharan African countries with a high prevalence of HIV-1. Lancet 2001;357(9267):1519–23, doi: 10.1016/S0140-6736(00)04639-0. [DOI] [PubMed] [Google Scholar]
- Ismail Nazir Ahmed, Mvusi Lindiwe, Ananta Nanoo, Andries Dreyer, Omar Shaheed V, Babatunde Sanni, et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect Dis 2018;18(7):779–87, doi: 10.1016/S1473-3099(18)30222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoye Deus, Ssengooba Willy, Musisi Kenneth, Kasule George W, Cobelens Frank GJ, Moses Joloba, et al. Variation and risk factors of drug resistant tuberculosis in Sub-Saharan Africa: a systematic review and meta-analysis. BMC Public Health 2015;15(1):1–13, doi: 10.1186/s12889-015-1614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maama-Maime Llang B, Mareka Mathabo, Ershova Julia V, Tlali Thabong E, Kao Kekeletso, Phalatse Mamakhetha, et al. Antituberculosis drug resistance survey in Lesotho, 2008–2009: lessons learned. PLoS One 2015;10(7):2008–9, doi: 10.1371/journal.pone.0133808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariandyshev Andrei, Eliseev Platon. Drug-resistant tuberculosis threatens WHO’s end-TB strategy. Lancet Infect Dis 2017;17(7):674–5, doi: 10.1016/S14733099(17)30246-3. [DOI] [PubMed] [Google Scholar]
- Menzies HJ, Moalosi G, Anisimova V, Gammino V, Sentle C, Bachhuber MA, et al. Increase in anti-tuberculosis drug resistance in Botswana: results from the fourth national drug resistance survey. Int J Tuberc Lung Dis 2014;18(9):1026–33, doi: 10.5588/ijtld.13.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe JZ, Makumbirofa S, Makamure B, Sandy Charles, Bara W, Mungofa S, et al. Drug-resistant tuberculosis in high-risk groups, Zimbabwe. Emerg Infect Dis 2014;20(1):135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyssonnier Vanina, Van Bui Thuy, Veziris Nicolas, Jarlier Vincent, Robert Jérôme. Rifampicin mono-resistant tuberculosis in France: a 2005–2010 retrospective cohort analysis. BMC Infect Dis 2014;14(January):18, doi: 10.1186/1471-2334-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health and Child Care. Zimbabwe national population-based tuberculosis prevalence survey Harare, Zimbabwe. 2014. [Google Scholar]
- Ministry of Health and Social Services: National Tuberculosis and Leprosy Programme. Namibia national tuberculosis drug resistance survey (2008–2009) Windhoek, Namibia. 2012. [Google Scholar]
- Mwinga Alwyn. Drug-resistant tuberculosis in Africa. Ann N Y Acad Sci 2006;106–12, doi: 10.1111/j.1749-6632.2001.tb11366.x. [DOI] [PubMed] [Google Scholar]
- National Institute for Communicable Diseases. South African tuberculosis drug resistance survey 2012–14 Pretoria, South Africa. 2014. [Google Scholar]
- Ridzon R, Whitney CG, McKenna MT, Taylor JP, Ashkar SH, Nitta AT, et al. Risk factors for rifampin mono-resistant tuberculosis. Am J Respir Crit Care Med 2013;157(6):1881–4, doi: 10.1164/ajrccm.157.6.9712009. [DOI] [PubMed] [Google Scholar]
- Schaaf HS, Botha P, Beyers N, Gie RP, Vermulen HA, Groenwald P, et al. The 5-year outcome of multidrug resistant tuberculosis patients in the Cape Province of South Africa. Trop Med Int Health 1996;1(5):718–22. [DOI] [PubMed] [Google Scholar]
- Stop TB Partnership. Mycobacteriology laboratory manual Geneva, Switzerland. 2014. [Google Scholar]
- STOP TB Partnership. The paradigm shift 2016–2020: global plan to end TB Geneva, Switzerland. 2015. [Google Scholar]
- Takarinda KC, Choto RC, Mutasa-Apollo T, Chakanyuka-Musanhu C, Timire C, Harries AD. Scaling up isoniazid preventive therapy in Zimbabwe: has operational research influenced policy and practice?. Public Health Action 2019;8(4):218–24, doi: 10.5588/pha.18.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarinda KC, Harries AD, Choto RC, Mutasa-Apollo T, Chakanyuka-Musanhu C. Routine implementation of isoniazid preventive therapy in HIV-infected patients in seven pilot sites in Zimbabwe. Public Health Action 2017;7(1):55–60, doi: 10.5588/pha.16.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Variava Ebrahim, Martinson Neil. Drug-resistant tuberculosis: the rise of the monos. Lancet Infect Dis 2018;18(7):705–6, doi: 10.1016/S1473-3099(18)30247-0. [DOI] [PubMed] [Google Scholar]
- Villegas Leonela, Otero Larissa, Sterling Timothy R, Huaman Moises A, Van der Stuyft Patrick, Gotuzzo Eduardo, et al. Prevalence, risk factors, and treatment outcomes of isoniazid- and rifampicin-mono-resistant pulmonary tuberculosis in Lima, Peru. PLoS One 2016;11(4)e0152933, doi: 10.1371/journal.pone.0152933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation. Guidelines for surveillance of drug resistance in tuberculosis. Geneva, Switzerland. 5th ed. 2015. https://www.doi.org/WHO/HTM/TB/2015.13. [Google Scholar]
- World Health Organisation. Global tuberculosis report 2015 Geneva, Switzerland. 2015. [Google Scholar]
- World Health Organisation. Global TB report 2016 Geneva, Switzerland. 2016. [Google Scholar]
- World Health Organisation. Global tuberculosis report 2017 Geneva, Switzerland. 2017. [Google Scholar]
- World Health Organization. Guidelines for surveillance of drug resistance in tuberculosis. 5th edition 2015. Geneva, Switzerland. [Google Scholar]
- Yang Xiujun, Yuan Yanli, Pang Yu, Wang Bo, Bai Yunlong, Wang Yanhua, et al. The burden of MDR/XDR tuberculosis in coastal plains population of China. PLoS One 2015;10(2):1–12, doi: 10.1371/journal.pone.0117361. [DOI] [PMC free article] [PubMed] [Google Scholar]


