Abstract
Regular moderate-to-vigorous physical activity (PA) and increased levels of cardiorespiratory fitness (CRF) or aerobic capacity are widely promoted as cardioprotective measures in the primary and secondary prevention of atherosclerotic cardiovascular (CV) disease (CVD). Nevertheless, physical inactivity and sedentary behaviors remain a worldwide concern. The continuing coronavirus (COVID-19) pandemic has been especially devastating to patients with known or occult CVD since sitting time and recreational PA have been reported to increase and decrease by 28% and 33%, respectively. Herein, in this first of a 2-part series, we discuss foundational factors in exercise programming, with specific reference to energy metabolism, contemporary PA recommendations, the dose-response relationship of exercise as medicine, the benefits of regular exercise training, including the exercise preconditioning cardioprotective phenotype, as well as the CV risks of PA. Finally, we discuss the ‘extreme exercise hypothesis,’ specifically the potential maladaptations resulting from high-volume, high-intensity training programs, including accelerated coronary artery calcification and incident atrial fibrillation. The latter is commonly depicted by a reverse J-shaped or U-shaped curve. On the other hand, longevity data argue against this relationship, as elite endurance athletes live 3–6 years longer than the general population.
Keywords: Physical activity, Cardiorespiratory fitness, Adenosine triphosphate, Exercise guidelines, Exercise risks and benefits, Exercise preconditioning, Extreme exercise maladaptations, Mitochondria, Mitophagy
Graphical abstract
Exercise training, as a subcategory of physical activity (PA), is defined as any structured exercise regimen with the objective of improving or maintaining cardiorespiratory fitness (CRF), muscle strength, health, functional independence, athletic performance, or combinations thereof. Aerobic capacity or CRF is typically expressed as mLO2/kg/min or metabolic equivalents (METs; 1 MET = 3.5 mL/kg/min) and can be directly determined using gas-exchange measurements or estimated from the attained treadmill speed, percent grade, and duration (minutes) or the cycle ergometer workload, expressed as kilogram meters per minute.
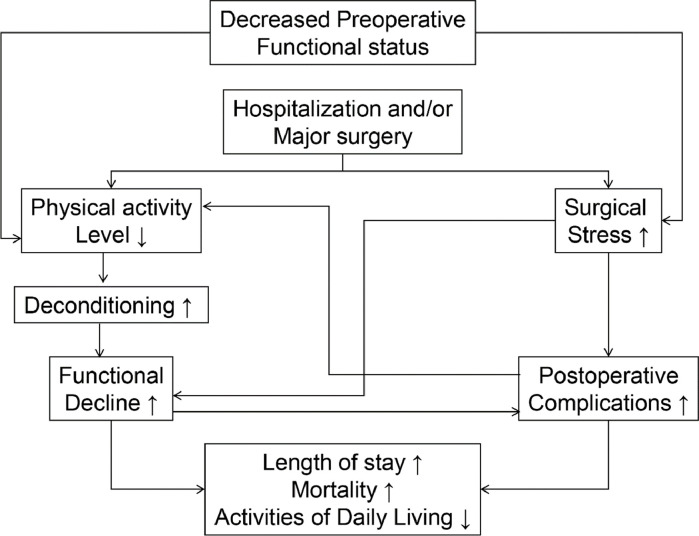
Substantial epidemiologic, clinical, and basic science evidence suggest that regular PA, structured exercise training, and higher levels of CRF prevent the development of atherosclerotic cardiovascular (CV) disease (CVD) and reduce the incidence of coronary heart disease (CHD) events [1,2]. Increased PA and/or CRF levels before hospitalization for acute coronary syndromes [3] and elective or emergent surgical procedures [2,4,5] also appear to confer more favorable short-term outcomes. Accordingly, the merits of exercise therapy before and after major surgery have been previously described (Fig. 1) [6]. Prophylactic exercise training, commonly referred to as prehabilitation, is the process of enhancing an individual's functional capacity to enable them to better tolerate a forthcoming stressor (e.g., major surgery). In addition, individuals with moderate-to-high levels of PA, CRF, or both, have lower annual healthcare costs [7]. Another provocative report linked lower intensities of peak daily energy expenditure, estimated from ambulatory electrocardiographic (ECG) recordings, with increased health care utilization and costs [8]. Recently, investigators reported that consistently meeting contemporary PA guidelines is associated with a reduced likelihood for hospitalization, intensive care unit admission, and death among patients with coronavirus disease (COVID-19) [9]. Higher levels of CRF also appear to confer more favorable COVID-19 outcomes [10].
Fig. 1.
Possible impact of decreased preoperative functional capacity on hospitalized patients undergoing emergent or elective surgery with specific reference to outcomes. Prehabilitation may be used to partially attenuate the deleterious impact of hospitalization and/or major surgery on functional capacity. Adapted from reference #6.
Collectively, these data suggest that failing to meet contemporary PA guidelines may impose harms similar to those of cigarette smoking and obesity [11]. Accordingly, additional concerted efforts are needed from patients, physicians, allied health professionals, medical systems, medical societies/associations, health care insurers, and governmental agencies to address the global health problem of physical inactivity [12].
Although this 2-part scientific statement (American Society for Preventive Cardiology) covers subject matter that is largely available in varied position statements, guidelines, and scientific advisories, we selected specific, timely clinical topics that are highly relevant to the preventive cardiology community and the patients they serve. Part 1 will focus on foundational factors in exercise prescription/programming, with specific reference to energy metabolism, general principles of exercise training, contemporary PA recommendations, the dose-response relationship of exercise as medicine, as well as the benefits and CV risks of exercise, including exercise preconditioning and potential maladaptations associated with extreme exercise regimens. Part 2 will cover PA and CRF as separate risk factors, the fundamentals and art of exercise prescription, including the concept of MET-min/week, minimum and goal intensities for exercise training, marathon running/triathlon participation, and high-intensity interval training. The salutary impact of structured exercise, lifestyle PA, or both on selected patient populations (i.e., diabetes mellitus, heart failure, peripheral arterial disease) will also be covered. Additional topics include: the medical screening of patients prior to their embarking on moderate-to-vigorous PA (MVPA) interventions; using technology to promote and reinforce PA programs; and research-based counseling strategies to enhance initiation of and participation in structured exercise/PA regimens. Finally, the practical implications of these data are succinctly summarized as clinical recommendations for the practicing clinician.
1. Mitochondria and myocyte bioenergetics of exercise
Increasing the intensity of exercise and rate of muscle contraction requires the provision of a continuous source of energy to maintain Na+/K+ gradients to drive calcium ion (a regulator of troponin conformation) clearance from the sarcoplasm of skeletal and cardiac myocytes and to dissociate actin-myosin cross bridges, thereby allowing the muscle to engage in appropriate rates of contraction-relaxation cycles. The mitochondrion is the myocyte's power generating station. Mitochondria, strategically placed in a reticulum within the sub-sarcolemmal and inner-myofibrillar spaces, are able to precisely match the energy needs of myocytes provided that oxygen delivery is adequate and sustained. Actively maintaining mitochondrial health and function is vital to attenuate age-related sarcopenia and deterioration in muscle function and efficiency. CVD is associated with deterioration in mitochondrial function (i.e., characterized by accelerated free radical leak) which contributes to poor outcomes [13], [14], [15], [16]. Chronic aerobic exercise is now widely acknowledged as a viable and lasting approach to preserving mitochondrial health and functionality, which correlates with improved muscle bioenergetics and long-term CV health.
1.1. Mitochondria and aging
Normally functioning muscle (skeletal, cardiac, smooth) mitochondria form a highly organized reticulum, providing the capacity to meet acute and changing energy demands of respective myocytes [17]. Like all components of a cell, mitochondria age and can accrue molecular injury and changes in their DNA. Mitochondrial function in the cell is maintained through a balance of biogenesis (de novo formation) and fusion, as well as fission and mitophagy. When mitochondria are defective, they can fuse with another mitochondrion, preserve the functional region, and eliminate that which is damaged or defective. Alternatively, mitochondria can undergo fission and split into two independent organelles that can subsequently be cleared by cellular machinery dedicated to the removal of organelles. Mitophagy is the highly orchestrated process by which the cell identifies a dysfunctional or poorly functional mitochondrion for destruction and elimination, which ultimately requires fusion with a lysosome. Under ideal circumstances, these processes are kept in balance to maintain an integrated mitochondrial pool and homeostasis between energy consumption and production. Impaired mitochondrial turnover and mitophagy are associated with atherosclerosis, cardiomyopathy, cardiac hypertrophy, and reperfusion injury, possibly because of impaired ATP production and increased production of reactive oxygen species (ROS) [15].
Clinical Implications. Aging and physical inactivity shift the balance of the mitochondrial pool toward fission and mitophagy, resulting in sarcopenia and reduced tissue responsiveness to energy demands. However, the following observations related to regular exercise are clinically relevant as it: (1) increases the number of mitochondria in myocytes and improves electron transport and oxidative phosphorylation; and (2) is associated with less ROS production and consequently lower levels of oxidative stress; and (3) increases mitophagy, which correlates with increased mitochondrial biogenesis and improved muscle performance [18], [19], [20], [21], [22]. Exercise not only helps to maintain functional motor strength and muscle mass but at the cellular level also helps myocytes maintain capacity for ATP biosynthesis and ensure that their mitochondrial pool is optimally functional. Healthier mitochondria may also be less predisposed to heightening oxidative tone and triggering myocyte apoptosis. In one study, patients with coronary artery disease engaged in an exercise training regimen for 8 months; total antioxidant capacity increased by up to 137% [23]. Continued exercise throughout life attenuates some loss of mitochondrial function and health as patients age. Moreover, with continued exercise, patients would be expected to better control body weight and fat stores, prevent the onset of metabolic syndrome, and maintain insulin sensitivity of skeletal muscle, among other cardioprotective benefits. To facilitate favorable CV adaptation and improvement, recent research-based PA guidelines have been published.
2. Contemporary physical activity recommendations
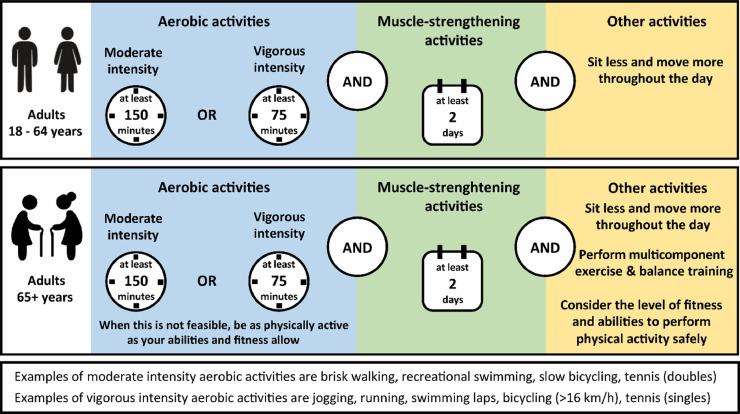
The 2020 World Health Organization (WHO) Guidelines on Physical Activity and Sedentary Behavior provide evidence-based public health recommendations regarding the amount (frequency, intensity, duration) and types (aerobic, strength, balance) of PA that offer significant health benefits and mitigate health risks [24] (Fig. 2). Together with the Guidelines on PA, sedentary behavior and sleep for children under 5 years of age [25], the new WHO guidelines now provide PA recommendations across the lifespan.
Fig. 2.
Contemporary physical activity recommendations for middle-aged and older adults, based on the WHO Guidelines on Physical Activity and Sedentary Behavior.
Adults are advised to engage at least in 150 to 300 min of moderate-intensity aerobic PA or 75 to 150 min of vigorous-intensity aerobic PA, or an equivalent combination thereof throughout the week. This recommendation differs from the previous version of the Guidelines, as it now includes a range (i.e., 150 to 300 min/week) rather than a minimum volume only (i.e., ≥150 min/week). Moreover, this change acknowledges that larger PA volumes yield dose-dependent health benefits. Therefore, adults may increase PA levels beyond the advised dose (i.e., up to 300 min/week of moderate-intensity PA or up to 150 min/week of vigorous-intensity PA) for additional health benefits. Another important change compared with the previous Guidelines is that the recommendation that PA should be performed in at least 10-minute bouts has been deleted. Indeed, more than a decade of granular data from reliable wearable fitness trackers has revealed the importance of accumulated bouts of very short duration PA (i.e., 1–2 min) [26], [27], [28], [29]. Accordingly, structured exercise/activity bouts of any duration now contribute to the cumulative PA volume [30,31].
Beyond aerobic activities, adults are advised to perform muscle-strengthening activities at a moderate or greater intensity that involve all major muscle groups on ≥2 days/week, as these provide additional health benefits [32]. Older adults (>65 years) should incorporate multicomponent PA that emphasizes functional balance and strength training at a moderate or greater intensity on ≥3 days/week to enhance functional capacity and prevent falls. The recommendations for multicomponent PA and strength training were previously only applied to older adults with poor mobility but now apply to all older adults irrespective of their functional abilities.
A new aspect of the Guidelines is the recommendation to limit sedentary behavior, based on increasing evidence that higher amounts of sedentary behavior are associated with lower CRF, poorer cardiometabolic health, an increased risk for adverse health outcomes such as the development of CVD, type 2 diabetes mellitus (DM), cancer and a higher (all-cause, CV and cancer) mortality risk [33]. Children and adolescents should limit the amount of time spent being sedentary, particularly recreational screen time. Middle-aged and older adults should limit the amount of time spent being sedentary, as replacing sedentary time with PA of any intensity (including light-intensity activities) provides health benefits. No quantitative threshold on the amount of sedentary behavior was provided, as this depends on the method to assess sedentary time, the health status of the individual, and the volume of habitual MVPA. Nevertheless, time spent sedentary should be decreased across all age groups as replacing sedentary time with PA is associated with better health outcomes [34].
There is strong evidence to support a dose-response association between time spent sedentary and health outcomes such as all-cause mortality, CVD mortality, and incident CVD [27]. Individuals reporting an objectively measured sedentary time >9.5 hrs/day have a significantly higher all-cause mortality risk compared with the reference group of 7.5 hrs/day [35]. Also, replacing 30–60 min/day of sedentary time with any type of PA was associated with risk reductions in various health outcomes, with stronger estimates for MVPA compared to light-intensity activities [29]. These outcomes highlight that lifestyle interventions to reduce sedentary time should be considered beyond interventions solely focusing on increases in PA, given their concurrent benefits.
3. The dose-response relationship of exercise as medicine
Individuals with a physically active lifestyle have a lower risk for the development and progression of many chronic diseases, including CVDs, several types of cancer, metabolic diseases, musculoskeletal disorders, neurological diseases, psychiatric conditions, and pulmonary diseases [36]. Hence, PA and structured exercise can be potent medicine to improve health status. To optimize and individualize the prescription of a physically active lifestyle, insight into the dose-response association between the volume and intensity of PA, sedentary time, and health outcomes is important.
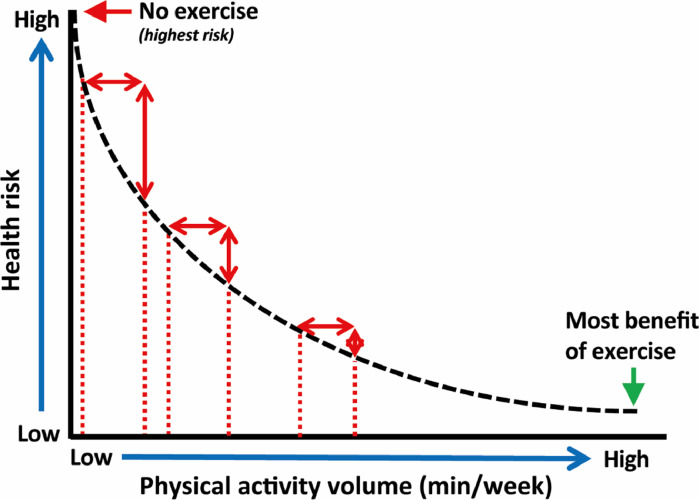
The relationship between PA volumes and CV outcomes is most often described as curvilinear. This means that the largest risk reductions will be obtained at the beginning of the curve by changing a habitually sedentary lifestyle to a mild or moderately active lifestyle (Fig. 3). Individuals who are already physically active, can further reduce their risk for adverse health outcomes by increasing their PA volumes, but the risk reduction per hour of activity gradually declines. Eventually, a plateau phase occurs in which additional PA volumes do not yield additional health benefits in the most active individuals.
Fig. 3.
Conceptual overview of the dose-response association between weekly physical activity volumes and health outcomes.
The WHO Guidelines for PA can probably be displayed in the middle of the curve, as the recommended exercise volumes should be feasible for the majority of the population, and the associated health benefits are at least moderate to large. This raises the question what the minimum and optimal exercise volumes are to improve health.
Large epidemiological studies have shown that exercise volumes below the recommendations are still associated with substantial risk reductions. As little as 15 min/day of moderate-intensity activities (i.e., brisk walking) [37] or 8-min/day of vigorous-intensity activities (i.e., running) [38] are associated with mortality risk reductions of 14% and 30%, respectively. As lack of time and/or an inactive lifestyle are important barriers to changing habitual PA patterns, such observations illustrate that even the busiest or most sedentary individuals can perform these minimal exercise volumes.
The optimal exercise volume is estimated at 3 to 5 times the lower end of the Guidelines recommendation range, which translates to 65 to 107 min/day of moderate-intensity activities or 33 to 54 min/day of vigorous-intensity activities, which correspond to exercise doses associated with a 39% lower mortality risk [39]. Although such exercise volumes are unrealistic for the majority of the population, recent studies suggest that lower thresholds may be applicable. Novel studies employing objective measures of PA, such as tri-axial accelerometers, are less prone to measurement errors and can also capture unstructured activities, which are often not included in questionnaires. A harmonized meta-analysis including accelerometry data from 36,383 individuals reported that the optimal exercise dose was achieved at 24 min/day of MVPA [35].
There is increasing interest regarding the impact of the intensity of activities on survival outcomes. The course of the dose-response association between PA volumes appears to be different for moderate versus vigorous-intensity activities [40]. For moderate-intensity activities, increasing exercise volumes yield greater health benefits, supporting the notion that more exercise is better. In contrast, an initial steep risk reduction is observed for vigorous-intensity activities below and at the Guidelines recommendation, and durations beyond 35–40 min do not further reduce mortality risks [37]. These observations demonstrate that for a given exercise duration or energy expenditure, vigorous versus moderate-intensity activities provide greater survival benefits [41], as previously reported [42,43].
Light-intensity activities (i.e., slow walking, lawn mowing, housework) are currently not included in the Guideline recommendations, but several studies demonstrate that these activities provide health benefits too. Increasing volumes of light-intensity activities are associated with gradual reductions in mortality risk, but the absolute volume to induce significant health benefits needs to be substantially higher compared with MVPA [26]. For example, the optimal light-intensity exercise dose was estimated at 375 min/day compared to only 24 min/day for MVPA [35]. Nonetheless, the message that even light-intensity activities contribute to health outcomes is promising, especially for vulnerable individuals such as patients with chronic diseases and the elderly, who may be (functionally) unable to perform MVPA.
Patients with chronic diseases, such as CVD, typically report low volumes of PA in combination with a high sedentary time which places them in the highest category for risk of death [27]. Interventions such as cardiac rehabilitation programs offer supervised exercise training to increase habitual PA volumes and CRF. Although participation in such programs is associated with a strong risk reduction for adverse outcomes [44], the PA improvements are small to modest and often transient as many patients relapse into an inactive lifestyle in the months following cessation of the program [45]. To fully benefit from prescribed exercise, a multi-dimensional program should be offered in which patients are encouraged to sit less, stand more, and gradually increase their time spent during light-, moderate- and vigorous-intensity activities to eventually improve and maintain CRF.
4. Cardioprotective mechanisms of regular aerobic exercise and improved cardiorespiratory fitness
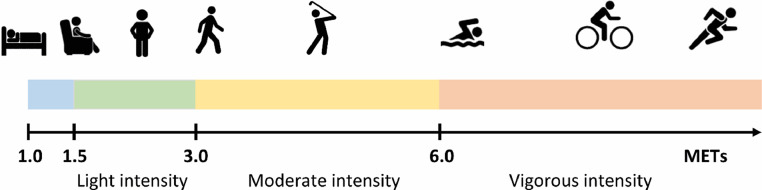
PA is defined as any bodily movement produced by skeletal muscles that result in energy expenditure beyond resting requirements, can be performed at different intensities, expressed as metabolic equivalents or METs. A MET-value of 1 equals the resting energy expenditure (3.5 mL of oxygen/ kg/minute), whereas a MET-value of 5 (i.e., brisk walking) represents a 5-fold increase in the metabolic demand associated with that activity. MET values between 1.5–2.9 represent light-intensity activities, 3–5.9 moderate-intensity activities, and ≥6 vigorous-intensity activities (Fig. 4). Contemporary PA guidelines recommend that young, middle-aged, and older adults regularly perform MVPA to improve CV health.
Fig. 4.
Examples of activities and their associated intensity across the continuum of metabolic equivalent of task (MET) scores.
Aerobic capacity (V̇O2max) or V̇O2peak is a strong predictor for CV mortality and morbidity [46]. Furthermore, the inverse association between CRF and health outcomes is present in a wide range of individuals, from the general population to persons with CV risk factors and those with and without CVD. As the addition of CRF to traditional CV risk factors improves the classification of risk for adverse outcomes, it has been suggested that fitness and regular PA be considered as clinical vital signs [2].
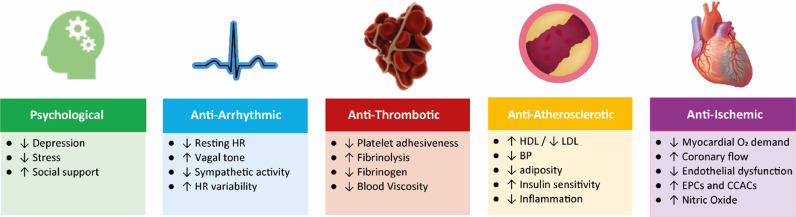
The underlying mechanisms responsible for the cardioprotective effects of regular PA and exercise training are complex and include a myriad of molecular responses and physiological adaptations [47]. Some of these changes immediately occur following a single bout of exercise, such as exercise preconditioning, reduced feelings of anxiety, decreased blood pressure, improved sleep, and enhanced insulin sensitivity, whereas long-term exercise training is needed to increase and maintain CRF, muscular strength, and CV remodeling. Regular PA has been associated with an improved psychological state and anti-atherosclerotic, anti-arrhythmic, anti-ischemic, and anti-thrombotic effects (Fig. 5). The exercise-induced improvements in these CV risk factors as well as changes in CV structure and function (e.g., coronary flow reserve, heart rate [HR] variability, endothelial function, capillary density, nitric oxide bioavailability), lower the risk for adverse health outcomes, including CV events, hypertension, DM, lipid disorders, several types of cancer, depression, dementia, musculoskeletal disorders and CV mortality [36].
Fig. 5.
Different domains of cardioprotective effects of habitual physical activity and exercise training. HR, heart rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BP, blood pressure; O2, oxygen; EPCs, endothelial progenitor cells; CCACs, cultured/circulating angiogenic cells.
4.1. Exercise preconditioning against ischemic insults
Given the high prevalence rates of CVD, including myocardial infarction, the role of exercise in the primary and secondary prevention of ischemic insults has gained heightened interest in recent decades. In this regard, exercise preconditioning refers to the phenomenon whereby recent bouts (i.e., days to weeks) of PA preempt, and partially negate, the pathological impact of an acute ischemic event. From a clinical perspective, exercise preconditioning prevents or attenuates the impact of transient ischemic insults, as evidenced by less threatening clinical and histopathological outcomes. For example, it is well established that improved outcomes relative to ventricular ectopy, threatening arrhythmias, myocardial stunning (and other markers of ventricular performance), circulating markers of cardiac tissue damage, and indices of acute myocardial infarction (AMI) are linked to exercise preconditioning [48] (Fig. 6).
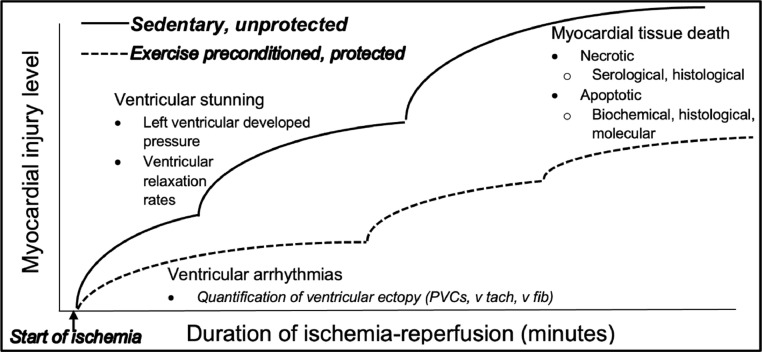
Fig. 6.
A comparison of exercise preconditioning versus sedentary hearts exposed to ischemia-reperfusion injury. Myocardial injury during an ischemic insult is proportional to the ischemic duration. Levels of injury are progressive and include electrical abnormalities, declines in ventricular pump function, and tissue death through necrotic and apoptotic mechanisms. Exercise preconditioning mitigates all forms of ischemic injury (dashed line). Adapted from reference [48], with permission.
One of the most remarkable features of exercise preconditioning is that the volume of exercise needed to elicit a robustly protected heart is relatively modest compared with the amount of training needed to achieve other health and fitness benefits (e.g., to prevent or combat obesity). The preconditioned phenotype can be evoked within hours to days after 1 to 3 bouts of structured exercise [49]. Moreover, this protection against the aforementioned forms of cardiac injury persists for more than a week after the last exercise bout [50]. Perhaps just as important as the fact that cardiac preconditioning occurs within hours of the exercise bout, the strenuousness of exercise needed to elicit protection is of moderate relative intensity (i.e., ∼50% of the V̇O2max) [51]. In this regard, convincing evidence from animal studies indicates that both moderate and high-intensity exercise are equally protective, suggesting that exercise preconditioning is threshold-dependent. Moreover, the relative nature of this protection means that moderate-intensity exercise for someone with a very low functional capacity (< 6 METs) is likely to be just as efficacious as a comparable intensity in a trained athlete (> 10 METs). Finally, the mode of exercise does not appear to be essential when it comes to eliciting a preconditioned heart. For example, preclinical investigations typically employ forced treadmill running, swimming, ladder climbing, and even some forms of strength training appear to produce equivalent levels of cardioprotection against surgically induced models of AMI. Finally, animal-based investigations indicate that intermittent PA (which approximates spontaneous PA and the execution of activities of daily living in persons with lower aerobic capacities, i.e., ∼5–15 ml/kg/min), can elicit a level of cardioprotection that matches that of a structured exercise regimen (equating to improved CV health and fitness) [48].
Perhaps most fundamental to the topic of exercise preconditioning is the observation from preclinical studies that hearts are equally protected whether from women or men, young or old [52,53]. That aged hearts are equally protected to their younger counterparts holds particular importance given that the incidence and severity of CVD are proportional to advancing age. Moreover, this finding is critically important when applied to aging human populations, who are physically inactive as the rule rather than the exception. Thus, the onset of cardioprotective benefits can be evoked rapidly, are not negated by decades of sedentary behavior, and do not require higher levels of CRF to protect the heart. Indeed, application of these findings to clinical populations suggests that the only prerequisite for exercise preconditioning is that one be medically stable to the extent that they can engage in continuous dynamic PA involving large muscle groups (or accumulate 30+ minutes of discontinuous PA) at a moderate intensity [48].
The cellular mechanisms responsible for exercise preconditioning are biochemical in nature and collectively reflect factors that strategically parallel the pathological foundations of ischemic injury and infarction. As with other aspects of exercise preconditioning, cellular protection mechanisms have been revealed primarily through preclinical animal studies in various rodent models. Importantly, however, many of these mechanistic findings have been preliminarily confirmed in humans, albeit through indirect observations. Specifically, the factors of cardioprotection mitigate the cellular pathology caused by cytosolic and mitochondrial calcium overload, free radical production leading to oxidative stress, and bioenergetic supply-demand mismatch. Accordingly, exercise preconditioned hearts exhibit protection of calcium handling proteins (e.g., sarcoplasmic endoplasmic reticulum calcium ATPase, SERCA2A), prevention of oxidative stress via upregulation of antioxidant enzymes (e.g., superoxide dismutase 2 and glutathione reductase), and by the preservation of mitochondrial function during ischemia and reperfusion challenges [54].
Collectively, this mechanistic protection prevents ischemic injury in a dynamic fashion, partially matching the evolving pathology of an ischemic insult. Accordingly, the emergence of ventricular dysrhythmias in the early minutes of an ischemic challenge is mitigated by unique cellular factors that differ from the mechanisms that prevent subsequent declines in ventricular pump function. Similarly, the biochemical factors responsible for preventing infarct damage are unique from those that mitigate electrical disturbances and ventricular stunning. Finally, the network of mechanisms responsible for exercise preconditioning reflects a division of protection that may be unique with respect to both necrotic and apoptotic forms of myocardial cell death [54].
As a final consideration, the biochemical factors responsible for exercise preconditioning appear to include mechanisms linked to receptor-mediated signaling processes. Although the signaling pathways have not been fully elucidated to date, early convincing evidence indicates delta-opioid receptors and interleukin-6 (IL-6) receptors (both soluble and membrane-bound) appear to be involved [55,56]. This finding is conceptually important for several reasons. First, receptor-based protection indicates there may be a mode of tissue-to-tissue communication whereby one organ (or organ system) is able to remotely precondition the heart. In this regard, early evidence suggests that the myocardium releases leu-enkephalins, which cardioprotect in an auto/paracrine fashion. Similarly, circulating IL-6, presumably released by active skeletal muscle, remotely preconditions the heart against ischemic insults. Whether these receptor-mediated pathways can be leveraged for pharmacologic interventions against ischemic injury remains an area of scientific debate. However, the evolving consensus suggests that the benefits of exercise-induced cardioprotection are not likely to yield a “druggable solution” for habitually sedentary populations [54].
5. The cardiovascular risks of physical activity
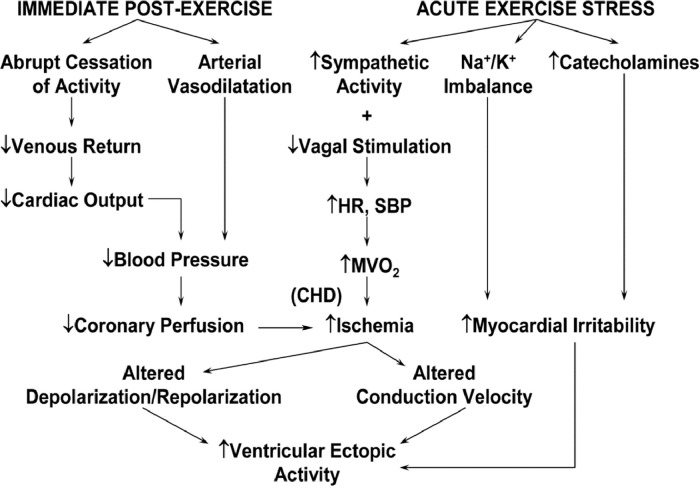
Although habitual PA reduces the risk of CVD, vigorous PA (defined as ≥60% of functional capacity or ≥6 METs) acutely increases the risk of sudden cardiac death (SCD), AMI, and hemorrhagic and ischemic stroke [57]. Possible triggering mechanisms for plaque rupture and acute coronary thrombosis [58] (Table 1) and/or threatening ventricular arrhythmias, mediated by decreased coronary perfusion, increased myocardial ischemia, heightened myocardial irritability, or combinations thereof, have been suggested (Fig. 7).
Table 1.
Potential Triggering Mechanisms of Acute Myocardial Infarction by Strenuous Physical Exertion*.
| Induces plaque rupture via: |
|
| Renders a fissured plaque more thrombogenic by: |
|
| Induces thrombogenesis directly via: |
|
Adapted from reference [58].
Fig. 7.
Physiological alterations accompanying acute exercise and recovery and their possible sequelae. HR, heart rate; SBP, systolic blood pressure; MV̇O2, myocardial oxygen consumption; CHD, coronary heart disease.
The pathology of exertion-related acute cardiac events varies with the victim's age. Structural CV abnormalities, most notably hypertrophic cardiomyopathy and coronary artery anomalies, are commonly cited as causes of SCD in younger athletes [59]. However, recent autopsy studies of exercise-related SCD in high school and college athletes have identified no structural cause at autopsy, a condition called either sudden arrhythmic death syndrome [60] or SCD with a structurally normal heart [61]. In contrast, atherosclerotic CVD is the most common autopsy finding in middle-aged and older adults [62]. Thus, the combination of vigorous PA and underlying atherosclerotic or structural heart disease, rather than exercise per se, seems to present the trigger for acute cardiac events associated with strenuous physical exertion.
5.1. Relative and absolute risks of exercise
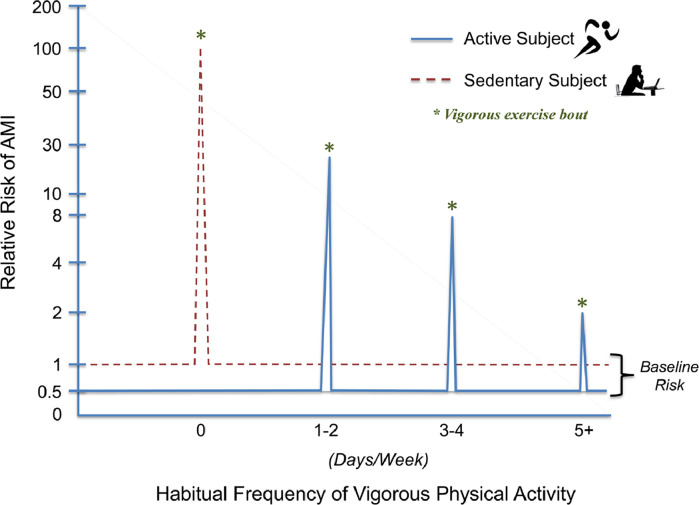
The relative risk (RR) for AMI and SCD during light-to-moderate intensity exercise is extremely low and similar to that at rest, whether estimated as events per participant or per hour of exercise [57]. In persons with known or occult CVD, vigorous PA, such as jogging or running, seems to be associated with a greater incidence of acute CV events compared with periods of light or no PA [57,63]. Vigorous PA increases the RR of acute cardiac events approximately 2-to-107 fold compared with non-vigorous exercise or rest, and the RR decreases with increasing frequency of regular vigorous exercise (Fig. 8) [64,65]. Nevertheless, despite the increased RR, the absolute risk of these events remains extremely low. For example, the rate of exercise-related nonfatal and fatal CV events in apparently healthy adults from fitness facilities is reported at 1 per 1,124,200 and 1 per 887,526 person-hours, respectively [66]. Moreover, the Physicians’ Health Study [67] and Nurses’ Health Study [68] reported only 1 SCD per 1.5 million hours of vigorous PA in men and 36.5 million hours of MVPA in women. A profile of the individual at increased risk for exercise-related acute CV events has emerged (Table 2) [62,69].
Fig. 8.
The relative risk for an acute myocardial infarction (AMI) is presented at rest (baseline) and during vigorous activity (days/week). No regular vigorous physical activity elevates the risk of an isolated, unaccustomed bout of vigorous exercise by ≥100-fold. Performing one to two days of weekly vigorous physical activity markedly lowers AMI risk during vigorous physical exertion. More frequent performance of vigorous physical activity further lowers exercise related AMI risk, although the relationship is not linear [64,65]. Vigorous exercise is delineated by the asterisks (*). Reprinted with permission from the American Heart Association.
Table 2.
Characteristics Associated with Exercise-Related Acute Cardiac Events.*.
| Clinical status |
|
| Exercise training participation |
|
| Exercise test data |
|
| Other |
|
| Hyperlipidemic |
5.2. Common activities associated with acute cardiac events
Highly strenuous activities, especially when sudden, unaccustomed, or involving high levels of anaerobic metabolism, are more likely to be associated with acute cardiac events. These include water, downhill or cross-country skiing, racquet sports, high-intensity interval training, and competitive sports activities (e.g., basketball), compared with other more moderate activities [57,63]. Neural and psychologic stimuli secondary to the competition may simultaneously increase sympathetic activity and catecholamine levels and lower the threshold for life-threatening ventricular arrhythmias [70]. Other recreational and domestic activities that are associated with increased cardiac demands and a greater incidence of CV events include marathon running [71] and triathlon participation [72], as well as deer hunting [73] and snow removal [74,75]. The latter pursuits involve isometric and/or heavy upper extremity exertion and substantial increases in left ventricular afterload in a cold environment. These responses, coupled with the body's normal thermoregulatory responses to cold, including increased platelet aggregability and plasma viscosity, coronary spasm, or vasoconstriction, and superimposed emotional/hyperadrenergic responses, may present a hazardous milieu for CV events.
5.3. Strategies to reduce exercise-related cv events
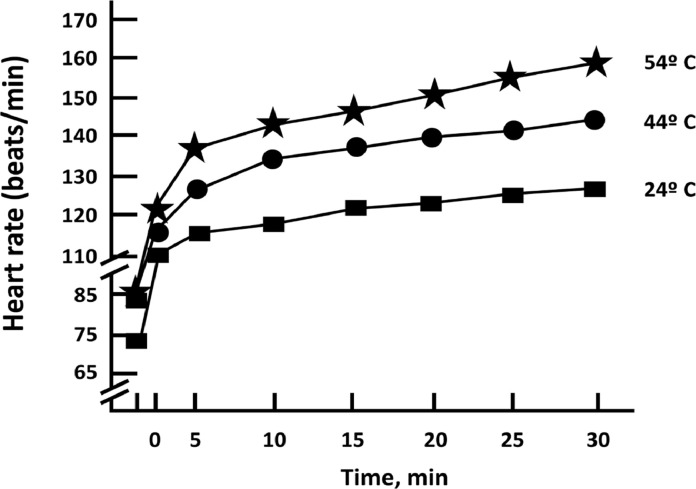
An important defense against exercise-related acute CV events in middle-aged and older adults is to maintain or enhance CRF via regular MVPA [57]. This recommendation is based on the observation that a disproportionate number of acute cardiac events occur in habitually sedentary subjects with known or occult coronary artery disease who were performing unaccustomed vigorous-to-high intensity PA [64]. Additional strategies to reduce exercise-related CV events, although unproven, include: advise inactive, unfit patients/clients to avoid unaccustomed, vigorous-to-near maximal leisure-time, domestic, and recreational PA (e.g., racquet sports, water or cross-country skiing, highly competitive sports [basketball], deer hunting, snow shoveling); advocate appropriate warm-up and cool-down procedures; counsel patients to heed warning signs/symptoms (e.g., chest pain or pressure, lightheadedness, heart palpitations/arrhythmias, unusual shortness of breath) by discontinuing exercise and seeking prompt medical assessment/clearance before resuming training; emphasize adherence to prescribed exercise HRs and perceived exertion levels (e.g., “fairly light” to “somewhat hard”); minimize competition and modify recreational games (e.g., one-bounce volleyball) to decrease the energy cost and HR response to play; and reduce the intensity of exercise under hyperthermic conditions and at altitudes of >1500 m until acclimated [63]. Exercisers who are not acclimated to heat and who are exposed to temperatures >24 °C experience tachycardic responses ― specifically added HR increases of 1 beat per min (bpm) per °C while exercising, and 2–4 bpm per °C with concomitant increased humidity (Fig. 9) [76].
Fig. 9.
Influence of environmental temperature on heart rate responses at a constant exercise work rate. Heart rate increases ∼1 beat per minute for each degree Celsius ( °C) increment in ambient temperature above 24 °C. (Adapted from Pandolf et al. [76]).
When previously sedentary individuals initiate an exercise program, it is strongly recommended to begin with a light-to-moderate intensity, specifically 2–3 METs, and gradually increase the intensity of exertion over time (i.e., 2–3 months), provided that perceived exertion approximates “fairly light” to “somewhat hard” and they remain asymptomatic. This “progressive transitional phase” should help eliminate the large spikes in relative CV risk that can occur when previously sedentary individuals perform unaccustomed vigorous PA (Fig. 8) [64,65]. Finally, for individuals with signs/symptoms of myocardial ischemia, which can be highly arrhythmogenic [77], the target HR for endurance exercise should be set safely below (≥10 bpm) the ischemic ECG or anginal threshold [78].
Prophylactic use of cardioprotective medications before competitive exercise. Although there are no definitive data indicating that cardioprotective medications prevent exercise-related acute CV events [63], some clinicians have suggested that recreational athletes with known or suspected atherosclerotic CVD may benefit from taking aspirin or β-blockers shortly before competitive exercise. Nevertheless, related reports [64,71] and the INTERHEART study [79] suggest there is insufficient evidence to recommend the prophylactic use of these medications before strenuous PA or competitive sports participation. Despite their proven effectiveness for secondary prevention, additional studies are needed regarding the risk-benefit ratio of this practice before it can be routinely recommended.
6. Extreme exercise hypothesis: potential maladaptations
The health benefits of regular exercise are indisputable. However, there is debate about the possibility that very high volumes of exercise may, over time, attenuate the health benefits obtained at moderate exercise volumes. The ‘extreme exercise hypothesis’ suggests that long-term high-volume, high-intensity exercise training may induce deleterious cardiac adaptations in some individuals (Fig. 10). Understanding these exercise-induced adaptations and their associated clinical significance is important to weigh the benefits and risks associated with high exercise volumes.
Fig. 10.
Overview of the potential deleterious effects of exercise on the heart.
Assessment of cardiac troponins (cTn) is the gold standard to assess myocardial injury in clinical practice. Elevated cTn concentrations have been reported following acute exercise bouts of different durations and intensities (i.e., running, cycling, swimming, walking, etc.) [80]. The extent of exercise-induced cTn elevations depends on multiple factors, of which the magnitude of cardiac work (expressed as exercise intensity or exercise duration) appears to be the strongest predictor. The proportion of athletes exceeding the 99th percentile (i.e., the upper reference limit) varies across studies but is >50% in most athletic populations [80]. The underlying mechanisms responsible for exercise-induced cTn elevations remain poorly understood, but a recent pilot study suggests that reversible cardiac injury may be responsible. Marathon runners demonstrated compromised cardiomyocyte integrity at 4 ± 2 h following a marathon run which was associated with increased cTn concentrations [81]. These findings suggest that exercise-induced increases in cardiomyocyte membrane permeability may allow cytosolic troponin fragments to leak from the cell into the circulation, a hypothesis that needs to be confirmed or refuted in future studies. As exercise-induced cTn elevations are transient and typically normalize within 72 h post-exercise, it is believed to be a physiological rather than a pathological response. However, a study among older long-distance walkers (n = 61 [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69] yrs) from the general population challenged this concept as individuals with a post-exercise cTn concentration above the upper reference limit had an increased risk for all-cause mortality and subsequent major CV events [82]. Whether these findings can be extrapolated to other types of exercise and younger athletes remains unclear.
Myocardial fibrosis is characterized by increased collagen volumes of the heart, secondary to cardiac injury or cardiac remodeling from myocardial ischemia, inflammation, hypertensive overload, or combinations thereof. A comparative overview of competitive athletes (n = 357) and their less active control counterparts (n = 270), revealed a substantially higher prevalence of myocardial fibrosis among the athletes (12.0% versus 1.5%) [63]. A high lifelong exercise exposure, quantified as years of training or number of completed marathons, was associated with myocardial fibrosis. However, the increased prevalence of myocardial fibrosis in older athletes appears most likely to be sex-specific. Although findings from studies in patient populations demonstrate that myocardial fibrosis is associated with an increased risk of myocardial stiffness, arrhythmias, and adverse clinical outcomes, these findings cannot necessarily be generalized to athletes, as the location (predominantly at the right ventricular insertion point) and magnitude of fibrosis is different from controls. Studies evaluating the clinical relevance of myocardial fibrosis in athletes are scarce, but a small case series reported non-sustained ventricular arrhythmias, symptomatic ventricular tachycardia, and progressive left ventricular dysfunction in 7 young asymptomatic athletes with myocardial fibrosis [83]. These findings indicate that additional studies are needed to clarify the potential consequences of myocardial fibrosis, if any, in selected athletes.
Atherosclerotic CHD is the leading cause of CV mortality and accounts for ±8.1 million deaths per year. Exercise training and habitual PA reduce the risk for CVD development and CV events, but several studies suggest that high-volume, high-intensity exercise training may accelerate coronary atherosclerosis. Aengevaeren et al. report on the dose-response relationship between lifelong exercise volumes and characteristics of coronary atherosclerosis [84]. The most active athletes (>2000 MET-min/wk) had a higher prevalence of coronary artery calcification scores (CACS) >0 (ORadjusted: 3.2 [95% CI, 1.6–6.6]) and any coronary plaques (ORadjusted: 3.3 [95% CI, 1.6–7.1]), compared to the least active athletes (<1000 MET-min/wk). Very vigorous-intensity exercise (>9 METs) was significantly associated with CACS >0 and plaque presence, whereas no association was found for exercise at moderate- to vigorous-intensities. Merghani et al. substantiated these findings in a cohort of British masters endurance athletes [85]. CACS (86 vs. 3 Agatston Units) and plaque prevalence (44% vs. 22%) were higher in male athletes versus controls. Both studies also reported that athletes demonstrated more calcified plaques and fewer mixed plaques compared to the control groups. This is important as calcified plaques are associated with a lower CV risk compared with mixed plaques, indicating that athletes have a more favorable plaque composition than controls. Results from the Cooper Clinic Longitudinal study show that CACS are higher in athletes and highly active/fit individuals. In the latter cohort, these data appear to have relevance to risk stratification, whereas similar data for elite athletes are lacking. Collectively, these data suggest that despite an elevated CACS, at any given CACS, higher levels of CRF and/or PA are associated with a lower subsequent mortality [86,87]. The underlying mechanisms of increased coronary atherosclerosis in athletes remain unknown, but it was hypothesized that exercise-induced deleterious CV responses (exercise-induced hypertension, mechanical stress, disrupted flow, altered coronary hemodynamics), changes in vitamins, minerals or hormones (lower vitamin D, lower magnesium, increased parathyroid hormone), inflammation, or other potential confounders (diet, psychological stress, genetics, performance-enhancing drugs, immune-modulating medication) might be responsible via innumerable combinations [84].
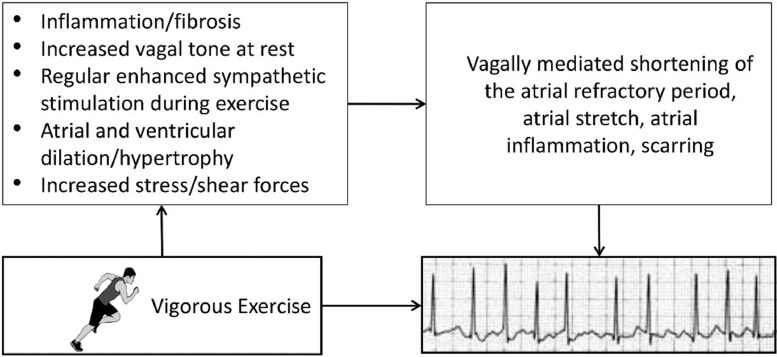
Atrial fibrillation (AF) is the most common arrhythmia in the general population. The association between habitual PA or exercise training and AF is complex and depicted by a reverse J-shaped curve. Inactivity and insufficient levels of PA have been associated with an increased risk for AF, whereas meeting the PA guidelines lowers the AF risk [88]. Individuals performing PA at the upper end of the PA spectrum, such as endurance athletes, had a 2-to-10-fold higher AF risk, primarily driven by the volume of lifetime exercise training at a (very) vigorous-intensity [63]. However, the increased risk appears most likely to be sex-specific. The underlying mechanisms of the increased AF risk in athletes are unknown but may relate to exercise-induced changes in autonomic function (i.e., increased vagal tone, sympathetic stimulation), structural adaptations (i.e., cardiac remodeling [dilatation/hypertrophy], myocardial fibrosis), and hemodynamics (i.e., increased shear forces, inflammation, oxidative stress) (Fig. 11). The clinical consequences of AF in athletes have recently been addressed. Long-distance cross-country skiers with a diagnosis of AF had a lower risk of stroke (HR: 0.73 [95% CI, 0.50–0.91]) and death (HR: 0.57 [95% CI, 0.49–0.065]) compared to non-skiers with AF [89], indicating that exercise should not be avoided in these individuals. Indeed, AF patients who performed more PA or had a higher CRF had a lower risk of all-cause mortality, CVD mortality, and CVD morbidity compared with their less active peers [90].
Fig. 11.
Potential mechanisms and associated sequelae for atrial fibrillation (AF) induced by strenuous endurance exercise over time. The combination of autonomic, structural, and hemodynamic effects of high-volume, high-intensity exercise likely impart some of the increased risk for AF [63]. Reprinted with permission from the American Heart Association.
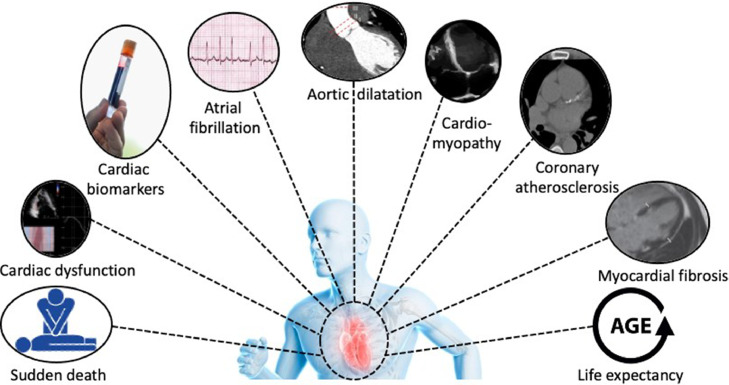
Other potential detrimental effects of acute and long-term exercise training on the heart, such as the risk of SCD, cardiac dysfunction, aortic dilatation, life expectancy, and the genotype to phenotype progression in individuals with inherited cardiomyopathies, are beyond the scope of the present clinical practice statement. Nevertheless, these topics have been extensively discussed in previously published reviews [63,91].
7. Clinical recommendations
-
•
Physical inactivity and sedentary behaviors remain a worldwide concern, exacerbated by the continuing COVID-19 pandemic, which has increased sitting time and decreased recreational PA by ∼30%, and perhaps even more in patients with CVD.
-
•
Clinicians should recognize that low PA and/or CRF levels may impose harms similar to those of cigarette smoking and obesity.
-
•
The largest risk reductions are obtained by individuals who vacate the bottom of the CRF/PA continuum – people who are initially in the least fit, least active subgroup (bottom 20%) who progress to the next quintile.
-
•
Exercise volumes below contemporary PA recommendations are still associated with substantial risk reductions.
-
•
Because larger PA volumes yield additional health benefits, adults are advised to limit sedentary behaviors and engage in ≥150 to 300 min of moderate-intensity aerobic PA or 75 to 150 min of vigorous-intensity PA, or equivalent combinations thereof, throughout the week.
-
•
The immediate cardioprotective benefits of exercise preconditioning are woefully underappreciated. Exercising at a moderate relative intensity (∼50% V̇O2max) prevents or attenuates the impact of transient ischemic insults, can be evoked within hours to days after 1 to 3 bouts of exercise, and this protection persists for more than 1 week after the last exercise bout. Accordingly, these benefits extend indefinitely for those who engage in regular exercise.
-
•
Unaccustomed vigorous PA, particularly when performed by inactive, unfit individuals with underlying atherosclerotic or structural heart disease, can acutely increase the risk of SCD and AMI.
-
•
Structural CV abnormalities, most notably hypertrophic cardiomyopathy or sudden arrhythmic death syndrome/SCD with a structurally normal heart and coronary artery anomalies, are commonly cited as causes of SCD in young athletes. In contrast, atherosclerotic CVD is the most common autopsy finding in middle-aged and older adults who die while exercising.
-
•
The favorable risk factor profiles and superb cardiac performance of long-distance runners, as well as the anti-aging effects of exercise, have likely contributed to the escalating numbers of adults who have embraced the notion that “more exercise is invariably better.”
-
•
Extreme endurance exercise training regimens are associated with potential cardiac maladaptations in some individuals, including accelerated CACS, elevated cardiac biomarker release, myocardial fibrosis, and AF, which may be described by a reverse J-shaped dose-response curve.
-
•
A higher prevalence of elevated CACS has been reported among endurance athletes versus matched inactive controls; however, the risk for adverse CV events is lower in fit/active persons compared with their unfit/inactive counterparts with the same CACS.
-
•
Similarly, individuals with low PA and/or CRF levels are 2–3 times more likely to die prematurely than their physically active/fitter counterparts when matched for age, sex, and coronary risk factor profiles.
-
•
As warning symptoms often precede exertion-related acute CV events, patients should be strongly advised that symptoms require immediate cessation of endurance training/competition and medical review.
-
•
There is insufficient evidence to justify routine CV screening (e.g., physician evaluation and exercise testing) in young asymptomatic athletes or healthy individuals as a preface to embarking on moderate-to-vigorous intensity exercise regimens.
-
•
There are no definitive data that taking cardioprotective medications, including aspirin and/or β-blockers, shortly before vigorous PA or competitive exercise, prevents exercise-related acute CV events.
-
•
The benefits of regular MVPA, and the associated improvements in CRF, far outweigh the risks for most individuals.
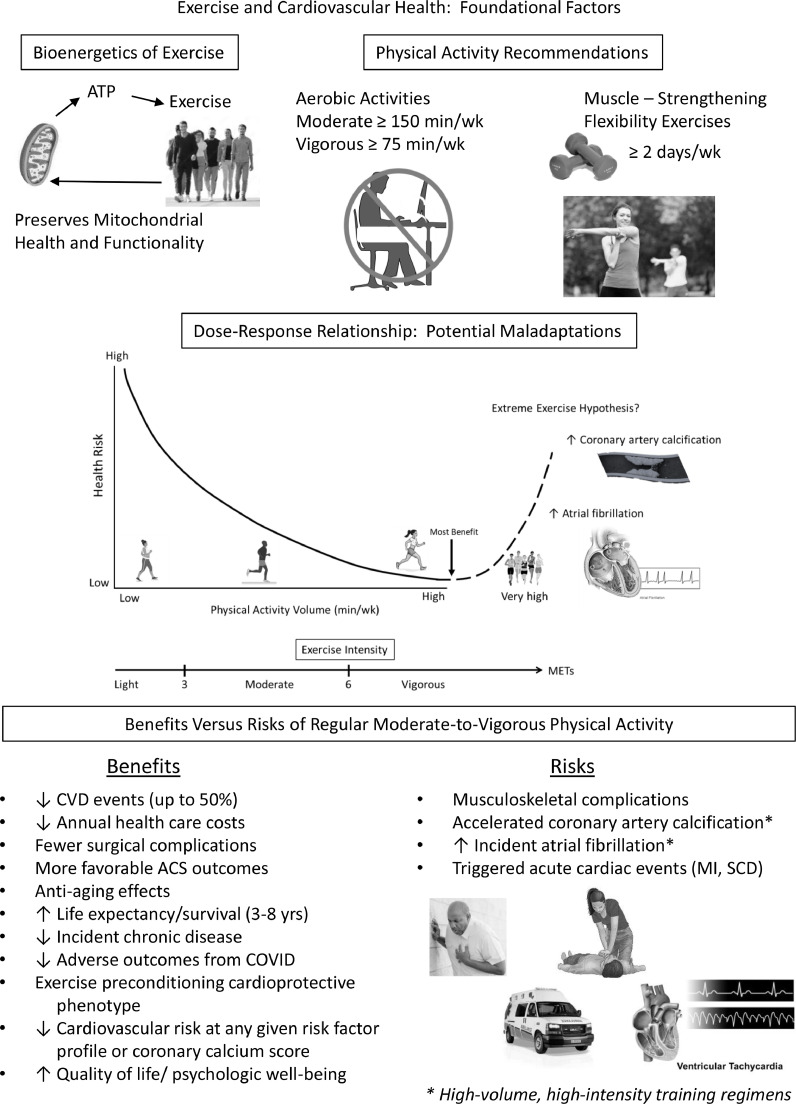
Key summary points on part I of this two-part scientific statement are highlighted in the Central Figure (Fig. 12), with specific reference to the bioenergetics of exercise, contemporary PA recommendations to promote CV health, the dose-response relationship between exercise and health, including the extreme exercise hypothesis, and the benefits vs risks of MVPA.
Fig. 12.
Central Figure, summarizing the foundational factors regarding exercise and cardiovascular health, with specific reference to the bioenergetics of exercise, contemporary physical activity recommendations, dose-response relationship between health risk and physical activity volumes, potential adaptations to extreme exercise regimens (i.e. reverse J-curve), and the benefits versus risks of MVPA.
8 Conclusions
Regular exercise increases the number of mitochondria in myocytes, improves electron transport and oxidative phosphorylation, lowers levels of oxidative stress, and increases mitochondrial resilience, potentially lessening the likelihood of cardiomyopathy, DM, LDL oxidation, hypertension, vasoconstriction, reduced vasoreactivity, and the development of atherosclerotic CVD. The recent WHO Guidelines on Physical Activity and Sedentary Behavior emphasize that larger PA volumes than previously recommended yield greater health benefits and that activity bouts of any duration contribute to the cumulative PA volume. Moreover, replacing sedentary time with PA of any duration or intensity appears to provide independent and additive health and survival benefits. Additional studies indicate that the relation between PA volumes and CV outcomes is most often described as curvilinear, with the largest risk reductions obtained at the beginning of the curve by changing a habitually sedentary lifestyle to one that embraces mild-to-moderate PA. Nevertheless, for the same exercise duration or energy expenditure, when comparing vigorous to moderate-intensity activity, the former appears to yield even greater health benefits.
Although regular MVPA reduces the likelihood of acute cardiac events, vigorous PA can increase the RR of SCD and AMI in susceptible individuals. The absolute rate of exercise-related SCD is extremely low and varies with the prevalence of disease in the study population. However, the incidence of both AMI and SCD is greatest in habitually sedentary individuals with known or occult CVD who perform unaccustomed, vigorous-to-high intensity exercise. Recent studies have also shown that extreme volumes and/or intensities of exercise are, over time, associated with several possible cardiac maladaptations. For example, a reverse J-shaped association is reported between exercise volumes and AF, with a reduced risk at light-to-moderate volumes and an increased risk at high volumes. Other potentially adverse CV manifestations with high-volume, high-intensity exercise regimens include accelerated CACS, exercise-induced cardiac biomarker release, myocardial fibrosis, and even a higher risk of SCD have been reported in endurance athletes. These fatalities have often been linked with underlying atherosclerotic CVD or inherited conditions such as long QT syndrome, hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and Brugada syndrome. Nevertheless, elite endurance athletes live 3 to 6 years longer than the general population [92], [93], [94], [95]. Finally, there are no definitive data that prophylactic use of selected cardioprotective medications before strenuous exercise or competition, most notably, aspirin or β-blockers, prevent exertion-related acute cardiac events.
Declaration of Competing Interest
The authors have no conflicts to report relative to this work.
Acknowledgments
The authors would like to thank Brenda White for her assistance with the preparation, formatting, and serial revisions of this scientific statement (part 1), diligently checking the placement and accuracy of our citations. We express our appreciation and gratitude to Dr. Esmée Bakker, Radboud Institute of Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands, for providing original Figs. 3 and 5 (and the rights to re-use them). These were included in her Ph.D. thesis titled, “Physical activity and sedentary behavior in the prevention of cardiovascular disease.”
References
- 1.Nocon M., Hiemann T., Müller-Riemenschneider F., Thalau F., Roll S., Willich S.N. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15(3):239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 2.Ross R., Blair S.N., Arena R., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a Scientific Statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 3.Pitasvos C., Kavouras S.A., Panagiotakos D.B., et al. Physical activity status and acute coronary syndromes survival The GREECS (Greek Study of Acute Coronary Syndromes) study. J Am Coll Cardiol. 2008;51(21):2034–2039. doi: 10.1016/j.jacc.2008.01.0153. [DOI] [PubMed] [Google Scholar]
- 4.McCullough P.A., Gallagher M.J., deJong A.T., et al. Cardiorespiratory fitness and short-term complications after bariatric surgery. Chest. 2006;130(2):517–525. doi: 10.1378/chest.130.2.517. [DOI] [PubMed] [Google Scholar]
- 5.Smith J.L., Verrill T.A., Boura J.A., Sakwa M.P., Shannon F.L., Franklin B.A. Effect of cardiorespiratory fitness on short-term morbidity and mortality after coronary artery bypass grafting. Am J Cardiol. 2013;112(8):1104–1109. doi: 10.1016/j/amjcard.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 6.Hoogeboom T.J., Dronkers J.J., Hulzebos E.H.J., van Meeteren N.L.U. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27(2):161–166. doi: 10.1097/ACO.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers J., Doom R., King R., et al. Association between cardiorespiratory fitness and health care costs: the Veterans Exercise Testing Study. Mayo Clin Proc. 2018;93(1):48–55. doi: 10.1016/jmayocp.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 8.George J., Abdulla R.K., Yeow R., et al. Daily energy expenditure and its relation to health care costs in patients undergoing ambulatory electrocardiographic monitoring. Am J Cardiol. 2017;119(4):658–663. doi: 10.1016/j.amjcard.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Sallis R., Young D.R., Tartof S.Y., et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021;55(19):1099–1105. doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- 10.Brawner C.A., Ehrman J.K., Bole S., et al. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc. 2021;96(1):32–39. doi: 10.1016/j.mayocp.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee I.-.M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T. Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallal P.C., Lee I.-.M. Prescription of physical activity: an undervalued intervention. Lancet. 2013;381(9864):356–357. doi: 10.1016/S0140-6736(12)61804-2. [DOI] [PubMed] [Google Scholar]
- 13.Poznyak A.V., Ivanova E.A., Sobenin I.A., Yet S.F., Orekhov A.N. The role of mitochondria in cardiovascular diseases. Biology (Basel) 2020;9(6):137. doi: 10.3390/biology9060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballinger S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38(10):1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Vásquez-Trincado C., García-Carvajal I., Pennanen C., et al. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. 2016;594(3):509–525. doi: 10.1113/JP271301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian R., Colucci W.S., Arany Z., et al. Unlocking the secrets of mitochondria in the cardiovascular system: path to a cure in heart failure—a Report from the 2018 National Heart, Lung, and Blood Institute Workshop. Circulation. 2019;140(14):1205–1216. doi: 10.1161/CIRCULATIONAHA.119.040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Memme J.M., Erlich A.T., Phukan G., Hood D.A. Exercise and mitochondrial health. J Physiol. 2021;599(3):803–817. doi: 10.1113/JP278853. [DOI] [PubMed] [Google Scholar]
- 18.Porter C., Reidy P.T., Bhattarai N., Sidossis L.S., Rasmussen B.B. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc. 2015;47(9):1922–1931. doi: 10.1249/MSS.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs R.A., Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol. 2013;114(3):344–350. doi: 10.1152/japplphysiol.01081.2012. (1985) [DOI] [PubMed] [Google Scholar]
- 20.Conley K.E. Mitochondria to motion: optimizing oxidative phosphorylation to improve exercise performance. J Exp Biol. 2016;219(pt 2):243–249. doi: 10.1242/jeb.126623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lira V.A., Okutsu M., Zhang M., et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27(10):4184–4193. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu N.N., Tian H., Chen P., Wang D., Ren J., Zhang Y. Physical exercise and selective autophagy: benefit and risk on cardiovascular health. Cells. 2019;8(11):1436. doi: 10.3390/cells8111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tofas T., Fatouros I.G., Draganidis D., et al. Effects of cardiovascular, resistance and combined exercise training on cardiovascular, performance and blood redox parameters in coronary artery disease patients: an 8-month training-detraining randomized intervention. Antioxidants (Basel) 2021;10(3):409. doi: 10.3390/antix10030409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Guidelines on physical activity and sedentary behaviour. Geneva; 2020 25 November 2020. Report No.: ISBN: 9789240015128.
- 25.World Health Organization . World Health Organization; Geneva: 2019. Guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age. 2 April 2019. Report No.: ISBN: 9789241550536. [PubMed] [Google Scholar]
- 26.Strain T., Wijndaele K., Dempsey P.C., et al. Wearable-device-measured physical activity and future health risk. Nat Med. 2020;26(9):1385–1391. doi: 10.1038/s41591-020-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunstan D.W., Dogra S., Carter S.E., Owen N. Sit less and move more for cardiovascular health: emerging insights and opportunities. Nat Rev Cardiol. 2021;18(9):637–648. doi: 10.1038/s41569-021-00547-y. [DOI] [PubMed] [Google Scholar]
- 28.Ekelund U., Tarp J., Fagerland M.W., et al. Joint associations of accelerometer measured physical activity and sedentary time with all-cause mortality: a harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med. 2020;54(24):1499–1506. doi: 10.1136/bjsports-2020-103270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Pozo-Cruz J., Garcia-Hermoso A., Alfonso-Rosa R.M., et al. Replacing sedentary time: meta-analysis of objective-assessment studies. Am J Prev Med. 2018;55(3):395–402. doi: 10.1016/j.amepre.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Fan J.X., Brown B.B., Hanson H., Kowaleski-Jones L., Smith K.R., Zick C.D. Moderate to vigorous physical activity and weight outcomes: does every minute count? Am J Health Promot. 2013;28(1):41–49. doi: 10.4278/ajhp.120606-QUAL-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glazer N.L., Lyass A., Esliger D.W., et al. Sustained and shorter bouts of physical activity are related to cardiovascular health. Med Sci Sports Exerc. 2013;45(1):109–115. doi: 10.1249/MSS.0b013e31826beae5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams M.A., Haskell W.L., Ades P.A., et al. for the American Heart Association Council on Clinical Cardiology; American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116(5):572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 33.Li S., Lear S.A., Rangarajan S., et al. Association of sitting time with mortality and cardiovascular events in high-income, middle-income, and low-income countries. JAMA Cardiol. 2022;15 doi: 10.1001/jamacardio.2022.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dempsey P.C., Biddle S.J.H., Buman M.P., et al. New global guidelines on sedentary behaviour and health for adults: broadening the behavioural targets. Int J Behav Nutr Phys Act. 2020;17(1):151. doi: 10.1186/s12966-020-01044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekelund U., Tarp J., Steene-Johannessen J., et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366:l4570. doi: 10.1136/bmj.I4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen B.K., Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 37.Wen C.P., Wai J.P., Tsai M.K., et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(1160749-6. [DOI] [PubMed] [Google Scholar]
- 38.Lee D.C., Pate R.R., Lavie C.J., Sui X., Church T.S., Blair S.N. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64(5):472–481. doi: 10.1016/j.jacc.2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arem H., Moore S.C., Patel A., et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eijsvogels T.M., Molossi S., Lee D.C., Emery M.S., Thompson P.D. Exercise at the extremes: the amount of exercise to reduce cardiovascular events. J Am Coll Cardiol. 2016;67(3):316–329. doi: 10.1016/j.jacc.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 41.Yang D., Bian Y., Zeng Z., Cui Y., Wang Y., Yu C. Associations between intensity, frequency, duration, and volume of physical activity and the risk of stroke in middle- and older-aged Chinese people: a cross-sectional study. Int J Environ Res Public Health. 2020;17(22):8628. doi: 10.3390/ijerph17228628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swain D.P., Franklin B.A. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97(1):141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 43.Franklin B.A., Kaminsky L.A., Kokkinos P. Quantitating the dose of physical activity in secondary prevention: relation of exercise intensity to survival. Mayo Clin Proc. 2018;93(9):1158–1163. doi: 10.1016/j.mayocp.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Eijsvogels T.M.H., Maessen M.F.H., Bakker E.A., et al. Association of cardiac rehabilitation with all-cause mortality among patients with cardiovascular disease in the Netherlands. JAMA Netw Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.11686. [DOI] [PubMed] [Google Scholar]
- 45.Bakker E.A., van Bakel B.M.A., Aengevaeren W.R.M., et al. Sedentary behaviour in cardiovascular disease patients: risk group identification and impact of cardiac rehabilitation. Int J Cardiol. 2021;326:194–201. doi: 10.1016/j.ijcard.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Kaminsky L.A., Arena R., Ellingsen O., et al. Cardiorespiratory fitness and cardiovascular disease - the past, present, and future. Prog Cardiovasc Dis. 2019;62(2):86–93. doi: 10.1016/j.pcad.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Bernardo B.C., Ooi J.Y.Y., Weeks K.L., Patterson N.L., McMullen J.R. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol Rev. 2018;98(1):419–475. doi: 10.1152/physrev.00043.2016. [DOI] [PubMed] [Google Scholar]
- 48.Quindry J.C., Franklin B.A. Exercise preconditioning as a cardioprotective phenotype. Am J Cardiol. 2021;148:8–15. doi: 10.1016/j.amjcard.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita N., Hoshida S., Otsu K., Asahi M., Kuzuya T., Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189(11):1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lennon S.L., Quindry J., Hamilton K.L., French J., Staib J., Mehta J.L., Powers S.K. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol. 2004;96(4):1299–1305. doi: 10.1152/japplphysiol.00920.2003. (1985) [DOI] [PubMed] [Google Scholar]
- 51.Lennon S.L., Quindry J.C., French J.P., Kim S., Mehta J.L., Powers S.K. Exercise and myocardial tolerance to ischaemia-reperfusion. Acta Physiol Scand. 2004;182(2):161–169. doi: 10.1111/j.1365-201X.2004.01346.x. [DOI] [PubMed] [Google Scholar]
- 52.Chicco A.J., Johnson M.S., Armstrong C.J., Lynch J.M., Gardner R.T., Fasen G.S., Gillenwater C.P., Moore R.L. Sex-specific and exercise-acquired cardioprotection is abolished by sarcolemmal KATP channel blockade in the rat heart. Am J Physiol Heart Circ Physiol. 2007;292(5):H2432–H2437. doi: 10.1152/ajpheart.01301.2006. [DOI] [PubMed] [Google Scholar]
- 53.Quindry J., French J., Hamilton K., Lee Y., Mehta J.L., Powers S. Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp Gerontol. 2005;40(5):416–425. doi: 10.1016/j.exger.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Quindry J.C., Franklin B.A. Cardioprotective exercise and pharmacologic interventions as complementary antidotes to cardiovascular disease. Exerc Sport Sci Rev. 2018;46(1):5–17. doi: 10.1249/JES.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 55.McGinnis G.R., Ballmann C., Peters B., Nanayakkara G., Roberts M., Amin R., Quindry J.C. Interleukin-6 mediates exercise preconditioning against myocardial ischemia reperfusion injury. Am J Physiol Heart Circ Physiol. 2015;308(11):H1423–H1433. doi: 10.1152/ajpheart.00850.2014. [DOI] [PubMed] [Google Scholar]
- 56.Miller L.E., McGinnis G.R., Peters B.A., Ballman C.G., Nanayakkara G., Amin R., Quindry J.C. Involvement of the δ-opioid receptor in exercise-induced cardioprotection. Exp. Physiol. 2015;100(4):410–421. doi: 10.1113/expphysiol.2014.083436. [DOI] [PubMed] [Google Scholar]
- 57.Thompson P.D., Franklin B.A., Balady G.J., et al. Exercise and acute cardiovascular events: placing the risks into perspective. A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 58.Thompson P.D. The cardiovascular complications of vigorous physical activity. Arch Intern Med. 1996;156(20):2297–2302. [PubMed] [Google Scholar]
- 59.Maron B.J., Gohman T.E., Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998;32(7):1881–1884. doi: 10.1016/s0735-1097(98)00491-4. [DOI] [PubMed] [Google Scholar]
- 60.Finocchiaro G., Papadakis M., Robertus J.-.L., et al. Etiology of sudden death in sports: insights from a United Kingdom Regional Registry. J Am Coll Cardiol. 2016;67(18):2108–2115. doi: 10.1016/j.jacc.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 61.Ullal A.J., Abdelfattah R.S., Ashley E.A., Froelicher V.F. Hypertrophic cardiomyopathy as a cause of sudden cardiac death in the young: a meta-analysis. Am J Med. 2016;129(5):486–496. doi: 10.1016/j.amjmed.2015.12.027. e2. [DOI] [PubMed] [Google Scholar]
- 62.Giri S., Thompson P.D., Kiernan F.J., et al. Clinical and angiographic characteristics of exertion-related acute myocardial infarction. JAMA. 1999;282(18):1731–1736. doi: 10.1001/jama.282.18.1731. [DOI] [PubMed] [Google Scholar]
- 63.Franklin B.A., Thompson P.D., Al-Zaiti S.S., et al. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective-an update: a Scientific Statement From the American Heart Association. Circulation. 2020;141(13):e705–e736. doi: 10.1161/CIR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 64.Mittleman M.A., Maclure M., Tofler G.H., Sherwood J.B., Goldberg R.J., Muller J.E. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigation. N Engl J Med. 1993;329(23):1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 65.Franklin B.A. Preventing exercise-related cardiovascular events: is a medical examination more urgent for physical activity or inactivity? Circulation. 2014;129(10):1081–1084. doi: 10.1161/CIRCULATIONAHA.114.007641. [DOI] [PubMed] [Google Scholar]
- 66.Goodman J.M., Burr J.F., Banks L., Thomas S.G. The acute risks of exercise in apparently healthy adults and relevance for prevention of cardiovascular events. Can J Cardiol. 2016;32(4):523–532. doi: 10.1016/j.cjca.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 67.Albert M.C., Mittleman M.A., Chae C.U., Lee I.M., Hennekens C.H., Manson J.E. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343(19):1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 68.Whang W., Manson J.E., Hu B.F., et al. Physical exertion, exercise, and sudden cardiac death in women. JAMA. 2006;295(12):1399–1402. doi: 10.1001/jama.295.12.1399. [DOI] [PubMed] [Google Scholar]
- 69.Hossack K., Hartwig R. Cardiac arrest associated with supervised cardiac rehabilitation. J Cardiac Rehabil. 1982;2:402–408. [Google Scholar]
- 70.Lown B., Verrier R.L., Rabinowitz S.H. Neural and psychologic mechanisms and the problem of sudden cardiac death. Am J Cardiol. 1977;39(6):890–902. doi: 10.1016/s0002-9149(77)80044-1. [DOI] [PubMed] [Google Scholar]
- 71.Kim J.H., Malhotra R., Chiampas G., et al. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366(2):130–140. doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- 72.Harris K.M., Creswell L.L., Haas T.S., et al. Death and cardiac arrest in U.S. triathlon participants, 1985 to 2016: a case series. Ann Intern Med. 2017;167(8):529–535. doi: 10.7326/M17-0847. [DOI] [PubMed] [Google Scholar]
- 73.Haapaniemi S., Franklin B.A., Wegner J.H., et al. Electrocardiographic responses to deer hunting activities in men with and without coronary artery disease. Am J Cardiol. 2007;100(2):175–179. doi: 10.1016/j.amjcard.2007.02.076. [DOI] [PubMed] [Google Scholar]
- 74.Franklin B.A., George P., Henry R., Gordon S., Timmis G.C., O'Neill W.W. Acute myocardial infarction after manual or automated snow removal. Am J Cardiol. 2001;87(11):1282–1283. doi: 10.1016/s0002-9149(01)01520-x. [DOI] [PubMed] [Google Scholar]
- 75.Chowdhury P.S., Franklin B.A., Boura J.A., et al. Sudden cardiac death after manual or automated snow removal. Am J Cardiol. 2003;92(7):833–835. doi: 10.1016/s0002-9149(03)00894-4. [DOI] [PubMed] [Google Scholar]
- 76.Pandolf K.B., Cafarelli E., Noble B.J., Metz K.F. Hyperthermia: effect on exercise prescription. Arch Phys Med Rehabil. 1975;56(12):524–526. [PubMed] [Google Scholar]
- 77.Hoberg E., Schuler G., Kunze B., et al. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990;65(9):583–589. doi: 10.1016/0002-9149(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 78.American College of Sports Medicine . 7th ed. Lippincott Williams & Wilkins; Baltimore: 2005. Guidelines for exercise testing and prescription; pp. 165–199. [Google Scholar]
- 79.Smyth A., O'Donnell M., Lamelas P., Teo K., Rangarajan S., Yusuf S., on behalf of the INTERHEART Investigators Physical activity and anger or emotional upset as triggers of acute myocardial infarction: the INTERHEART Study. Circulation. 2016;134(15):1059–1067. doi: 10.1161/CIRCULATIONAHA.116.023142. [DOI] [PubMed] [Google Scholar]
- 80.Aengevaeren V.L., Baggish A.L., Chung E.H., et al. Exercise-induced cardiac troponin elevations: from underlying mechanisms to clinical relevance. Circulation. 2021;144(24):1955–1972. doi: 10.1161/CIRCULATIONAHA.121.056208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aengevaeren V.L., Froeling M., Hooijmans M.T., et al. Myocardial injury and compromised cardiomyocyte integrity following a marathon run. JACC Cardiovasc Imaging. 2020;13(6):1445–1447. doi: 10.1016/j.jcmg.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 82.Aengevaeren V.L., Hopman M.T.E., Thompson P.D., et al. Exercise-induced cardiac troponin I increase and incident mortality and cardiovascular events. Circulation. 2019;140(10):804–814. doi: 10.1161/CIRCULATIONAHA.119.041627. [DOI] [PubMed] [Google Scholar]
- 83.Schnell F., Claessen G., La Gerche A., et al. Subepicardial delayed gadolinium enhancement in asymptomatic athletes: let sleeping dogs lie? Br J Sports Med. 2016;50(2):111–1117. doi: 10.1136/bjsports-2014-094546. [DOI] [PubMed] [Google Scholar]
- 84.Aengevaeren V.L., Mosterd A., Sharma S., et al. Exercise and coronary atherosclerosis: observations, explanations, relevance, and clinical management. Circulation. 2020;141(16):1338–1350. doi: 10.1161/CIRCULATIONAHA.119.044467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Merghani A., Maestrini V., Rosmini S., et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation. 2017;136(2):126–137. doi: 10.1161/CIRCULATIONAHA.116.026964. [DOI] [PubMed] [Google Scholar]
- 86.Radford N.B., DeFina L.F., Leonard D., et al. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle-age men: results from the Cooper Center Longitudinal Study. Circulation. 2018;137(18):1888–1895. doi: 10.1161/CIRCULATIONAHA.117.032708. [DOI] [PubMed] [Google Scholar]
- 87.DeFina L.F., Radford N.B., Barlow C.E., et al. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019;4(2):174–181. doi: 10.1001/jamacardio.2018.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khurshid S., Weng L.C., Al-Alusi M.A., et al. Accelerometer-derived physical activity and risk of atrial fibrillation. Eur Heart J. 2021;42(25):2472–2483. doi: 10.1093/eurheartj/ehab250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Svedberg N., Sundstrӧm J., James S., Hållmarker U., Hambraeus K., Andersen K. Long-term incidence of atrial fibrillation and stroke among cross-country skiers. Circulation. 2019;140(11):910–920. doi: 10.1161/CIRCULATIONAHA.118.39461. [DOI] [PubMed] [Google Scholar]
- 90.Garnvik L.E., Malmo V., Janszky I., et al. Physical activity, cardiorespiratory fitness, and cardiovascular outcomes in individuals with atrial fibrillation: the HUNT study. Eur Heart J. 2020;41(15):1467–1475. doi: 10.1093/eurheartj/ehaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eijsvogels T.M.H., Fernandez A.B., Thompson P.D. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol Rev. 2016;96(1):99–125. doi: 10.1152/physrev.00029.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kettunen J.A., Kujala U.M., Kaprio J., et al. All-cause and disease-specific mortality among male, former elite athletes: an average 50-year follow-up. Br J Sports Med. 2015;49(13):893–897. doi: 10.1136/bjsports-2013-093347. [DOI] [PubMed] [Google Scholar]
- 93.Garatachea N., Santos-Lozano A., Sanchis-Gomar F., et al. Elite athletes live longer than the general population: a meta-analysis. Mayo Clin Proc. 2014;89(9):1195–1200. doi: 10.1016/j.mayocp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Clarke P.M., Walter S.J., Hayen A., Mallon W.J., Heijmans J., Studdert D.M. Survival of the fittest: retrospective cohort study of the longevity of Olympic medallists in the modern era. Br J Sports Med. 2015;49(13):898–902. doi: 10.1136/bjsports-2015-e8308rep. [DOI] [PubMed] [Google Scholar]
- 95.Marijon E., Tafflet M., Antero-Jacquemin J., et al. Mortality of French participants in the Tour de France (1947-2012) Eur Heart J. 2013;34(40):3145–3150. doi: 10.1093/eurheartj/eht347. [DOI] [PubMed] [Google Scholar]