Abstract
The prescription of exercise for individuals with and without cardiovascular disease (CVD) should be scientifically-based yet adapted to the patient. This scientific statement reviews the clinical and physiologic basis for the prescription of exercise, with specific reference to the volume of physical activity (PA) and level of cardiorespiratory fitness (CRF) that confer significant and optimal cardioprotective benefits. Recommendations are provided regarding the appropriate intensity, frequency, and duration of training; the concept of MET-minutes per week; critical components of the exercise session (warm-up, conditioning phase, cool-down); methodologies for establishing the training intensity, including oxygen uptake reserve (V̇O2R), target heart rate derivation and rating perceived exertion; minimum and goal intensities for exercise training; and, types of training activities, including resistance training, adjunctive lifestyle PA, marathon/triathlon training, and high-intensity interval training. In addition, we discuss the rationale for and value of exercise training programs for patients with peripheral artery disease, diabetes mellitus, and heart failure.
Keywords: Physical activity, Cardiorespiratory fitness, Exercise prescription, Metabolic equivalents, Oxygen uptake reserve, Marathon/triathlon training, High-intensity interval training, Peripheral artery disease, Diabetes mellitus, Heart failure
Abbreviations: physical activity (PA), cardiorespiratory fitness (CRF), cardiovascular disease (CVD),, coronary heart disease (CHD), rating of perceived exertion (RPE),oxygen uptake reserve (VO2R) , high intensity interval training (HIIT)
Graphical Abstract
The volume of regular physical activity (PA) and level of cardiorespiratory fitness (CRF), expressed as mL/kg/min or as metabolic equivalents (METs; 1 MET = 3.5 mLO2/kg/min), are inversely related to the risk of coronary heart disease (CHD). In fact, for the primary and secondary prevention of cardiovascular disease (CVD), each 1 MET increase in CRF confers an ∼16% decrease in mortality, which compares favorably with the survival benefit provided by commonly prescribed cardiovascular (CV) medications after acute myocardial infarction (AMI) [1]. Higher levels of PA and/or CRF before hospitalization for acute coronary syndromes and elective or emergent surgical procedures also appear to yield more favorable short-term outcomes [2].
In this scientific statement (Part II), we expand on previous review articles and guidelines and those topics covered in Part I [3], with specific reference to CRF and PA as distinct risk factors, how to prescribe exercise, including the intensity, frequency, duration, and type of training activity, as well as the concept of MET-minutes per week. Additional topics include oxygen consumption reserve (V̇O2R), rating of perceived exertion (RPE), minimum exercise training intensity to maximize survival benefits, the cardioprotective value of progressing the exercise intensity, complementary exercise interventions such as resistance training, lifestyle PA, marathon/triathlon training, and the advantages, limitations and unknowns of high-intensity interval training (HIIT). Finally, we discuss the rationale for and value of exercise training programs for individuals with peripheral artery disease (PAD), type 2 diabetes mellitus (DM), and heart failure (HF), or combinations thereof. Because physical inactivity and low CRF are modifiable risk factors, clinicians should routinely assess and prescribe structured exercise and increased lifestyle PA to the patients they counsel.
1. Cardiorespiratory fitness and physical activity
1.2. As separate risk factors
Numerous epidemiologic analyses combined with evidence of biologic plausibility support a cause-and-effect relationship between increased PA and/or CRF levels and reduced CVD mortality. Consequently, low levels of CRF have now been designated as a clinical vital sign, risk factor for CVD [1], [2], [3], and as a strong prognostic indicator in exercise-based cardiac rehabilitation (CR) [4,5].
Exercise training, as a subcategory of PA, is defined as any structured intervention to increase or maintain CRF, decrease the incidence of chronic disease, or improve physical performance and/or health outcomes. PA or structured exercise is typically assessed by questionnaire, step counts (pedometers), accelerometry, or via a relatively new fitness metric termed the personalized activity intelligence (PAI) score [6]. PAI is derived from the cumulative fluctuations in heart rate (HR) over the most recent 7 days, to provide an approximation of the relative intensity of PA and associated energy expenditure. Studies in both primary and secondary prevention have shown that participants with a weekly PAI score ≥100 demonstrate a lower risk of all-cause mortality [7], [8], [9]. Aerobic capacity or CRF can be directly measured during cardiopulmonary exercise testing and expressed as mLO2/kg/min or METs, estimated from the attained treadmill grade and speed or cycle ergometer work rate (kg∙m∙min−1), adjusted for duration.
2.2. Physical activity: epidemiologic and cohort studies
In a comprehensive meta-analysis of 43 studies evaluating the relationship between PA and CHD incidence, the relative risk of CHD associated with physical inactivity ranged from 1.5 to 2.4, with a median value of 1.9 [10]. Moreover, the relative risk of a sedentary lifestyle appeared to be similar in magnitude to that associated with other major CHD risk factors. Another systematic review and meta-analysis of 33 PA studies, including 883,372 participants, reported pooled risk reductions of 35% and 33% for CVD and all-cause mortality, respectively [11]. More recently, researchers analyzed data from 2 major ongoing studies, the Nurses’ Health Study (n = 78,865) and the Health Professionals Follow-up Study (n = 44,354), to estimate the impact of lifestyle on life expectancy in the U.S. population [12]. During up to 34 years of follow-up, the most physically active cohorts of men and women demonstrated 7- to 8-year gains in life expectancy!
2.3. Physical activity versus cardiorespiratory fitness: comparative risk reductions
Numerous studies now suggest that CRF is one of the strongest prognostic markers in persons with and without chronic disease, including CVD [13], [14], [15], [16]. Higher levels of CRF are associated with a reduced risk of developing hypertension, DM, atrial fibrillation, chronic kidney disease, and major adverse CV events, including HF, acute myocardial infarction (AMI), stroke, and coronary artery bypass grafting [17].
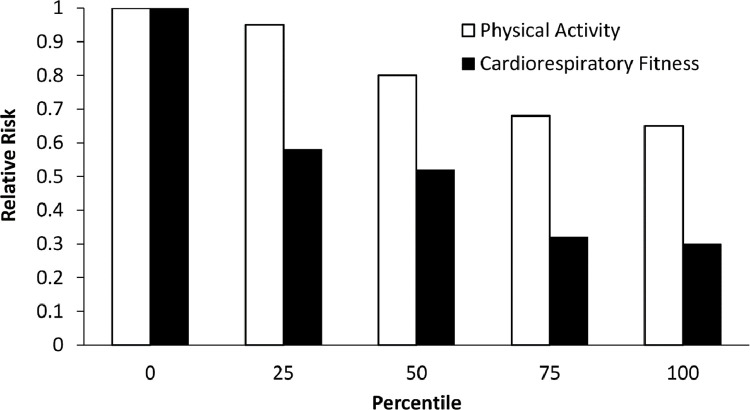
Williams [18] reported that the risks of CHD and CVD decrease linearly in association with increasing percentiles of PA (Fig. 1). In contrast, there was a marked decrease in disease risk when the lowest was compared with the next-lowest category of CRF. Beyond this distinction, the reductions in risk paralleled those observed with increasing PA but were essentially twice as great for CRF. Four important findings emerged from this report. First, being unfit warrants consideration as an independent risk factor. Second, a below-average level of CRF or aerobic capacity (V̇O2max) decreases the relative CVD risk to a greater extent than a comparable level (same percentile) of PA. Third, the primary beneficiaries of a regular exercise regimen appear to be those comprising the least fit category. Fourth, for both PA and CRF, there is little or no additional relative risk reduction by moving from the 75th to the 100th percentile, suggesting a plateau in relative risk.
Fig. 1.
The risks of CHD and CVD decrease in association with increasing percentiles of physical activity and cardiorespiratory fitness, corresponding to 30% and 64% in the most active and fit individuals, respectively. Interestingly, little or no additional benefit occurs when moving from the 75th to the 100th percentile, that is, “good” to “excellent,” suggesting a plateau in relative risk. Adapted from reference #18.
In a similar study, Myers et al. [19] compared estimated CRF versus self-reported PA patterns in predicting all-cause mortality in 6213 consecutive men (mean ± SD age = 59 ± 11 years) who were referred for exercise testing. Of these, 842 had completed a PA questionnaire. The predictive power of exercise capacity (peak METs) and energy expenditure during PA, expressed as kilocalories per week, for all-cause mortality were determined over a mean ± SD follow-up of 5.5 ± 2 years. Estimated exercise capacity based on the peak attained treadmill speed and grade, using age-specific quartiles, was a stronger predictor of mortality than was self-reported PA. These 2 variables were stronger predictors of mortality than established risk markers such as cigarette smoking, hypertension, DM, previous myocardial infarction, or a history of HF. Interestingly, a 1000-kcal/week increase in PA was similar to a 1 MET increase in CRF; both conferred a mortality benefit of 20%.
2.3.1. Accelerometry-reported PA data
To evaluate the dose-response associations of total PA, sedentary time, and intensities of PA assessed by accelerometry with all-cause mortality, investigators analyzed data from 8 studies including 36,383 middle-aged and older adults (mean age, 62.6 years; 73% women) who were followed for an average of 5.8 years [20]. At ∼225 min/week of moderate-to-vigorous PA (MVPA) the hazard ratio for accelerometry was 0.45 for all-cause mortality [21]. The investigators concluded that higher levels of total PA, at any intensity, and less time spent sedentary, were associated with a lower risk of death.
2.4. How much exercise is enough? The concept of MET-minutes/week
In addition to contemporary PA recommendations, the PAI weekly goal of ≥100 [6], or the use of technology to promote increased PA [22], the concept of MET-minutes per week has been widely promulgated [23]. This metric enables clinicians and patients to translate guideline-driven MVPA recommendations (≥500 to 1,000 MET-minutes per week) into achievable goals by quantifying accumulated exercise each week in a single formula: METs per activity x number of minutes/session x days/week = MET-minutes per week. For example, 60 min of walking at a 3-mph pace (3.4 METs), 3 days/week = 612 MET-min per week. Alternatively, 30 min of singles tennis (∼7 METs), 3 days/week = 630 MET-min per week. Or, for the recreational jogger, 20 min of jogging at a 5-mph pace (7.5 METs), 4 days/week = 600 MET-min per week. Accordingly, all 3 of these exercise regimens would meet the minimum criteria (500 MET-min per week) for an effective exercise dosage.
3. Exercise prescription/programming
Although detailed descriptions of exercise prescription/programing are available elsewhere [24], this section is directed toward components of the exercise session, the appropriate intensity, frequency, and duration of exercise, relevant contemporary guidelines, methodologies, “prescriptive pearls” for the clinician, and training activities that have been shown to elicit favorable physiologic, clinical, and health outcomes. If the current mantra “exercise is medicine” is embraced, underdosing and overdosing are possible. Accordingly, exercise may have a typical dose-response curve with a plateau in benefit or even adverse effects at more extreme levels in individuals with known or occult CVD [25].
3.1. Components of the physical conditioning session
Exercise training sessions should include a preliminary warm-up (10 min), a cool-down (5–8 min), and an optional recreational activity (10–15 min). A conditioning phase (30–60 min), interspersed between the warm-up and cool-down, should primarily involve endurance exercise complemented by flexibility/resistance training.
3.1.1. Warm-up
Warm-up exercises facilitate the transition from rest to endurance training, stretching postural muscles and increasing blood flow. This preliminary phase may also reduce the risk of exercise-related CV complications. Sudden strenuous exertion without prior warm-up has been shown to elicit ischemic ST-segment depression and/or ventricular ectopy in up to 70% of healthy men with normal electrocardiographic (ECG) responses to maximal exercise testing [26]. These abnormalities were generally attenuated or eliminated when the exertion was preceded by a warm-up (jogging in place). Thus, the warm-up should include calisthenic exercise followed by activities that increase the HR to within 20 bpm of the minimum target HR range prescribed for endurance training.
3.1.2. Cool-down
The cool-down involves slow walking or low-intensity exercise (e.g., pedaling) and provides a gradual recovery from the endurance or conditioning phase. It permits appropriate circulatory adjustments and return of the rate-pressure product to near resting values; enhances venous return, thereby reducing the potential for postexercise hypotension and dizziness; facilitates the dissipation of body heat; promotes more rapid removal of lactic acid than stationary recovery, and combats the potential, deleterious effects of the postexercise rise in plasma catecholamines [27].
Omission of a cool-down in the immediate postexercise period may result in a transient decrease in venous return, possibly reducing coronary blood flow when the HR and systolic blood pressure may still be high. Consequences may include angina pectoris, ischemic ST-segment depression, malignant ventricular arrhythmias, or combinations thereof. Of 61 CV events reported during the exercise training of patients with CVD, at least 44 (72%) occurred during either the warm-up or cool-down phases [28].
3.1.3. Conditioning phase
The conditioning phase should follow the warm-up and includes an endurance component as well as resistance and flexibility training. Endurance training, which improves the patients’ cardiorespiratory responses to submaximal and maximal exercise, should be prescribed in terms of intensity, duration, frequency, and type of activity.
3.2. Exercise training intensity methodologies
The prescribed exercise intensity, expressed as a percentage of the CRF (peak or maximal METs) or aerobic capacity, should be above a minimum level required to induce a “training effect,” yet below the metabolic load that evokes significant symptoms, ECG or blood pressure abnormalities. For deconditioned/inactive individuals or patients with CVD, the minimum or threshold intensity for improving CRF corresponds to ∼60%–70% of the highest HR achieved during peak or symptom-limited exercise testing [29,30]. However, considerable evidence suggests that the threshold increases in direct proportion to the initial level of fitness or habitual PA [24].
For previously sedentary patients embarking on a physical conditioning program who have not undergone exercise testing, the standing resting HR plus ∼10 to 20 bpm is recommended for the initial exercise intensity, using symptomatology and RPE (category-ratio scale [0-10], 2 [weak] to 4 [somewhat strong]; category scale [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], 11 [fairly light] to 13 [somewhat hard]) as adjunctive intensity modulators. Over time, the exercise intensity should be gradually increased to 50%–80% of the exercise capacity or level of CRF, which approximates 70%–85% of the highest HR attained during peak or symptom-limited exercise testing [24]. Additional exercise intensity methodologies and modulators include target HR, the concept of V̇O2R, RPE, and the MET method of activity prescription.
3.2.1. Heart rate
Since HR and oxygen consumption are linearly related during dynamic exercise involving large muscle groups, a predetermined training or target heart rate (THR) has become widely employed as an index of exercise intensity. Two commonly used methods of establishing the THR are: (1) the maximal HR reserve (HRR) method [31], in which THR = (maximal HR – resting HR) x 60% to 80% + resting HR, and (2) the relative submaximal reserve method, which involves computing the THR as a standardized percentage of the measured maximal HR [24]. This method is based on previous studies that have shown remarkably similar regressions of percent V̇O2max on the percent of maximal HR (i.e., 60% – 80% V̇O2max ∼70% – 85% of maximal HR), irrespective of potential confounders.
Although the formula “220-age” has been widely recommended to estimate maximal HR, there is considerable variability (SD ± 10–12 bpm) in using this method for exercise prescription in apparently healthy people and even greater inaccuracy in individuals with CHD [24]. Moreover, the validity of this equation has never been established in a sample that included a sufficient number of older adults; accordingly, it's utility has been challenged [32,33]. Often, patients with CVD may be taking prescribed medications (e.g., beta-blockers, selected calcium channel blockers) that can markedly blunt the rise in HR during exercise. Concomitant DM and the associated disturbances in CV autonomic nervous system regulation in other cardiac patients may also decrease the chronotropic response to progressive levels of PA. Consequently, using this formula or other age-sex-predicted maximal HR regressions [32,34] may overestimate or underestimate the HR for exercise training in some patient subsets. For these reasons, if a “true” maximal HR has not been determined during exercise testing, we counsel patients to rely more on exertional symptoms and RPE to regulate their exercise intensity. It should be noted that patients with CHD may derive considerable physiologic benefit from an exercise training program in the presence of beta-blocking drugs, despite therapeutic doses and a reduced training heart rate [35], [36], [37].
3.2.2. The concept of oxygen consumption reserve (V̇O2R)
Although it was traditionally believed that a given percentage of the HR reserve corresponded to the same percentage of aerobic capacity or V̇O2max [38], more recent studies have shown that it more closely approximates the same percentage of the V̇O2R [39]. This methodology relates HR reserve to a level of metabolism that starts at a resting level (i.e., 1 MET) rather than from zero. An additional advantage is increased accuracy in establishing training workloads for low-fit patients. To calculate the target V̇O2 (TV̇O2) based on V̇O2R, the following equation is used:
For example, what is the TV̇O2 at 40% of V̇O2R for a patient with a 5-MET exercise capacity?
3.2.3. Rating of perceived exertion (RPE)
This objective rating of overall bodily exertion provides a useful and important complement to HR as an adjunctive intensity modulator during exercise training. The RPE category scale, first introduced by Borg [40], consists of 15 grades from 6 to 20: 7 = very, very light; 9 = very light; 11 = fairly light; 13 = somewhat hard; 15 = hard; 17 = very hard; 19 = very, very hard. During the initial 8-10 weeks of exercise training, ratings of 11-13 (“fairly light” to “somewhat hard”) are strongly recommended. Thereafter, exercise rated between 13 and 15, that is, between “somewhat hard” and “hard,” is generally considered appropriate, provided that the patient remains asymptomatic and adheres to the THR. This methodology may be particularly helpful when counseling patients with arrhythmias that preclude an accurate assessment of HR during exercise, including those with atrial fibrillation [41].
3.2.4. The MET method of activity prescription
The metabolic costs of many household, occupational, and recreational activities have been defined in terms of kilocalorie expenditure per minute or by oxygen consumption, expressed on a relative basis as mL/kg/min or as METs. To facilitate exercise and activity prescription, a comprehensive compendium of physical activities has been developed [42], with an abbreviated version shown in Table 1. Consequently, this resource is often used to identify and prescribe activities that are sufficiently below the highest MET level achieved during exercise testing.
Table 1.
Estimated energy expenditure (METs) of daily activities.
| PHYSICAL ACTIVITY | |
|---|---|
| Light intensity activities | <3 |
| Sleeping | 0.9 |
| Watching television | 1.0 |
| Writing, desk work, typing | 1.8 |
| Walking, 1.7 mph (2.7 km/h), level ground, strolling, very slow | 2.3 |
| Walking, 2.5 mph (4 km/h) | 2.9 |
| Moderate intensity activities | 3 to 5.9 |
| Bicycling, stationary, 50 watts*, very light effort | 3.0 |
| Walking, 3.0 mph (4.8 km/h) | 3.3 |
| Calisthenics, home exercise, light or moderate effort | 3.5 |
| Walking, 3.4 mph (5.5 km/h) | 3.6 |
| Bicycling, <10 mph (16 km/h), leisure, to work or for pleasure | 4.0 |
| Bicycling, stationary, 100 watts, light effort | 5.5 |
| Vigorous intensity activities | ≥6 |
| Singles tennis | 6.5 |
| Jogging (5 mph) | 7.5 |
| Squash racquets | 8.5 |
| Running (6 mph) | 10.0 |
*Note: 1 watt ∼6 kg∙m∙min−1; Adapted from Ref. [42].
Although the MET method is frequently employed by clinicians when prescribing exercise, there are some limitations when using it as a guide to recreational or vocational counseling [43]. One limitation is the assumption that 1 MET = 3.5 mLO2/kg/min. Studies in selected populations demonstrate that this value significantly overestimates directly measured resting oxygen consumption and caloric expenditure by, on average, 30% to 35% [44,45]. Second, the published MET requirements of varied activities represent average energy expenditures. These values may vary considerably, depending on how the individual performs the activity, their skill and mechanical efficiency, and the degree of competition. Third, the oxygen costs or MET requirements listed in the compendium of physical activities [42] were derived from continuous steady-state work (≥3-min bouts). In contrast, activities of daily living are often performed intermittently rather than continuously. Thus, the MET method of activity prescription may considerably underestimate the patients’ capacity for vocational or leisure-time activities. Finally, one cannot assume that all occupational work demanding aerobic requirements similar to that achieved during exercise testing elicits similar cardiac demands and vice versa. Additional variables at work include the stresses of emotional and cognitive demands, environmental influences (altitude, temperature, wind-chill factor, and humidity), and the activation of muscle groups not used during the exercise test, particularly the upper extremities.
3.2.5. Using the HR index equation to estimate METs
Given the linear relationship between HR and V̇O2, HR may be used to estimate METs during structured exercise or PA. Wicks et al. [46] reported a simple formula to estimate oxygen uptake (METs) during PA in patients with and without CHD, including those taking β-blockers, using the HR index equation (Table 2).
Table 2.
Changes in heart rate to estimate energy expenditure (mets) during daily activities*.
| The energy cost of any activity, expressed as METs, can be estimated from the resting and exercise heart rates using the equation: |
|---|
| METs = (6 x Heart Rate Index) – 5 |
| where the Heart Rate Index equals the activity heart rate divided by the resting heart rate. |
| Example #1 A tennis player's resting heart rate of 60 beats per minute (bpm) is increased to 120 bpm during a tennis match. His MET level is estimated as follows: 120 bpm/60 bpm = 2.0 Heart Rate Index which is multiplied by 6, yielding 12, from which we subtract 5, yielding an estimated 7 METs. |
| (120/60 × 6) – 5 = (2 × 6) – 5 = 7 METs |
| Example #2 A recreational walker with a resting heart rate of 70 bpm walks at 105 bpm. Her estimated MET level is…… |
| (105/70 × 6) – 5 = (1.5 × 6) – 5 = 4 METs |
3.3. Relative and absolute criteria for classifying the intensity of physical activity: implications for the patient with CHD
Cardiorespiratory endurance training serves as the foundation for most early outpatient (phase II) cardiac rehabilitation (CR) training programs [47]. Importantly, the exercise workloads prescribed to phase II cardiac patients vary significantly based upon their functional capacity. Although a significant percentage of patients referred to phase II CR initiate their program with a V̇O2peak ∼5-6 METs, some may be as low 2-3 METs, whereas others can exceed 10 METs. Based on this heterogenous patient population, contemporary approaches to phase II exercise prescription should consider both relative and absolute criteria for quantifying exercise intensity.
In patients with a very low functional capacity (e.g., 2-3 METs), the traditional approach to exercise prescription becomes particularly challenging. For example, 40% V̇O2peak in a patient with a 3 MET capacity equates to a workload of 1.2 METs, narrowly exceeding their resting metabolic rate. Similarly, 80% V̇O2peak is 2.4 METs for the same patient and would likely require the use of multiple brief bouts (e.g., 1-3 min) of discontinuous activity, regardless of whether they achieve the prescribed minimum intensity for a single session. In support of this approach, ventilatory threshold exhibits a physiologic nadir at 4-5 METs [48,49]. Thus, it should be expected that patients with a very low functional capacity would be unable to complete a traditional phase II CR exercise session without premature fatigue and/or use of brief work: rest intervals.
3.4. Goal intensities for exercise training: age-, sex-, and fitness-adjusted targets
Although most middle-aged and older adults initiate exercise-based rehabilitation programs at ≤3 METs, they often fail to increase the intensity of their exercise regimen [50]. This failure to advance their training MET level likely prevents them from achieving the maximal possible reduction in their risk of CVD. Several lines of evidence support the benefits for up-titrating the exercise intensity over time.
First, MVPA which corresponds to any activity ≥3 METs, has been consistently shown to reduce the health risks associated with chronic diseases, including CVD [51]. Second, an increasing body of research strongly suggests that the gradual progression of exercise intensities, from moderate-to-vigorous to high-intensity training regimens (in selected individuals), may result in even greater cardioprotective and survival benefits [6,52,53], which are likely attributed, at least in part, to higher levels of CRF, expressed as METs. Third, an exercise capacity or CRF ≤5 METs is associated with the poorest prognosis, signifying the least fit population cohort (i.e., the bottom quintile or 20%) [19,54,55]. Fourth, research suggests that up to ∼10 METs, each 1 MET increase in exercise capacity is associated with a 15% reduction in mortality [56]. Finally, since the additive survival benefits when progressing from “good” to “excellent” CRF levels are small [54,57], achieving “good” fitness levels should be a primary goal or objective.
Collectively, these data suggest that endurance training programs should be designed to achieve 2 objectives: a level of CRF >5 METs; and, because aerobic capacity can be influenced by age, sex, regular PA, and chronic disease, it is important to establish individualized goal exercise training intensities, expressed as METs, which are likely to confer “good” fitness levels associated with more favorable health outcomes and increased long-term survival [58].
3.5. Minimum exercise training intensity to maximize survival benefits?
A key objective during the initial weeks of exercise training is to gradually increase the intensity of exercise so that, at a minimum, individuals can emerge from the least fit population cohort, or bottom 20%, which corresponds to an exercise capacity or level of CRF ≤5 METs [19,54,55]. Empiric experience suggests that an exercise capacity >5 METs can be achieved by regularly exercising above 3 METs. Using the treadmill, irrespective of age, sex, weight, or fitness, this corresponds to walking at 2.0 mph, 3.5% grade, or on the level (0% grade) at 3.0 mph [43]. Accordingly, both of these workloads ∼3.4 METs. On the other hand, for the stationary cycle ergometer, work rates (kilogram meters per minute [kg∙m∙min−1]), expressed as METs, are weight dependent. The minimum work rates based on increasing body weight to achieve an aerobic requirement of ∼3.5 METs are shown in Table 3 [24]. For outdoor bicycling, the speed corresponding to 3 to 4 METs is ∼6 miles/hour [42].
Table 3.
Minimum work rates (kg∙m∙min−1) to achieve an energy expenditure of ∼3.5 metabolic equivalents (METs) on the stationary cycle ergometer at progressive body weights*.
| Body Weight | Minimum Work Rate | |
|---|---|---|
| kg | lb. | kg∙m∙min—1) |
| 50 | 110 | 200 |
| 60 | 132 | 250 |
| 70 | 154 | 300 |
| 80 | 176 | 350 |
| 90 | 198 | 400 |
| 100 | 220 | 450 |
| 110 | 242 | 500 |
| 120 | 264 | 550 |
| 130 | 286 | 600 |
*The estimated energy expenditure of ∼3.5 METs is achieved after 3 or more minutes at this work rate. Adapted from Ref. [24].
3.6. Prescribing age-, sex-, and fitness-adjusted intensities for training
To develop target exercise training intensity recommendations for attaining “good” (or higher) levels of CRF, the Fitness Registry and the Importance of Exercise: A National Database (FRIEND) was employed [59]. This registry consists of directly measured CRF on men (n = 4098) and women (n = 2762), aged 30 _ 79 years, without known CVD or chronic obstructive pulmonary disease. Tests that were terminated due to inadequate effort (peak respiratory exchange ratio <1.0) or because of abnormal signs/symptoms (e.g., increasing anginal symptoms, ischemic ST-segment depression >2 mm, threatening arrhythmias), rather than volitional fatigue, were excluded. Age-and sex-adjusted “good” fitness levels were calculated at the 60th percentile, and the associated vigorous training intensities likely to achieve these CRF levels (or higher), corresponding to 60% – 80% of the V̇O2R. The V̇O2R was calculated as ([V̇O2max – 1] x 60, 70 or 80%) +1, where V̇O2max was expressed as METs, assuming 1 MET equals rest.
Table 4 provides “good” fitness levels and recommended aerobic training requirements (METs) to achieve these for men and women (aged 30 – 79 years), corresponding to 60% – 80% of the V̇O2R [58]. This analysis uniquely provides age-, sex-, and fitness-adjusted aerobic training intensities that are compatible with attaining cardioprotective levels of CRF. Depending on their age, men should ideally be training between 5.0 and 10.5 METs and women between 3.7 and 7.6 METs. In our experience, if patients can progress to training intensities that are 60% – 80% of the V̇O2R, without adverse signs/symptoms or excessive RPEs (i.e., ≥15 [“hard work”] on the Borg 6 – 20 RPE scale), it is likely that they can attain fitness levels that are compatible with decreased mortality and increased survival. For example, using the FRIEND database, “good” fitness for a 65-year-old man approximates ≥8.7 METs. Accordingly, a training intensity of 5.6 – 7.2 METs or ∼6.4 METs (70% V̇O2R), achieved over time, should enable this patient to attain “good” fitness during subsequent exercise testing. This training intensity approximates singles tennis, graded treadmill walking (3.0 mph, 7.5% grade) or, for a 70 – kg patient, exercising at 600 kg∙m∙min−1 on the stationary cycle ergometer (Table 5). Age- and sex-based regression equations corresponding to 70% V̇O2R for progressively aged men and women (30 – 79 years), that is, signifying the training MET requirements needed to achieve “good” fitness levels, are shown below [58]:
Table 4.
“Good” fitness levels for middle-aged and older men and women and the training aerobic requirements associated with these cardiorespiratory fitness levels*.
| Age groups (years) | ||||||
|---|---|---|---|---|---|---|
| 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | ||
| Good | ||||||
| Men | fitness | ≥12.9 | ≥11.5 | ≥10.0 | ≥8.7 | ≥7.7 |
| Training | ||||||
| METs | 8.1—10.5 | 7.3—9.4 | 6.4—8.2 | 5.6—7.2 | 5.0—6.4 | |
| Good | ||||||
| Women | fitness | ≥9.2 | ≥8.2 | ≥7.2 | ≥6.1 | ≥5.5 |
| Training | ||||||
| METs | 5.9—7.6 | 5.3—6.8 | 4.7—6.0 | 4.1—5.1 | 3.7—4.6 | |
*Age- and sex-adjusted “good” levels of cardiorespiratory fitness (METs) for men and women and the recommended goal training intensity (60% — 80% V̇O2R, expressed as METs) to achieve these fitness levels. Adapted from Ref. [58].
Table 5.
Approximate energy expenditure in METs* during stationary cycle ergometry.
| Body Weight | Power Output or Work Rate (kg∙m∙min−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| kg | lb | 300 | 450 | 600 | 750 | 900 | 1050 | 1200 |
| 50 | 110 | 5.1 | 6.6 | 8.2 | 9.7 | 11.3 | 12.8 | 14.3 |
| 60 | 132 | 4.6 | 5.9 | 7.1 | 8.4 | 9.7 | 11.0 | 12.3 |
| 70 | 154 | 4.2 | 5.3 | 6.4 | 7.5 | 8.6 | 9.7 | 10.8 |
| 80 | 176 | 3.9 | 4.9 | 5.9 | 6.8 | 7.8 | 8.8 | 9.7 |
| 90 | 198 | 3.7 | 4.6 | 5.4 | 6.3 | 7.1 | 8.0 | 8.9 |
| 100 | 220 | 3.5 | 4.3 | 5.1 | 5.9 | 6.6 | 7.4 | 8.2 |
*1 MET = 3.5 mL/kg/min; Estimated values are based on completion of the 3-min exercise stage. Adapted from Ref. [24].
Men
Women
Although not all patients will achieve “good” CRF levels for their age and sex, most will be able to increase their level of CRF beyond the “bottom” quintile or 20%. By achieving this goal, patients are more likely to derive the greatest relative reduction in mortality with increasing levels of fitness (i.e., progressing from ≤5 METs to >5 METs) and the most impactful increase in survival [19].
3.7. Exercise duration and frequency
The duration of exercise required to elicit a significant training effect varies inversely with the intensity; the greater the intensity (up to ∼80% of exercise capacity), the shorter the duration of exercise necessary to achieve favorable adaptation and improvement in CRF [24]. Exercise training for 10 to 15-min periods improves CRF, and 30-min sessions are even more effective. However, for novice exercisers, there is little additional aerobic benefit thereafter, and with sessions lasting ≥45 min, the incidence of orthopedic injury increases disproportionately [60].
Although traditional recommendations [61] suggest that accumulated MVPA bouts should last ≥10 min to achieve the 30-min daily minimum, more recent studies suggest that even shorter periods of PA, accrued over time, can produce CV and metabolic health benefits [62,63]. Other reports suggest that replacing sedentary time with even brief periods of light-intensity PA (∼2 min/hour) may confer a survival benefit [64].
Deconditioned patients may respond to slightly less than twice-weekly exercise; however, 3 to 4 vigorous intensity (≥60% V̇O2max) workouts per week appear to be the optimal training frequency. Additional benefits of vigorous-intensity training ≥5 sessions per week seem to be minimal, whereas the incidence of musculoskeletal injury increases disproportionately [60]. On the other hand, to promote and maintain health, moderate-intensity PA (40% – 59% V̇O2max) for a minimum of 30 min on 5 days each week, or vigorous PA (≥60% V̇O2max) for a minimum of 20 min on 3 days each week, is recommended [51].
3.8. Types of training activities: exercise modalities
The most effective exercises for the endurance or conditioning phase include outdoor walking, level or graded treadmill walking, jogging, running, stationary cycle ergometry, outdoor cycling, swimming, jumping rope, rowing, arm ergometry, and combined arm-leg ergometry. The energy costs of walking vs. running vs. outdoor bicycling, as well as treadmill walking and stationary cycle ergometry, 5 of the most popular training modalities, are detailed below. Complementary activities include adjunctive resistance training and increased lifestyle activity. Vigorous-to-high intensity PA, particularly when unaccustomed, and some competitive endurance sports (e.g., marathon running and triathlon participation) are associated with a greater incidence of acute cardiac events [65,66]. Moreover, only limited data are available regarding the benefit:risk ratio of high-volume, high-intensity exercise training regimens/competition in patients with ischemic and other forms of CVD. Recent studies have also shown that large exercise volumes and vigorous-to-high exercise intensities are associated with potential cardiac maladaptations, including accelerated coronary artery calcification and atrial fibrillation [67].
3.8.1. Energy expenditure during walking, running, and bicycling
Because of the popularity of walking, running, and outdoor bicycling, there is interest in the energy expenditure per unit distance for each activity. Running a given distance expends more calories than walking the same distance. The gross caloric cost of walking and running is ∼1.15 and 1.70 kcal/kg/1.6 km, respectively [24,42]. Moreover, unless the person walks or runs at extremely slow or fast paces, the caloric cost per distance is relatively independent of speed. Although many obese persons may be unable to run, a substantial energy expenditure can result by moving their heavier body weight through distance walking. Outdoor bicycling is an energy-efficient method of covering distance. The gross caloric expenditure of bicycling, also relatively independent of speed, is ∼0.60 kcal/kg/1.6 km [24,42], where 1.6 km equals 1 mile. For a given distance, bicycling uses approximately one half and one third the kcal of walking and running, respectively. Accordingly, the energy cost of bicycling 4.8 km is the approximate equivalent of walking 2.4 km or running 1.6 km. To burn calories for weight control, a rather long bicycling distance must be covered [68].
3.8.2. Estimating METs during level and graded treadmill walking
The “Rule of 2 and 3 mph” has been suggested in estimating “steady-state” energy expenditure during treadmill walking [43]. Level walking at 2 and 3 mph approximate 2 and 3 METs, respectively. At a 2-mph speed, each 3.5% grade increment adds an additional MET to the energy expenditure. For individuals who can walk at 3 mph, each 2.5% increase in treadmill grade adds an additional MET. Thus, a 5 MET workload can be achieved by either 2.0 mph, 10.5% grade, or 3.0 mph, 5.0% grade.
3.8.3. Energy expenditures in METs during cycle ergometry
For individuals using a stationary cycle ergometer, the energy cost, expressed as METs, depends upon the work rate (kilogram meters per minute [kg∙m∙min−1]) and the person's body weight (kg or pounds [lb]). For example, a 100-kg person exercising at 900 kg∙m∙min−1 would be working at 6.6 METs, whereas the same work rate for a 60-kg person would correspond to approximately 9.7 METs (Table 5) [24].
3.8.4. Resistance training
Resistance training provides an effective method for increasing muscle strength and endurance, favorably modifying selected risk factors, and enhancing psychosocial well-being. It is also comparable or superior to endurance training for enhancing bone mineral density, increasing muscle mass and strength, improving insulin sensitivity, and augmenting the basal metabolic rate [69]. Moreover, resistance training has been shown to decrease the rate-pressure product when any given load is lifted [70,71], reducing cardiac demands during daily activities such as carrying packages or lifting moderate-to-heavy objects. Although the traditional weight-training prescription involved performing each exercise 3 times (e.g., 3 sets of 10 – 15 repetitions per set), it appears that 1 set provides similar improvements in muscle strength and endurance, at least for the novice exerciser. Such regimens should include 8 – 10 different exercises at a load that permits 8 – 15 repetitions per set [69].
3.8.5. Lifestyle or incidental physical activity
Randomized controlled trials (RCTs) have shown that an alternative approach to structured exercise, that is, increased lifestyle PA, has similar effects on CRF, body composition, and coronary risk factors as a conventional exercise program [72,73]. More recently, in the Coronary Artery Risk Development in Young Adults (CARDIA) study of 2110 Black and White men and women (aged 38 – 50 yrs) with a mean follow-up of 10.8 years, investigators reported that participants taking ≥7000 steps/day, compared with <7000 steps/day, had a 50% to 70% lower risk of mortality [74]. Interestingly, there was no association of step intensity with mortality. These findings have important implications for public health, suggesting a viable alternative to habitually sedentary individuals who are not ready to comply with a structured exercise regimen.
3.8.6. Marathon running and triathlon participation
Since the early 1970s, increasing numbers of patients have embraced long-distance running and marathon participation in response to the emergence of PA as a possible protective intervention in CHD [75]. However, marathon running and triathlon participation do not necessarily prevent progression of atherosclerotic CVD or sudden cardiac death after AMI [76], [77], [78]. Indeed, autopsy-proved coronary atherosclerosis and structural heart disease, most notably, hypertrophic cardiomyopathy, have been reported in marathon runners and triathletes who died while competing [65,66].
Several considerations preclude the widespread advocacy of marathon running and/or triathlon participation in CR. First, both activities are associated with acute cardiac events each year [65,66]. Second, the associated high-volume, high-intensity training regimens far exceed the threshold necessary for CV conditioning [78]. Third, training for and participation in marathons has limited applicability to patients with CVD. In one study, only 13 of 623 patients (2%) attending an exercise-based CR program subsequently completed ≥1 marathon [79]. Finally, marathon running and triathlon participation pose increased risks of acute cardiac events where cardiopulmonary-resuscitation personnel and equipment may be unavailable [78].
3.9. High-intensity interval training: risks, benefits, and recommendations
Is HIIT appropriate for persons with documented CVD? Interest in this question has resulted in recent clinically-driven investigations, primarily focused on the application to phase II CR. Empirical findings suggest limited evidence that HIIT may provide phase II CR patients with some training advantages over those who participate in the more ‘traditional’ moderate-intensity continuous training (MICT). Within the literature, conventional exercise training has recently been described as MICT to differentiate it from HIIT-based rehabilitation. In summarizing this formative body of literature, it is essential to emphasize the following points:
-
1)
Conclusions should be derived from RCTs that include HIIT and MICT exercise interventions.
-
2)Conclusions must be informed by outcome measures that include:
-
a)Exercise capacity/performance (e.g., maximal and sub-maximal intensity)
-
b)CVD risk factor reduction (e.g., lipid profiles, fasting blood glucose, etc.)
-
c)Metrics of disease recurrence/worsening (e.g., revascularization rates)
-
d)Metrics of morbidity and mortality that are based on long-term follow-up investigations (e.g., 5-year survival rates)
-
e)Conclusions may also include factors related to exercise adherence and patient depression metrics (e.g., PHQ9 surveys and related metrics)
-
a)
-
3)
Conclusions should reflect the current state of the research sub-field, which is in its infancy. This point is evidenced by the fact that, to date, few relevant RCTs comparing HIIT and MICT in CR settings have been conducted. Furthermore, most of these investigations have been conducted in low-risk patients and male-centric populations. Finally, the patient hours represented within the collective body of literature fall short of statistical estimates for anticipated number of observational hours likely needed to elicit acute cardiac events during HIIT [80,81].
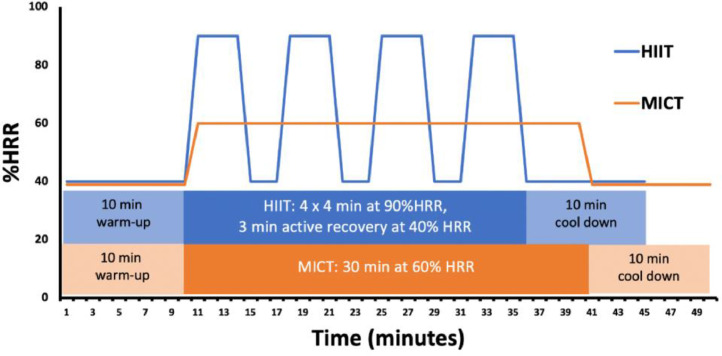
Despite these scientific constraints, the growing body of available research is bolstered by the fact that most HIIT exercise interventions adhere to a fairly homogenous training protocol (Fig. 2). While outliers exist, most existing RCTs utilize 3-4 exercise intervals with durations of 2-4 min. The intensity assigned to HIIT groups is between 90% and 100% of aerobic capacity and quantified relative to HRpeak, HRR, HRmax, or peak power output. The HIIT recovery intervals are 2, 3 min of active recovery, often performed at ∼40% HRR [80].
Fig. 2.
Representative training schedules for HIIT and MICT programs in phase II cardiac rehabilitation. While variations in both HIIT and MICT exist, common approaches include 4 high intensity intervals, interspersed with moderate-intensity active recovery periods. As shown, both HIIT and MICT programs shorten the warm-up and cool-down periods of an equivalent duration. While some programs shorten the warm-up and cool-down duration in the MICT protocol to match the total duration of the corresponding HIIT program, most examples within the literature reflect a time savings for patients assigned to the high-intensity protocol.
Based on these findings, it is important to re-emphasize that due to a lack of data and observation hours in various populations with CVD, it is currently premature to conclude that HIIT is a safe and superior CR intervention compared with MICT. Further supporting this conclusion, few published studies provide a comprehensive assessment of the critical categorical measures outlined previously. Perhaps most notably absent from these essential observations are replicative findings of cardiac function using state-of-the-science techniques for cardiac imaging, differentiating HIIT versus MICT adaptations and outcomes. Moreover, linking these cardiac performance measures to the other outcome variables across multiple populations and risk profiles remains a fundamental barrier to determining HIIT efficacy within cardiac patients. Further limiting scientific consensus on the topic, outcome findings from the existing HIIT-MICT comparisons are largely equivocal for all but one of the variable groupings described previously [80].
Despite the uncertainties surrounding HIIT for outpatient CR, it is reasonable to conclude, based on the existing data, that HIIT offers advantages over MICT relative to improvements in both V̇O2peak and corresponding submaximal exercise performance parameters. Specifically, numerous RCT investigations have reported that HIIT enhances aerobic capacity by an additional 0.5 METs compared with patients assigned to MICT [80]. For cardiac patients that exhibit these incremental improvements in V̇O2peak, the additional increase in aerobic capacity corresponds to statistically significant 8% and 5% decreases in all-cause mortality and CV mortality, respectively [82]. However, these HIIT-related increases in exercise capacity are not universally observed in RCT comparisons of HIIT-MICT. Gains in aerobic capacity in patients who perform HIIT are also tempered by the observation that improvements are comparable to MICT when normalized for exercise-dependent caloric expenditure [83].
Perhaps the most pressing concern about the application of HIIT to cardiac patients is the increased potential to precipitate an exertion-related fatal cardiac event [80,81]. Current preliminary estimates suggest that it takes 23,182 h of high-intensity exercise training in this population to elicit one untoward cardiac event [84]. As indicated earlier, the phase II patient hours collectively observed in the HIIT-MICT trials published to date represent only a fraction of those expected to result in a cardiac event. Moreover, these estimates should be contextualized to the patient risk profile, as defined by contemporary risk stratification criteria. How the risk of HIIT applies to varied CVD diagnoses, including HF patients with preserved or reduced ejection fraction and in those with multiple co-morbidities, are also understudied. Similarly, the benefits of HIIT as applied to under-represented populations, based on sex, race, and ethnicity, are largely unknown and further highlight the need to withhold conclusions about the safety and effectiveness of HIIT as compared to MICT [81].
4. Exercise and type 2 diabetes mellitus
4.1. Physical activity, fitness, and risk of incident diabetes
The burden of DM is increasing worldwide, and an estimated 463 million individuals are currently living with diabetes [85]. The increasing burden of DM is partly due to the growing prevalence of lifestyle related cardiometabolic risk factors such as obesity and physical inactivity. Multiple epidemiological cohort studies have demonstrated the protective association between higher levels of PA and CRF and lower risk of DM [86,87]. In the CARDIA study, higher levels of CRF in young adulthood were associated with a lower risk of DM in middle age [88]. A pooled dose-response meta-analysis of 15 cohort studies reported that a 1-MET higher fitness level was associated with an 8% lower risk of DM [89].
4.2. Fitness change, exercise training, and prevention of diabetes
Changes in CRF levels with aging have been associated with the risk of DM. In the CARDIA cohort, a greater decline in CRF in young adulthood was associated with a greater risk of DM [90]. Several trials have evaluated whether lifestyle interventions can reduce the development of DM among at-risk individuals [91,92]. The Diabetes Prevention Program (DPP) was a seminal RCT that reported a 58% reduction in risk of DM among individuals with pre-DM who underwent an intensive lifestyle intervention (vs. placebo/usual care) that involved substantive changes in dietary intake and regular PA [92]. Over a follow-up period of 12 years, there was a 6% decrease in diabetes incidence per 6 MET-h/week increase in time-dependent PA [93]. Similarly, the Finnish Diabetes Prevention Study (FDPS) also reported a decreased 3-year risk of diabetes by 58% in participants with prediabetes who were randomized to a lifestyle intervention promoting increased MVPA (≥150 min per week) and healthy dietary changes [91,94]. Similar to the DPP trial, the FDPS also demonstrated a 70% reduction in diabetes incidence after adjustment for baseline confounders in individuals who met their PA activity goal (>4 hr-week) but did not meet the accompanying weight loss goal (>5% weight loss) [95]. Another systematic review that included 16 studies reported that diet and PA promotion (vs. usual care) reduced the risk of incident DM (RR: 0.59; 95% CI, 0.52 to 0.66) while also improving other cardiometabolic risk factors [96].
Exercise training interventions also appear to have a legacy effect in preventing DM and insulin resistance. In the STRRIDE-Reunion trial, participants who underwent an 8-month exercise training intervention in middle age had lower fasting insulin levels at 10-year follow-up than their control group counterparts [97]. Greater improvements in CRF with short-term training was associated with higher CRF 10 years later [98]. Collectively, these data suggest that even short-term participation in exercise training may be beneficial in reducing the aging-related cardiometabolic disease burden.
4.3. Physical activity, fitness, and risk of cardiovascular events in DM
CVD is an important downstream complication of DM and accounts for >75% of total mortality among patients with DM [99]. In a pooled analysis from 19 prospective observational cohort studies, physical inactivity in the generalized population was associated with a 24% higher risk of CHD (HR, 1.24; 95% CI, 1.13–1.36) and 42% higher risk of diabetes (HR, 1.42; 95% CI, 1.25–1.61) [100]. Furthermore, previous studies have shown that regular PA plays an important role in preventing macrovascular complications and all-cause mortality in patients with DM. A pooled analysis of 10 population-based cohort studies showed an inverse association of PA with CVD or all-cause mortality in patients with diabetes [101]. Compared with inactive participants, those who achieved less than the recommended activity or met the recommended PA had a 26% and 35% lower all-cause mortality [101]. Similarly, a meta-analysis of 17 studies reported that 1 MET-h/day incrementally higher PA was associated with 9.5% and 7.9% reductions in all-cause mortality and CVD risk, respectively [102]. Accordingly, the American Heart Association (AHA) and American Diabetes Association (ADA) recommend that individuals with DM engage in ≥150 min per week of moderate-intensity aerobic PA or ≥90 min of vigorous aerobic exercise per week to reduce the CVD risk [103].
There are several physiologic mechanisms through which higher PA and CRF levels can lower the risk of atherosclerotic CVD events. First, PA can reduce CV risk by a favorable effect on other coronary risk factors. Higher PA levels are associated with lower levels of atherosclerotic risk factors, including elevated blood pressure, insulin resistance, glucose intolerance, elevated triglyceride concentrations, low HDL, and obesity [104,105]. Another mechanism through which PA can be protective against CVD in DM is through its direct favorable effects on cardiac structure and function. To this end, in a meta-analysis of 6 clinical trials, exercise training in individuals with DM significantly improved several measures of cardiac function, including early diastolic velocity, systolic function as measured by the global longitudinal strain, and CRF signified by increased V̇O2 peak [106].
4.4. Exercise training for the management of DM
Several RCTs have assessed the effects of exercise training on glycemic control and other cardiometabolic parameters in patients with DM. The Look AHEAD trial, a large, randomized trial, evaluated a lifestyle intervention in older adults with DM compared with a diabetes support and education control group [107]. The intensive lifestyle intervention group, which included dietary modification and unsupervised exercise, achieved significantly greater improvements in CRF, blood glucose control, weight loss, blood pressure, and quality of life (QOL) [108]. However, major CV events were similar in both groups, which may be partially explained by the greater use of cardioprotective medications in the diabetes support and education group [107]. Two RCTs, the Diabetes Aerobic and Resistance Exercise (DARE) study and the Health Benefits of Aerobic and Resistance Training in individuals with DM study demonstrated that combined aerobic and resistance training is more effective in improving glycemic control than either exercise intervention alone [109,110]. Moreover, the DARE study also showed that the individual effects of aerobic and resistance training on HBA1C were comparable to the greatest cardiometabolic benefits achieved by the combined exercise training (aerobic +resistance) approach.
Finally, the Italian Diabetes and Exercise Study demonstrated the effectiveness of combined aerobic and resistance training compared to counseling in improving glycemic control [111]. These studies reiterate the importance of incorporating some form of exercise in treatment strategies for individuals with diabetes. Although the treatment for DM commonly involves oral hypoglycemic medication and dietary modification, these methods do not improve CRF, which is crucial to increase exercise tolerance, glycemic control, and survival among individuals with diabetes.
5. Exercise and heart failure
5.1. Physical activity, fitness, and risk of heart failure
Physical inactivity is garnering attention as a major risk factor for the development of HF. Epidemiological studies have consistently linked low levels of PA with increased HF risk. Observational studies have also shown that moderate-to-high levels of PA and/or CRF are associated with a lower risk of HF in a linear, graded fashion [112], [113], [114], [115]. Furthermore, the associations between PA and CRF and HF risk are stronger and more dose-dependent for HF with preserved ejection fraction (HFpEF) than for HF with reduced ejection fraction (HFrEF) [114,116]. Observational studies have also shown that long-term increases in PA and CRF are associated with a reduced risk of HF [114,117]. However, exercise training and lifestyle PA interventions have failed to demonstrate a reduction in the risk of HF in limited RCTs. In the landmark Look AHEAD trial, participants with DM were randomized to an intensive lifestyle change arm vs. standard of care to evaluate whether the former intervention, including exercise training, reduced the risk of CVD [118]. There was no reduction in the risk of HF or its subtypes among Look AHEAD participants that underwent the intensive lifestyle intervention. However, increased CRF over time was associated with a lower risk of HF, suggesting that exercise training resulting in significant, sustained improvement in CRF may favorably modify the risk of HF [114].
5.2. Role of exercise training in patients with heart failure
Among individuals with HF, exercise training may improve QOL, increase exercise capacity, and reduce the risk of HF hospitalization. In the landmark HF-ACTION trial, individuals with stable HFrEF were randomized to supervised aerobic exercise training versus usual care. Although mortality rates were similar in both groups, participants in the intervention arm experienced improved QOL and lower HF hospitalization rates [119]. Based on these encouraging findings, which support the notion that supervised exercise training is beneficial, useful and effective, the current American College of Cardiology (ACC)/AHA guidelines issued a Class I recommendation for exercise-based CR in patients with HFrEF.
Despite the existing guidelines that support CR, the enrollment and participation in CR among patients with HFrEF remains very low [119]. The Centers for Medicare and Medicaid Services expanded their coverage for CR reimbursement to include patients with chronic stable HFrEF in 2014. However, since then, the referral and enrollment rates for CR among patients with HFrEF have remained at <5% [119]. This implementation gap highlights the need for novel approaches to CR for patients with HFrEF. Such approaches could include early initiation of exercise or physical conditioning interventions and the transition from supervised CR to home-based exercise training. Accordingly, the recent REHAB-HF trial demonstrated the feasibility, safety, and efficacy of an early, progressive rehabilitation intervention that included multiple physical-function domains. It was initiated during the in-hospital stay of patients with acute decompensated HF and maintained at home [120]. The physical function intervention was associated with significant improvement in functional status, frailty burden, and QOL. Future studies evaluating the efficacy of such pragmatic, multi-domain physical function interventions to prevent recurrent HF hospitalizations and early mortality in patients with HF are needed, especially considering the relatively high prevalence of non-responders to exercise training in this patient subset.
Whether exercise training modifies the risk of adverse outcomes among patients with HFpEF is less well-established [121]. In a pooled analysis of RCTs evaluating the effect of exercise on adverse outcomes among patients with HFpEF, exercise training was associated with improvement in exercise capacity and QOL [122]. Furthermore, the optimal type of exercise training among patients of HFpEF remains unclear. In a recent study, investigators evaluated whether HIIT was associated with greater improvement in exercise capacity among patients with HFpEF compared with MICT or PA counseling [123,124]. Investigators reported that the improvement in exercise capacity was comparable with MICT vs. HIIT. Future studies with larger sample sizes and longer follow-up are needed to evaluate the optimal exercise training regimen for patients with HFpEF to improve clinical outcomes.
6. Exercise and peripheral arterial disease (PAD)
6.1. Physical activity, fitness, and risk of PAD
PAD affects >200 million people worldwide and is defined as an ankle-brachial index (ABI) ≤0.9 [125]. Traditional risk factors for atherosclerotic CVD such as cigarette smoking, DM, age, and renal insufficiency play a prominent role in the development of PAD. However, the impact of regular PA and improved CRF on the prevention and treatment of PAD has recently been reported. Collins et al. found that low levels of self-reported PA in African Americans were associated with an increased risk of PAD as assessed by ABI <0.9 [126]. Wilson et al. demonstrated that lifetime recreational activity positively correlated to ABI, and reduced PA was associated with prevalent PAD [127]. More recently, using the MESA cohort database, which included community-dwelling adults without clinically evident CVD, investigators reported that higher self-reported intentional exercise was protective for incident PAD (assessed by ABI) over a relatively short follow-up (∼3 years) [128]. Besides self-reported PA behavior, objective measures of sedentary time—assessed using an accelerometer—are also associated with low ABI values in an asymptomatic population [129].
6.2. Prognostic roles of PA and CRF in PAD
Intermittent claudication is the most common clinical manifestation of PAD and is defined as exertional calf pain that occurs during exercise and resolves within 10 min of rest. Notably, the pathophysiologic basis of the functional limitation in PAD is incompletely understood and includes both anatomic and vascular abnormalities. Due to fixed stenosis, heightened metabolic demands from increased lower extremity muscle contraction are unable to be met, leading to muscle ischemia. Concomitantly, endothelial dysfunction leads to impaired vasodilation of calf resistance vessels in the microcirculation [130]. Lastly, inflammation, nerve impairment, and calf muscle dysfunction all contribute to the functional limitations in PAD [131].
Evaluating the magnitude of functional impairment is a critical component of clinical care for patients with PAD, as claudication can have significant adverse effects on ambulation and QOL. Importantly, functional impairment is present even in patients who do not have exertional leg symptoms [132]. Patients with claudication also demonstrate significantly reduced V̇O2 peak than those without PAD [133]. The impaired functional capacity has prognostic value in PAD with higher rates of all-cause and CV mortality [134,135].
6.3. Exercise training and PA interventions in the management of PAD
Treatment of patients with PAD focuses on reducing CV events and symptom burden of the lower extremities. Exercise training is a critical non-invasive treatment modality to improve QOL, as well as pleiotropic physiologic effects with reported reductions in inflammatory markers, increases in calf muscle capillary density, and a partial restoration in peripheral vasodilation [136]. Treadmill-based supervised exercise training (SET) is highly effective in patients with and without claudication. An early meta-analysis based on nonrandomized studies provided a foundation for intermittent treadmill exercise therapy for PAD [137]. A subsequent meta-analysis of 25 RCTs substantiated significant improvements in claudication onset time and symptom-limited peak walking duration [138].
SET has also been evaluated relative to endovascular therapy per se for the treatment of claudication. Spronk et al. compared a 24-week SET intervention to balloon angioplasty followed by stent use, if indicated, and demonstrated greater improvement with SET than endovascular therapy alone [139]. These findings were corroborated in a network meta-analysis (180 m improvement with SET vs. 85 m improvement with endovascular therapy) [140]. Additionally, the IRONIC trial compared revascularization versus SET in addition to optimal medical therapy, and initial follow-up at 1- and 2-years reported improved health-related QOL only in the revascularization group. However, after 5-years of follow-up, the revascularization strategy versus SET plus optimal medical therapy yielded comparable improvements in health-related QOL [141]. In the recent CLEVER trial, SET and endovascular therapy, when combined with optimal medical therapy, were associated with greater improvements in functional outcomes compared with optimal medical therapy alone with no difference in therapeutic efficacy between SET vs. endovascular therapy [142]. Combined SET plus endovascular therapy has also been evaluated and appears to offer the greatest benefit in functional outcomes. In a pooled analysis, patients treated with SET and endovascular therapy as compared to SET alone had higher maximum walk distance (weighted mean difference 98.9 feet) and lower risk of revascularization or amputation (odds ratio 0.09, 95% confidence interval: 0.40) [143].
Prescribing a supervised exercise program involves counseling the patient with PAD to walk until the onset of claudication, followed by time for recovery [144]. Current recommendations suggest a frequency of 3 sessions per week and a program duration of ≥12 weeks, although implementation is highly variable [145]. The optimal intensity of exercise training for patients with PAD remains controversial. Studies have shown that intensity of training may be related to the improvement in V̇O2peak but not walking distance. A systemic review and meta-analysis of exercise training trials in PAD demonstrated a significantly greater improvement in exercise capacity with HIIT vs. MICT [146].
Despite being a Class I ACC/AHA recommendation, patient referrals and participation in SET for patients with PAD remain low. In a recent analysis of Medicare beneficiaries with intermittent claudication, only 1.3% were enrolled in SET between 2017 and 2018 [147]. Even in the trial setting, up to 70% of eligible patients with PAD decline participation in SET interventions. To combat these barriers to participation, more accessible alternatives such as home-based exercise therapy have been suggested. Evidence for the efficacy of home-based exercise or PA behavioral interventions for improving exercise capacity in patients with PAD is equivocal. Some trials have demonstrated a significant improvement in functional capacity with home-based exercise training [148,149], whereas others have not [150,151]. More recently, home-based exercise intervention with high-intensity exercise training has been shown to significantly improve 6-min walk distance compared with low-intensity training and a non-exercise control group [152]. Furthermore, the effect of low-intensity training on 6-min walk distance was comparable to that of the non-exercise control group. The current ACC/AHA guidelines provide a class IIA recommendation for home-based exercise training as a viable treatment option in patients with PAD [145]. Based on the existing evidence, home-based interventions that incorporate high-intensity training and include occasional in-person meetings with an exercise coach may be most effective for improving functional outcomes [153].
In conclusion, a low level of PA is a known risk factor for the development of PAD. Additionally, a reduced exercise capacity in patients with PAD is associated with worse clinical outcomes. SET is a cornerstone treatment for symptomatic PAD to improve walking ability and overall QOL. However, many patients are not able to access SET, and novel alternatives, such as home-based therapy, are needed to combat the growing burden of symptomatic PAD.
7. Clinical recommendations
-
•
Each 1 MET increase in CRF confers an ∼16% decrease in mortality, which compares favorably with survival benefit provided by aspirin, statins, β-blockers, and angiotensin-converting enzyme inhibitors after AMI.
-
•
The concept of MET-minutes per week enables clinicians and patients to translate guideline-driven MVPA recommendations (≥500 to 1,000 MET-minutes per week) into achievable goals by quantifying accumulated exercise each week in a single formula: METs per activity x number of minutes/session x days/week = MET-minutes per week.
-
•
For previously inactive patients embarking on a physical conditioning program who have not undergone exercise testing, the resting HR (standing) plus 10 to 20 bpm is recommended for the initial exercise intensity, using symptomatology and RPE (≤ “somewhat hard”) as adjunctive intensity modulators.
-
•
Any duration of PA, even 1 – 2 min bouts, accrued over time, can elicit CV and metabolic health benefits.
-
•
Vigorous exercise appears to be more effective than moderate-intensity exercise in reducing CV risk. Similarly, when comparing increasing percentiles of self-reported PA versus CRF, the reductions in risk are greater for CRF.
-
•
MVPA, which corresponds to any activity ≥3 METs, has been consistently shown to reduce the health risks associated with numerous chronic diseases. This also signifies the “threshold” training intensity that allows individuals to emerge from the least fit, least active population cohort, or bottom 20%, which appears to confer the greatest relative reduction in mortality.
-
•
Little additional survivor benefit occurs when CRF levels increase from “good” to “excellent,” suggesting a plateau in the reduced relative risk for CVD. Accordingly, the optimal CV benefits of exercise are most likely to be achieved by the gradual progression of exercise intensity, that is, to attain the age-/sex-recommended training MET levels likely to achieve “good” CRF.
-
•
For deconditioned or inactive individuals, the minimum or threshold intensity for improving CRF is ∼30-45% V̇O2R, corresponding to ∼60-70% of the highest HR achieved during peak or symptom-limited exercise testing.
-
•
Level walking at 2 and 3 mph approximates 2 and 3 METs, respectively. At a 2-mph speed, each 3.5% grade increment adds an additional MET to the gross energy expenditure. For the 3-mph pace, each 2.5% increase in treadmill grade adds an additional MET.
-
•
A simple method for estimating oxygen uptake during PA, expressed as METs, employs the resting and exercise heart rates using the heart rate index equation: METs = (6 x Heart Rate Index) —5, where the heart rate index equals the activity heart rate divided by the resting heart rate.
-
•
Because resistance training is comparable or superior to endurance training in enhancing bone mineral density, muscle mass and strength, insulin sensitivity, and basal metabolism, it should be recommended to complement any physical conditioning program.
-
•
RCTs have shown that an alternative approach to structured exercise, that is, increased lifestyle PA, provides similar beneficial health outcomes. Accordingly, clinicians should counsel patients to integrate increased PA into daily living.
-
•
Although numerous studies purport that HIIT elicits slightly greater increases in CRF (by ∼0.5 MET) than MICT, while simultaneously providing a less time-consuming training alternative, concerns regarding the safety of repeated near-maximal exercise bouts in middle-aged and older patients with known or suspected CHD suggest that it should be cautiously prescribed or proscribed, especially in unsupervised, nonmedical settings.
-
•
Supervised and home-based exercise programs provide independent and additive benefits to comprehensive medical interventions aimed at preventing and treating medically stable patients with PAD, DM, and HF, or combinations thereof.
7.1. Exercise interventions: controversial issues and special considerations
Three relevant topics that merit brief discussion in this 2-part scientific statement include: medical screening of patients prior to their embarking on MVPA regimens; using technology to promote and reinforce PA programs; and research-based counseling strategies to enhance initiation of and adherence to structured exercise and/or increased lifestyle PA.
The value of adjunctive medical screening procedures, including physician evaluation, with or without exercise testing, as a preface to MVPA, remains controversial. The US Preventive Services Task 2018 recommendations advised against routine screening with exercise testing to prevent CV events [154], including higher-risk populations with DM [155]. Asymptomatic patients who might benefit from exercise testing before beginning a PA program, especially if vigorous exercise is contemplated, include previously sedentary individuals with multiple risk factors, an elevated coronary artery calcium score, a family history of premature CHD, or those whom the clinician suspects may be ignoring symptoms or not giving an accurate history.
Digital tools such as mobile games on smartphones and tablets, various apps that promote PA, and activity trackers may reduce barriers to regular PA, increase access to fitness programs, and provide daily goal reminders [22]. Self-monitoring techniques or devices (e.g., pedometers, accelerometers, PAI, HR monitors) can be helpful in this regard. Active-play video gaming [156], with reported aerobic requirements of ∼1.5-5.6 METs, corresponding to slow, moderate, and extremely fast walking speeds, can also be used to meet daily or weekly PA requirements and serve as a gateway to structured exercise regimens [157]. Collectively, these data suggest that using technology, a contributor to the physical inactivity epidemic, can also be part of the solution.
Finally, research-based counseling strategies should be used to facilitate healthy behavior change, including initiating and complying with a structured exercise program, increased lifestyle PA, or both [158], [159], [160]. These include: assessing patient readiness to change; the 5A's approach to behavior modification [158,160]; motivational interviewing, and overcoming inertia with downscaled goals. Accordingly, patients need to realize that they have the single greatest influence over their destiny relative to wellness and health promotion [161]. An enduring axiom of success in the field of personal achievement states, “The universe rewards action.” This also represents a key tenet underlying successful lifestyle modification and attaining salutary health outcomes.
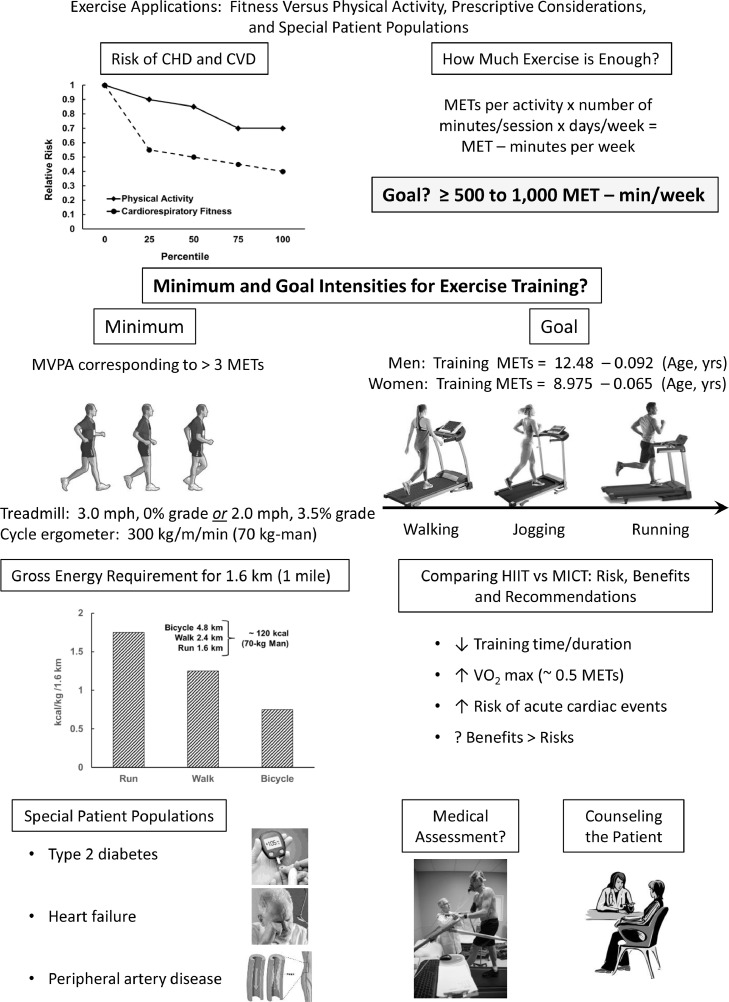
Key summary points on part II of this scientific statement are highlighted in the Central Figure (Fig. 3), with specific reference to the relations between PA, CRF and CVD, the recommended exercise dosage expressed as MET-min/week, minimum and goal intensities for exercise training, energy requirement for walking vs. running vs. outdoor bicycling, the comparative benefits and risks of HIIT vs. MICT, and the role of exercise training for special patient populations. Related medical assessment for exercise participation and counseling strategies are also discussed, including the use of technology to promote PA.
Fig. 3.
Central Figure, summarizing the key relations between increasing levels of physical activity, cardiorespiratory fitness, and risk of cardiovascular disease, prescriptive considerations, and special patient populations, with specific reference to medical assessments and patient counseling.
8. Conclusions
Although physical inactivity represents a leading cause of death worldwide [162], the beneficial effects of structured exercise and/or increased lifestyle PA are often underestimated by many clinicians and the public at large. Consequently, the health burden of physical inactivity continues to grow with technologic advances, suboptimal community landscape planning, and inadequate emphasis during most clinical encounters. The latter represents missed opportunities to counsel individuals using proven behavioral interventions to combat our increasingly hypokinetic environment [163].
Behavioral lifestyle choices are consistently reported to be the single greatest determinant of premature death, approximating all other health modulators combined [161]. Indeed, common characteristics of nonagenarians and centenarians in 3 widely separated regions of the world (Sardinians, Adventists, and Okinawans) include daily PA [164]. It has been suggested that “a prescription to walk 30 min per day could be one of the most important prescriptions a patient could receive” [165]. Clinicians and allied health professionals play a trusted and influential role in counseling their patients to be more physically active. These efforts should be complemented by making self-responsibility (e.g., meeting certain incentivized health metrics such as regular MVPA) a greater priority in the evolving health care coverage environment [166].
The prescription of exercise has become increasingly scientific. Guidelines are available regarding the appropriate intensity, frequency, and duration of training. Moreover, detailed attention has focused on the methods underlying the target HR derivation, prescribed training intensities (METs), adjunctive intensity modulators (e.g., RPE), continuous versus interval training, and the merits and limitations of selected exercise modalities. However, the key beneficiary is often overlooked ― the patient. Consequently, maintaining the commitment can be challenging, leading to a decline in exercise adherence and effectiveness.
Exercise recommendations should additionally consider several commonsense questions. Can the patient accomplish the exercise prescription without feeling exhausted? Can the prescribed exercise intensity be attained realistically and comfortably without adverse signs or symptoms? Are patients advised to adjust arbitrary HR and workload recommendations, including prescribed exercise intensities (METs), according to the RPE and symptomatology? Are they counseled to “listen to their body” as an adjunct intensity guide? Are the recommended activities perceived by the patient as invigorating and/or enjoyable?
When used in conjunction with cardioprotective medications, regular MVPA provides independent and additive cardiovascular and survival benefits [167]. Accordingly, for the vast majority of patients who are not physically active, the prescription remains unfilled.
Declaration of Competing Interest
The authors have no conflicts to report relative to this work.
Acknowledgment
The authors would like to thank Brenda White for her assistance with the preparation, formatting, and serial revisions of this scientific statement (part II), diligently checking the placement and accuracy of our citations.
References
- 1.Boden WE, Franklin BA, Wenger NK. Physical activity and structured exercise for patients with stable ischemic heart disease. JAMA. 2013;309(2):143–144. doi: 10.1001/jama.2012.128367. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Blair SN, Arena R, et al. on behalf of the American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 3.Franklin BA, Eijsvogels TMH, Pandey A, Quindry J, Toth PP. Physical activity, cardiorespiratory fitness, and cardiovascular health. A Clinical Practice Statement of the American Society for Preventive Cardiology, Part I. Am J Prev Cardiol (In press). [DOI] [PMC free article] [PubMed]
- 4.De Schutter A, Kachur S, Lavie CJ, et al. Cardiac rehabilitation fitness changes and subsequent survival. Eur Heart J Qual Care Clin Outcomes. 2018;4(3):173–179. doi: 10.1093/ehjqcco/qcy018. [DOI] [PubMed] [Google Scholar]
- 5.Carbone S, Kim Y, Kachur S, et al. Peak oxygen consumption achieved at the end of cardiac rehabilitation predicts long-term survival in patients with coronary heart disease. Eur Heart J Qual Care Clin Outcomes. 2022;8(3):361–367. doi: 10.1093/ehjqcco/qcab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nes BM, Gutvik CR, Lavie CJ, Nauman J, Wisløff U. Personalized activity intelligence (PAI) for prevention of cardiovascular disease and promotion of physical activity. Am J Med. 2017;130(3):328–336. doi: 10.1016/j.amjmed.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Kieffer SK, Zisko N, Coombes JS, Nauman J, Wisløff U. Personal activity intelligence and mortality in patients with cardiovascular disease: the HUNT study. Mayo Clin Proc. 2018;93(9):1191–1201. doi: 10.1016/j.mayocp.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Nauman J, Sui X, Lavie CJ, et al. Personal activity intelligence and mortality – Data from the Aerobics Center Longitudinal Study. Prog Cardiovasc Dis. 2021;64:121–126. doi: 10.1016/j.pcad.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Nauman J, Franklin BA, Nes BM, et al. Association between personal activity intelligence and mortality: Population-based China Kadoorie Biobank Study. Mayo Clin Proc. 2022;97(4):668–681. doi: 10.1016/j.mayocp.2021.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health. 1987;8:253–287. doi: 10.1146/annurev.pu.08.050187.001345. [DOI] [PubMed] [Google Scholar]
- 11.Nocon M, Hiemann T, Müller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15(3):239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138(4):345–355. doi: 10.1161/CIRCULATIONAHA.117.032047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair SN, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- 14.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106(6):666–671. doi: 10.1161/01.cir.0000024413.15949.ed. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh T, Mertens DJ, Hamm LF, et al. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol. 2003;42(12):2139–2143. doi: 10.1016/j.jacc.2003.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Kokkinos P, Faselis C, Samuel IBH, et al. Cardiorespiratory fitness and mortality risk across the spectra of age, race, and sex. J Am Coll Cardiol. 2022;80(6):598–609. doi: 10.1016/j.jacc.2022.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Kokkinos P, Narayan P, Myers J, Franklin B. Cardiorespiratory fitness and the incidence of chronic disease. J Clinic Exerc Physiol. 2018;7(2):37–45. [Google Scholar]
- 18.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33(5):754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers J, Kaykha A, George S, et al. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004;117(12):912–918. doi: 10.1016/j.amjmed.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 20.Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonized meta-analysis. BMJ. 2019;366:I4570. doi: 10.1136/bmj.I4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekelund U, Dalene KE, Tarp J, Lee I-M. Physical activity and mortality: what is the dose response and how big is the effect? Br J Sports Med. 2020;54(19):1125–1126. doi: 10.1136/bjsports-2019-101765. [DOI] [PubMed] [Google Scholar]
- 22.Chaddha A, Jackson EA, Richardson CR, Franklin BA. Technology to help promote physical activity. Am J Cardiol. 2017;119(1):149–153. doi: 10.1016/j.amjcard.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine . 142-166. Wolters Kluwer; PhiladelphiaPA: 2021. pp. 226–275. (ACSM's Guidelines for Exercise Testing and Prescription). [Google Scholar]
- 25.Franklin BA, Billecke S. Putting the benefits and risks of aerobic exercise in perspective. Curr Sports Med Rep. 2012;11(4):201–208. doi: 10.1249/JSR.0b013e31825dabd4. [DOI] [PubMed] [Google Scholar]
- 26.Barnard RJ, Gardner GW, Diaco NV, MacAlpin RN, Kattus AA. Cardiovascular responses to sudden strenuous exercise — heart rate, blood pressure, and ECG. J Appl Physiol. 1973;34(6):833–837. doi: 10.1152/jappl.1973.34.6.833. [DOI] [PubMed] [Google Scholar]
- 27.Dimsdale JE, Hartley LH, Guiney T, Ruskin JN, Greenblatt D. Postexercise peril. Plasma catecholamines and exercise. JAMA. 1984;251(5):630–632. doi: 10.1001/jama.251.5.630. [DOI] [PubMed] [Google Scholar]
- 28.Haskell WL. Cardiovascular complications during exercise training of cardiac patients. Circulation. 1978;57(5):920–924. doi: 10.1161/01.cir.57.5.920. [DOI] [PubMed] [Google Scholar]
- 29.Swain DP, Franklin BA. VO2 reserve and the minimal intensity for improving cardiorespiratory fitness. Med Sci Sports Exerc. 2002;34(1):152–157. doi: 10.1097/00005768-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Swain DP, Franklin BA. Is there a threshold intensity for aerobic training in cardiac patients? Med Sci Sports Exerc. 2002;34(7):1071–1075. doi: 10.1097/00005768-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35(3):307–315. [PubMed] [Google Scholar]
- 32.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhu N, Suarez-Lopez JR, Sidney S, et al. Longitudinal examination of age-predicted symptom-limited exercise maximum HR. Med Sci Sports Exerc. 2010;42(8):1519–1527. doi: 10.1249/MSS.0b013e3181cf8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz C Noel, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: the St. James Women Take Heart Project. Circulation. 2010;122(2):130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- 35.Pratt CM, Welton DE, Squires WG, Jr, Kirby TE, Hartung GM, Miller RR. Demonstration of training effect during chronic beta-adrenergic blockade in patients with coronary artery disease. Circulation. 1981;64(6):1125–1129. doi: 10.1161/01.cir.64.6.1125. [DOI] [PubMed] [Google Scholar]
- 36.Vanhees L, Fagard R, Amery A. Influence of beta-adrenergic blockade on the hemodynamic effects of physical training in patients with ischemic heart disease. Am Heart J. 1984;108(2):270–275. doi: 10.1016/0002-8703(84)90611-2. [DOI] [PubMed] [Google Scholar]
- 37.Froelicher V, Sullivan M, Myers J, Jensen D. Can patients with coronary artery disease receiving beta blockers obtain a training effect? Am J Cardiol. 1985;55(10):155D–161D. doi: 10.1016/0002-9149(85)91073-2. [DOI] [PubMed] [Google Scholar]
- 38.Davis JA, Convertino VA. A comparison of heart rate methods for predicting endurance training intensity. Med Sci Sports. 1975;7(4):295–298. [PubMed] [Google Scholar]
- 39.Swain DP, Leutholtz BC, King ME, Haas LA, Branch JD. Relationship between % heart rate reserve and % VO2 reserve in treadmill exercise. Med Sci Sports Exerc. 1998;30(2):318–321. doi: 10.1097/00005768-199802000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 41.Keteyian SJ, Ehrman JK, Fuller B, Pack QR. Exercise testing and exercise rehabilitation for patients with atrial fibrillation. J Cardiopulm Rehabil Prev. 2019;39(2):65–72. doi: 10.1097/HCR.0000000000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ainsworth BE, Haskell WL, Herrmann SD, et al. Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. 2011. [DOI] [PubMed] [Google Scholar]
- 43.Franklin BA, Brinks J, Berra K, Lavie CJ, Gordon NF, Sperling LS. Using metabolic equivalents in clinical practice. Am J Cardiol. 2018;121(3):382–387. doi: 10.1016/j.amjcard.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 44.Byrne NM, Hills AP, Hunter GR, Weinsier RL, Schutz Y. Metabolic equivalent: one size does not fit all. J Appl Physiol. 2005;99(3):1112–1119. doi: 10.1152/japplphysiol.00023.2004. [DOI] [PubMed] [Google Scholar]
- 45.Lavie CJ, Milani RV. Metabolic equivalent (MET) inflation — not the MET we used to know. J Cardiopulm Rehabil Prev. 2007;27(3):149–150. doi: 10.1097/01.HCR,0000270692.09258.6a. [DOI] [PubMed] [Google Scholar]
- 46.Wicks JR, Oldridge NB, Nielsen LK, Vickers CE. HR index — a simple method for the prediction of oxygen uptake. Med Sci Sports Exerc. 2011;43(10):2005–2012. doi: 10.1249/MSS.0b013e318217276e. [DOI] [PubMed] [Google Scholar]
- 47.Myers J. Human Kinetics; Champaign, IL: 2021. Physical activity and exercise. In: Guidelines for cardiac rehabilitation programs; pp. 61–68. [Google Scholar]
- 48.Paterson DH, Cunningham DA, Koval JJ, St Croix CM. Aerobic fitness in a population of independently living men and women aged 55-86 years. Med Sci Sports Exerc. 1999;31(12):1813–1820. doi: 10.1097/00005768-19912000-00018. [DOI] [PubMed] [Google Scholar]
- 49.Gaskill SE, Walker AJ, Serfass RA, et al. Changes in ventilatory threshold with exercise training in a sedentary population: the HERITAGE Family Study. Int J Sports Med. 2001;22(8):586–592. doi: 10.1055/s-2001-18522. [DOI] [PubMed] [Google Scholar]
- 50.Squires RW, Kaminsky LA, Porcari JP, Ruff JE, Savage PD, Williams MA. Progression of exercise training in early outpatient cardiac rehabilitation: an official statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2018;38(3):139–146. doi: 10.1097/HCR.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 51.Haskell WL, Lee I-M, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 52.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97(1):141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 53.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 54.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, KH Cooper, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 55.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation. 2003;108(13):1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 56.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 57.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JD. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 58.Franklin BA, Arena R, Kaminsky LA, Peterman JE, Kokkinos P, Myers J. Maximizing the cardioprotective benefits of exercise with age-, sex-, and fitness-adjusted target intensities for training. Eur J Prevent Cardiol. 2022;29(1):e1–e3. doi: 10.1093/eurjpc/zwaa094. [DOI] [PubMed] [Google Scholar]
- 59.Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc. 2015;90(11):1515–1523. doi: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollock ML, Gettman LR, Milesis CA, Bah MD, Durstine L, Johnson RB. Effects of frequency and duration of training on attrition and incidence of injury. Med Sci Sports. 1977;9(1):31–36. [PubMed] [Google Scholar]
- 61.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 62.Fan JX, Brown BB, Hanson H, Kowaleski-Jones L, Smith KR, Zick CD. Moderate to vigorous physical activity and weight outcomes: does every minute count? Am J Health Promot. 2013;28(1):41–49. doi: 10.4278/ajhp.120606-QUA-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glazer NL, Lyass A, Esliger DW, et al. Sustained and shorter bouts of physical activity are related to cardiovascular health. Med Sci Sports Exerc. 2013;45(1):109–115. doi: 10.1249/MSS.0b013e31826beae5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beddhu S, Wei G, Marcus RL, Chonchol M, Greene T. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin J Am Soc Nephrol. 2015;10(7):1145–1153. doi: 10.2215/CJN.08410814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JH, Malhotra R, Chiampas G, et al. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366(2):130–140. doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- 66.Harris KM, Creswell LL, Haas T, et al. Death and cardiac arrest in U.S. triathlon participants, 1985 to 2016: a case series. Ann Intern Med. 2017;167(8):529–535. doi: 10.7326/M17-0847. [DOI] [PubMed] [Google Scholar]
- 67.Franklin BA, Thompson PD, Al-Zaiti SS, et al. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective – an update. A Scientific Statement from the American Heart Association. Circulation. 2020;141(13):e705–e736. doi: 10.1161/CIR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 68.Franklin BA, Rubenfire M. Losing weight through exercise. JAMA. 1980;244(4):377–379. [PubMed] [Google Scholar]
- 69.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;117(5):572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 70.McCartney N, McKelvie RS, Martin J, Sale DG, MacDougall JD. Weight-training-induced attenuation of the circulatory response of older males to weight lifting. J Appl Physiol. 1993;74(3):1056–1060. doi: 10.1152/jappl.1993.74.3.1056. [DOI] [PubMed] [Google Scholar]
- 71.Grafe K, Bendick P, Burr M, Boura J, Franklin BA. Effects of resistance training on vascular and hemodynamic responses in patients with coronary artery disease. Res Q Exerc Sport. 2018;89(4):457–464. doi: 10.1080/02701367.2018.1519385. [DOI] [PubMed] [Google Scholar]
- 72.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, 3rd, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281(4):327–334. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- 73.Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281(4):335–340. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 74.Paluch AE, Gabriel KP, Fulton JE, et al. Steps per day and all-cause mortality in middle-aged adults in the Coronary Artery Risk Development in Young Adults Study. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kavanagh T, Shephard RH, Pandit V. Marathon running after myocardial infarction. JAMA. 1974;229(12):1602–1605. [PubMed] [Google Scholar]
- 76.Noakes TD, Opie LH, Rose AG, Kleynhans PH, Schepers NJ, Dowdeswell R. Autopsy-proved coronary atherosclerosis in marathon runners. N Engl J Med. 1979;301(2):86–89. doi: 10.1056/NEJM197907123010205. [DOI] [PubMed] [Google Scholar]
- 77.Handler JB, Asay RW, Warren SE, Shea PM. Symptomatic coronary artery disease in a marathon runner. JAMA. 1982;248(6):717–719. [PubMed] [Google Scholar]
- 78.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: anatomic and physiologic determinants. Ann NY Acad Sci. 1977;301:484–494. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 79.Kavanagh T, Shephard RJ, Kennedy J. Characteristics of postcoronary marathon runners. Ann NY Acad Sci. 1977;301:455–465. doi: 10.1111/j.1749-6632.1977.tb38222.x. [DOI] [PubMed] [Google Scholar]
- 80.Quindry JC, Franklin BA, Chapman M, Humphrey R, Mathis S. Benefits and risks of high-intensity interval training in patients with coronary artery disease. Am J Cardiol. 2019;123(8):1370–1377. doi: 10.1016/j.amjcard.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Franklin BA, Quindry J. High level physical activity in cardiac rehabilition: Implications for exercise training and leisure-time pursuits. Prog Cardiovasc Dis. 2021 doi: 10.1016/j.pcad.2021.12.005. Dec29;S0033-0620(21)00137-7. [DOI] [PubMed] [Google Scholar]
- 82.Martin BJ, Arena R, Haykowsky M, Hauer T, Austford LD, Knudtson M, Aggarwal S, Stone JA, Investigators APPROACH. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc. 2013;88(5):455–463. doi: 10.1016/j.mayocp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Gomes-Neto M, Duräes AR, Correia dos Reis HF, Neves VR, Martinez BP, Carvalho VO. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24(16):1696–1707. doi: 10.1177/2047487317728370. [DOI] [PubMed] [Google Scholar]
- 84.Rognmo O, Moholdt T, Bakken H, Hole T, Mølstad Myhr NE, Grimsmo J, Wisløff U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126(12):1436–1440. doi: 10.1161/CIRCULATIONAHA.112.123117. [DOI] [PubMed] [Google Scholar]
- 85.Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108072. [DOI] [PubMed] [Google Scholar]
- 86.Laaksonen DE, Lindström J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes. Diabetes. 2005;54(1):158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 87.Carr DB, Utzschneider KM, Boyko EJ, et al. A reduced-fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra-abdominal fat and improves insulin sensitivity but not β-cell function. Diabetes. 2005;54(2):340–347. doi: 10.2337/diabetes.54.2.340. [DOI] [PubMed] [Google Scholar]
- 88.Chow LS, Odegaard AO, Bosch TA, et al. Twenty year fitness trends in young adults and incidence of prediabetes and diabetes: the CARDIA study. Diabetologia. 2016;59(8):1659–1665. doi: 10.1007/s00125-016-3969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarp J, Støle AP, Blond K, Grøntved A. Cardiorespiratory fitness, muscular strength and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetologia. 2019;62(7):1129–1142. doi: 10.1007/s00125-019-4867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carnethon MR, Sternfeld B, Schreiner PJ, et al. Association of 20-year changes in cardiorespiratory fitness with incident type 2 diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Fitness Study. Diabetes Care. 2009;32(7):1284–1288. doi: 10.2337/dc08-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lindström J, Peltonen M, Eriksson JG, et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologia. 2013;56(2):284–293. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- 92.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kriska AM, Rockette-Wagner B, Edelstein SL, et al. The impact of physical activity on the prevention of type 2 diabetes: Evidence and lessons learned from the Diabetes Prevention Program, a long-standing clinical trial incorporating subjective and objective activity measures. Diabetes Care. 2021;44(1):43–49. doi: 10.2337/dc20-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindström J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26(12):3230–3236. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 95.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 96.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: A systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):437–451. doi: 10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson JL, Slentz CA, Ross LM, Huffman KM, Kraus WE. Ten-year legacy effects of three eight-month exercise training programs on cardiometabolic health parameters. Front Physiol. 2019;10:452. doi: 10.3389/fphys.2019.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pandey A, Johnson JL, Slentz CA, et al. Short-term changes in cardiorespiratory fitness in response to exercise training and the association with long-term cardiorespiratory fitness decline: the STRRIDE Reunion Study. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005;28(4):799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- 100.Kivimäki M, Singh-Manoux A, Pentti J, et al. Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ. 2019;365:I1495. doi: 10.1136/bmj.I1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sadarangani KP, Hamer M, Mindell JS, Coombs NA, Stamatakis E. Physical activity and risk of all-cause and cardiovascular disease mortality in diabetic adults from Great Britain: pooled analysis of 10 population-based cohorts. Diabetes Care. 2014;37:1016–1023. doi: 10.2337/dc13-1816. [DOI] [PubMed] [Google Scholar]
- 102.Kodama S, Tanaka S, Heianza Y, et al. Association between physical activity and risk of all-cause mortality and cardiovascular disease in patients with diabetes: a meta-analysis. Diabetes Care. 2013;36(2):471–479. doi: 10.2337/dc12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30(1):162–172. doi: 10.2337/dc07.9917. [DOI] [PubMed] [Google Scholar]
- 104.Thompson PD, Buchner D, Piña IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 105.Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33(6 Suppl):S484–S492. doi: 10.1097/00005768-200106001-00018. [DOI] [PubMed] [Google Scholar]
- 106.Anand V, Garg S, Garg J, Bano S, Pritzker M. Impact of exercise training on cardiac function among patients with type 2 diabetes: A SYSTEMATIC REVIEW AND META-ANALYSIS. J Cardiopulm Rehabil Prev. 2018;38(6):358–365. doi: 10.1097/HCR.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 107.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pi-Sunyer X. The Look AHEAD Trial: a review and discussion of its outcomes. Curr Nutr Rep. 2014;3(4):387–391. doi: 10.1007/s13668-014-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 110.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304(20):2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balducci S, Zanuso S, Nicolucci A, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170(20):1794–1803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- 112.Pandey A, Garg S, Khunger M, et al. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation. 2015;132(19):1786–1794. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 113.Berry JD, Pandey A, Gao A, et al. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6(4):627–634. doi: 10.1161/CIRCHEARTFAILURE.112.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pandey A, Patel KV, Bahnson JL, et al. Association of intensive lifestyle intervention, fitness, and body mass index with risk of heart failure in overweight or obese adults with type 2 diabetes mellitus: an analysis from the Look AHEAD Trial. Circulation. 2020;141(16):1295–1306. doi: 10.1161/CIRCULATIONAHA.119.044865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pandey A, Patel KV, Vaduganathan M, et al. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6(12):975–982. doi: 10.1016/j.jchf.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69(9):1129–1142. doi: 10.1016/j.jacc.2016.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Florido R, Kwak L, Lazo M, et al. Six-year changes in physical activity and the risk of incident heart failure: ARIC Study. Circulation. 2018;137(20):2142–2151. doi: 10.1161/CIRCULATIONAHA.117.030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pandey A, Keshvani N, Zhong L, et al. Temporal trends and factors associated with cardiac rehabilitation participation among medicare beneficiaries with heart failure. JACC Heart Fail. 2021;9(7):471–481. doi: 10.1016/j.jchf.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 120.Kitzman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385(3):203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pandey A, Shah SJ, Butler J, et al. Exercise intolerance in older adults with heart failure with preserved ejection fraction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78:1166–1187. doi: 10.1016/j.jacc.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12(7):706–715. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mueller S, Winzer EB, Duvinage A, et al. Effect of high-intensity interval training, moderate continuous training, or guideline-based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2021;325(6):542–551. doi: 10.1001/jama.2020.26812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pandey A, Kitzman DW. Searching for the optimal exercise training regimen in heart failure with preserved ejection fraction. JAMA. 2021;325(6):537–539. doi: 10.1001/jama.2020.26347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 126.Collins TC, Slovut DP, Newton R, Jr., et al. Ideal cardiovascular health and peripheral artery disease in African Americans: Results from the Jackson Heart Study. Prev Med Rep. 2017;7:20–25. doi: 10.1016/j.pmedr.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wilson AM, Sadrzadeh-Rafie AH, Myers J, et al. Low lifetime recreational activity is a risk factor for peripheral arterial disease. J Vasc Surg. 2011;54(2):427–432. doi: 10.1016/j.jvs.2011.02.052. 432 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Delaney JA, Jensky NE, Criqui MH, Whitt-Glover MC, Lima JA, Allison MA. The association between physical activity and both incident coronary artery calcification and ankle brachial index progression: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2013;230(2):278–283. doi: 10.1016/j.atherosclerosis.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kulinski JP, Sanghavi M, Ayers CR, et al. Association between abnormal ankle-brachial index and accelerometer-derived sedentary and exercise time in the asymptomatic general population. Vasc Med. 2015;20(4):332–338. doi: 10.1177/1358863X15573837. [DOI] [PubMed] [Google Scholar]
- 130.Liao JK, Bettmann MA, Sandor T, Tucker JI, Coleman SM, Creager MA. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res. 1991;68(4):1027–1034. doi: 10.1161/01.res.68.4.1027. [DOI] [PubMed] [Google Scholar]
- 131.Grenon SM, Chong K, Alley H, et al. Walking disability in patients with peripheral artery disease is associated with arterial endothelial function. J Vasc Surg. 2014;59(4):1025–1034. doi: 10.1016/j.jvs.2013.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McDermott MM, Ferrucci L, Liu K, et al. Leg symptom categories and rates of mobility decline in peripheral arterial disease. J Am Geriatr Soc. 2010;58(7):1256–1262. doi: 10.1111/j.1532-5415.2010.02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bauer TA, Brass EP, Nehler M, Barstow TJ, Hiatt WR. Pulmonary VO2 dynamics during treadmill and arm exercise in peripheral arterial disease. J Appl Physiol (1985) 2004;97(2):627–634. doi: 10.1152/japplphysiol.00612.2003. [DOI] [PubMed] [Google Scholar]
- 134.Gardner AW, Montgomery PS, Parker DE. Physical activity is a predictor of all-cause mortality in patients with intermittent claudication. J Vasc Surg. 2008;47(1):117–122. doi: 10.1016/j.jvs.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McDermott MM, Tian L, Liu K, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51(15):1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duscha BD, Robbins JL, Jones WS, et al. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arterioscler Thromb Vasc Biol. 2011;31(11):2742–2748. doi: 10.1161/ATVBAHA.111.230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274(12):975–980. [PubMed] [Google Scholar]
- 138.Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg. 2012;56(4):1132–1142. doi: 10.1016/j.jvs.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 139.Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospital-based exercise training–randomized controlled trial. Radiology. 2009;250(2):586–595. doi: 10.1148/radiol.2501080607. [DOI] [PubMed] [Google Scholar]
- 140.Saratzis A, Paraskevopoulos I, Patel S, et al. Supervised exercise therapy and revascularization for intermittent claudication: Network Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc Interv. 2019;12(12):1125–1136. doi: 10.1016/j.jcin.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 141.Djerf H, Millinger J, Falkenberg M, Jivegard L, Svensson M, Nordanstig J. Absence of long-term benefit of revascularization in patients with intermittent claudication: five-year results from the IRONIC randomized controlled trial. Circ Cardiovasc Interv. 2020;13(1) doi: 10.1161/CIRCINTERVENTIONS.119.008450. [DOI] [PubMed] [Google Scholar]
- 142.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol. 2015;65(10):999–1009. doi: 10.1016/j.jacc.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pandey A, Banerjee S, Ngo C, et al. Comparative efficacy of endovascular revascularization versus supervised exercise training in patients with intermittent claudication: Meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2017;10(7):712–724. doi: 10.1016/j.jcin.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 144.McDermott MM. Exercise training for intermittent claudication. J Vasc Surg. 2017;66(5):1612–1620. doi: 10.1016/j.jvs.2017.05.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gerhard-Herman MD, Gornik HL, Barrett C, et al. AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;69(11):e71–e126. doi: 10.1016/j.jacc.2016.11.007. 2017. [DOI] [PubMed] [Google Scholar]
- 146.Parmenter BJ, Dieberg G, Smart NA. Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports Med. 2015;45(2):231–244. doi: 10.1007/s40279-014-0261-z. [DOI] [PubMed] [Google Scholar]
- 147.Divakaran S, Carroll BJ, Chen S, Shen C, Bonaca MP, Secemsky EA. Supervised exercise therapy for symptomatic peripheral artery disease among medicare beneficiaries between 2017 and 2018: Participation rates and outcomes. Circ Cardiovasc Qual Outcomes. 2021;14(8) doi: 10.1161/CIRCOUTCOMES.121.007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McDermott MM, Guralnik JM, Criqui MH, et al. Home-based walking exercise in peripheral artery disease: 12-month follow-up of the GOALS randomized trial. J Am Heart Assoc. 2014;3(3) doi: 10.1161/JAHA.113.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123(5):491–498. doi: 10.1161/CIRCULATIONAHA.110.963066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Collins TC, Lunos S, Carlson T, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care. 2011;34(10):2174–2179. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319(16):1665–1676. doi: 10.1001/jama.2018.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McDermott MM, Spring B, Tian L, et al. Effect of low-intensity vs high-intensity home-based walking exercise on walk distance in patients with peripheral artery disease: the LITE randomized clinical trial. JAMA. 2021;325(13):1266–1276. doi: 10.1001/jama.2021.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Treat-Jacobson D, McDermott MM, Bronas UG, et al. Optimal exercise programs for patients with peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(4):e10–e33. doi: 10.1161/CIR.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 154.Curry SJ, Krist AH, Owens DK, et al. Screening for cardiovascular disease risk with electrocardiography: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(22):2308–2314. doi: 10.1001/jama.2018.6848. [DOI] [PubMed] [Google Scholar]
- 155.Jonas DE, Reddy S, Middleton JC, et al. Screening for cardiovascular disease risk with resting or exercise electrocardiography: Evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(22):2315–2328. doi: 10.1001/jama.2018.6897. [DOI] [PubMed] [Google Scholar]
- 156.Miyachi M, Yamamoto K, Ohkawara K, Tanaka S. METs in adults while playing active video games: metabolic chamber study. Med Sci Sports Exerc. 2010;42(6):1149–1153. doi: 10.1249/MSS.0b013e3181c51c78. [DOI] [PubMed] [Google Scholar]
- 157.Lieberman DA, Chamberlain B, Medina E, Franklin BA, Sanner BM, Vafiadis DK. The power of play: Innovations in Getting Active Summit 2011: a science panel proceedings report from the American Heart Association. Circulation. 2011;123(21):2507–2516. doi: 10.1161/CIR.0b013e318219661d. [DOI] [PubMed] [Google Scholar]
- 158.Lavie CJ, Franklin BA, Ferdinand KC. Improving behavioral counseling for primary cardiovascular disease prevention. JAMA Cardiol. 2022 doi: 10.1001/jamacardio.2022.2259. Jul 26Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 159.Spring B, Ockene JK, Gidding SS, et al. Better population health behavior change in adults: a call to action. Circulation. 2013;128(19):2169–2176. doi: 10.1161/01.cir.0000435173.25936.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Franklin BA, Myers J, Kokkinos P. Importance of lifestyle modification on cardiovascular risk reduction: counseling strategies to maximize patient outcomes. J Cardiopulm Rehabil Prev. 2020;40(3):138–143. doi: 10.1097/HCR.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 161.Schroeder SA., Shattuck Lecture We can do better―improving the health of the American people. N Engl J Med. 2007;357(12):1221–1228. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- 162.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)6103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sallis R, Franklin B, Joy L, Ross R, Sabgir D, Stone J. Strategies for promoting physical activity in clinical practice. Prog Cardiovasc Dis. 2015;57(4):375–386. doi: 10.1016/j.pcad.2014.10.1003. [DOI] [PubMed] [Google Scholar]
- 164.Buettner D, Skemp S. Blue Zones: Lessons from the world's longest lived. Am J Lifestyle Med. 2016;10(5):318–321. doi: 10.1177/1559827616637066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Berra K, Rippe J, Manson JE. Making physical activity counseling a priority in clinical practice: the time for action is now. JAMA. 2015;314(24):2617–2618. doi: 10.1001/jama.2015/16244. [DOI] [PubMed] [Google Scholar]
- 166.Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate. J Am Coll Cardiol. 2015;65(4):389–395. doi: 10.1016/j.jacc.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 167.Kokkinos PF, Faselis C, Myers J, Panagiotakos D, Doumas M. Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: a cohort study. Lancet. 2013;381(9864):394–399. doi: 10.1016/S0140-6736(12)61426-3. [DOI] [PubMed] [Google Scholar]