Graphical abstract
Abstract
The Malpighian (renal) tubule is capable of transporting fluid at remarkable rates. This review will focus on recent insights into the mechanisms by which these high rates are achieved and controlled, with particular reference to the tubules of Drosophila melanogaster, in which the combination of physiology and genetics has led to particularly rapid progress. Like many vertebrate epithelia, the Drosophila tubule has specialized cell types, with active cation transport confined to a large, metabolically active principal cell; whereas the smaller intercalated stellate cell controls chloride and water shunts to achieve net fluid secretion. Recently, the genes underlying many of these processes have been identified, functionally validated and localized within the tubule. The imminent arrival of new types of post-genomic data (notably single cell sequencing) will herald an exciting era of new discovery.
Current Opinion in Insect Science 2021, 47:31–37
This review comes from a themed issue on Molecular physiology section
Edited by Aylin Rodan and Julian Dow
For a complete overview see the Issue and the Editorial
Available online 8th March 2021
https://doi.org/10.1016/j.cois.2021.02.018
2214-5745/© 2021 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
A model for tubule function
Insect survival depends on maintaining a constant internal environment in the face of external variation. Much of this homeostatic heavy lifting is performed by the Malpighian (renal) tubules, in conjunction with the hindgut. Tubule function has been extensively reviewed over the last decade, both as a transport phenomenon, but also with reference to possible modelling of human renal diseases [1, 2, 3, 4, 5, 6, 7]. Such comprehensive coverage allows this short article to focus on recent insights into our understanding of insect renal tubules in general, and those of Drosophila melanogaster in particular. However, space will not allow coverage of some other interesting growth areas, such as the role of tubules in maintaining ionic homeostasis during insect adaptation to cold [8, 9, 10], or the promise of modelling human diseases such as nephrolithiasis [11, 12, 13, 14, 15, 16, 17, 18].
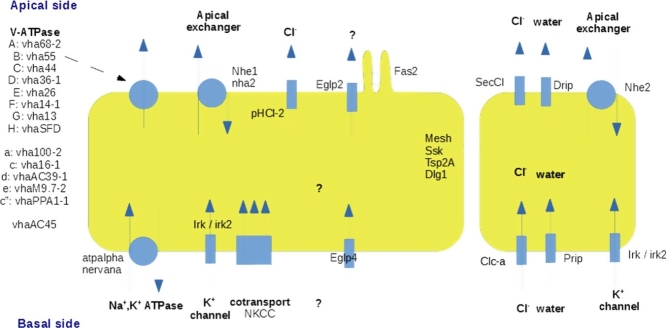
The basic model, discussed below, segregates active cation transport into a large, metabolically active principal cell, energized by the large H+ motive V-ATPase complex, while chloride flows through channels in smaller stellate cells to balance the charge. This accomplishes a net flux of salt, and so osmotically obliged water follows to accomplish a bulk flow of approximately isotonic saline (Figure 1).
Figure 1.
Overview of the two-cell model for insect tubule fluid secretion and its control.
This article will address each of these stages in turn.
Cation transport
The role of H+ V-ATPases in tubule function is well-documented, and mutation of any of the 13 genes encoding the plasma membrane isoform of the V-ATPase confers lethality and a characteristic tubule phenotype [19]. The V-ATPase is thought to drive an apical K+/H+ exchanger of the NHA family [20], accomplishing an apical secretion of K+. Basolateral entry of K+ could be through several routes: for example through the Na+, K+ ATPase [21] or the NKCC cotransport [22]. However, the tubule expresses very high levels of all three inward rectifier K+ channels, that allow flux of K+ into, but not out of, the cell; these have been localized to principal cells, and tubule fluid secretion has accordingly been shown to be potently inhibited by antidiabetic sulphonylurea drugs [23]. Irk1 and Irk2 play semi-redundant roles in secretion, as knockdown of both is required to significantly impact secretion rates, whereas Irk3 knockdowns were without effect [24]. Similarly, the Aedes aegypti orthologues Kir1 and Kir2B were expressed on the basolateral membranes of principal cells, and the intracellular Kir3 did not allow K+ currents when expressed in Xenopus oocytes [25]. This is intriguing, because Irk3 is expressed strongly and uniquely in Malpighian tubules of Drosophila. Similar tubule enrichment was observed in the Hemipteran bedbug, Cimex lenticularis, where knockdown of the Irk3 orthologue did not impact survival [26]. In the lepidopteran pest Chilo suppresalis (the Asiatic rice borer), knockdown of inward rectifiers with Flonicamid, or of the tubule-enriched CsKir2B by dsRNA, reversibly inhibited growth [27].
Although there are other routes for basolateral K+ entry, the effect of combined knockdown of Irk1 and Irk2 suggests that these are major players in basolateral K+ entry. A slightly different model was suggested for Aedes; there, Irk1 and Irk2 localise to the basolateral membranes of principal and stellate cells, respectively [25]; stellate cells could provide K+ to neighbouring principal cells via gap junctions. By contrast Irk3 is intracellular, which would also explain the lack of impact of Irk3 knockdowns in Drosophila secretion assays. Nonetheless, the phylogenetically conserved tubule-specific expression of Irk3 and its orthologues suggest that it plays an important role in tubule function. Given that the tubule contains multiple specialized vesicles, for example to store visual pigment [28], a functional role for Irk3 may be found with more subtle phenotypes.
Chloride flux
Chloride flux is under direct control of kinin and tyramine signalling, both of which act through intracellular calcium signals in stellate cells [29] to collapse the normally luminal positive potential in seconds by activating the chloride shunt conductance [30, 31, 32]. The route for stimulated chloride flux has been controversial, but has been resolved in two recent papers. Chloride entry is via the Clc-a chloride channel, which was localized immunocytochemically to the basolateral membrane of only stellate cells. Knockdown of the Clc-a gene in stellate cells did not affect basal secretion rates, but abolished kinin-stimulated secretion. Knockdown also led to a characteristic bloated phenotype in adult flies, as urination was compromised [33]. Using a transgenic intracellular chloride sensor, ClopHensor [34], it was possible to demonstrate a transient in intracellular choride within the stellate cells when they were stimulated [33]. Similar work has identified the pentameric cys-loop chloride channel SecCl as the apical partner to Clc-a in stellate cells [35]. There is thus a complete transepithelial pathway for hormone-sensitive chloride movement across stellate cells. Nonetheless, all tubule cell types are likely to have finite chloride permeability. Another pentameric chloride channel, pHCl-2, has been localized to the apical surface of principal cells, where it is thought to be gated by extracellular (luminal) pH, rather than by chemical ligands. Knockdown only slightly impacts cAMP-induced fluid secretion [36], suggesting that this channel may play a somewhat subtle, regulatory role.
Water flux
Now that there is a sound understanding of cation transport and balancing chloride flux, it remains to be established how osmotically obliged water follows the net salt flux. Tubules are known to express genes for several members of the Major Intrinsic Protein (MIP) family, that includes the classical aquaporins as well as the aquaglyceroporins, that also facilitate fluxes of several small organic solutes. In principle, then, water could flow transcellularly through the principal cells or the stellate cells, or intercellularly through the septate junctions (see next section), or through any combination of these. To address this, a recent paper identified the route of water flux by incubating tubules with an impermeant high molecular weight fluorescent dextran, that could be drawn into intercellular clefts, or into basal infoldings of either cell type. As tubules do not allow such large molecules to pass, they would accumulate at the site of water flux. In this way, it was shown that water moves preferentially through the stellate cells [37]. Tubules express very high levels of four members of the MIP family, two classical aquaporins (Drip and Prip), and two aquaglyceroporins (Eglp2 and Eglp4). Functional studies in Xenopus oocytes showed that Drip and Prip were classical water channels, whereas Eglp2 and Eglp4 additionally allowed flux of small molecules such as urea and glycerol [37]. Immunocytochemistry showed that Prip was basolateral, and Drip apical, in just the stellate cells; whereas Eglp4 was basal, and Eglp2 apical, in just the principal cells [37]. Cell-specific knockdowns confirmed the roles of aquaporins in tubule water flux. Although the possibility that some water flows through the principal cells cannot be excluded, there is a clear, functioning transcellular route for water through the stellate cells.
Although these precise genetic interventions are not easy beyond Drosophila, there is also evidence for a functional role for MIPs in other species. The aquaporin family of the Dengue vector mosquito A. aegypti has been characterised [38], and an aquaglyceroporin has been localised to principal cells [39]. An aquaporin has also been demonstrated in midgut and tubules of the malaria vector Anopheles gambiae [40]. It seems likely, then, that a transcellular route for water flux may be general, especially considering the formidable barrier of the septate junctions.
Junctions, cytoarchitecture and the paracellular route
Like all epithelia, intercellular spaces in Malpighian tubules are guarded by tight junctions to prevent leakage of solutes, and so dissipation of gradients established by vectorial transport. In insects they are termed septate junctions, based on their electron microscopic appearance. Tubules, in common with midgut, have smooth (rather than pleated) septate junctions. Recent progress (reviewed elsewhere in this volume [64] has identified several key players in tubule junctions. Knockdown of known septate junction proteins Mesh [41] or Tetraspanin 2A [42] in tubule principal cells results in measurably leaky epithelia with compromised fluid secretion and bloated phenotype. Similar effects were seen with knockdown of Snakeskin, encoding another junctional protein [43]. Additionally, progressive disruption in epithelial organisation was observed with aging [43]. The overall impression therefore, is that the septate junction is indeed a functional analogue of the vertebrate tight junction, and that its barrier function is necessary to allow successful secretion.
Successful junctions are required for correct epithelial organization, and one conspicuous property of the principal cells is an abundant field of apical microvilli, loaded with V-ATPase and each containing a long mitochondrion. Given that the tubule secretes faster on a per-cell volume basis than any other epithelium in biology, the microvilli are likely to be under significant shear stress, and may require structural reinforcement. A well-known protein in morphogenesis, Fasciclin II, plays this role [44]. Knockdown of Fas2 shortens the microvilli, whereas overexpression lengthens them, compared to wild-type. Fluid secretion is proportional to microvillar length, providing direct evidence for what had previously been assumed; that microvilli extend surface area and allow higher transport rates in an epithelium [44].
Regulation
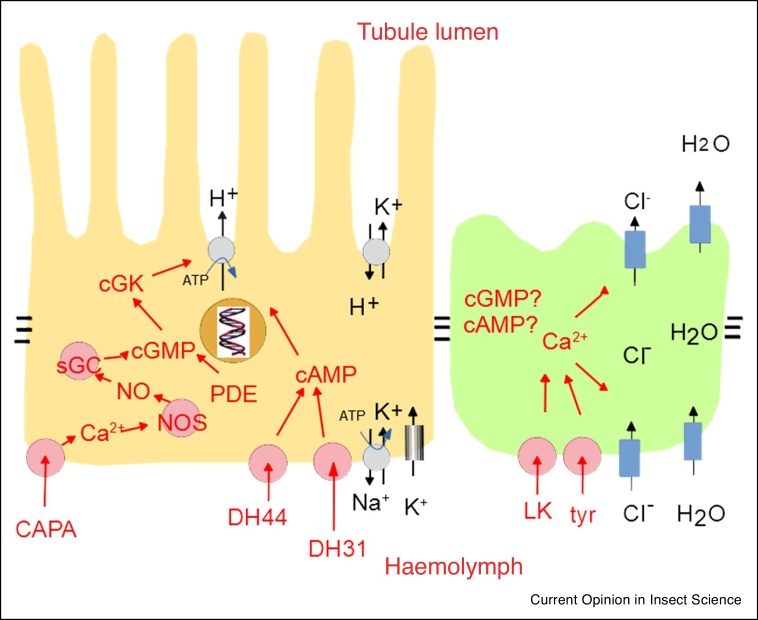
The hormonal control of tubule secretion by neuropeptides Capa, kinin, DH31, DH44 and Nplp1-4 have been well-reviewed elsewhere [1,2]. However, in mammalian kidney there are also internal homeostatic mechanisms. For example, in vertebrate nephron, autophosphorylation of the ‘with no lysine’ (WNK) kinase is reduced by chloride. WNK phosphorylates two further kinases, SPAK and OSR1, which then phosphorylate the sodium chloride cotransporter NCC and the sodium–potassium-2 chloride cotransporters NKCC1 and 2 [45]. There are Drosophila orthologues for most of these components, so could a similar system apply? In Drosophila tubule, WNK is activated by hypotonicity, which would act to stimulate fluid secretion and so correct the imbalance. Knockdown of Drosophila WNK or SPAK/OSR1/fray reduces K+ flux through the tubule, and it was shown that Fray phosphorylates NKCC in vitro [46]. The scaffold protein Mo25 also impacts on WNK signalling in tubule [47]. Although the tubule is directly controlled by the nervous system, mechanisms of direct intracellular homeostasis are now being uncovered.
Comparative
The genetic tools and genomic resources unique to Drosophila have hugely accelerated research into tubule function, but it is critical to establish whether the models being developed in fly have more general applicability. There is evidence, for example, that functional properties associated with stellate cells in Drosophila are associated with similar subpopulations of cells in other organisms. The kinin receptor, highly specific to stellate cells in fly, shows a similar distribution in Anopheles and Aedes [48]. Similarly, tiptop, a paralogue of the Teashirt transcription factor that drives the stellate cell fate in flies [49,50], is found in specialized cell subtypes in in the beetle Tribolium castaneum and the cricket Gryllus bimaculatus [49].
Given that the diuretic neuropeptides Capa, DH31 and DH34 are all thought to act to stimulate fluid secretion by activating principal cell V-ATPase, and kinin by activating chloride flux in stellate cells, a direct test of the two-cell model would be to map receptors in tubules of other insects. Both immunocytochemistry and in situ hybridization would require species-specific informatics and generation of reagents; however, direct labelling of neuropeptides with a fluor allowed visualization of cell-specific binding to tubules across representatives of 90% of insect biodiversity [51]. In this way, it was shown that kinin indeed labelled a subpopulation of small cells in tubules of the endopterygote Diptera, Hymenoptera and Lepidoptera; but labelled tubules of the exopterygote Orders Hemiptera and Orthoptera more generally. It thus seems that the two-cell model may be general to the ‘higher’ insect Orders. Conspicuously though, the Coleoptera showed no binding of kinin, nor the presence of kinin or its receptor in any species studied at the time [51]; it has since become clear that only very few beetles from the suborder Adephaga have kinin signalling, and it may have been lost secondarily through the rest of the Order [52, 53, 54].
Single cell sequencing
Drosophila has provided clear evidence for the segregation of active cation transport from the anion and water shunts (see updated model in graphical abstract), but the transcriptomic signature of this separation is masked by the necessity for whole tissue transcriptomics. However, the relatively new technique of single-cell sequencing can radically change this landscape. In a recent paper, the lower tubules of Drosophila were painstakingly dissected in order to characterize their stem cell population [55]. The physical separation of lower tubules from main segments is necessarily approximate, and the authors provided evidence that they might have adventitiously included some main segment principal and stellate cells [55]. The original dataset was heavily filtered to enrich for the small stem cells; so realizing that principal and stellate cells differed hugely in both ploidy and mitochondrial content, we reanalysed their kindly provided raw datasets with fewer filtering restrictions. This produced more cells of each type; and we identified the main segment principal cells as expressing high CAPAR but low Aph4 (a marker of lower tubule in Drosophila and other insects [56]), and stellate cells as containing high lkr [29], Drip [37] and Clc-a [33]. The most enriched genes in each cell type (relative to the average lower tubule transcriptome) are shown in Table 1.
Table 1.
Provisional single cell transcriptomics of main segment principal and stellate cells of adult Drosophila
| Principal cells |
Stellate cells |
||||
|---|---|---|---|---|---|
| Gene | Log FC | Function | Gene | Log FC | Function |
| CG8492 | 4.4 | Lysozyme | tutl | 7.7 | Cell–cell adhesion mediator |
| Hs3st-A | 4.1 | Heparan sulfate 3-O sulfotransferase-A | CG6282 | 7.6 | Oxidoreductase |
| Chinmo | 3.9 | Nucleic acid binding | twz | 6.4 | Potassium channel |
| CG42235 | 3.7 | Symporter | NetB | 6.0 | Neuron guidance |
| lncRNA:CR43768 | 3.7 | — | Nha2 | 5.9 | Na+/H+ exchanger |
| Cpr92A | 3.6 | Structural constituent of cuticle | dac | 5.7 | Transcription factor |
| List | 3.5 | Neurotransmitter:sodium symporter | Octalpha2R | 5.5 | Biogenic amine receptor |
| CG14397 | 3.4 | — | CG30116 | 5.1 | — |
| CG4928 | 3.4 | Potassium channel regulator | Doc2 | 5.1 | Transcription factor |
| CG8785 | 3.3 | Amino acid transmembrane transporter | Alk | 5.0 | Transmembrane receptor protein tyrosine kinase |
| CG4467 | 3.3 | Metalloaminopeptidase | Ih | 4.8 | Potassium channel |
| nompC | 3.2 | Calcium channel | tsh | 4.8 | Transcription factor |
| Snmp2 | 3.2 | Scavenger receptor | ETHR | 4.8 | Neuropeptide receptor |
| Dh31-R | 3.2 | Diuretic hormone receptor | Ldh | 4.7 | Lactate dehydrogenase |
| ouib | 3.2 | Transcription factor | CG4607 | 4.6 | Carbohydrate:proton symporter |
| Pu | 3.2 | GTP cyclohydrolase I | tok | 4.5 | Zinc metallopeptidase |
| CG7342 | 3.2 | Transmembrane transporter | Doc1 | 4.5 | Ectodermal transciption factor |
| Slc45-1 | 3.2 | Sucrose transporter | Pde1c | 4.5 | Phosphodiesterase |
| CG8028 | 3.1 | Monocarboxylic acid transporter | SK | 4.4 | Potassium channel |
| wtrw | 3.1 | Calcium channel | asRNA:CR43454 | 4.4 | — |
| CG7882 | 3.1 | Transmembrane transporter | CG2145 | 4.4 | Endoribonuclease |
| ppk13 | 3.1 | Sodium channel | Lim3 | 4.3 | Transcription factor |
| CG2187 | 3.0 | Symporter | Ten-a | 4.3 | Cell adhesion |
| CG16727 | 3.0 | Transmembrane transporter | CG13323 | 4.2 | Transcription regulator |
| Ptx1 | 3.0 | Transcription factor | ed | 4.1 | Cell adhesion |
| Ugt302K1 | 3.0 | Hexosyl transferase | svp | 4.0 | Transcriptional regulator |
| CG31663 | 3.0 | Transporter | Or94b | 3.9 | Odorant receptor |
| Cyp6t3 | 2.9 | Oxidoreductase | CG44325 | 3.9 | Septate junction |
| CG4286 | 2.9 | — | CG9674 | 3.9 | Glutamate synthase |
| CG34426 | 2.9 | Chitin binding | Lkr | 3.9 | Kinin neuropeptide receptor |
| Selected enriched >2x | Selected enriched >2x | ||||
|---|---|---|---|---|---|
| Irk1 | 2.8 | Potassium channel | Nep2 | 3.8 | Peptidase |
| NaPi-T | 2.2 | Phosphate transporter | hth | 3.3 | Transcription factor |
| Eglp4 | 2.1 | Aquaglyceroporin | Ace | 3.0 | Acetylcholine esterase |
| cad | 2.1 | Transcription factor | norpA | 2.5 | Phospholipase C |
| Fas3 | 2.1 | Cell adhesion | Pde11 | 2.1 | Phosphodiesterase |
| pHCl-2 | 2.0 | Chloride channel | rdgA | 2.0 | Diacylglycerol kinase |
| subdued | 2.0 | Chloride channel | sdt | 2.0 | Junctional polarity |
Genes are ranked by their log fold change above an average of all cells in the lower tubule dataset [55]. Below the top 30, further genes with significant enrichments above 2.0x are selected for interest.
While it should be emphasized that this is an imperfect analysis (a nuclear single cell transriptome for the whole tubule is imminent as part of the FlyCellAtlas project), we now have a first view of what makes principal and stellate cells unique. Confidence in the dataset is increased by the observation of enriched genes previously characterized in tubule, for example DH31R [57], Irk1 [24], NaPi-T [58], Eglp4 [37] and pHCl-2 [35] in principal cells and Nha2, tsh, lkr and Nep2 in stellate cells. However, there are also surprises; for example, an enrichment of genes associated with the termination of cell signals (Pde1c [59], Pde11 [59], Nep2 [60], Ace [61], norpA [62] and rdgA [63]) in stellate cells.
Perhaps the most exciting outcome, however, is that many of the genes with expression most highly enriched in principal and stellate cells have yet to be named (Table 1), suggesting that, even in this extraordinarily well-studied epithelium, there is much still to be learned. As more detailed post-genomic datasets come onstream, there is likely to be an exciting step change in our understanding of this remarkable tissue, and thus of insect success.
Conflict of interest statement
Nothing declared.
Acknowledgement
This work was funded by BBSRC grants BB/P008097/1 and BB/P024297/1.
References
- 1.Beyenbach K.W., Skaer H., Dow J.A.T. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–374. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 2.Cohen E., Sawyer J.K., Peterson N.G., Dow J.A.T., Fox D.T. Physiology, development, and disease modeling in the Drosophila excretory system. Genetics. 2020;214:235–264. doi: 10.1534/genetics.119.302289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies S.A., et al. Humana Press; New York, NY: 2019. Kidney Organogenesis 203-221. [Google Scholar]
- 4.Dow J.A.T., Halberg K.A., Terhzaz S., Davies S.A. In: Model Animals in Neuroendocrinology: From Worm to Mouse to Man. Ludwig M., Levkowitz G., editors. John Wiley & Sons; 2018. pp. 81–100. [Google Scholar]
- 5.Dow J.A.T., Pandit A., Davies S.A. New views on the Malpighian tubule from post-genomic technologies. Curr Opin Insect Sci. 2018;29:7–11. doi: 10.1016/j.cois.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Rodan A.R. The Drosophila Malpighian tubule as a model for mammalian tubule function. Curr Opin Nephrol Hypertens. 2019;28:455–464. doi: 10.1097/MNH.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautam N.K., Verma P., Tapadia M.G. Drosophila Malpighian tubules: a model for understanding kidney development, function, and disease. Results Probl Cell Differ. 2017;60:3–25. doi: 10.1007/978-3-319-51436-9_1. [DOI] [PubMed] [Google Scholar]
- 8.Andersen M.K., MacMillan H.A., Donini A., Overgaard J. Cold tolerance of Drosophila species is tightly linked to the epithelial K(+) transport capacity of the Malpighian tubules and rectal pads. J Exp Biol. 2017;220:4261–4269. doi: 10.1242/jeb.168518. [DOI] [PubMed] [Google Scholar]
- 9.MacMillan H.A., Andersen J.L., Davies S.A., Overgaard J. The capacity to maintain ion and water homeostasis underlies interspecific variation in Drosophila cold tolerance. Sci Rep. 2015;5 doi: 10.1038/srep18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terhzaz S., et al. Renal neuroendocrine control of desiccation and cold tolerance by Drosophila suzukii. Pest Manag Sci. 2018;74:800–810. doi: 10.1002/ps.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landry G.M., et al. Cloning, function, and localization of human, canine, and Drosophila ZIP10 (SLC39A), a Zn(2+) transporter. Am J Physiol Renal Physiol. 2019;316:F263–F273. doi: 10.1152/ajprenal.00573.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossano A.J., Romero M.F. Optical quantification of intracellular pH in Drosophila melanogaster Malpighian tubule epithelia with a fluorescent genetically-encoded pH indicator. J Vis Exp. 2017 doi: 10.3791/55698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W.C., et al. Melamine-induced urolithiasis in a Drosophila model. J Agric Food Chem. 2012;60:2753–2757. doi: 10.1021/jf204647p. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y.H., et al. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. Kidney Int. 2011;80:369–377. doi: 10.1038/ki.2011.80. [DOI] [PubMed] [Google Scholar]
- 15.Ghimire S., et al. Targeted renal knockdown of Na(+)/H(+) exchanger regulatory factor Sip1 produces uric acid nephrolithiasis in Drosophila. Am J Physiol Renal Physiol. 2019;319:F930–F940. doi: 10.1152/ajprenal.00551.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata T., et al. In vivo Drosophila genetic model for calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol. 2012;303:F1555–1562. doi: 10.1152/ajprenal.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho C.Y., et al. Effects of commercial citrate-containing juices on urolithiasis in a Drosophila model. Kaohsiung J Med Sci. 2013;29:488–493. doi: 10.1016/j.kjms.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Wu S.Y., et al. An emerging translational model to screen potential medicinal plants for nephrolithiasis, an independent risk factor for chronic kidney disease. Evid Based Complement Altern Med. 2014;2014 doi: 10.1155/2014/972958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan A.K., Du J., Davies S.A., Dow J.A.T. Genome-wide survey of V-ATPase genes in Drosophila reveals a conserved renal phenotype for lethal alleles. Physiol Genomics. 2005;22:128–138. doi: 10.1152/physiolgenomics.00233.2004. [DOI] [PubMed] [Google Scholar]
- 20.Chintapalli V.R., et al. Transport proteins NHA1 and NHA2 are essential for survival, but have distinct transport modalities. Proc Natl Acad Sci U S A. 2015;112:11720–11725. doi: 10.1073/pnas.1508031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torrie L.S., et al. Resolution of the insect ouabain paradox. Proc Natl Acad Sci U S A. 2004;101:13689–13693. doi: 10.1073/pnas.0403087101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodan A.R., Baum M., Huang C.L. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol Cell Physiol. 2012;303:C883–C894. doi: 10.1152/ajpcell.00201.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans J.M., Allan A.K., Davies S.A., Dow J.A.T. Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophila melanogaster renal function. J Exp Biol. 2005;208:3771–3783. doi: 10.1242/jeb.01829. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y., Baum M., Huang C.L., Rodan A.R. Two inwardly rectifying potassium channels, Irk1 and Irk2, play redundant roles in Drosophila renal tubule function. Am J Physiol Regul Integr Comp Physiol. 2015;309:R747–R756. doi: 10.1152/ajpregu.00148.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piermarini P.M., et al. Localization and role of inward rectifier K(+) channels in Malpighian tubules of the yellow fever mosquito Aedes aegypti. Insect Biochem Mol Biol. 2015;67:59–73. doi: 10.1016/j.ibmb.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Mamidala P., Mittapelly P., Jones S.C., Piermarini P.M., Mittapalli O. Molecular characterization of genes encoding inward rectifier potassium (Kir) channels in the bed bug (Cimex lectularius) Comp Biochem Physiol B Biochem Mol Biol. 2013;164:275–279. doi: 10.1016/j.cbpb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Meng X., et al. Flonicamid and knockdown of inward rectifier potassium channel gene CsKir2B adversely affect the feeding and development of Chilo suppressalis. Pest Manag Sci. 2020 doi: 10.1002/ps.6232. [DOI] [PubMed] [Google Scholar]
- 28.Tearle R. Tissue specific effects of ommochrome pathway mutations in Drosophila melanogaster. Genet Res. 1991;57:257–266. doi: 10.1017/s0016672300029402. [DOI] [PubMed] [Google Scholar]
- 29.Radford J.C., Davies S.A., Dow J.A.T. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem. 2002;277:38810–38817. doi: 10.1074/jbc.M203694200. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell M.J., et al. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol. 1998;274:R1039–R1049. doi: 10.1152/ajpregu.1998.274.4.R1039. [DOI] [PubMed] [Google Scholar]
- 31.Blumenthal E.M. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol. 2003;284:C718–C728. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- 32.Cabrero P., Richmond L., Nitabach M., Davies S.A., Dow J.A.T. A biogenic amine and a neuropeptide act identically: tyramine signals through calcium in Drosophila tubule stellate cells. Proc Biol Sci. 2013;280 doi: 10.1098/rspb.2012.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabrero P., et al. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci U S A. 2014;111:14301–14306. doi: 10.1073/pnas.1412706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arosio D., et al. Simultaneous intracellular chloride and pH measurements using a GFP-based sensor. Nat Methods. 2010;7:516–518. doi: 10.1038/nmeth.1471. [DOI] [PubMed] [Google Scholar]
- 35.Feingold D., et al. secCl is a cys-loop ion channel necessary for the chloride conductance that mediates hormone-induced fluid secretion in Drosophila. Sci Rep. 2019;9:7464. doi: 10.1038/s41598-019-42849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feingold D., Starc T., O’Donnell M.J., Nilson L., Dent J.A. The orphan pentameric ligand-gated ion channel pHCl-2 is gated by pH and regulates fluid secretion in Drosophila Malpighian tubules. J Exp Biol. 2016;219:2629–2638. doi: 10.1242/jeb.141069. [DOI] [PubMed] [Google Scholar]
- 37.Cabrero P., et al. Specialized stellate cells offer a privileged route for rapid water flux in Drosophila renal tubule. Proc Natl Acad Sci U S A. 2020;117:1779–1787. doi: 10.1073/pnas.1915943117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drake L.L., et al. The Aquaporin gene family of the yellow fever mosquito, Aedes aegypti. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misyura L., Yerushalmi G.Y., Donini A. A mosquito entomoglyceroporin, Aedes aegypti AQP5, participates in water transport across the Malpighian tubules of larvae. J Exp Biol. 2017;220:3536–3544. doi: 10.1242/jeb.158352. [DOI] [PubMed] [Google Scholar]
- 40.Liu K., Tsujimoto H., Cha S.J., Agre P., Rasgon J.L. Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation. Proc Natl Acad Sci U S A. 2011;108:6062–6066. doi: 10.1073/pnas.1102629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonusaite S., et al. The septate junction protein Mesh is required for epithelial morphogenesis, ion transport, and paracellular permeability in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol. 2020;318:C675–C694. doi: 10.1152/ajpcell.00492.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beyenbach K.W., et al. The septate junction protein Tetraspanin 2A is critical to the structure and function of Malpighian tubules in Drosophila melanogaster. Am J Physiol Cell Physiol. 2020;318:C1107–C1122. doi: 10.1152/ajpcell.00061.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dornan A.J., Halberg K.A., Beuter L.-K., Davies S.A., Dow J.A.T. The septate junction protein Snakeskin is critical for epithelial barrier function and tissue homeostasis in the Malpighian tubules of adult Drosophila. bioRxiv. 2020 doi: 10.1101/2020.12.14.422678. [DOI] [Google Scholar]
- 44.Halberg K.A., et al. The cell adhesion molecule Fasciclin2 regulates brush border length and organization in Drosophila renal tubules. Nat Commun. 2016;7 doi: 10.1038/ncomms11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodan A.R. WNK-SPAK/OSR1 signaling: lessons learned from an insect renal epithelium. Am J Physiol Renal Physiol. 2018;315:F903–F907. doi: 10.1152/ajprenal.00176.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y., Schellinger J.N., Huang C.L., Rodan A.R. Hypotonicity stimulates potassium flux through the WNK-SPAK/OSR1 kinase cascade and the Ncc69 sodium-potassium-2-chloride cotransporter in the Drosophila renal tubule. J Biol Chem. 2014;289:26131–26142. doi: 10.1074/jbc.M114.577767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Q., et al. Intracellular chloride and scaffold protein Mo25 cooperatively regulate transepithelial ion transport through WNK signaling in the Malpighian tubule. J Am Soc Nephrol. 2018;29:1449–1461. doi: 10.1681/ASN.2017101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu H.L., Kersch C., Pietrantonio P.V. The kinin receptor is expressed in the Malpighian tubule stellate cells in the mosquito Aedes aegypti (L.): a new model needed to explain ion transport? Insect Biochem Mol Biol. 2011;41:135–140. doi: 10.1016/j.ibmb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denholm B., et al. The tiptop/teashirt genes regulate cell differentiation and renal physiology in Drosophila. Development. 2013;140:1100–1110. doi: 10.1242/dev.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denholm B., et al. Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr Biol. 2003;13:1052–1057. doi: 10.1016/s0960-9822(03)00375-0. [DOI] [PubMed] [Google Scholar]
- 51.Halberg K.A., Terhzaz S., Cabrero P., Davies S.A., Dow J.A.T. Tracing the evolutionary origins of insect renal function. Nat Commun. 2015;6 doi: 10.1038/ncomms7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pandit A.A., Davies S.-A., Smagghe G., Dow J.A. Evolutionary trends of neuropeptide signaling in beetles-a comparative analysis of Coleopteran transcriptomic and genomic data. Insect Biochem Mol Biol. 2019;114 doi: 10.1016/j.ibmb.2019.103227. [DOI] [PubMed] [Google Scholar]
- 53.Veenstra J.A. Coleoptera genome and transcriptome sequences reveal numerous differences in neuropeptide signaling between species. PeerJ. 2019;7 doi: 10.7717/peerj.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ragionieri L., Predel R. The neuropeptidome of Carabus (Coleoptera, Adephaga: Carabidae) Insect Biochem Mol Biol. 2020;118 doi: 10.1016/j.ibmb.2019.103309. [DOI] [PubMed] [Google Scholar]
- 55.Wang C., Spradling A.C. An abundant quiescent stem cell population in Drosophila Malpighian tubules protects principal cells from kidney stones. eLife. 2020;9 doi: 10.7554/eLife.54096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cabrero P., Pollock V.P., Davies S.A., Dow J.A.T. A conserved domain of alkaline phosphatase expression in the Malpighian tubules of dipteran insects. J Exp Biol. 2004;207:3299–3305. doi: 10.1242/jeb.01156. [DOI] [PubMed] [Google Scholar]
- 57.Johnson E.C., et al. A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J Exp Biol. 2005;208:1239–1246. doi: 10.1242/jeb.01529. [DOI] [PubMed] [Google Scholar]
- 58.Rose E., et al. Endocrine regulation of MFS2 by branchless controls phosphate excretion and stone formation in Drosophila renal tubules. Sci Rep. 2019;9:8798. doi: 10.1038/s41598-019-45269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies S.A., et al. Cell signalling mechanisms for insect stress tolerance. J Exp Biol. 2014;217:119–128. doi: 10.1242/jeb.090571. [DOI] [PubMed] [Google Scholar]
- 60.Thomas J.E., et al. Drosophila melanogaster NEP2 is a new soluble member of the neprilysin family of endopeptidases with implications for reproduction and renal function. Biochem J. 2005;386:357–366. doi: 10.1042/BJ20041753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall L.M., Spierer P. The Ace locus of Drosophila melanogaster: structural gene for acetylcholinesterase with an unusual 5’ leader. EMBO J. 1986;5:2949–2954. doi: 10.1002/j.1460-2075.1986.tb04591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollock V.P., et al. NorpA and itpr mutants reveal roles for phospholipase C and inositol (1,4,5)- trisphosphate receptor in Drosophila melanogaster renal function. J Exp Biol. 2003;206:901–911. doi: 10.1242/jeb.00189. [DOI] [PubMed] [Google Scholar]
- 63.Raghu P., et al. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron. 2000;26:169–179. doi: 10.1016/s0896-6273(00)81147-2. [DOI] [PubMed] [Google Scholar]
- 64.Jonusaite S., Rodan A.R. Molecular basis for epithelial morphogenesis and ion transport in the Malpighian tubule. Curr Opin Insect Sci. 2021;47:7–11. doi: 10.1016/j.cois.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]