Abstract
Polymorphonuclear cells (PMNs) from healthy donors and differentiated HL-60 cells were compared in an opsonophagocytic assay using fluorescent latex beads coated with Streptococcus pneumoniae polysaccharide conjugates. Serum-specific phagocytosis was efficiently mediated by both sources of cells, as measured by flow cytometry, but the mean number of beads ingested per cell was three- to fivefold higher when PMNs were used than when HL-60 cells were used. Nevertheless, differentiated HL-60 cells could be a convenient and standardized source of cells to evaluate the functionality of specific antibodies to vaccine candidates as a coating on fluorescent beads.
In the search for new vaccines, investigators often have to select a few candidates among a large number of antigens and formulations. A good way to evaluate the potential of such candidates is to measure the specific immune responses against the different antigens in convalescent-phase or resistant hosts after natural infection with the corresponding pathogen. Besides classical enzyme-linked immunosorbent assay techniques, functional assays, such as opsonophagocytic assays, have been demonstrated to be more relevant to analyze the role of antibodies in protection, in particular in the case of Streptococcus pneumoniae (pneumococcal) or Neisseria meningitidis (meningococcal) infections (1–5). An opsonophagocytic assay using fluorescent beads coated with different antigenic structures has been developed by A. Lehmann and coworkers (1), with polymorphonuclear cells (PMNs) from healthy donors as effector cells; opsonophagocytic activity is measured by flow cytometric analysis, the end points being the percentage of fluorescent cells, the mean number of beads per each phagocyte (designated by M in this study), and the product of these two values, the phagocytosis product (PP) (2, 3). In parallel, the group of G. M. Carlone has developed opsonophagocytic assays using differentiated HL-60 cells. In this assay, live bacteria and, more recently, fluorescently labeled and fixed bacteria were used (4, 5). Opsonophagocytic activity is measured in the former case by viable count, while in the latter case, flow cytometry is used.
In the present study, we evaluated the combined use of antigen-coated fluorescent beads as targets and HL-60 cells as phagocytes compared to PMNs from healthy donors. Streptococcus pneumoniae polysaccharides from serotypes 4 and 14 conjugated to tetanus toxoid (TT [Pn4-TT and Pn14-TT, respectively]) were used as antigens. The assay was set up with rabbit positive sera (demonstrated in the department to be opsonic with human cells in a viable opsonophagocytic assay) and negative sera (directed against an irrelevant conjugate) in order to have well-identified negative controls, because it was difficult to obtain and select human sera without specific antipneumococcal antibodies. Nevertheless, several human sera, including a reference serum from Sandoz (Sandoglobuline; Sandoz, Rueil Malmaison, France), were tested in a second step. Rabbit sera heated at 56°C were obtained from animals hyperimmunized with Pn4 polysaccharide coupled to diphtheria toxoid (DT) instead of TT to avoid unwanted reactions against the carrier protein (anti-Pn4-DT [positive serum]), or hyperimmunized with an irrelevant Haemophilus influenzae type b (HiB) polysaccharide conjugate (anti-HiB [negative serum]). Antigens and sera were prepared in the Research and Development facilities of Aventis Pasteur in Marcy l'Etoile, France. S. pneumoniae serotype 4 bacteria were heat inactivated (1 h at 60°C) and labeled with fluorescein isothiocyanate (FITC) (Sigma, St. Louis, Mo.) to be used as a positive antigen control in the assay. Conjugates were adsorbed to fluorescent beads (Fluoresbrite Plain Microspheres; Polysciences, Warrington, Pa.) with diameters of 1, 3, and 6 μm, as described in reference 1, and the coating efficiency was estimated by protein dosage in the supernatant (bicinchoninic acid protein assay) to be about 20%; despite the evaluation of several different conditions, it has not been possible to reach higher coating values. PMNs were obtained from healthy donors by using Polymorphprep (Nycomed, Oslo, Norway), while HL-60 cells were obtained from the American Type Culture Collection and differentiated as described by Romero-Steiner et al. (5). The general conditions of the assay were based on those described in references 1 to 3 when fluorescent beads and PMNs were used combined with those described in references 4 and 5 when HL-60 cells were used. However, some modifications were introduced, and the influence of different parameters was evaluated, including time of opsonization (from 5 to 45 min), time of phagocytosis (from 15 to 45 min), amount of exogenous human complement serum (Sigma; reference no. S1764 [not reactive against pneumococcal antigens in our assays]), and amount of specific antiserum. In addition, labeling of PMNs with anti-CD13-phycoerythrin (PE) (labeling nonlymphocytes, granulocytes, and monocytes) and of HL-60 cells with anti-CD32-PE (labeling differentiated HL-60 cells) fluorescent antibodies (Immunotech, Marseille, France) allowed a more accurate determination of the gates in the flow cytometric analysis. This was performed on a FACScan fluorescence-activated cell sorter (Becton Dickinson) by using the Cellquest program; 5,000 events were analyzed on CD13+ gated PMNs, while 10,000 events were analyzed on CD32+ gated HL-60 cells. All in all, the optimal conditions for both PMNs and HL-60 cells were as follows: opsonization time of 30 min at 37°C with 100 μl of beads (final bead/cell ratio of 30) in the presence of 3 μl of human complement (Sigma; 1/166 final dilution [no phagocytosis observed in the absence of complement]) and 15 μl of serum (1/33 final dilution), followed by 15 min of phagocytosis at 37°C with 2.5 × 106 PMNs or HL-60 cells for 100 μl of beads (final volume of 500 μl in phosphate-buffered saline–glucose–bovine serum albumin buffer). These gave the best results in terms of sensitivity, specificity, and reproducibility. The results obtained under these conditions with rabbit serum and representative of at least five different and independent experiments for both PMNs and HL-60 cells when using 1-μm-diameter beads or labeled bacteria are presented in Fig. 1 and Table 1.
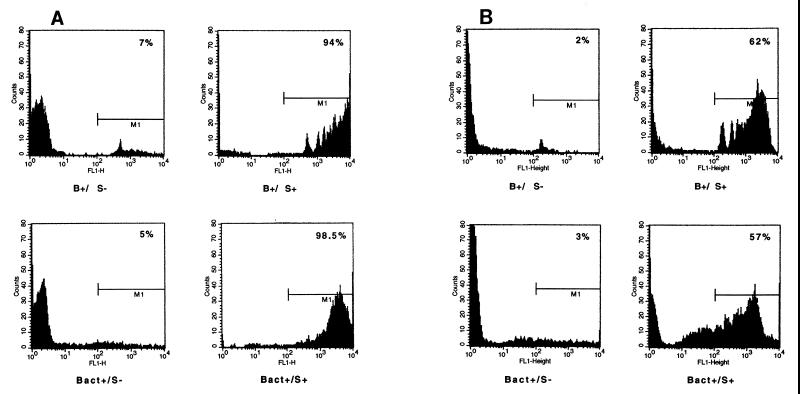
FIG. 1.
Flow cytometric analysis of opsonophagocytic activity mediated by PMNs (A) or HL-60 cells (B) in the presence of Pn4 bacteria (Bact+), Pn4-TT-coated beads (B+), anti-Pn4-DT serum (S+), or anti-Hib conjugate negative serum (S−). One-micrometer-diameter beads were used.
TABLE 1.
Percentages of fluorescent cells, M, and PP determined after flow cytometric analysis as presented in part in Fig. 1a
| Expt | Fluorescence for:
|
% Fluorescent cellsb | M | PP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S+ | S− | 1-μm-diam beads
|
3-μm-diam beads
|
FITC-labeled Pn4 bacteria | PMNs | HL-60 cells | ||||||
| B+ | B− | B+ | B− | |||||||||
| a | + | − | + | − | − | − | − | + | − | 94 (89 ± 7.3) | 5.4 | 507 |
| − | + | + | − | − | − | − | + | − | 7 (11.8 ± 4.6) | 1 | 7 | |
| + | − | − | + | − | − | − | + | − | 14.5 (16.3 ± 3) | 0.95 | 14 | |
| − | + | − | + | − | − | − | + | − | 10 (24 ± 8) | 1.1 | 11 | |
| b | + | − | + | − | − | − | − | − | + | 62 (57.6 ± 4.5) | 1.88 | 116.5 |
| − | + | + | − | − | − | − | − | + | 2 (2.6 ± 1.3) | 0.2 | 0.4 | |
| + | − | − | + | − | − | − | − | + | 4 (5.4 ± 2) | 0.2 | 0.8 | |
| − | + | − | + | − | − | − | − | + | 9 (7.9 ± 3.6) | 0.24 | 2.2 | |
| c | + | − | − | − | + | − | − | + | − | 32 | 9 | 288 |
| − | + | − | − | + | − | − | + | − | 2.5 | 8.7 | 22 | |
| + | − | − | − | − | + | − | + | − | 6.5 | 9 | 58.5 | |
| − | + | − | − | − | + | − | + | − | 10 | 9 | 90 | |
| d | + | − | − | − | + | − | − | − | + | 29 | 5.5 | 159.5 |
| − | + | − | − | + | − | − | − | + | 11 | 5.1 | 56.1 | |
| + | − | − | − | − | + | − | − | + | 7 | 5 | 35 | |
| − | + | − | − | − | + | − | − | + | 11.5 | 5 | 57.5 | |
| e | + | − | − | − | − | − | + | + | − | 98 (96 ± 2.5) | 3.8 | 372.5 |
| − | + | − | − | − | − | + | + | − | 12 (6.7 ± 3) | 1.2 | 14.4 | |
| f | + | − | − | − | − | − | + | − | + | 57 (38.3 ± 18.5) | 1.1 | 63 |
| − | + | − | − | − | − | + | − | + | 3 (3 ± 0) | 1.15 | 3.45 | |
Experiments were carried out in presence of Pn4 bacteria (Bact+), Pn4-TT-coated beads (B+), Pn14-TT-coated beads (B−), anti-Pn4-DT serum (S+), or anti-HiB conjugate-negative serum (S−). PMNs or HL-60 cells were used with 1-μm beads (a and b, respectively), 3-μm beads (c and d, respectively), or FITC-labeled bacteria (e and f, respectively).
Values in parentheses are means ± standard deviations.
For each assay, four conditions were systematically tested (i.e., one test, and three negative controls): (i) Pn4-TT-coated beads in the presence of anti-Pn4-DT serum (B+/S+), (ii) Pn4-TT beads in the presence of heterologous anti-HiB serum (B+/S−), (iii) Pn14-TT beads with anti-Pn4-DT serum (B−/S+), and (iv) Pn14-TT beads in the presence of heterologous anti-Hib serum (B−/S−). Results corresponding to the first two conditions are illustrated in Fig. 1, while all end points are presented in Table 1. In addition, heat-inactivated and FITC-labeled bacteria (Bact+, S. pneumoniae serotype 4) were used as a positive control in the presence of anti-Pn4-DT or heterologous anti-Hib serum. Results were expressed as in references 1 to 3 by the percentage of fluorescent cells, M (calculated by dividing the mean particle fluorescence of the phagocytes by the fluorescence of single beads), and PP (corresponding to the product of the first two values). The test was considered internally to be positive and specific when the percent or PP values obtained in the assay were at least four times higher than the values of each of the three negative controls.
As shown in Fig. 1, it appears that both PMNs and HL-60 cells can ingest labeled bacteria as already described by other authors (1, 4) and can also ingest fluorescent beads in the presence of homologous serum (Bact+/S+ and B+/S+), whereas this was not the case in the presence of heterologous serum (Bact+/S− and B+/S−). Percentages of fluorescent cells were on the same order of magnitude for PMNs and HL-60, both for bacteria (98.5 and 57%, respectively) and antigen-coated beads (94 and 62%, respectively), although the values were higher for PMNs. These values are similar to those obtained with labeled bacteria by Martinez et al. (4) using HL-60 cells and 1/8 to 1/32 dilutions of specific serum. However, as presented in Table 1 for one representative experiment, the mean number of beads or bacteria per cell and consequently the phagocytosis product was three to five times higher for PMNs than for HL-60 cells, showing the greater capacity of the former cells to phagocytize beads under the conditions of the assay. Differences between test conditions and each negative control were highly significant when using 1-μm-diameter beads (P < 0.0003 for PMNs and P < 0.0001 for HL-60 cells in Student's t test). However, higher background level was observed when PMNs were used, with some negative controls reaching values as high as 30% fluorescent cells in some experiments (data not shown). Table 1 also shows that 1-μm-diameter beads were more efficiently and more specifically ingested than 3-μm-diameter beads, which gave a high background level in negative controls; unlike 1-μm beads, 3-μm beads, inducing clearly less favorable results, have not been used extensively (only two independent experiments) with HL-60 cells, and standard deviations are thus not presented in the table for these beads. Six-micrometer-diameter beads were not extensively tested due to the very low level of phagocytosis observed in preliminary experiments (data not shown).
Different human sera, as well as a reference serum from Sandoz (Sandoglobuline), were used at least twice (with similar results) in independent experiments with HL-60 cells. Table 2 shows that 41 to 53% fluorescent cells were obtained according to the sera in the presence of Pn14-TT-coated beads, and phagocytosis products were in the same range of magnitude (mean of about 50%). The reactivity of these sera was less important in the presence of Pn4-TT-coated beads (from 5 to 21% fluorescent cells), as with the corresponding FITC-labeled Pn4 bacteria (from 11 to 16% fluorescent cells). Unfortunately, we did not have access to pre- and postexposure or pre- and postvaccination human sera, and no real negative serum could be used in these experiments, as in the previous ones with rabbit sera.
TABLE 2.
Percentages of fluorescent cells, M, and PP determined after flow cytometric analysis as defined in Table 1a
| Serum | Fluorescence for:
|
% Fluorescent cells | M | PP | ||
|---|---|---|---|---|---|---|
| Pn4 | Pn14 | FITC-labeled Pn4 bacteria | ||||
| 1 | + | − | − | 8 | 0.4 | 3.2 |
| − | + | − | 45 | 1.12 | 50.4 | |
| − | − | + | 11 | 0.76 | 8.4 | |
| 2 | + | − | − | 8.5 | 0.3 | 2.5 |
| − | + | − | 37 | 0.8 | 29.5 | |
| − | − | + | 13 | 0.6 | 8 | |
| 3 | + | − | − | 6 | 0.2 | 1.2 |
| − | + | − | 43 | 0.8 | 34.4 | |
| − | − | + | 14 | 0.6 | 9 | |
| Sandoglobuline | + | − | − | 21 | 0.38 | 8 |
| − | + | − | 50 | 1 | 50 | |
| − | − | + | 16 | 0.64 | 10 | |
HL-60 cells were used as phagocytes with four different human sera or a pool of sera to opsonize beads (sera 1, 2, and 3 and Sandoglobuline). Pn4, beads coated with Pn4 conjugate; Pn14, beads coated with Pn14 conjugate.
In conclusion, our results indicate that HL-60 cells can ingest fluorescent beads coated with antigenic preparations in the presence of specific antisera. Although the number of beads ingested was higher when PMNs from different donors were used, HL-60 cells presented less background activity. All in all, the good specificity of the assay should allow the use of this more convenient and standardized HL-60 cell line to screen a potentially large number of antigens and corresponding antisera in a functional assay, in particular if a higher coating efficiency can be reached with these antigens. This would be of great value in vaccine research.
Acknowledgments
We acknowledge Marie-José Quentin Millet for constant support and Jean-Michel Chapsal for providing the pneumococcal conjugates.
REFERENCES
- 1.Lehmann A K, Halstensen A, Holst J, Bassoe C F. Functional assays for evaluation of serogroup B meningococcal structures as mediators of human opsonophagocytosis. J Immunol Methods. 1997;200:55–68. doi: 10.1016/s0022-1759(96)00185-8. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann A K, Halstensen A, Bassoe C F. Flow cytometric quantitation of human opsonin-dependent phagocytosis and oxidative burst responses to meningococcal antigens. Cytometry. 1998;33:406–413. doi: 10.1002/(sici)1097-0320(19981201)33:4<406::aid-cyto3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann A K, Halstensen A, Aaberge I S, Holst J, Michaelsen T E, Sornes S, Wetzler L M, Guttormsen H. Human opsonins induced during meningococcal disease recognize outer membrane proteins PorA and PorB. Infect Immun. 1999;67:2552–2560. doi: 10.1128/iai.67.5.2552-2560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez J E, Romero-Steiner S, Pilishvili T, Barnard S, Schinsky J, Goldblatt D, Carlone G M. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin Diagn Lab Immunol. 1999;6:581–586. doi: 10.1128/cdli.6.4.581-586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]