Abstract
Exposure to hazardous wastes, especially petroleum wastes hydrocarbon (PWHCs), can damage human health and biological diversity. A huge amount of petroleum waste along with persistent organic pollutants is being generated during exploration and processing of crude oil. The dumping of petroleum waste hydrocarbons in an open pit contaminates the soil which can cause severe threats to human health and agro-geo-environmental ecosystem. The current study aimed to evaluate the mode of occurrence, composition, environmental, and health impacts of petroleum waste by using recent literature. The extracted results show that oil emulsion contains 48% oil, suspension 23%, settled emulsion 42%, and sludge emulsion 36%. The study discusses the possible biological techniques for rehabilitation of petroleum waste-contaminated areas. Several physical and chemical techniques are available for remediation of petroleum waste, but they are either costly or environmentally not feasible. Whereas, biological remediation namely, Bioremediation (Biostimulation and Bioaugmentation), Phytoremediation (Phytodegradation, Rhizoremediation, Phytovolatilization, and Rhizo-filtration) is a cheap and environmentally friendly way to remove petroleum waste hydrocarbons from contaminated soil and water. Some important enzymes (i.e., peroxidase, nitrilase, nitroreductase, phosphatase) and plant species i.e., Acacia and Chloris species are prominent methods to remediate the PWHCs. The knowledge assembled in this review is expected to create new doors for researchers to develop more efficient techniques to control the harmful impacts of PWHCs on the environment and health.
Keywords: Petroleum wastes, Phytoremediation, Rhizo-filtration, Remediation, Environ-health impacts
Petroleum wastes; Phytoremediation; Rhizo-filtration; Remediation; Environ-health impacts.
1. Introduction
Hydrocarbon and its wastes are the aggregations of various materials, including crude oil, emulsion, solids, and slurry. The exploration, processing, and transportation of hydrocarbon and petroleum wastes may damage the surrounding environment, aquatic, and terrestrial biota as well as human health (Shahzad et al., 2020; Karam and Al-Wazzan, 2021). The most fragile contents of the environment namely, air, soil, and water are essential factors for the biogeochemical ecosystem and are exposed to hazardous nature of hydrocarbon (Nwaichi et al., 2014; Turner et al., 2021). Soil contamination occurs due to sporadic discharge and dumping of hydrocarbon and petroleum wastes. Thus, the petroleum wastes that affected soil do not support flora and flora and remained unfertile over decades before being remediated (Khan et al., 2018). Similarly, the overexploitation of petroleum resources also contaminates the water resources, especially small agricultural channels, rivers, and streams located nearby the oil rigs and processing units (Balogh and Watson, 2020). Due to lower density, oily wastes can float over the water surface and thus affect photosynthetic plants/ organisms in water bodies and soil (because of settling on the surface). Whereas denser petroleum waste penetrates downward; causing groundwater contamination and reducing the soil porosity (Sayed et al., 2021). The contaminated water with hydrocarbon and persistent organic wastes drastically affects human health and other living organisms (Ahmed and Fakhruddin, 2018). Some petroleum waste is volatile and affects the air quality and causes other types of air pollution i.e., smog, which needs to formulate anti-air pollution strategies (Chen et al., 2020). The availability of hydrocarbon and petroleum wastes in air may also cause nose and throat allergies, vomiting and significantly affect the lungs of humans and other living organisms (Müller and Sedláčková, 2003). Exposure to a high concentration of hydrocarbon may cause malignant and non-malignant lungs disorder (Connellan, 2017). It is documented officially, that any type of exportation, processing, preparation, or operation activities that generate pollutants or contaminate the surrounding environment may need to be remediated or reduce the pollutant below the permissible level (GoP, 1997; Sattar et al., 2021).
More than 100 thousand sites all over the world are categorized as hazardous for living organisms, particularly humans. Most of these are mainly from the disposal of untreated petroleum hydrocarbons (Bujang et al., 2013). Complete remediation of such sites over short period has remained a problem, mostly because of cost involved in the disposal and processing. Other possibilities include lack of best remediation technique that can degrade the diverse nature of petroleum waste hydrocarbons over a short period (Jasmine and Mukherji, 2014). The spilling and open dumping of liquid oily wastes in the surrounding environment and their exploration and identification is one of the major problems. The purpose of this study was: 1) To explore the source and composition of petroleum waste in contaminated soils at dumping sites. 2) To evaluate the impacts of petroleum waste on human health, soil microbiota, land degradation, and aquatic life. 3) To discuss the possible available biological techniques for rehabilitation of petroleum waste-contaminated soils. The effective petroleum waste remediation action plan includes; the collection of petroleum waste from the source, analyzing, processing, and remediation of petroleum hydrocarbons through different physical, chemical, and biological methods (Figure 1).
Figure 1.
Overall summarization and action plan of the PWHCs from origination to remediation.
2. Mode of occurrence of petroleum hydrocarbons
It was well reported that hydrocarbon is comprised of various hazardous materials depending upon the source, organic materials, geological environment, and decomposition rate (Ahmadun et al., 2009; Waples, 2013; Srivastava et al., 2019). This is more likely because petroleum is derived from residual organic matter or biomass of biogenic origin including terrestrial plants-C4 and marine algae-C3 or phytoplankton (Welte and Tissot, 1984). Moreover, marine algae have high content of organic materials, especially nitrogenous and lipid compounds, however, the terrestrial plants have high compositions of lignin, cellulose, and other carbon-containing materials (Cloern et al., 2002; Mannino and Rodger, 2004). The structure and behavior of hydrocarbon derived from algal or terrestrial plants differ from one another and produce various types of pollutants during the exploration. The variation in petroleum compounds is because of their decomposition environment, temperature, biomarkers, maturity level, and presence/availability of organic materials (Thangalazhy et al., 2012). The maturity biomarker is mostly associated with polysaccharides (i.e., aromatic hydrocarbon, steroids, hopanoids, and porphyrins), and terpenes. However, the non-biomarker maturity level is commonly attributed to aldehyde, aromatic, and isoprenoids (Thangalazhy et al., 2012). The presence and availability of maturity biomarkers and non-biomarker are attributed to organic materials derived from terrestrial and marine plants. During Ordovician era, the algal growth (C3-plants) was dominant, where the hydrocarbon was abundant in n-alkanes with high content of odd n-alkane while there was less concentration of n-dodecane (Hatch et al., 1987; Colcord et al., 2019). However, the aromatic hydrocarbon i.e., phenanthrene and naphthalene were abundant in oil derived from both marine and terrestrial plant materials (Table 1).
Table 1.
An indication of the carbon source for the C range present in Petroleum (adapted from Welte and Tissot (1984)).
| Biomarker | C Range | Indication |
|---|---|---|
| n-Alkanes CPI˂1 |
Co–C21 | Marine, lacustrine algal source, C15, C17, C19 dominant |
| C25–C37 | Terrestrial plant wax source C27, C29, C31 dominant | |
| C12–C24 and C20–C34 | Bacterial source: oxic, anoxic, marine, lacustrine Saline, anoxic environment: carbonates, evaporites |
|
| Acyclic isoprenoids Head to tail Pristane Phytane Head-to-Head Botryococcene |
C19 | Chlorophyll, α-tocopherol, oxic, sub-oxic environments |
| C20 | Chlorophyll, Phytanyl ethers of methanogens, anoxic, saline | |
| C25, C30, C40, C34 | Archaebacterial, bacterial cell-wall lipids, lacustrine, Brackish | |
| Sesquiterpenoids, Cadalene, Eudesmane |
C15 | Terrestrial plants |
| Diterpenoids, Abletane, plmarane, Kaurane, retene. |
C19–C20 | High plant resins |
| Tricyclic terpenes | C19–C45 | Diagenetic products of bacterial and algal cell-wall lipids |
| Tetracyclic Terpenes | C24–C27 | Degradation of pentacyclic triterpenoids |
| Hopanes | C27–C40 | Bacteria |
| Norhopanes | C27–C28 | Anoxic marine |
| 2- and 3- methyl hopanes | C28–C38 | Carbonate rocks |
| Benzohopenoids | C32–C35 | Carbonate environments |
| Hexahydrobenzohopenoids | C32–C35 | Anoxic, carbonate anhydrite |
| Gammacerane | C30 | Hypersaline environments |
| Oleananes, Lupanes | C30 | Late cretaceous and tertiary flowering plants |
| Bicadinane | C30 | Gymnosperm tree resins |
| β-Carotane Steranes 24-n-Propylsterane 4-Methylsteranes Dinosteranes |
Cn C19–C23 C28–C30 C30 C28–C30 C30 |
Arid, hypersaline Eukaryote organisms, plants, and animals Restricted to marine sediments Marine and lacustrine dinoflagellates Marine, Triassic, or Younger |
Asif (2010) documented that the biogenic derived oil in Kohat and Potwar Plateau originated from marine/terrestrial materials, which are categorized as Kerogen type II and III. In this region, the crude oil was dominated by saturated hydrocarbons, while other organic compounds i.e., aromatic hydrocarbon were available in minor amounts. The saturated hydrocarbons in crude oil are derived from organic matter, while aromatic hydrocarbons in crude oil are originated from olefinic and naphthenic compounds associated with biogenic decay of organic materials (Nissenbaum et al., 1985; Roushdy et al., 2010; Waples, 2013; Wilkes et al., 2020).
3. Petroleum waste composition
It is well understood that during exploration of crude oil, a huge amount of petroleum waste is generated, which shows the characteristics and composition of source rocks (Hu et al., 2013; Ozdemir et al., 2020). The chemical characteristic of petroleum is always similar to crude oil and somewhat to the source rocks which they are derived from. However, the major portion of petroleum is crude oil, while in trace amounts, emulsion, water, soil, and clay are available (Table 2). The generation of petroleum waste during exploration and its dumping in open pits may cause hazardous impacts on humans. Similarly, petroleum wastes may cause carcinogenic impacts because of the presence of carcinogenic chemicals (Benzene, anthracene, Cr+6, Pyrene, As) (Mansur et al., 2015). The composition of wastes in petroleum may differ from area to area and the geologic environment underneath the earth (Table 2).
Table 2.
The mode of occurrence and composition of Petroleum waste hydrocarbon around the world.
| Region | Type | Appearance | Oil % |
Water % |
Solids % |
References |
|---|---|---|---|---|---|---|
| USA | Slop oil | Emulsion | 48 | 40 | 12 | (Bider and Hunt, 1982) |
| Sludge | Suspension | 23 | 53 | 24 | ||
| Lybia | Crude oil tank bottom sludge | Emulsion | 42.08 | 2.9 | 55.02 | (Mansur et al., 2015) |
| World | Petroleum waste | Emulsion | 30–50 | 30–50 | 10–12 | (Hu et al., 2013) |
| India | Oily sludge | Emulsion | 35.59 | 55.13 | 9.246 | (Kumar and Raj Mohan, 2013) |
| Pakistan | Crude oil | Emulsion | 0.2–2.3 | 0–3.6 | (OGDCL, 2017) |
The major composition of petroleum wastes, namely water 55.2%, light hydrocarbon 23.2%, waxes 10.5%, clay 9%, and asphaltenes 1.9% (Islam, 2015). Specifically, the composition of petroleum wastes generated during exploration was given in Table 2. The data revealed that petroleum wastes are comprised of aromatic, saturated hydrocarbon, nitrogen, aliphatic, amines, and oxides. However, the composition is varying from region to region depending on the biogenic and geologic environment (Ozdemir et al., 2020). Singh et al. (2017) documented that the world petroleum reservoir is dominated by aromatic and aliphatic hydrocarbon. The aromatic and aliphatic hydrocarbon dominated with 75–85% fractions (i.e., gasoline, kerosene, naphtha, diesel, crude oil, and bitumen), while sulfur, nitrogen, and oxygen are present in minor/low concentrations (De Junet et al., 2013). The aforementioned data revealed that aliphatic hydrocarbon, namely paraffin, alkanes, alkenes, and aromatic hydrocarbon (anthracene, pyrene, naphthalene, and chrysene) are also dominant in liquid petroleum wastes, which may cause serious environmental pollution and disturbance in an ecosystem (Connell et al., 1980; De Junet et al., 2013). The environmental pollution caused by petroleum exploration, and processing needs to be remediated by using advanced biological techniques to limit the environmental consequences (Serafim et al., 2008).
4. Impacts of petroleum waste contamination
4.1. Effect on human health
Several health issues like respiratory system disorders, miscarriages and infertility in women, birth defects, skin rashes, and childhood leukemia are associated with petroleum hydrocarbon contamination. Besides, there are high chances of carcinogenic, mutagenic, and teratogenic disorders, in most cases, it can damage deoxyribonucleic acid (Rengarajan et al., 2015). Moreover, petroleum contamination in the surrounding environment is a significant source of concern with a detrimental impact on human health. Such compounds enter the human body through different routes, i.e., skin, inhalation, dermal, and ingestion (Figure 2). The toxicity of petroleum-waste hydrocarbon is mainly dependent on the concentration and duration of exposure (Rengarajan et al., 2015; Murawski et al., 2021). For instance, even short-term exposure to an elevated level of some volatile petroleum hydrocarbons (such as toluene) is toxic to the central nervous system and can cause dizziness and headache. In addition, some adverse health symptoms like respiratory problems, skin and eyes irritation, and headache are reported in the workers exposed to petroleum hydrocarbons for long term (Levy and Nassetta, 2011). International Program on Chemical Safety (IPCS) 2000 suggested that some chronic symptoms like vomiting, diarrhea, abdominal pain, nausea, confusion, and headache can be experienced by a person when exposed shortly to organic pollutants (WHO, 1984).
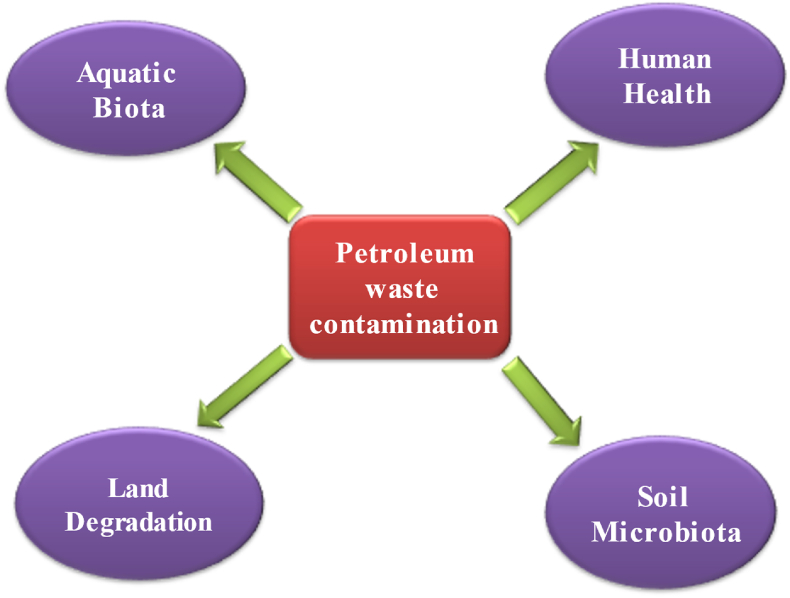
Figure 2.
Impacts of petroleum wastes on the surrounding biotic and abiotic environment.
4.2. Effects of petroleum hydrocarbons on soil microbiota
Petroleum waste hydrocarbons contaminated soils may lose their functions i.e., soil fertility, porosity, regulation of water supply, degradation of decayed material, and providing habitat to microorganisms. Contaminated soils may lose or threaten several microbial species, which will ultimately affect the biodiversity of soil microorganisms (Mafiana et al., 2021). Ren et al. (2015) explored the effect of pyrene pollution on bacterial population. Their results depicted that bacterial population and diversity in pyrene-polluted soils were adversely affected. Moreover, the growth of several species of microorganism (i.e., belonging to phyla Chlorflexi, Alphaproteobacteria, Actinobacteria, Deltaproteobacteria, and Crenarchaeota) was potentially decreased (Ren et al., 2015) (Figure 2, Table 3).
Table 3.
Bioremediation of Petroleum wastes hydrocarbons using different biological species.
| Source | Bacterial Species | References |
|---|---|---|
| Petroleum Refinery Effluents (PAH) | Pseudomonas sp. | (Liu et al., 2021) |
| Petroleum Hydrocarbon | Pseudomonas, Stenotrophomonas, Achromobacter, Mesorhizobium, and Brucella (genera's) | (Zhang et al., 2021) |
| Oily sludge | Pseudomonas aeruginosa | (Varjani et al., 2020) |
| Oily sludge | Bacillus cereus, Bacillus altitudinis, Commomonas (Delftia sp.), and Stenotrophomonas maltophilia | (Shahzad et al., 2020) |
| Oil refinery sludge | Bacillus, Coprothermobacter, Rhodobacter, Pseudomonas, chromobacter, Desulfitobacter, Desulfosporosinus, Methanobacterium, Methanosaeta, | (Roy et al., 2018) |
| Oily sludge | Gordonia alkaliphila and Gordonia paraffinivorans | (Qi et al., 2017) |
| Oil contaminated soils (TPH) | Pseudomonas stutzeri BP10 and Aspergillus niger PS9 | (Kumari et al., 2016) |
| Total Petroleum Hydrocarbons | Pseudomonas sp. | (Xu et al., 2016) |
| Crude oil | Pseudomonas aeruginosa | (Varjani et al., 2015) |
| Petroleum hydrocarbons | Proteobacteria, Actinobacteria and Firmicutes (Phyla) | (Fuentes et al., 2014) |
| Oily Sludge | Bacillus, Burkholderia, Paenibacillus, Pseudomonas, Bacillus, Stenotrophomonas Enterobacteria Pseudomonas, Bacillus, Pandoraeaand Kocuria | (Roy et al., 2014) |
| Crude Oil | Acinetobacter baumannii | (Kim et al., 2009) |
| Petroleum contaminated soils (TEM, PAH, alkanes) | Pseudomonas sp. BS2201, BS2203 and Brevibacillus sp. BS2202 (Nitrate reducing bacterial strains) | (Grishchenkov et al., 2000) |
4.3. Land degradation
Quite numerous incidents happened in the world because of flushing/dumping of petroleum wastes in an open environment. However, the release of PWHCs' may also occur because of accidental leakage of the storage tank and pipelines. Due to this, several fertile agricultural lands were converted to unfertile and degraded soil fertility (Zabbey and Olsson, 2017). In 2003, an oil spillage accident occurred in Karachi, Pakistan, where about 15,000 tons of oil spilled into the marine environment from a crude oil tanker. The land was badly affected, which ultimately affected flora and fauna. Moreover, marine and wildlife were also affected. During transportation, oil spilled accidents may affect agricultural land nearby the highways. Similarly, several acres of agricultural land are annually getting contaminated with petroleum waste nearby the waste pit because of leakage from waste pit (Siddiqui et al., 2016; Sattar et al., 2021). Therefore, it is necessary to control the further loss of agricultural land and need reclamation of these land at any cost. The natural degradation process and rehabilitating of contaminated land is a slower process than the contamination of useable land (Shahzad et al., 2020). For the reclamation and rehabilitation of such sites, it is mandatory to either avoid further degradation of contaminated lands or to adopt some suitable remediation technology to remove petroleum waste from contaminated soils (Figure 2).
4.4. Effects on aquatic ecosystem
Petroleum seepage in surface water causes an adverse impact on the aquatic ecosystem (Figure 2). It not only causes marine pollution but also contaminates the fishing areas and drinking water sources (Charles et al., 2021). Besides, groundwater gets contaminated with petroleum hydrocarbons when oil percolates into soil and reaches the groundwater aquifers. Several scientific studies explored the negative impacts of petroleum hydrocarbons on aquatic biota (Dupuis and Francisco, 2015; Luke and Odokuma, 2021). The Immuno-toxic effects of petroleum hydrocarbons (i.e., polyaromatic hydrocarbons) on mammals and fishes have been examined, and reported that acute exposure to PWHCs can affect the production of eggs in fish and their normal growth (Hellou et al., 2006). Moreover, several studies demonstrated the susceptibility of zooplankton to petroleum hydrocarbon exposure. The mortality of zooplankton is dependent on duration of exposure rather than the concentration of PWHCs. For soil and water safety from oil wastes, it is essential to use the bioremediation technique to detoxify/remove the toxicants from soil (Payne et al., 2014; Kurylenko and Izosimova, 2016).
4.5. Removal of petroleum waste
The presence of toxic and complex compounds in petroleum waste contaminated soils makes it difficult to completely remove PWHCs through the natural process of degradation. Up till now, several methods have been established to reclaim contaminated sites, including physical, chemical, and biological processes (Abdullah et al., 2020; Ossai et al., 2020; Sayed et al., 2021).
The selection of a suitable method for rehabilitation of contaminated sites is mainly dependent on composition and concentration of petroleum hydrocarbons. Among the above-mentioned techniques, physical and chemical processes are more efficient but they are either costly or eco-toxic. Whereas, biological processes are economical and environmentally friendly but take more time to reduce contamination from the soil surface.
5. Bioremediation
Bioremediation is an effective natural method for the degradation of pollutants (like PWHCs) using microorganisms. In microbial remediation, microorganisms attach themselves to petroleum hydrocarbons and degrade them by using them as a source of food. The process of bioremediation can be enhanced by adding microbial culture (bio-degraders) and fertilizers. However, the rate of biodegradation of different compounds is different (Namukuye, 2021; Zhen et al., 2021).
Microbes in the soil perform various activities i.e., chemical, and biological processes to degrade PWHCs effectively. However, microbes can play an essential role in the biogeochemical cycles (Sridhar et al., 2021). The microbial activities may be catabolic or metabolic using an enzyme to catalyze organic wastes in soil (Fierer et al., 2021). Microbial population and their communities are quite variable in different ecosystems. The microbial degradation of petroleum wastes may also depend on climatic conditions, biotic and abiotic factors, especially the availability of nitrogen, carbon, and oxygen.
It was reported that petroleum wastes especially in agricultural soil affect the soil, food quality, and yield. However, petroleum wastes in the soil can be degraded by microbes naturally, but the process will be very slow (Sengupta and Pal, 2021). The petroleum materials are less dense and may accumulate on the soil surface. Further, it can block the soil porosity and permeability which reduce microbial activity and plant growth (Hewelke et al., 2018). By blocking soil porosity and permeability, an anxious condition is created where oxygen availability for microbes in soil reduces which stops the microbial activity (Das and Kazy, 2014). Thus, bioremediation (by inoculating biodegrading microbes) can trigger the degradation of PWC’s contaminated soils. However, consortium of Bacillus altitudinis (KF859970), Bacillus cereus (KR232400), and Comamonas (KF859971) can trigger oil waste degradation, especially alkanes group compounds (Shahzad et al., 2020) (Table 3). They further reported that the lag phase was minimized to 03 days with consortiums and 14 days without using consortiums. However, Maddela et al. (2017) reported that 87.5% of petroleum wastes were degraded using a synthetic microbial consortium of Bacillus cereus, Bacillus thuringiensis along with Geomyces sp. HV, and Geomyces pannorum HR stain.
Although bioremediation is thought to be an effective strategy for overcoming all the obstacles to remediating petroleum hydrocarbon successfully, like other techniques of remediation. However, it also has some limitations, such as inability to completely remediate and remove PWHCs from contaminated soils. Due to persistent nature of some compounds present in PWHC's contaminated soils, some hydrocarbons can be degraded completely; some resist the natural process of degradation, whereas some compounds are non-biodegradable (Artham and Doble, 2008; Patel et al., 2011). Similarly, saturated subsurface remediation (in anoxic conditions) may not be achieved effectively through this method. Additionally, this method cannot prevent groundwater contamination from PWHCs. Despite this, bioremediation with its limitations is widely accepted and applied. However, the improvement in bioremediation with genetically modified microorganisms, plants, and microorganisms producing surfactants is gaining slight attention among researchers.
6. Techniques of bioremediation
6.1. Biostimulation
Biostimulation provides optimized ecological conditions for bioremediation process in contaminated sites. This process is adopted when there is a need to speed up the natural degradation of contaminated sites (Zhou et al., 2019). As discussed above, when hydrocarbon-degrading microorganisms are injected into contaminated soils, PWHCs breakdown takes place. However, in contaminated soils, the degradation of PWHCs with nutrients improves up to the maximum level (Table 4). When nutrients are added to PWHC’s contaminated soils, however, more degradation can be achieved. The provision of oxygen, nutrients, and electron acceptors to bacteria (engaged in bioremediation) stimulates their development and activities. For this purpose, subsurface biostimulation systems are developed, and additives are injected through injection wells. However, some inconsistent evidence was also found in the literature regarding the PWHC's degradation with nutrient and microbial consortium (Table 4). It was reported that consortiums along with nutrients had no observed impacts on petroleum waste hydrocarbon degradation (Siddiqui et al., 2016). However, a consortium along with nutrients triggered the degradation of PWHCs in contaminated soil effectively (Wu et al., 2017).
Table 4.
Various studies report the effectiveness of Bioaugmentation and Biostimulation of Petroleum hydrocarbons.
| Contamination | Location | Technique applied | Reference | |
|---|---|---|---|---|
| 1 | Light crude oil contaminating a sandy beech | Marine oil wastes at sand beach, Delaware, USA | Bioaugmentation and biostimulation | (Venosa et al., 1996) |
| 2 | Crude oil and heavy fuel oil | Sea Empress oil spill, UK | Biostimulation | (Swannell et al., 1999) |
| 3 | Heavy crude oil | Japan | Bioaugmentation | (Tsutsumi et al., 2000) |
| 4 | Crude oil degradation | The Grande Terre, Kerguelen islands | Biostimulation | (Delille et al., 2002) |
| 5 | Petroleum Hydrocarbons | Crude oil spill site in Liaohe Oilfield, Liaoning Province, China. | Bioaugmentation | (Xu and Lu, 2010) |
| 6 | heavy crude oil/total petroleum hydrocarbons | Xingang port of Dalian City in China | Bioaugmentation | (Cai et al., 2016) |
| 7 | Crude oil | the hyper-arid region in Israel | Biostimulation and Bioaugmentation | (Banet et al., 2021) |
6.2. Bioaugmentation
Petroleum waste can also be remediated through bioaugmentation effectively. In this process, genetically engineered or indigenous microbes are added to the petroleum waste contaminated soils to degrade petroleum waste hydrocarbons (Cunningham and Philp, 2000). Bioaugmentation is an effective tool to enhance the degradation rate of hydrocarbons by increasing the total population of indigenous microbes (Wu et al., 2017). Bioaugmentation can enhance the rate of degradation by introducing non-indigenous microorganisms to contaminated sites. For the degradation of pollutants like petroleum hydrocarbons, Biostimulation is encouraged. This usually involves modifying the pH of contaminated site, adding limiting nutrients to obtain an optimal C:N:P ratio, and increasing soil moisture. However, the bacteria that degrade petroleum hydrocarbons are exposed to hazardous oil spills, they attempt to release a suitable amount of biosurfactant which convert all type of hydrocarbon to some other substance, as a result minimizes the harmful impacts of compound (Table 4).
6.3. Land farming
Land-farming is an efficient tool used to degrade the PWHCs' contaminated soil. The contaminated soils are rotated periodically for aeration and mixed with soil nutrients to boost up enzymatic actions of microbes to increase the natural degradation process of petroleum hydrocarbons (Besalatpour et al., 2011; Wang et al., 2016). The land farming technique is usually preferred over landfilling and incineration as it involves less energy consumption, and a lower risk of water pollution (both surface and groundwater) due to the immobility of hydrocarbons through the soil, regulatory compliance, and compatibility with soil properties and climatic conditions (Besalatpour et al., 2011).
7. Phytoremediation
The remediation of oil wastes through microorganisms was further improved by the addition of plants. The microbial remediation of petroleum waste is considered bioremediation, while the oil waste remediation through plants is called phytoremediation. Thus both methods, either phytoremediation or bioremediation can reduce the petroleum waste in contaminated soil (Aliku et al., 2021). Phytoremediation is an environmentally friendly and economic tool to degrade and detoxify petroleum waste hydrocarbons. In this process, green plants are used to remove, disintegrate, stabilize, mineralize, or degrade the toxic pollutants from soil and water (Nero, 2021). Several plant species (i.e., Mirabilis Jalapa L, Sebastiania commersoniana, Festuca arundinacea, Panicum virgatum) are reported as potential phytoremediators of petroleum hydrocarbons (Yavari et al., 2015) (Table 3). Such plants show good tolerance in petroleum hydrocarbon contaminated soils. Various mechanisms are involved in phytoremediation process and were given in Figure 3.
Figure 3.
Different mechanisms are mediated by plants during the phytoremediation process.
7.1. Phytodegradation
Phytodegradation is the process of degradation of contaminants using plant enzymes. However, organic substances can be degraded via phytodegradation either inside the plant or in rhizosphere. Solvents in the groundwater, petroleum, and aromatic compounds in soils are examples of such contaminants that can be removed from the environment using this technology. Some reported plants utilizing enzymes responsible for the degradation of organic contaminants are dehalogenase, peroxidase, nitrilase, nitroreductase, and phosphatase (Yadav et al., 2018) (Figure 3).
7.2. Rhizoremediation
Rhizoremediation is the most efficient way to degrade petroleum hydrocarbons (Alotaibi et al., 2021). Rhizoremediation involves both bioremediation and phytoremediation processes to degrade oily wastes in the surrounding environment. Rhizospheric soils provide a favorable environment for the growth and multiplication of petroleum hydrocarbons degrading microbes. The cumulative impact of secretion of root exudates (i.e., amino acids, organic acids, and sugars) and the presence of petroleum hydrocarbons degrading microbes enhance the rate of degradation. Hoang et al. (2021) investigated the rhizoremediation potential of Acacia pyrifolia, Banksia seminuda, Hakea prostrata, Triodiawiseana, Acacia inaequilatera, Hardenbergia Violacea, Acacia stellaticeps, and Chloris truncata reduced the extent of total petroleum wastes in contaminated soil (Table 3). Furthermore, with the addition of Hakea prostrata, Banksia seminuda, and Chloris truncata, plants were efficient to degrade TPHs (total petroleum hydrocarbons) in diesel contaminated soils (Figure 3).
7.3. Phyto-volatilization
Phytovolatilization of petroleum hydrocarbons takes place when the pollutants (more specifically the VOC) in soil are uptaken by plant roots and evaporated through leaves by the process of evapotranspiration. The contaminants are converted to a gaseous state and thus released. This process is mostly applied in marshy environment or it needs to construct wetlands (Herath and Vithanage, 2015) (Figure 3). The overall graphical summary and process of remediation were given in Figure 1.
8. Factors affecting the biological degradation of petroleum waste hydrocarbons
The major factors, namely soil pH, temperature, viscosity, and oxygen can greatly affect the biological degradation of PWCs. The accomplishment of bioremediation is dependent on the presence of suitable biodegrading species of microbes, adaptation, composition of pollutants, and nutrient availability (Cameotra and Makkar, 2010). Besides above-mentioned factors, the efficiency of bioremediation further depends upon soil pH, temperature, oxygen supply, salinity, and viscosity of pollutants (Margesin and Schinner, 2001; Sharma, 2020).
The oily waste degradation efficiency of microorganisms also depends upon pH and the presence of calcite and dolomite. Literature shows that most PAH (polyaromatic hydrocarbons) degrading microbes perform effectively at neutral pH, whereas, fungi are reported to be tolerant in an acidic environment. Literature shows maximum degradation of PWHCs occurs at a pH range of 6–9 (Al-Daher et al., 1998; Sharma, 2020).
Temperature plays a significant role in the biodegradation of petroleum waste hydrocarbons. Literature shows that the occurrence, growth, and function of microorganisms in a medium are highly dependent on temperature. Eltoukhy et al. (2020); Gomez et al. (2021) reported when temperature is dropped to a level below the optimum growth temperature; the microorganisms responsible for the degradation are no more available. Moreover, literature shows that viscosity of petroleum wastes increases and volatility decreases at low temperatures, which ultimately slows down the degradation rate of petroleum waste hydrocarbons. Whereas, by increasing temperature, the solubility of petroleum waste hydrocarbons increases and viscosity decreases, so the solid phase hydrocarbons are converted to liquid phase. The maximum degradation occurs at a temperature range of 30–40 °C (Das and Tiwari, 2018).
It is well accepted that distribution pattern of petroleum waste hydrocarbons is different in the terrestrial environment; mainly depends upon (a) viscosity of PWHCs and (b) texture of the soil they are spilled on (Angeli and Hewitt, 2000). Highly viscous oil will be distributed horizontally on the land surface. Whereas the less viscous will penetrate vertically, their downward movement will depend upon the nature of soil they are spilled over; whether it's sandy, silty, or clay soil. Moreover, viscosity of petroleum waste may also play a significant role in the plant's growth. The viscosity index of ∼35 shows lighter oil, which has a high potential of infiltration (regardless of the specific soil type) and groundwater contamination (Fingas and Brown, 2018; Fu et al., 2021; Jahromi et al., 2021).
The lighter petroleum waste blocks the soil porosity and creates anoxic environments in subsurface soil. The leaching of light petroleum waste in subsurface soil may also affect moisture content, gaseous and ion exchange capacity in subsurface soils. An anoxic environment and deficient moisture contents in subsurface soil, affect the microbial community and nutrient availability (Patel et al., 2021; Wang et al., 2022) (Figure 4).
Figure 4.
Petroleum extraction, dumping in open pits, and their impacts on the environment.
It is well documented that bioremediation accelerates the degradation of PWHCs under aerobic conditions (at the surface). Under anoxic conditions, the indigenous hydrocarbon-degrading microbial population decreases in number and diversity (Zhao et al., 2018; Fenibo, 2021). For instance, Roy et al. (2018); Mai et al. (2021) conducted a bioremediation study on petroleum hydrocarbons, and their study concluded that bioremediation (under aerobic conditions) can effectively degrade petroleum wastes up to 15 m. However, at a higher depth, the degradation rate of petroleum wastes is minimized. Additionally, the effective degradation of PWHCs in soil is still under consideration. The researcher, therefore suggests, applying the soil flushing technique for the removal of PWHCs from subsurface soils rather than biostimulation by injecting air. However, installation of an injection well for oxygen supply is costly and may not be suggested for oily contaminated soil especially contaminated soil with PWHCs.
9. Conclusion
Petroleum waste released from oil industry/refinery is a global environmental issue. Petroleum waste hydrocarbons are carcinogenic, immune-toxic, mutagenic, and teratogenic in nature and may cause potentially harmful impacts on humans and other living organisms. Such compounds enter the human body through different routes, i.e., skin, inhalation, and ingestion. During last few decades, the environmental degradation of PWHCs has gained huge attention. The major contents in liquid petroleum wastes were water 55.2%, light hydrocarbon 23.2%, waxes 10.5%, clay 9%, and asphaltenes 1.9%. Microorganisms are known for bio-transformation and bio-degradation of different types of environmental contaminants except for PWHCs. The most common eco-friendly biological technique used for the enhancement of natural degradation process is bioremediation. Other than biological techniques, some physical and chemical technologies are also developed for the degradation and removal of petroleum contaminants from the environment. Whereas, bioremediation is cheap, eco-friendly, intriguing, and a potential remediation technique for the rehabilitation of oily contaminated soils, and up to some extent it can remediate PWHCs. However, bioremediation is also hampered by several physicochemical, biological, and environmental variables (limiting agents). Therefore, it is difficult to predict the same results of bioremediation in different lab and field conditions. Moreover, little information is available about the genetic, enzymatic, and chemical processes of PWHC's breakdown in anaerobic conditions. This study contributes to the identification of different effective biological techniques to reduce the harmful impacts of petroleum waste on the environment and health. The present study also describes the role of microbial consortium in combination with nutrients to degrade PWHCs giving ambiguous results, which need further investigation. The knowledge assembled in this review is expected to create new doors for researchers to develop more efficient techniques and control the harmful impacts of PWHCs on the environment and health.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Dr Rahib Hussain was supported by Higher Education Commision, Pakistan [134/IPFP-II(Batch-I)/SRGP/NAHE/HEC/2020/227].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdullah S.R.S., Al-Baldawi I.A., Almansoory A.F., Purwanti I.F., Al-Sbani N.H., Sharuddin S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: application, mechanisms, challenges and opportunities. Chemosphere. 2020;247 doi: 10.1016/j.chemosphere.2020.125932. [DOI] [PubMed] [Google Scholar]
- Ahmadun F.l.R., Pendashteh A., Abdullah L.C., Biak D.R.A., Madaeni S.S., Abidin Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard Mater. 2009;170(2-3):530–551. doi: 10.1016/j.jhazmat.2009.05.044. [DOI] [PubMed] [Google Scholar]
- Ahmed F., Fakhruddin A. A review on environmental contamination of petroleum hydrocarbons and its biodegradation. Int. J. Environ. Sci. Nat. Resour. 2018;11(3):1–7. [Google Scholar]
- Al-Daher R., Al-Awadhi N., El-Nawawy A. Bioremediation of damaged desert environment using the windrow soil pile system in Kuwait. Environ. Int. 1998;24(1-2):175–180. [Google Scholar]
- Aliku C.B., Madu C.N., Aliku O. Organic stimulants for enhancing phytoremediation of crude oil polluted soil: a study on cowpea. Environ. Pollut. 2021;287 doi: 10.1016/j.envpol.2021.117674. [DOI] [PubMed] [Google Scholar]
- Alotaibi F., Hijri M., St-Arnaud M. Overview of approaches to improve rhizoremediation of petroleum hydrocarbon-contaminated soils. Appl. Microbiol. 2021;1(2):329–351. [Google Scholar]
- Angeli P., Hewitt G. Flow structure in horizontal oil–water flow. Int. J. Multiphas. Flow. 2000;26(7):1117–1140. [Google Scholar]
- Artham T., Doble M. Biodegradation of aliphatic and aromatic polycarbonates. Macromol. Biosci. 2008;8(1):14–24. doi: 10.1002/mabi.200700106. [DOI] [PubMed] [Google Scholar]
- Asif M. University of Engineering and Technology; Lahore, Pakistan: 2010. Geochemical Applications of Polycyclic Aromatic Hydrocarbons in Crude Oils and Sediments from Pakistan; p. 173. [Google Scholar]
- Balogh J.C., Watson J.R. CRC Press; 2020. Role and Conservation of Water Resources, Golf Course Management & Construction; pp. 39–104. [Google Scholar]
- Banet G., Turaani A., Farber R., Armoza-Zvuloni R., Rotem N., Stavi I., Cahan R. The effects of biostimulation and bioaugmentation on crude oil biodegradation in two adjacent terrestrial oil spills of different age, in a hyper-arid region. J. Environ. Manag. 2021;286 doi: 10.1016/j.jenvman.2021.112248. [DOI] [PubMed] [Google Scholar]
- Besalatpour A., Hajabbasi M., Khoshgoftarmanesh A., Dorostkar V. Landfarming process effects on biochemical properties of petroleum-contaminated soils. Soil Sediment Contam. 2011;20(2):234–248. [Google Scholar]
- Bider W.L., Hunt R.G. US Enviromental Protection Agency, Office of solid waste; 1982. Industrial Resource Recovery Practices: Petroleum Refinderies and Releated Industries. [Google Scholar]
- Bujang M., Ibrahim N.A., Rak A.E. Biodegradation of oily wastewater by pure culture of Bacillus cereus. ARPN J. Agric. Biol. Sci. 2013;8(2):1–8. [Google Scholar]
- Cai B., Ma J., Yan G., Dai X., Li M., Guo S. Comparison of phytoremediation, bioaugmentation and natural attenuation for remediating saline soil contaminated by heavy crude oil. Biochem. Eng. J. 2016;112:170–177. [Google Scholar]
- Cameotra S.S., Makkar R.S. Biosurfactant-enhanced bioremediation of hydrophobic pollutants. Pure Appl. Chem. 2010;82(1):97–116. [Google Scholar]
- Charles F., Salami S., Dashak D., Chimezie-Nwosu H. An analytical investigation study of potential human health risks caused by petroleum-contaminated surface water containing various toxic heavy metals at the Okpoka creek, Niger-delta, Nigeria. Int. Res. J. Pure Appl. Chem. 2021:1–11. [Google Scholar]
- Chen T., Xue L., Zheng P., Zhang Y., Liu Y., Sun J., Han G., Li H., Zhang X., Li Y. Volatile organic compounds and ozone air pollution in an oil production region in northern China. Atmos. Chem. Phys. 2020;20(11):7069–7086. [Google Scholar]
- Cloern J.E., Canuel E.A., Harris D. Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system. Limnol. Oceanogr. 2002;47(3):713–729. [Google Scholar]
- Colcord D.E., Shilling A.M., Freeman K.H., Njau J.K., Stanistreet I.G., Stollhofen H., Schick K.D., Toth N., Brassell S.C. Aquatic biomarkers record Pleistocene environmental changes at Paleolake Olduvai, Tanzania. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019;524:250–261. [Google Scholar]
- Connell D., Miller G., Farrington J. Petroleum hydrocarbons in aquatic ecosystems—behavior and effects of sublethal concentrations: Part 1. Crit. Rev. Environ. Sci. Technol. 1980;11(1):37–104. [Google Scholar]
- Connellan S.J. Lung diseases associated with hydrocarbon exposure. Respir. Med. 2017;126:46–51. doi: 10.1016/j.rmed.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Cunningham C., Philp J. Comparison of bioaugmentation and biostimulation in ex situ treatment of diesel contaminated soil. Land Contam. Reclamat. 2000;8(4):261–269. [Google Scholar]
- Das R., Kazy S.K. Microbial diversity, community composition and metabolic potential in hydrocarbon contaminated oily sludge: prospects for in situ bioremediation. Environ. Sci. Pollut. Res. 2014;21(12):7369–7389. doi: 10.1007/s11356-014-2640-2. [DOI] [PubMed] [Google Scholar]
- Das P., Tiwari P. Valorization of packaging plastic waste by slow pyrolysis. Resour. Conserv. Recycl. 2018;128:69–77. [Google Scholar]
- De Junet A., Basile-Doelsch I., Borschneck D., Masion A., Legros S., Marol C., Balesdent J., Templier J., Derenne S. Characterisation of organic matter from organo-mineral complexes in an Andosol from Reunion Island. J. Anal. Appl. Pyrol. 2013;99:92–100. [Google Scholar]
- Delille D., Delille B., Pelletier E. Effectiveness of bioremediation of crude oil contaminated subantarctic intertidal sediment: the microbial response. Microb. Ecol. 2002;44:118–126. doi: 10.1007/s00248-001-1047-z. [DOI] [PubMed] [Google Scholar]
- Dupuis A., Francisco U.M. A literature review on the aquatic toxicology of petroleum oil: an overview of oil properties and effects to aquatic biota. Can. Sci. Advis. Secretariat, Research Documents. 2015;2015/007 [Google Scholar]
- Eltoukhy A., Jia Y., Nahurira R., Abo-Kadoum M., Khokhar I., Wang J., Yan Y. Biodegradation of endocrine disruptor Bisphenol A by Pseudomonas putida strain YC-AE1 isolated from polluted soil, Guangdong, China. BMC Microbiol. 2020;20(1):1–14. doi: 10.1186/s12866-020-1699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenibo E.O. Suitability of bacteria in bioremediation techniques common for petroleum-related pollution. Asia J. Appl. Microbiol. 2021;8(1):1–18. [Google Scholar]
- Fierer N., Wood S.A., de Mesquita C.P.B. How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 2021;153 [Google Scholar]
- Fingas M., Brown C.E. A review of oil spill remote sensing. Sensors. 2018;18(1):91. doi: 10.3390/s18010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P., Zhu R., Jia J., Hu Y., Wu C., Cieszczyk P., Holmberg H.-C., Gong L. Aerobic exercise promotes the functions of brown adipose tissue in obese mice via a mechanism involving COX2 in the VEGF signaling pathway. Nutr. Metab. 2021;18(1):1–14. doi: 10.1186/s12986-021-00581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes S., Méndez V., Aguila P., Seeger M. Bioremediation of petroleum hydrocarbons: catabolic genes, microbial communities, and applications. Appl. Microbiol. Biotechnol. 2014;98:4781–4794. doi: 10.1007/s00253-014-5684-9. [DOI] [PubMed] [Google Scholar]
- Gomez E.J., Delgado J.A., Gonzalez J.M. Influence of water availability and temperature on estimates of microbial extracellular enzyme activity. PeerJ. 2021;9 doi: 10.7717/peerj.10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GoP . Gazette of Pakistan; Islamabad: 1997. Pakistan Environmental Protection Act, Government of Pakistan.https://na.gov.pk/uploads/documents/Pakistan=Environmental-Protection-Act-1997.pdf retrived on 4th Feb, 2022. [Google Scholar]
- Grishchenkov V., Townsend R., McDonald T., Autenrieth R., Bonner J., Boronin A. Degradation of petroleum hydrocarbons by facultative anaerobic bacteria under aerobic and anaerobic conditions. Process Biochem. 2000;35:889–896. [Google Scholar]
- Hatch J.R., Jacobson S.R., Witzke B.J., Risatti J.B., Anders D.E., Watney W.L., Newell K.D., Vuletich A.K. Possible late Middle Ordovician organic carbon isotope excursion: evidence from Ordovician oils and hydrocarbon source rocks, mid-continent and east-central United States. AAPG Bull. 1987;71(11):1342–1354. [Google Scholar]
- Hellou J., Leonard J., Collier T., Ariese F. Assessing PAH exposure in feral finfish from the Northwest Atlantic. Mar. Pollut. Bull. 2006;52(4):433–441. doi: 10.1016/j.marpolbul.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Herath I., Vithanage M. Springer; 2015. Phytoremediation in Constructed Wetlands, Phytoremediation; pp. 243–263. [Google Scholar]
- Hewelke E., Szatyłowicz J., Hewelke P., Gnatowski T., Aghalarov R. The impact of diesel oil pollution on the hydrophobicity and CO2 efflux of forest soils. Water, Air. Soil Pollut. 2018;229(2):1–11. doi: 10.1007/s11270-018-3720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang S.A., Lamb D., Seshadri B., Sarkar B., Choppala G., Kirkham M., Bolan N.S. Rhizoremediation as a green technology for the remediation of petroleum hydrocarbon-contaminated soils. J. Hazard Mater. 2021;401 doi: 10.1016/j.jhazmat.2020.123282. [DOI] [PubMed] [Google Scholar]
- Hu G., Li J., Zeng G. Recent development in the treatment of oily sludge from petroleum industry: a review. J. Hazard Mater. 2013;261:470–490. doi: 10.1016/j.jhazmat.2013.07.069. [DOI] [PubMed] [Google Scholar]
- Islam B. Petroleum sludge, its treatment and disposal: a review. Int. J. Chem. Sci. 2015;13(4):1584–1602. [Google Scholar]
- Jahromi H., Adhikari S., Roy P., Shelley M., Hassani E., Oh T.-S. Synthesis of novel biolubricants from waste cooking oil and cyclic oxygenates through an integrated catalytic process. ACS Sustain. Chem. Eng. 2021;9(40):13424–13437. [Google Scholar]
- Jasmine J., Mukherji S. Evaluation of bioaugmentation and biostimulation effects on the treatment of refinery oily sludge using 2 n full factorial design. Environ. Sci. J. Integr. Environ. Res.: Process. Impacts. 2014;16(8):1889–1896. doi: 10.1039/c4em00116h. [DOI] [PubMed] [Google Scholar]
- Karam Q., Al-Wazzan Z. Toxicity of petroleum hydrocarbons to Brachyuran crabs: a review of deleterious effects of oil-related xenobiotics on life stages. J. Mar. Biol. Assoc. U. K. 2021:1–16. [Google Scholar]
- Khan M.A.I., Biswas B., Smith E., Naidu R., Megharaj M. Toxicity assessment of fresh and weathered petroleum hydrocarbons in contaminated soil-a review. Chemosphere. 2018;212:755–767. doi: 10.1016/j.chemosphere.2018.08.094. [DOI] [PubMed] [Google Scholar]
- Kim Y.M., Ahn C.K., Woo S.H., Jung G.Y., Park J.M. Synergic degradation of phenanthrene by consortia of newly isolated bacterial strains. J. Biotechnol. 2009;144:293–298. doi: 10.1016/j.jbiotec.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Kumar B., Raj Mohan B. Petroleum oily sludge and the prospects of microwave for its remediation. Int. J. Eng. Res. Technol. 2013;2:359–370. [Google Scholar]
- Kumari B., Singh S., Singh D. Induced degradation of crude oil mediated by microbial augmentation and bulking agents. Int. J. Environ. Sci. Technol. 2016;13:1029–1042. [Google Scholar]
- Kurylenko V., Izosimova O. Study of the impact of petroleum hydrocarbons on sea organisms. Journal of Ecological Engineering. 2016;17(1) [Google Scholar]
- Levy B.S., Nassetta W.J. The adverse health effects of oil spills: a review of the literature and a framework for medically evaluating exposed individuals. Int. J. Occup. Environ. Health. 2011;17(2):161–168. doi: 10.1179/107735211799031004. [DOI] [PubMed] [Google Scholar]
- Liu Y., Hu H., Zanaroli G., Xu P., Tang H. A Pseudomonas sp. strain uniquely degrades PAHs and heterocyclic derivatives via lateral dioxygenation pathways. J. Hazard Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123956. [DOI] [PubMed] [Google Scholar]
- Luke M.E., Odokuma L.O. Acute toxicity of crude oil from NNPC and artisanal refineries in Niger Delta on selected aquatic biota. GSC Biol. Pharm. Sci. 2021;15(3):16–24. [Google Scholar]
- Maddela N.R., Scalvenzi L., Venkateswarlu K. Microbial degradation of total petroleum hydrocarbons in crude oil: a field-scale study at the low-land rainforest of Ecuador. Environ. Technol. 2017;38(20):2543–2550. doi: 10.1080/09593330.2016.1270356. [DOI] [PubMed] [Google Scholar]
- Mafiana M.O., Kang X.-H., Leng Y., He L.-F., Li S.-W. Petroleum contamination significantly changes soil microbial communities in three oilfield locations in Delta State, Nigeria. Environ. Sci. Pollut. Res. 2021:1–15. doi: 10.1007/s11356-021-12955-1. [DOI] [PubMed] [Google Scholar]
- Mai C.T.N., Linh N.V., Lich N.Q., Ha H.P., Van Quyen D., Tang D.Y.Y., Show P.L. Advanced materials for immobilization of purple phototrophic bacteria in bioremediation of oil-polluted wastewater. Chemosphere. 2021;278 doi: 10.1016/j.chemosphere.2021.130464. [DOI] [PubMed] [Google Scholar]
- Mannino A., Rodger H.H. Black carbon in estuarine and coastal ocean dissolved organic matter. Limnol. Oceanogr. 2004;49(3):735–740. [Google Scholar]
- Mansur A.A., Pannirselvam M., Al-Hothaly K.A., Adetutu E.M., Ball A.S. Recovery and characterization of oil from waste crude oil tank bottom sludge from Azzawiya oil refinery in Libya. J. Adv. Chem. Eng. 2015;5(1):27200–27227. [Google Scholar]
- Margesin R., Schinner F. Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl. Microbiol. Biotechnol. 2001;56(5):650–663. doi: 10.1007/s002530100701. [DOI] [PubMed] [Google Scholar]
- Müller F., Sedláčková M.K.I. Contamination of soils and groundwater by petroleum hydrocarbons and volatile organic compounds–Case study: ELSLAV BRNO. Bull. Geosci. 2003;78(3):225–239. [Google Scholar]
- Murawski S.A., Grosell M., Smith C., Sutton T., Halanych K.M., Shaw R.F., Wilson C.A. Impacts of petroleum, petroleum components, and dispersants on organisms and populations. Oceanography. 2021;34(1):136–151. [Google Scholar]
- Namukuye A. Makerere University; 2021. Bioremediation of Soil Contaminated Crude Oil by Agaricomycetes. [Google Scholar]
- Nero B.F. Phytoremediation of petroleum hydrocarbon-contaminated soils with two plant species: Jatropha curcas and Vetiveria zizanioides at Ghana Manganese Company Ltd. Int. J. Phytoremediation. 2021;23(2):171–180. doi: 10.1080/15226514.2020.1803204. [DOI] [PubMed] [Google Scholar]
- Nissenbaum A., Goldberg M., Aizenshtat Z. Immature condensate from southeastern Mediterranean coastal plain, Israel. AAPG Bull. 1985;69(6):946–949. [Google Scholar]
- Nwaichi E., Wegwu M., Nwosu U. Distribution of selected carcinogenic hydrocarbon and heavy metals in an oil-polluted agriculture zone. Environ. Monit. Assess. 2014;186(12):8697–8706. doi: 10.1007/s10661-014-4037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGDCL Oil and gas development company limited (OGDCL), Pakistan, Annual Report (2017) 2017. https://ogdcl.com/sites/default/files/Annual-Report-201720171002153148931_0.pdf retrived on March 3, 2022.
- Ossai I.C., Ahmed A., Hassan A., Hamid F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: a review. Environ. Technol. Innovat. 2020;17 [Google Scholar]
- Ozdemir A., Karataş A., Palabiyik Y., Yaşar E., Sahinoglu A. Oil and gas exploration in Seferihisar Uplift (Western Turkey) containing an operable-size gold deposit: geochemical evidence for the presence of a working petroleum system. Geomech. Geophys. Geo-Energy Geo-Resour. 2020;6(1):1–22. [Google Scholar]
- Patel P.N., Parmar K.G., Nakum A.N., Patel M.N., Patel P.R., Patel V.R., Sen D.J. Biodegradable polymers: an ecofriendly approach in newer millennium. Asian J. Biomed. Pharm. Sci. 2011;1(3):1–17. [Google Scholar]
- Patel H.K., Kalaria R.K., Jokhakar P.H., More B.S., Khimani M.R., Patel C.R., Dudhagara P.R. Elsevier; 2021. Membrane Reactors, Membrane-Based Hybrid Processes for Wastewater Treatment; pp. 227–255. [Google Scholar]
- Payne S.J., King C.K., Zamora L.M., Virtue P. Temporal changes in the sensitivity of coastal Antarctic zooplankton communities to diesel fuel: a comparison between single-and multi-species toxicity tests. Environ. Toxicol. Chem. 2014;33(4):882–890. doi: 10.1002/etc.2522. [DOI] [PubMed] [Google Scholar]
- Qi Y.-B., Wang C.-Y., Lv C.-Y., Lun Z.-M., Zheng C.-G. Removal capacities of polycyclic aromatic hydrocarbons (PAHs) by a newly isolated strain from oilfield produced water. Int. J. Environ. Res. Publ. Health. 2017;14:215. doi: 10.3390/ijerph14020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Ren W., Teng Y., Li Z. Evident bacterial community changes but only slight degradation when polluted with pyrene in a red soil. Front. Microbiol. 2015;6:22. doi: 10.3389/fmicb.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan T., Rajendran P., Nandakumar N., Lokeshkumar B., Rajendran P., Nishigaki I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015;5(3):182–189. [Google Scholar]
- Roushdy M., El Nady M., Mostafa Y., El Gendy N.S., Ali H. Biomarkers characteristics of crude oils from some oilfields in the Gulf of Suez, Egypt. J. Am. Sci. 2010;6(11):911–925. [Google Scholar]
- Roy A., Pal S., Kazy S.K., Sarkar P., Sar P., Ghoshal A.K. Characterization of culturable bacterial communities in petroleum hydrocarbon contaminated sludge of oil refineries and oil exploration sites. J. Environ. Res. Dev. 2014;8:451. [Google Scholar]
- Roy A., Dutta A., Pal S., Gupta A., Sarkar J., Chatterjee A., Saha A., Sarkar P., Sar P., Kazy S.K. Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour. Technol. 2018;253:22–32. doi: 10.1016/j.biortech.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Sattar S., Jehan S., Siddiqui S. Potentially toxic metals in the petroleum waste contaminated soils lead to human and ecological risks in Potwar and Kohat Plateau, Pakistan: Application of multistatistical approaches. Environ. Technol. Innov. 2021;22 [Google Scholar]
- Sayed K., Baloo L., Sharma N.K. Bioremediation of Total Petroleum Hydrocarbons (TPH) by bioaugmentation and biostimulation in water with floating oil spill containment booms as bioreactor basin. Int. J. Environ. Res. Publ. Health. 2021;18(5):2226. doi: 10.3390/ijerph18052226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta K., Pal S. A review on microbial diversity and genetic markers involved in methanogenic degradation of hydrocarbons: futuristic prospects of biofuel recovery from contaminated regions. Environ. Sci. Pollut. Res. 2021:1–20. doi: 10.1007/s11356-021-13666-3. [DOI] [PubMed] [Google Scholar]
- Serafim A., Lopes B., Company R., Ferreira A., Bebianno M. Comparative petroleum hydrocarbons levels and biochemical responses in mussels from hydrothermal vents (Bathymodiolus azoricus) and coastal environments (Mytilus galloprovincialis) Mar. Pollut. Bull. 2008;57(6-12):529–537. doi: 10.1016/j.marpolbul.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Shahzad A., Siddiqui S., Bano A., Sattar S., Hashmi M.Z., Qin M., Shakoor A. Hydrocarbon degradation in oily sludge by bacterial consortium assisted with alfalfa (Medicago sativa L.) and maize (Zea mays L.) Arabian J. Geosci. 2020;13(17):1–12. [Google Scholar]
- Sharma I. IntechOpen; 2020. Bioremediation techniques for polluted environment: concept, advantages, limitations, and prospects. (Trace Metals in the Environment-New Approaches and Recent Advances). [Google Scholar]
- Siddiqui S., Sattar S., Bano A., Shahzad A. Quantifying the effect of microbial consortium and alfalfa to accelerate the degradation of oily sludge. J. Himal. Earth Sci. 2016;49(1):117. [Google Scholar]
- Singh P., Jain R., Srivastava N., Borthakur A., Pal D., Singh R., Madhav S., Srivastava P., Tiwary D., Mishra P.K. Current and emerging trends in bioremediation of petrochemical waste: a review. Crit. Rev. Environ. Sci. Technol. 2017;47(3):155–201. [Google Scholar]
- Sridhar A., Ponnuchamy M., Kumar P.S., Kapoor A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: a review. Environ. Chem. Lett. 2021;19(2):1715–1735. doi: 10.1007/s10311-020-01126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M., Srivastava A., Yadav A., Rawat V. IntechOpen; 2019. Source and Control of Hydrocarbon Pollution, Hydrocarbon Pollution and its Effect on the Environment. [Google Scholar]
- Swannell R., Mitchell D., Lethbridge G., Jones D., Heath D., Hagley M., Jones M., Petch S., Milne R., Croxford R. A field demonstration of the efficacy of bioremediation to treat oiled shorelines following the Sea Empress incident. Environ. Technol. 1999;20:863–873. [Google Scholar]
- Thangalazhy G.S., Adhikari S., Chattanathan S.A., Gupta R.B. Catalytic pyrolysis of green algae for hydrocarbon production using H+ ZSM-5 catalyst. Bioresour. Technol. 2012;118:150–157. doi: 10.1016/j.biortech.2012.05.080. [DOI] [PubMed] [Google Scholar]
- Tsutsumi H., Kono M., Takai K., Manabe T., Haraguchi M., Yamamoto I., Oppenheimer C. Bioremediation on the shore after an oil spill from the Nakhodka in the Sea of Japan. III. Field tests of a bioremediation agent with microbiological cultures for the treatment of an oil spill. Mar. Pollut. Bull. 2000;40:320–324. [Google Scholar]
- Turner N.R., Parkerton T.F., Renegar D.A. Toxicity of two representative petroleum hydrocarbons, toluene and phenanthrene, to five Atlantic coral species. Mar. Pollut. Bull. 2021;169 doi: 10.1016/j.marpolbul.2021.112560. [DOI] [PubMed] [Google Scholar]
- Varjani S.J., Rana D.P., Jain A.K., Bateja S., Upasani V.N. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int. Biodeterior. Biodegrad. 2015;103:116–124. [Google Scholar]
- Varjani S., Upasani V.N., Pandey A. Bioremediation of oily sludge polluted soil employing a novel strain of Pseudomonas aeruginosa and phytotoxicity of petroleum hydrocarbons for seed germination. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.139766. [DOI] [PubMed] [Google Scholar]
- Venosa A.D., Suidan M.T., Wrenn B.A., Strohmeier K.L., Haines J.R., Eberhart B.L., King D., Holder E. Bioremediation of an experimental oil spill on the shoreline of Delaware Bay. Environ. Sci. Technol. 1996;30:1764–1775. [Google Scholar]
- Wang S.-Y., Kuo Y.-C., Hong A., Chang Y.-M., Kao C.-M. Bioremediation of diesel and lubricant oil-contaminated soils using enhanced landfarming system. Chemosphere. 2016;164:558–567. doi: 10.1016/j.chemosphere.2016.08.128. [DOI] [PubMed] [Google Scholar]
- Wang C., Dippold M.A., Blagodatskaya E., Dorodnikov M. Oxygen matters: short-and medium-term effects of aeration on hydrolytic enzymes in a paddy soil. Geoderma. 2022;407 [Google Scholar]
- Waples D.W. Springer Science & Business Media; 2013. Geochemistry in Petroleum Exploration. [Google Scholar]
- Welte D., Tissot P. Springer; 1984. Petroleum Formation and Occurrence. [Google Scholar]
- WHO . World Health Organization; 1984. International Program on Chemical Safety.https://iris.paho.org/bitstream/handle/10665.2/6639/25908.pdf?sequence=1&isAllowed=y retrived on Jan 28 2022. [Google Scholar]
- Wilkes H., Jarling R., Schwarzbauer J. 2020. Hydrocarbons and Lipids: an Introduction to Structure, Physicochemical Properties, and Natural Occurrence. Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; pp. 3–48. [Google Scholar]
- Wu M., Ye X., Chen K., Li W., Yuan J., Jiang X. Bacterial community shift and hydrocarbon transformation during bioremediation of short-term petroleum-contaminated soil. Environ. Pollut. 2017;223:657–664. doi: 10.1016/j.envpol.2017.01.079. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lu M. Bioremediation of crude oil-contaminated soil: comparison of different biostimulation and bioaugmentation treatments. J. Hazard Mater. 2010;183:395–401. doi: 10.1016/j.jhazmat.2010.07.038. [DOI] [PubMed] [Google Scholar]
- Xu G., Liu H., Li M., Li Z., Peng Z., Zuo L., He X., Liu W., Cai L. In situ bioremediation of crude oil contaminated site: a case study in Jianghan oil field, China. Petrol. Sci. Technol. 2016;34:63–70. [Google Scholar]
- Yadav K.K., Gupta N., Kumar A., Reece L.M., Singh N., Rezania S., Khan S.A. Mechanistic understanding and holistic approach of phytoremediation: a review on application and future prospects. Ecol. Eng. 2018;120:274–298. [Google Scholar]
- Yavari S., Malakahmad A., Sapari N.B. A review on phytoremediation of crude oil spills. Water Air Soil Pollut. 2015;226(8):1–18. [Google Scholar]
- Zabbey N., Olsson G. Conflicts–oil exploration and water. Glob. Chall. 2017;1(5) doi: 10.1002/gch2.201600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Bao D., Li M., Tang Q., Wu M., Zhou H., Liu L., Qu Y. Bioremediation of petroleum hydrocarbons by alkali–salt-tolerant microbial consortia and their community profiles. J. Appl. Chem. Biotechnol. 2021;96:809–817. [Google Scholar]
- Zhao F., Li P., Guo C., Shi R.-J., Zhang Y. Bioaugmentation of oil reservoir indigenous Pseudomonas aeruginosa to enhance oil recovery through in-situ biosurfactant production without air injection. Bioresour. Technol. 2018;251:295–302. doi: 10.1016/j.biortech.2017.12.057. [DOI] [PubMed] [Google Scholar]
- Zhen L., Hu T., Lv R., Wu Y., Chang F., Gu J. Succession of microbial communities and synergetic effects during bioremediation of petroleum hydrocarbon-contaminated soil enhanced by chemical oxidation. J. Hazard Mater. 2021;410 doi: 10.1016/j.jhazmat.2020.124869. [DOI] [PubMed] [Google Scholar]
- Zhou H., Huang X., Bu K., Wen F., Zhang D., Zhang C. Fungal proliferation and hydrocarbon removal during biostimulation of oily sludge with high total petroleum hydrocarbon. Environ. Sci. Pollut. Res. 2019;26(32):33192–33201. doi: 10.1007/s11356-019-06432-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.