Abstract
Acute myeloid leukemia (AML) is one of the most prevalent and acute blood cancers with a poor prognosis and low overall survival rate, especially in the elderly. Although several new AML markers and drug targets have been recently identified, the rate of long-term cancer eradication has not improved significantly due to the presence and drug resistance of AML cancer stem cells (CSCs). Here we develop a novel computational pipeline to analyze the transcriptomic profiles of AML cancer (stem) cells and identify novel candidate AML CSC markers and drug targets. In our novel pipeline we apply a top-down meta-analysis strategy to integrate The Cancer Genome Atlas data with CSC datasets to infer cell stemness features. As a result, a set of genes termed the “AML key CSC genes” along with all the available drugs/compounds that could target them were identified. Overall, our novel computational pipeline could retrieve known cancer drugs (Carfilzomib) and predicted novel drugs such as Zonisamide, Amitriptyline, and their targets amongst the top ranked drugs and drug targets for targeting AML. Additionally, the pipeline applied in this study could be used for the identification of CSC-specific markers, drivers and their respective targeting drugs in other cancer types.
Keywords: AML, Cancer stem cells, Drug repurposing, Zonisamide, Carfilzomib, Amitriptyline, CA1, MAOB, TNFSF4
AML; Cancer stem cells; Drug repurposing; Zonisamide; Carfilzomib; Amitriptyline; CA1; MAOB; TNFSF4.
1. Introduction
Acute myeloid leukemia (AML) is the most prevalent acute leukemia in adults, with a 35–40% 5-year overall survival rate for patients younger than 60 years and median overall survival of about one year, with older patients usually having a poorer prognosis (De Kouchkovsky and Abdul-Hay, 2016; Thomas and Majeti, 2017). The incidence of AML is high, with over 20,000 cases per year in the United States alone (De Kouchkovsky and Abdul-Hay, 2016). Genomic mutations in the genes involved in differentiation and hematopoietic proliferation and large chromosomal translocations are the major players in AML development (De Kouchkovsky and Abdul-Hay, 2016). Recent technological advancements have enabled faster identification of the molecular basis of AML progression including genomic, epigenomic, transcriptomic, and proteomic markers, which are useful in diagnosis, prognosis and monitoring of AML and have led to the discovery of several therapeutic agents for various indications in AML (Kantarjian et al., 2021; Prada-Arismendy et al., 2017).
However, despite all of these advancements and the success of chemotherapy and adjuvant therapies in shrinking leukemic blasts, the long-term outcomes have not enhanced notably for over 3 decades. The cancer stem cell (CSC) model suggests that both CSCs and non-stem-like cancer cells need to be eradicated to achieve long-term remissions (Thomas and Majeti, 2017). AML CSCs, first reported nearly 2 decades ago, are defined as leukemic cells with a transcriptomic and epigenomic signature resembling that of haematopoietic stem cells (HSCs) and normal multipotent haematopoietic progenitors, and are capable of both self-renewal and proliferation and generation of progenies. Consequently these cells show a sustained survival in engraftments into immunocompromised mice as well as optimized ex vivo co-culture systems (Thomas and Majeti, 2017; Vetrie et al., 2020). Accordingly, the CSCs that remain after initial treatments result in a poor long-term prognosis and cancer recurrence. Though several drugs such as Valemetostat and Rapamycin have been proposed for targeting drug-resistant AML CSCs, most of them have not entered clinical trials yet (van Gils et al., 2021). Furthermore, single-cell RNA-sequencing has demonstrated that AML CSCs are composed of distinct dormant and active compartments (Sachs et al., 2020). Dormant CSCs, which are the major cause of cancer recurrence (Damen et al., 2021), could be transformed into active ones through various pathways such as the activation of Wnt signaling pathway (Yang et al., 2020a). This highlights the importance of characterization and targeting of both sub-classes of AML CSC for thorough eradication of cancer cells and prevention of cancer relapse. However, to date the markers and drivers of different sub-classes of AML CSC have not been systematically characterized and no drug has been confirmed to be used in the clinics for specific targeting of different sub-classes of CSCs.
Several human AML cell lines such as K562, HL-60, and KG-1 have also been generated thus far which contain both cancer stem and non-stem cells, and provide model systems for studying the cellular and molecular biology, characteristics, and perhaps therapy of human AML in vitro (Koeffler and Golde, 1980). From a transcriptomic viewpoint, KG1 is one of AML cell lines that best resembles primary AML tissues and also has the AML FAB subtype closest to the microarray primary sample subtypes we examine in this study (Yu et al., 2019).
The molecular profiles of cancer cells could be investigated from a variety of different aspects, one of which is the holistic and top-down approach using systems biology techniques. Bottom-up systems biology approaches that involve gene-level interrogations, literature mining, and dynamic modellings have thus far been applied to many cancer types to study their CSC characteristics (Mertins, 2014). However, due to the challenges in separation of CSCs from non-stem-like cancer cells, amongst many other challenges in dealing with CSCs (Dobbin and Landen, 2013), very few studies have been performed on the non-genomic cell high-throughput profiles of CSCs. Furthermore, although some bottom-up systems biology approaches and mathematical modelling have been performed on AML leukemic stem cells (Vaidya, 2014), to the best of our knowledge, no comprehensive top-down systems biology study has been performed so far on the omics profiles of AML CSCs. The benefit of such an approach is that the biological system of an omics profile of cells is inspected from a holistic viewpoint and could be separated into functional modules that are assembled to collaboratively form a complete system. In this regard, the transcriptomic profiles of cancer cells could be inspected to extract significant associations between genes in order to reconstruct such functional networks. By definition, a functional module is composed of biomolecules that perform a particular function (Kurata et al., 2014). This top-down approach offers the opportunity to interrogate the characteristics of a biological systems at the pathway level rather than focusing on single genes. Also, such association networks could be extended by projecting them to the drug-target interaction data to detect all the available drugs that could target their most influential molecules, a process termed as “drug repurposing”. Hence, several candidate drugs could be identified at considerably lower cost and time to either induce cancer cell death or suppress various cancer hallmarks such as uncontrolled cell proliferation (Sleire et al., 2017). Additionally, association networks and their functional modules can be further inspected to identify their most influential building blocks for further filtration as well as identification of key drivers of a process, consequently identifying the most efficient drugs.
To date, most of the studies on the stemness characteristics of AML cells such as self-renewal and proliferation and CSC-related drug resistance have been performed in the context of mutation profiles of cancer cells. In this approach, the functional molecular profiles of cells (i.e. their transcriptome and proteome) are not considered, which makes it impossible to directly infer the phenotypic and functional features of CSCs. In this study, we applied a novel computational pipeline by recruiting several bioinformatics and systems biology techniques to interrogate the transcriptomic profiles of AML cancer (stem) cells with the purpose of identifying novel candidate AML CSC markers and drug targets and to reposition already available drugs to them. The selection of the right candidate compound is though challenging and no non-anti-cancer repurposed drug has been approved for AML management so far (Valli et al., 2020). Here, we contend that our data-/meta-analysis and filtration pipeline together with the applied scoring functions have opened up new avenues and enabled the identification of important candidate AML CSC drug targets and drugs, which were further confirmed using in vitro assays.
2. Results
2.1. CSC study pipeline
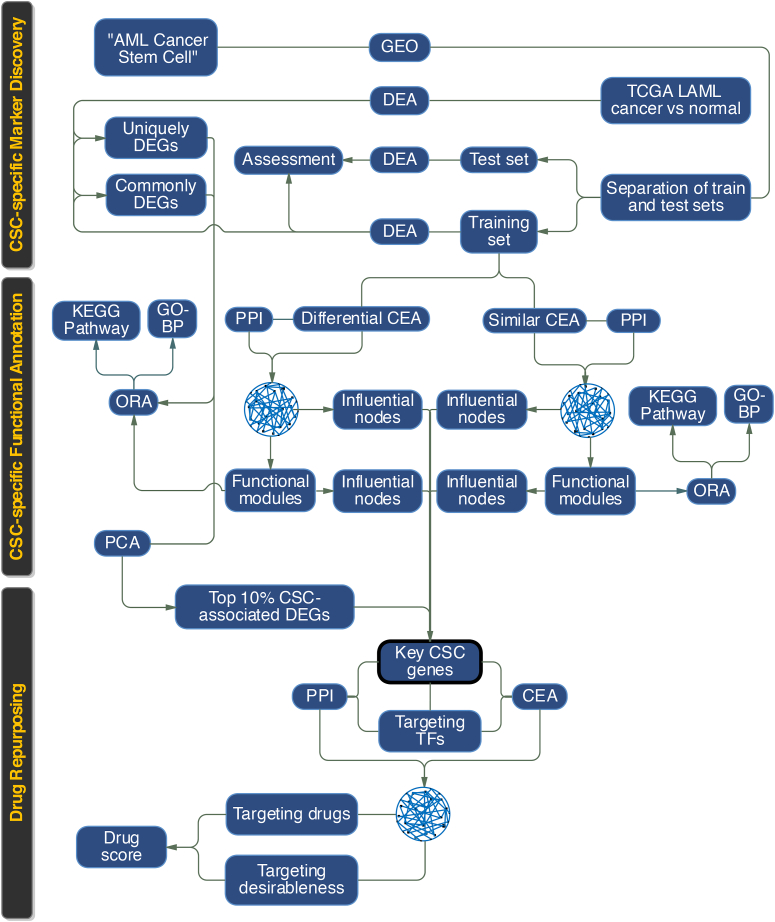
To study AML CSCs, characterize their distinctive sets of markers and drivers and their functional roles, and to evaluate their druggability as well as their targeting desirableness we developed a novel computational pipeline schematically outlined in Figure 1. This pipeline could be divided into three major consecutive sections;
-
1)
CSC-specific marker discovery based on the intersection analysis of differentially expressed genes (DEGs) of CSC vs non-stem-like cancer cells, hereinafter called general tumor cells (GTCs), and The Cancer Genome Atlas (TCGA) cancer vs normal samples. This step firstly helps identification of CSC-associated distinctive genes compared with GTCs, and subsequently filtering out those genes that have the same expression variation in normal vs. cancer. In other words, a gene that is up-regulated, for instance, in CSC vs. GTC, and is up-regulated in TCGA normal vs. cancer as well will be filtered out;
-
2)
CSC-specific functional annotation according to the similar/differential association-based network analysis followed by the over-representation analysis (ORA) of functional modules of networks as well as CSC-specific markers on biological processes and pathways;
-
3)
drug repurposing via the assessment of druggability of the association network of CSCs and their targeting transcription factors (TFs).
Figure 1.
Schematic outline of research purposes and workflows. The abbreviations used in the figure are described in the following. GEO: gene expression omnibus; TCGA: the cancer genome atlas; DEA: differential expression analysis; CSC: cancer stem cell; DEGs: differentially expressed genes; CEA: co-expression analysis; PPI: protein-protein interaction; PCA: principal component analysis; ORA: over-representation analysis; TFs: transcription factors.
2.2. Optimal datasets to study AML CSCs
To apply our pipeline for the study of AML CSCs, and to elicit the features that are specific to AML CSCs rather than being common across all AML cancer cells, we used AML transcriptomic datasets that included the distinctive expression profiles of AML CSCs and GTCs. The cell surface expression of CD34 and CD38 is usually considered as the immunophenotypic characteristics of AML CSCs and for their distinction from GTCs (Spyrou and Papapetrou, 2020). However, as different studies have detected different expression levels of these two markers for the detection of AML CSCs, besides several other markers that are being used for this purpose (Yabushita et al., 2018), currently there is no universal marker or combination of markers for the isolation of AML CSCs (Spyrou and Papapetrou, 2020). Having said that, functional validation of marker-based isolated AML CSCs based on xenotransplantation assays is considered as the “gold standard” for the precise separation of AML CSCs (Hokland et al., 2019; Spyrou and Papapetrou, 2020). Accordingly, a single microarray dataset (GSE30375) was selected based on a rigorous search and filtration strategy and was obtained from the gene expression omnibus (GEO) repository. Particularly, this dataset was the only human primary sample-based transcriptomic dataset that its corresponding samples were primarily sorted and separated into AML CSCs and GTCs and their AML-initiating activity was functionally validated based on xenotransplant assays, and that its respective samples/patients had not undergone any treatment, administration or gene knockout. We filtered out datasets generated from the treatment and administration studies so as to accurately extract AML CSC-specific features inherent to the biology of CSCs rather than standing out due to perturbations. The selected transcriptomic dataset contains the microarray-based intensity profiles of total RNA extracted from CSCs and GTCs that were initially separated on the basis of surface expression of CD34 and CD38 molecules using Fluorescence-activated Cell Sorting (FACS) and were further functionally validated by xenotransplantation assays (Eppert et al., 2011). The systems biology approaches adapted in our pipeline facilitated the interrogation of the transcriptomic profile of AML CSCs in a holistic and systematic way, and the meta-analysis of TCGA data and AML CSC dataset enabled the accurate distinction of CSC-associated genes.
2.3. Several genes were differentially expressed in AML CSCs compared with GTCs
We initially performed a differential expression analysis on the transcriptomic profiles of CSCs versus GTCs based on the limma model (Ritchie et al., 2015). This analysis would facilitate the identification of genes that have a differential expression and behaviour in CSCs compared with GTCs and is served as a steppingstone for downstream analyses for the filtration and prioritization of CSC-associated markers and key drivers. Also, the differential expression analysis of TCGA cancer vs normal samples would help to further filter CSC-specific genes, as explained above. The differential expression analyses demonstrated that 425 and 10,388 genes were significantly differentially expressed (p.adj <0.05 and |log2FC| > 1) in CSCs compared with general tumor cells (GTCs, those cancer cells that do not have stemness features) as well as in TCGA heterogenous tumor cell (HTC, combination of CSCs and GTCs) samples compared with normal ones, respectively (Raw data output 1 at https://doi.org/10.26180/21259491.v1). The differential expression analysis of the TCGA LAML dataset was done using the limma method, p. adj <0.05 and |log2FC| > 1 available on the GEPIA2 web server. Moreover, the intersection analyses showed that while 91 genes were commonly differentially expressed in the same direction in AML CSC/GTC microarray and TCGA bulk RNA-seq datasets, 108 genes were uniquely differentially expressed in CSCs vs GTCs but not in the TCGA dataset (Raw data output 2 at https://doi.org/10.26180/21259491.v1). We hypothesize that these uniquely DEGs, hereinafter termed as “CSC-specific DEGs”, are characteristic features of AML CSCs.
Moreover, to ascertain the validity of these results we performed a limma-model-based differential expression analysis on a randomly selected subset of AML CSC/GTC samples. This subset was primarily selected based on the 80–20 principle and was set aside as the test set before performing any analysis. Notably, the differential expression assessments showed that ∼94 percent of the training set-based DEGs including all CSC-specific DEGs were also differentially expressed with the same direction in the test dataset (Raw data output 3 at https://doi.org/10.26180/21259491.v1).
The unsupervised principal component analysis (PCA) of the expression data of both uniquely and commonly DEGs demonstrated that the transcriptomic profile of these genes could separate CSCs from GTCs (Figure 2A,B). Also, the PCA-based rotation analysis of these genes indicated that the co-variations of the expression profiles of some of these DEGs (over-expressed ones) were associated with CSC samples (Figure 2C,D). Similarly, the expression the co-variations of under-expressed DEGs were characteristic features of GTCs (Figure 2C,D). Additionally, the unsupervised hierarchical clustering illustrated that samples of CSCs and GTCs are separated from each other according to both uniquely (Supplementary figure S1) and commonly (Supplementary figure S2) DEGs.
Figure 2.
Scattering of expression profiles of AML cells and biological processes associated with AML CSC-specific DEGs. A. Scatter plot of PC1 and PC2 of principle component analysis of CSC and GTC samples based on the expression profile of CSC-specific DEGs. B. Scatter plot of PC1 and PC2 of principle component analysis of CSC and GTC samples based on the expression profile of commonly DEGs. C. Scatter plot of the principal components one and two of rotation values of CSC-specific DEGs. D. Scatter plot of the principal components one and two of rotation values of commonly DEGs. E. The biological processes significantly associated with AML CSC-specific DEGs. The parent biological processes are ordered based on the average combined scores of all of their corresponding child terms. The color of dots represents the statistical significance of the over-representation of AML CSC-specific DEGs on the respective biological processes. Pure red dots indicate the biological processes with a higher statistical significance. PC, DEGs, CSC, and GTC are abbreviations of principal component, differentially expressed genes, cancer stem cell, and general tumor cell, respectively.
To assess the biological processes that are either specific to AML CSCs or common between CSCs and GTCs, an ORA was performed on the CSC-associated DEGs and commonly DEGs, separately. This demonstrated that CSC-associated DEGs (Figure 2E) and commonly DEGs (Supplementary figure S3) were significantly (p-value < 0.01) enriched in different biological processes (BPs), meaning that different processes drive the specific characteristics of AML CSCs. More precisely, while the BPs enriched in CSC-associated DEGs (e.g. positive regulation of killing of cells of other organism (Djomehri et al., 2020), as the most significant one) were stemness characteristics of cancer cells, those of commonly DEGs (e.g. regulation of cell population proliferation (Silva et al., 2021; van Dijk et al., 2021; Zhou and Chen, 2021), as the most significant one) were relevant to general characteristics of AML cells.
2.4. Evaluation of the specificity of CSC-associated features to AML
To assess if our identified CSC markers, drivers and their corresponding pathways are specific to AML, we sought to compare our AML results against CSC-features obtained from other cancer-types. To achieve this, we searched the GEO database for transcriptomic datasets from other cancer types, having both CSCs and GTCs profiles. The breast cancer microarray dataset GSE52327 (Raw data output 4 at https://doi.org/10.26180/21259491.v1) was the only one that met the same selection criteria as the AML dataset. The CSCs and GTCs in this dataset were initially FACS-sorted and separated based on the cell marker ALDH and were subsequently functionally validated using primary xenografts (Liu et al., 2014). By applying the same differential expression analysis depicted in Figure 1 to this dataset, we first identified 120 significant DEGs (padj <0.05) between CSCs and GTCs in breast cancer (Raw data output 5 at https://doi.org/10.26180/21259491.v1), highlighting the differential transcriptomic profiles of CSCs and GTCs in breast cancer. Of those, 52 genes overlapped with DEGs between CSCs and GTCs in AML but interestingly, 373 out of 425 (88%) AML CSC-associated DEGs were specific to AML. These results indicate the majority of AML-CSC predicted driver genes were AML-specific.
Second, to further explore the cancer-type specificity for these features, we classified significantly DEGs between breast CSCs and GTCs into uniquely and commonly DEGs based on the TCGA BRCA dataset. 80/120 DEGs from the breast had concordant deferential expression direction between the microarray breast cancer and TCGA BRCA datasets. Of these 80 DEGs, 77 were uniquely DEGs in breast microarray but not TCGA dataset. Of these 77 DEGs, only 1 overlapped with the 108 uniquely DEGs in AML, and none overlapped with the 91 commonly DEGs in AML. This further highlights the cancer type-specificity of CSC-associated markers and drivers.
Third, we investigated the similarities or differences in the biological processes driven by the CSC-associated DEGs in each cancer type. To achieve this, we performed ORA on the 77 breast CSC-associated DEGs (i.e. uniquely DEGs; Raw data output 6 at https://doi.org/10.26180/21259491.v1), using the same methodology used for the AML dataset. We found that CSC-associated DEGs of breast cancer (Supplementary figure S4) and AML (Figure 2E) are enriched in different biological processes, that are all relevant to cancer (stem) cell function (Davis et al., 2008, 2021). For instance, the “GO:0060558; regulation of calcidiol 1-monooxygenase activity”, had the second highest average combined score in GO-BPs associated with uniquely DEGs in the AML dataset, and is not enriched in the breast cancer dataset. This biological pathway is linked with vitamin D function, deficiency of which has been reported in AML patients (Seyedalipour et al., 2017). In addition, the expression level of vitamin D receptor and the amount of active vitamin D inside AML cells is known to control their stemness (Paubelle et al., 2020). On the other hand, the “GO:0051283; negative regulation of sequestering of calcium ion”, had the second highest average combined score in GO-BPs associated with uniquely DEGs in breast cancer dataset, and not enriched in AML dataset. This pathway has been linked with breast CSC functioning. Indeed, increased cytosolic calcium ion stimulates breast CSC enrichment through activation of PYK2-SRC-STAT3 signaling pathway (Lu et al., 2017) as well as elevated collaboration with Wnt signaling pathway (Yang et al., 2020a).
Altogether, these analyses support the interpretation that the features predicted are specific to AML CSCs.
2.5. Several genes were differentially co-expressed in AML CSCs versus GTCs
To systematically interrogate the gene sets and biological processes and pathways that are either specifically or commonly active in CSCs versus GTCs, we performed co-expression network analysis. First, we found 1692 genes that were statistically significantly differentially co-expressed (p-value < 0.05) between CSC and GTC samples. Also, these genes had high levels of correlation (r > 0.91 and p. adj <0.02) with each other within the set of CSC samples (Raw data output 7 at https://doi.org/10.26180/21259491.v1). Hereinafter, we term this set of differentially co-expressed genes (CEGs) as “CSC-specific CEGs”. Moreover, the protein-protein interaction (PPI) analysis using the STRING database (Szklarczyk et al., 2019) demonstrated that several of these CSC-specific CEGs had significant physical interactions (experimental interaction score >0.7) with each other (Raw data output 7 at https://doi.org/10.26180/21259491.v1).
Second, we found 1108 genes that were statistically significantly commonly co-expressed (r > 0.7 and p. adj <0.02) with each other in CSCs and GTCs (Raw data output 7 at https://doi.org/10.26180/21259491.v1). Furthermore, according to the PPI analyses, several of these commonly CEGs were involved in protein-protein interactions (experimental interaction score >0.7) with each other (Raw data output 7 at https://doi.org/10.26180/21259491.v1). These results indicate that different multi-omics (i.e. transcriptomic and proteomic) regulatory networks could be functioning in different subclasses of AML cells which we sought to investigate next.
2.6. Different biological processes and pathways were differentially and commonly modulated in different subclasses of AML cells
Initially, two differential and common multi-omics networks were reconstructed from the integration of differential and common co-expression and PPI data, respectively. Then, the functional modules of each multi-omics network were identified using Markov Clustering (MCL), a random walk-based graph clustering algorithm, which indicated that variant functional modules were associated with different subclasses of AML cells. Subsequently, an ORA was performed on the functional modules of the multi-omics network of CSC-specific CEGs to identify their associated biological processes and KEGG pathways. The results demonstrated that several biological processes and pathways were specifically modulated (p.adj <0.05) in AML CSCs (Figure 3), which were associated with leukemic stem cell activity (e.g. Tyrosine metabolism (Luo et al., 2020; Reikvam et al., 2016); module 1).
Figure 3.
Functional modules, biological processes and pathways of the association network of differentially co-expressed genes. The heading above each module is the first ranked KEGG pathway associated with that module. The border color of nodes is set according to the differential expression of genes in CSCs compared with GTCs; grey color indicates genes with no significant differential expression, red color is indicative of overexpression, and blue color represents under-expressed genes. The shape of nodes is illustrative of differential expression type of genes; diamond nodes indicate genes that are commonly differentially expressed in AML CSCs and TCGA HTCs with the same direction of differential expression, rectangle nodes shows genes that are uniquely differentially expressed in CSCs, and circle nodes indicate nodes that are not significantly differentially expressed between CSCs and GTCs. It should also be noted that the genes that are commonly differentially expressed in CSCs and TCGA HTCs with opposite directions of differential expression were also considered as genes with no significant differential expression. The colors of each node correspond to the GO-BP terms they are associated with according to the over-representation analysis. However, nodes with light blue color indicate nodes with no association with any of the selected GO-BP terms. The edge line shape corresponds to the type of connection; the solid line is indicative of co-expression, while, sinewave lines demonstrate PPI between nodes. CEGs, CSC, GTC, HTC, and GO-BP are abbreviations of co-expressed genes, cancer stem cell, general tumor cell, heterogenous tumor cell, and gene-set enrichment analysis, respectively. All of the non-modulated nodes, as well as modules with less than 10 nodes, are not shown in the network. Nodes of each module are sorted according to their degrees.
By contrast, the ORA based on the functional modules of the integrative network of commonly CEGs revealed the shared biological processes and pathways between AML CSCs and GTCs relevant to both stemness (e.g. T cell receptor signaling pathway (Majeti et al., 2009) and hemoglobin metabolic process (Handschuh, 2019); module 3) and general (e.g. Ribosome pathway (Huang et al., 2019; Vegi et al., 2016); module 2) characteristics of AML cells (Supplementary figure S5). These biological processes and their respective key driver genes could be considered for co-targeting of AML CSCs and GTCs.
Next, the topology of integrative networks was analyzed to identify intra- and inter-modular most influential nodes (genes). Accordingly, 49 genes in the multi-omics networks of differentially CEGs and their modules were identified as the most influential nodes. These most influential genes along with the top 10% of the CSC-associated DEGs (10 genes) according to their PCA-derived rotation (loading) values were considered as “AML key CSC genes” and their connectivity were inspected using the STRING database and visualized using the Cytoscape software (Shannon et al., 2003) (Figure 4; Supplementary table S1). This illustrates that the candidate AML key CSC genes are associated with each other (mean interaction score: 0.46).
Figure 4.
The connectivity network of AML key CSC genes. The connectivity and interaction of AML key CSC genes with each other based on the STRING database. These AML key CSC were stemmed from multi-omics association networks and principal component analyses. CSC is the abbreviation of cancer stem cell.
2.7. AML key CSC genes were functionally relevant to AML
In order to further assess the relevance/importance of the predicted key CSC genes to AML, we performed in silico functional assays to comprehensively evaluate the essentiality of these genes for AML cell viability and development. For this we interrogated the AML key CSC genes in databases of CRISPR-based functional assays. The functional (essentiality) assessment of these genes using the BioGRID ORCS database (Oughtred et al., 2021) demonstrated that the 49 out of 59 of these genes (83%) were significantly associated with the development or drug resistance of AML/leukemia cancer cell lines (Supplementary table S2). Furthermore, interrogation of the loss-of-function experiments via CRISPR studies on the DepMap portal (Ghandi et al., 2019) revealed the importance of the majority of these genes for AML cell viability: 39 out of 59 of the AML key CSC genes (66%) showed a significant dependence score (negative score) to the viability of cells in over 50% of AML cell lines (Supplementary figure S6), supporting that the genes are required for the viability of AML cancer cell lines.
As an example, the evaluation of genomic alterations of TNFSF4 on the DepMap portal showed that its copy numbers were increased in 51 AML cell lines but were not decreased in any cell line (0.921 < Copy Number log2 (relative to ploidy +1) < 1.39). Of note, TNFSF4 was also significantly up-regulated in both AML CSCs versus GTCs and TCGA AML samples versus normal ones. Furthermore, according to the COSMIC database (Tate et al., 2019), while none of the samples of the TCGA AML dataset showed a copy number loss, only one of the samples showed a copy number gain (TCGA-GR-A4D9-01) in TNFSF4. This might indicate that in vivo TNFSF4 is up-regulated through non-genomic associated alterations. The interrogation of the DepMap portal also demonstrated that TNFSF4 contains protein-changing mutations in two blood (leukemia) cell lines including PL21 and JURKAT. In addition, the interrogation of TNFSF4 on the Cancer Gene and Pathway Explorer (CGPE) (Liu et al., 2021a) web server indicated that TNFSF4 is highly active in several relevant KEGG pathways such as Acute Myeloid Leukemia, other well-known cancer-related pathways (e.g. JAK-STAT signalling pathway), and pathways relevant to the stemness level of hematopoietic cells (e.g. Hematopoietic cell lineage) in AML cell lines (Supplementary figure S7). Altogether, alterations and high level of activity and expression of TNFSF4, as an instance of AML key CSC gene, are functionally important for the development, regulation, and progression of AML primary and in vitro (cell line) samples and their characteristics including stemness. This highlights the priority of predicted AML key CSC genes in future AML treatment and drug designing/discovery studies.
2.8. AML key CSC genes could characterize different subclasses of CSCs
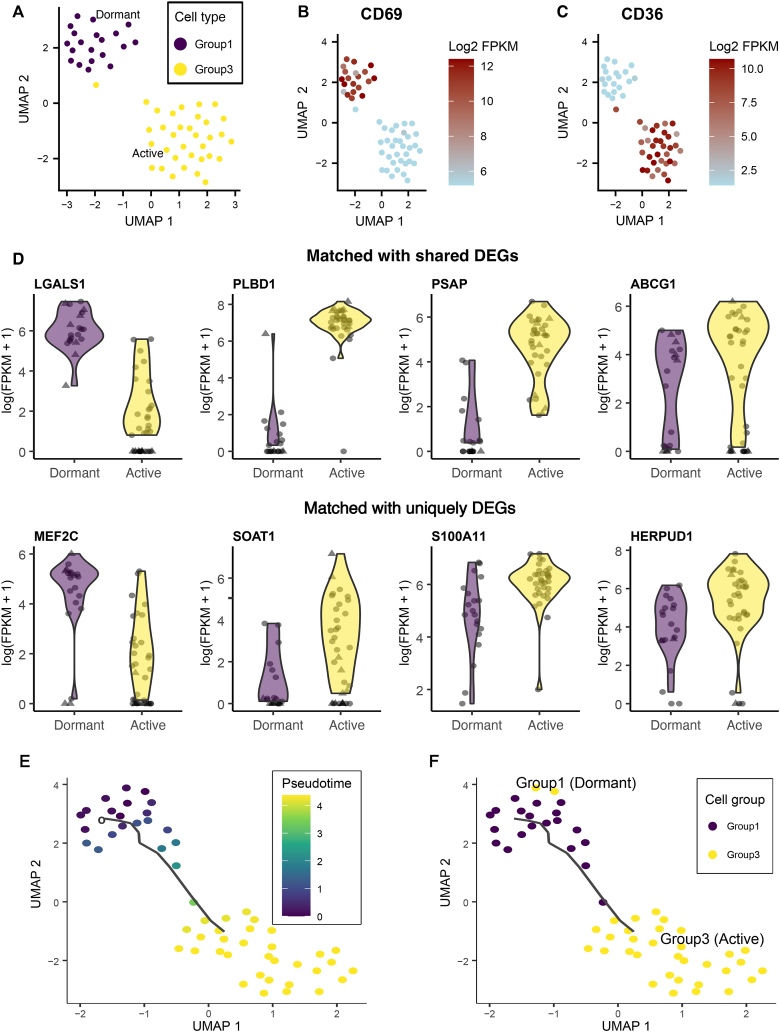
Given that AML CSCs come into two subclasses with distinctive features important for various cancer hallmarks such as cancer relapse, we next investigated whether our AML CSC features could discriminate AML dormant vs active subclasses. We hypothesize that AML key CSC genes that overlap with DEGs between dormant and active CSCs may be used to potentially characterize different AML CSC subtypes. To identify such commonalties, we initially interrogated a mouse scRNA-seq dataset of AML CSCs subclasses (Sachs et al., 2020) to see if dormant and active CSCs are separable based on their transcriptomic profiles. This dataset was the only available dataset for investigating the transcriptomic profiles of different subclasses of AML CSCs. The AML CSCs in this model have been previously characterized and classified into different groups according to their cell surface markers including group 1 (dormant CSCs; CD69high) and group 3 (active CSCs; CD36high) cells. First, using the Uniform Manifold Approximation and Projection (UMAP) confirmed transcriptomic profiles of cells in these two groups, primary labelled by the original study, are distinctive from each other and the cells within each group cluster together (Figure 5A). Also, the gene expression analysis of the cell surface markers used for distinguishing dormant vs active CSCs, namely CD69 and CD36 respectively, showed the specific expression of these genes in their corresponding cell subclass (Figure 5B,C).
Figure 5.
Distinct transcriptional profile of different subsets of AML CSCs. A. Separate clustering of different subsets of AML single-cells, including group1 (dormant AML CSCs) and group3 (active AML CSCs), according to their transcriptional profiles. B. Gene expression of CD69, marker of dormant AML CSCs, across the dataset. C. Gene expression of CD36, marker of active AML CSCs, across the dataset. D. Common DEGs between AML CSCs vs. GTCs and AML dormant vs. active CSCs. Shared DEGs are those DEGs that are common between AML CSCs vs. GTCs and TCGA AML vs. normal samples. Uniquely DEGs refer to those DEGs that are specifically differentially expressed in AML CSCs vs. GTCs but not in TCGA AML vs. normal samples. E. Pseudotime analysis based on the transcriptomic profile of all AML single-cells including all group1 and group3 cells. F. Cell group-based coloring of all AML single-cells scattered according to their pseudotime analysis. Group 1 and Group 3 are representative of AML dormant and active CSCs, respectively. DEGs, GTCs, and TCGA are the abbreviations of differentially expressed genes, general tumor cells, and The Cancer Genome Atlas, respectively.
The differential expression analysis demonstrated that 579 genes were significantly differentially expressed (p-value < 0.05) between AML dormant and active CSCs, 532 of which had known human homologs (Raw data output 8 at https://doi.org/10.26180/21259491.v1). Despite the differences between the microarray and the scRNA-seq datasets, eight out of these 532 genes were common with DEGs (#425) previously obtained from microarray data analyses (Figure 5D), suggesting that these DEGs could be potential markers for distinguishing different subclasses of AML CSCs. Also, 795 genes were uniquely expressed in either dormant or active AML CSCs (Raw data output 9 at https://doi.org/10.26180/21259491.v1). We then performed an intersection analysis between these AML CSC subclass-specific genes and the “AML key CSC genes”. Interestingly, 4 out of 59 AML key CSC genes including GAS2, IL1RL1, GINS1, and RAD51 were common with group 1 (dormant CSC) uniquely expressed genes, and one of them, namely DKK3, was common with group 3 (active CSC) uniquely expressed genes. Altogether corresponding to 8.5% of AML key CSC genes. These results indicate the existence of characteristic genes of different subclasses of AML CSCs in our selected AML key CSC genes. We recommend these genes to be considered in future targeting and essentiality experiments on different subclasses of AML CSCs.
Next, we performed a pseudotime analysis of the transcriptomic alterations of cells and compared that with cell type-based uniformal projection of the transcriptomic profile of cells on a 2D space. This demonstrated that dormant AML CSCs (group 1 cells) could be transformed to active (group 3) ones across the pseudotime (Figure 5E,F). It is further highlighting the importance of studying and targeting of both active and dormant CSCs for the cancer eradication and relapse prevention.
2.9. AML key CSC genes and associated transcription factors regulating them can be targeted by available drugs and chemical compounds
Considering the importance of AML key CSC genes at various levels (i.e. their differential expression, influence on the regulatory network, and AML specificity, as explained above), we sought to identify drugs that could target them. In order to increase the efficiency of our drug repurposing analysis, we expanded our search to upstream TFs regulators of AML key CSC genes as well. In fact, TFs, amongst all upstream gene regulators, are the major general influencers of the gene expression level, and this network expansion would complement the association network with an additional layer of regulatory information.
First, we extended the connectivity network of AML key CSC genes to all upstream transcription factors (TFs) known to regulate these AML key CSC genes. Interactions were obtained from the ENCODE Transcription Factor 2012 database (Gerstein et al., 2012). We identified 69 TFs upstream of the 35 AML key CSC genes, such as CD34, CDK1, and CA1. To evaluate the relevance of these TFs to the context of the study, we performed an intersection analysis on these TFs and uniquely/commonly DEGs obtained from the microarray dataset as well as the differentially/uniquely expressed genes resulted from the scRNA-seq data. Accordingly, 3 TFs were common with uniquely/commonly DEGs of the microarray dataset, and 8 TFs were shared with DEGs between dormant and active AML CSCs or the uniquely expressed genes in either subclass of AML CSCs (Raw data output 10 at https://doi.org/10.26180/21259491.v1). This corresponds to ∼16 percent of all TFs targeting AML key CSC genes, reinforcing their validity and indicating that these transcription factors could be involved in regulating the functionality of different subclasses of AML CSCs.
Second, we conducted a drug repurposing on all AML key CSC genes and their respective TFs based on drug-target interaction analyses using all the available drugs and compounds in the DrugBank v. 4.2 and ChEMBL v. 23 databases. These analyses illustrated that 346 drugs and 2,346 bioactive drug-like small molecules were predicted to target AML key CSC genes and their corresponding TFs (Supplementary figure S8).
To select top candidates for experimental validation, we assigned a targeting desirableness score (Raw data output 11 at https://doi.org/10.26180/21259491.v1) and a CSC-specific targeting desirableness score (Raw data output 12 at https://doi.org/10.26180/21259491.v1) to all of the AML key CSC genes and their corresponding TFs. For this we applied two in-house scoring functions (see Methods), which consider all the expression dysregulations, co-expressions, TF-target interactions (Supplementary figure S9) and PPI (Raw data output 13 at https://doi.org/10.26180/21259491.v1). Literature review of the top 10 ranked genes/TFs confirmed their association with AML and/or other cancers’ cancer (stem) cells as well as with haematopoietic stem cells (Raw data output 14 at https://doi.org/10.26180/21259491.v1). After ranking the genes/TFs, a drug score (Raw data output 15 at https://doi.org/10.26180/21259491.v1) and a CSC-specific drug score (Raw data output 16 at https://doi.org/10.26180/21259491.v1) were assigned to each of the drugs and bioactive drug-like small molecules (see Methods). This analysis identified the top three drugs: Zonisamide, Carfilzomib, and Amitriptyline to be selected for experimental validation in order to evaluate their impact on different subclasses of AML cancer (stem) cells (Raw data output 17 at https://doi.org/10.26180/21259491.v1).
2.10. The proliferation of KG1 AML cells dropped after drug treatments
Carfilzomib is an already approved drug for the treatment of haematological malignancies (Hosseini et al., 2019). Also, the efficiency of carfilzomib on AML treatment (Peng et al., 2020; Wartman et al., 2016) and targeting of AML CSCs (van der Helm et al., 2015) has been previously demonstrated. Thus, carfilzomib served both as a confirmation of our computational pipeline and as a positive control to be compared with the two other identified candidate drugs. Additionally, although Amitriptyline is not an approved cancer drug it may have a supportive role in cancer treatment by inhibiting glutathione S-transferase pi and alpha (Kulaksiz-Erkmen et al., 2013). To assess the viability and proliferation of the cells in different drug concentrations, the AlamarBlue assay was used. The drug treatments with Zonisamide and Carfilzomib showed a consistently negative impact from 100nM to 1mM on the proliferation of KG1 AML cells (Figure 6-A). Also, the analysis of variance (ANOVA) followed by Tukey's HSD test showed that the viability of Carfilzomib- and Zonisamide-treated ones in all except 100nM doses were significantly lower than the control group (samples with no drug treatment). This might indicate the ineffectiveness of these drugs at very low concentrations. On the other hand, while the application of low (100nM) and medium (100uM) concentrations of Amitriptyline showed the same pattern (i.e. continuous reduction of cell viability) consistent with the other two drugs, its higher dosage (1mM) resulted in the re-enhancement of cell viability, probably due to its insolubility at high concentrations. It should also be noted that only at 100uM concentrations the viability of Amitriptyline-treated cells was significantly lower than the control group (Figure 6-A). Accordingly, amongst all concentrations we performed downstream drug treatment assays based on 100uM concentration of all drugs.
Figure 6.
Drug repurposing for targeting AML key CSC genes. A. The impact of the top three drugs selected based on our drug scoring function on the proliferation and viability of AML cells in the KG1 cell line. The box plots include the data of three biological replicates for each drug as well as the control sample. B. The impact of drugs on different cell cycle stages of cancer (KG1) cells based on flow cytometry analyses.
2.11. Carfilzomib and Amitriptyline similarly affected the cell cycle
The cell cycle assay of treated and untreated KG1 cells using RNase and Propidium Iodide (PI)-based flow analysis illustrated that Zonisamide does not affect the cell cycle stages of cells after 16h treatment at 100uM concentrations resembling those of control samples (Figure 6-B). In contrast, Carfilzomib and Amitriptyline showed similar noticeable effects on the cell cycle. Specifically, Carfilzomib and Amitriptyline treatment at 100uM concentrations for 16h enhanced the number of dead cells (sub-G0) and considerably reduced the number of cells at the G0-G1 stage, indicating that these drugs might affect dormant cancer (stem) cells which their cell cycle is halted at G0-G1 stage (Aguirre-Ghiso, 2007; Santos-de-Frutos and Djouder, 2021).
3. Discussion
In this paper, we applied a ‘reverse-engineering’ strategy by recruiting bioinformatics and systems biology techniques to infer the regulatory interactions among AML CSC-associated genes and to identify novel candidate AML CSC-specific markers, drug targets, and drugs. To the best of our knowledge this is the first study that applies a top-down systems biology technique to integrate the TCGA data with CSC datasets to precisely extract AML cell stemness features. At the core of the pipeline adopted in this study we used the association (co-expression) analysis and its relevant network-based downstream analyses. Though co-expression between genes does not necessarily imply physical interactions it can imply their associations and involvements in the same pathways. Additionally, such association studies could help identify functional modules of related genes in a system which could be subsequently used for assessing the behaviour of the system according to functional assays (Bansal et al., 2007).
The differential expression of genes between AML CSCs and GTCs implies the differential activity of pathways and biological processes in these two cell types. Though, the DEGs that are uniquely differentially expressed in CSCs versus GTCs (Raw data output 2 at https://doi.org/10.26180/21259491.v1) but not in the TCGA HTCs versus normal samples are supposed to be more specific characteristics of AML CSCs. Similarly, differentially CEGs (Figure 3) which are uniquely co-expressed in CSCs but not in GTCs could be indicative of the AML CSC-specific pathways. In this regard, Tyrosine metabolism (Sun et al., 2020), Hippo signalling pathway (Liu et al., 2021b), and the Proteoglycans in cancer (Ahrens et al., 2020) are amongst the currently known pathways to be associated with CSC functioning. Accordingly, the pathways and biological processes enriched by differentially CEGs could be further focused on in future studies concerning AML CSC functioning. Having said that, biological processes enriched by commonly CEGs (Supplementary figure S5) may also be considered for studying the common features of AML CSCs and GTCs. These differential expression and association investigations hold the basics of AML key CSC gene selection which their importance for the viability and functioning of AML cells was subsequently confirmed using in vitro assays and in silico CRISPR-based functional assessments (according to the BioGRID ORCS and DepMap databases). Additionally, the fact that several of DEGs between dormant and active CSCs of a different study based on a different platform were common with our selected “AML key CSC genes” re-confirms our strategy for selecting CSC-specific genes. Trajectory pseudotime analysis illustrated in Figure 5E,F indicated that dormant AML CSCs can be transformed into active ones according to the alteration of their transcriptomic profiles. This highlights the importance of characterization and targeting of both active and dormant CSCs for the treatment of AML and the prevention of its relapse. In this regard, the common genes between our selected AML key CSC genes and the DEGs between dormant and active AML CSCs, namely GAS2, IL1RL1, GINS1, DKK3, and RAD51, are highly worth being considered in the future functional (knock-out/down and over-expression) assays for assessing the AML CSC functioning.
According to the applied targeting desirableness and drug scoring functions, three drugs including Zonisamide, Carfilzomib and Amitriptyline were finally selected as candidates for targeting AML cancer (stem) cells in KG1 cell line. Previous transcriptomic studies based on microarray (GSE54484) (Farren et al., 2014) and RNA-seq (GSE74928) (Mitton et al., 2016) technologies have demonstrated that all of the targets of these three selected drugs, namely PSMB8, MAOB, CA1, HTR2A, and CHRM3, are expressed in KG1 cell line. The experimental assays showed that the candidate drugs do impact the viability, proliferation, and cell cycle of AML cells. More specifically, AlamarBlue assays showed that Zonisamide and Carfilzomib consistently and negatively influence the viability of the AML cells in the KG1 cell line. AlamarBlue assay has been used for the evaluation of the impact of drugs on cell viability of many other cancer cell lines as well (Brooks et al., 2019; Rampersad, 2012). However, the effect of Amitriptyline reverses from 100uM to 1mM and results in the re-enhancement of cell viability, perhaps due to physical dissolution from the medium in high drug concentrations (Figure 6-A). Also, all three drugs have their best efficacy at medium concentrations (100uM), with Zonisamide's effect being statistically more significant compared with others. Though, the impact of these drugs on the viability of normal cells need to be examined in future studies to assess the toxicity effect of these drugs in normal conditions. The following paragraph briefly discuss the mechanism of action of three drugs in the context of their targets.
Carfilzomib is an FDA (US Food and Drug Administration) approved proteasome inhibitor for the treatment of multiple myeloma (MM) (Niewerth et al., 2013). It was also shown that Carfilzomib has antiproliferative and proapoptotic effects on human AML cells (Stapnes et al., 2007). Carfilzomib primarily targets the immunoproteasome PSMB8 (Monoamine Oxidase B) and affect cell proliferation and AML progression via modulating the PI3K/AKT signalling pathway (Lei et al., 2020). The in vitro proliferation assays illustrated the effect of Carfilzomib on reduction of AML cell proliferations compared with control (Figure 6A). The in vitro cell cycle assays (Figure 6B) also confirmed the impact of Carfilzomib on promotion of cell death (SubG0 cells) and reduction of cell proliferation (G0-M cells). This information confirms the importance of Carfilzomib, the second ranked drug according to our pipeline and scoring functions, and its direct target for administering AML and its treatment. Zonisamide directly targets MAOB (Monoamine Oxidase B) and CA1 (Carbonic Anhydrase 1) proteins. Several mutations in the gene MAOB have been previously reported in AML patients (https://cancer.sanger.ac.uk/cosmic) (Welch et al., 2012) and its up-regulation could enhance oxidative stress levels which results in chronic inflammation and subsequently cancer (Edmondson, 2014; Reuter et al., 2010). Also, MAOB could induce the progression of epithelial-to-mesenchymal transition (EMT), a characteristic of CSCs (Yang et al., 2020b), highlighting the significance of targeting MAOB for suppressing cancer stemness (Yin et al., 2018). knockdown of CA1 leads to the inhibition of STAT3 and NF-κB signaling pathways in-vitro and in vivo (Feng et al., 2020) and, consequently, suppresses cancer growth through induction of apoptosis (Hoesel and Schmid, 2013; Yamamoto and Gaynor, 2001). Amitriptyline targets HTR2A (5-Hydroxytryptamine Receptor 2A) and CHRM3 (Cholinergic Receptor Muscarinic 3) proteins. HTR2A may have mitogenic activities in blood cancers through modulation of the serotonin levels (Krevvata et al., 2014; Sridhar et al., 2009). Our cell cycle assays also indicated the impact Amitriptyline on the cell cycle and proliferation of KG1 AML cells (Figure 6B), similar to that of Carfilzomib. CHRM3 is one of the muscarinic G-protein coupled receptors (Spindel, 2012) that its expression is associated with cancer cell proliferation, metastasis and drug resistance (Feng et al., 2012; Wang et al., 2015). In vivo studies indicated that functional Chrm3 is required for the initiation of intestinal tumors and its genetic and pharmacological ablation attenuates tumor formation and growth (Raufman et al., 2011), highlighting the potential role of this gene in cancer stemness. Also, CHRM3 is significantly differentially expressed in human AML CSCs compared with healthy human hematopoietic stem/progenitor cells (Shingai et al., 2021). Our in vitro assays also demonstrated that CHRM3's targeting drug, namely Amitriptyline, at medium concentrations (100uM) significantly supresses the proliferation of AML cells (Figure 6A).
In addition to the top candidate drugs and the drugable genes, the “yet to be drugged” AML key CSC genes also worth considering for future AML functional assays and drug designing/development studies. The association between many of the AML key CSC genes and AML and/or other cancers and their stemness features is already confirmed. In silico functional assays on AML key CSC genes, and more comprehensively on TNFSF4 as an instance, revealed the functional relevance of these genes to the viability and proliferation of AML cells. TNFSF4 is an established risk locus for systemic lupus erythematosus (SLE) (Graham et al., 2008; Han et al., 2009; Lessard et al., 2016; Yang et al., 2010). It has been shown that haematological abnormalities are the common clinical manifestations of the SLE, and several studies are reporting the association between AML and SLE (Lofstrom et al., 2009; Mahutondji Massi, 2018; Ramadan et al., 2012). These data suggest the potential role of defective immunological mechanisms especially through the aberration of TNFSF4 in the development of AML. It has been also reported that PD1-positive/OX40-positive T cells are more frequent in AML bone marrow aspirates (Williams et al., 2019), and leukemic cells of most of the adult T-cell leukemia patients essentially express OX40, which may be crucial for cell adhesion and leukemic cell infiltration (Imura et al., 1997). Consequently, down-regulation of TNFSF4 (OX40 ligand) would suppress the aforementioned phenomenon in AML development. Moreover, suppression of TNFSF4 results in the enhancement of cytokine production, cytotoxicity, NK-cell activation against primary AML cells (Nuebling et al., 2018).
To conclude, in this study we examined the transcriptomic profiles of AML (cancer) stem cells and proposed a set of genes as the “AML key CSC genes”. We also introduced several genes as being specific characteristics of either AML dormant or active CSCs. According to the experimental assays, apart from Carfilzomib that is an already known medicine for blood cancers including AML, Zonisamide is proposed as a candidate drug for targeting AML cancer (stem) cells by suppressing its direct targets, namely CA1 and MAOB. Though this should be noted that most of the result of this study are based on bioinformatics analyses done using R language as well as several online bioinformatics resources. Consequently, further experimental studies are required to validate the results of this project. Furthermore, as AML is a heterogenous cancer, some AML subtypes might not benefit from the results of this project, which requires further studies and experiments. Having said that, this study opens up avenues for the study of the molecular mechanisms of action of Zonisamide and its targets in the context of AML. Other candidate “AML key CSC genes” and drugs including Amitriptyline are also worth being considered and examined in future studies. Also, the pipeline used in this study could be applied to all the other cancer types for the identification of CSC-specific markers and driver genes.
4. Methods
4.1. Data preparation
To gather the required data for our study, the keyword “AML cancer stem cells” was searched in the GEO database to find appropriate GEO series with the following options; Organism: Homo sapiens, Study type: Expression profiling by array/Expression profiling by high throughput sequencing. Also, the following criteria must be fulfilled for filtering the search results and selection of suitable datasets for downstream analyses; the datasets should have comprised the expression data of both CSCs and GTCs of the same tissue that has not undergone any treatment, administration or gene knockout; the leukemia-initiating activity of separated groups of cells/samples should have been functionally validated; and all samples must have been obtained from primary sources, not cancer cell lines. Accordingly, of the 11 series found based on the aforementioned keyword, 1 GEO series (GSE30375) met our desired criteria and was selected for downstream analyses. Additionally, those samples that were inconsistent with our selection criteria were filtered out. Furthermore, as only one dataset was identified according to the filtering criteria, we utilized the 80/20 principle to select the training and test sets for differential expression analysis. Therefore, about 20 percent of the paired samples of AML were randomly designated as the test set and the rest were selected as the training set (Raw data output 18 at https://doi.org/10.26180/21259491.v1). Also, all the downstream analyses after the differential expression analysis were done on the training set.
To further validate the results of microarray data analysis and to identify genes characteristics of different subclasses of AML CSCs, namely active and dormant CSCs, we retrieved a scRNA-seq dataset from the GEO repository (GSE140896). Additionally, to assess the specificity of our top candidates and to evaluate our results against a negative control, we used the same selection criteria used for selecting the AML dataset to identify appropriate datasets of other cancer types. Accordingly, a breast cancer microarray dataset was selected and retrieved from the GEO repository (GSE52327).
4.2. Differential expression analysis
Differential expression analysis was performed on training and test sets, separately, by fitting a linear model to the expression data of each probe followed by computing the contracts of fitted models and moderated empirical Bayes statistics using the limma R package (Ritchie et al., 2015). Subsequently, the genes with p. adj <0.05 and absolute log2 fold change (|log2FC|) > 1 were selected as the significant DEGs. Next, significant DEGs of the training set were compared with those obtained from the analysis of the test set to assess the reliability of differential expression results. However, as the number of samples in the test set was too low, the differential expression results of the test set were not filtered based on p. adj and only the direction of differential expression was assessed.
To enhance the specificity of our study and discriminate CSC-specific markers in each dataset, the intersection of DEGs in CSCs (compared with GTCs) and TCGA HTCs (compared with NCs) were obtained and those genes with an opposite direction of differential expression in two contexts were excluded from the list of CSC-associated DEGs. To this purpose, the differential expression analysis of the TCGA LAML dataset was done using the GEPIA2 web server, based on the limma method, p. adj <0.05 and |log2FC| > 1, and all of the differentially expressed genes were retrieved and used for the intersection analysis. GEPIA is a web server for RNA-seq data analysis that integrates 8,587 normal and 9,736 tumors samples from the GTEx and the TCGA projects using a standard pipeline and consequently reduces the inefficiency of differential analysis by balancing the normal and tumor data. Regarding the breast cancer dataset, we used the same exact differential expression analysis workflow applied to the AML microarray dataset to accurately identify DEGs and to classify them.
4.3. Unsupervised component analysis and clustering
The expression data of those DEGs that were uniquely differentially expressed in CSCs (compared with GTCs) but were not differentially expressed in TCGA HTCs (compared with NCs) were selected and put through an unsupervised PCA to see if their expression profile could separate CSC samples from GTC ones. Also, the expression data of these genes were clustered based on the unsupervised hierarchical clustering according to the “complete” method. Likewise, the same unsupervised component analysis and clustering were exerted on the expression data of commonly DEGs to see if the expression profile of these genes can force the discrete clustering of samples of different groups. Additionally, a PCA was done on the combined expression data of uniquely and commonly DEGs and the top 10 percent of CSC-associated DEGs with the highest rotation values were selected for inclusion in the set of “key CSC genes”.
4.4. Association analysis of different subsets of AML relevant genes
The co-expression of genes in CSC samples and GTC ones, as well as differential co-expression of genes between these two sample sets, were calculated employing the DiffCorr R package (Fukushima, 2013). Considering that different samples and replicates of the same biological condition (same sample set) would have different expression profiles, the co-expression analysis was done for CSC and GTC samples separately, followed by applying a stringent threshold for selection of significantly CEGs to identify the truly CEGs within each condition. The diversity in the expression profiles of different replicates of the same biological condition was further confirmed by the identification of lots of non-CEGs in each condition. In these analyses, the Pearson method, local False Discovery Rate (FDR), and Fisher's z-test were used for the calculation of correlation, multiple testing correction, and identification of significant differentially CEGs, respectively. Differential co-expression analysis is a method for the identification of alterations of co-expression patterns between two cell types or experimental conditions. Also, the common co-expression of non-differentially CEGs in CSC and GTC samples were calculated using the Hmisc R package (https://CRAN.R-project.org/package=Hmisc). Likewise, the Pearson method and local FDR were applied for the calculation of correlations and adjusted p-values, respectively. A common co-expression analysis is a method, opposite to differential co-expression analysis, for the identification of common co-expression patterns between two cell types or experimental conditions. Next, all of the significant differentially CEGs with Pearson correlation coefficient (r) > 0.7 and p. adj <0.02 in CSC samples were selected and the highly confident experimentally validated physical protein-protein interactions (PPI) between any pair of them were retrieved from the STRING database (Szklarczyk et al., 2019). Also, all of the KEGG pathways involving each of the selected differentially CEGs were retrieved from the Comparative Toxicogenomics Database (Davis et al., 2019) based on the enrichment method. Lastly, the association network of the selected differentially CEGs in CSCs was reconstructed involving the integrated results of co-expression, PPI, pathway enrichment, and differential expression analyses utilizing the Cytoscape v. 3.7.1 (Shannon et al., 2003). On the other hand, the same workflow was applied on the commonly co-expressed data and an integrated association network was reconstructed based on the commonly CEGs.
4.5. Computational functional annotation of different subsets of AML associated genes
The MCL, a graph clustering algorithm based on random walks in the simulated stochastic graph flows, was used for the identification of functional modules of the association networks utilizing the Cytoscape plugin AutoAnnotate (Kucera et al., 2016). Also, the AutoAnnotate plugin was used to randomly walk through modules using the adjacent words algorithm to find the first ranked pathway associated with each functional module. This is a pathway-based functional similarity calculation method that suggests that all of the genes in each module might directly/indirectly be involved in the selected pathway. Moreover, BiNGO (Maere et al., 2005) and GOlorize (Garcia et al., 2007) Cytoscape plugins were used for the ORA and visualization of GO-BPs, respectively. For this purpose, a hypergeometric test was used for the ORA, and Benjamini and Hochberg (BH) method was used for multiple-testing correction and calculation of FDR. Next, a hierarchical clustering layout was applied on the GO-BP network of each module separately to identify the most semantically specific GO-BP terms. In the end, one of the most semantically specific and most relevant terms of each module was selected to color the nodes of modules according to those selected terms. Moreover, the uniquely and commonly DEGs were separately subjected to the ORA to find the GO-BPs they are involved in. To this purpose, the R package enrichR (https://cran.r-project.org/package=enrichR) was used and GO-BO terms associated with each set of DEGs were retrieved. Then, the GO-BP terms were filtered by their statistical significance and a similarity matrix of the selected terms was achieved using the rrvgo R package (https://github.com/ssayols/rrvgo). Subsequently, GO-BP terms were summarized considering both their levels of similarity and statistical significance in order to identify the parent terms. Lastly, an average combined score and statistical significance were achieved for each parent term according to its corresponding child terms.
4.6. Selection of AML key CSC genes
The significant genes outputted from all of our upstream analyses were shortlisted to perform a more specific association analysis, drug repurposing as well as an experimental validation of top candidate genes and drugs. Accordingly, the topology of the association networks of differentially and commonly CEGs of CSCs were analyzed using the R package influential (https://cran.r-project.org/package=influential) (Salavaty et al., 2020) and the top 10 nodes with the highest IVI values were selected for downstream analyses. Also, the topology of the extracted network of each of the selected modules of association networks was separately analyzed and the top one and two nodes with the highest IVI values were selected as the most influential nodes of the modules with less than 20 nodes and over 20 nodes, respectively. Altogether, all of the influential nodes of the association networks and their sub-networks (modules), as well as the top 10 percentage of CSC-associated DEGs with the highest rotation values (based on the PCA analysis of combined uniquely and commonly DEGs) were listed and termed as “AML key CSC genes”. Moreover, the connectivity and interaction of AML key CSC genes with each other were inspected using the STRING database and visualized by means of the Cytoscape software. The BioGRID ORCS v.1.1.9 was interrogated to investigate the functional importance of these genes in AML development and drug resistance according to the CRISPR screening studies. Also, the importance of AML key CSC genes for the viability of AML cancer cell lines was evaluated using the DepMap portal. Moreover, the interaction between any of these AML key CSC genes and all TFs was interrogated. To this end, the CyTargetLinker v. 4.1.0 (Kutmon et al., 2013), a Cytoscape plugin, was recruited and all of the TFs targeting any of the AML key CSC genes were identified according to the ENCODE Transcription Factor 2012 (Gerstein et al., 2012). The ENCODE Transcription Factor 2012 is a known database of TF-target interactions based on ChIP-seq experiments.
4.7. Calculation of targeting desirableness and drug scores
To calculate the targeting desirableness of AML key CSC genes and their respective TFs, firstly, an association network was reconstructed based on the co-expressions and PPIs between any pair of nodes in the interaction network of AML key CSC genes and their targeting TFs. The co-expression and PPI data were obtained from the co-expression analysis of training set samples and the STRING database, respectively, with the same aforementioned criteria. Subsequently, the topology of the network was analyzed and the IVI values of all of the nodes were calculated using the R package influential (Salavaty et al., 2020). Then, the targeting desirableness score of each node was calculated according to the following scoring function (Equation 1).
| (1) |
Where IVI is indicative of the IVI value of gene/TF, DE stands for differentially expressed, and Associated network score was calculated based on the network of origin (influential node of the association network of commonly CEGs = 4; influential node of the association network of differentially CEGs = 2; not an influential node = 1). Additionally, a text mining based on the literature in the PubMed database was performed using the Coremine Medical web server (www.coremine.com/medical) to investigate if there is any report about the association of top 10 nodes of the association network of AML key CSC genes and their targeting TFs according to both targeting desirableness and CSC-specific targeting desirableness scores with “AML CSC”, “cancer” in general, and “normal stem cells”.
To identify the drugs and compounds targeting any of the AML key CSC genes and their corresponding TFs, firstly, the association network and the interaction network of AML key CSC genes and their corresponding TFs were merged. Next, the merged network was extended by all of the available drugs and bioactive drug-like small molecules targeting any of the nodes within the network according to the DrugBank v. 4.2 and ChEMBL v. 23 databases. Lastly, the score of each of the drugs and drug-like small molecules was calculated according to the following formula (Equation 2);
| (2) |
Similarly, the CSC-specific targeting desirableness score of each node of the same association network of AML key CSC genes and their corresponding TFs was calculated according to the following scoring function (Equation 3).
| (3) |
Where IVI is indicative of the IVI value of desired gene/TF, DE stands for differentially expressed, and Associated network score were calculated based on the network of origin (influential node of the association network of commonly CEGs = 2, influential node of the association network of differentially CEGs = 4, not an influential node = 1). Also, the CSC-specific score of each drug within the previously reconstructed merged network was calculated according to the following scoring function (Equation 4).
| (4) |
4.8. Assessment of subclasses of AML key CSC genes
To further validate the results of microarray data analysis, more precisely study the AML key CSC genes, and to identify genes characteristics of different subclasses of AML CSCs, namely active and dormant CSCs, AML key CSC genes and their targeting TFs were meta-analyzed against an AML scRNA-seq dataset (GSE140896) downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo). This dataset includes characterized subsets of AML stem cells obtained from a murine leukemia model (Sachs et al., 2020). The cells within this dataset have been previously characterized and clustered into three different groups. Also, using in vivo CellTrace labelling-based proliferation and leukemia reconstitution-based self-renewal assays, the authors have demonstrated that only group1 cells (dormant AML CSCs) which included CD69High AML CSCs were capable of self-renewal and were poorly proliferative. On the other hand, group3 cells (active AML CSCs), including CD36High AML CSCs, were highly proliferative but unable to transplant leukemia (Sachs et al., 2020). Accordingly, we interrogated the transcriptomic profile of these two groups of cells, namely groups 1 and 3. It is also worth mentioning that the authors of the dataset generated two sets of samples, one set of samples for training and another one for testing the results. However, as two datasets have resulted from the same sequencing platform, same read length and the same normalization pipeline, and because of the low number of cells within each set, we combined the training and test set cells to create a larger dataset and increase the accuracy of our downstream analyses.
First, the Fragments Per Kilobase Million (FPKM) values of genes previously downloaded from the GEO database were log2 transformed to bring the expression data closer to a normal distribution. Then, a nonlinear dimensionality reduction based on the Uniform Manifold Approximation and Projection (UMAP) was performed on the expression data using the R package Seurat (Hao et al., 2021) to see if cells of group1 and group3 separately cluster together. Also, the expression of the cell surface markers, namely CD69 and CD36, used for distinguishing dormant versus active CSCs, respectively, were interrogated in the dataset and visualized. Next, we performed a pseudotime analysis to see if either group of cells could potentially be transformed to the other. For this purpose, we recruited the functions of the R package Monocle 3 (Qiu et al., 2017; Trapnell et al., 2014) and performed a pseudotime analysis on the original FPKM values followed by a cell type-based coloring of the trajectory of cells across pseudotime.
Besides, differential expression analysis was performed to identify the genes that are significantly differentially expressed in group1 versus group3 cells. To this end, the fold changes of genes were calculated according to a modified version of the SingleScore method. The SingleScore is a method developed by the authors of the under-investigation scRNA-seq dataset for the assessment of the transcriptomic profile of a single-cell dataset based on FPKM values (Sachs et al., 2020). First, the L2 Norm (normalized transcriptomic profile) of each cell was calculated according to the following formula (Equation 5).
| (5) |
Then, the L2-normalized expression value of each gene in every single cell was calculated using the following formula (Equation 6).
| (6) |
Subsequently, the expression fold change of each gene in every single cell of group1 versus group3 was calculated based on the following formula (Equation 7).
| (7) |
Where and represent the median normalized expression value of a gene among group1 and group3 cells that express that gene, respectively. Lastly, the average fold change of each gene across all group1 cells versus group3 cells was calculated using base R functions. In parallel, the statistical significance of differential expression of genes was calculated using the scDD R package (Korthauer et al., 2016). The scDD is a scRNA-seq specialized R package that employs a statistical approach to powerfully detect all DEGs even subtle differences in gene expression distributions, and is flexible to a variety of different gene expression formats.
Additionally, the overlap between scRNA-seq derived significant genes and microarray derived AML key CSC genes and their targeting TFs were interrogated in order to select right candidates for specific targeting of dormant and active AML CSCs. To this end, the human homolog of the genes in the scRNA-seq were identified using the gprofiler2 R package (https://cran.r-project.org/package=gprofiler2). Then, an intersection analysis was performed on the human homolog of scRNA-seq derived significant DEGs, group1 uniquely expressed genes, and group3 uniquely expressed genes, and the microarray derived AML key CSC genes.
4.9. Experimental validation of top candidate drugs
With the purpose of targeting both dormant and active AML CSCs as well as AML GTCs, amongst all of the drugs/small chemical compounds three FDA-approved drugs were selected for experimental validation according to their (CSC-specific) drug scores (Raw data output 17 at https://doi.org/10.26180/21259491.v1).
Cell viability and proliferation assay. The KG1 cells were cultured in IMDM 20% fetal bovin serum (FBS) for two days until the cell confluency reached 80%. The cells were collected and centrifuged at 1200 rpm for 5 min. Then, 5000 cells (suspended in 200 ul of media) were inputted into each well of a 96-well black plate and left to grow for 4 h. Next, three drugs including Carfilzomib (PR-171, S2853), Zonisamide (S1445), and Amitriptyline (S5947) were added to the cells in different wells and concentrations (1 mM, 100 uM, and 100 nM) and the plate was incubated for 18 h. Subsequently, the proliferation of cells was evaluated by adding 20 ul AlamarBlue (DAL1025) to each well. The plate was incubated further for 4 h at 37 °C. Finally, the fluorescence signal was read at excitation 560nm and emission 590nm on PerkinElmer's EnSpire.
Cell cycle assay. The KG1 cell line was used to evaluate the impact of drug treatments on the cycle progression. Firstly, a 300k/ml suspension of cells was cultured in a 6 well plate with/without treatment for 24hrs. Then, cells were harvested and washed twice in cold PBS. Subsequently, cells were pelleted and fixed in 500μl ice cold 70% ethanol (added to the pellet in a dropwise manner while vortexing the tube). During the fixation period, Ethanol was kept fresh from Absolute Ethanol and MilliQ H2O, and the tubes were placed in the -20 °C freezer until use. Finally, the lids of tubes were double clicked and stored at 4 °C overnight in the fridge for 16 h.
To perform a flow analysis the cells were treated with RNase and PI. To this end, cells were washed twice at room temperature with PBS280, treated with RNase I, incubate for 45 min at room temp, and 150ul of PI (25 ug/ml, 1/40) was directly added to tubes (without any wash) and analysed on the BD LSR II Flow cytometer.
Declarations
Author contribution statement
Adrian Salavaty: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sara Alaei Shehni: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Mirana Ramialison: Analyzed and interpreted the data; Wrote the paper.
Peter David Currie: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Professor Peter David Currie and Mirana Ramialison were supported by National Health and Medical Research Council [1136567 & 1180905].
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2022.e11093.
Acknowledgements
The authors would like to thank Professor Graham Lieschke for his constructive feedback on the manuscript. The results shown in this study are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/. This work was supported by Monash University, a National Health and Medical Research Fellowship (1136567) to PDC, an NHMRC grant (1180905) to MR, and an Australian Government Research Training Program (RTP) Scholarship to AS. The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government. The Novo Nordisk Foundation Center for Stem Cell Medicine (ERP, DAE, MR) is supported by Novo Nordisk Foundation grants (NNF21CC0073729).
Contributor Information
Mirana Ramialison, Email: mirana.ramialison@mcri.edu.au.
Peter D. Currie, Email: peter.currie@monash.edu.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Aguirre-Ghiso J.A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens T.D., Bang-Christensen S.R., Jørgensen A.M., Løppke C., Spliid C.B., Sand N.T., Clausen T.M., Salanti A., Agerbæk M.Ø. The role of Proteoglycans in cancer metastasis and circulating tumor cell analysis. Front. Cell Dev. Biol. 2020;8:749. doi: 10.3389/fcell.2020.00749. 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal M., Belcastro V., Ambesi-Impiombato A., di Bernardo D. How to infer gene networks from expression profiles. Mol. Syst. Biol. 2007;3:78. doi: 10.1038/msb4100120. 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks E.A., Galarza S., Gencoglu M.F., Cornelison R.C., Munson J.M., Peyton S.R. Applicability of drug response metrics for cancer studies using biomaterials. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374:20180226. doi: 10.1098/rstb.2018.0226. 20180226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen M.P.F., van Rheenen J., Scheele C.L.G.J. Targeting dormant tumor cells to prevent cancer recurrence. FEBS J. 2021;288:6286–6303. doi: 10.1111/febs.15626. [DOI] [PubMed] [Google Scholar]
- Davis A.P., Grondin C.J., Johnson R.J., Sciaky D., McMorran R., Wiegers J., Wiegers T.C., Mattingly C.J. The comparative Toxicogenomics database: update 2019. Nucleic Acids Res. 2019;47:D948–D954. doi: 10.1093/nar/gky868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.P., Grondin C.J., Johnson R.J., Sciaky D., Wiegers J., Wiegers T.C., Mattingly C.J. Comparative Toxicogenomics database (CTD): update 2021. Nucleic Acids Res. 2021;49:D1138–D1143. doi: 10.1093/nar/gkaa891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.P., Murphy C.G., Rosenstein M.C., Wiegers T.C., Mattingly C.J. The Comparative Toxicogenomics Database facilitates identification and understanding of chemical-gene-disease associations: arsenic as a case study. BMC Med. Genom. 2008;1:48. doi: 10.1186/1755-8794-1-48. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kouchkovsky I., Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50. e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djomehri S.I., Gonzalez M.E., da Veiga Leprevost F., Tekula S.R., Chang H.-Y., White M.J., Cimino-Mathews A., Burman B., Basrur V., Argani P., et al. Quantitative proteomic landscape of metaplastic breast carcinoma pathological subtypes and their relationship to triple-negative tumors. Nat. Commun. 2020;11:1723. doi: 10.1038/s41467-020-15283-z. 1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbin Z.C., Landen C.N. Isolation and characterization of potential cancer stem cells from solid human tumors–potential applications. Curr. Protoc. Pharmacol. 2013;63 doi: 10.1002/0471141755.ph1428s63. Unit 14.28-Unit 14.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D.E. Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: biological implications. Curr. Pharmaceut. Des. 2014;20:155–160. doi: 10.2174/13816128113190990406. [DOI] [PubMed] [Google Scholar]
- Eppert K., Takenaka K., Lechman E.R., Waldron L., Nilsson B., van Galen P., Metzeler K.H., Poeppl A., Ling V., Beyene J., et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- Farren M.R., Carlson L.M., Netherby C.S., Lindner I., Li P.-K., Gabrilovich D.I., Abrams S.I., Lee K.P. Tumor-induced STAT3 signaling in myeloid cells impairs dendritic cell generation by decreasing PKCβII abundance. Sci. Signal. 2014;7:ra16. doi: 10.1126/scisignal.2004656. ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Zhong M., Tang Y., Liu X., Liu Y., Wang L., Zhou H. The role and underlying mechanism of exosomal CA1 in chemotherapy resistance in diffuse large B cell lymphoma. Mol. Ther. Nucleic Acids. 2020;21:452. doi: 10.1016/j.omtn.2020.06.016. 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y.-J., Zhang B.-Y., Yao R.-Y., Lu Y. Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells. Hepatobiliary Pancreat. Dis. Int. 2012;11:418–423. doi: 10.1016/s1499-3872(12)60201-x. [DOI] [PubMed] [Google Scholar]
- Fukushima A. DiffCorr: an R package to analyze and visualize differential correlations in biological networks. Gene. 2013;518:209–214. doi: 10.1016/j.gene.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Garcia O., Saveanu C., Cline M., Fromont-Racine M., Jacquier A., Schwikowski B., Aittokallio T. GOlorize: a Cytoscape plug-in for network visualization with Gene Ontology-based layout and coloring. Bioinformatics. 2007;23:394. doi: 10.1093/bioinformatics/btl605. 396. [DOI] [PubMed] [Google Scholar]
- Gerstein M.B., Kundaje A., Hariharan M., Landt S.G., Yan K.-K., Cheng C., Mu X.J., Khurana E., Rozowsky J., Alexander R., et al. Architecture of the human regulatory network derived from ENCODE data. Nature. 2012;489:91–100. doi: 10.1038/nature11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandi M., Huang F.W., Jané-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R., Barretina J., Gelfand E.T., Bielski C.M., Li H., et al. Next-generation characterization of the cancer cell line encyclopedia. Nature. 2019;569:503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.S.C., Graham R.R., Manku H., Wong A.K., Whittaker J.C., Gaffney P.M., Moser K.L., Rioux J.D., Altshuler D., Behrens T.W., Vyse T.J. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat. Genet. 2008;40:83–89. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.-W., Zheng H.-F., Cui Y., Sun L.-D., Ye D.-Q., Hu Z., Xu J.-H., Cai Z.-M., Huang W., Zhao G.-P., et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat. Genet. 2009;41:1234. doi: 10.1038/ng.472. 1237. [DOI] [PubMed] [Google Scholar]
- Handschuh L. Not only mutations matter: molecular picture of acute myeloid leukemia emerging from transcriptome studies. JAMA Oncol. 2019;2019:7239206. doi: 10.1155/2019/7239206. 7239206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573. doi: 10.1016/j.cell.2021.04.048. 3587.e3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesel B., Schmid J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokland P., Woll P.S., Hansen M.C., Bill M. The concept of leukaemic stem cells in acute myeloid leukaemia 25 years on: hitting a moving target. Br. J. Haematol. 2019;187:144–156. doi: 10.1111/bjh.16104. [DOI] [PubMed] [Google Scholar]
- Hosseini M.S., Mohammadi M.H., Vahabpour Roudsari R., Jafari L., Mashati P., Gharehbaghian A. Proteasome inhibition by carfilzomib induced apotosis and autophagy in a T-cell acute lymphoblastic leukemia cell line. Iran. J. Pharm. Res. (IJPR) 2019;18:132–145. doi: 10.22037/ijpr.2020.112692.13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Liao X., Li J., Wei J., Su X., Lai X., Liu B., Zhu F., Huang Y., Li Q. Genome-scale integrated analysis to identify prospective molecular mechanisms and therapeutic targets in isocitrate dehydrogenase 2 R140Q-mutated acute myeloid leukemia. Oncol. Rep. 2019;41:2876–2888. doi: 10.3892/or.2019.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura A., Hori T., Imada K., Kawamata S., Tanaka Y., Imamura S., Uchiyama T. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: implication for the possible involvement of OX40 in leukemic cell infiltration. Blood. 1997;89:2951–2958. [PubMed] [Google Scholar]
- Kantarjian H., Kadia T., DiNardo C., Daver N., Borthakur G., Jabbour E., Garcia-Manero G., Konopleva M., Ravandi F. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11:41. doi: 10.1038/s41408-021-00425-3. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H.P., Golde D.W. Human myeloid leukemia cell lines: a review. Blood. 1980;56:344–350. [PubMed] [Google Scholar]
- Korthauer K.D., Chu L.-F., Newton M.A., Li Y., Thomson J., Stewart R., Kendziorski C. A statistical approach for identifying differential distributions in single-cell RNA-seq experiments. Genome Biol. 2016;17:222. doi: 10.1186/s13059-016-1077-y. 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krevvata M., Silva B.C., Manavalan J.S., Galan-Diez M., Kode A., Matthews B.G., Park D., Zhang C.A., Galili N., Nickolas T.L., et al. Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood. 2014;124:2834. doi: 10.1182/blood-2013-07-517219. 2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera M., Isserlin R., Arkhangorodsky A., Bader G.D. AutoAnnotate: a Cytoscape app for summarizing networks with semantic annotations. F1000Res. 2016;5:1717. doi: 10.12688/f1000research.9090.1. 1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaksiz-Erkmen G., Dalmizrak O., Dincsoy-Tuna G., Dogan A., Ogus I.H., Ozer N. Amitriptyline may have a supportive role in cancer treatment by inhibiting glutathione S-transferase pi (GST-π) and alpha (GST-α) J. Enzym. Inhib. Med. Chem. 2013;28:131. doi: 10.3109/14756366.2011.639017. 136. [DOI] [PubMed] [Google Scholar]
- Kurata H., Maeda K., Onaka T., Takata T. BioFNet: biological functional network database for analysis and synthesis of biological systems. Briefings Bioinf. 2014;15:699–709. doi: 10.1093/bib/bbt048. [DOI] [PubMed] [Google Scholar]
- Kutmon M., Kelder T., Mandaviya P., Evelo C.T.A., Coort S.L. CyTargetLinker: a cytoscape app to integrate regulatory interactions in network analysis. PLoS One. 2013;8:e82160. doi: 10.1371/journal.pone.0082160. e82160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M., Jingjing Z., Tao J., Jianping M., Yuanxin Z., Jifeng W., Lianguo X., Lidong Z., Ying W. LncRNA HCP5 promotes LAML progression via PSMB8-mediated PI3K/AKT pathway activation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393:1025–1032. doi: 10.1007/s00210-019-01788-y. [DOI] [PubMed] [Google Scholar]
- Lessard C.J., Sajuthi S., Zhao J., Kim K., Ice J.A., Li H., Ainsworth H., Rasmussen A., Kelly J.A., Marion M., et al. Identification of a systemic lupus erythematosus risk locus spanning ATG16L2, FCHSD2, and P2RY2 in Koreans. Arthritis Rheumatol. 2016;68:1197–1209. doi: 10.1002/art.39548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Dong C., Liu Y., Wu H. CGPE: an integrated online server for cancer gene and pathway exploration. Bioinformatics. 2021;37:2201–2202. doi: 10.1093/bioinformatics/btaa952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Liu X., Song G. The Hippo pathway: a master regulatory network important in cancer. Cells. 2021;10:1416. doi: 10.3390/cells10061416. 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Cong Y., Wang D., Sun Y., Deng L., Liu Y., Martin-Trevino R., Shang L., McDermott S.P., Landis M.D., et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofstrom B., Backlin C., Sundstrom C., Hellstrom-Lindberg E., Ekbom A., Lundberg I.E. Myeloid leukaemia in systemic lupus erythematosus–a nested case-control study based on Swedish registers. Rheumatology. 2009;48:1222. doi: 10.1093/rheumatology/kep204. 1226. [DOI] [PubMed] [Google Scholar]
- Lu H., Chen I., Shimoda L.A., Park Y., Zhang C., Tran L., Zhang H., Semenza G.L. Chemotherapy-Induced Ca2+ release stimulates breast cancer stem cell enrichment. Cell Rep. 2017;18:1946–1957. doi: 10.1016/j.celrep.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Luo M., Li J.-F., Yang Q., Zhang K., Wang Z.-W., Zheng S., Zhou J.-J. Stem cell quiescence and its clinical relevance. World J. Stem Cell. 2020;12:1307–1326. doi: 10.4252/wjsc.v12.i11.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maere S., Heymans K., Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- Mahutondji Massi R. Association of acute myeloid leukemia and systemic lupus erythematosus: a case report. Am. J. Intern. Med. 2018;6:25–28. [Google Scholar]
- Majeti R., Becker M.W., Tian Q., Lee T.-L.M., Yan X., Liu R., Chiang J.-H., Hood L., Clarke M.F., Weissman I.L. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3396–3401. doi: 10.1073/pnas.0900089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins S.D. Cancer stem cells: a systems biology view of their role in prognosis and therapy. Anti Cancer Drugs. 2014;25:353–367. doi: 10.1097/CAD.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton B., Chae H.D., Hsu K., Dutta R., Aldana-Masangkay G., Ferrari R., Davis K., Tiu B.C., Kaul A., Lacayo N., et al. Small molecule inhibition of cAMP response element binding protein in human acute myeloid leukemia cells. Leukemia. 2016;30:2302–2311. doi: 10.1038/leu.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewerth D., Dingjan I., Cloos J., Jansen G., Kaspers G. Proteasome inhibitors in acute leukemia. Expert Rev. Anticancer Ther. 2013;13:327–337. doi: 10.1586/era.13.4. [DOI] [PubMed] [Google Scholar]
- Nuebling T., Schumacher C.E., Hofmann M., Hagelstein I., Schmiedel B.J., Maurer S., Federmann B., Rothfelder K., Roerden M., Dörfel D., et al. The immune checkpoint modulator OX40 and its ligand OX40L in NK-cell immunosurveillance and acute myeloid leukemia. Cancer Immunol Res. 2018;6:209–221. doi: 10.1158/2326-6066.CIR-17-0212. [DOI] [PubMed] [Google Scholar]
- Oughtred R., Rust J., Chang C., Breitkreutz B.-J., Stark C., Willems A., Boucher L., Leung G., Kolas N., Zhang F., et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021;30:187–200. doi: 10.1002/pro.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paubelle E., Zylbersztejn F., Maciel T.T., Carvalho C., Mupo A., Cheok M., Lieben L., Sujobert P., Decroocq J., Yokoyama A., et al. Vitamin D receptor controls cell stemness in acute myeloid leukemia and in normal bone marrow. Cell Rep. 2020;30:739–754. doi: 10.1016/j.celrep.2019.12.055. e734. [DOI] [PubMed] [Google Scholar]
- Peng M.Y., Abaza Y., McDermott M., Mead M., Slamon D.J., Larson S. Activity of carfilzomib (CFZ) in acute myeloid leukemia (AML) as a single agent and in novel combinations. Blood. 2020;136:6–7. [Google Scholar]
- Prada-Arismendy J., Arroyave J.C., Röthlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017;31:63–76. doi: 10.1016/j.blre.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Qiu X., Mao Q., Tang Y., Wang L., Chawla R., Pliner H.A., Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan S.M., Fouad T.M., Summa V., Hasan S.K., Lo-Coco F. Acute myeloid leukemia developing in patients with autoimmune diseases. Haematologica. 2012;97:805–817. doi: 10.3324/haematol.2011.056283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersad S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raufman J.-P., Shant J., Xie G., Cheng K., Gao X.-M., Shiu B., Shah N., Drachenberg C.B., Heath J., Wess J., Khurana S. Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice. Carcinogenesis. 2011;32:1396–1402. doi: 10.1093/carcin/bgr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reikvam H., Hatfield K., Bruserud Ø. The pretransplant systemic metabolic profile reflects a risk of acute graft versus host disease after allogeneic stem cell transplantation. Metabolomics. 2016;12:12. doi: 10.1007/s11306-015-0880-x. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs K., Sarver A.L., Noble-Orcutt K.E., LaRue R.S., Antony M.L., Chang D., Lee Y., Navis C.M., Hillesheim A.L., Nykaza I.R., et al. Single-cell gene expression analyses reveal distinct self-renewing and proliferating subsets in the leukemia stem cell compartment in acute myeloid leukemia. Cancer Res. 2020;80:458–470. doi: 10.1158/0008-5472.CAN-18-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavaty A., Ramialison M., Currie P.D. Integrated value of influence: an integrative method for the identification of the most influential nodes within networks. Patterns. 2020;1:100052. doi: 10.1016/j.patter.2020.100052. 100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-de-Frutos K., Djouder N. When dormancy fuels tumour relapse. Commun Biol. 2021;4:747. doi: 10.1038/s42003-021-02257-0. 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedalipour F., Mansouri A., Vaezi M., Gholami K., Heidari K., Hadjibabaie M., Ghavamzadeh A. High prevalence of vitamin D deficiency in newly diagnosed acute myeloid leukemia patients and its adverse outcome. Int. J. Hematol. Oncol. Stem Cell Res. 2017;11:209–216. [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai Y., Yokota T., Okuzaki D., Sudo T., Ishibashi T., Doi Y., Ueda T., Ozawa T., Nakai R., Tanimura A., et al. Autonomous TGFβ signaling induces phenotypic variation in human acute myeloid leukemia. Stem Cell. 2021;39:723–736. doi: 10.1002/stem.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.C.L., Coelho-Silva J.L., Traina F., Machado-Neto J.A. ARHGAP6 expression IS associated with molecular RISK and impacts clinical outcomes in acute myeloid leukemia. Hematol Transfus Cell Ther. 2021;43:S150–S151. doi: 10.1016/j.htct.2023.06.004. [DOI] [PubMed] [Google Scholar]
- Sleire L., Førde H.E., Netland I.A., Leiss L., Skeie B.S., Enger P.Ø. Drug repurposing in cancer. Pharmacol. Res. 2017;124:74–91. doi: 10.1016/j.phrs.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Spindel E.R. Muscarinic receptor agonists and antagonists: effects on cancer. Handb. Exp. Pharmacol. 2012:451–468. doi: 10.1007/978-3-642-23274-9_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyrou N., Papapetrou E.P. Studying leukemia stem cell properties and vulnerabilities with human iPSCs. Stem Cell Res. 2020;50:102117. doi: 10.1016/j.scr.2020.102117. 102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar K., Ross D.T., Tibshirani R., Butte A.J., Greenberg P.L. Relationship of differential gene expression profiles in CD34+ myelodysplastic syndrome marrow cells to disease subtype and progression. Blood. 2009;114:4847–4858. doi: 10.1182/blood-2009-08-236422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapnes C., Døskeland A.P., Hatfield K., Ersvaer E., Ryningen A., Lorens J.B., Gjertsen B.T., Bruserud O. The proteasome inhibitors bortezomib and PR-171 have antiproliferative and proapoptotic effects on primary human acute myeloid leukaemia cells. Br. J. Haematol. 2007;136:814–828. doi: 10.1111/j.1365-2141.2007.06504.x. [DOI] [PubMed] [Google Scholar]
- Sun L., Zhang L., Chen J., Li C., Sun H., Wang J., Xiao H. Activation of tyrosine metabolism in CD13+ cancer stem cells drives relapse in hepatocellular carcinoma. Cancer Res Treat. 2020;52:604–621. doi: 10.4143/crt.2019.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J.G., Bamford S., Jubb H.C., Sondka Z., Beare D.M., Bindal N., Boutselakis H., Cole C.G., Creatore C., Dawson E., et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Majeti R. Biology and relevance of human acute myeloid leukemia stem cells. Blood. 2017;129:1577–1585. doi: 10.1182/blood-2016-10-696054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Cacchiarelli D., Grimsby J., Pokharel P., Li S., Morse M., Lennon N.J., Livak K.J., Mikkelsen T.S., Rinn J.L. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. Can systems biology approach help in finding more effective treatment for acute myeloid leukemia? Syst Synth Biol. 2014;8:165–167. doi: 10.1007/s11693-014-9147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli D., Gruszka A.M., Alcalay M. Has drug repurposing fulfilled its promise in acute myeloid leukaemia? J. Clin. Med. 2020;9:1892. doi: 10.3390/jcm9061892. 1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm L.H., Bosman M.C.J., Schuringa J.J., Vellenga E. Effective targeting of primitive AML CD34+ cells by the second-generation proteasome inhibitor carfilzomib. Br. J. Haematol. 2015;171:652–655. doi: 10.1111/bjh.13418. [DOI] [PubMed] [Google Scholar]
- van Dijk A.D., Hoff F.W., Qiu Y.H., Chandra J., Jabbour E., de Bont E.S.J.M., Horton T.M., Kornblau S.M. Loss of H3K27 methylation identifies poor outcomes in adult-onset acute leukemia. Clin. Epigenet. 2021;13:21. doi: 10.1186/s13148-021-01011-x. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gils N., Denkers F., Smit L. Escape from treatment; the different faces of leukemic stem cells and therapy resistance in acute myeloid leukemia. Front. Oncol. 2021;11:659253. doi: 10.3389/fonc.2021.659253. 659253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegi N.M., Klappacher J., Oswald F., Mulaw M.A., Mandoli A., Thiel V.N., Bamezai S., Feder K., Martens J.H.A., Rawat V.P.S., et al. MEIS2 is an oncogenic partner in AML1-ETO-positive AML. Cell Rep. 2016;16:498–507. doi: 10.1016/j.celrep.2016.05.094. [DOI] [PubMed] [Google Scholar]
- Vetrie D., Helgason G.V., Copland M. The leukaemia stem cell: similarities, differences and clinical prospects in CML and AML. Nat. Rev. Cancer. 2020;20:158–173. doi: 10.1038/s41568-019-0230-9. [DOI] [PubMed] [Google Scholar]
- Wang N., Yao M., Xu J., Quan Y., Zhang K., Yang R., Gao W.-Q. Autocrine activation of CHRM3 promotes prostate cancer growth and castration resistance via CaM/CaMKK-mediated phosphorylation of akt. Clin. Cancer Res. 2015;21:4676–4685. doi: 10.1158/1078-0432.CCR-14-3163. [DOI] [PubMed] [Google Scholar]
- Wartman L.D., Fiala M.A., Fletcher T., Hawkins E.R., Cashen A., DiPersio J.F., Jacoby M.A., Stockerl-Goldstein K.E., Pusic I., Uy G.L., et al. A phase I study of carfilzomib for relapsed or refractory acute myeloid and acute lymphoblastic leukemia. Leuk. Lymphoma. 2016;57:728–730. doi: 10.3109/10428194.2015.1076930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch J.S., Ley T.J., Link D.C., Miller C.A., Larson D.E., Koboldt D.C., Wartman L.D., Lamprecht T.L., Liu F., Xia J., et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P., Basu S., Garcia-Manero G., Hourigan C.S., Oetjen K.A., Cortes J.E., Ravandi F., Jabbour E.J., Al-Hamal Z., Konopleva M., et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer. 2019;125:1470–1481. doi: 10.1002/cncr.31896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabushita T., Satake H., Maruoka H., Morita M., Katoh D., Shimomura Y., Yoshioka S., Morimoto T., Ishikawa T. Expression of multiple leukemic stem cell markers is associated with poor prognosis in de novo acute myeloid leukemia. Leuk. Lymphoma. 2018;59:2144–2151. doi: 10.1080/10428194.2017.1410888. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Gaynor R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Shi P., Zhao G., Xu J., Peng W., Zhang J., Zhang G., Wang X., Dong Z., Chen F., Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Targeted Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Shen N., Ye D.-Q., Liu Q., Zhang Y., Qian X.-X., Hirankarn N., Ying D., Pan H.-F., Mok C.C., et al. Genome-Wide association study in asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLoS Genet. 2010;6:e1000841. doi: 10.1371/journal.pgen.1000841. e1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-C., Chien M.-H., Lai T.-C., Su C.-Y., Jan Y.-H., Hsiao M., Chen C.-L. Monoamine oxidase B expression correlates with a poor prognosis in colorectal cancer patients and is significantly associated with epithelial-to-mesenchymal transition-related gene signatures. Int. J. Mol. Sci. 2020;21:2813. doi: 10.3390/ijms21082813. 2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Li J., Liao C.-P., Jason Wu B. Monoamine oxidase deficiency causes prostate atrophy and reduces prostate progenitor cell activity. Stem Cell. 2018;36:1249–1258. doi: 10.1002/stem.2831. [DOI] [PubMed] [Google Scholar]
- Yu K., Chen B., Aran D., Charalel J., Yau C., Wolf D.M., van 't Veer L.J., Butte A.J., Goldstein T., Sirota M. Comprehensive transcriptomic analysis of cell lines as models of primary tumors across 22 tumor types. Nat. Commun. 2019;10:3574. doi: 10.1038/s41467-019-11415-2. 3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Chen B. Prognostic significance of ferroptosis-related genes and their methylation in AML. Hematology. 2021;26:919–930. doi: 10.1080/16078454.2021.1996055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.