OBJECTIVES:
Respiratory virus infections cause significant morbidity and mortality ranging from mild uncomplicated acute respiratory illness to severe complications, such as acute respiratory distress syndrome, multiple organ failure, and death during epidemics and pandemics. We present a protocol to systematically study patients with severe acute respiratory infection (SARI), including severe acute respiratory syndrome coronavirus 2, due to respiratory viral pathogens to evaluate the natural history, prognostic biomarkers, and characteristics, including hospital stress, associated with clinical outcomes and severity.
DESIGN:
Prospective cohort study.
SETTING:
Multicenter cohort of patients admitted to an acute care ward or ICU from at least 15 hospitals representing diverse geographic regions across the United States.
PATIENTS:
Patients with SARI caused by infection with respiratory viruses that can cause outbreaks, epidemics, and pandemics.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Measurements include patient demographics, signs, symptoms, and medications; microbiology, imaging, and associated tests; mechanical ventilation, hospital procedures, and other interventions; and clinical outcomes and hospital stress, with specimens collected on days 0, 3, and 7–14 after enrollment and at discharge. The primary outcome measure is the number of consecutive days alive and free of mechanical ventilation (VFD) in the first 30 days after hospital admission. Important secondary outcomes include organ failure-free days before acute kidney injury, shock, hepatic failure, disseminated intravascular coagulation, 28-day mortality, adaptive immunity, as well as immunologic and microbiologic outcomes.
CONCLUSIONS:
SARI—Preparedness is a multicenter study under the collaboration of the Society of Critical Care Medicine Discovery, Resilience Intelligence Network, and National Emerging Special Pathogen Training and Education Center, which seeks to improve understanding of prognostic factors associated with worse outcomes and increased resource utilization. This can lead to interventions to mitigate the clinical impact of respiratory virus infections associated with SARI.
Keywords: clinical research networks, COVID-19, hospitalized patients, observational study, severe acute respiratory syndrome coronavirus 2
Lower respiratory tract infections are one of the leading causes of global years of life lost across all ages (1). The 2009–2010 H1N1 pandemic highlighted the need for more global disease burden data for severe influenza, which prompted the expansion of severe acute respiratory infection (SARI) surveillance efforts and the development of a surveillance framework and pandemic-preparedness efforts by the World Health Organization (WHO) (2). WHO created a case definition for SARI as an acute respiratory illness of recent onset (within 10 d) that includes fever (≥38°C) and cough and requires hospitalization (2). Important causes of SARI include seasonal and pandemic influenza viruses and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen responsible for causing the COVID-19 pandemic, which has resulted in morbidity and mortality at an extraordinary scale globally (3). The true impact and epidemiology of viral SARIs requiring hospitalization and ICU admission are still not fully understood (4–6). Given these challenges, the Severe Acute Respiratory Infection—Preparedness (SARI-PREP) multicenter consortium was established to provide the research infrastructure needed for rapidly collecting and evaluating prospective data on SARI clinical epidemiology and outcomes, including biologic specimens and laboratory testing profiles, and contemporary hospital-level stress data. Results of this study may aid in the ongoing development of diagnostic and treatment modalities for SARIs. Given the predominant etiology of current SARIs across our sites, the subsequent introduction and analyses focus on SARS-CoV-2. The study will, however, actively collect data on all SARIs.
A number of single or multisite observational studies (7–11) have been conducted to investigate factors and treatments associated with COVID-19 outcomes. Other studies have used narrower populations to more thoroughly investigate transmission, etiology, and immunological profiles associated with COVID-19 (12, 13). However, substantial challenges remain in nationwide coordination of efforts (14). Further, hospital stress—defined as threats to standard clinical operations and need for increased daily resource use—is increasingly being recognized as a factor impacting care delivery and clinical outcomes in patients admitted with SARI (15–17). The COVID-19 pandemic is another reminder that clinical research networks need to be ready to respond immediately to public health emergencies (14).
The current study seeks to address these challenges through a U.S. nationwide cohort of academic and public acute care hospitals that leverages multiple consortiums and existing research networks to enroll severely ill and critically ill SARI patients. This work will allow understanding of the pathogenesis of SARI and integrated analyses of clinical characteristics, clinical laboratory findings, virology, immunology, proteomics, and transcriptomics associated with SARI. We will also investigate the variance in current treatment and outcomes of hospitalized patients with SARI in order to design and implement interventions to improve outcomes. In addition, we will study the relationship between hospital stress and changes in care delivery on clinical outcomes of SARI patients at the study sites.
SARI-PREP CONSORTIUM FORMATION
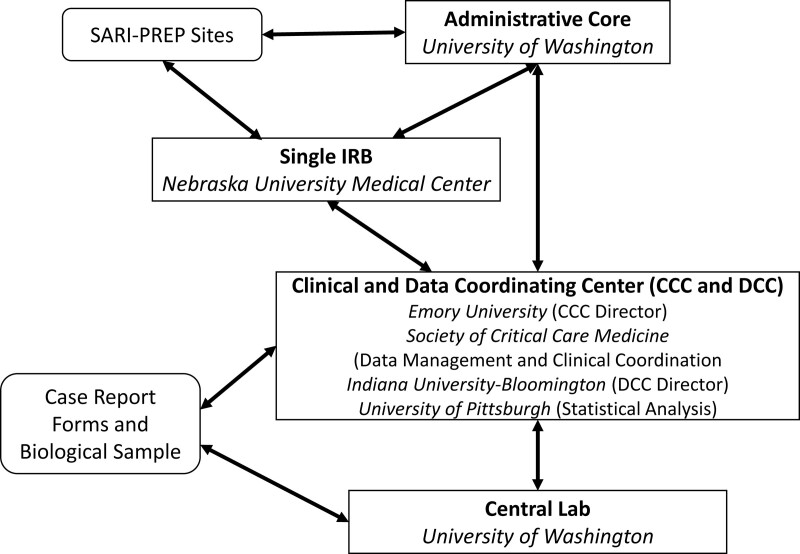
SARI-PREP is a collaboration between clinical research networks with expertise in infectious diseases, pandemic preparedness, critical care, and special pathogens, including the National Emerging Special Pathogen Training and Education Center, as well as with two large research consortiums with past collaboration: the Society of Critical Care Medicine (SCCM) Discovery and the Resilience Intelligence Network. The central institutional review board (IRB) for “SARI-PREP” (IRB 544-20-FB) was approved by the University of Nebraska Medical Center.
Study sites will be recruited from these clinical research networks, ensuring broad geographic and hospital diversity across the United States. Study enrollment to date includes 15 participating hospital locations (Supplemental Table 1, http://links.lww.com/CCX/B73) with over 700 patients currently enrolled (as of June 1, 2021). We anticipate establishing 20 clinical sites from academic and community settings (with the goal of representing all 10 U.S. Department of Health and Human Services regions), with a target cohort size of at least 1,200 adult patients by spring 2022. We will include clinical sites in areas with high caseload and areas that will allow for enrollment of populations at higher risk for SARI, for poor SARI-related outcomes, including underrepresented racial and ethnic minority populations. Direct invitations from the leadership group as well as open invitations using the Society were used in attempt to optimize community hospital involvement. The likely disproportionate number of large academic hospitals and institutions on the east and west coast will admittedly introduce some lack of generalizability but also optimize feasibility.
STUDY SUBJECTS, DATA COLLECTION, AND STUDY DESIGN
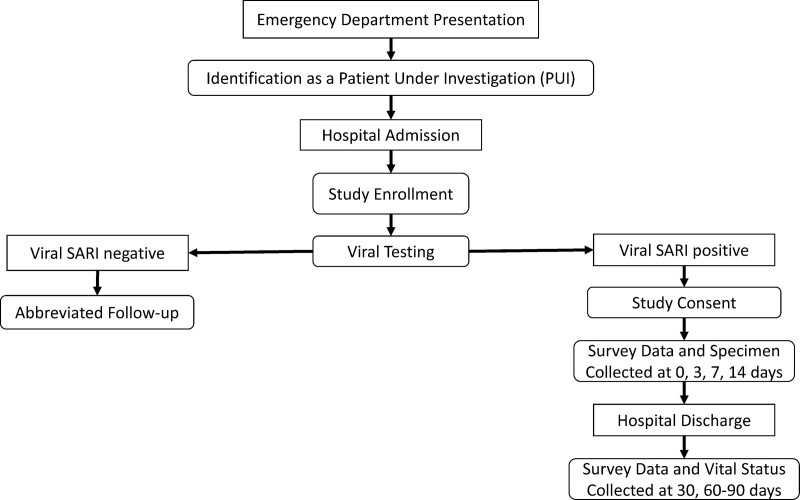
SARI-PREP is a prospective cohort study in which we will enroll, sample, and follow greater than 1,200 hospitalized patients greater than or equal to 18 years old with laboratory-confirmed viral SARI due to SARS-CoV-2, influenza A and B viruses, or other viral respiratory pathogens. Figures 1 and 2 provide a schematic of the network structure and clinical procedures, respectively. Inclusion criteria for a patient under investigation (PUI), based on daily screening of admitted patients at each study site, include: admission to an acute care ward or ICU and a clinical syndrome of lower respiratory tract infection defined by fever, cough, radiographic infiltrates, oxygen saturation as measured by pulse oximetry (Spo2) less than or equal to 94% on room air, or new or increased supplemental oxygen requirement. Patients in the PUI cohort will have abbreviated data collection including demographics and comorbidities and will be followed for laboratory confirmation of viral SARI. Subsequent inclusion in the case cohort will be based on inclusion in the PUI cohort plus laboratory-confirmed respiratory viral infection identified in respiratory specimens. Upon enrollment, each case will undergo the full study protocol. Detailed daily and weekly information will be collected regarding any additional infections, as well as antimicrobial/ antifungal therapies. A detailed SARI-PREP case report form (CRF) will be completed, and serial specimen collection will be initiated. Investigators will also seek consent for ongoing review of the electronic health record to ascertain readmissions and other long-term outcomes. End-of-life issues are also being collected daily, including documenting a family conference or palliative care consult, and associated care decisions.
Figure 1.
Severe acute respiratory infection (SARI) preparedness network structure.
Figure 2.
Severe acute respiratory infection-preparedness (SARI-PREP) patient enrollment and clinical procedures. CCC = Clinical Coordinating Center, DCC = Data Coordinating Center, IRB = institutional review board.
Exclusion criteria for the PUI and case cohorts include: persons less than 18 years old, prisoners or wards of the state, patients with the inability to consent and lack of availability of legal surrogate, and patients with a do-not-resuscitate/do-not-intubate status at admission.
Data collection will occur in two phases: phase 1 will use existing data collected from a subset of study sites (three sites, approximately n = 400), which had ongoing prospective data collection from relevant ICU patients using similar CRFs as SARI-PREP (18, 19) and patient specimens that could be shared with SARI-PREP through data use agreements and transfer of data. The rationale for phase 1 was to leverage data from hospitals with high SARI case volume during earlier phases of the COVID-19 pandemic. Phase 2 data will be collected prospectively using SARI-PREP CRFs at all study sites. As part of phase 2, to understand the impact of hospital stress—threats to standard clinical operations, such as whether patient caseload surge affects clinical management and patient outcomes, study site Principal investigators will be surveyed weekly to collect data about levels of hospital stress on capacity, staffing, supplies, and equipment to assess the influence of measures of stress on patient-level outcomes. The SARI-PREP hospital stress survey is based on the tool developed by Discovery PREP for assessing health system stress during seasonal influenza. For the COVID-19 era, additional questions were included. In total, the questions ask for responses gauging weekly staffing changes, process of care changes, drug shortages (e.g., vasopressors and neuromuscular blocking agents), device shortages (e.g., renal replacement therapy [RRT] and mechanical ventilators), shortages of personal protective equipment (e.g., N95 respirators), and interfacility transfers (e.g., the inability to transfer patients to a higher level of care). Hospital stress metrics will be assessed across the spectrum of acute care including the emergency department (ED), wards, and ICUs, and Principal investigators will additionally be queried to determine if hospital operations are perceived to be abnormal or if there is perceived potentially avoidable patient harm.
SPECIMEN COLLECTION STRATEGY
Biospecimens will be collected with the goals of identifying the molecular markers of risk for poor inhospital and postdischarge clinical outcomes in hospitalized patients with viral SARI and understanding the immune response and recovery from viral SARI. Current proposed analyses include a discovery approach using plasma proteomics and then validating candidate proteins using immunoassays, and collection of peripheral blood transcriptomics and peripheral blood humoral profiling. When contemporaneous viral outbreaks occur in enrolled SARI patients as determined by testing of respiratory specimens at hospital clinical and public health laboratories (e.g., SARS-CoV-2 and Influenza A), we will compare measurements between patients with the different viral pathogens to better understand the biological processes that are specific to these viral infections. This information might inform the tailoring of treatments to patients with viral SARI based on their unique immune and inflammatory profiles.
STUDY GOALS
The primary study goal was to identify which patient characteristics, such as race, ethnicity, age, gender, presence of comorbidities, and use of outpatient medications, are associated with disease severity and clinical outcomes, including risk for respiratory failure and death, in patients admitted with viral SARI, and to quantify the magnitude of those associations. Given the evolution of different variants, analyses will also model differences across time as different variants of SARS-CoV-2 were prevalent. Hypotheses for secondary analyses span four domains: 1) clinical manifestations; 2) adaptive immunity, virology, and coinfections; 3) molecular markers specific to SARS-CoV-2 infection; and 4) the relationship between hospital stress and outcomes. The secondary goals are further detailed in Supplemental Tables 1.a, 1.b, and 1.c, http://links.lww.com/CCX/B73.
OUTCOMES
The primary study outcome is the number of consecutive days alive and free of mechanical ventilation in the first 30 days after hospital admission and study enrollment, recorded to the nearest calendar day. Secondary outcomes of interest include organ failure-free days before acute kidney injury (AKI), shock, hepatic failure, and disseminated intravascular coagulation (DIC). Other outcomes that will be measured are 28- and 90-day survival, hospital and ICU length of stay, need for vasopressors or RRT, and development of adaptive immunity (e.g., anti-SARS-CoV-2 antibodies). Illness severity will also be assessed using the WHO ordinal scale for respiratory failure (20).
DATA ELEMENTS
SARI-PREP will investigate the current standards of care for patients with SARI, including collection of data on treatments, patient characteristics, and patient outcomes. Data elements will include clinical characteristics, and clinical laboratory and research assay findings, including virology, immunology, proteomics, and transcriptomics results. We will investigate the variance in current treatment and outcomes of hospitalized patients with SARI in order to design and implement future interventions to improve outcomes. Table 1 displays the core dataset elements and their associated description and timing. The timing and the volume and aliquots of biological specimens are summarized in Supplemental Tables 3 and 4 (http://links.lww.com/CCX/B73). Supplemental Table 5 (http://links.lww.com/CCX/B73) displays the elements, values, and timing of the data collection for the at-risk cohort. Biospecimens will be stored frozen at −80°C at the University of Washington biorespository for a minimum of 6 years for potential additional testing and analyses.
TABLE 1.
Core Dataset Elements and Collection Dates
| Data | Description | Days Collected |
|---|---|---|
| Core data I | Inclusion, testing, and location | Day 0 |
| Core data II | Demographics, symptoms, preadmission medications, and history | Day 0 |
| Core data III | Microbiology and host response and miscellaneous tests | Days 0–29 |
| Core data IV | Daily imaging and mechanical ventilation | Days 0–29 |
| Core data V | Daily processes of care and Ventilator-associated Pneumonia bundle | Days 0–29 |
| Core data VI | Daily fluid, vasopressors, and hospital medications | Days 0–29 |
| Core data VII | Outcomes | Discharge or death |
| Enhanced data 1 | Daily vitals, neurologic examination, and labs | Days 0–29, 3- and 6-mo follow-up |
| Enhanced data 2 | Daily Sequential Organ Failure Assessment score and events | Days 0–29 |
| Full data A | Apache II score | Day 1 |
| Full data B | Echocardiogram and electrocardiogram | Days 0–29 |
ANALYTIC PLAN AND STATISTICAL CONSIDERATIONS
Enrollment will be tracked with monthly counts of enrolled participants and their follow-up outcomes and status. Participant counts will also be reported in a transparent fashion using the Consolidated Standards of Reporting Trials diagram. The statistical analysis will include descriptive elements and assessments of correlations and associations. Correlations and associations between different types of clinical interventions and/or testing measures and clinical outcomes and complications will be calculated. Relationships will be quantified through both unadjusted measures and using linear and generalized linear models (21) adjusted for baseline clinical characteristics. Data will be graphically described through scatter, line, and box plots, with displays of changes in incidence or outcomes over time. Counts and contingency tables will also be presented to summarize distributions and associations. Ninety-five percent CIs and p values will be displayed to quantify variability and chance. The course of specimen testing, any related clinical interventions, and subsequent clinical outcomes will also be described. Model formulation for the regression analysis will be driven by clinical significance; a more specific outline of statistical models will be specified in advance of the actual analysis to maximize reproducibility and generalizability. Assumptions for each of the regression models will be assessed using appropriate diagnostic graphical displays and statistics.
Statistical models for follow-up articles will depend on the research question, for example, different sets of variables or interactions may be forced into the model depending on clinical knowledge. A logistic model will be used to evaluate the degree of associations between dysregulation of inflammation, innate immunity, adaptive immunity, endothelial injury, apoptosis, and risk for severe hypoxemic respiratory failure and death. A proportional hazards model will be used to evaluate association of these measures with survival time and respiratory failure-free days. Linear models will be used to evaluate associations with degree of respiratory failure (i.e.8-point ordinal scale) and degree of organ dysfunction in viral SARI. Finally, a logistic model will be used to evaluate associations between circulating inflammatory markers (on ICU admission) and severe organ dysfunction (acute respiratory distress syndrome, AKI, shock, and DIC). The models will be formed by identifying variables with significant results in the unadjusted model or variables deemed to be clinically important and then individually testing each additional variable against that beginning model. In addition, as described above for the epidemiologic hypotheses, tree models (22) and random forests (23) will also be used to conduct a more exploratory analyses in a nonparametric fashion using the same set of covariates and outcomes. For all analyses, we will describe all models and variables that were tested, and how (e.g., with which statistic), to provide a transparent accounting of the final interpretation.
For virologic and serologic analyses, different types of generalized linear (e.g., logistic, linear, Poisson, or negative binomial) models or survival models (depending on the outcome distribution) will be used to evaluate associations between early (and time to) development of a humoral immune response to viral infection, level of antibodies, and clinical outcomes, severity of illness, or immune responses. Similar models will be used to assess associations between administration of antiviral therapy or immune-modulating therapy and the above-described outcomes. Associations between viral and bacterial coinfections at admission, such as influenza A and B viruses, Respiratory syncytial virus, Streptococcus pneumoniae, Staphylococcus aureus, and the above-described outcomes, will also be modeled with the same approaches. Finally, differences in risk for hospital-acquired infections between influenza and COVID-19 patients will be compared with logistic models adjusted for duration of mechanical ventilation and corticosteroid administration.
For hospital stress, similar generalized linear models and survival models will be used to assess the association between ICU capacity stress and processes of care and clinical outcomes during both phases 1 and 2. ICU capacity stress, measured daily, will be defined in two ways: 1) all COVID-19 plus non-COVID-19 SARI ICU patients, based on the presence in an ICU or ICU-designated bed and 2) all COVID-19 plus non-COVID-19 SARI ICU-eligible patients based on utilization of high-flow nasal cannula, noninvasive ventilation, invasive mechanical ventilation, or continuous vasopressor infusion, regardless of level of care/bed designation. The inclusion of measurements based on resource utilization rather than ICU bed allocation seeks to minimize bias from ICU admission thresholds that may be different among hospitals or within hospitals over time. Both ICU capacity stress measures will be standardized to pre-COVID ICU bed capacity based on routinely staffed beds by facility during the prior year. All adult ICUs, including ICU-designated surge locations, regardless of discipline will be included. The exposure variable for a given primary study population enrolled SARI patient will be the mean of all daily ICU capacity stress measurements across their acute care encounter. Adjustment variables will include demographics, severity of illness, comorbidities, facility, and longitudinal time. Prespecified sensitivity analyses will stratify based on SARI pathogen identified and peak respiratory support level. Phase 2 analyses will additionally use the prospective hospital stress survey data to provide additional stress metrics that could increase discrimination; measure correlation between stress survey qualitative measures and above quantitative ICU capacity stress; measure daily quantitative ICU capacity stress variability within the weekly qualitative stress survey; interrogate divergent stress between hospital, ICU, ED locations; and interrogate relevance of deviations from standard operating procedures.
POWER AND SAMPLE SIZE
The study will have a target enrollment of 1,200 participants (depending on SARI incidence over the study period). Given the exploratory nature of this study, the wide range of research questions that are critical to investigate throughout the pandemic (and beyond), and the use of several different models with different tests based on different outcome distributions, we have opted not to calculate a specific power statistic. In general, the power of the different tests is limited by the tests of binary outcomes, since continuous, time-to-event, or count outcomes yield more information and greater power for a fixed effect size and a fixed sample size. The lowest power would be for a rare exposure or treatment and an outcome with a 50% probability (or incidence). As a conservative example, an exposure or treatment that is present for 20% of the participants, n = 1,000, yields 80% power to detect a difference of 50% versus 60% in a binary outcome but yields 97% power to detect the same 10% absolute difference between 10% versus 20% in the outcome. The given sample size also provides sufficient data to model a large number of covariates in the regression model based on the usual, but conservative, guideline for 10 events per variable in the model (24).
DATA GOVERNANCE, RESEARCH, AND REPORTING
The SARI-PREP Data Coordinating Center (DCC) and Clinical Coordinating Center (CCC) use the SCCM Discovery communications infrastructure for site recruitment and management, data management, and analysis. The DCC and CCC monitor site performance and communicate with sites on a regular basis. The DCC and CCC use REDCap Cloud (25) to provide an enrollment dashboard that tracks site progress, which is summarized on a publicly facing SCCM website. Additionally, the SARI-PREP Executive Committee will determine appropriateness of updated article and secondary or ancillary analysis publications. The study protocol was approved by the central IRB at the University of Nebraska Medical Center and by the IRB at each participating site. The Centers for Disease Control and Prevention determined that the proposed information collection is not conducted or sponsored by the Federal government, and the study protocol did not require Human Research Protection Office review under “45 CFR 46.102(l).”
FUTURE DIRECTIONS
SARI-PREP is a U.S. platform of consortiums and hospitals to address important clinical questions regarding patients with SARI. The current SARI-PREP study will continue prospective enrollment of hospitalized patients to evaluate the natural history, prognostic biomarkers, and characteristics, including hospital stress, associated with clinical outcomes and severity. The study also aims to identify the biological basis for SARI and associated outcomes, and determine the long-term sequelae of SARI. Beyond this study, SARI-PREP can be leveraged to create a working platform for future surveillance and real-time public health emergency research, as well as for clinical trials of interventions to improve clinical management.
Supplementary Material
Footnotes
Drs. Postelnicu and Srivastava are co-first authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the Centers for Disease Control Foundation Project “Clinical Research Networks to Improve Clinical Management of Hospitalized COVID-19 Patients”; National Clinical Trial number NCT04786301.
Dr. Anesi receives research funding from the Agency for Healthcare Research and Quality and reports payments for authoring COVID-19 chapters for UpToDate and for expert witness consulting. Dr. Segal receives grant funding from the National Institutes of Health (Method to Extend Research in Time R37 CA244775) paid to institution, and Dr. Wyles receives funding from Gilead Sciences paid to institution (ended August 31, 2021). The remaining authors have disclosed that they do not have any potential conflicts of interest.
The views expressed are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, et al. : Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzner J, Qasmieh S, Mounts AW, et al. : Revision of clinical case definitions: Influenza-like illness and severe acute respiratory infection. Bull World Health Organ 2018; 96:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. COVID-19 Weekly Epidemiological Update Edition 54. Published August 24, 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---24-august-2021. Accessed. October 29, 2021.
- 4.Ljungström LR, Jacobsson G, Claesson BEB, et al. : Respiratory viral infections are underdiagnosed in patients with suspected sepsis. Eur J Clin Microbiol Infect Dis 2017; 36:1767–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartman L, Zhu Y, Edwards KM, et al. : Underdiagnosis of influenza virus infection in hospitalized older adults. J Am Geriatr Soc 2018; 66:467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen C, Kaku S, Tutera D, et al. : Viral respiratory infections of adults in the intensive care unit. J Intensive Care Med 2016; 31:427–441 [DOI] [PubMed] [Google Scholar]
- 7.Bennett TD, Moffitt RA, Hajagos JG, et al. : The national COVID cohort collaborative: Clinical characterization and early severity prediction. medRxiv Preprint posted online Jan 23, 2021. doi: 10.1101/2021.01.12.21249511 [Google Scholar]
- 8.Cobb NL, Sathe NA, Duan KI, et al. : Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc 2021; 18:632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Greco M, Zanella A, et al. ; COVID-19 Lombardy ICU Network: Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliva A, Ceccarelli G, Borrazzo C, et al. : Comparison of clinical features and outcomes in COVID-19 and influenza pneumonia patients requiring intensive care unit admission. Infection 2021; 49:965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouze A, Martin-Loeches I, Povoa P, et al. : Early bacterial identification among intubated patients with COVID-19 or influenza pneumonia: A European multicenter comparative cohort study. Am J Respir Criti Care Med 2021; 204:546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathew D, Giles JR, Baxter AE, et al. ; UPenn COVID Processing Unit: Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020; 369:eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coles C, Millar EV, Burgess T, et al. : The acute respiratory infection consortium: A multi-site, multi-disciplinary clinical research network in the department of defense. Mil Med 2019; 184:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angus DC, Gordon AC, Bauchner H: Emerging lessons from COVID-19 for the US clinical research enterprise. JAMA 2021; 325:1159–1161 [DOI] [PubMed] [Google Scholar]
- 15.Xie Y, Bowe B, Maddukuri G, et al. : Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: Cohort study. BMJ 2020; 371:m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anesi GL, Liu VX, Gabler NB, et al. : Associations of intensive care unit capacity strain with disposition and outcomes of patients with sepsis presenting to the emergency department. Ann Am Thorac Soc 2018; 15:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anesi GL, Chowdhury M, Small DS, et al. : Association of a novel index of hospital capacity strain with admission to intensive care units. Ann Am Thorac Soc 2020; 17:1440–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatraju PK, Morrell ED, Zelnick L, et al. : Comparison of host endothelial, epithelial and inflammatory response in ICU patients with and without COVID-19: A prospective observational cohort study. Crit Care 2021; 25:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulaiman I, Chung M, Angel L, et al. : Microbial signatures in the lower airways of mechanically ventilated COVID19 patients associated with poor clinical outcome. medRxiv Preprint posted online Feb 26, 2021. doi: 10.1101/2021.02.23.21252221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klok FA, Boon GJ, Barco S, et al. : The Post-COVID-19 functional status scale: A tool to measure functional status over time after COVID-19. Eur Respir J 2020; 56:2001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinbaum DG, Kupper LL, Nizam A, et al. : Applied Regression Analysis and Other Multivariable Methods. Belmont, CA, Cengage Learning, 2013 [Google Scholar]
- 22.Breiman L, Friedman J, Stone CJ, et al. : Classification and Regression Trees. CRC; Press, 1984 [Google Scholar]
- 23.Breiman L: Random forests. Machine learning 2001; 45:5–32 [Google Scholar]
- 24.Vittinghoff E, McCulloch CE: Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007; 165:710–718 [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.