IMPORTANCE:
The role of early, serial measurements of protein biomarkers in sepsis-induced acute respiratory distress syndrome (ARDS) is not clear.
OBJECTIVES:
To determine the differences in soluble receptor for advanced glycation end-products (sRAGEs), angiopoietin-2, and surfactant protein-D (SP-D) levels and their changes over time between sepsis patients with and without ARDS.
DESIGN, SETTING, AND PARTICIPANTS:
Prospective observational cohort study of adult patients admitted to the medical ICU at Grady Memorial Hospital within 72 hours of sepsis diagnosis.
MAIN OUTCOMES AND MEASURES:
Plasma sRAGE, angiopoietin-2, and SP-D levels were measured for 3 consecutive days after enrollment. The primary outcome was ARDS development, and the secondary outcome of 28-day mortality. The biomarker levels and their changes over time were compared between ARDS and non-ARDS patients and between nonsurvivors and survivors.
RESULTS:
We enrolled 111 patients, and 21 patients (18.9%) developed ARDS. The three biomarker levels were not significantly different between ARDS and non-ARDS patients on all 3 days of measurement. Nonsurvivors had higher levels of all three biomarkers than did survivors on multiple days. The changes of the biomarker levels over time were not different between the outcome groups. Logistic regression analyses showed association between day 1 SP-D level and mortality (odds ratio, 1.52; 95% CI, 1.03–2.24; p = 0.03), and generalized estimating equation analyses showed association between angiopoietin-2 levels and mortality (estimate 0.0002; se 0.0001; p = 0.04).
CONCLUSIONS AND RELEVANCE:
Among critically ill patients with sepsis, sRAGE, angiopoietin-2, and SP-D levels were not significantly different between ARDS and non-ARDS patients but were higher in nonsurvivors compared with survivors. The trend toward higher levels of sRAGE and SP-D, but not of angiopoietin-2, in ARDS patients may indicate the importance of epithelial injury in sepsis-induced ARDS. Changes of the biomarker levels over time were not different between the outcome groups.
Keywords: acute respiratory distress syndrome, biomarkers, sepsis
Key Points
Question: Are there differences in protein biomarkers and their changes over time between sepsis patients who develop ARDS and those who do not?
Findings: The levels of soluble receptor for advanced glycation end-products (sRAGE), angiopoietin-2, and surfactant protein D (SP-D) and their changes over the first three days of enrollment were similar between ARDS and non-ARDS patients. Higher levels of these biomarkers, especially Ang-2 and SP-D, were associated with mortality in patients with sepsis.
Meaning: Among sepsis patients, levels of sRAGE, angiopoietin-2, and SP-D were not significantly different between ARDS and non-ARDS patients, but larger studies and deeper mechanistic understanding are needed.
The acute respiratory distress syndrome (ARDS) is a severe form of acute inflammatory lung injury associated with high mortality (1). ARDS is a markedly heterogeneous syndrome, with a wide variety of predisposing conditions that result in different clinical phenotypes (2). The heterogeneity of ARDS is thought to contribute to the lack of a reliable diagnostic test or a specific pharmacologic therapy for ARDS despite decades of research (2, 3). In order to address these problems, protein biomarkers have been used to help understand ARDS heterogeneity and phenotypes. Protein biomarkers can be measured from various body compartments such as plasma and the lungs and can be used to help understand the pathophysiologic mechanisms in the development and progression of ARDS (4). In particular, sRAGE is thought to be a causal intermediate in sepsis-induced ARDS (5). Previous studies have demonstrated a correlation between sRAGE and the presence and severity of ARDS, as well as increased mortality (6–10). Angiopoietin-2 and SP-D are additional biomarkers that can also help distinguish different subtypes of ARDS (11–16).
However, several methodological limitations exist in prior studies of ARDS biomarkers. First, many prior studies were retrospective, often using biospecimens and data from prior ARDS clinical trials. Second, many prior studies did not differentiate patients based on the heterogeneous etiologies of ARDS. In particular, patients with sepsis-induced ARDS have worse clinical outcomes and demonstrate different biomarker profiles compared with those with ARDS from other causes (17, 18), suggesting differences in pathophysiology that warrant targeted investigation. Third, many prior studies measured the biomarker levels only at a single time point, and only a few studies monitored the longitudinal changes of the biomarkers prospectively. Monitoring the changes in biomarker levels over time can provide useful information about the dynamic changes and responses to treatment interventions in sepsis (19) and ARDS (8, 15).
The objective of this study was to determine the differences in plasma sRAGE, angiopoietin-2, and SP-D levels and their changes over time between sepsis patients with and without ARDS, in order to examine the potential biological differences between the two groups. We sought to address the methodologic limitations in prior studies by conducting a prospective cohort study consisting only of patients with sepsis, and performing serial measurements of sRAGE, angiopoietin-2, and SP-D over the first 3 days of enrollment. The hypothesis was that the ARDS patients will have higher absolute sRAGE, angiopoietin-2, and SP-D levels and have greater increases in the biomarker levels over time, compared with the non-ARDS patients.
MATERIALS AND METHODS
Study Information
This was a prospective observational cohort study conducted in the medical ICU (MICU) at Grady Memorial Hospital, Atlanta, GA, between September 16, 2020, and November 8, 2021. This study was reviewed and approved by the Institutional Review Board (IRB) at Emory University, Atlanta, GA (study title: “Biomarkers and Metabolomics in Sepsis-Induced ARDS”, approval number: “STUDY00001060”, approval date: July 10, 2020) and by the Research Oversight Committee (ROC) at Grady (study title: “Examining the Association between Plasma Biomarkers and Metabolic Profiles and ARDS Development in Patients with Sepsis”, approval number: “#000-1060”, approval date: September 9, 2020). Informed consent was obtained from each participant or their legally authorized representatives. For eligible patients who were unable to consent and whose legally authorized representatives could not be reached, a waiver of informed consent was also permitted by the Emory IRB and Grady ROC given minimal risk to the participants. The study procedures were followed in accordance with the ethical standards of the Emory IRB and Grady ROC and with the Helsinki Declaration of 1975.
Screening and Enrollment
The Grady MICU was screened daily for eligible patients. Patients were eligible if they were admitted to the MICU within 72 hours of diagnosis of sepsis or septic shock, as defined by the Sepsis-3 definition (20). There was no time limit on being admitted to the MICU as long as the patient was within 72 hours of a new diagnosis of sepsis or septic shock. Patients were excluded if they were under 18 years old, pregnant, or incarcerated; already had ARDS at the time of screening; were not candidates for full resuscitation or pursuing comfort measures only; or declined participation in the study.
Study Protocol
Serial blood samples were collected from each participant once daily on days 1, 2, and 3 of study enrollment (first blood sample on the day of enrollment as soon as possible after obtaining or waiving informed consent and then 24 ± 3 and 48 ± 3 hr after the first blood sample collection). The timing of the blood sample collections was chosen in order to capture the biomarker levels before or around the time of ARDS onset, based on literature reporting that the majority of ARDS cases develop within the first 2–3 days of acute hypoxic respiratory failure or hospital admission (1, 21). Blood was centrifuged to isolate the plasma, which was frozen and stored at –80°C until analysis. Levels of sRAGE, angiopoietin-2, and SP-D were measured from each of the plasma samples using commercially available enzyme-linked immunosorbent assay (ELISA) kits (sRAGE: BioVendor, Asheville, NC; angiopoietin-2 and SP-D: R&D Systems, Minneapolis, MN) (Supplemental Table S1, http://links.lww.com/CCX/B76).
Participants were followed for up to 28 days for the primary outcome of ARDS development according to the Berlin definition (22), with specific criteria for participants receiving oxygen support with heated and humidified high-flow nasal cannula (HFNC) and additional diagnostic considerations outlined in Supplemental Table S1 (http://links.lww.com/CCX/B76). The ARDS diagnosis was first determined by the primary investigator with experience in ARDS research (P.Y.). Any patient who received mechanical ventilation required verification of ARDS diagnosis by the senior investigator (A.M.E.). Secondary outcomes included 28-day all-cause in-hospital mortality (including in-hospital death and discharge to hospice), ventilator-free days, and ICU-free days.
Additional clinical information including demographics, medical comorbidities, severity of illness scores (Sequential Organ Failure Assessment [23] and Acute Physiology and Chronic Health Evaluation-II [24] scores), primary and secondary sources of infection, ventilator settings, duration of mechanical ventilation, ICU and hospital length of stays, and the final disposition status were recorded. In order to reduce bias, the investigators assessing the ARDS diagnosis and clinical outcomes were blinded to the biomarker measurements until completion of clinical data entry, and the investigators performing the biomarker measurements were blinded to the clinical information until completion of biomarker measurements.
Statistical Analysis and Analytical Methods
Based on preliminary data from an internal study showing a difference in sRAGE level between ARDS and non-ARDS patients of 2,822 pg/mL and sd of 3,468 pg/mL, expected ARDS occurrence rate of 20% resulting in 1:4 enrollment ratio of ARDS to non-ARDS patients, significance level of 0.05, and power of 0.80, the calculated sample size needed was 75. This calculation was extrapolated to angiopoietin-2, SP-D, and for serial measurements, given lack of preliminary data related to these aspects of the study.
Simple descriptive statistics were used for comparisons of baseline demographics and clinical data between ARDS and non-ARDS patients. Two-sample independent t test was used for comparing normally distributed continuous variables; Wilcoxon rank-sum test, for comparing nonnormally distributed continuous variables; and chi-square or Fisher exact test, for comparing categorical variables. The absolute sRAGE, angiopoietin-2, and SP-D levels were found to be nonnormally distributed and were log-transformed to approximate a normal distribution and then compared using two-sample t test. The absolute changes in the sRAGE, angiopoietin-2, and SP-D levels from day 1 to days 2 and 3 were calculated. The changes in the biomarker levels were also nonnormally distributed, but these values were not log-transformed and were compared as-is using the Wilcoxon rank-sum test.
Multivariable logistic regression (LR) and generalized estimating equation (GEE) models were used to examine the association between the biomarker levels and the outcome variables, adjusting for potential confounders. For LR models, the main exposure variables of interest were the absolute sRAGE, angiopoietin-2, and SP-D levels on day 1 and the changes of the three biomarker levels from day 1 to days 2 and 3. Due to significant correlation between the three biomarker levels, each of the biomarker variables were input individually into separate LR models. For GEE models, the sRAGE, angiopoietin-2, and SP-D levels over the 3 days were analyzed as repeated measurements within each subject to account for the correlation from longitudinal sampling.
For covariate selection, age, sex, and race were included by convention. The following covariates were also considered: primary source of infection (COVID-19 vs pulmonary [pneumonia or aspiration pneumonia] vs nonpulmonary [all other sources of infection]), vasopressor requirement, renal replacement therapy requirement, tidal volume per ideal body weight, positive end-expiratory pressure, and ARDS diagnosis (when modeling mortality as the outcome). From this list, covariates for inclusion in the final model were selected based on clinical reasoning, results of the univariate analyses, likelihood ratio tests for sequential addition of the covariates to the model, and model fit considerations. The final model for ARDS included age, sex, race, and primary source of infection, in addition to the biomarker variable. The final model for mortality included age, sex, race, and vasopressor use as covariates, in addition to the biomarker variable. For the GEE models, the time variable indicating the day of sample collection and the interaction term between the biomarker levels and the time variable were also considered. However, these terms were not significant with estimates and ses rounding to 0.0000 and were not included the final models.
Significance level of α equals to 0.05 was used for all statistical tests. All data analyses and statistical tests were performed in SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
A total of 111 critically ill patients with sepsis were enrolled between September 16, 2020, and November 8, 2021 (Supplemental Fig. S1, http://links.lww.com/CCX/B76). The primary outcome of ARDS developed in 21 patients (18.9%), with median time from day 1 blood draw to ARDS onset of 24 hours (interquartile range [IQR], 8–42 hr). ARDS and non-ARDS patients were similar with regard to their demographics, chronic medical comorbidities, and severity of illness scores at the time of enrollment (Table 1). ARDS patients had a higher proportion of pulmonary sources of infection, including pneumonia, aspiration pneumonia, and COVID-19 (Table 1). A higher proportion of ARDS patients required invasive mechanical ventilation (IMV) compared with non-ARDS patients (n = 20 [95.2%] in ARDS vs n = 59 [65.6%] in non-ARDS, p = 0.007). One ARDS patient fulfilled the Berlin criteria while receiving noninvasive ventilation, but did not require IMV. Overall mortality was not significantly different between ARDS versus non-ARDS patients (n = 10 [47.6%] in ARDS group vs n = 35 [38.9%] in non-ARDS group; p = 0.46), but ARDS patients had significantly fewer 28-day ventilator-free days (median [IQR] 8 [0–22] vs 20.5 [6–28] d; p = 0.02) and 28-day ICU-free days (median [IQR] 1 [0–21] vs 16.5 [3–24] d; p = 0.02) (Table 2).
TABLE 1.
Baseline Characteristics of the Study Participants at the Time of Study Enrollment
| Characteristic | Total (N = 111) | ARDS (N = 21; 18.9%) | Non-ARDS (N = 90; 81.1%) | p |
|---|---|---|---|---|
| Age (yr), median (IQR) | 65 (55–74) | 62 (52–71) | 65 (55–75) | 0.44a |
| Sex, n (%) | 0.51b | |||
| Male | 67 (60.4) | 14 (66.7) | 53 (58.9) | |
| Race, n (%) | 0.07b | |||
| Black | 88 (79.3) | 13 (61.9) | 75 (83.3) | |
| White | 16 (14.4) | 5 (23.8) | 11 (12.2) | |
| Other | 7 (6.3) | 3 (14.3) | 4 (4.44) | |
| Body mass index (kg/m2), median (IQR) | 25.4 (21.8–30.0) | 24.4 (22.4–31.0) | 25.4 (21.5–29.9) | 0.66a |
| Sequential Organ Failure Assessment score, median (IQR) | 8 (6–11) | 8 (6–11) | 8 (6–11) | 0.77a |
| Acute Physiology and Chronic Health Evaluation-II score, median (IQR) | 21 (16–26) | 22 (16–24) | 21 (17–26) | 0.79a |
| Medical comorbidities, n (%) | > 0.05c | |||
| Dementia | 20 (18.0) | 3 (14.3) | 17 (18.9) | |
| Stroke | 25 (22.5) | 4 (19.1) | 21 (23.3) | |
| Congestive heart failure | 29 (26.1) | 2 (9.5) | 27 (30.0) | |
| Coronary artery disease and/or myocardial infarction | 11 (9.9) | 0 (0.0) | 11 (12.2) | |
| Atrial fibrillation | 19 (17.1) | 1 (4.8) | 18 (20.0) | |
| Hypertension | 63 (56.8) | 11 (52.4) | 52 (57.8) | |
| Chronic lung disease | 31 (27.9) | 5 (23.8) | 26 (28.9) | |
| Cirrhosis | 6 (5.4) | 1 (4.8) | 5 (5.6) | |
| Chronic kidney disease | 23 (20.7) | 3 (14.3) | 20 (22.2) | |
| End-stage renal disease | 8 (7.2) | 1 (4.8) | 7 (7.8) | |
| Diabetes mellitus | 42 (37.8) | 10 (47.6) | 32 (35.6) | |
| Malignancy | 11 (9.9) | 2 (9.5) | 9 (10.0) | |
| HIV | 7 (6.3) | 2 (9.5) | 5 (5.6) | |
| Primary infection, n (%) | 0.02c | |||
| Pneumonia | 26 (23.4) | 5 (23.8) | 21 (23.3) | |
| Aspiration | 14 (12.6) | 5 (23.8) | 9 (10.0) | |
| COVID-19 | 19 (17.1) | 8 (38.1) | 11 (12.2) | |
| Urine | 24 (21.6) | 2 (9.5) | 22 (24.4) | |
| Gastrointestinal /abdominal | 5 (4.5) | 0 (0.0) | 5 (5.6) | |
| Skin/soft tissue | 14 (12.6) | 0 (0.0) | 14 (15.6) | |
| Other | 9 (8.1) | 1 (4.8) | 8 (8.9) |
ARDS = acute respiratory distress syndrome, IQR = interquartile range.
aWilcoxon rank-sum test.
bχ2.
cFisher exact test were used to calculate the p values.
TABLE 2.
Clinical Course and Outcomes of the Study Participants
| Outcome | Total (N = 111) | ARDS (N = 21; 18.9%) | Non-ARDS (N = 90; 81.1%) | p |
|---|---|---|---|---|
| Vasopressor requirement, n (%) | 84 (75.7) | 17 (81.0) | 67 (74.4) | 0.53a |
| Renal replacement therapy, n (%) | 27 (24.3) | 3 (14.3) | 24 (26.7) | 0.23a |
| Invasive mechanical ventilation, n (%) | 79 (71.2) | 20 (95.2) | 59 (65.6) | 0.007a |
| Initial tidal volume per ideal body weight (mL/kg), median (IQR) | 6.21 (5.85–6.95) | 5.88 (5.43–6.53) | 6.26 (5.92–7.04) | 0.08b |
| Initial positive end-expiratory pressure (cm H2O), median (IQR) | 8 (8) | 8 (8–12) | 8 (8) | 0.01b |
| Worst Pao2/Fio2 ratio, median (IQR) | 132 (181–250) | 118 (78–166) | 202 (143–262) | < 0.001b |
| Mortality, n (%) | 45 (40.5) | 10 (47.6) | 35 (38.9) | 0.46a |
| 28-d ventilator-free days (d), median (IQR) | 19 (2–26) | 8 (0–22) | 20.5 (6–28) | 0.02b |
| 28-d ICU-free days (days), median (IQR) | 15 (0–24) | 1 (0–21) | 16.5 (3–24) | 0.02b |
ARDS = acute respiratory distress syndrome, IQR = interquartile range.
aχ2.
bWilcoxon rank-sum test were used to calculate the p values.
Protein Biomarker Analysis by ARDS Diagnosis
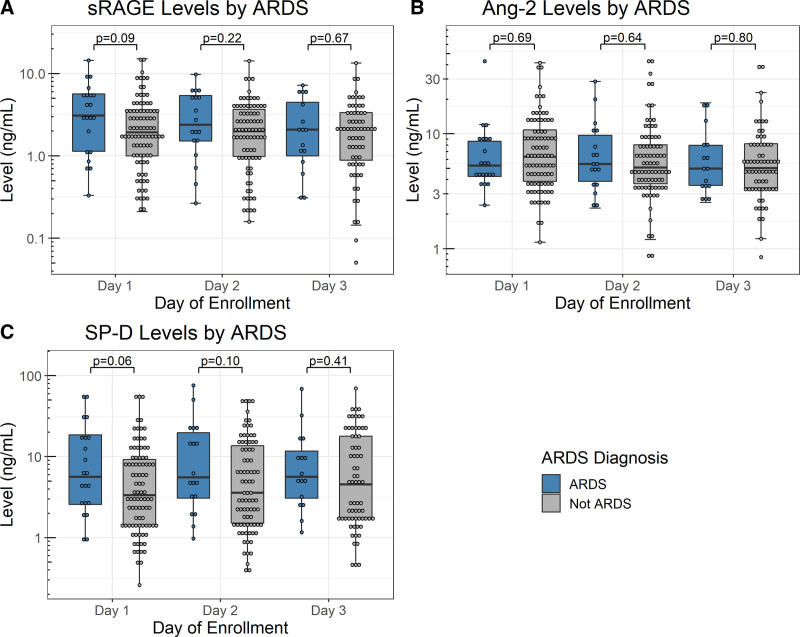
The absolute levels of sRAGE, angiopoietin-2, and SP-D were not significantly different between ARDS and non-ARDS patients on all 3 days of measurement (Fig. 1; and Supplemental Table S2, http://links.lww.com/CCX/B76). The absolute sRAGE and SP-D levels trended higher in ARDS patients than in non-ARDS patients, but there was a significant overlap between the groups. The mean differences in the absolute sRAGE and SP-D levels between ARDS versus non-ARDS patients were greater on day 1 and became smaller on subsequent days. ARDS patients had a greater change in angiopoietin-2 level from day 1 to day 2 compared with non-ARDS patients, but there was a significant overlap between the groups; the changes in sRAGE or SP-D levels over time were not significantly different between ARDS patients and non-ARDS patients (Supplemental Fig. S2, http://links.lww.com/CCX/B76).
Figure 1.
Levels of soluble receptor for advanced glycation end-products (sRAGE) (A), angiopoietin-2 (Ang-2) (B), and surfactant protein-D (SP-D) (C) on each day by acute respiratory distress syndrome (ARDS) diagnosis. Each panel shows the absolute levels and distribution of the three biomarkers on each day of collection. Number of patients for each day was as follows: 111 on day 1 (21 ARDS vs 90 non-ARDS), 100 on day 2 (18 ARDS vs 82 non-ARDS), and 83 on day 3 (16 ARDS vs 67 non-ARDS).
Protein Biomarker Analysis by Mortality Status
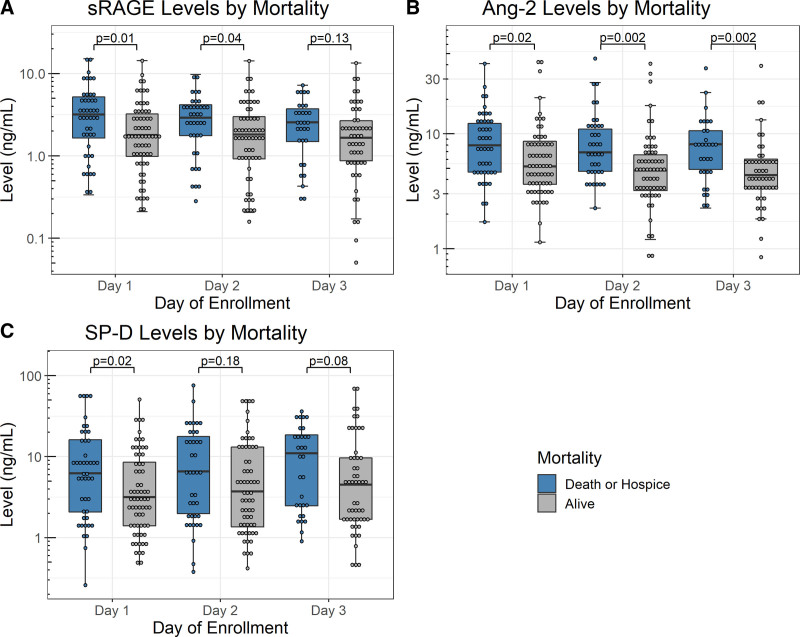
Nonsurvivors had significantly higher absolute levels of sRAGE on days 1 and 2, higher absolute levels of angiopoietin-2 on all 3 days, and higher absolute SP-D on day 1 (Fig. 2; and Supplemental Table S3, http://links.lww.com/CCX/B76). In particular, angiopoietin-2 levels, which were comparable between ARDS versus non-ARDS patients, showed a significant difference between nonsurvivors versus survivors that became more pronounced with time. The changes of the biomarker levels over time were not significantly different between nonsurvivors and survivors regardless of the time points (Supplemental Fig. S3, http://links.lww.com/CCX/B76).
Figure 2.
Levels of soluble receptor for advanced glycation end-products (sRAGE) (A), angiopoietin-2 (Ang-2) (B), and surfactant protein-D (SP-D) (C) on each day by mortality status. Each panel shows the absolute levels and distribution of the three biomarkers on each day of collection. Number of patients for each day was as follows: 111 on day 1 (45 death/hospice vs 66 alive), 100 on day 2 (39 death/hospice vs 61 alive) and 83 on day 3 (31 death/hospice vs 52 alive).
Additional Analyses
There were two patients in the non-ARDS group who were not considered to have ARDS solely because they received HFNC without subsequently requiring positive-pressure ventilation. When these two patients were reclassified into the ARDS group, the differences in the absolute SP-D level on day 1 (mean ± sd 1.979 ± 1.229 log [ng/mL] in ARDS vs 1.333 ± 1.203 log [ng/mL] in non-ARDS; p = 0.02) and day 2 (mean ± sd 2.120 ± 1.266 vs 1.415 ± 1.267 log [ng/mL]; p = 0.03) as well as the change in angiopoietin-2 from day 1 to 2 (median [IQR] 0.321 [–0.881 to 1.457] vs –0.609 [–2.006 to 0.146] ng/mL; p = 0.02) were statistically significant.
In subgroup analyses examining patients with COVID-19 as the primary source of infection (n = 19; 8 ARDS and 11 non-ARDS), none of the absolute biomarker levels or the changes of the biomarker levels over time were significantly different between ARDS and non-ARDS patients (Supplemental Table S4, http://links.lww.com/CCX/B76). Analyzing the percent changes of the biomarker levels rather than the absolute changes yielded similar results.
Multivariable Analyses
In LR analyses for the overall cohort, absolute SP-D level on day 1 was significantly associated with mortality (adjusted odds ratio, 1.52; 95% CI, 1.03–2.24; p = 0.03) after adjusting for age, sex, race, and vasopressor requirement (Table 3). The other biomarker variables were not significantly associated with ARDS development or mortality in LR analyses. All LR models had good fit by Hosmer-Lemeshow goodness-of-fit test (p > 0.05 for all models).
TABLE 3.
Results From Multivariable Logistic Regression Analyses, With Separate Models for Each Biomarker Variable
| Biomarker Variables | Adjusted OR for Acute Respiratory Distress Syndrome Developmenta | 95% CI | p |
|---|---|---|---|
| log (sRAGE level), day 1 | 1.55 | 0.81–2.94 | 0.18 |
| Δ sRAGE, day 1 to 2 | 0.73 | 0.36–1.45 | 0.36 |
| Δ sRAGE, day 1 to 3 | 0.80 | 0.46–1.40 | 0.43 |
| log (Ang-2 level), day 1 | 1.21 | 0.53–2.74 | 0.65 |
| Δ Ang-2, day 1 to 2 | 1.04 | 0.92–1.17 | 0.57 |
| Δ Ang-2, day 1 to 3 | 0.99 | 0.88–1.11 | 0.82 |
| log (SP-D level), day 1 | 1.53 | 0.97–2.42 | 0.07 |
| Δ SP-D, day 1 to 2 | 1.04 | 0.97–1.11 | 0.32 |
| Δ SP-D, day 1 to 3 | 1.01 | 0.96–1.07 | 0.72 |
| Biomarker Variables | Adjusted OR for Mortalityb | 95% CI | p |
| log (sRAGE level), day 1 | 1.57 | 0.99–2.48 | 0.06 |
| Δ sRAGE, day 1 to 2 | 1.04 | 0.58–1.88 | 0.90 |
| Δ sRAGE, day 1 to 3 | 0.94 | 0.57–1.55 | 0.81 |
| log (Ang-2 level), day 1 | 1.65 | 0.85–3.20 | 0.14 |
| Δ Ang-2, day 1 to 2 | 1.08 | 0.96–1.21 | 0.18 |
| Δ Ang-2, day 1 to 3 | 1.04 | 0.95–1.14 | 0.41 |
| log (SP-D level), day 1 | 1.52 | 1.03–2.24 | 0.03 |
| Δ SP-D, day 1 to 2 | 0.96 | 0.91–1.02 | 0.23 |
| Δ SP-D, day 1 to 3 | 0.96 | 0.91–1.02 | 0.21 |
Ang-2 = angiopoietin-2, OR = odds ratio, SP-D = surfactant protein-D, sRAGE = soluble receptor for advanced glycation end-products.
aEach logistic regression model for acute respiratory distress syndrome development adjusts for age (continuous), sex (male or female), race (Black, White, or other), and primary source of infection (COVID-19, pulmonary infection [pneumonia or aspiration pneumonia], or other [all other sources of infection]), in addition to the biomarker-related variable in that row.
bEach logistic regression model for mortality adjusts for age (continuous), sex (male or female), race (Black, White, or other), and vasopressor use (yes or no), in addition to the biomarker-related variable in that row.
Each row of the table represents separate logistic regression models, each adjusting for the biomarker-related variable in that row only, plus the covariates detailed below. Only the results for the biomarker-related variable from each model is presented in the table.
In GEE analyses, the time variable indicating the day of sample collection and the interaction term between the biomarker levels and the time variable were not significant and were not included in the final models. In the final GEE models, angiopoietin-2 levels were weakly associated with mortality (estimate 0.0002; se 0.0001; p = 0.04), but none of the biomarker levels were significantly associated with ARDS development (Table 4).
TABLE 4.
Results From Generalized Estimating Equation Analyses, With Separate Models for Each Biomarker Variable
| Biomarker Variables | Estimate (se) for Acute Respiratory Distress Syndrome Developmenta | p |
|---|---|---|
| log (sRAGE levels) | 0.0002 (0.0002) | 0.26 |
| log (Ang-2 levels) | 0.0001 (0.0001) | 0.32 |
| log (SP-D levels) | 0.0001 (0.0001) | 0.13 |
| Biomarker Variables | Estimate (se) for Mortalityb | p |
| log (sRAGE levels) | 0.0004 (0.0002) | 0.06 |
| log (Ang-2 levels) | 0.0002 (0.0001) | 0.04 |
| log (SP-D levels) | 0.0001 (0.0001) | 0.14 |
Ang-2 = angiopoietin-2, sRAGE = soluble receptor for advanced glycation end-products, SP-D = surfactant protein-D.
Each row of the table represents separate generalized estimating equation models, each adjusting for the biomarker-related variable in that row only, plus the covariates detailed below. Only the results for the biomarker-related variable from each model is presented in the table. Time variable indicating the day of sample collection and the interaction term between the biomarker levels and the time variable were not significant, and were not included in the final models.
aEach generalized estimating equation model for acute respiratory distress syndrome development adjusts for age (continuous), sex (male or female), race (Black, White, or other), and primary source of infection (COVID-19, pulmonary infection [pneumonia or aspiration pneumonia], or other [all other sources of infection]), in addition to the biomarker-related variable in that row.
bEach generalized estimating equation model for mortality adjusts for age (continuous), sex (male or female), race (Black, White, or other), and vasopressor use (yes or no), in addition to the biomarker-related variable in that row.
DISCUSSION
In this prospective observational cohort study of critically ill patients with sepsis, sRAGE, angiopoietin-2, and SP-D levels were not significantly different between patients who developed ARDS and those who did not develop ARDS. The sRAGE, angiopoietin-2, and SP-D levels were significantly higher in non-survivors compared with survivors during the first 3 days of enrollment, and multivariable models showed associations between SP-D and angiopoietin-2 levels and mortality. However, the temporal changes of the three biomarker levels over time were not significantly different between ARDS patients compared with non-ARDS patients and between nonsurvivors compared with survivors.
Although the biomarker levels were not significantly different between ARDS and non-ARDS patients, some observations can be made from the results. sRAGE and SP-D, both markers of lung epithelial injury, trended higher in ARDS patients compared with non-ARDS patients, whereas angiopoietin-2, a marker of endothelial injury, was similar between ARDS and non-ARDS patients. Although it is possible that the high proportion of pulmonary sources of infection in the ARDS group contributed to the markers of epithelial injury being elevated in these patients (14), our results are similar to those of a prior study of sepsis patients by Ware et al (18): when compared with patients without ARDS, those with ARDS had higher levels of sRAGE, SP-D, and other markers of epithelial injury and inflammation, but not of angiopoietin-2. Interestingly, angiopoietin-2 level was significantly higher in nonsurvivors compared with survivors in our cohort on all three days of measurement. Prior studies (25, 26) reported the association of higher angiopoietin-2 levels with mortality and pulmonary dysfunction in sepsis, although these studies did not specifically analyze the angiopoietin-2 levels based on the presence of ARDS. Another study by van der Heijden et al (11) reported that higher angiopoietin-2 levels correlated with ARDS and mortality, albeit in a mixed population of both sepsis and nonsepsis patients, whereas Calfee et al (27) has reported that the prognostic performance of angiopoietin-2 for clinical outcomes was weaker in infection-related acute lung injury (ALI) than in noninfection-related ALI. Taken together, these results suggest that endothelial injury is a hallmark of sepsis that is present regardless of ARDS status and contributes to sepsis-related mortality, but concomitant lung epithelial injury may play a more prominent and direct role in the progression from sepsis to sepsis-induced ARDS development. Although these results must be interpreted with caution given the lack of statistical significance and the observational nature of our study, they may serve as pilot data for future studies to better characterize the biological differences between sepsis patients with and without ARDS. Further investigation with larger sample size and more sophisticated analyses of biomarkers (such as multiomics) may help understand the pathophysiologic mechanisms in the development of sepsis-induced ARDS, as well as their implications for therapeutic targets.
In addition, the differences in sRAGE and SP-D levels between ARDS versus non-ARDS patients were the greatest on the first day than on subsequent days, and examining the changes of the biomarker levels over time was not useful for distinguishing the outcomes of interest. This suggests the importance of measuring these biomarkers early in the course of sepsis in order to maximize their diagnostic and prognostic utility. In fact, the timing of the biospecimen sampling may be a limitation in this study, as many patients satisfied the sepsis definition within the first 3 hours of initial presentation to the emergency department or hospital and likely already had sepsis for an unknown period of time prior to admission. Our screening protocol also identified a substantial number of patients (n = 34) who were excluded because they already had ARDS at the time of screening. It is possible that earlier initiation of biospecimen sampling is necessary to detect more significant differences in the absolute biomarker levels. On the contrary, some prior studies have used serial biospecimens collected as late as 28 days after enrollment (7), and a longer follow-up period than was used in this study may be necessary for a more complete understanding of the variability and the trajectories of the biomarkers over time.
There was also a significant overlap of the biomarker levels between ARDS versus non-ARDS patients. There are several possible explanations for these findings. First, many non-ARDS patients in the analysis required IMV and had Pao2/Fio2 ratios less than 300, suggesting that these patients may have had severe lung injury with elevated biomarker levels without meeting the ARDS definition. Furthermore, majority of patients requiring IMV received relatively low tidal volumes regardless of ARDS status. This could have further attenuated the differences in biomarker levels between the outcome groups, as prior studies have reported a greater decline or a smaller rise of biomarker levels over time with low tidal volume ventilation (8, 15, 16). Second, this study may not have sufficiently controlled for the heterogeneity of sepsis itself, especially with the significant proportion of COVID-19 patients included in the study. Last, plasma may not accurately reflect the localized pathology within the lungs in ARDS, and biospecimen sampling from the lungs or the alveolar spaces could be considered for a more direct examination of ARDS pathophysiology.
This study has several additional limitations. First, this was a single-center study conducted at an urban safety net hospital consisting predominantly of African-American patients, and generalizability may be limited. Second, the overall sample size and the number of ARDS patients were both small, and the sample size calculation was extrapolated from prior data examining one-time measurement of sRAGE. Therefore, the statistical power was likely limited for angiopoietin-2 and SP-D measurements, serial measurements of the biomarkers, and multivariable models that resulted in somewhat variable results for angiopoietin-2 and SP-D. The small sample size also limited our ability to perform subgroup analyses or other analytical methods to control for the heterogeneity within the cohort. Third, our analyses did not correct for multiple comparisons or for batch effects in the ELISA analyses. As discussed previously, a longer period of follow-up biomarker measurements may have allowed for a better understanding of the trajectories of biomarkers over time. Last, ARDS frequently developed before the serial sample collections were completed. Therefore, the ability to perform analyses incorporating time-to-event data and to interpret the results in the context of causal, prognostic, or predictive relationships with ARDS development was limited.
CONCLUSIONS
In conclusion, in this prospective observational cohort study of critically ill patients with sepsis, sRAGE, angiopoietin-2, and SP-D levels and their changes over the first 3 days of study enrollment were not different between ARDS versus non-ARDS patients. Higher levels of the three biomarkers were associated with mortality in critically ill patients with sepsis, although this was not the primary aim of the study. The results suggest different involvement of epithelial and endothelial injuries in ARDS development and mortality in sepsis, but further investigation is needed to better understand these pathophysiologic mechanisms as well as the role of protein biomarkers in the clinical management of sepsis-induced ARDS.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Yang, Martin, A.M.E contributed to conceptualization and design. Drs. Yang, Iffrig, and Harris contributed to data acquisition. Dr. Yang contributed to data analysis, with consultation of the Biostatistics, Epidemiology, and Research Design program from the Georgia Clinical and Translational Science Alliance. Dr. Yang contributed to article preparation. Drs. Yang, Iffrig, Harris, Holder, Martin, and Esper contributed to article review and editing. Drs. Holder, Martin, and Esper contributed to project supervision. All authors reviewed the results and approved the final version of the article.
Dr. Yang was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number TL1TR002382/UL1TR002378 (2020–2021) and the National Heart, Lung, and Blood Institute of the NIH under Award Number 5T32HL116271-08 (2021–2022). Dr. Holder is supported by the National Institute of General Medical Sciences of the NIH under Award Number K23GM37182. Dr. Holder reports receiving speaker fees from Baxter International and have received consulting fees from Philips. The remaining authors have no financial disclosures or conflicts of interest to report.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 2.Reilly JP, Calfee CS, Christie JD: Acute respiratory distress syndrome phenotypes. Semin Respir Crit Care Med 2019; 40:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Laorden MI, Lorente JA, Flores C, et al. : Biomarkers for the acute respiratory distress syndrome: How to make the diagnosis more precise. Ann Transl Med 2017; 5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terpstra ML, Aman J, van Nieuw Amerongen GP, et al. : Plasma biomarkers for acute respiratory distress syndrome: A systematic review and meta-analysis*. Crit Care Med 2014; 42:691–700 [DOI] [PubMed] [Google Scholar]
- 5.Jones TK, Feng R, Kerchberger VE, et al. : Plasma sRAGE acts as a genetically regulated causal intermediate in sepsis-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabaudon M, Blondonnet R, Pereira B, et al. : Plasma sRAGE is independently associated with increased mortality in ARDS: A meta-analysis of individual patient data. Intensive Care Med 2018; 44:1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabaudon M, Futier E, Roszyk L, et al. : Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med 2011; 39:480–488 [DOI] [PubMed] [Google Scholar]
- 8.Calfee CS, Ware LB, Eisner MD, et al. ; NHLBI ARDS Network: Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008; 63:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Sato E, Fujiwara N, et al. : Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box 1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin Biochem 2011; 44:601–604 [DOI] [PubMed] [Google Scholar]
- 10.Fremont RD, Koyama T, Calfee CS, et al. : Acute lung injury in patients with traumatic injuries: Utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma 2010; 68:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. : Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 2008; 63:903–909 [DOI] [PubMed] [Google Scholar]
- 12.Agrawal A, Matthay MA, Kangelaris KN, et al. : Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med 2013; 187:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Pabon M, Choi AMK, et al. : Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: Validation in US and Korean cohorts. BMC Pulm Med 2017; 17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calfee CS, Janz DR, Bernard GR, et al. : Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015; 147:1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Determann RM, Royakkers AA, Haitsma JJ, et al. : Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med 2010; 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisner MD, Parsons P, Matthay MA, et al. ; Acute Respiratory Distress Syndrome Network: Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003; 58:983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheu CC, Gong MN, Zhai R, et al. : Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest 2010; 138:559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware LB, Koyama T, Zhao Z, et al. : Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care 2013; 17:R253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney T, Shidham A, Wong H, et al. : A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci Transl Med 2015; 13(Suppl 7):287ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shari G, Kojicic M, Li G, et al. : Timing of the onset of acute respiratory distress syndrome: A population-based study. Respir Care 2011; 56:576–582 [DOI] [PubMed] [Google Scholar]
- 22.The ARDS Definition Task Force: Acute respiratory distress syndrome: The Berlin definition. JAMA 2012; 307(Suppl 23):2526–2533 [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Moreno R, Takala J, et al. : The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 24.Knaus WA, Draper EA, Wagner DP, et al. : APACHE II: A severity of disease classification system. Crit Care Med 1985; 13:818–829 [PubMed] [Google Scholar]
- 25.van der Heijden M, Pickkers P, van Nieuw Amerongen GP, et al. : Circulating angiopoietin-2 levels in the course of septic shock: Relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med 2009; 35:1567–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricciuto DR, dos Santos CC, Hawkes M, et al. : Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med 2011; 39:702–710 [DOI] [PubMed] [Google Scholar]
- 27.Calfee CS, Gallagher D, Abbott J, et al. ; NHLBI ARDS Network: Plasma angiopoietin-2 in clinical acute lung injury: Prognostic and pathogenetic significance. Crit Care Med 2012; 40:1731–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.