Abstract
The antibody response in patients with American cutaneous leishmaniasis was analyzed by immunoblotting with soluble and insoluble antigens of Leishmania braziliensis. The recognition of the 27- and/or 30-kDa soluble antigens was considered relevant for the diagnosis of cutaneous leishmaniasis. Immunoblotting was found to be significantly more sensitive and specific than indirect immunofluorescence and enzyme-linked immunosorbent assay.
Leishmaniasis comprises a spectrum of diseases found all over the world in tropical and subtropical regions. The severity of disease varies from benign self-healing cutaneous lesions to severe mutilating mucocutaneous lesions or visceral infections. In Brazil, American cutaneous leishmaniasis (ACL), caused by Leishmania braziliensis, is widely distributed from south of the Amazon basin to the southeast (World Health Organization, Special Programme for Research and Training in Tropical Diseases [http://www.who.int/tdr/index.html]). This species is the most important causative agent of ACL in northeast Brazil. In a study site in the state of Pernambuco in northeast Brazil, cross-sectional and retrospective epidemiological surveys of infections and/or clinical symptoms confirmed a high current force of infection (0.092/year) and an approximately 10-fold increase in transmission during the last 10 years (2). Thus, more reliable methods of diagnosis are important to direct appropriate control approaches.
The definitive diagnosis of leishmaniasis relies on the clinical manifestations of the disease together with the detection of the intracellular stages of the parasite by examination of smears or biopsies of skin lesions and culturing of specimens. However, often the presumptive diagnosis cannot be confirmed by the identification of the parasite. In this situation, serodiagnosis appears to be a valid diagnostic alternative (11). During the past few years, particular emphasis has been given to characterization of Leishmania antigenic components with a goal of identifying specific antigens for diagnosis. Most of the papers concerning the use of immunoblotting in the diagnosis of leishmaniasis have reported on visceral leishmaniasis (VL) (5, 14, 15) that courses with high levels of anti-leishmania antibodies. In cutaneous leishmaniasis, anti-leishmania antibodies may also be detected in serum, although normally they are present at low levels. Immunoblotting is not widely adopted for diagnosing ACL due to the complexity and inconsistency of the patterns reported (5, 14, 15, 19) and thus needs to be better evaluated and improved. In the present communication, we have identified relevant antigens of L. braziliensis by immunoblotting and have demonstrated the utility of the approach for serodiagnosis of ACL.
A total of 96 serum samples were studied from three groups of subjects: (i) 58 patients with clinical cutaneous leishmaniasis (22 females and 36 males; mean age, 33 years; range, 10 to 83) living in the Amaraji Municipality, Pernambuco State, Brazil, an area in which L. braziliensis is endemic (3); (ii) 8 healthy individuals living in a leishmaniasis-free area (5 females and 3 males; mean age, 29 years; range, 21 to 53); and (iii) 30 patients with other infectious diseases (11 females and 19 males; mean age, 30 years; range, 2 to 83): malaria (n = 5), schistosomiasis (n = 5), syphilis (n = 2), sporotrichosis (n = 2), chromomycosis (n = 1), tuberculosis (n = 5), Chagas' disease (n = 5), and VL (n = 5). Diagnosis of cutaneous leishmaniasis was based upon the collective analysis of a set of elements: presence of typical lesions, compatible epidemiological history, direct parasite detection, and clinical response to specific treatment.
Ten micrograms (each) of soluble and insoluble antigens of L. braziliensis per lane was separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (12) with a Mini Gel apparatus (Sigma, St. Louis, Mo.). To prepare the antigens, L. braziliensis (MHOM/BR/75/M-2903) promastigotes were resuspended in aqueous solution containing 1 mM phenylmethylsulfonyl fluoride (Sigma) and 1 mM EDTA (Sigma) before cell lysis by sonication. After removal of debris, the antigenic mixture was ultracentrifuged at 100,000 × g for 1 h at 4°C. The pellet (insoluble fraction) and supernatant (soluble fraction) protein contents were determined by using a modified Bradford method (20). Polypeptides from the gels were electroblotted onto 0.45-μm nitrocellulose membranes with a semidry blotter at 200 mA for 90 min (Sigma), following instructions of the supplier. For the immunodetection, strips were cut from previously blotted membranes and were blocked for 90 min with 5% skimmed milk in phosphate-buffered saline (PBS). Strips were then washed with PBS–0.05% Tween 20 (PBS-T) (10 min three times each), followed by incubation with sera diluted 1:100 in PBS-T for 12 h at 4°C. After incubation with the primary antibodies, the strips were washed as described and incubated with anti-human immunoglobulin G (Fc-specific) peroxidase conjugate (A-0170; Sigma) diluted 1:5,000 in PBS-T for 1 h at room temperature. After washing, the blot strips were developed by using 0.7 mg of 4-chloronaphthtol per ml as the substrate. Enzyme-linked immunosorbent assay (ELISA) was performed essentially according to Hommel et al. (8). The plates were coated with 0.25 μg of soluble L. braziliensis antigens, and the human sera were used in a 1:100 dilution. The cutoff was based on the results obtained with healthy individuals (mean absorbance plus 1.96 standard deviations). Indirect immunofluorescence (IIF) was performed with promastigotes of L. braziliensis, exactly as described by Camargo (4). The sensitivity and specificity of the diagnostic tests were calculated according to Feinstein (7). These indexes were compared using the McManus test, assigning statistical significance to differences with P values of <0.05 (Statistica for Windows; StatSoft, Tulsa, Okla.).
The sera of 58 patients with ACL reacted with several antigens, resulting in multiple bands. Seven antigens, more frequently recognized and well differentiated, were selected for diagnostic analysis: the 66-, 30-, and 27-kDa soluble antigens and the 60-, 48-, 19-, and 16-kDa insoluble antigens (Fig. 1). Figure 2 shows a representative Western blot of sera from some patients with active cutaneous leishmaniasis. As can be observed in Fig. 3, a consistent diagnostic antigenic pattern was identified in which the soluble 30- and 27-kDa antigens were the most frequently recognized (88 and 91%, respectively) by sera from patients with ACL. The use of soluble and insoluble antigenic fractions was essential for obtaining a simple and reproducible diagnostic profile, since the identification of diagnostic bands using whole-parasite homogenate was difficult (data not shown). In most of the reports where immunoblotting was used for the diagnosis of cutaneous leishmaniasis, whole-cell lysates were used as antigens (6, 10, 17, 18). The antigenic patterns obtained were complex, variable, and difficult to analyze, resulting in poor performance, with specificity in some cases not reaching 65% (19). Mengistu et al. (17) were not able to demonstrate a distinct pattern of reactivity in patients with active cutaneous leishmaniasis or a specific pattern of reactivity with Leishmania aethiopica antigens. The sera of two leprosy patients reacted with the L. aethiopica antigens in the immunoblot assay. There was also variation in the antigenic pattern described by Jaffe et al. (10), although the 5- and 50-kDa antigens were implicated as possibly relevant for the diagnosis of cutaneous leishmaniasis. Our results are also different from the ones reported by Isaza, Restrepo, and Mosca (9), which showed that sera from patients with ACL caused by Leishmania panamensis specifically recognized fractions of 120 (76.7%), 123 and 129 (69.7%), 138 (61%), 141 (53%), and 78 (44%) kDa. Possibly, the above reported differences for the antigenic recognition pattern may be ascribed to the species of leishmaniasis involved, differences in the antigenic preparation, and methodological procedures.
FIG. 1.
Immunoblot patterns of clinical cutaneous leishmaniasis. The most frequently recognized bands are shown. S, soluble antigenic fraction; I, insoluble antigenic fraction. The numbers indicate the molecular weights in kilodaltons.
FIG. 2.
Immunoblot showing the recognition patterns of patients with active cutaneous leishmaniasis. Lanes: 1 and 2, patient GIA-1; 3 and 4, patient GIA-4; 5 and 6, patient GIA-13; 7 and 8, patient GIA-14; 9 and 10, patient GIA-15. Lanes 11 and 12 correspond to positive serum samples, and lanes 13 and 14 represent normal serum samples. In lanes 1, 3, 5, 7, 9, 11, and 13, soluble antigens were used, while in lanes 2, 4, 6, 8, 10, and 12, insoluble antigens were used. M, molecular weight markers (in kilodaltons).
FIG. 3.
Recognition frequency of the diagnostic antigens by sera of patients with ACL, Chagas' disease, and VL.
Figure 3 showed that antibodies from patients with Chagas' disease and VL cross-react with antigens of L. braziliensis. In Fig. 3, for simplicity, the combined antigen recognition frequency of patients with Chagas' disease and VL was shown. Nonetheless, none of the VL patients recognized the 66- and 19-kDa antigens. On the other hand, additional antigens were exclusively recognized by patients with Chagas' disease (36- and 27-kDa insoluble antigens) and VL (20-kDa insoluble antigen and 70-, 36-, and 19-kDa soluble antigens) (data not shown).
Based on the immunoblotting band patterns of the several groups of individuals studied, we proposed the following criteria for the serodiagnosis of Leishmania infection: (i) the presence of the 27-kDa peptide alone or in combination with other bands of the diagnostic pattern; (ii) the presence of the 30-kDa peptide alone or in combination with other bands of the diagnostic pattern; and (iii) the presence of the 66-, 48-, 19-, and 16-kDa peptides, which supports but is insufficient for diagnosis.
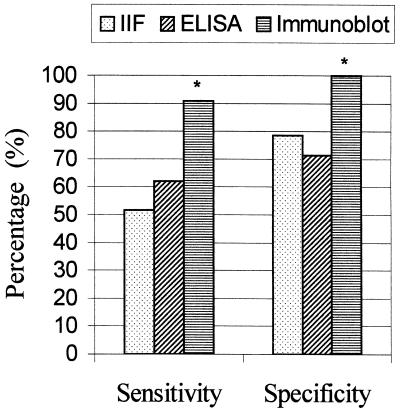
The sensitivity and specificity of the immunoblot blot test was compared with IIF and ELISA. For the calculation of the sensitivity, sera from 58 patients with clinical cutaneous leishmaniasis were used, while for the determination of specificity, sera from 38 individuals without leishmaniasis (healthy individuals and patients with other infectious diseases) were analyzed. Sera from patients with VL and Chagas' disease were excluded from calculation of the specificity of the assays, as these sera cross-react with L. braziliensis antigens (Fig. 3). The sensitivity of the immunoblotting test was 91% and the specificity was 100%. The sensitivity and specificity showed by the IIF assay were 52 and 79%, respectively. The ELISA presented a sensitivity of 62% and a specificity of 71%. Immunoblotting was compared to IIF and ELISA (Fig. 4) and found to be significantly more sensitive and specific (P < 0.01), in agreement with other investigations (9, 15).
FIG. 4.
Sensitivity and specificity of the ELISA and IIF and immunoblot assays for the diagnosis of active cutaneous leishmaniasis. An asterisk (∗) indicates that the result for a group is significantly different from the results for the other groups (P < 0.01).
One of the problems of immunodiagnosis of Leishmania infection has been the presence of cross-reacting antigens between different species within a family (3, 5, 9, 11, 13, 16) or between phylogenetically distant organisms such as Mycobacterium (21, 22). This issue is particularly relevant in regions in which leishmaniasis coexists with these infections. In the present study, the IIF test and ELISA were positive for all sera from VL and Chagas' disease patients, whereas cross-reaction with the 27- and 30-kDa antigens on immunoblotting was only observed in 5 of 10 patients with the above-mentioned diseases. Although clear distinction between ACL, VL, and Chagas' disease based upon immunoblotting was not possible, the identification of a few antigens uniquely recognized by patients with VL and Chagas' disease may be useful for the differential diagnosis in conjunction with additional diagnostic elements. Regarding malaria, schistosomiasis, syphilis, sporotrichosis, chromomycosis, and tuberculosis, no cross-reaction was observed by immunoblotting; nevertheless, this occurred by ELISA and IIF.
In conclusion, immunoblot analysis with soluble and insoluble antigenic fractions of L. braziliensis allowed the identification of potentially diagnostic antigens, recognized by a high proportion of ACL patients. The immunoblot assay was clearly more sensitive and specific than the ELISA and IIF test, indicating that it could be a useful diagnostic approach. We are currently cloning the genes encoding the identified proteins in order to use the recombinant antigens in ELISA.
Acknowledgments
We are grateful to Jeffrey Shaw and Siddhartha Mahanty for valuable comments and suggestions.
REFERENCES
- 1.Arias J, Beltrãn F, Desjeux P, Walton B. Epidemiologia y control de la leishmaniasis en las Américas, por país o territorio. 1996. pp. 1–52. . Organización Panamericana de la Salud, Washington, D.C. [Google Scholar]
- 2.Brandão-Filho S P, Camppbell-Lundrum D, Brito M E F, Shaw J J, Davies C R. Epidemiological surveys confirm increasing burden of cutaneous leishmaniasis in North-East Brazil. Trans R Soc Trop Med Hyg. 1999;93:488–494. doi: 10.1016/s0035-9203(99)90346-2. [DOI] [PubMed] [Google Scholar]
- 3.Brandão-Filho S P, Carvalho F G, Brito M E F, Almeida F A, Nascimento L A. American cutaneous leishmaniasis in Pernambuco, Brazil: eco-epidemiological aspects in “Zona da Mata” region. Mem Inst Oswaldo Cruz. 1994;89:445–449. doi: 10.1590/s0074-02761994000300028. [DOI] [PubMed] [Google Scholar]
- 4.Camargo M E. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev Inst Med Trop São Paulo. 1966;8:227–235. [PubMed] [Google Scholar]
- 5.Cardeñosa N, Riera C, Cortés P, March F, Muñoz C, Portús M, Prats G. Detection and characterization by immunoblot analysis of potentially diagnostic Leishmania infantum polypeptides in human visceral leishmaniasis. Parasite Immunol. 1995;17:509–516. doi: 10.1111/j.1365-3024.1995.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 6.Delgado D, Guevara P, Silva S, Belfort E, Ramirez J L. Follow-up of a human accidental infection by Leishmania (Viannia) braziliensis using conventional immunologic techniques and polymerase chain reaction. Am J Trop Med Hyg. 1996;55:267–272. doi: 10.4269/ajtmh.1996.55.267. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein A R. Clinical biostatistics. Saint Louis, Mo: The C. V. Mosby Co.; 1977. pp. 1–468. [Google Scholar]
- 8.Hommel M, Peters W, Ranque J, Quilici M, Lanotte G. The micro-ELISA technique in the serodiagnosis of visceral leishmaniasis. Ann Trop Med Parasitol. 1978;72:213–218. doi: 10.1080/00034983.1978.11719308. [DOI] [PubMed] [Google Scholar]
- 9.Isaza D M, Restrepo M, Mosca W. Immunoblot analysis of Leishmania panamensis antigens in sera of patients with American cutaneous leishmaniasis. J Clin Microbiol. 1997;35:3043–3047. doi: 10.1128/jcm.35.12.3043-3047.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffe C L, Shor R, Trau H, Passawell J H. Parasite antigens recognized by patients with cutaneous leishmaniasis. Clin Exp Immunol. 1990;80:77–82. doi: 10.1111/j.1365-2249.1990.tb06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kar K. Serodiagnosis of leishmaniasis. Crit Rev Microbiol. 1995;21:123–152. doi: 10.3109/10408419509113537. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli V K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Malchiodi E L, Chiaramonte M G, Taranto N J, Zwirnwe N W, Margini R A. Cross-reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp., use of immunoblotting and ELISA with a purified antigen (Ag 163 B6) Clin Exp Immunol. 1994;97:417–423. doi: 10.1111/j.1365-2249.1994.tb06104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marty P, Lelièvre A, Quaranta J F, Suffia I, Eulalio M, Gari-Toussaint M, Le Fichoux Y, Kubar J. Detection by Western blot of four antigens characterizing acute clinical leishmaniasis due to Leishmania infantum. Trans R Soc Trop Med Hyg. 1995;89:690–691. doi: 10.1016/0035-9203(95)90447-6. [DOI] [PubMed] [Google Scholar]
- 15.Mary C, Lamouroux D, Dunan S, Quilici M. Western blot analysis of antibodies to Leishmania infantum antigens: potential of the 14-kD and 16-kD antigens for diagnosis and epidemiologic purposes. Am J Trop Med Hyg. 1992;47:764–771. doi: 10.4269/ajtmh.1992.47.764. [DOI] [PubMed] [Google Scholar]
- 16.Marzochi M C A, Coutinho S G, Sabroza P C, Sousa W J. Reação de imunofluorescência indireta e intradermoreação para leishmaniose tegumentar americana em moradores na área de Jacarepaguá (Rio de Janeiro). Estudo Comparativo dos resultados observados em 1974 e 1978. Rev Inst Med Trop São Paulo. 1980;22:149–155. [PubMed] [Google Scholar]
- 17.Mengistu G, Akuffo H, Fehniger T E, Negese Y, Nilsen R. Comparison of parasitological and immunological methods in the diagnosis of leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. 1992;86:154–157. doi: 10.1016/0035-9203(92)90548-q. [DOI] [PubMed] [Google Scholar]
- 18.Monroy-Ostria A, Sosa-Cabrera T, Rivas-Sanchez B, Ruiz-Tuyu R, Mendoza-Gonzalez A R, Favila-Castillo L. Seroepidemiological studies of cutaneous leishmaniasis in the Campeche state of Mexico. Mem Inst Oswaldo Cruz. 1997;92:21–26. doi: 10.1590/s0074-02761997000100004. [DOI] [PubMed] [Google Scholar]
- 19.Montoya Y, Leon C, Talledo M, Nolasco O, Padilla C, Munoz-Najar U, Barker D C. Recombinant antigens for specific and sensitive serodiagnosis of Latin American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg. 1997;91:674–676. doi: 10.1016/s0035-9203(97)90520-4. [DOI] [PubMed] [Google Scholar]
- 20.Read S M, Northcote D H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981;116:53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- 21.Reed S G, Badaro R, Lloyd R M. Identification of specific and cross-reactive antigens of Leishmania donovani chagasi by human infection sera. J Immunol. 1987;138:1596–1601. [PubMed] [Google Scholar]
- 22.Smrkovski L L, Larson C L. Antigenic cross-reactivity between Mycobacterium bovis (BCG) and Leishmania donovani. Infect Immun. 1977;18:561–562. doi: 10.1128/iai.18.2.561-562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]