Structured Abstract
Objective
Primary treatment of hydrocephalus with endoscopic third ventriculostomy and choroid plexus cauterization (ETV+CPC) is well described in the neurosurgical literature, reporting a wide range of success and complication rates. The purpose of this study is to describe the safety and efficacy of ETV revision after initial ETV+CPC failure.
Methods
Prospectively collected data in the Hydrocephalus Clinical Research Network (HCRN) Core Data Project (Registry) were reviewed. Children who underwent ETV+CPC as initial treatment for hydrocephalus between 2013 and 2019, and in whom the initial ETV+CPC was completed (not abandoned) were included. Log-rank survival analysis was used to compare time to failure (any other surgical treatment for hydrocephalus or death related to hydrocephalus) of the initial ETV+CPC procedure versus the ETV revision (the primary analysis), using random effect modeling to account for subjects being included in both the initial and revision groups. Secondary analyses compared ETV revision to shunt placement after failure of initial ETV+CPC using log-rank test, and shunt failure after ETV+CPC versus after ETV revision. Cox regression was used to identify predictors of failure among children treated with ETV revision.
Results
We identified 521 ETV+CPC procedures meeting our inclusion criteria. Ninety-one children underwent ETV revision after ETV+CPC failure. ETV revision had lower one-year success compared to initial ETV+CPC (29.5% versus 45%, p<0.001). ETV revision after initial ETV+CPC failure had a lower success rate (29.5% versus 77.8%, p<0.001) compared to shunting. Shunt survival after the initial ETV+CPC failure was not significantly different than shunt survival after ETV revision failure (p=0.963). Complication rates were similar for all surgeries examined (initial ETV+CPC, ETV revision, VPS after ETV+CPC, and VPS after ETV revision). Only young age was found to be predictive of ETV revision failure (p=0.02).
Conclusions
ETV revision has a significantly lower one-year success rate compared to initial ETV+CPC and compared to VPS after ETV+CPC. Complication rates are similar for all studied procedures. Younger age, but not time since initial ETV+CPC, is a risk factor for ETV revision failure.
Keywords: Hydrocephalus, Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization, ETV Revision, Hydrocephalus Clinical Research Network
Introduction
Endoscopic Third Ventriculostomy with Choroid Plexus Cauterization (ETV+CPC) has shown promising results as a primary treatment for hydrocephalus in infants in East Africa.1,2 Subsequent investigation of ETV+CPC in North America by the Hydrocephalus Clinical Research Network (HCRN) and other investigators reported varying success rates that were somewhat lower than earlier reports from Africa.3 In 2018, the HCRN reported an initial ETV+CPC success rate of 36%.4 More recently, the HCRN reported a success rate of 45% at 18 months, perhaps attributable to more rigorous patient selection, including evaluation of prepontine adhesions on MRI.5 Some children who fail initial ETV or ETV+CPC may be amenable to treatment with ETV revision, that is reopening of a closed ETV site. There are a few published reports of ETV revision 6–13. However, these are almost exclusively in patients who underwent ETV alone, not ETV+CPC. Therefore, most of the patients included in these series are older children or young adults, and as such, have a higher likelihood of ETV success.14 Only one previous study has focused on ETV revision after ETV+CPC.6 This study assessed both ETV and ETV+CPC patients together, thus complicating any conclusions about ETV after ETV+CPC. Furthermore, it was performed in an East African setting where hydrocephalus etiology is most commonly post-infectious, thus limiting its applicability to the North American population. Investigation of outcomes in this “ETV revision after failed ETV+CPC” patient population would help surgeons provide more accurate pre-operative counseling about procedural risks and the potential for continuing hydrocephalus treatment without implanted hardware.
The purpose of this analysis was to compare ETV revision to initial ETV+CPC in both failure and complication rates. We hypothesized that ETV revision would have a higher failure rate (lower success) than initial ETV+CPC. In addition, we compared failure rates and complications of ETV revision to ventriculoperitoneal shunt (VPS) placement in children who had previously undergone ETV+CPC. Finally, we aimed to identify variables associated with successful ETV revision following initial ETV+CPC.
Methods
Study Design
The study population includes children with new-onset hydrocephalus who underwent ETV+CPC surgery between May 2013 and December 2019 (ETV+CPC was first offered at HCRN sites in 2013). Children underwent an ETV+CPC procedure at one of 13 HCRN centers (Children’s Hospital of Alabama, Birmingham, AL; Primary Children’s Hospital, Salt Lake City, UT; Seattle Children’s Hospital, WA; Children’s Hospital of Pittsburgh, PA; St. Louis Children’s Hospital, MO; Texas Children’s Hospital, Houston, TX; Sick Kids Hospital, Toronto, Canada; Monroe Carrell Jr. Children’s Hospital, Nashville, TN; British Columbia Children’s Hospital, Vancouver, BC; Alberta Children’s Hospital, Calgary, AB; Children’s Hospital of Los Angeles, Los Angeles, CA; Children’s Hospital Colorado, Aurora, CO; Nationwide Children’s Hospital, Columbus, OH). Data were collected prospectively into an observational HCRN registry, which tracks all hydrocephalus surgeries at each HCRN center from date of joining HCRN to present. Most centers did not require informed consent for Registry inclusion, which allowed for comprehensive enrollment and construction of a representative sample. IRB approval was obtained from each clinical site as well as data coordinating center 15.
The HCRN Registry database was reviewed for all patients who had undergone ETV+CPC in the period specified. Children were included if their first treatment for hydrocephalus was ETV+CPC. Children for whom ETV was planned, but was not completed, were excluded (e.g. if ETV was found to be impossible during the initial attempt, and a shunt was placed instead of completing ETV+CPC, the child was excluded).
Three separate groups were then identified for analysis: 1) children in whom initial ETV+CPC was successful, and no further treatment for hydrocephalus was required; 2) children whose ETV+CPC failed and went on to have ETV revision as their next hydrocephalus-related surgery; and 3) children whose ETV+CPC failed and then had a VPS placed as their next hydrocephalus surgery.
Outcomes
The primary analysis is a comparison of the time to failure between initial ETV+CPC and ETV revision following a failed ETV+CPC. ETV+CPC and ETV revision failure was defined as a composite outcome of need for a subsequent shunt placement, another ETV revision, or death due to hydrocephalus. Additional survival analyses were performed to compare ETV revision versus VPS placement as treatment after failure of initial ETV+CPC. Finally, we identified post-procedural (during the hospital stay) and short-term (within 6 months of surgery) complications after ETV revision. Complications were categorized as any new neurological deficit, CSF leak (defined as any episode of CSF leak, regardless of treatment), wound infection, diabetes insipidus and other complications (venous thrombosis, hyponatremia, urinary tract infection, sepsis, pneumonia, cardiac arrest, pressure ulcer, intracranial fluid collection, meningitis, seizure, pseudomeningocele, hemorrhage).
Statistical Methods
Patient characteristics, and post-procedural and short-term complications are presented as counts and percentages for categorical variables, and median, first, and third quartiles for continuous variables. Associations between patient characteristics for initial ETV+CPC and ETV revision were assessed using Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
Kaplan-Meier curves of time to failure were created for both the primary and secondary analyses. First, initial ETV+CPC was compared to ETV revision. Second, among children who had ETV+CPC failure, those who had ETV revision were compared to those treated with VPS. Finally, survival of VPS placed after ETV revision was compared to survival of VPS after ETV+CPC (with no intervening ETV revision). Log-rank test of significance was used to compare survival/failure curves. Subjects were censored at the time of relocation out of HCRN-participating sites or death not related to hydrocephalus.
A Cox regression model with a random effect for subject was created to determine if there was a significant difference in time to failure between initial ETV+CPC procedures and ETV revisions following failed initial ETV+CPC. The random effect for subject component of the model was included to account for the fact that a subject can be in both the initial ETV+CPC and ETV revision group. Procedure type was the only covariate besides the random effect. Poisson regression with robust error estimates was used to compare rates for each individual complication between initial ETV+CPC procedures and ETV revisions. A compound symmetry covariance structure was used to account for the correlation between outcomes from subjects that were in both cohorts.
To analyze factors associated with failure of ETV revision, a Cox regression model was created. Candidate predictors included corrected age at revision procedure, sex, etiology of hydrocephalus (post-IVH secondary to prematurity, myelomeningocele, aqueductal stenosis, and other etiology), frontal occipital horn ratio, presence of bleeding during the procedure, method of dilation of the ETV site, and time after initial ETV+CPC. All variables associated with time to failure of ETV revision (p-value < 0.10) in a univariable analysis with no more than 10% missing values were included as candidate predictors in a multivariable Cox regression model. A bi-directional stepwise selection procedure with a criterion of p < 0.15 was used to determine which candidate predictors were included in the final model. The proportional hazard assumption was determined plausible on all final candidate predictors. Corrected age and etiology were forced into the multivariable model because they are well established as predictors of ETV+CPC failure.4,5,16
P-values were reported based on a 2-sided alternative and are considered significant when p<0.05. All analyses were conducted using SAS 9.4 (SAS Institute; Cary, NC).
Results
The final cohort of children undergoing initial ETV+CPC procedures included 521 patients (Figure 1). Of these, 234 were successful, requiring no further hydrocephalus treatment at time of most recent follow up in the HCRN registry. Of the 287 children whose initial ETV+CPC failed, 91 had ETV revision as their next treatment, and 196 had VPS placement. Median follow up from initial ETV+CPC procedure was 4.5 years (IQR 2.7–6.2). Most ETV revisions (79 of 91, 87%) were performed by the surgeon who performed the original ETV+CPC.
Figure 1:
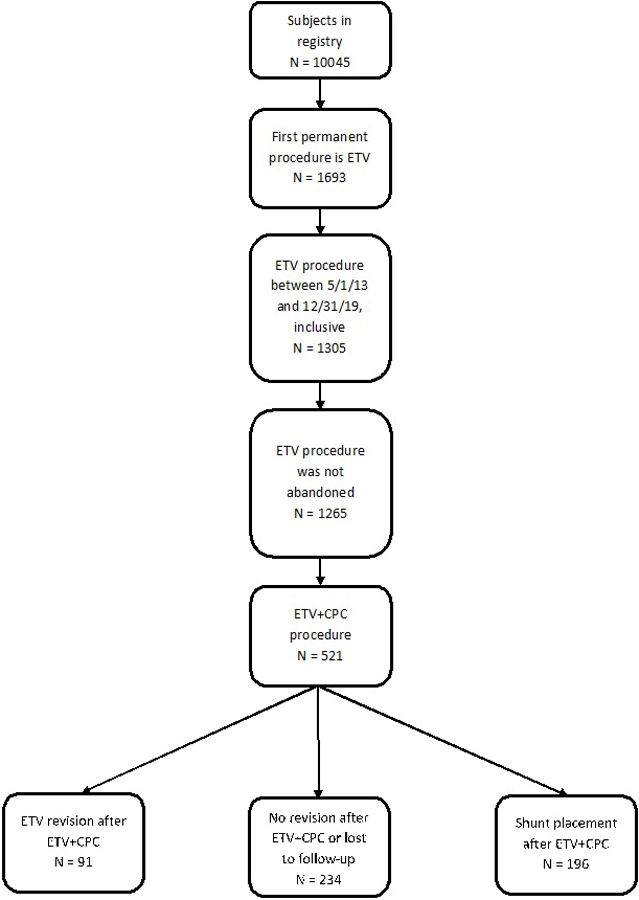
Participant flow diagram
Comparison of Initial ETV+CPC to ETV Revision
The median age at ETV revision was 3.8 months (IQR 2.1–7.7); 53.8% were boys; 78.6% were white, non-Hispanic. The most common etiology of hydrocephalus was myelomeningocele (34.1%), followed by post-hemorrhagic hydrocephalus associated with prematurity (22.0%) and aqueductal stenosis (20.9%). (Table 1). There was not a significant difference between the ETV revision group and the initial ETV+CPC group for any of these variables except age (median age 2.1 versus 3.8 months, p<0.001). Frontal-occipital ratio (FOHR) at repeat ETV was larger than at initial ETV+CPC (0.63 vs. 0.59, p<0.001). Bilateral complete CPC was performed in 76.8% of initial ETV+CPC. There was no difference in the number or severity of bleeding events during the operations. Balloon catheter dilation was more likely to be used in ETV revision (30.6% vs. 17.9%, p=0.035). There was no difference in the rate of complications between ETV+CPC and ETV revision (Table 2).
TABLE 1.
Patient characteristics
| Initial ETV+CPC (n = 521) |
ETV revision following initial ETV+CPC (n = 91) |
Shunt placement following initial ETV+CPC (n = 196) |
P-value for comparison of initial ETV+CPC vs ETV revision | |
|---|---|---|---|---|
| Corrected age at procedure (months) | 2.1 [0.4, 5.8] | 3.8 [2.1, 7.7] | 3.5 [1.3, 6.4] | <.0011 |
| Male | 304 (58.3%) | 49 (53.8%) | 128 (65.3%) | 0.4242 |
| Race 3 | 0.5532 | |||
| White | 369 (77.5%) | 66 (78.6%) | 131 (73.6%) | |
| Black or African American | 89 (18.7%) | 17 (20.2%) | 42 (23.6%) | |
| Other | 18 (3.8%) | 1 (1.2%) | 5 (2.8%) | |
| Etiology of hydrocephalus | 0.4772 | |||
| Post-IVH secondary to prematurity | 98 (18.8%) | 20 (22.0%) | 56 (28.6%) | |
| Myelomeningocele | 158 (30.3%) | 31 (34.1%) | 36 (18.4%) | |
| Aqueductal stenosis | 104 (20.0%) | 19 (20.9%) | 38 (19.4%) | |
| Other etiology | 161 (30.9%) | 21 (23.1%) | 66 (33.7%) | |
| Post-Infectious | 8 | 1 | 4 | |
| Spontaneous ICH/IVH/SAH | 28 | 5 | 14 | |
| Posterior fossa tumor | 4 | 0 | 1 | |
| Supratentorial tumor | 3 | 0 | 1 | |
| Midbrain tumor/lesion | 2 | 0 | 0 | |
| Post-head injury | 4 | 1 | 1 | |
| Encephalocele | 9 | 0 | 4 | |
| Posterior fossa cyst | 33 | 5 | 13 | |
| Other intracranial cyst | 6 | 0 | 3 | |
| Communicating congenital hydrocephalus | 34 | 3 | 15 | |
| Other congenital | 11 | 3 | 4 | |
| Craniosynostosis | 6 | 0 | 3 | |
| Other | 13 | 3 | 3 | |
| Frontal occipital horn ratio 4 | 0.59 (0.53, 0.67) | 0.63 (0.58, 0.69) | 0.66 [0.57, 0.72] | <.0011 |
| Bleeding during procedure | 0.1112 | |||
| None | 288 (55.3%) | 61 (67.0%) | ||
| Mild | 206 (39.5%) | 25 (27.5%) | ||
| Moderate | 16 (3.1%) | 4 (4.4%) | ||
| Severe | 11 (2.1%) | 1 (1.1%) | ||
| Dilation method 5 | 0.0352 | |||
| Forceps | 7 (1.3%) | 2 (2.4%) | ||
| Fogarty | 64 (12.3%) | 4 (4.7%) | ||
| Neuroballoon | 93 (17.9%) | 26 (30.6%) | ||
| Spreader | 1 (0.2%) | 0 (0.0%) | ||
| Endoscope | 301 (57.9%) | 44 (51.8%) | ||
| Multiple | 54 (10.4%) | 9 (10.6%) |
Wilcoxon rank-sum test with unpooled variance estimated comparing initial ETV+CPC and ETV revision following initial ETV+CPC.
Fisher’s exact test comparing inital ETV+CPC and ETV revision following initial ETV+CPC.
Missing on 45 patients.
Missing on 3 patient at initial ETV+CPC and 9 patients at shunt placement following initial ETV+CPC.
Missing on 6 patients at ETV revision following initial ETV+CPC.
TABLE 2.
Post-procedural and short-term complications associated with initial ETV+CPC and ETV revision following initial ETV+CPC
| Post-procedural (during hospital stay) | Short-term (within 6 months of surgery) | |||
|---|---|---|---|---|
| Initial ETV+CPC (n = 521) |
ETV Revision following initial ETV+CPC (n = 91) |
Initial ETV+CPC (n = 301) |
ETV Revision following initial ETV+CPC (n = 38) |
|
| New neurologic deficit | 5 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CSF leak | 8 (1.5%) | 2 (2.2%) | 3 (1.0%) | 0 (0%) |
| Wound infection | 1 (0.2%) | 0 (0%) | 1 (0.3%) | 0 (0%) |
| Diabetes insipidus | 2 (0.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
The overall success rate one year after ETV+CPC was 45% (228 of 508). Among the 91 children who underwent ETV revision, 29.5% were successful one year after surgery (26 of 88; 3 did not have 1-year follow up). Kaplan-Meier survival curve with Cox regression model showed a significant difference in survival between these two surgeries (p<0.001) (Figure 2).
Figure 2:
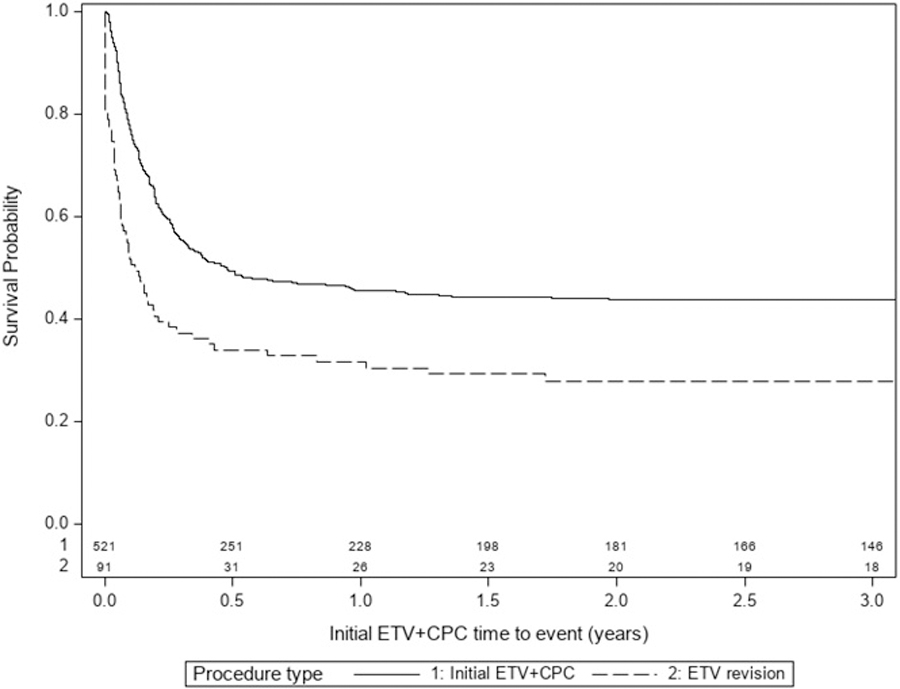
Survival curve for initial ETV+CPC vs ETV revision
Cox regression model of factors associated with time to failure of ETV revision showed only one significant association: older age at the time of the procedure was associated with lower risk of failure (HR 0.94, 95% CI 0.89–0.99, p=0.02 in multivariable model). Compared to IVH-related hydrocephalus, all other etiologies had lower hazard ratio for failure, but these did not reach statistical significance. In addition, the time from the initial ETV+CPC to ETV revision (survival time of ETV+CPC) showed no correlation with ETV revision failure (HR 0.64, 95% CI 0.33–1.25, p=0.19). Results from the Cox model are shown in Table 3.
TABLE 3.
Univariable and multivariable associations with time to failure in ETV revisions following initial ETV+CPC
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Corrected age at procedure (months) | 0.94 (0.89,0.99) | 0.019 | 0.94 (0.89, 0.99) | 0.02 |
| Sex | 0.257 | |||
| Female | Reference | |||
| Male | 1.32 (0.82, 2.15) | |||
| Race | ||||
| White | Reference | |||
| Black or African American | 1.34 (0.75, 2.40) | |||
| Other | 3.04 (0.41, 22.51) | |||
| Etiology of hydrocephalus | 0.232 | 0.3 | ||
| Post-IVH secondary to prematurity | Reference | |||
| Myelomeningocele | 0.60 (0.32, 1.13) | 0.54 (0.28, 1.04) | ||
| Aqueductal stenosis | 0.91 (0.45, 1.83) | 0.77 (0.38, 1.56) | ||
| Other etiology | 0.55 (0.27, 1.13) | 0.64 (0.31, 1.33) | ||
| Frontal occipital horn ratio (HR is for an increase of 0.1) | 1.10 (0.81, 1.50) | 0.522 | ||
| Bleeding during procedure | 0.154 | |||
| None | Reference | |||
| Mild | 1.35 (0.80, 2.29) | |||
| Moderate | 2.51 (0.88, 7.16) | |||
| Severe | 4.49 (0.60, 33.75) | |||
| Dilation method | ||||
| Forceps | 0.57 (0.08, 4.19) | |||
| Fogarty | 1.05 (0.32, 3.44) | |||
| Neuroballoon | 0.75 (0.42, 1.34) | |||
| Endoscope | Reference | |||
| Multiple | 0.72 (0.32, 1.63) | |||
| Initial ETV+CPC time to event (years) | 0.64 (0.33, 1.25) | 0.188 | ||
Corrected age at revision procedure (clinical suspicion) and etiology of hydrocephalus (clinical suspicion) were entered as candidate predictors into the multivariable model.
HR=Hazard ratio
Comparison of ETV Revision to VPS after ETV+CPC failure
One hundred ninety-six patients underwent shunt placement at the time of failure of their ETV+CPC; 77.8% of children with VPS had no further hydrocephalus surgery during the first year after shunt insertion, compared to 29.5% of the ETV revision patients. Figure 3 shows the Kaplan-Meier survival curves comparing ETV revision to VP shunt placement after initial ETV+CPC failure. VP shunt placement had a higher success rate than an ETV revision based on the log-rank test (p<0.001). There was no difference in the rate of complications between ETV revision and VPS placement in the setting of hydrocephalus treatment after ETV+CPC failure (Table 4).
Figure 3:
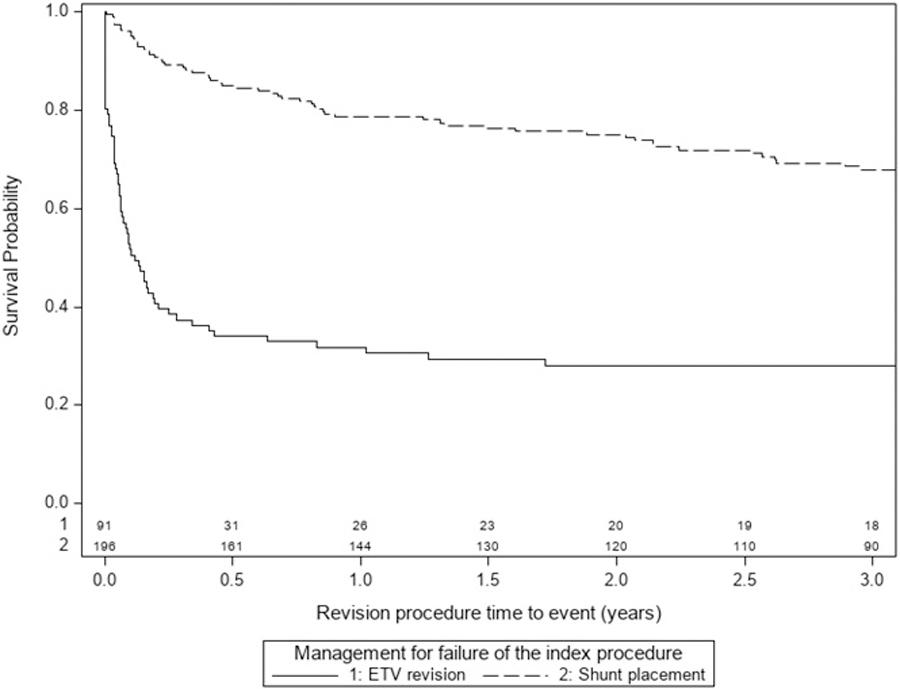
Survival curve for ETV revision vs VPS placement for the treatment of failed initial ETV+CPC
TABLE 4.
Post-procedural and short-term complications associated with shunt placement and ETV revision following initial ETV+CPC
| Post-procedural (during hospital stay) | Short-term (within 6 months of surgery) | |||
|---|---|---|---|---|
| Shunt placement following initial ETV+CPC (n = 196) |
ETV Revision following initial ETV+CPC (n = 91) |
Shunt placement following initial ETV+CPC (n = 136) |
ETV Revision following initial ETV+CPC (n = 38) |
|
| New neurologic deficit | 1 (0.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CSF leak | 4 (2.0%) | 2 (2.2%) | 0 (0%) | 0 (0%) |
| Wound infection | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Diabetes insipidus | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Comparison of VPS placed after ETV Revision to VPS after ETV+CPC with no ETV Revision
Fifty-nine children had VPS placement after ETV revision. Compared to 196 who had VPS placement after initial ETV+CPC failure, this cohort showed no difference in survival of the VPS (p=0.963) (Figure 4).
Figure 4:
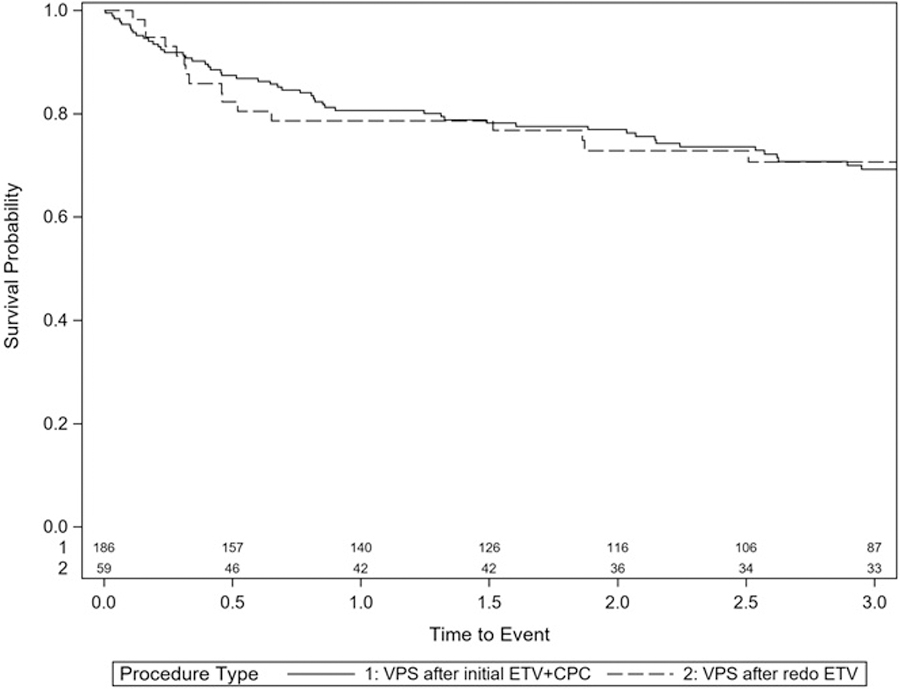
Survival curve for VPS after failed ETV revision compared to VPS after failed initial ETV+CPC
Discussion
The purpose of this study is to examine the success of ETV revision after initial ETV+CPC failure in young children. ETV revision is successful in 29.5% of patients, a significantly lower success rate than initial ETV+CPC. However, given that all children who underwent ETV revision had already failed ETV+CPC, it might be expected that the likelihood of success would be low. No patient had a new neurologic deficit, wound infection, or new diabetes insipidus after ETV revision. The frequency of complications is similar to initial ETV+CPC. These data help inform the decision to offer ETV revision in the setting of failed ETV+CPC, considering the balance of the risk of complication with the likelihood of success.
When comparing children undergoing initial ETV+CPC to those with ETV revision, we see a significant difference in age, as expected: children undergoing ETV revision are older than those who undergo initial ETV+CPC. We also observe a difference in ventricle size, as measured by FOHR. At the time of ETV revision, children had larger median FOHR than at initial ETV. This finding may also be expected since ventricles could continue to enlarge after unsuccessful ETV+CPC. Indeed, patients may be selected for ETV revision in part because their ventricles continued to enlarge.
Compared to the only previous dedicated study of ETV revision after ETV+CPC, we report a lower success rate (29.5% versus approximately 60%).6 However, in the prior study, 56% of 215 included children had post-infectious hydrocephalus (PIH), and children with PIH were noted to have higher likelihood of successful ETV revision. Marano et al. also showed that longer time to failure of the initial ETV correlated with higher likelihood of ETV revision success, a relationship that was not significant in the present analysis. The only variable to show significant association with ETV revision success in Cox PH modelling was age at the time of procedure. The hazard ratio (HR) of 0.94 (95% CI 0.89–0.99) indicates a lower hazard for failure with each month of increasing age. It is well established that younger age is the most important risk factor for ETV failure.
The question faced by clinicians is what treatment to offer a child whose initial ETV+CPC has failed. The most pertinent data to inform this decision is the comparison between ETV revision and VPS insertion. Among children with initial ETV+CPC failure, we compared those who had shunt placement as the next treatment to those who underwent redo ETV. There is a significantly higher success rate of shunting at all time points, with only 20% of shunts placed in this circumstance failing within three years. This rate of shunt failure is lower than what has been published in most large series (median survival of initial VP shunt 2–3 years)17–19. Previous series examining shunt survival have not shown any difference between shunts placed as primary treatment compared to those placed after failed ETV+CPC 20. We also showed complication rates for ETV revision similar to VPS placement in this scenario.
With a success rate for ETV revision at 1 year of only 29.5%, most children who underwent ETV revision subsequently had VPS placement. Our comparison of VPS after ETV+CPC to VPS after ETV revision showed similar failure rates. In summary, when faced with a patient who has failed ETV+CPC, ETV revision carries a 29.5% chance of success within the first year, a similar complication profile to other hydrocephalus surgeries, and no change to the performance of a subsequent VPS.
The ETV Success Score (ETVSS) is a commonly used instrument to estimate the likelihood of ETV success. However, it has not previously been applied to ETV revision. ETVSS is determined by three factors, age, hydrocephalus etiology, and history of shunt. When considering an ETV revision compared to an initial ETV, the only one of these factors that may have changed is age. Older age would lead to higher ETVSS, indicating higher likelihood of ETV success. However, as shown here, we observe significantly lower success among ETV revision when compared to initial ETV. In conclusion, use of ETVSS for estimation of success of ETV revision is not likely to be helpful.
Limitations
These analyses are based on registry data, and therefore limited to the information collected in the registry. However, the HCRN registry is a prospective cohort with processes in place such as centralized training, surgeon first-hand involvement in data collection, data validation through quality control and queries to ensure accuracy and validity of data. Selection bias may be present in this sample, as surgeons have different thresholds for offering ETV+CPC or ETV revision procedure. This sample only represents Northern America and results may not generalize to other regions of the world.
Conclusions
ETV revision has a low one-year success rate (29.5%) and complication rates comparable to those of initial ETV+CPC and VPS. Older age was the only risk factor that showed significant association with ETV revision success: older age correlating with higher likelihood of success. Importantly, time between initial ETV+CPC and ETV revision showed no relationship to successful ETV revision. Finally, we observed no difference in VPS survival comparing shunts placed after ETV+CPC to those placed after ETV revision. These data can inform decision making and counseling of parents at the time of ETV+CPC failure.
Acknowledgments
The authors would like to thank their colleagues for their past and ongoing support of HCRN: D Brockmeyer, M Walker, R Bollo, S Cheshier, J Blount, J Johnston, L Acakpo-Satchivi, WJ Oakes, P Dirks, J Rutka, M Taylor, P Dirks, D Curry, G Aldave, R Dauser, A Jea, S Lam, H Weiner, T Luerssen, R Ellenbogen, J Ojemann, A Lee, A Avellino, S Greene, E Tyler-Kabara, T Abel, TS Park, J Strahle, S McEvoy, M Smyth, N Tulipan, A Singhal, P Steinbok, D Cochrane, W Hader, C Gallagher, M Benour, E Kiehna, J G McComb, P Chiarelli, A Robison, A Alexander, M Handler, T Hankinson, C Wilkinson, L Governale, A Drapeau, J Leonard, E Sribnick, A Shaikhouni, E Ahn, A Cohen, M Groves, S Robinson, C M Bonfield, C Shannon.
In addition, our work would not be possible without the outstanding support of the dedicated personnel at each clinical site and the data coordinating center. Special thanks go to: L Holman, J Clawson, P Martello, N Tattersall, T Bach (Salt Lake City); T Caudill, P Komarova, A Bey (Birmingham); H Ashrafpour, M Lamberti-Pasculli, L O’Connor (Toronto); E Santisbon, E Sanchez, S Martinez, S Ryan (Houston); K Hall, C Gangan, J Klein, A Anderson, G Bowen (Seattle); S Thambireddy, K Diamond, A Luther (Pittsburgh); A Morgan, H Botteron, D Morales, M Gabir, D Berger, D Mercer (St. Louis); M Stone, A Wiseman, J Stoll, D Dawson, S Gannon (Nashville); A Cheong, R Hengel (British Columbia); R Rashid, S Ahmed (Calgary); J Yea, A Loudermilk (Baltimore); N Chapman, N Rea, C Cook (Los Angeles); S Staulcup (Colorado); S Saraswat, A Sheline (Columbus); and N Nunn, M Langley, V Wall, D Austin, B Conley, V Freimann, L Herrera, B Miller (Utah Data Coordinating Center).
Disclosures
The HCRN is thankful for the following sources of funding:
private philanthropy and the Hydrocephalus Association.
NINDS grant 1RC1NS068943-01
None of the sponsors participated in design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of this manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the sponsors.
Abbreviations:
- CPC
choroid plexus cauterization
- ETV
endoscopic third ventriculostomy
- FOHR
frontooccipital horn ratio
- HCRN
Hydrocephalus Clinical Research Network
- VPS
ventriculoperitoneal shunt
Footnotes
Previous Presentations: Oral presentation at 2018 AANS/CNS Section on Pediatric Neurosurgery
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg Dec 2005;103(6 Suppl):475–81. doi: 10.3171/ped.2005.103.6.0475 [DOI] [PubMed] [Google Scholar]
- 2.Warf BC, Mugamba J, Kulkarni AV. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus in Uganda: report of a scoring system that predicts success. J Neurosurg Pediatr Feb 2010;5(2):143–8. doi: 10.3171/2009.9.Peds09196 [DOI] [PubMed] [Google Scholar]
- 3.Stone SSD, Warf BC. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. Journal of neurosurgery Pediatrics. 2014;14(5):439–446. doi: 10.3171/2014.7.peds14152 [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni AV, Riva-Cambrin J, Rozzelle CJ, et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infant hydrocephalus: a prospective study by the Hydrocephalus Clinical Research Network 2018;21(3):214. doi: 10.3171/2017.8.Peds17217 [DOI] [PubMed] [Google Scholar]
- 5.Riva-Cambrin J, Kestle JRW, Rozzelle CJ, et al. Predictors of success for combined endoscopic third ventriculostomy and choroid plexus cauterization in a North American setting: a Hydrocephalus Clinical Research Network study. J Neurosurg Pediatr May 31 2019:1–11. doi: 10.3171/2019.3.Peds18532 [DOI] [PubMed] [Google Scholar]
- 6.Marano PJ, Stone SS, Mugamba J, Ssenyonga P, Warf EB, Warf BC. Reopening of an obstructed third ventriculostomy: long-term success and factors affecting outcome in 215 infants. J Neurosurg Pediatr Apr 2015;15(4):399–405. doi: 10.3171/2014.10.Peds14250 [DOI] [PubMed] [Google Scholar]
- 7.Hellwig D, Giordano M, Kappus C. Redo third ventriculostomy. World Neurosurg Feb 2013;79(2 Suppl):S22.e13–20. doi: 10.1016/j.wneu.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Surash S, Chumas P, Bhargava D, Crimmins D, Straiton J, Tyagi A. A retrospective analysis of revision endoscopic third ventriculostomy. Childs Nerv Syst Dec 2010;26(12):1693–8. doi: 10.1007/s00381-010-1176-0 [DOI] [PubMed] [Google Scholar]
- 9.Oertel J, Vulcu S, Eickele L, Wagner W, Cinalli G, Rediker J. Long-Term Follow-Up of Repeat Endoscopic Third Ventriculostomy in Obstructive Hydrocephalus. World Neurosurg Mar 2017;99:556–565. doi: 10.1016/j.wneu.2016.12.072 [DOI] [PubMed] [Google Scholar]
- 10.Siomin V, Weiner H, Wisoff J, et al. Repeat endoscopic third ventriculostomy: is it worth trying? Childs Nerv Syst Sep 2001;17(9):551–5. doi: 10.1007/s003810100475 [DOI] [PubMed] [Google Scholar]
- 11.Mahapatra A, Mehr S, Singh D, Tandon M, Ganjoo P, Singh H. Ostomy closure and the role of repeat endoscopic third ventriculostomy (re-ETV) in failed ETV procedures. Neurol India Nov-Dec 2011;59(6):867–73. doi: 10.4103/0028-3886.91367 [DOI] [PubMed] [Google Scholar]
- 12.Peretta P, Cinalli G, Spennato P, et al. Long-term results of a second endoscopic third ventriculostomy in children: retrospective analysis of 40 cases. Neurosurgery Sep 2009;65(3):539–47; discussion 547. doi: 10.1227/01.Neu.0000350228.08523.D1 [DOI] [PubMed] [Google Scholar]
- 13.Moreira I, Pereira J, Oliveira J, Salvador SF, Vaz R. Endoscopic re-opening of third ventriculostomy: Case series and review of literature. Clin Neurol Neurosurg Jun 2016;145:58–63. doi: 10.1016/j.clineuro.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni A, Drake J, Mallucci C, Sgouros S, Roth J, Constantini S. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr 2009;155:254–259. [DOI] [PubMed] [Google Scholar]
- 15.Tamber MS, Kestle JRW, Reeder RW, et al. Temporal trends in surgical procedures for pediatric hydrocephalus: an analysis of the Hydrocephalus Clinical Research Network Core Data Project. J Neurosurg Pediatr Dec 18 2020:1–8. doi: 10.3171/2020.7.Peds20142 [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni AV, Riva-Cambrin J, Holubkov R, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network 2016;18(4):423. doi: 10.3171/2016.4.Peds163 [DOI] [PubMed] [Google Scholar]
- 17.Kestle JR, Drake JM, Cochrane DD, et al. Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg Feb 2003;98(2):284–90. doi: 10.3171/jns.2003.98.2.0284 [DOI] [PubMed] [Google Scholar]
- 18.Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery Aug 1998;43(2):294–303; discussion 303–5. doi: 10.1097/00006123-199808000-00068 [DOI] [PubMed] [Google Scholar]
- 19.Riva-Cambrin J, Kestle JR, Holubkov R, et al. Risk factors for shunt malfunction in pediatric hydrocephalus: a multicenter prospective cohort study. J Neurosurg Pediatr Apr 2016;17(4):382–90. doi: 10.3171/2015.6.Peds14670 [DOI] [PubMed] [Google Scholar]
- 20.Warf BC, Bhai S, Kulkarni AV, Mugamba J. Shunt survival after failed endoscopic treatment of hydrocephalus. J Neurosurg Pediatr Dec 2012;10(6):463–70. doi: 10.3171/2012.9.Peds1236 [DOI] [PubMed] [Google Scholar]


