Abstract
Nuclear clearance and cytoplasmic mislocalization of the essential RNA binding protein, TDP-43, is a pathologic hallmark of amyotrophic lateral sclerosis, frontotemporal dementia, and related neurodegenerative disorders collectively termed “TDP-43 proteinopathies.” TDP-43 mislocalization causes neurodegeneration through both loss and gain of function mechanisms. Loss of TDP-43 nuclear RNA processing function destabilizes the transcriptome by multiple mechanisms including disruption of pre-mRNA splicing, the failure of repression of cryptic exons, and retrotransposon activation. The accumulation of cytoplasmic TDP-43, which is prone to aberrant liquid–liquid phase separation and aggregation, traps TDP-43 in the cytoplasm and disrupts a host of downstream processes including the trafficking of RNA granules, local translation within axons, and mitochondrial function. In this review, we will discuss the TDP-43 therapy development pipeline, beginning with therapies in current and upcoming clinical trials, which are primarily focused on accelerating the clearance of TDP-43 aggregates. Then, we will look ahead to emerging strategies from preclinical studies, first from high-throughput genetic and pharmacologic screens, and finally from mechanistic studies focused on the upstream cause(s) of TDP-43 disruption in ALS/FTD. These include modulation of stress granule dynamics, TDP-43 nucleocytoplasmic shuttling, RNA metabolism, and correction of aberrant splicing events.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01260-5.
Keywords: Amyotrophic lateral sclerosis, Frontotemporal dementia, TDP-43, Autophagy, Stress granules, RNA
Introduction
TAR DNA binding protein 43 kD (TDP-43) is an essential RNA-binding protein that plays a crucial role in RNA processing and stability (reviewed in [1, 2]). In 2006, TDP-43 was identified as a major component of ubiquitinated inclusions in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) and found to be mislocalized from the nucleus to the cytoplasm in neurons and glia in affected CNS regions [3, 4]. Soon after, TDP-43 mutations were discovered in families with inherited ALS [5–8] and FTD [9, 10] suggesting a primary role for TDP-43 disruption in disease pathogenesis. Numerous in vitro and in vivo studies subsequently demonstrated that TDP-43 mutations and overexpression trigger neurodegeneration, through both a loss of nuclear RNA processing regulation and a gain of toxicity from cytoplasmic aggregates (reviewed in [1, 11, 12]). Despite the clinical heterogeneity of sporadic and familial ALS and FTD, TDP-43 pathology is observed in up to 97% and 45% of cases at autopsy, respectively, making TDP-43 an important therapeutic target (reviewed in [13]).
Thus far, TDP-43 disease-causing mutations appear specific to ALS and FTD. However, TDP-43 mislocalization and aggregation have been reported in other CNS disorders including Alzheimer’s disease and other dementias [14–16], chronic traumatic encephalopathy [17], stroke [18], multiple sclerosis [19], and the newly described entities limbic-predominant age-related TDP-43 encephalopathy (LATE) and cerebral age-related TDP-43 with sclerosis (CARTS) (reviewed in [20]). In addition, sarcoplasmic mislocalization and aggregation of TDP-43 is seen in inclusion body myositis and other myopathies with rimmed vacuoles [21–23], as well as in multisystem proteinopathies involving bone, muscle, and brain [24]. It remains unclear whether TDP-43 disruption is a primary contributor to the pathogenesis of these disorders, or a secondary consequence of cellular dysfunction. Nevertheless, TDP-43 proteinopathy may also be a therapeutic target in disorders beyond ALS/FTD.
Past efforts to develop TDP-43-targeting therapies in ALS and FTD have been recently comprehensively reviewed [25–27]. Unfortunately, as in the broader field of neurodegenerative disease, few drugs have advanced to clinical trial, and no TDP-43-directed therapies to date have been successful in slowing or reversing the human disease. However, there is an unprecedented number of ongoing clinical trials in ALS/FTD, including trials targeting TDP-43 proteinopathy. Moreover, there have been exciting advancements in preclinical studies of TDP-43 biology. In this review, we will summarize our current understanding of TDP-43 disruption in ALS/FTD, then discuss the background and rationale for current TDP-43-targeting therapies that have progressed to clinical trials. Finally, we will discuss novel strategies identified by recent high-throughput genetic and pharmacologic screens and hypothesis-driven studies on TDP-43 pathomechanisms.
TDP-43 in Health and Disease
Functional Domains and Nucleocytoplasmic Shuttling
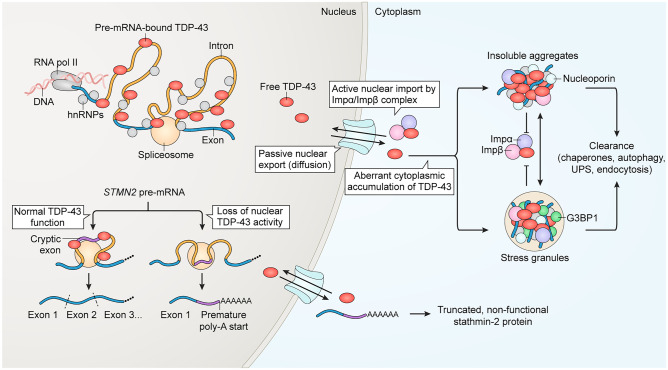
TDP-43 is an essential, ubiquitously expressed member of the heterogenous nuclear ribonucleoprotein (hnRNP) family. Like many hnRNP proteins, TDP-43 continuously shuttles between the nucleus and the cytoplasm but is primarily nuclear at steady state [28]. In the nucleus, TDP-43 preferentially binds GU-rich motifs in a large repertoire of pre-mRNAs and other nuclear RNAs and regulates RNA processing (Fig. 1). Functions include splicing modulation [29, 30], the repression of cryptic exons [31–33], regulation of alternative polyadenylation [34], silencing retrotransposons [35, 36], promoting microRNA biogenesis [37], and suppressing double-stranded RNA accumulation [38]. Numerous cytoplasmic functions for TDP-43 have also been identified, including the regulation of mRNA stability and trafficking, miRNA processing, translation, stress granule dynamics and others (recently reviewed [39]).
Fig. 1.
Overview of TDP-43 nuclear/cytoplasmic localization and its links to cytoplasmic aggregation and nuclear loss of function in pre-mRNA splicing. In the nucleus, TDP-43 accumulates at GU-rich sequences primarily within the distal introns of thousands of genes, where it acts to modulate splicing. The loss of nuclear TDP-43, triggered by nuclear clearance or disease-causing mutations, unmasks cryptic splice sites that permit the aberrant inclusion of cryptic exons within mRNA. For example, the aberrant inclusion of the STMN2 cryptic exon 2a generates a premature poly-adenylation start site, resulting in the synthesis of non-functional truncated stathmin-2 protein and loss of its crucial function in axonal regeneration. The predominantly nuclear localization of TDP-43 at steady state results from its active nuclear import via importin α and importin β and export via passive diffusion through nuclear pore complexes. In disease, accumulating cytoplasmic TDP-43 forms progressively insoluble aggregates, through a pathway that can proceed by both SG-dependent and -independent pathways. Both TDP-43 aggregates and SGs sequester importins and nucleoporins, which may hamper TDP-43 nuclear re-import. Clearance of cytoplasmic TDP-43 aggregates is mediated by multiple pathways, including via chaperones, autophagy, the ubiquitin–proteasome system (UPS), and endocytosis. For references, see text
TDP-43 contains multiple distinct domains (reviewed in [2]). The N-terminal portion of TDP-43 consists of a compact N-terminal domain (NTD) which mediates TDP-43 self-oligomerization [40], followed by a bipartite nuclear localization sequence (NLS) and tandem RNA recognition motifs (RRM1 and RRM2). The C-terminal domain consists of a prion-like intrinsically disordered region (IDR) which facilitates TDP-43 liquid–liquid-phase separation (LLPS) into condensates such as ribonucleoprotein (RNP) granules and stress granules (SG) (reviewed in [41]). The IDR, particularly a short, partially helical domain which harbors several ALS mutations, critically dictates TDP-43 RNA binding and regulatory function [31, 42–44]. However, LLPS is not an absolute requirement for TDP-43 splicing regulation [45]. Notably, TDP-43 undergoes multiple post-translational modifications that affect virtually all aspects of its function, including phosphorylation, acetylation, SUMOylation, PARylation, ubiquitination, and proteolytic cleavage (reviewed in [2, 46]).
Our mechanistic understanding of TDP-43 nucleocytoplasmic transport continues to evolve (recently discussed in [47]). A dynamic balance between nuclear import and export maintains the predominantly nuclear localization of TDP-43 in healthy cells (Fig. 1). Active, NLS-dependent nuclear import of TDP-43 is mediated by the nuclear import receptors importin α and β, which facilitate the passage of TDP-43 through the nuclear pore complex (NPC) along the RanGTP concentration gradient [28, 48]. The nuclear export of TDP-43 was initially thought to occur via binding of the exportin-1 receptor (XPO1 or CRM1) to a predicted nuclear export sequence (NES) in the RRM2 domain. However, multiple studies have demonstrated that the predicted NES is non-functional and inhibition or ablation of XPO1 does not alter TDP-43 nuclear export [49–51]. Data from these studies and our recent work suggest that TDP-43 leaves the nucleus by passive diffusion, and that TDP-43 binding to nuclear RNAs limits its availability for passive export [52].
Loss and Gain of Function in Disease
TDP-43 nuclear clearance and cytoplasmic aggregation in neurons and glia in affected CNS regions of ALS and FTD have been widely observed in sporadic and familial cases, with the notable exceptions of patients with inherited SOD1, FUS, and tau mutations (reviewed in [13]). The typical pathology involves TDP-43 nuclear clearance and cytoplasmic aggregation of polyubiquitinated, hyperphosphorylated protein [3, 4, 53, 54], including full-length TDP-43 and C-terminal fragments (reviewed in [55]). A subset of cells also shows nuclear clearance without cytoplasmic aggregation [56, 57]. While much is known about factors that promote and perpetuate TDP-43 cytoplasmic mislocalization, aggregation, and loss of nuclear function in ALS/FTD, the upstream initiating event that starts this cascade, particularly in sporadic disease, remains unknown.
Extensive evidence suggests that TDP-43 nuclear loss of function (LOF) critically disrupts pre-mRNA splicing regulation, leading to the failure of repression of cryptic exons and destabilizing hundreds to thousands of transcripts in a species- and cell-specific manner [29–32, 34, 58]. Further studies of neuronal targets of TDP-43 splicing regulation, including stathmin-2 (STMN2) [33] (Fig. 1) and UNC13A [59, 60], have now implicated TDP-43 LOF-induced splicing defects with axonal and synaptic disruption. Notably, some TDP-43 disease-causing mutations disrupt splicing function without affecting TDP-43 localization [61, 62], further highlighting the importance of TDP-43 nuclear LOF in disease pathogenesis. Other RNA processing functions of TDP-43 have also been implicated in neuronal dysfunction and disease, including a role of TDP-43-regulated miRNAs in neurite outgrowth [37] and growing data linking retrotransposon activation with TDP-43 mislocalization and neuropathology [35, 63].
Cytoplasmic accumulation and aggregation of TDP-43 contributes to multiple toxic gains of function (GOF). Most notably, TDP-43 aggregation appears to promote cytoplasmic trapping, either causing or exacerbating TDP-43 nuclear clearance. Various perturbations can trigger cytoplasmic TDP-43 aggregation, including overexpression of wild-type TDP-43, TDP-43-ΔNLS, C-terminal fragments, or disease-causing mutations [64–68]. Indeed, the bulk of TDP-43 ALS/FTD-causing mutations cluster in the C-terminal IDR (reviewed by [11]) and increase the propensity of TDP-43 to aggregate [69–71]. Experimental aggregation can also be induced by prolonged or repetitive exposure to experimental stressors [72–75], optogenetic-clustering [76–78], or seeding cells with preformed aggregates of TDP-43 or other prion-like proteins [79, 80]. Under certain conditions, these experimental perturbations promote deleterious and irreversible TDP-43 LLPS from a liquid-like state to fibrous aggregates, through both SG-dependent and independent pathways [74, 75, 81, 82] (Fig. 1). Once nucleated, TDP-43 aggregation appears to worsen via a feed-forward cascade. Past a ‘point of no return’ (which remains incompletely understood), mature, fibrous aggregates of TDP-43 appear to become unavailable for nuclear import, thus propagating the vicious cycle of cytoplasmic trapping, nuclear clearance, and nuclear LOF. Sequestration of nuclear transport machinery by TDP-43 aggregate—including nuclear transport receptors and nucleoporins—may further amplify the nuclear import blockade (Fig. 1) [79, 83].
Besides promoting the loss of nuclear TDP-43, the accumulation of cytoplasmic TDP-43 has been shown to compromise neuronal homeostasis by multiple mechanisms (reviewed by [84]). Cytoplasmic accumulation of TDP-43 induces a distinct gene expression pattern [85] and inhibits local translation in distal axons and neuromuscular junctions (NMJs) [86]. TDP-43 also accumulates in mitochondria, causing mitochondrial dysfunction [87] and stimulating inflammation via the cGAS/STING pathway [88, 89]. The relative contribution of TDP-43 LOF versus GOF in disease has been a topic of ongoing discussion. Current evidence suggests that TDP-43 aggregate formation contributes to both LOF and GOF to drive neuronal degeneration [84]. Indeed, studies comparing the contribution of TDP-43 LOF and GOF in disease models have concluded that there is likely concurrent involvement of both processes [90–92].
Finally, there is evidence for dysregulation of the synthesis and clearance of TDP-43 in disease that further amplifies TDP-43 cytoplasmic mislocalization and aggregation. TDP-43 autoregulation, a negative feedback loop by which TDP-43 regulates the splicing and stability of its own pre-mRNA [93] is disrupted, increasing TDP-43 synthesis and adding to the cytoplasmic burden of TDP-43 available to aggregate [47, 94]. TDP-43 aggregates are cleared by multiple routes, including chaperone-mediated pathways, autophagy, the ubiquitin–proteasome system (UPS), and endocytosis (Fig. 1) [95–99]. In disease models, TDP-43 impairs these clearance pathways through both LOF and GOF mechanisms, increasing pathologic TDP-43 accumulation [97, 99–104].
TDP-43 Therapy Development Pipeline
In the 15 years since TDP-43 proteinopathy was identified in ALS/FTD, numerous therapeutic approaches have been tested in cell and animal models to attenuate TDP-43 pathology, and that literature has been recently reviewed [25–27]. This section will highlight the major classes of TDP-43-directed therapies being tested in current or upcoming clinical trials in ALS (listed in Table 1). No clinical trials of TDP-43-targeting agents are currently registered for FTD. Importantly, this section and the remainder of the review will focus on strategies that directly target or modify TDP-43 protein or its localization, aggregation, or clearance. Numerous indirect approaches have also been tested in TDP-43 models, targeting oxidative stress, inflammation, glutamatergic signaling, ER stress, and other pathways, which are outside the scope of this review.
Table 1.
Clinical trials of TDP-43-directed therapies in ALS
| Drug | Mechanism of action | Statusa | NCT number | Results |
|---|---|---|---|---|
| Autophagy induction | ||||
| Rapamycin | mTOR inhibitor |
Phase 2 (Sept 2017-ongoing) |
NCT03359538 | Ongoing |
| Monepantel | mTOR inhibitor |
Phase 1 (not yet recruiting) |
NCT04894240 | N/A |
| Tamoxifen | mTOR-independent autophagy induction |
Phase 2 (3 trials completed) |
Did not achieve stated outcomes |
|
| Bosutinib | Src/c-Abl inhibitor, activates autophagy |
Phase 1 (March 2019-ongoing) |
NCT04744532 | Ongoing |
|
Trehalose (SLS-005) |
TFEB-mediated autophagy activation |
Phase 2 (Feb 2022-ongoing) |
NCT05136885 | Ongoing |
| Stress granule modulator | ||||
|
ATXN2 ASO (BIIB105) |
Ataxin-2 knockdown; inhibit SG maturation and TDP-43 recruitment to SG |
Phase 1 (Sept 2020-ongoing) |
NCT04494256 | Ongoing |
| Kinase inhibitor | ||||
| Tideglusib | GSK-3β inhibitor, reduce TDP-43 phosphorylation |
Phase 2 (not yet recruiting) |
NCT05105958 | N/A |
| Other/mixed mechanism | ||||
| Ibudilast (MN116) |
Nonselective phosphodiesterase inhibitor with anti-inflammatory effects; TFEB-mediated autophagy activation |
Phase 1/2 Phase 2 Phase 2/3 |
No biomarker change [121]; Ongoing |
|
| Colchicine | Induce HSPB8 expression to activate autophagy and modulate SG dynamics |
Phase 2 (April 2019-ongoing) |
NCT03693781 | Ongoing |
| Withania somnifera | NF-kB inhibitor |
Phase 2 (Oct 2021-ongoing) |
NCT05031351 | Ongoing |
| 3K3A-APC | Activates PAR1-dependent signaling to induce autophagosome formation, reduce inflammation, and other activities |
Phase 2 (Nov 2021-ongoing) |
NCT05039268 | N/A |
NCT National Clinical Trial registration number (clinicaltrials.gov), ASO antisense oligonucleotide, mTOR mammalian target of rapamycin, SG stress granule, N/A not applicable
aStatus as of manuscript submission
Autophagy Activation
Macroautophagy, a key degradative pathway for the delivery of cytoplasmic material to lysosomes, plays a major role in clearing protein aggregates in neurodegenerative diseases, including TDP-43 (reviewed in [105]). TDP-43 aggregates have also been shown to inhibit autophagy [100, 101], further exacerbating TDP-43 cytoplasmic accumulation. More than a dozen autophagy-activating compounds, including drugs that are FDA-approved for other indications, have shown efficacy in clearing protein aggregates in neurodegenerative disease models, including TDP-43 (reviewed in [105]), and a subset has shown efficacy in vivo as discussed below. To date, autophagy activators are the largest category of TDP-43-directed therapies that have progressed to human clinical trials. Therapies being tested in clinical trial act by multiple mechanisms including mammalian target of rapamycin (mTOR)-dependent and independent pathways.
mTOR Inhibitors
Rapamycin (also known as sirolimus), which functions as an inhibitor of mTOR, a sensor of the cellular nutrient state that negatively regulates autophagy, is FDA-approved for the prevention of organ rejection following kidney transplant (reviewed in [106]). Rapamycin has been studied in numerous neurodegenerative disease models based on its role as an autophagy activator. When administered to FTLD-U mice that overexpress wild-type TDP-43 in forebrain neurons, rapamycin attenuated cognitive impairment, slowed neuronal loss, reduced cytosolic TDP-43 inclusions, and decreased levels of insoluble, full-length, and cleaved TDP-43 [107]. These effects were associated with autophagy activation as measured by LC3 (microtubule-associated protein 1 light chain 3) puncta in the hippocampus, an increased LC3-II/LC3-I ratio (indicative of increased autophagosome formation), and a reduction in SQSTM1/p62 levels, a marker of autophagic flux. A phase 2 trial of rapamycin in ALS (63 participants) began in September 2017 and is ongoing (NCT03359538). Of note, the veterinary drug Monepantel was recently found to have off-target activity inhibiting mTOR and has shown favorable safety in early cancer trials [108]. A phase 1 trial of oral Monepantel in ALS (12 participants) is registered but not yet recruiting as of the time of submission (NCT04894240).
mTOR-Independent Agents
Tamoxifen, an oral selective estrogen receptor modulator that is FDA-approved for the prevention and treatment of specific types of breast cancer, activates autophagy through an mTOR-independent pathway [109]. Like rapamycin, tamoxifen treatment of FTDL-U mice reduced TDP-43-positive cortical inclusions and activated autophagy markers [107]. Three small phase 2 trials of tamoxifen in ALS have been completed, with results published for two of them [110, 111]. Although some beneficial trends were reported, these studies were underpowered to detect a difference between tamoxifen and placebo, leaving open the question of potential benefit in disease.
In 2017, bosutinib, an FDA-approved drug for chronic myeloid leukemia and an inhibitor of the nonreceptor tyrosine kinases Src and c-Abl, was identified in a screen for drugs that prolong survival of mutant SOD1 induced pluripotent stem cell (iPSC)-derived motor neurons [112]. Bosutinib appeared to confer its beneficial effect by activating autophagy as evidenced by an increase in p62 and the LC3-II/LC3-I ratio in parallel with reduced misfolded SOD1. Other effects included improved ATP levels and altered expression of TCA cycle and electron transport chain genes. A modest benefit was observed following bosutinib administration to SOD1 mutant mice. Subsequent testing in sporadic ALS and TDP-43-mutant iPSC-derived neurons showed improvement in survival and reduction of aberrant high and low molecular weight TDP-43 species. Administration of bosutinib and the related drug nilotinib to wild-type TDP-43- and TDP-43 A315T-overexpressing mice was also reported to reduce TDP-43 levels, although the mechanism was attributed in this case to parkin modulation [113]. A phase 1 trial of bosutinib in ALS (24 participants) began in March 2019 and is ongoing (NCT04744532).
TFEB Induction
Trehalose is a natural disaccharide used in food production that induces autophagy by inducing nuclear translocation of transcription factor EB (TFEB), a master regulator of autophagolysosomal gene expression [114, 115]. Mechanistic studies show that trehalose causes transient lysosomal damage and enlargement, which likely activates protein phosphatase 3 (PPP3CB)-mediated dephosphorylation of TFEB, triggering its nuclear translocation [115]. TFEB-independent effects of trehalose have also been reported, including induction of the lysosomal regulator progranulin [116] and the autophagy-associated chaperone HSPB8 [115], suggesting pleiotropic effects in promoting aggregate clearance. Trehalose enhanced the autophagic degradation of wild-type and mutant TDP-43 in SH-SY5Y cells [114] and promoted the degradation of insoluble C-terminal fragments of TDP-43 in NSC34 cells [115]. Trehalose attenuated neurodegeneration in mutant SOD1 ALS mice [117] and mouse models of Huntington’s and Parkinson’s disease (reviewed in [118]); however, there are no published reports to date of testing in in vivo models of TDP-43 proteinopathy. Trehalose is CNS penetrant but highly degraded in the GI tract, precluding oral administration [119]. Weekly intravenous infusion of trehalose (SLS-005) was recently announced as a treatment arm of the ongoing Healey ALS Platform trial (160 participants) and began recruiting participants in February 2022 (NCT05136885).
Ibudilast (MN-116), a nonselective phosphodiesterase inhibitor that attenuates CNS microglial activation and neuroinflammation, was also recently shown to enhance autophagy and clearance of TDP-43 aggregates via a TFEB-mediated mechanism, broadening its potential therapeutic mechanisms in disease [120]. Two small clinical trials of ibudilast have been completed. In a phase 1/2 dose-finding and pharmacokinetics study of 35 patients, discontinuations were frequent and ibudilast did not alter biomarkers, including a PET readout of glial activation and serum neurofilament light chain levels [121] (NCT02714036). An additional phase 2 study in 70 patients was completed, but results are not yet published (NCT02238626). A phase 2/3 study of ibudilast in 230 patients began in May 2020 and is ongoing (NCT04057898).
Stress Granule Modulation
Stress granules (SG), cytoplasmic RNP complexes of which TDP-43 is a known constituent and regulator [122–126], form in response to cellular stress and play a crucial role in transiently altering translation as part of the cellular stress response (reviewed in [127]). Growing data suggest that aberrant SG persistence may serve as a nidus for the maturation of TDP-43 aggregates (reviewed in [128]). Therefore, modulation of SG dynamics to limit formation, TDP-43 recruitment, and speed dissolution is widely regarded as a therapeutic target for TDP-43 proteinopathy.
In 2010, an unbiased screen for modifiers of TDP-43 toxicity in yeast identified PBP1, the yeast ortholog of the SG constituent ataxin-2 (ATXN2) as a potent enhancer of TDP-43 toxicity, findings that were replicated in Drosophila [129]. When crossed with Atxn2 knockout mice, TDP-43Tg/Tg mice that overexpress wild-type human TDP-43 (hTDP-43) in neurons and exhibit severe, early onset motor dysfunction and death showed remarkable phenotypic improvement and prolongation of lifespan, along with a modest reduction in full-length and C-terminal TDP-43 fragments and phospho-TDP-43-positive inclusions [130]. Intracerebroventricular injection of Atxn2-ASOs also markedly attenuated disease in TDP-43Tg/Tg mice. In human cell lines, TDP-43 and ATXN2 interact in cytoplasmic complexes in an RNA-dependent manner [129], and ATXN2 knockdown inhibits SG maturation and TDP-43 recruitment to SG following arsenite treatment [130], suggesting a key role for ATXN2 in promoting SG-mediated cytoplasmic aggregation of TDP-43. Further genetic investigation of ATXN2 in ALS patients led to the discovery that intermediate-length ATXN2 CAG trinucleotide repeat expansions are a risk factor for ALS [129, 131]. A phase 1 multicenter trial of ATXN2 ASOs (BIIB105, 70 participants) began in September 2020 and is ongoing (NCT04494256).
Kinase Inhibitors
TDP-43 phosphorylation broadly regulates its normal functions, and hyperphosphorylation is consistently observed in postmortem tissue, generating interest in modulating kinases as a therapeutic strategy (reviewed in [132]). Glycogen synthase kinase 3β (GSK-3β), one of multiple kinases that phosphorylate TDP-43, is upregulated in ALS postmortem tissue and GSK-3β inhibitors confer beneficial effects in in vitro and in vivo models (recently reviewed [133]). One small molecule inhibitor of GSK-3β, kenpaullone, was identified in a screen for compounds that promote the survival of mouse embryonic stem cell-derived motor neurons, including wild-type and mutant SOD1 motor neurons [134]. In this same study, kenpaullone also promoted the survival of TDP-43 M337V iPSC-derived motor neurons. However, the effects were likely augmented by inhibition of HGK kinases, as highly specific inhibitors of GSK-3β failed to support neuronal survival. Tideglusib, an orally administered competitive GSK-3β inhibitor, has been shown to attenuate TDP-43 hyperphosphorylation in ALS patient-derived lymphoblast cell lines and TDP-43-A315T mice [133]. Based on a similar role for GSK-3β in the phosphorylation of tau, Tideglusib was previously tested in phase 2 trials in progressive supranuclear palsy (n = 146 patients) [135] and Alzheimer’s disease (n = 306 patients) [136]. Although Tideglusib was well tolerated, neither trial met primary or secondary clinical endpoints. A phase 2 multicenter trial of tideglusib in ALS (98 participants) has been registered but is not yet recruiting (NCT05105958).
Other/Mixed Mechanism
Colchicine was identified in a screen for drugs that increase the expression of the chaperone heat shock protein B8 (HSPB8), which as part of the HSPB8-BAG3-HSP70 complex promotes clearance of TDP-43 aggregates and C-terminal fragments in vitro [137, 138]. Colchicine also augments pro-autophagy TFEB signaling [137]. Interestingly, the HSPB8-BAG3-HSP70 complex was also found to regulate SG composition and disassembly [139], suggesting that colchicine-induced HSPB8 expression could also modulate SG dynamics, although that remains to be tested. Thus, it has been proposed that HSPB8 induction by colchicine could improve TDP-43 proteostasis both by attenuating SG-mediated aggregate formation and augmenting autophagic clearance [140]. Overexpression of the fly homolog of HSPB8 in Drosophila attenuated neurodegeneration in models of TDP43-ΔNLS and C-terminal fragment overexpression [138], supporting an in vivo benefit of augmenting HSPB8 activity. No published studies to date have evaluated the effectiveness of colchicine in in vivo models of TDP-43 proteinopathy. Colchicine is FDA-approved for the treatment of gout, pericarditis, and other indications, based on inhibition of microtubule polymerization and systemic anti-inflammatory effects [141, 142]. A phase 2 study of colchicine in ALS (54 participants) was initiated in April 2019 and is ongoing (NCT03693781) [140].
Investigation of changes in the TDP-43 interactome in LPS-stimulated mouse microglial BV-2 cells uncovered a novel, gain of function interaction between TDP-43 and p65 NF-κB that was confirmed in TDP-43 mouse models and ALS postmortem spinal cord and mapped to the N-terminal region of TDP-43 [143]. Mechanistic studies demonstrated that TDP-43 acts as a coactivator of NF-κB and augments glial LPS-induced proinflammatory cytokine release. Withaferin A, a synthetic NF-κB inhibitor derived from the root extract of Withania somnifera [144], attenuated cell death induced by TDP-43 overexpression in neuron/microglia cocultures, reduced expression of GFAP (a marker of astrocyte activation and target of NF-κB signaling), and modestly improved NMJ innervation in wild type hTDP-43-overexpressing mice [143]. Withaferin A also attenuated TDP-43 inclusions and improved cognitive function in TDP-43 G348C mutant mice [145]. Administration of Withafera somnifera root extract to TDP-43 A315T mutant mice improved motor and cognitive phenotypes and attenuated TDP-43 mislocalization and aggregation in spinal motor neurons [146]. The precise mechanism of action related to TDP-43 localization and aggregation remains to be determined, although NF-κB negatively regulates autophagy, and autophagy activation was detected in Withaferin A-treated TDP-43 G348C mutant mice [145]. A phase 2 trial of Withania somnifera (75 participants) began recruiting in October 2021 (NCT05031351).
Activated protein C (APC) has long been of therapeutic interest in neurodegenerative disease and stroke based on neuroprotective properties independent of its anticoagulant effects [147]. APC activates G-protein coupled signaling via the protease-activated receptor 1 (PAR1) leading to diverse homeostatic effects including anti-inflammatory signaling, blood–brain barrier modulation, and stimulating neurogenesis. Initial tests of 3K3A-APC, a mutant form of APC that lacks anticoagulant effects, showed therapeutic benefit in mutant SOD1 mice by a mechanism thought to involve transcriptional downregulation of SOD1 [148] and blood-spinal cord barrier repair [149]. A subsequent study in a mouse model of lung injury showed that 3K3A-APC also enhances autophagy by inducing the expression of LC3, ATG5, and Rab7 [150]. 3K3A-APC rescued defects in autophagosome formation in C9ORF72 and sporadic ALS iPS-derived neurons as measured by LC3 puncta, LAMP2 vesicles, and induction of the autophagy-regulating factors ATG5 and ATG10. In parallel, 3K3A-APC attenuated TDP-43 cytoplasmic mislocalization by a mechanism proposed to involve enhanced authophagic clearance, although this was not further investigated [151]. A phase 2 trial of intravenous 3K3A-APC in 16 ALS patients began recruiting in November 2021 (NCT05039268).
High-Throughput Screening for Genetic and Pharmacologic Modifiers
As is evident from the above discussion, therapies currently being tested in patients are largely focused on accelerating the clearance of TDP-43 aggregates once already formed. This is partly due to the opportunity to repurpose existing, FDA-approved therapies which greatly accelerates the therapy development timeline, but also reflects our limited understanding of the upstream events that initiate the cascade of TDP-43 disruption in disease. Fortunately, this landscape is rapidly changing with advances in preclinical studies, including the results of unbiased high-throughput screens (HTS) for genomic and pharmacologic regulators of TDP-43 toxicity. This section will discuss recent systematic screens that point to key pathways and compounds that may enable earlier intervention in the TDP-43 pathologic cascade.
Genetic Screens for Modifiers of TDP-43 Toxicity
Three model organisms—yeast, C. elegans, and Drosophila—have been used to probe the genome for suppressors of toxicity induced by the expression of wild-type and mutant TDP-43 (Table 2). These screens, and follow-up studies in mice and human cells, showcase the value of forward genetic screens in identifying therapeutic targets for TDP-43 proteinopathy.
Table 2.
High-throughput genetic screens for modifiers of TDP-43 proteinopathy
| Design and scope | #Hits and functional categories | Featured hitsa | Validation in other models or human tissue | Ref |
|---|---|---|---|---|
| Yeast | ||||
|
5500 overexpressed genes; wild-type hTDP-43 toxicity |
13 suppressors/27 enhancers: RNA metabolism Cell cycle Transport Protein modification process |
Direct hits: PBP1 (ATXN2) |
ATXN2 knockdown improved eye degeneration and survival in flies, ATXN2 inclusions reported in ALS spinal motor neurons | [129] |
| Stress response |
Via interaction analysis: PABPC1 EIF2A |
SG modulation via genetic and pharmacologic inhibition of eIF2α phosphorylation (PERK inhibitor GSK2606414) rescued TDP-43 toxicity in fly, primary neurons | [152] | |
|
Genome-wide loss of function; wild-type hTDP-43 toxicity (two screens: non-essential and essential genes) |
8 suppressors/6 enhancers: RNA metabolism RNP granule Translation Mitochondrial function |
DBR1 PBP1 (ATXN2) |
Rescued mutant TDP-43 toxicity in human neuronal cell line and primary rat neurons | [154] |
|
4653 diploid deletion mutants; Wild-type and mutant hTDP-43 toxicity (M337V and Q331K) |
52 suppressors, 13 validated: Electron transport chain Nucleotide excision repair Coated pit Cellular metabolic process Organic substance transport |
SDH (SDHA) POR2 (VDAC3) HSC82 (HSP90AB1) |
Pharmacologic inhibition not beneficial in N2a cells, but SDHA and VDAC3 inhibition reduced proinflammatory cytokine release and mitochondrial dysfunction in primary astrocytes | [155] |
| C. elegans | ||||
| RNAi kinome screen of 453 predicted kinases and phosphatases; pan-neuronal hTDP-43 M337V-induced motor deficits | 12 kinases | CDC-7 | CDC-7 inhibitor PHA767491 decreased pTDP-43 in EA-treated NSC-34 cells and C. elegans and attenuated neuronal loss | [156] |
| Genome-wide RNAi screen of 16,757 genes; pan-neuronal hTDP-43 M337V-induced motor deficits |
46 hits, 9 validated: Extracellular matrix/cytoskeleton Energy production/metabolism Proteostasis Nucleic acid function |
hse5 (GLCE) | GLCE LOF improved motor function via effect on synaptic transmission. GLCE siRNA reduced pTDP-43 in EA-treated HEK293 cells. GLCE is reduced in FTLD-TDP frontal cortex | [161] |
| Drosophila | ||||
| ~ 3000-gene deficiency screen (chromosomes 2–3); motor neuron WT hTDP-43-induced lethality | 1 of 29 deficiency segments selected for finer mapping | Wnd (DLK) | OE of the Wnd/DLK target bsk/JNK improved survival while p38b OE shortened survival. p38 inhibitors improved lifepan. Bsk and p38b manipulation produced reciprocal effects on TDP-43-induced markers of oxidative stress and neuroinflammation | [164] |
| 3189 EMS-mutagenized lines (X chromosome); hTDP-43 Q331K-induced NMJ denervation |
3 hits: chromatin remodeling RNA metabolism kinase activity |
hat-trick (ARID4A, 4B) xmas-2 (GANP) sgg (GSK3) |
N/A | [169] |
| RNAi screen of 84 histone-modifying genes; WT hTDP-43-induced rough-eye phenotype | 31 modifiers (4 strong) |
lid (H3K4me3 demethylase) Chd1 (CHD2) |
Upregulation of Chd1 restored stress gene induction in Drosophila. TDP-43 OE in HEK293 cells decreased CHD2; CHD2 expression is reduced in FTD patient temporal cortex | [172] |
| 15,500 insertional mutants (~ 50% of genome); mutant hTDP-43 M337V- and hFUS R521C-induced rough-eye phenotype |
553 TDP-43 modifiers, 432 in common with FUS modifiers nucleic acid binding cytoskeletal protein signaling pathway oxidoreductase pathway |
SF2 (SRSF1) lilli (AFF2/FMR2) dGLE1 (GLE1) 7 PLD1/2-pathway genes |
SF2 and lilli LOF suppressed fly TBPH-N493D NMJ denervation. Gle1 LOF suppressed TDP-43 aggregation. PLD1/2 deletion modestly improved motor function in SOD1 mutant mice | [173] |
| 2933 mutant lines; modifiers of WT hTDP-43-induced rough eye phenotype |
12 enhancers, 23 suppressors hits/categories not specified |
Ell, aka Su(Tpl) (ELL2) Little (LEC) and super (SEC) transcription elongation complexes |
Ell knockdown rescued motor deficits and snRNA upregulation in hTDP-43 flies. LEC (U12 snRNA) and SEC (lncRNA Hsrω/SatIII) noncoding RNAs were dysregulated by TDP-43 OE. SatIII lncRNA was increased in FTD patient motor cortex | [179] |
| shRNA screen of ~ 2700 genes; motor neuron WT hTDP-43-induced motor impairment |
32 suppressors: Mediator complex chromatin remodeling nucleocytoplasmic transport |
SF2 (SRSF1) msk (IMP7) Chd1 (CHD1/2) Su(Tpl) (ELL2) |
Motor neuron-specific CRISPR-KO of SF2 and msk rescued motor impairment in Drosophila | [177] |
| Human cells | ||||
|
siRNA whole genome screen (18,230 genes) in HeLa cells; TDP-43 N/C localization |
60 siRNAs promoted cytoplasmic TDP-43 localization Nucleocytoplasmic transport Calcium signaling |
Itp (ITPR1) | Itp (ITPR1) mutations improved motor function and extended lifespan in Drosophila with MN-directed hTDP-43 expression | [182] |
hTDP-43 human TDP-43, pTDP-43 phospho-TDP-43, WT wild type, TBPH Drosophila TDP-43, SG stress granule, LOF loss of function, EA ethacrynic acid, OE overexpression, N/C nuclear/cytoplasmic
aHuman orthologs in parentheses
Yeast
As introduced above, in 2010, Elden et al. [129] screened a library of 5500 overexpressed genes in S. cerevisiae for modification of cytotoxicity from human TDP-43 expression. 40 modifiers were identified, 13 suppressors and 27 enhancers, including the yeast ortholog of the SG protein ataxin-2. Ataxin-2 knockdown strongly ameliorated TDP-43 toxicity in yeast, Drosophila, and mice, prompting a phase 1 clinical trial of ataxin-2 ASOs. Additional modifiers identified in the screen were enriched for RNA binding proteins and genes involved in RNA metabolism, including multiple constituents of SGs [152]. Further interactome analysis uncovered common links between modifier genes and the yeast ortholog of the human EIF2A translation initiation factor, which plays a key role in SG formation. Subsequent studies in Drosophila identified the fly homologs of additional SG regulators, PABC1 (cytoplasmic poly-A binding protein) and PERK (the protein kinase R-like endoplasmic reticulum kinase), which phosphorylates the key integrated stress response protein eIF2α [153], as enhancers of TDP-43 toxicity. Treatment with the PERK inhibitor GSK2606414 decreased eIF2α phosphorylation and rescued TDP-43 toxicity in flies and primary rat neurons. Taken together, these studies identified suppression of SG formation, such as through ATXN2 knockdown or inhibition of eIF2α phosphorylation, as a candidate therapeutic strategy for TDP-43-dependent toxicity.
The Gitler lab [154] also carried out complimentary genome-wide loss of function screens for modifiers of hTDP-43 toxicity in yeast. Among 14 top modifiers, a taxin-2 was again identified as a suppressor, and loss of DBR1, which encodes an RNA lariat debranching enzyme, was strongly protective in both screens. Follow-up testing showed that DBR1 knockdown protected against TDP-43 Q331K toxicity in human neuroblastoma M17 cells and primary rat neurons. The protective effect required the loss of DBR1 activity in cleaving the 2′-hydroxyl-5′-phosphate bond that closes the circular intronic RNA lariat formed during pre-mRNA splicing. In the absence of DBR1, circular RNA lariats accumulated in the cytoplasm, where they interacted with TDP-43. The authors proposed a mechanism by which lariat RNAs competitively bind to cytoplasmic TDP-43, preventing it from harmful interactions with other essential cellular RNAs [154].
Most recently, a screen in homozygous diploid yeast deletion mutants expressing wild type or mutant human TDP-43 (M337V and Q331K) revealed additional genes whose deletion suppressed TDP-43 toxicity [155]. The screen identified 52 modifiers, none of which overlapped with previous wild-type TDP-43 screens [129, 152]. Following subsequent validation and network analysis, orthologs of three genes were chosen for further investigations: SDHA (succinate dehydrogenase flavoprotein subunit A), HSP90AB1 (Heat Shock Protein 90 Alpha Family Class B Member 1), and VDAC3 (Voltage-Dependent Anion Channel 3). Pharmacological inhibition of SDHA or VDAC32 (but not HSP90AB1) suppressed TDP-43 wild-type and M337V-induced proinflammatory cytokine release and mitochondrial dysfunction in primary astrocyte cultures, although none of the inhibitors affected TDP-43 Q331K-induced toxicity.
C. elegans
In 2013, Liachko et al. [156] performed a kinome screen to identify genes responsible for deleterious TDP-43 phosphorylation. The RNAi screen targeted 453 predicted kinases and phosphatases in the C. elegans genome while monitoring the rescue of motor impairment induced by pan-neuronal expression of hTDP-43 M337V. The screen identified 12 candidate kinases, including the orthologs of human CDC7 and TTBK1/2, as enhancers of hTDP-43 M337V toxicity. CDC7 directly phosphorylated hTDP-43 in vitro and the CDC7 inhibitor PHA767491 attenuated ethacrynic acid-induced TDP-43 phosphorylation in NSC-43 cells. PHA767491 also decreased TDP-43 phosphorylation and TDP-43 M377V-induced neurodegeneration in C.elegans, although doses were limited by cell cycle disruption. TTKB1 and TTKB2 also directly phosphorylate hTDP-43 in vitro [157]. TTKB1/2 overexpression induced TDP-43 phosphorylation and cytoplasmic mislocalization in cultured cells and caused neurodegeneration in C.elegans [158]. In addition, TTKB1/2 immunohistochemistry in FTD frontal cortex showed increased expression and colocalization with TDP-43 inclusions [157]. Together, these findings support TDP-43 phosphorylation by CDC7 and TTBK1/2 as candidate therapeutic targets for TDP-43 proteinopathy [159, 160], although no agents have yet advanced to human trial.
A subsequent genome-wide RNAi screen in C. elegans expressing pan-neuronal hTDP-43 M337V identified 46 suppressor RNAi clones that improved motor function, 9 of which have human homologs and were validated by deletion or LOF mutations [161]. Of these, LOF mutants of the heparan sulfate-modifying enzyme hse-5 (human glucuronic acid epimerase (GLCE)), attenuated motor deficits and accumulation of phospho-TDP-43. Through its epimerase function, GLCE supports the formation of mature heparan sulfate, a component of the extracellular matrix and cell surface [162]. Hse-5 LOF mutants did not improve survival or protect against TDP-43-induced neuronal loss in C. elegans but promoted axonal outgrowth and partially restored synaptic function. GLCE knockdown by RNAi protected against phospho-TDP-43 accumulation in HEK293 cells. The frontal cortex of FTLD-TDP patients also showed reduced GLCE levels, supporting the potential involvement of GLCE in TDP-43-associated neurotoxicity, although by an unclear mechanism. A potential link of the reduced GLCE expression to suppression of endocytosis and autophagy was proposed [163].
Drosophila
In 2014, Zhan and colleagues performed a deficiency screen covering approximately 3000 genes in chromosomes 2 and 3 to identify genes that increased the survival of Drosophila overexpressing human TDP-43 in motor neurons [164]. A region that conferred a two-fold increase in median survival was further interrogated, resulting in the identification of 23 candidate suppressors, including the MAP3K Wnd (human dual leucine kinase, DLK), a key upstream regulator in the stress-activated protein kinase pathway known to modulate neuromuscular junction development and axonal injury. Follow-up testing identified opposing effects of manipulating downsteam Wnd targets, with beneficial effects of JNK overexpression and deleterious effects of p38 overexpression. Conversely, p38 knockdown or treatment of TDP-43 overexpressing flies with the p38 small molecule inhibitor SB202190 were beneficial, and their effects correlated with changes in oxidative stress and innate immunity pathways. DLK knockdown or small molecule inhibition was subsequently found to be beneficial in Alzheimer’s disease and mutant SOD1-ALS mice [165] and identified in a CRISPRi-screen for genes that modify survival of human iPSC-derived neurons [166], suggesting a potential broad regulatory role for DLK in neuronal survival. Although not a TDP-43-specific target, DLK inhibition continues to be of therapeutic interest in neurodegeneration [167]. A small molecule DLK inhibitor, GDC-0134, was tested in a phase 1 trial of 49 ALS patients but safety concerns precluded further testing [168].
Sreedharan et al. [169] developed an innovative mosaic analysis approach and carried out a forward genetic screen for modifiers of NMJ denervation in Drosophila. The screen examined 3189 EMS-mutagenized lines on the X chromosome for suppressors of hTDP-43 Q331K-induced NMJ denervation. Three suppressors were identified and validated, including LOF mutants in a novel gene, hat-trick, containing chromatin organization modifier domains and predicted to correspond to ARID4A and ARID4B, members of the mammalian chromatin-remodeling complex [170]. Additional suppressors included xmas-2 (GANP), which regulates transcription by binding to RNA Pol II and scaffolds formation of the TREX-2 complex during mRNA export. Notably, mutations in the gene encoding GANP have been identified in patients with peripheral neuropathy and intellectual disability [171]. A final weak modifier was shaggy (sgg), the ortholog of human GSK3, a kinase that phosphorylates TDP-43 and is a target of interest in ALS clinical trials (Table 1, discussed above). Both hat-trick and xmas-2 LOF mutations markedly reduced TDP-43 expression by a post-transcriptional mechanism, which could have contributed to their beneficial effects. It was unclear if xmas-2 mutations preserved innervation via RNA export modulation or by a separate pathway, as mutations in other RNA export genes did not alter TDP-43 toxicity. Further follow-up testing of these targets in mammalian disease models is needed.
Berson et al. [172] subsequently performed a focused RNAi screen targeting 84 histone-modifying genes to investigate the effects of chromatin regulation on TDP-43-toxicity in the fly eye. 31 modifiers were identified, six of which modulated the methylation of histone H3 on lysine 4 (H3K4me3), a marker associated with active gene expression. Genes promoting methylation suppressed toxicity, while demethylation enhanced toxicity. However, the effects were not directly dependent on H3K4me3 levels but rather appeared to be mediated by regulation of TDP-43 functional interactions, including with the chromatin remodeling protein, Chd1 (human CHD2). TDP-43 interaction with Chd1 impaired the cellular stress response, notably the upregulation of heat shock proteins. TDP-43 overexpression in HEK cells induced the loss of CHD2 and decreased CHD2 expression was observed in FTD temporal cortex. Thus, the induction of CHD2 or other approaches for modulating chromatin dynamics may be a potential strategy to oppose TDP-43 disruption of the cellular stress response.
Using the Exelixis Collection of 15,500 insertional mutation strains (covering about half of the Drosophila genome), Kankel et al. [173] performed independent whole-genome screens for modifiers of the rough-eye phenotype induced by hTDP-43 M337V and hFUS R521C. 553 and 637 hits were identified for mutant TDP-43 and FUS toxicity, respectively, with 432 modifiers in common (most of which acted in the same manner, i.e., suppressor vs. enhancer), suggesting considerable pathogenic overlap. Nucleic acid-binding genes were the most prevalent class of modifiers (64 genes), including 11 mRNA processing factors involved in splicing and polyadenylation, and 10 RRM-containing RNA binding proteins, including the DBR1 RNA lariat debranching enzyme and ataxin-1, showing intriguing parallels with the yeast screens discussed above. Like ataxin-2, ataxin-1 intermediate-length expansions are also a genetic risk factor for ALS [174]. Secondary validation studies in c9orf72 and dTDP-43 flies corroborated many of the hits. dTDP-43 N493D (human N378D)-induced NMJ denervation was suppressed by LOF alleles for the essential splicing factor SF2 (SRSF1) and the transcription elongation factor lilli (AFF2/FMR2). A subset of suppressors also attenuated TDP-43 aggregation including the RNA export protein GLE1, which is mutated in fetal motor neuron disease and rare ALS kindreds [175, 176]. A cluster of hits in the phospholipase D pathway, broadly involved in cell signaling and membrane phospholipid biosynthesis, was tested further in mice based on the availability of targeting compounds and parallel interest from other neurodegenerative diseases. Phospholipase D1/D2 knockout in SOD1 mutant mice conferred a modest and transient improvement in motor dysfunction but did not extend survival.
Most recently, Azpurua et al. [177] carried out an shRNA-based screen for suppressors of age-dependent motor dysfunction induced by the expression of wild-type hTDP-43 in Drosophila motor neurons. Using negative geotaxis (the innate ability of the flies to climb upward on vertical surfaces) as a behavioral readout, they screened ~ 2700 genes. Knockdown of 32 transcripts significantly reduced motor impairment, including genes involved in chromatin remodeling, nucleocytoplasmic transport, and the mediator complex, a hub for transcriptional regulation [178]. Chromatin remodeling factors Chd1 and moira overlapped with a previous screen [172], although interestingly, Chd1 shRNA exerted opposite effects in motor neurons vs. glia. Overall, the chromatin-related hits suggested that silenced chromatin helps to reduce TDP-43 toxicity, which remains to be tested in mammalian cells. Other overlapping hits included the transcription elongation factor, Su(Tpl) (also known as Ell), also identified in another Drosophila screen [179], where it was demonstrated to promote the TDP-43-induced aberrant expression of small nucleolar RNAs. Nucleocytoplasmic transport-related hits included the nuclear import receptor msk/importin-7, which in Drosophila mediates the import of phospho-MAPK, and in mammalian cells has been shown to import and act as a chaperone for histone H1 and some ribosomal proteins, together with importin β (reviewed in [180, 181]). Three proteins in the mRNA export pathway were also identified—the orthologs of human UAP56, RBM15, and NXF1. Finally, the core spliceosomal factor SF2/SRSF1 was again identified and validated in follow-up assays to attenuate neuronal and glial TDP-43 toxicity.
Human Cell Lines
Only one screen thus far has probed genome-wide causes of TDP-43 disruption in human cells. In 2012, Kim et al. [182] performed an unbiased RNAi-based genome-wide screen in HeLa cells, combined with automated microscopy in fixed and immunostained cells, to search for modifiers of endogenous TDP-43 and FUS nucleocytoplasmic localization. Whereas FUS localization was surprisingly not affected by any RNAi targets, the knockdown of 60 genes increased TDP-43 cytoplasmic localization. As expected, the hits involved several proteins involved in nucleocytoplasmic transport including importin β (KPNB1), Nup54, NupL1, and the Ran GTPase. The ER-resident IP3 receptor ITPR1, with a critical role in cellular calcium signaling but no known role in nuclear transport, was also a strong hit. ITPR1 is highly expressed in the brain, and mutations cause spinocerebellar ataxia 15 (SCA15) [183]. Follow-up studies showed that ITPR1 knockdown induced cytoplasmic TDP-43 accumulation in primary rat cortical neurons and inactivating ITPR1 mutations improved the lifespan and climbing activity of Drosophila overexpressing hTDP-43 in motor neurons [182]. TDP-43 knockdown increased ITPR1 expression, and RNA immunoprecipitation demonstrated that TDP-43 binds to the 3’ proximal UG-repeat region in intron 4 of ITPR1, suggesting an autoregulatory loop between TDP-43, ITPR1, and calcium homeostasis. Consistent with these results, a recent study in Drosophila also reported a critical role for calcium, importin-α3, and calpain in regulating TDP-43 nuclear import [184].
High-Throughput Pharmacologic Screens for TDP-43-Modifying Compounds
Numerous high-throughput pharmacologic screens have searched for compounds targeting specific stages of TDP-43 proteinopathy, including TDP-43 aggregation, SG dynamics, TDP-43 cytoplasmic shuttling, and TDP-43 phosphorylation (Table 3). Several screens identified the same classes of compounds, including FDA-approved drugs, despite differing designs. These compounds, in particular, may be appropriate for further in vivo testing and clinical trial consideration. Toxicity may be a limiting factor for some approaches; nevertheless, these screens provide encouraging leads on classes of molecules that will be indispensable for further mechanistic studies and may be further optimized to improve specificity and tolerability.
Table 3.
High-throughput pharmacologic screens for modifiers of TDP-43 proteinopathy
| Screen assay | Analysis method |
#Hits/ #Screened |
Major categories of hits | Lead/validated compounds | Disease model validation | Ref |
|---|---|---|---|---|---|---|
| Spontaneous nuclear TDP-43 aggregates in sALS iPSC-derived motor neurons | Microscopy |
38/ 1757 |
CDK inhibitors JNK inhibitors Triptolide Cardiac glycosides |
Digoxin Lanatoside C Proscillardin A |
Confirmed efficacy in iPSC-derived cortical neurons | [185] |
| Arsenite-induced TDP-43-GFP aggregates in PC12 cells | Microscopy |
16/ 75,000 |
not discussed | LDN-0130436 | Reduced motor deficits in WT and mutant TDP-43-expressing C.elegans | [187] |
| Arsenite-induced SG formation in HEK293 cells and iPSC-derived motor neurons | Microscopy |
~100/ ~3000–6000 |
Cardiac glycosides Planar molecules that intercalate with nucleic acids |
Mitoxantrone Quinacrine Doxorubicin Daunorubicin |
Reduced TDP-43 SG recruitment and improved survival in mouse neurons expressing TDP43 M337V | [188] |
| MG132-induced TDP43-mKate2 cytoplasmic translocation in iPSC-derived motor neurons | Microscopy |
7/ 946 |
Cardiac glycosides Planar topoisomerase inhibitors STAT3 inhibitor |
Stattic niclosamide |
STAT3 inhibitors also suppressed arsenite-induced cytoplasmic foci | [199] |
| CK-1δ-dependent TDP-43 phosphorylation | Chemical optimization screen | N/A | N-(benzothiazolyl)-2-phenylacetamides | Multiple derivatives | Reduced pTDP-43 in HEK293 cells, prolonged survival in hTDP-43 flies | [203] |
| CDC7-dependent TDP-43 phosphorylation | Chemical optimization screen | N/A | benzylmercapto-purines | Multiple derivatives | Reduced pTDP-43 in C. elegans, improved motor function and pTDP-43 clearance in A315T mice | [205] |
| TDP-43 RRM1 domain | In silico |
1/ 50,000 |
N/A | rTDRD01 | Reduced motor deficits in A298S Drosophila | [206] |
| TDP-43 N-terminal domain | In silico |
1/ 50,000 |
N/A | nTRD22 | Promoted TDP-43-GFP clearance in rat neurons. Reduced motor deficits in WT hTDP-43 Drosophila | [207] |
hTDP-43 human TDP-43, pTDP-43 phospho-TDP-43, WT wild type, SG stress granule, iPSC induced pluripotent stem cell, N/A not applicable
aHuman orthologs in parentheses
Modulators of TDP-43 Aggregation
Burkhardt et al. [185] reprogrammed control and sALS patient-derived fibroblasts into iPSCs, differentiated them into motor neurons, and screened for compounds that suppress spontaneously occurring intranuclear TDP-43 aggregates, which were observed in three of 16 cell lines evaluated. Nuclear aggregates were speculated but not confirmed to be associated with nuclear TDP-43 LOF. Automated confocal microscopy in fixed and immunostained cells was applied to quantify nuclear TDP-43 aggregation. Among 1757 drugs (including an FDA-approved library), they identified four classes of compounds that reduced the frequency of TDP-43 nuclear aggregates: (1) cyclin-dependent kinase inhibitors, (2) c-Jun N-terminal kinase inhibitors, (3) Triptolide (a pleiotropic natural compound that has been of interest in cancer [186]), and (4) cardiac glycosides (digoxin, Lanatoside C and Proscillaridin A). Because glycosides inhibit the Na+/K+-ATPase, which consumes high ATP levels, it was proposed that increased intracellular ATP may oppose TDP-43 aggregation, although glycosides also modulate calcium signaling and other pathways.
Boyd et al. [187] utilized high content microscopy to identify suppressors of arsenite-induced TDP-43 aggregation. A rat neuronal PC12 cell line inducibly expressing TDP-43-GFP was treated with arsenite and a library of 75,000 compounds, including FDA-approved drugs. The screen identified 16 compounds that were highly active in reducing the predominantly nuclear TDP-43 aggregates induced by sodium arsenite, without inducing cell death. One of the compounds (LDN-0130436) also ameliorated locomotor deficits in C. elegans overexpressing WT and mutant hTDP-43. No follow-up studies of this compound have been published to date.
Stress Granule Modulation
Fang et al. [188] took a broader approach and screened for suppressors of arsenite-induced SG formation in HEK293 cells and iPSC-derived MNs, with a secondary analysis of TDP-43 SG recruitment. To enable SG visualization, CRISPR was used to introduce GFP into the endogenous locus of the core SG constituent, G3BP1. The screens, performed in libraries of ~ 3–6,000 compounds, identified 40 (HEK) and 27 (neuron) compounds that reduced total SG number, and another 50 compounds that altered SG size or density. Hits included a large group of cardiac glycosides that inhibit the Na + /K + -ATPase, including those identified by Burkhardt and colleagues to reduce nuclear TDP-43 puncta [185] (e.g., digoxin, digitoxin, ouabain, lanatoside C, proscillaridin A). Here, it was speculated that glycosides might act by modulating intracellular ion or solute concentrations, including ATP which has been shown to regulate RBP phase transitions [189]. Glycoside efficacy has been reported in other unrelated settings, including mutant SOD1 astrocyte-induced motor neuron death [190] and a polyglutamine-expanded androgen receptor model of spinal bulbar muscular atrophy [191]. Interestingly, digoxin was identified as potentially beneficial in a recent case–control analysis of prescription drugs that may slow ALS progression from the U.S. Medicare database [192]. Whether there is a unifying explanation for these observations remains unclear.
Additionally, Fang et al. [188] identified a group of ~ 20 planar molecules that interact or intercalate with nucleic acids, including mitoxantrone, quinacrine, doxorubicin, and daunorubicin. A subset of the planar compounds disrupted TDP-43 association with SGs and increased survival of mouse primary neurons expressing TDP-43 M337V. Remarkably, the planar molecules attenuated SG formation without altering other features of the integrated stress response, such as eIF2α phosphorylation and translational arrest, suggesting a direct effect on SG formation itself. Previous studies demonstrate the ability of selected planar molecules to alter LLPS of RNA [193, 194] or prion protein condensates [195], raising the possibility that such molecules may bind to essential RNA or protein sites that mediate SG nucleation. Because many are DNA or RNA intercalating drugs and inhibit topoisomerase activity, planar compounds such as doxorubicin and daunorubicin are primarily used as chemotherapeutic agents [196, 197]. Mitoxantrone was previously used as an immunosuppressive agent for advanced multiple sclerosis, but dosing was limited by significant risk of cardiotoxicity and secondary malignancy [198].
TDP-43 Cytoplasmic Shuttling
As with the genetic screens discussed above, there is a surprising lack of pharmacologic screens for modifiers of TDP-43 nucleocytoplasmic localization. A single 2021 study by Kato and Sakamoto [199] used human iPSC-derived motor neurons expressing TDP-43-mKate2 to screen for compounds suppressing TDP-43 cytoplasmic translocation induced by prolonged (three days) proteasome inhibition with MG132. In this microscopy-based screen of 946 compounds, seven compounds were identified from now-familiar categories, including Na + /K + -ATPase inhibitors (digoxin, ouabain), planar topoisomerase inhibitors (mitoxantrone, epirubicin, doxorubicin, daunorubicin), and a STAT3 inhibitor, Stattic. In follow-up experiments, both Stattic and niclosamide, another STAT3 inhibitor with a long history of use for parasitic infections [200], suppressed MG132- and arsenite-induced cytoplasmic TDP-43 mislocalization. Niclosamide also suppressed ROS generation, caspase activity, and increased neuronal branch points and was proposed to act by upregulating autophagy and mitophagy, including via the PINK1-parkin-ubiquitin pathway. Niclosamide was reported to have beneficial effects in mice overexpressing FUS [201], but no studies in in vivo models of TDP-43 proteinopathy have yet been reported.
Inhibition of Kinase-Dependent TDP-43 Phosphorylation
Two focused screens have been carried out to optimize the structure and selectivity of kinases known to phosphorylate TDP-43. TDP-43 contains 29 direct phosphorylation sites for casein kinase-1 (CK-1) [202]. Salado et al. applied a forward chemical genetics approach to develop inhibitors of the CK-1δ isoform [203], which has also been implicated in Alzheimer’s and Parkinson’s disease [204]. Screening a small heterocyclic compound library for inhibitors of CK-1δ activity in vitro, the compound MR-3.15 was identified and then extensively derivatized, yielding several N-(benzothiazolyl)-2-phenylacetamides as potent CK-1δ inhibitors. Further characterization of the most potent inhibitors showed that they acted through competitive inhibition of ATP and demonstrated their selectivity towards CK-1δ and ability to reduce TDP-43 phosphorylation in HEK293 cells. A subset of benzothiazole compounds modestly extended the lifespan of Drosophila expressing hTDP-43 in adult neurons.
Most recently, Rojas-Prats and colleagues sought to develop a more potent inhibitor of CDC-7 kinase, which directly phosphorylates TDP-43 [205]. They tested a curated library of bioactive compounds to identify benzylmercaptopurine as an optimal scaffold for a derivatization-based approach. Out of 39 derivatives tested, one was active in reducing TDP-43 phosphorylation in C. elegans, and another ameliorated behavioral defects and induced clearance of phospho-TDP-43 from the spinal cord of TDP-43 A315T mice.
TDP-43-Targeting Small Molecules
Two in silico computational screens have been carried out to identify small molecule binders of TDP-43. In the first approach, the Chembridge library (~ 50,000 compounds) was interrogated for molecules docking to the TDP-43 RRM1 domain, leading to the identification of compound rTDRD01 [206]. Subsequent tests showed that, while rTDRD01 bound to recombinant TDP-43 RRM, it did not prevent TDP-43 binding to (UG)6 RNA in vitro. rTDRD01 significantly reduced locomotor defects in the hTDP-43(A298S) Drosophila ALS model, by a mechanism that remains to be determined. In a second study, a similar approach was used to identify binders of the TDP-43 NTD, identifying nTRD22 [207]. Interestingly, although in vitro tests confirmed binding to the NTD, they also showed that nTRD22 blocked (UG)6 RNA binding to the adjacent RRM, indicating an allosteric effect. nTRD22 mitigated climbing defects in a wild-type hTDP-43 Drosophila model and promoted TDP-43-GFP clearance in rat primary neurons. It remains unclear if the NTD binding or allosteric block of TDP-43 -RNA binding underlines the beneficial in vivo effect of nTRD22, and how that mechanism disrupts TDP-43 toxicity in the flies.
Emerging Strategies from Preclinical Studies
Together, the above screens provide further support for existing drugs in clinical trials, such as autophagy-inducing agents and kinase inhibitors. In addition, they highlight key areas of TDP-43 biology that may lie upstream of aggregation and neurotoxicity, including RNA metabolism, chromatin remodeling, and SG dynamics. Here we will discuss preclinical studies aiming to target these and other upstream pathways, providing important leads for future therapy development.
Modulating LLPS and SG Dynamics
In addition to TDP-43, mutations have been identified in a growing number of hnRNPs and related RNA binding proteins (RBPs) in familial ALS and FTD, including FUS, Matrin-3, hnRNPA1, hnRNPA2/B1, EWS, TAF15, and TIA1, many of which contain prion-like IDRs capable of LLPS (reviewed in [208]). Intriguingly, this group of ALS/FTD-associated RNA binding proteins show considerable structural and functional overlap, frequently interact in RNP granules, and are all known constituents of SGs [126]. This has prompted intense investigation into the phase separation of RNA•RBP condensates and generated a keen interest in therapeutic modulation to prevent the aberrant transition of phase-separated condensates into insoluble aggregates in disease (recently reviewed [41, 209, 210]). Still, the feasibility of preventing excessive or maladaptive interactions while preserving functionally essential LLPS, particularly in patients, remains to be determined. Moreover, both SG-dependent and -independent pathways for TDP-43 cytoplasmic aggregation have been demonstrated [76, 79, 188, 211], leaving open the question of whether SG modulation alone will be sufficient to combat aggregate formation in disease. The efficacy of ataxin-2 reduction in TDP-43 yeast, Drosophila, and mice [129, 130] is encouraging in this regard.
LLPS Modulation
Following the initial identification of research tool compounds such as 1,6-hexanediol, an aliphatic alcohol that dissolves membraneless organelles and other phase-separated condensates in vitro [212–214], additional compounds have been found that modify TDP-43 LLPS behavior, including in living cells. Babinchak and colleagues sought to use the fluorescent molecule, 4,4’-dianilino-1,1’-binaphthyl-5,5’-disulfonic acid (bis-ANS), which binds hydrophobic regions of proteins, to monitor the behavior of the TDP-43 low complexity domain, and unexpectedly observed strong induction of LLPS [215]. Congo red, a similar dye used to detect amyloid deposition, showed similar TDP-43 LLPS-inducing behavior. In cells, bis-ANS was insufficient to induce de novo TDP-43 SG formation but promoted SG persistence following arsenite treatment. Such an effect is opposite to that desired in a therapeutic compound, but the result instructs the potential design of small molecules targeting the TDP-43 hydrophobic domains. RNA modulates TDP-43 LLPS in biochemical assays [216, 217] and living cells [76, 218], with sequence, length, and concentration-dependent effects that are increasingly well delineated [217]. Mann et al. tested the ability of a 34-nucleotide GU-rich “bait oligonucleotide” to alter light-induced (optogenetic) TDP-43 phase separation and aggregation and observed marked inhibition of TDP-43 aggregation and protection against neuronal cell death [76]. Unlike ASO therapies that bind target RNA transcripts and cause RNase-H-mediated degradation of a disease protein (SOD1, ATXN2, C9orf72), the bait oligonucleotide is an RNA motif that binds the target protein (clip34NT to TDP-43) to modulate LLPS and aggregation, rather than expression level. These studies provide conceptual support for protein- and RNA-based strategies to mitigate aberrant TDP-43 LLPS. Given the requirement of LLPS for essential TDP-43 functions, including the regulation of splicing [42] and translation [219], it is crucial that LLPS-modulating therapies do not abolish TDP-43 LLPS, but instead promote homeostatic LLPS and oppose the formation of insoluble aggregates.
Stress Granule Dynamics
In addition to ATXN2 ASOs, now in clinical trial (Table 1), a growing number of SG-modulating compounds have been identified. As discussed above, a recent HTS identified a class of planar molecules, including mitoxantrone, quinacrine, and daunorubicin, that inhibited arsenite-induced SG formation, attenuated TDP-43 recruitment to SGs, reduced TDP-43 cytoplasmic puncta in TDP-43 mutant iPSC-derived motor neurons, and protected against cell death in mouse primary neurons overexpressing TDP-43 M337V [188]. The proposed mechanism involves direct interaction of the planar compounds with SGs, antagonizing RNA•RBP interactions during SG assembly. Several other indirect methods to modulate stress granule formation are at various stages of investigation. The most well-known of these agents, including the integrated stress response inhibitor (ISRIB) [220] and the PERK inhibitor GSK2606414 [152], modulate the integrated stress response that drives SG formation. Parker and colleagues also demonstrated that bis (thiosemicarbazonato)-copper complexes, developed initially to facilitate tumor imaging, block paraquat-induced, TDP-43-containing SG formation in vitro and reduced C-terminal TDP-43 accumulation in SH-SY5Y cells, likely by inhibiting ERK phosphorylation [221].
Targeting TDP-43 Aggregation
Direct TDP-43 Binding Strategies
With advances in our structural understanding of TDP-43 functional domains (reviewed in [2]), multiple direct TDP-43-targeting molecules have been developed to mitigate aggregation and aberrant accumulation of TDP-43. These include small molecules targeting the RRM and N-terminal domains identified through in silico screening, including rTRD01 [206] and nTRD22 [207], both of which decreased RNA binding in vitro and were protective in Drosophila, albeit by mechanisms that remain to be clarified. Viral delivery of a single-chain antibody (VH7Vk9) targeting RRM1 was protective in TDP-43 mutant mice by disrupting TDP-43 binding to NF-κB and reducing neuroinflammation [222]. VH7Vk9 reduced overall TDP-43 levels and attenuated TDP-43 aggregation and mislocalization, although interestingly did not interfere with TDP-43-RNA binding. Single-chain variable fragment intrabodies (3B12A intrabodies) to misfolded TDP-43 were also developed and demonstrated to accelerate the proteasomal degradation of aggregated TDP-43 in vitro and in vivo in the embryonic brain following in utero electroporation [223]. TDP-43 IDR-targeting strategies have also shown anti-aggregation effects, including acridine derivatives (e.g., AIM4) [224] and a polyglutamine binding peptide (QBP1) [225].
NIR Chaperones and Other Disaggregases
In 2018, multiple groups reported the ability of beta-karyopherin nuclear import receptors (NIR), including importins and transportins, to prevent aberrant cytoplasmic LLPS of RNA binding protein import cargoes, including TDP-43, FUS, and others (reviewed by [226]). These findings demonstrate the powerful chaperone capacity of NIRs, in addition to their canonical function in promoting the transport of RBP cargoes across the NPC. Remarkably, NIRs could also reverse cargo aggregation once formed, acting as Ran-dependent disaggregases, and extracting RBPs from SGs where NIRs also reside [227, 228]. Variants of the yeast protein disaggregase Hsp104 were also recently identified by systematic screening and shown to rescue TDP-43 toxicity [229]. Compared to small molecule- or RNA-based therapeutics, the neuronal delivery of the protein or peptide-based therapies is more challenging. Still, these studies lay essential groundwork for future development efforts.
Preventing TDP-43 Mitochondrial Accumulation
Evidence in disease models and postmortem tissue suggests that cytoplasmic TDP-43 accumulates within mitochondria and perturbs multiple aspects of mitochondrial function [89, 230]. Competitive inhibition experiments using synthetic peptides (PM1 and PM3) fused with Tat protein transduction domains showed that intramitochondrial TDP-43 localization depends on three N-terminal mitochondrial import sequences [87]. When subcutaneously infused into hTDP-43 A315T mice, PM1 peptides crossed the blood–brain barrier, reduced neuronal loss, and improved motor function. Chronic administration of PM1 by subcutaneous mini osmotic pumps alleviated TDP-43 proteinopathy, neurodegeneration, and cognitive deficits in a mouse model of Alzheimer’s disease [231]. A recent study showed that TDP-43 induces the release of mitochondrial DNA to the cytoplasm, triggering the native immune response via the cGAS/STING-NFκB pro-inflammatory pathway which recognizes cytoplasmic ssDNA [88]. Sting knockout and treatment of TDP-43 A315T mice with the STING inhibitor H-151 ameliorated neuronal loss and improved motor function, confirming the efficacy of genetic and pharmacological suppression of cGAS/STING signaling in vivo.
Conclusion and Future Directions
ALS is a heterogeneous disorder that nearly universally converges on TDP-43 proteinopathy and has thus far proven irreversible. Biological and technical limitations of successful TDP-43 therapeutic targeting are many, as recently discussed [232]. These include the essential function of TDP-43 (precluding its downregulation), the far-reaching LOF and GOF consequences of TDP-43 mislocalization, and the still unknown initiating cause of the TDP-43 pathogenic cascade. There is an unprecedented number of ongoing clinical trials targeting TDP-43 proteinopathy in ALS, although most aim to accelerate the clearance of cytoplasmic TDP-43 aggregates by inducing autophagy or other downstream clearance pathways without altering upstream factors that will continue to propagate TDP-43 pathology. Predictions of success are further complicated by preclinical models that largely rely on overexpression of either wild-type or rare mutant forms of TDP-43 and may not adequately model the human disease.
Encouragingly, genetic screening efforts in yeast, C. elegans, and Drosophila, with confirmation in mammalian cells, have revealed additional and likely more immediate pathways modulating TDP-43 toxicity. Those pathways include the control of SG dynamics, RNA metabolism, chromatin remodeling, and nucleocytoplasmic transport, among others. The first of this newer generation of therapeutics, ATXN2 ASOs, are now in clinical trial. Pharmacologic HTS targeting TDP-43 aggregation and SG recruitment have converged on two classes of FDA-approved drugs (cardiac glycosides and planar topoisomerase inhibitors) that attenuate TDP-43 proteinopathy in cell culture models, although these drugs have not yet been tested in vivo and may have dose-limiting toxicity. Recent discoveries of TDP-43-targeting small molecules, antibodies, peptides, and chaperones foreshadow exciting opportunities for more direct TDP-43 targeting.
Numerous additional challenges and opportunities exist. TDP-43 is now well-established to play far-reaching roles in RNA metabolism, and RNA in turn critically modulates TDP-43 nuclear localization, LLPS, and aggregation. Genes involved in RNA metabolism, splicing, and transport have consistently been over-represented in genome-wide screens, indicating potential interactions along and perhaps even upstream of the TDP-43 pathogenic cascade. RNA dysregulation is therefore an important topic of continued investigation in the pathogenesis of ALS/FTD (recently reviewed [233, 234]), that also may provide inroads for therapy development (reviewed in [235]). For example, overexpression of the nonsense-mediated decay factor UPF1 was found to ameliorate TDP-43 GOF toxicity in primary neurons, by a mechanism that remains unknown [236]. Viral UPF1 delivery in a TDP-43 rat model also attenuated motor impairment [237]. Efforts are also underway to develop ASOs to prevent key TDP-43 LOF missplicing events such as STMN2 truncation [33] or UNC13A cryptic exon inclusion [59, 60], inspired by ASOs that correct missplicing events in neurodevelopmental disorders such as spinal muscular atrophy [238] and Duchenne muscular dystrophy [239]. Given TDP-43’s prolific role in splicing regulation across the transcriptome, it is unknown whether targeting individual missplicing events will be sufficient to mitigate neurotoxicity. Encouragingly, restoration of STMN2 expression potently rescued axonal degeneration in TDP-43-depleted iPSC-derived neurons [33]. Increasingly detailed insights into TDP-43•RNA interactions suggest multiple other potential targets for RNA-based intervention, including nucleocytoplasmic shuttling [52] and cytoplasmic aggregation [76, 210].
Finally, due to inherent difficulties of modeling TDP-43 proteinopathy in patient iPSC-derived neurons and the technical hurdles for high-throughput screening in this model, there are no published genome-wide screens of modifiers of TDP-43 proteinopathy in human iPSC-derived neurons to date. However, this is likely to change following recent CRISPR-based methodologic advances [166, 240, 241]. Such data will undoubtedly spur innovations and provide additional insight into upstream events and therapeutic targets at the apex of the ALS pathogenic cascade.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge the graphical expertise of Natalia Nedelsky. The Hayes laboratory is supported by the NIH (K08NS104273, R01NS123538, R03NS127011), the Robert Packard Center for ALS Research, and the Guy McKhann Scholar award.
Required Author Forms
34 provided by the authors are available with the online version of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prasad A, Bharathi V, Sivalingam V, Girdhar A, Patel BK. Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front Mol Neurosci. 2019;12:25. doi: 10.3389/fnmol.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.François-Moutal L, Perez-Miller S, Scott DD, Miranda VG, Mollasalehi N, Khanna M. Structural insights Into TDP-43 and effects of post-translational modifications. Front Mol Neurosci. 2019;12:1199. doi: 10.3389/fnmol.2019.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 4.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Bioph Res Co. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 5.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Velde CV, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 6.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 Mutations in Familial and Sporadic Amyotrophic Lateral Sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deerlin VMV, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borroni B, Bonvicini C, Alberici A, Buratti E, Agosti C, Archetti S, et al. Mutation within TARDBP leads to Frontotemporal Dementia without motor neuron disease. Hum Mutat. 2009;30:E974–E983. doi: 10.1002/humu.21100. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs GG, Murrell JR, Horvath S, Haraszti L, Majtenyi K, Molnar MJ, et al. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Movement Disord. 2009;24:1842–1847. doi: 10.1002/mds.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buratti E. Functional significance of TDP-43 mutations in disease. Adv Genet. 2015;91:1–53. doi: 10.1016/bs.adgen.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Broeck LV, Callaerts P, Dermaut B. TDP-43-mediated neurodegeneration: towards a loss-of-function hypothesis? Trends Mol Med. 2014;20:66–71. doi: 10.1016/j.molmed.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youmans KL, Wolozin B. TDP-43: a new player on the AD field? Exp Neurol. 2012;237:90–95. doi: 10.1016/j.expneurol.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai T, Mackenzie IRA, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 17.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thammisetty SS, Pedragosa J, Weng YC, Calon F, Planas A, Kriz J. Age-related deregulation of TDP-43 after stroke enhances NF-κB-mediated inflammation and neuronal damage. J Neuroinflammation. 2018;15:312. doi: 10.1186/s12974-018-1350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masaki K, Sonobe Y, Ghadge G, Pytel P, Lépine P, Pernin F, et al. RNA-binding protein altered expression and mislocalization in MS. Neurol Neuroimmunol Neuroinflammation. 2020;7. [DOI] [PMC free article] [PubMed]
- 20.de Boer EMJ, Orie VK, Williams T, Baker MR, Oliveira HMD, Polvikoski T, et al. TDP-43 proteinopathies: a new wave of neurodegenerative diseases. J Neurology Neurosurg Psychiatry. 2021;92:86–95. doi: 10.1136/jnnp-2020-322983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weihl CC, Temiz P, Miller SE, Watts G, Smith C, Forman M, et al. TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J Neurology Neurosurg Psychiatry. 2008;79:1186. doi: 10.1136/jnnp.2007.131334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salajegheh M, Pinkus JL, Taylor JP, Amato AA, Nazareno R, Baloh RH, et al. Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle Nerve. 2009;40:19–31. doi: 10.1002/mus.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Küsters B, van Hoeve BJA, Schelhaas HJ, ter Laak H, van Engelen BGM, Lammens M. TDP-43 accumulation is common in myopathies with rimmed vacuoles. Acta Neuropathol. 2009;117:209–211. doi: 10.1007/s00401-008-0471-2. [DOI] [PubMed] [Google Scholar]
- 24.Taylor JP. Multisystem proteinopathy Intersecting genetics in muscle, bone, and brain degeneration. Neurology. 2015;85:658–660. doi: 10.1212/WNL.0000000000001862. [DOI] [PubMed] [Google Scholar]
- 25.Apontes, P. Chapter 9 - New opportunities for treatment of neurodegenerative disease through the modulation of TDP-43, in TDP-43 and Neurodegeneration. (eds. V. Kumar & M.K. Jaiswal) p183-250 (Academic Press, 2022).
- 26.Buratti E. Targeting TDP-43 proteinopathy with drugs and drug-like small molecules. Brit J Pharmacol. 2021;178:1298–1315. doi: 10.1111/bph.15148. [DOI] [PubMed] [Google Scholar]
- 27.Palomo V, Tosat-Bitrian C, Nozal V, Nagaraj S, Martin-Requero A, Martinez A. TDP-43: a key therapeutic target beyond amyotrophic lateral sclerosis. Acs Chem Neurosci. 2019;10:1183–1196. doi: 10.1021/acschemneuro.9b00026. [DOI] [PubMed] [Google Scholar]
- 28.Ayala YM, Zago P, D’Ambrogio A, Xu YF, Petrucelli L, Buratti E, et al. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. [DOI] [PubMed] [Google Scholar]
- 29.Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14:459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling JP, Pletnikova O, Troncoso JC, Wong PC. TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science. 2015;349:650–655. doi: 10.1126/science.aab0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan Q, Yalamanchili HK, Park J, Maio AD, Lu HC, Wan YW, et al. Extensive cryptic splicing upon loss of RBM17 and TDP43 in neurodegeneration models. Hum Mol Genet. 2016;ddw337. [DOI] [PMC free article] [PubMed]
- 33.Melamed Z, López-Erauskin J, Baughn MW, Zhang O, Drenner K, Sun Y, et al. Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat Neurosci. 2019;22:180–190. doi: 10.1038/s41593-018-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rot G, Wang Z, Huppertz I, Modic M, Lenče T, Hallegger M, et al. High-resolution RNA maps suggest common principles of splicing and polyadenylation regulation by TDP-43. Cell Rep. 2017;19:1056–1067. doi: 10.1016/j.celrep.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam OH, Rozhkov NV, Shaw R, Kim D, Hubbard I, Fennessey S, et al. Postmortem cortex samples identify distinct molecular subtypes of ALS: retrotransposon activation, oxidative stress, and activated glia. Cell Rep. 2019;29:1164–1177.e5. doi: 10.1016/j.celrep.2019.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romano G, Klima R, Feiguin F. TDP-43 prevents retrotransposon activation in the Drosophila motor system through regulation of Dicer-2 activity. Bmc Biol. 2020;18:82. doi: 10.1186/s12915-020-00816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawahara Y, Mieda-Sato A. TDP-43 promotes microRNA biogenesis as a component of the Drosha and Dicer complexes. Proc National Acad Sci. 2012;109:3347–3352. doi: 10.1073/pnas.1112427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saldi TK, Ash PE, Wilson G, Gonzales P, Garrido-Lecca A, Roberts CM, et al. TDP-1, the Caenorhabditis elegans ortholog of TDP-43, limits the accumulation of double-stranded RNA. Embo J. 2014;33:2947–2966. doi: 10.15252/embj.201488740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birsa N, Bentham MP, Fratta P. Cytoplasmic functions of TDP-43 and FUS and their role in ALS. Semin Cell Dev Biol. 2019;99:193–201. doi: 10.1016/j.semcdb.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Afroz T, Hock EM, Ernst P, Foglieni C, Jambeau M, Gilhespy LAB, et al. Functional and dynamic polymerization of the ALS-linked protein TDP-43 antagonizes its pathologic aggregation. Nat Commun. 2017;8:45. doi: 10.1038/s41467-017-00062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portz B, Lee BL, Shorter J. FUS and TDP-43 phases in health and disease. Trends Biochem Sci. 2021;46:550–563. doi: 10.1016/j.tibs.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conicella AE, Dignon GL, Zerze GH, Schmidt HB, D’Ordine AM, Kim YC, et al. TDP-43 α-helical structure tunes liquid–liquid phase separation and function. Proc National Acad Sci. 2020;117:5883–5894. doi: 10.1073/pnas.1912055117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallegger M, Chakrabarti AM, Lee FCY, Lee BL, Amalietti AG, Odeh HM, et al. TDP-43 condensation properties specify its RNA-binding and regulatory repertoire. Cell. 2021;184:4680–4696.e22. doi: 10.1016/j.cell.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conicella AE, Zerze GH, Mittal J, Fawzi NL. ALS mutations disrupt phase separation mediated by α-helical structure in the TDP-43 low-complexity c-terminal domain. Structure. 2016;24:1537–1549. doi: 10.1016/j.str.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt HB, Barreau A, Rohatgi R. Phase separation-deficient TDP43 remains functional in splicing. Nat Commun. 2019;10:4890. doi: 10.1038/s41467-019-12740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buratti E. TDP-43 post-translational modifications in health and disease. Expert Opin Ther Tar. 2018;22:279–293. doi: 10.1080/14728222.2018.1439923. [DOI] [PubMed] [Google Scholar]
- 47.Tziortzouda P, Bosch LVD, Hirth F. Triad of TDP43 control in neurodegeneration: autoregulation, localization and aggregation. Nat Rev Neurosci. 2021;22:197–208. doi: 10.1038/s41583-021-00431-1. [DOI] [PubMed] [Google Scholar]
- 48.Nishimura AL, Zupunski V, Troakes C, Kathe C, Fratta P, Howell M, et al. Nuclear import impairment causes cytoplasmic trans-activation response DNA-binding protein accumulation and is associated with frontotemporal lobar degeneration. Brain. 2010;133:1763–1771. doi: 10.1093/brain/awq111. [DOI] [PubMed] [Google Scholar]
- 49.Archbold HC, Jackson KL, Arora A, Weskamp K, Tank EMH, Li X, et al. TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia. Sci Rep. 2018;8:4606. doi: 10.1038/s41598-018-22858-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinarbasi ES, Cağatay T, Fung HYJ, Li YC, Chook YM, Thomas PJ. Active nuclear import and passive nuclear export are the primary determinants of TDP-43 localization. Sci Rep. 2018;8:7083. doi: 10.1038/s41598-018-25008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ederle H, Funk C, Abou-Ajram C, Hutten S, Funk EBE, Kehlenbach RH, et al. Nuclear egress of TDP-43 and FUS occurs independently of Exportin-1/CRM1. Sci Rep. 2018;8:7084. doi: 10.1038/s41598-018-25007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan L, Zaepfel BL, Aksenova V, Dasso M, Rothstein JD, Kalab P, et al. Nuclear RNA binding regulates TDP-43 nuclear localization and passive nuclear export. Cell Rep. 2022;In press. [DOI] [PMC free article] [PubMed]
- 53.Davidson Y, Kelley T, Mackenzie IRA, Pickering-Brown S, Plessis DD, Neary D, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 54.Bodansky A, Kim JMH, Tempest L, Velagapudi A, Libby R, Ravits J. TDP-43 and ubiquitinated cytoplasmic aggregates in sporadic ALS are low frequency and widely distributed in the lower motor neuron columns independent of disease spread. Amyotroph Lateral Sc. 2010;11:321–327. doi: 10.3109/17482961003602363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berning BA, Walker AK. The pathobiology of TDP-43 C-terminal fragments in ALS and FTLD. Front Neurosci-switz. 2019;13:335. doi: 10.3389/fnins.2019.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vatsavayai SC, Yoon SJ, Gardner RC, Gendron TF, Vargas JNS, Trujillo A, et al. Timing and significance of pathological features in C9orf72 expansion-associated frontotemporal dementia. Brain. 2016;139:3202–3216. doi: 10.1093/brain/aww250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nana AL, Sidhu M, Gaus SE, Hwang JHL, Li L, Park Y, et al. Neurons selectively targeted in frontotemporal dementia reveal early stage TDP-43 pathobiology. Acta Neuropathol. 2019;137:27–46. doi: 10.1007/s00401-018-1942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeong YH, Ling JP, Lin SZ, Donde AN, Braunstein KE, Majounie E, et al. Tdp-43 cryptic exons are highly variable between cell types. Mol Neurodegener. 2017;12:13. doi: 10.1186/s13024-016-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma XR, Prudencio M, Koike Y, Vatsavayai SC, Kim G, Harbinski F, et al. TDP-43 represses cryptic exon inclusion in the FTD–ALS gene UNC13A. Nature. 2022;603:124–130. doi: 10.1038/s41586-022-04424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown AL, Wilkins OG, Keuss MJ, Hill SE, Zanovello M, Lee WC, et al. TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature. 2022;603:131–137. doi: 10.1038/s41586-022-04436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, et al. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci. 2013;110:E736–E745. doi: 10.1073/pnas.1222809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White MA, Kim E, Duffy A, Adalbert R, Phillips BU, Peters OM, et al. TDP-43 gains function due to perturbed autoregulation in a Tardbp knock-in mouse model of ALS-FTD. Nat Neurosci. 2018;21:552–563. doi: 10.1038/s41593-018-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu EY, Russ J, Cali CP, Phan JM, Amlie-Wolf A, Lee EB. Loss of Nuclear TDP-43 Is Associated with Decondensation of LINE Retrotransposons. Cell Rep. 2019;27:1409–1421.e6. doi: 10.1016/j.celrep.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang YT, Kuo PH, Chiang CH, Liang JR, Chen YR, Wang S, et al. The truncated C-terminal RNA recognition motif of TDP-43 protein plays a key role in forming proteinaceous aggregates*. J Biol Chem. 2013;288:9049–9057. doi: 10.1074/jbc.M112.438564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen AKH, Lin RYY, Hsieh EZJ, Tu PH, Chen RPY, Liao TY, et al. Induction of amyloid fibrils by the C-terminal fragments of TDP-43 in amyotrophic lateral sclerosis. J Am Chem Soc. 2010;132:1186–1187. doi: 10.1021/ja9066207. [DOI] [PubMed] [Google Scholar]
- 67.Chang Z, Deng J, Zhao W, Yang J. Amyloid-like aggregation and fibril core determination of TDP-43 C-terminal domain. Biochem Bioph Res Co. 2020;532:655–661. doi: 10.1016/j.bbrc.2020.08.096. [DOI] [PubMed] [Google Scholar]
- 68.Walker AK, Spiller KJ, Ge G, Zheng A, Xu Y, Zhou M, et al. Functional recovery in new mouse models of ALS/FTLD after clearance of pathological cytoplasmic TDP-43. Acta Neuropathol. 2015;130:643–660. doi: 10.1007/s00401-015-1460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo W, Chen Y, Zhou X, Kar A, Ray P, Chen X, et al. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nat Struct Mol Biol. 2011;18:822–830. doi: 10.1038/nsmb.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nonaka T, Kametani F, Arai T, Akiyama H, Hasegawa M. Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum Mol Genet. 2009;18:3353–3364. doi: 10.1093/hmg/ddp275. [DOI] [PubMed] [Google Scholar]
- 71.Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity*. J Biol Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ratti A, Gumina V, Lenzi P, Bossolasco P, Fulceri F, Volpe C, et al. Chronic stress induces formation of stress granules and pathological TDP-43 aggregates in human ALS fibroblasts and iPSC-motoneurons. Neurobiol Dis. 2020;145:105051. doi: 10.1016/j.nbd.2020.105051. [DOI] [PubMed] [Google Scholar]
- 73.Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, et al. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111:1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- 74.Babinchak WM, Haider R, Dumm BK, Sarkar P, Surewicz K, Choi JK, et al. The role of liquid–liquid phase separation in aggregation of the TDP-43 low-complexity domain. J Biol Chem. 2019;294:6306–6317. doi: 10.1074/jbc.RA118.007222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hans F, Glasebach H, Kahle PJ. Multiple distinct pathways lead to hyperubiquitylated insoluble TDP-43 protein independent of its translocation into stress granules. J Biol Chem. 2020;295:673–689. doi: 10.1074/jbc.RA119.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mann JR, Gleixner AM, Mauna JC, Gomes E, DeChellis-Marks MR, Needham PG, et al. RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43. Neuron. 2019;102:321–338.e8. doi: 10.1016/j.neuron.2019.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otte CG, Fortuna TR, Mann JR, Gleixner AM, Ramesh N, Pyles NJ, et al. Optogenetic TDP-43 nucleation induces persistent insoluble species and progressive motor dysfunction in vivo. Neurobiol Dis. 2020;146:105078. doi: 10.1016/j.nbd.2020.105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asakawa K, Handa H, Kawakami K. Optogenetic modulation of TDP-43 oligomerization accelerates ALS-related pathologies in the spinal motor neurons. Nat Commun. 2020;11:1004. doi: 10.1038/s41467-020-14815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gasset-Rosa F, Lu S, Yu H, Chen C, Melamed Z, Guo L, et al. Cytoplasmic TDP-43 de-mixing independent of stress granules drives inhibition of nuclear import, loss of nuclear TDP-43, and cell death. Neuron. 2019;102:339–357.e7. doi: 10.1016/j.neuron.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Porta S, Xu Y, Restrepo CR, Kwong LK, Zhang B, Brown HJ, et al. Patient-derived frontotemporal lobar degeneration brain extracts induce formation and spreading of TDP-43 pathology in vivo. Nat Commun. 2018;9:4220. doi: 10.1038/s41467-018-06548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y, Cohen TJ. Aggregation of the nucleic acid–binding protein TDP-43 occurs via distinct routes that are coordinated with stress granule formation. J Biol Chem. 2019;294:3696–3706. doi: 10.1074/jbc.RA118.006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corbet GA, Wheeler JR, Parker R, Weskamp K. TDP43 ribonucleoprotein granules: physiologic function to pathologic aggregates. RNA Biol. 2021;1–11. [DOI] [PMC free article] [PubMed]
- 83.Chou CC, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I, Liu F, et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci. 2018;21:228–239. doi: 10.1038/s41593-017-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hergesheimer RC, Chami AA, de Assis DR, Vourc’h P, Andres CR, Corcia P, et al. The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: a resolution in sight? Brain. 2019;142:1176–1194. doi: 10.1093/brain/awz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amlie-Wolf A, Ryvkin P, Tong R, Dragomir I, Suh E, Xu Y, et al. Transcriptomic changes due to cytoplasmic TDP-43 expression reveal dysregulation of histone transcripts and nuclear chromatin. PLoS ONE. 2015;10:e0141836. doi: 10.1371/journal.pone.0141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Altman T, Ionescu A, Ibraheem A, Priesmann D, Gradus-Pery T, Farberov L, et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat Commun. 2021;12:6914. doi: 10.1038/s41467-021-27221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang W, Wang L, Lu J, Siedlak SL, Fujioka H, Liang J, et al. The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat Med. 2016;22:869–878. doi: 10.1038/nm.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu CH, Davidson S, Harapas CR, Hilton JB, Mlodzianoski MJ, Laohamonthonkul P, et al. TDP-43 triggers mitochondrial DNA release via mPTP to activate cGAS/STING in ALS. Cell. 2020;183:636–649.e18. doi: 10.1016/j.cell.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucini CB, Braun RJ. Mitochondrion-Dependent Cell Death in TDP-43 Proteinopathies. Biomed. 2021;9:376. doi: 10.3390/biomedicines9040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miguel L, Frébourg T, Campion D, Lecourtois M. Both cytoplasmic and nuclear accumulations of the protein are neurotoxic in Drosophila models of TDP-43 proteinopathies. Neurobiol Dis. 2011;41:398–406. doi: 10.1016/j.nbd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 91.Cascella R, Capitini C, Fani G, Dobson CM, Cecchi C, Chiti F. Quantification of the relative contributions of loss-of-function and gain-of-function mechanisms in TAR DNA-binding protein 43 (TDP-43) proteinopathies*. J Biol Chem. 2016;291:19437–19448. doi: 10.1074/jbc.M116.737726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mihevc SP, Baralle M, Buratti E, Rogelj B. TDP-43 aggregation mirrors TDP-43 knockdown, affecting the expression levels of a common set of proteins. Sci Rep-UK. 2016;6:33996. doi: 10.1038/srep33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ayala YM, Conti LD, Avendaño-Vázquez SE, Dhir A, Romano M, D’Ambrogio A, et al. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011;30:277–288. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koyama A, Sugai A, Kato T, Ishihara T, Shiga A, Toyoshima Y, et al. Increased cytoplasmic TARDBP mRNA in affected spinal motor neurons in ALS caused by abnormal autoregulation of TDP-43. Nucleic Acids Res. 2016;44:5820–5836. doi: 10.1093/nar/gkw499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Fan H, Ying Z, Li B, Wang H, Wang G. Degradation of TDP-43 and its pathogenic form by autophagy and the ubiquitin-proteasome system. Neurosci Lett. 2010;469:112–116. doi: 10.1016/j.neulet.2009.11.055. [DOI] [PubMed] [Google Scholar]
- 96.Cascella R, Fani G, Capitini C, Rusmini P, Poletti A, Cecchi C, et al. Quantitative assessment of the degradation of aggregated TDP-43 mediated by the ubiquitin proteasome system and macroautophagy. Faseb J. 2017;31:5609–5624. doi: 10.1096/fj.201700292RR. [DOI] [PubMed] [Google Scholar]
- 97.Ormeño F, Hormazabal J, Moreno J, Riquelme F, Rios J, Criollo A, et al. Chaperone mediated autophagy degrades TDP-43 protein and is affected by TDP-43 aggregation. Front Mol Neurosci. 2020;13:19. doi: 10.3389/fnmol.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scotter EL, Vance C, Nishimura AL, Lee YB, Chen HJ, Urwin H, et al. Differential roles of the ubiquitin proteasome system and autophagy in the clearance of soluble and aggregated TDP-43 species. J Cell Sci. 2014;127:1263–1278. doi: 10.1242/jcs.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu G, Coyne AN, Pei F, Vaughan S, Chaung M, Zarnescu DC, et al. Endocytosis regulates TDP-43 toxicity and turnover. Nat Commun. 2017;8:2092. doi: 10.1038/s41467-017-02017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bose JK, Huang CC, Shen CKJ. Regulation of autophagy by neuropathological protein TDP-43*. J Biol Chem. 2011;286:44441–44448. doi: 10.1074/jbc.M111.237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen S, Zhang X, Song L, Le W. Autophagy dysregulation in amyotrophic lateral sclerosis. Brain Pathol. 2012;22:110–116. doi: 10.1111/j.1750-3639.2011.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee S, Jeon YM, Cha SJ, Kim S, Kwon Y, Jo M, et al. PTK2/FAK regulates UPS impairment via SQSTM1/p62 phosphorylation in TARDBP/TDP-43 proteinopathies. Autophagy. 2019;16:1–17. doi: 10.1080/15548627.2019.1686729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leibiger C, Deisel J, Aufschnaiter A, Ambros S, Tereshchenko M, Verheijen BM, et al. TDP-43 controls lysosomal pathways thereby determining its own clearance and cytotoxicity. Hum Mol Genet. 2018;27:1593–1607. doi: 10.1093/hmg/ddy066. [DOI] [PubMed] [Google Scholar]
- 104.Yin P, Bai D, Zhu L, Deng F, Guo X, Li B, et al. Cytoplasmic TDP-43 impairs the activity of the ubiquitin-proteasome system. Exp Neurol. 2021;345:113833. doi: 10.1016/j.expneurol.2021.113833. [DOI] [PubMed] [Google Scholar]
- 105.Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93:1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 106.Bové J, Martínez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 107.Wang IF, Guo BS, Liu YC, Wu CC, Yang CH, Tsai KJ, et al. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc National Acad Sci. 2012;109:15024–15029. doi: 10.1073/pnas.1206362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mislang A, Mollard R, Rico GT, Fairlie WD, Lee EF, Harris TJ, et al. A preliminary assessment of oral monepantel’s tolerability and pharmacokinetics in individuals with treatment-refractory solid tumors. Cancer Chemoth Pharm. 2020;86:589–594. doi: 10.1007/s00280-020-04146-5. [DOI] [PubMed] [Google Scholar]
- 109.Bedlack R, Stephens J, Barkhaus PE, Bereman M, Caress JB, Crayle J, et al. ALSUntangled 59: Tamoxifen. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22:1–4. doi: 10.1080/21678421.2021.1876731. [DOI] [PubMed] [Google Scholar]
- 110.Chen PC, Hsieh YC, Huang CC, Hu CJ. Tamoxifen for amyotrophic lateral sclerosis: A randomized double-blind clinical trial. Medicine. 2020;99:e20423. doi: 10.1097/MD.0000000000020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Babu S, Macklin EA, Jackson KE, Simpson E, Mahoney K, Yu H, et al. Selection design phase II trial of high dosages of tamoxifen and creatine in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;21:1–9. doi: 10.1080/21678421.2019.1672750. [DOI] [PubMed] [Google Scholar]
- 112.Imamura K, Izumi Y, Watanabe A, Tsukita K, Woltjen K, Yamamoto T, et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9:eaaf3962. doi: 10.1126/scitranslmed.aaf3962. [DOI] [PubMed] [Google Scholar]
- 113.Wenqiang C, Lonskaya I, Hebron ML, Ibrahim Z, Olszewski RT, Neale JH, et al. Parkin-mediated reduction of nuclear and soluble TDP-43 reverses behavioral decline in symptomatic mice. Hum Mol Genet. 2014;23:4960–4969. doi: 10.1093/hmg/ddu211. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y, Liu FT, Wang YX, Guan RY, Chen C, Li DK, et al. Autophagic modulation by trehalose reduces accumulation of TDP-43 in a cell model of amyotrophic lateral sclerosis via TFEB activation. Neurotox Res. 2018;34:109–120. doi: 10.1007/s12640-018-9865-7. [DOI] [PubMed] [Google Scholar]
- 115.Rusmini P, Cortese K, Crippa V, Cristofani R, Cicardi ME, Ferrari V, et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy. 2018;15:631–651. doi: 10.1080/15548627.2018.1535292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holler CJ, Taylor G, McEachin ZT, Deng Q, Watkins WJ, Hudson K, et al. Trehalose upregulates progranulin expression in human and mouse models of GRN haploinsufficiency: a novel therapeutic lead to treat frontotemporal dementia. Mol Neurodegener. 2016;11:46. doi: 10.1186/s13024-016-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Castillo K, Nassif M, Valenzuela V, Rojas F, Matus S, Mercado G, et al. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy. 2013;9:1308–1320. doi: 10.4161/auto.25188. [DOI] [PubMed] [Google Scholar]
- 118.Lee HJ, Yoon YS, Lee SJ. Mechanism of neuroprotection by trehalose: controversy surrounding autophagy induction. Cell Death Dis. 2018;9:712. doi: 10.1038/s41419-018-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee GC, Lin CH, Tao YC, Yang JM, Hsu KC, Huang YJ, et al. The potential of lactulose and melibiose, two novel trehalase-indigestible and autophagy-inducing disaccharides, for polyQ-mediated neurodegenerative disease treatment. Neurotoxicology. 2015;48:120–130. doi: 10.1016/j.neuro.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y, Wang H, Ying Z, Gao Q. Ibudilast enhances the clearance of SOD1 and TDP-43 aggregates through TFEB-mediated autophagy and lysosomal biogenesis: The new molecular mechanism of ibudilast and its implication for neuroprotective therapy. Biochem Bioph Res Co. 2020;526:231–238. doi: 10.1016/j.bbrc.2020.03.051. [DOI] [PubMed] [Google Scholar]
- 121.Babu S, Hightower BG, Chan J, Zürcher NR, Kivisäkk P, Tseng CEJ, et al. Ibudilast (MN-166) in amyotrophic lateral sclerosis- an open label, safety and pharmacodynamic trial. Neuroimage Clin. 2021;30:102672. doi: 10.1016/j.nicl.2021.102672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aulas A, Stabile S, Velde CV. Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP. Mol Neurodegener. 2012;7:1. doi: 10.1186/1750-1326-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McDonald KK, Aulas A, Destroismaisons L, Pickles S, Beleac E, Camu W, et al. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet. 2011;20:1400–1410. doi: 10.1093/hmg/ddr021. [DOI] [PubMed] [Google Scholar]
- 124.Khalfallah Y, Kuta R, Grasmuck C, Prat A, Durham HD, Velde CV. TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types. Sci Rep. 2018;8:7551. doi: 10.1038/s41598-018-25767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderweyde T, Vanderwyde T, Citro A, et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS ONE. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–498. doi: 10.1016/j.cell.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Protter DSW, Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26:668–679. doi: 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wolozin B, Ivanov P. Stress granules and neurodegeneration. Nat Rev Neurosci. 2019;20:649–666. doi: 10.1038/s41583-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Becker LA, Huang B, Bieri G, Ma R, Knowles DA, Jafar-Nejad P, et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature. 2017;544:367–371. doi: 10.1038/nature22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sproviero W, Shatunov A, Stahl D, Shoai M, van Rheenen W, Jones AR, et al. ATXN2 trinucleotide repeat length correlates with risk of ALS. Neurobiol Aging. 2017;51:178.e1–178.e9. doi: 10.1016/j.neurobiolaging.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eck RJ, Kraemer BC, Liachko NF. Regulation of TDP-43 phosphorylation in aging and disease. Geroscience. 2021;43:1605–1614. doi: 10.1007/s11357-021-00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martínez-González L, Gonzalo-Consuegra C, Gómez-Almería M, Porras G, de Lago E, Martín-Requero Á, et al. Tideglusib, a non-ATP competitive inhibitor of GSK-3β as a drug candidate for the treatment of amyotrophic lateral sclerosis. Int J Mol Sci. 2021;22:8975. doi: 10.3390/ijms22168975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang YM, Gupta SK, Kim KJ, Powers BE, Cerqueira A, Wainger BJ, et al. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell. 2013;12:713–726. doi: 10.1016/j.stem.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tolosa E, Litvan I, Höglinger GU, Burn D, Lees A, Andrés MV, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014;29:470–478. doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 136.Lovestone S, Boada M, Dubois B, Hüll M, Rinne JO, Huppertz HJ, et al. A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimer’s Dis. 2015;45:75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- 137.Crippa V, D’Agostino VG, Cristofani R, Rusmini P, Cicardi ME, Messi E, et al. Transcriptional induction of the heat shock protein B8 mediates the clearance of misfolded proteins responsible for motor neuron diseases. Sci Rep-UK. 2016;6:22827. doi: 10.1038/srep22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crippa V, Cicardi ME, Ramesh N, Seguin SJ, Ganassi M, Bigi I, et al. The chaperone HSPB8 reduces the accumulation of truncated TDP-43 species in cells and protects against TDP-43-mediated toxicity. Hum Mol Genet. 2016;25:3908–3924. doi: 10.1093/hmg/ddw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ganassi M, Mateju D, Bigi I, Mediani L, Poser I, Lee HO, et al. A surveillance function of the HSPB8-BAG3-HSP70 chaperone complex ensures stress granule integrity and dynamism. Mol Cell. 2016;63:796–810. doi: 10.1016/j.molcel.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 140.Mandrioli J, Crippa V, Cereda C, Bonetto V, Zucchi E, Gessani A, et al. Proteostasis and ALS: protocol for a phase II, randomised, double-blind, placebo-controlled, multicentre clinical trial for colchicine in ALS (Co-ALS) BMJ Open. 2019;9:e028486. doi: 10.1136/bmjopen-2018-028486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ben-Chetrit E, Bergmann S, Sood R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology. 2006;45:274–282. doi: 10.1093/rheumatology/kei140. [DOI] [PubMed] [Google Scholar]
- 142.Gasparyan AY, Ayvazyan L, Yessirkepov M, Kitas GD. Colchicine as an anti-inflammatory and cardioprotective agent. Expert Opin Drug Met. 2015;11:1781–1794. doi: 10.1517/17425255.2015.1076391. [DOI] [PubMed] [Google Scholar]
- 143.Swarup V, Phaneuf D, Dupré N, Petri S, Strong M, Kriz J, et al. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor κB–mediated pathogenic pathways. J Exp Med. 2011;208:2429–2447. doi: 10.1084/jem.20111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oh JH, Lee TJ, Park JW, Kwon TK. Withaferin A inhibits iNOS expression and nitric oxide production by Akt inactivation and down-regulating LPS-induced activity of NF-κB in RAW 264.7 cells. Eur J Pharmacol. 2008;599:11–17. doi: 10.1016/j.ejphar.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 145.Kumar S, Phaneuf D, Julien JP. Withaferin-A treatment alleviates TAR DNA-binding protein-43 pathology and improves cognitive function in a mouse model of FTLD. Neurotherapeutics. 2021;18:286–296. doi: 10.1007/s13311-020-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dutta K, Patel P, Rahimian R, Phaneuf D, Julien JP. Withania somnifera reverses transactive response DNA binding protein 43 proteinopathy in a mouse model of amyotrophic lateral sclerosis/frontotemporal lobar degeneration. Neurotherapeutics. 2017;14:447–462. doi: 10.1007/s13311-016-0499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 2018;132:159–169. doi: 10.1182/blood-2018-02-769026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, et al. Blood–spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci. 2014;111:E1035–E1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yen YT, Yang HR, Lo HC, Hsieh YC, Tsai SC, Hong CW, et al. Enhancing autophagy with activated protein C and rapamycin protects against sepsis-induced acute lung injury. Surgery. 2013;153:689–698. doi: 10.1016/j.surg.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 151.Shi Y, Hung ST, Rocha G, Lin S, Linares G, Staats KA, et al. Identification and therapeutic rescue of autophagosome and glutamate receptor defects in C9ORF72 and sporadic ALS neurons. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed]
- 152.Kim HJ, Raphael AR, LaDow ES, McGurk L, Weber R, Trojanowski JQ, et al. Therapeutic modulation of eIF2α-phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet. 2014;46:152–160. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moon SL, Sonenberg N, Parker R. Neuronal Regulation of eIF2α function in health and neurological disorders. Trends Mol Med. 2018;24:575–589. doi: 10.1016/j.molmed.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 154.Armakola M, Higgins MJ, Figley MD, Barmada SJ, Scarborough EA, Diaz Z, et al. Inhibition of RNA lariat debranching enzyme suppresses TDP-43 toxicity in ALS disease models. Nat Genet. 2012;44:1302–1309. doi: 10.1038/ng.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim JH, Rahman MH, Park D, Jo M, Kim HJ, Suk K. identification of genetic modifiers of TDP-43: inflammatory activation of astrocytes for neuroinflammation. Cells. 2021;10:676. doi: 10.3390/cells10030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liachko NF, McMillan PJ, Guthrie CR, Bird TD, Leverenz JB, Kraemer BC. CDC7 inhibition blocks pathological TDP-43 phosphorylation and neurodegeneration. Ann Neurol. 2013;74:39–52. doi: 10.1002/ana.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liachko NF, McMillan PJ, Strovas TJ, Loomis E, Greenup L, Murrell JR, et al. The tau tubulin kinases TTBK1/2 promote accumulation of pathological TDP-43. Plos Genet. 2014;10:e1004803. doi: 10.1371/journal.pgen.1004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taylor LM, McMillan PJ, Liachko NF, Strovas TJ, Ghetti B, Bird TD, et al. Pathological phosphorylation of tau and TDP-43 by TTBK1 and TTBK2 drives neurodegeneration. Mol Neurodegener. 2018;13:7. doi: 10.1186/s13024-018-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nozal V, Martínez-González L, Gomez-Almeria M, Gonzalo-Consuegra C, Santana P, Chaikuad A, et al. TDP-43 modulation by tau-tubulin kinase 1 inhibitors: a new avenue for future amyotrophic lateral sclerosis therapy. J Med Chem. 2022;65:1585–1607. doi: 10.1021/acs.jmedchem.1c01942. [DOI] [PubMed] [Google Scholar]
- 160.Vaca G, Martinez-Gonzalez L, Fernandez A, Rojas-Prats E, Porras G, Cuevas EP, et al. Therapeutic potential of novel Cell Division Cycle Kinase 7 inhibitors on TDP-43-related pathogenesis such as Frontotemporal Lobar Degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) J Neurochem. 2021;156:379–390. doi: 10.1111/jnc.15118. [DOI] [PubMed] [Google Scholar]
- 161.Liachko NF, Saxton AD, McMillan PJ, Strovas TJ, Keene CD, Bird TD, et al. Genome wide analysis reveals heparan sulfate epimerase modulates TDP-43 proteinopathy. Plos Genet. 2019;15:e1008526. doi: 10.1371/journal.pgen.1008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Meneghetti MCZ, Hughes AJ, Rudd TR, Nader HB, Powell AK, Yates EA, et al. Heparan sulfate and heparin interactions with proteins. J Roy Soc Interface. 2015;12:20150589. doi: 10.1098/rsif.2015.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schultheis N, Jiang M, Selleck SB. Putting the brakes on autophagy: The role of heparan sulfate modified proteins in the balance of anabolic and catabolic pathways and intracellular quality control. Matrix Biol. 2021;100:173–181. doi: 10.1016/j.matbio.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 164.Zhan L, Xie Q, Tibbetts RS. Opposing roles of p38 and JNK in a Drosophila model of TDP-43 proteinopathy reveal oxidative stress and innate immunity as pathogenic components of neurodegeneration. Hum Mol Genet. 2015;24:757–772. doi: 10.1093/hmg/ddu493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pichon CEL, Meilandt WJ, Dominguez S, Solanoy H, Lin H, Ngu H, et al. Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Sci Transl Med. 2017;9. [DOI] [PubMed]
- 166.Tian R, Gachechiladze MA, Ludwig CH, Laurie MT, Hong JY, Nathaniel D, et al. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. Neuron. 2019;104:239–255.e12. doi: 10.1016/j.neuron.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Siu M, Ghosh AS, Lewcock JW. Dual Leucine Zipper Kinase Inhibitors for the Treatment of Neurodegeneration. J Med Chem. 2018;61:8078–8087. doi: 10.1021/acs.jmedchem.8b00370. [DOI] [PubMed] [Google Scholar]
- 168.Katz JS, Rothstein JD, Cudkowicz ME, Genge A, Oskarsson B, Hains AB, et al. A Phase 1 study of GDC-0134, a dual leucine zipper kinase inhibitor, in ALS. Ann Clin Transl Neur. 2022;9:50–66. doi: 10.1002/acn3.51491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sreedharan J, Neukomm LJ, Brown RH, Freeman MR. Age-dependent TDP-43-mediated motor neuron degeneration requires GSK3, hat-trick, and xmas-2. Curr Biol. 2015;25:2130–2136. doi: 10.1016/j.cub.2015.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin C, Song W, Bi X, Zhao J, Huang Z, Li Z, et al. Recent advances in the ARID family: focusing on roles in human cancer. Oncotargets Ther. 2014;7:315–324. doi: 10.2147/OTT.S57023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Woldegebriel R, Kvist J, Andersson N, Õunap K, Reinson K, Wojcik MH, et al. Distinct effects on mRNA export factor GANP underlie neurological disease phenotypes and alter gene expression depending on intron content. Hum Mol Genet. 2020;29:1426–1439. doi: 10.1093/hmg/ddaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Berson A, Sartoris A, Nativio R, Deerlin VV, Toledo JB, Porta S, et al. TDP-43 Promotes Neurodegeneration by Impairing Chromatin Remodeling. Curr Biol. 2017;27:3579–3590.e6. doi: 10.1016/j.cub.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kankel MW, Sen A, Lu L, Theodorou M, Dimlich DN, McCampbell A, et al. Amyotrophic lateral sclerosis modifiers in Drosophila reveal the phospholipase D pathway as a potential therapeutic target. Genetics. 2020 doi: 10.1534/genetics.119.302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Conforti FL, Spataro R, Sproviero W, Mazzei R, Cavalcanti F, Condino F, et al. Ataxin-1 and ataxin-2 intermediate-length PolyQ expansions in amyotrophic lateral sclerosis. Neurology. 2012;79:2315–2320. doi: 10.1212/WNL.0b013e318278b618. [DOI] [PubMed] [Google Scholar]
- 175.Nousiainen HO, Kestilä M, Pakkasjärvi N, Honkala H, Kuure S, Tallila J, et al. Mutations in mRNA export mediator GLE1 result in a fetal motoneuron disease. Nat Genet. 2008;40:155–157. doi: 10.1038/ng.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kaneb HM, Folkmann AW, Belzil VV, Jao LE, Leblond CS, Girard SL, et al. Deleterious mutations in the essential mRNA metabolism factor, hGle1, in amyotrophic lateral sclerosis. Hum Mol Genet. 2015;24:1363–1373. doi: 10.1093/hmg/ddu545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Azpurua J, El-Karim EG, Tranquille M, Dubnau J. A behavioral screen for mediators of age-dependent TDP-43 neurodegeneration identifies SF2/SRSF1 among a group of potent suppressors in both neurons and glia. PLoS Genet. 2021;17:e1009882. doi: 10.1371/journal.pgen.1009882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Bio. 2015;16:155–166. doi: 10.1038/nrm3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chung CY, Berson A, Kennerdell JR, Sartoris A, Unger T, Porta S, et al. Aberrant activation of non-coding RNA targets of transcriptional elongation complexes contributes to TDP-43 toxicity. Nat Commun. 2018;9:4406. doi: 10.1038/s41467-018-06543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kimura M, Imamoto N. Biological significance of the importin-β family-dependent nucleocytoplasmic transport pathways. Traffic (Copenhagen, Denmark) 2014;15:727–748. doi: 10.1111/tra.12174. [DOI] [PubMed] [Google Scholar]
- 181.Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Mol Cell. 2004;16:319–330. doi: 10.1016/j.molcel.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 182.Kim SH, Zhan L, Hanson KA, Tibbetts RS. High-content RNAi screening identifies the Type 1 inositol triphosphate receptor as a modifier of TDP-43 localization and neurotoxicity. Hum Mol Genet. 2012;21:4845–4856. doi: 10.1093/hmg/dds321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. doi: 10.1016/S1474-4422(10)70183-6. [DOI] [PubMed] [Google Scholar]
- 184.Park JH, Chung CG, Park SS, Lee D, Kim KM, Jeong Y, et al. Cytosolic calcium regulates cytoplasmic accumulation of TDP-43 through Calpain-A and Importin α3. Elife. 2020;9:e60132. doi: 10.7554/eLife.60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Burkhardt MF, Martinez FJ, Wright S, Ramos C, Volfson D, Mason M, et al. A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells. Mol Cell Neurosci. 2013;56:355–364. doi: 10.1016/j.mcn.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Noel P, Hoff DDV, Saluja AK, Velagapudi M, Borazanci E, Han H. Triptolide and its derivatives as cancer therapies. Trends Pharmacol Sci. 2019;40:327–341. doi: 10.1016/j.tips.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 187.Boyd JD, Lee-Armandt JP, Feiler MS, Zaarur N, Liu M, Kraemer B, et al. A High-Content Screen Identifies Novel Compounds That Inhibit Stress-Induced TDP-43 Cellular Aggregation and Associated Cytotoxicity. J Biomol Screen. 2013;19:44–56. doi: 10.1177/1087057113501553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fang MY, Markmiller S, Vu AQ, Javaherian A, Dowdle WE, Jolivet P, et al. Small-molecule modulation of TDP-43 recruitment to stress granules prevents persistent TDP-43 accumulation in ALS/FTD. Neuron. 2019;103:802–819.e11. doi: 10.1016/j.neuron.2019.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patel A, Malinovska L, Saha S, Wang J, Alberti S, Krishnan Y, et al. ATP as a biological hydrotrope. Science. 2017;356:753–756. doi: 10.1126/science.aaf6846. [DOI] [PubMed] [Google Scholar]
- 190.Gallardo G, Barowski J, Ravits J, Siddique T, Lingrel JB, Robertson J, et al. An α2-Na/K ATPase/α-adducin complex in astrocytes triggers non–cell autonomous neurodegeneration. Nat Neurosci. 2014;17:1710–1719. doi: 10.1038/nn.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Piccioni F, Roman BR, Fischbeck KH, Taylor JP. A screen for drugs that protect against the cytotoxicity of polyglutamine-expanded androgen receptor. Hum Mol Genet. 2004;13:437–446. doi: 10.1093/hmg/ddh045. [DOI] [PubMed] [Google Scholar]
- 192.Pfeiffer RM, Mayer B, Kuncl RW, Check DP, Cahoon EK, Rivera DR, et al. Identifying potential targets for prevention and treatment of amyotrophic lateral sclerosis based on a screen of medicare prescription drugs. Amyotroph Lateral Scler Frontotemporal Degener. 2019;21:1–11. doi: 10.1080/21678421.2019.1682613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kapuscinski J, Darzynkiewicz Z. Relationship between the pharmacological activity of antitumor drugs Ametantrone and mitoxantrone (Novatrone) and their ability to condense nucleic acids. Proc Natl Acad Sci. 1986;83:6302–6306. doi: 10.1073/pnas.83.17.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jain A, Vale RD. RNA phase transitions in repeat expansion disorders. Nature. 2017;546:243. doi: 10.1038/nature22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Korth C, May BCH, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc National Acad Sci. 2001;98:9836–9841. doi: 10.1073/pnas.161274798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van der Zanden SY, Qiao X, Neefjes J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021;288:6095–6111. doi: 10.1111/febs.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gong Q, Zhou L, Xu S, Li X, Zou Y, Chen J. High doses of daunorubicin during induction therapy of newly diagnosed acute myeloid leukemia: a systematic review and meta-analysis of prospective clinical trials. PLoS ONE. 2015;10:e0125612. doi: 10.1371/journal.pone.0125612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cocco E, Marrosu MG. The current role of mitoxantrone in the treatment of multiple sclerosis. Expert Rev Neurother. 2014;14:607–616. doi: 10.1586/14737175.2014.915742. [DOI] [PubMed] [Google Scholar]
- 199.Kato Y, Sakamoto K. Niclosamide affects intracellular TDP-43 distribution in motor neurons, activates mitophagy, and attenuates morphological changes under stress. J Biosci Bioeng. 2021;132:640–650. doi: 10.1016/j.jbiosc.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 200.Chen W, Mook RA, Premont RT, Wang J. Niclosamide: Beyond an antihelminthic drug. Cell Signal. 2018;41:89–96. doi: 10.1016/j.cellsig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Milani M, Mammarella E, Rossi S, Miele C, Lattante S, Sabatelli M, et al. Targeting S100A4 with niclosamide attenuates inflammatory and profibrotic pathways in models of amyotrophic lateral sclerosis. J Neuroinflamm. 2021;18:132. doi: 10.1186/s12974-021-02184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kametani F, Nonaka T, Suzuki T, Arai T, Dohmae N, Akiyama H, et al. Identification of casein kinase-1 phosphorylation sites on TDP-43. Biochem Bioph Res Co. 2009;382:405–409. doi: 10.1016/j.bbrc.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 203.Salado IG, Redondo M, Bello ML, Perez C, Liachko NF, Kraemer BC, et al. Protein kinase CK-1 inhibitors as new potential drugs for amyotrophic lateral sclerosis. J Med Chem. 2014;57:2755–2772. doi: 10.1021/jm500065f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Perez DI, Gil C, Martinez A. Protein kinases CK1 and CK2 as new targets for neurodegenerative diseases. Med Res Rev. 2011;31:924–954. doi: 10.1002/med.20207. [DOI] [PubMed] [Google Scholar]
- 205.Rojas-Prats E, Martinez-Gonzalez L, Gonzalo-Consuegra C, Liachko NF, Perez C, Ramírez D, et al. Targeting nuclear protein TDP-43 by cell division cycle kinase 7 inhibitors: A new therapeutic approach for amyotrophic lateral sclerosis. Eur J Med Chem. 2021;210:112968. doi: 10.1016/j.ejmech.2020.112968. [DOI] [PubMed] [Google Scholar]
- 206.François-Moutal L, Felemban R, Scott DD, Sayegh MR, Miranda VG, Perez-Miller S, et al. Small molecule targeting TDP-43’s RNA recognition motifs reduces locomotor defects in a Drosophila model of amyotrophic lateral sclerosis (ALS) ACS Chem Biol. 2019;14:2006–2013. doi: 10.1021/acschembio.9b00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mollasalehi N, Francois-Moutal L, Scott DD, Tello JA, Williams H, Mahoney B, et al. An allosteric modulator of RNA binding targeting the N-terminal domain of TDP-43 yields neuroprotective properties. ACS Chem Biol. 2020;15:2854–2859. doi: 10.1021/acschembio.0c00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xue YC, Ng CS, Xiang P, Liu H, Zhang K, Mohamud Y, et al. Dysregulation of RNA-binding proteins in amyotrophic lateral sclerosis. Front Mol Neurosci. 2020;13:78. doi: 10.3389/fnmol.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Darling AL, Shorter J. Combating deleterious phase transitions in neurodegenerative disease. Biochimica Et Biophysica Acta Bba - Mol Cell Res. 2021;1868:118984. doi: 10.1016/j.bbamcr.2021.118984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mann JR, Donnelly CJ. RNA modulates physiological and neuropathological protein phase transitions. Neuron. 2021;109:2663–2681. doi: 10.1016/j.neuron.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fernandes N, Nero L, Lyons SM, Ivanov P, Mittelmeier TM, Bolger TA, et al. Stress granule assembly can facilitate but is not required for TDP-43 cytoplasmic aggregation. Biomol. 2020;10:1367. doi: 10.3390/biom10101367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ribbeck K, Görlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 2002;21:2664–2671. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shulga N, Goldfarb DS. Binding dynamics of structural nucleoporins govern nuclear pore complex permeability and may mediate channel gating. Mol Cell Biol. 2003;23:534–542. doi: 10.1128/MCB.23.2.534-542.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Alberti S, Gladfelter A, Mittag T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell. 2019;176:419–434. doi: 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Babinchak WM, Dumm BK, Venus S, Boyko S, Putnam AA, Jankowsky E, et al. Small molecules as potent biphasic modulators of protein liquid-liquid phase separation. Nat Commun. 2020;11:5574. doi: 10.1038/s41467-020-19211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.French RL, Grese ZR, Aligireddy H, Dhavale DD, Reeb AN, Kedia N, et al. Detection of TAR DNA-binding protein 43 (TDP-43) oligomers as initial intermediate species during aggregate formation. J Biol Chem. 2019;294:6696–6709. doi: 10.1074/jbc.RA118.005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Grese ZR, Bastos AC, Mamede LD, French RL, Miller TM, Ayala YM. Specific RNA interactions promote TDP-43 multivalent phase separation and maintain liquid properties. Embo Rep. 2021;22:e53632. doi: 10.15252/embr.202153632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Maharana S, Wang J, Papadopoulos DK, Richter D, Pozniakovsky A, Poser I, et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science. 2018;360:918–921. doi: 10.1126/science.aar7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gao J, Wang L, Ren X, Dunn JR, Peters A, Miyagi M, et al. Translational regulation in the brain by TDP-43 phase separation. J Cell Biol. 2021;220:e202101019. doi: 10.1083/jcb.202101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sidrauski C, McGeachy AM, Ingolia NT, Walter P. The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. Elife. 2015;4:e05033. doi: 10.7554/eLife.05033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Parker SJ, Meyerowitz J, James JL, Liddell JR, Nonaka T, Hasegawa M, et al. Inhibition of TDP-43 accumulation by bis(thiosemicarbazonato)-copper complexes. PLoS ONE. 2012;7:e42277. doi: 10.1371/journal.pone.0042277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pozzi S, Thammisetty SS, Codron P, Rahimian R, Plourde KV, Soucy G, et al. Virus-mediated delivery of antibody targeting TAR DNA-binding protein-43 mitigates associated neuropathology. J Clin Investig. 2019;129:1581–1595. doi: 10.1172/JCI123931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tamaki Y, Shodai A, Morimura T, Hikiami R, Minamiyama S, Ayaki T, et al. Elimination of TDP-43 inclusions linked to amyotrophic lateral sclerosis by a misfolding-specific intrabody with dual proteolytic signals. Sci Rep-uk. 2018;8:6030. doi: 10.1038/s41598-018-24463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Prasad A, Raju G, Sivalingam V, Girdhar A, Verma M, Vats A, et al. An acridine derivative, [4,5-bis{(N-carboxy methyl imidazolium)methyl}acridine] dibromide, shows anti-TDP-43 aggregation effect in ALS disease models. Sci Rep-uk. 2016;6:39490. doi: 10.1038/srep39490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mompeán M, de Mingo DR, Hervás R, del Carmen Fernández-Ramírez M, Carrión-Vázquez M, Laurents DV. Molecular mechanism of the inhibition of TDP-43 amyloidogenesis by QBP1. Arch Biochem Biophys. 2019;675:108113. doi: 10.1016/j.abb.2019.108113. [DOI] [PubMed] [Google Scholar]
- 226.Guo L, Fare CM, Shorter J. Therapeutic dissolution of aberrant phases by nuclear-import receptors. Trends Cell Biol. 2019;29:308–322. doi: 10.1016/j.tcb.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Guo L, Kim HJ, Wang H, Monaghan J, Freyermuth F, Sung JC, et al. Nuclear-import receptors reverse aberrant phase transitions of RNA-binding proteins with prion-like domains. Cell. 2018;173:677–692.e20. doi: 10.1016/j.cell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yoshizawa T, Ali R, Jiou J, Fung HYJ, Burke KA, Kim SJ, et al. Nuclear import receptor inhibits phase separation of FUS through binding to multiple sites. Cell. 2018;173:693–705.e22. doi: 10.1016/j.cell.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tariq A, Lin J, Jackrel ME, Hesketh CD, Carman PJ, Mack KL, et al. Mining disaggregase sequence space to safely counter TDP-43, FUS, and α-synuclein proteotoxicity. Cell Rep. 2019;28:2080–2095.e6. doi: 10.1016/j.celrep.2019.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lau DHW, Hartopp N, Welsh NJ, Mueller S, Glennon EB, Mórotz GM, et al. Disruption of ER−mitochondria signalling in fronto-temporal dementia and related amyotrophic lateral sclerosis. Cell Death Dis. 2018;9:327. doi: 10.1038/s41419-017-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gao J, Wang L, Gao C, Arakawa H, Perry G, Wang X. TDP-43 inhibitory peptide alleviates neurodegeneration and memory loss in an APP transgenic mouse model for Alzheimer’s disease. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2020;1866:165580. doi: 10.1016/j.bbadis.2019.165580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kim G, Gautier O, Tassoni-Tsuchida E, Ma XR, Gitler AD. ALS genetics: gains, losses, and implications for future therapies. Neuron. 2020;108:822–842. doi: 10.1016/j.neuron.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Butti Z, Patten SA. RNA Dysregulation in amyotrophic lateral sclerosis. Front Genet. 2019;9:712. doi: 10.3389/fgene.2018.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zaepfel BL, Rothstein JD. RNA is a double-edged sword in ALS pathogenesis. Front Cell Neurosci. 2021;15:708181. doi: 10.3389/fncel.2021.708181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nussbacher JK, Tabet R, Yeo GW, Lagier-Tourenne C. Disruption of RNA metabolism in neurological diseases and emerging therapeutic interventions. Neuron. 2019;102:294–320. doi: 10.1016/j.neuron.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Barmada SJ, Ju S, Arjun A, Batarse A, Archbold HC, Peisach D, et al. Amelioration of toxicity in neuronal models of amyotrophic lateral sclerosis by hUPF1. Proc Natl Acad Sci. 2015;112:7821–7826. doi: 10.1073/pnas.1509744112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jackson KL, Dayton RD, Orchard EA, Ju S, Ringe D, Petsko GA, et al. Preservation of forelimb function by UPF1 gene therapy in a rat model of TDP-43-induced motor paralysis. Gene Ther. 2015;22:20–28. doi: 10.1038/gt.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 239.Echevarría L, Aupy P, Goyenvalle A. Exon-skipping advances for Duchenne muscular dystrophy. Hum Mol Genet. 2018;27:R163–R172. doi: 10.1093/hmg/ddy171. [DOI] [PubMed] [Google Scholar]
- 240.Kampmann M. A CRISPR approach to neurodegenerative diseases. Trends Mol Med. 2017;23:483–485. doi: 10.1016/j.molmed.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kampmann M. CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chem Biol. 2018;13:406–416. doi: 10.1021/acschembio.7b00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.