Abstract
We evaluated the effect of repetitive trans-spinal magnetic stimulation (rTSMS) in patients with Parkinson’s disease (PD) in a randomised, single-blind study. Participants were hospitalised and administered a single trial of rTSMS or sham treatment 2 days a week for 4 weeks. In addition, all participants underwent rehabilitation 5 days a week for 4 weeks. The primary outcome was the difference between the two groups in the mean change from baseline to post-training in the total score on the Unified Parkinson’s Disease Rating Scale (UPDRS). Secondary endpoints included the differences between the two groups in the mean change on the UPDRS part III (motor) score and the Timed Up and Go (TUG) score. Eligible participants were randomly assigned to either the rTSMS group (n = 50) or sham group (n = 50). The between-group difference in mean change in the total UPDRS score was 10.28 (95% confidence interval (CI), 4.42 to 16.13; P = 0.014) immediately after intervention from baseline, 5.04 (95% CI, − 5.41 to 15.50; P = 0.024) 3 months after intervention from baseline and 2.38 (95% CI, 7.18 to 11.85; P = 0.045) 6 months after intervention from baseline. Significant differences between groups in UPDRS part III and TUG scores were maintained more strictly than those in the UPDRS total score. These results strongly indicate that rTSMS promotes the effect of rehabilitation on motor function in patients with PD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01213-y.
Keywords: Parkinson’s disease, rTSMS, Repetitive trans-spinal magnetic stimulation, SCS, Spinal cord stimulation, TUG, Timed up and Go, UPDRS
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder caused by the loss of dopaminergic neurons in the nigrostriatal system [1]. Various pharmacological approaches, of which l-dopa administration is the most effective, have been used to improve the motor symptoms by supplementing the striatal dopamine deficiency [2]. Despite its initial efficacy, long-term administration of l-dopa results in a decrease or fluctuation in the clinical response and causes several adverse effects [2]. Most patients with PD continue to experience a wide range of motor and non-motor symptoms [3, 4]. Patients in the late stages of PD have axial motor symptoms, such as gait dysfunction, freezing of gait (FOG) and postural instability [5–7]. Axial symptoms are largely resistant to dopamine replacement therapy [8–10] and frequently lead to falls and fall-related injuries [11]. Deep brain stimulation (DBS) of the subthalamic nucleus or globus pallidus pars interna is another treatment option. DBS is an accepted treatment modality for medically intractable symptoms of PD [10]. Although axial signs might initially respond to DBS, such improvements are usually not sustained over the long term [10, 12].
A subset of patients shows a severe postural abnormality called camptocormia that is a significant disability in daily life, and the estimated prevalence varies widely from 3 to 17.6% in patients with PD [5]. Camptocormia, usually resistant to antiparkinsonian drugs as well as other treatments, is usually progressive and causes significant disability in these patients, increasing the risk of fall and related injuries and reducing the quality of life. Spinal cord stimulation (SCS) has been used for several decades as a minimally invasive neuromodulation strategy for the treatment of patients with chronic pain [13]. SCS was recently shown to be particularly effective in patients with Parkinson’s disease related to pain and/or postural abnormalities [14–16]. In a previous study, we focused on repetitive trans-spinal magnetic stimulation (rTSMS), which demonstrated a significant reduction in lumbago through antinociceptive effects similar to SCS [17]. We reported short-term outcomes of rTSMS on camptocormia in patients with PD [18]. During the study period, we observed that rTSMS was also effective for improving the motor symptoms as well as postural abnormalities for a few days. In this study, we planned to investigate the long-term effects of rTSMS on motor symptoms in a randomised, single-blind, sham-controlled trial among patients with PD.
Patients and Methods
Patients
The study participants were recruited from patients with PD attending the Tokushima National Hospital. They were initially screened by a neurologist. Potentially eligible patients who met the initial inclusion criteria were referred to our research laboratory, where they were re-evaluated. Eligibility criteria included a clinical diagnosis of PD with a disease severity rating of stages 3–4 on the Hoehn and Yahr scale [5] (age 50–85 years), with stable medication (antiparkinsonian drugs that have not been changed in at least the last 6 months), medical clearance for participation and willingness to participate in the treatment and outcome testing. The patients fulfilled the established criteria for the diagnosis of Parkinson’s disease according to the Parkinson’s Disease Society of UK Brain Bank [19]. Patients with the following characteristics were excluded from the study: ongoing DBS therapy, implanted pacemakers, history of lumbar spine surgery and metal implants, current participation in any other behavioural or pharmacologic studies or presence of debilitating conditions that would hamper full participation. Additionally, patients who received additional antiparkinsonian drugs or switched therapy during the rTSMS/sham stimulation were considered as dropouts.
Randomisation and Masking
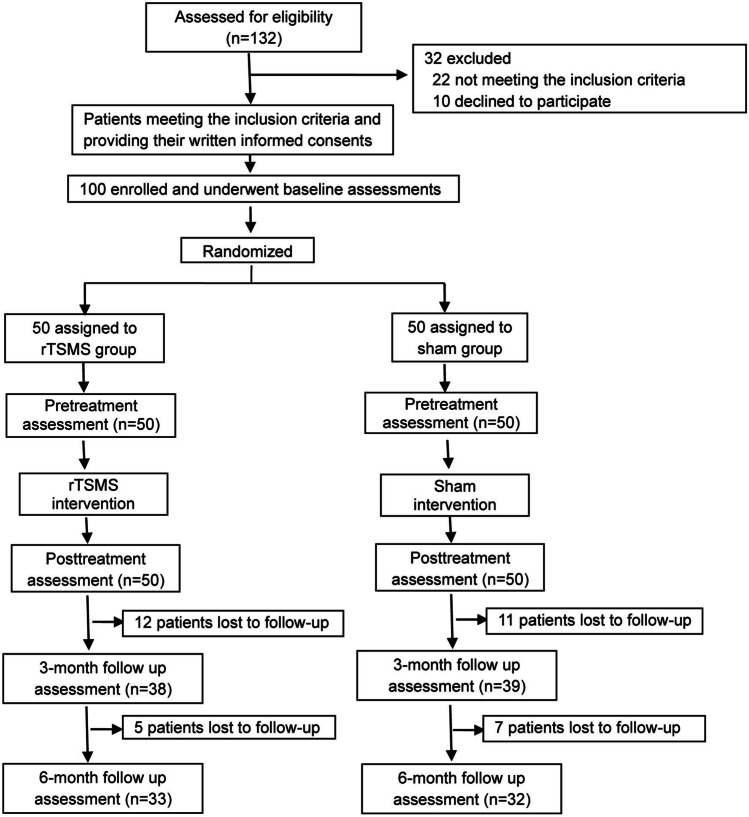
As shown in Fig. 1, we recruited 132 patients, and eligible participants (n = 100) were randomised to one of the two intervention groups in a ratio of 1:1 through a permuted block randomisation with implemented concealment of allocation. Randomisation was computer generated (Stata version 12) after obtaining informed consent and baseline assessments for 100 participants. The baseline data included age, sex and Hoehn and Yahr scale. This was followed by assessments for motor disability, including Unified Parkinson’s Disease Rating Scale (UPDRS) scores [20]. The randomisation sequence was generated by a team from the clinical trial centre, which was not associated with the present study. The randomisation schedule was maintained by a research assistant, who delivered it to the operator in a sealed envelope. The assessors who were not involved in the treatment program and were not informed about the patient groups transmitted the data with names of participants replaced by numbers to the data centre. The study investigators, patients, physiotherapists, occupational therapists, nurses and caregivers, but not the operator of the magnetic stimulator, were blinded to the group allocations. Blinding was strictly maintained by emphasising to the assessors the importance of minimising assessment bias and regular checking of the blinding status. Participants were instructed not to reveal their rehabilitation program to the study assessors throughout the study. In the data centre, the analysts worked with anonymised data. Treatment allocation remained masked until the database was locked.
Fig. 1.
Trial profile. rTSMS, repetitive trans-spinal magnetic stimulation
Procedures
We assigned patients to either the sham group or the rTSMS group as outlined schematically in Fig. 1. Magnetic stimulation was performed according to a previous report with minor modifications [18]. Briefly, we used a MagPro® (Medtronic Inc., USA) generator connected to a butterfly coil (MC-B70; MagVenture) or a placebo butterfly coil (MC-P-B70; MagVenture). The coil was centred on the skin over the spine. The target of magnetic stimulation was focused on Th12-L1, aiming at lumbar spinal cord enlargement, which is a promising target of SCS for treatment of PD [21]. The sham coil looks, acts and sounds like the active coil but prevents the magnetic field from reaching the target tissue through interception by a built-in shield plate. Subjects were seated with upper limbs relaxed, and rTSMS at 5 Hz was delivered in 10 trains of 1-s duration with 10-s inter-train intervals (50 total stimuli delivered). Patients were informed that rTSMS or sham stimulation would be applied to the centre of their lower back, but which treatment was actually applied was not disclosed. To minimise the inherent fluctuations in response, all participants received magnetic stimulation from 3:00 to 3:30 PM. In our previous study, we observed that magnetic stimulation had an effect on postural abnormalities for approximately 3 days. In addition, the patients appeared to have improved gait during 3 days. Hence, we set the magnetic or sham stimulation to twice a week (Monday and Thursday) for 4 weeks in this study.
Rehabilitation
Participants were hospitalised and received rehabilitation by a personal unit until they were discharged from the hospital 4 weeks later. Patients in the rTSMS and sham groups were scheduled to undergo a total of 75 h of rehabilitation during the 4-week hospital stay. All participants received a similar regimen involving the physical therapist, occupational therapist and speech-language-hearing therapist. The program included two categories of training: multidisciplinary rehabilitation consisting of conventional training of passive and active movements, as reported previously [22]. The schedule for rehabilitation had a fixed daily routine. Speech-language therapy, physical therapy and occupational therapy were provided from 9:00 AM to 10:00 AM, 1:00 PM to 2:00 PM and 2:00 PM to 3:00 PM, respectively.
Outcomes
The primary endpoint was the difference between the rTSMS and sham groups in the mean change in the total score on the UPDRS from baseline to 0 months, 3 months and 6 months after the last active/sham stimulation. The UPDRS includes subscales of mental function, activities of daily living and motor function; total scores on the scale range from 0 to 176, with higher scores indicating more severe disease [20]. Secondary endpoints were the difference between the rTSMS and sham groups in the mean change in the Timed Up and Go (TUG) score and UPDRS motor (part III) score from baseline to month 0, month 3 and month 6. The TUG test, specifically designed to measure functional mobility including the three anchors of the concept, i.e. gait, balance and postural transitions [23, 24], measures in seconds the time that a patient takes to stand up from a chair, walk 300 cm, make a 180° turn around a traffic cone and return to his or her original sitting position. Additional secondary endpoints included the difference in the mean change from baseline to post-intervention in gait speed, stride, the number of steps, the score of Simple Test for Evaluating Hand Function [25] (STEF, a simple evaluation method test for functional motor assessment of the upper arm, comprises 10 subtests, and the time required to complete each test is divided into 10 stages, scoring from 1 to 10 points), the score on flexion angle of the thoracolumbar spine in the standing position obtained by measuring the angle between the vertical plane and a line passing through the trochanter and the edge of the acromion, and the Frontal Assessment Battery (FAB) (a brief battery of six neuropsychological tasks designed to assess frontal lobe function at bedside; scores range from 0 to 18, with lower scores indicating greater frontal lobe dysfunction) [26]. For gait analysis, we used a sheet-type loading force sensor (MW-1000; Anima Inc., Tokyo, Japan). Patients walked for 10 m three times on a flat force plate, and the average walking speed, the stride length and the number of steps were measured. All motor functions, including UPDRS, TUG and gait, were assessed when the patients were in the ON state. Assessment at the time of post-intervention “0 M” was performed 4 days after the last active/sham stimulation.
Statistical Analysis
We used linear mixed model analysis based on multiple imputations to evaluate the robustness of the results and address the missing data. Linear mixed model analyses for repeated measures were performed for each of the outcome measures, with group and time entered as fixed effects. Post hoc analyses with the Bonferroni correction for multiple comparisons were performed when statistical significance was observed in the repeated measures analysis. Two-sided P values less than 0.05 were considered statistically significant. Analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 25. A sample size was calculated to detect a between-group difference of 12 in the primary outcome, UPDRS with a standard deviation of 16.3, a type I error of 5% and a power of 80% [22]. At least 43 patients were needed in each group to allow a dropout rate of 25%.
Monitoring of Adverse Events
Safety during the intervention phase was monitored by (i) recording of adverse events that occurred during stimulation, (ii) daily records by rehabilitation therapists and nurses and (iii) structured weekly screening by the intervention therapists for any adverse events (AEs) lasting longer than 48 h related to therapy. The study investigators monitored the safety information and decided to continue or discontinue the study depending on the severity of AEs. During the follow-up phase, all participants were instructed to report any AEs to our coordinator, such as falls, pain or neuropsychiatric symptoms. They were also questioned about the presence of any AEs at every visit.
Retention and Adherence
Retention was defined by (i) the proportion of participants who attended the first post-intervention assessment and (ii) the proportion of participants who completed all follow-up assessments compared to the number who completed baseline assessments. Adherence considered the consistency of participant attendance at the intervention sessions.
Data Availability
Appropriate anonymised data can be made available to qualified investigators on reasonable request. For access, data requestors will need to sign a data access agreement.
Standard Protocol Approvals, Registrations and Patient Consents
We conducted the study in accordance with the International Conference on Harmonisation Guidelines on Good Clinical Practice and the Declaration of Helsinki. The study was approved by the Tokushima National Hospital Ethical Review Board (No. 23–04), and all participants provided written informed consent prior to the initiation of the trial. This trial is registered with the UMIN Clinical Trials Registry (UMIN000014159).
Results
Patients
Potential study participants were recruited from June 2014 to December 2018. Individuals considered eligible after screening were provided informed consent forms followed by a baseline evaluation. Those who meet all the study criteria were informed about the research procedures. After completing baseline assessments and providing informed consent, 100 patients with PD were enrolled and randomised to the rTSMS or sham groups (Fig. 1). Table 1 presents the demographic and baseline clinical characteristics of the study population. The treatment groups were similar with respect to age (P = 0.15), disease duration (P = 0.06), levodopa equivalent daily dose (LEDD) (P = 0.63), UPDRS score (total UPDRS, P = 0.95; part 1, P = 0.344; part 2, P = 0.52; part 3, P = 0.17; part 4, P = 0.34), TUG score (P = 0.34), STEF scores (right, 0.050 and left, 0.60), flexion angle of the thoracolumbar spine (P = 0.17) and FAB (P = 0.054).
Table 1.
Baseline characteristics of two groups
| rTSMS group (n = 50) | Sham group (n = 50) | P value | |
|---|---|---|---|
| Age, years (SD) | 68.66 (8.69) | 70.98 (7.30) | 0.15 |
| Sex, male/female | 24/26 | 23/27 | |
| Disease duration (SD) | 7.68 (5.44) | 5.74 (5.05) | 0.06 |
| Modified Hoehn and Yahr Scale | |||
| Score 2 | 1 | 0 | |
| Score 2.5 | 0 | 1 | |
| Score 3 | 19 | 16 | |
| Score 3.5 | 19 | 24 | |
| Score 4 | 10 | 9 | |
| Score 4.5 | 1 | 0 | |
| Use of drugs | |||
| Levodopa | 45 | 46 | |
| Dopamine agonist | 27 | 25 | |
| COMT inhibitor | 9 | 4 | |
| MAO-β blockers | 13 | 13 | |
| Amantadine | 1 | 1 | |
| LEDD (SD) | 557.7 (166.8) | 532.0 (193.1) | 0.63 |
| UPDRS total (SD) | 74.94 (24.33) | 75.22 (23.23) | 0.95 |
| UPDRS I (0–16) (SD) | 5.72 (3.35) | 6.30 (2.68) | 0.34 |
| UPDRS II (0–52) (SD) | 21.18 (7.81) | 22.12 (6.81) | 0.52 |
| UPDRS III (0–108) (SD) | 46.24 (14.57) | 45.24 (15.03) | 0.73 |
| UPDRS IV (0–23) (SD) | 1.80 (2.32) | 1.56 (2.20) | 0.54 |
| TUG (SD) | 10.43 (4.29) | 11.36 (5.51) | 0.34 |
| STEF, right (SD) | 84.06 (19.02) | 83.88 (13.018) | 0.050 |
| STEF, left (SD) | 83.36 (20.82) | 85.28 (15.07) | 0.60 |
| Flexion angle of spine (SD) | 19.5 (12.1) | 23.2 (14.2) | 0.17 |
| FAB (SD) | 14.80 (2.25) | 13.63 (3.20) | 0.054 |
rTSMS repetitive trans-spinal magnetic stimulation, COMT catechol-O-methyltransferase, MAO monoamine oxidases, LEDD levodopa equivalent daily dose, UPDRS Unified Parkinson’s Disease Rating Scale, TUG Timed Up and Go test, STEF simple test for evaluating hand function, FAB Frontal Assessment Battery
Outcomes
The change in total UPDRS score, the primary endpoint, from baseline to post-training (0 M) was 21.78 ± 18.03 (mean ± standard deviation) in the rTSMS group and 11.50 ± 10.47 in the sham group (difference 10.28 points; 95% confidence interval (CI), 4.42 to 16.13; P = 0.014) (Table 2 and Fig. 2). The change in the scores from baseline to month 3 (3 M) was 23.48 ± 19.78 in the rTSMS group and 16.93 ± 42.23 in the sham group (difference 5.04 points; 95% CI, − 5.41 to 15.50; P = 0.024). The change in the scores from baseline to month 6 (6 M) was 15.30 ± 20.22 in the rTSMS group and 14.75 ± 45.49 in the sham group (difference 2.38 points; 95% CI, − 7.08 to 11.85; P = 0.045). For secondary endpoints, the change in the TUG score from baseline to post-training was 2.36 ± 2.26 in the rTSMS group and 0.10 ± 2.84 in the sham group (difference 2.26 points; 95% CI, 1.24 to 3.28; P = 0.001). The change in the scores from baseline to month 3 was 1.83 ± 1.88 in the rTSMS group and 1.043 ± 6.41 in the sham group (difference 2.88 points; 95% CI, 0.34 to 5.4; P = 0.001). The change in the scores from baseline to month 6 was 1.77 ± 2.07 in the rTSMS group and 0.17 ± 2.66 in the sham group (difference 1.60 points; 95% CI, 0.04 to 3.15; P = 0.028). The differences in the UPDRS part III score between the groups from baseline to post-training (9.02 points; 95% CI, 4.64 to 13.4), month 3 (5.60 points; 95% CI, − 1.30 to 12.50) and month 6 (6.93 points; 95% CI, − 0.89 to 14.76) were also significant (P = 0.016, 0.015 and 0.017, respectively) (Table 2). The difference between the groups in the mean change of gait speed and stride from baseline to post-training was significant (P = 0.014 and 0.014, respectively); however, the difference in the number of steps was not significant (Table S1). The difference between the groups in the mean change in STEF, the flexion angle of the spine or FAB score from baseline to post-training was not significant (Table S1).
Table 2.
Primary and secondary outcomes
| Outcome | rTSMS group* | Sham group* | Difference between the groups (95% CI) | P value |
|---|---|---|---|---|
| Primary endpoints | ||||
| Change from baseline to post-intervention in total UPDRS score | ||||
| Baseline | 74.94 (24.33) | 75.22 (23.23) | ||
| Post-intervention | 53.16 (21.43) | 63.72 (20.96) | ||
| Difference | 21.78 (18.03) | 11.50 (10.47) | 10.28 (4.42 to 16.13) | 0.014 |
| Change from baseline to month 3 in total UPDRS score | ||||
| Month 3 | 51.61 (21.34) | 56.81 (16.18) | ||
| Difference | 23.48 (19.78) | 16.93 (42.23) | 5.04 (− 5.41 to 15.50) | 0.024 |
| Change from baseline to month 6 in total UPDRS score | ||||
| Month 6 | 59.17 (20.95) | 61.18 (17.38) | ||
| Difference | 15.30 (20.22) | 14.75 (45.49) | 2.38 (− 7.08 to 11.85) | 0.045 |
| Secondary endpoints | ||||
| Change from baseline to post-intervention in TUG score | ||||
| Baseline | 10.43 (4.29) | 11.36 (5.51) | ||
| Post-intervention | 8.06 (2.86) | 11.26 (5.28) | ||
| Difference | 2.36 (2.26) | 0.10 (2.84) | 2.26 (1.24 to 3.28) | 0.001 |
| Change from baseline to month 3 in total TUG score | ||||
| Month 3 | 9.59 (5.35) | 12.12 (8.58) | ||
| Difference | 1.83 (1.88) | 1.043 (6.41) | 2.88 (0.34 to 5.40) | 0.001 |
| Change from baseline to month 6 in total TUG core | ||||
| Month 6 | 9.63 (3.95) | 10.70 (6.19) | ||
| Difference | 1.77 (2.07) | 0.17 (2.66) | 1.60 (0.04 to 3.15) | 0.028 |
| Change from baseline to post-intervention in UPDRS part III score | ||||
| Baseline | 46.24 (14.58) | 45.24 (15.01) | ||
| Post-intervention | 31.71 (13.73) | 39.1 (12.84) | ||
| Difference | 15.16 (14.02) | 6.14 (6.86) | 9.02 (4.64 to 13.40) | 0.016 |
| Change from baseline to month 3 in UPDRS part III score | ||||
| Month 3 | 30.56 (11.24) | 33.52 (8.11) | ||
| Difference | 13.93 (14.99) | 8.33 (10.28) | 5.60 (− 1.30 to 12.50) | 0.015 |
| Change from baseline to month 6 in UPDRS part III score | ||||
| Month 6 | 32.60 (13.77) | 34.90 (10.22) | ||
| Difference | 14.36 (16.60) | 7.43 (6.93) | 6.93 (− 0.89 to 14.76) | 0.017 |
rTSMS repetitive trans-spinal magnetic stimulation, UPDRS Unified Parkinson’s Disease Rating Scale, TUG Timed Up and Go test
*Data are represented as mean (standard deviation)
Fig. 2.
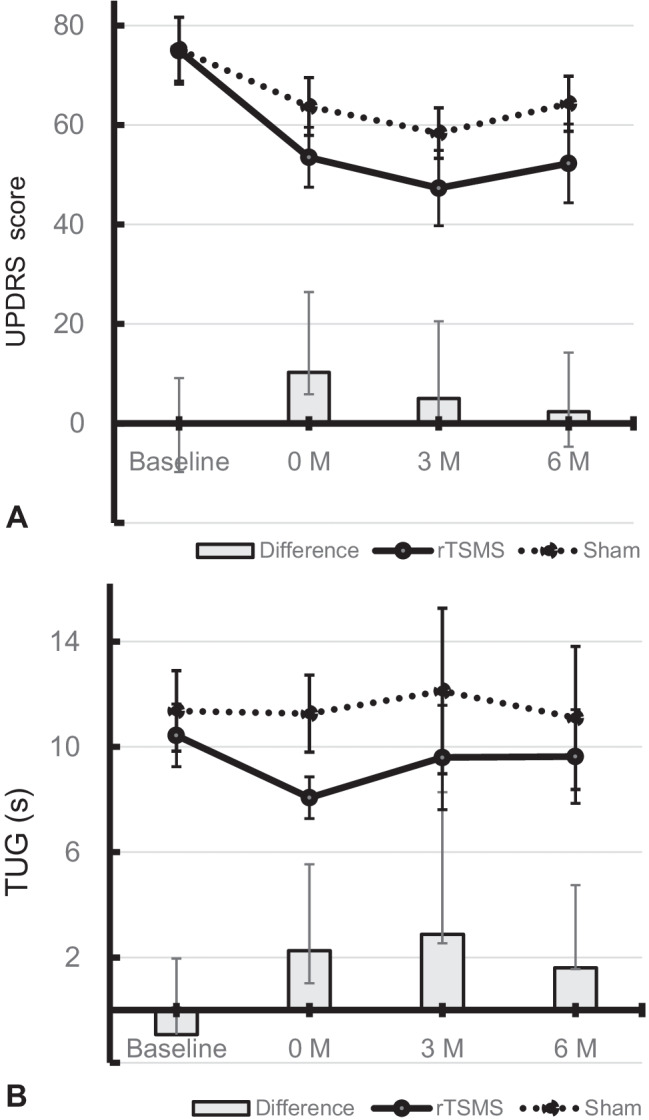
The transition of total scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) (A) and the Timed Up and Go (TUG) (B) during the entire study period. Columns represent the difference between groups. Vertical bars denote 95% confidence intervals
Safety Outcomes
AEs occurred during or as a direct result of exercise. Over the course of the study, 21 AEs were reported by 11 subjects (6, rTSMS group; 5, sham group). AEs were included: falls/injuries (n = 2; n = 5), back pain (n = 2; n = 3), arm pain (n = 2; n = 1), worsening of insomnia (n = 0; n = 1) and dizziness (n = 2: n = 1), respectively. All AEs were easily managed by short rest or symptom-specific drugs.
Retention, Attendance and Adherence
All patients received a total of eight sessions of magnetic or sham stimulation over a 4-week period, and we obtained the pre- and post-intervention assessment data for each patient. According to the rehabilitation management system used at our facility, the records of rehabilitation performed by speech-language therapists, physical therapists and occupational therapists revealed excellent compliance rates (98% in the rTSMS group and 99.3% in the sham group). Of the 100 subjects randomised, 66% (33/50) in the rTSMS group versus 64% (32/50) in the sham group completed the primary 6-month follow-up assessment. Seventeen subjects were lost to follow-up in the rTSMS group (2, medical unrelated reasons; 15, personal reasons) and 18 subjects in the sham group (1, unrelated medical reasons; 17, personal reasons). Antiparkinsonian medications were not changed for patients in both groups during the stimulation period. Three patients in the rTSMS group and five patients in the sham group had their antiparkinsonian medication changed or additional drugs added during the follow-up period.
Discussion
In the present study, rTSMS/sham stimulation was performed twice a week for 4 weeks in addition to daily multidisciplinary rehabilitation for PD patients. The intervention reduced the total UPDRS in the rTSMS group by about 21.8 points, compared with 11.5 points in the sham group. The improvement in the sham group seems to mostly reflect the effect of the rehabilitation, since our 4-week multidisciplinary rehabilitation improved the total UPDRS by 10.9 points [22]. The rTSMS group had significantly improved total UPDRS score and UPDRS part III score for 6 months after the intervention compared to the sham group. The change in total UPDRS score from baseline to post-intervention was 10.28 points between groups, which is assessed as a moderate clinical important difference [27]. However, the difference decreased during the follow-up period and was lost after 6 months. In contrast, the moderate clinically important difference in UPDRS part III was maintained throughout the 6-month period, indicating that rTSMS preferentially improved the motor function of PD. Since about half of the improvement in UPDRS scores was attributed to the effects of rehabilitation, it would be preferable to conclude that rTSMS significantly enhances the effect of rehabilitation on the motor function of PD.
During the previous study that examined the effect of rTSMS on posture in patients with PD complicated with camptocormia [18], we noticed that some patients with gait freezing walked well immediately after receiving rTSMS (Video Files 1 and 2). These patients were able to perform their daily activities with ease for a few days. In recent times, functional mobility (FM) has been emphasised as an indicator of the quality of daily life in PD. FM incorporates a person’s physiological ability to move independently and safely in various environments to accomplish functional activities or tasks and to participate in the activities of daily living at home, at work and in the community [23]. In this study, we adopted TUG, specifically designed to measure FM including gait, balance and postural transitions, as a secondary endpoint [23, 28, 29]. We found that a 4-week rTSMS protocol significantly decreased the TUG score and UPDRS part III score for 6 months. The decrease in stride length and walking speed, which are characteristic of gait impairment in PD [28], improved following rTSMS; however, the number of steps, STEF, flexion angle of the spine or FAB did not improve. We previously reported that rTSMS significantly reduced the thoracolumbar spine flexion angle in patients with PD and camptocormia [18]. In this study, camptocormia was observed only in 3 patients in the rTSMS group and 4 patients in the sham group, and the difference in flexion angle was not significant between the two groups. This suggests that rTSMS has no apparent effect on flexion angle in patients without abnormal bending posture.
SCS is one of the most common neuromodulation techniques for refractory neuropathic pain. This antinociceptive effect of electrical stimulation might result from inhibition of pain transmission by concomitant afferent inputs, termed gate control [30]. The therapeutic effects of SCS on PD have received much attention in recent years. The restoration of locomotor function in rodent models of PD has been validated in a number of studies since it was first reported in 2009 [31]. In those models, dopaminergic neuronal cell count [32], tissue dopamine level [33] and expression of tyrosine hydroxylase (TH) [34] in the striatum and substantia nigra increased in the SCS group compared to that in the control group, indicating that SCS promotes neurogenesis. Several studies have also shown the effectiveness of SCS in PD; SCS has shown therapeutic effects on parkinsonism [35], gait dysfunction [36–39] and abnormal postures [14, 16]. It should be noted that SCS is effective in treating gait abnormalities in patients who are at an advanced stage [36] or who have previously been treated with DBS [39], due to its unique ability to improve anticipatory postural adjustment and FOG [37, 38]. SCS stimulates the sensory ascending pathway and disrupts pathological neuronal hyper-oscillations in the basal ganglia via activation of the reticular formation in the brainstem in a manner that mimics the effects caused by pharmacological dopamine replacement therapy or deep brain stimulation [16, 30, 40].
While the SCS can stimulate the conduction pathways of the dorsal columns through the dura mater of the spinal cord [35], the conventional magnetic stimulator stimulates mainly the motor axons of the nerve roots at the level of the exit from the spinal column [41, 42]. However, rTSMS affects not only peripheral motor nerves, but also synaptic transmission at least at the level of the spinal cord. Lumbar repetitive magnetic stimulation reduces spastic tone as well as electrical stimulation [43] and may reduce corticocortical inputs onto corticospinal neurons and promotes a surround inhibition in the spinal cord and nerve axons [44]. So far, little is known about the effects of rTSMS on the ascending sensory pathway, but the fact that rTSMS is as effective as SCS in the treatment of neuropathic pain [45] suggests that rTSMS stimulates the ascending pathway in the spinal cord via the nerve roots. The mechanism of action of rTSMS is currently unknown, but by analogy to SCS, disruption of akinetic corticostriatal activity might be involved.
The present study has several limitations. First, we were not able to mask our intervention methods because we could not use true sham stimulation. Most participants feel a twitching sensation in the paraspinal muscles around the target site. Furthermore, they feel the contraction of the leg muscles caused by the excitation of the motor axons by rTSMS but not by sham stimulation. Although it was not explained to the patients that rTSMS produced twitching sensation, we could not completely rule out the possibility that the participants were aware of the assigned intervention. This means that a placebo effect cannot be ruled out, and we consider this study to be a single blind study. Second, patients who received rTSMS improved not only FM but also FOG, which is characteristic of PD; however, this study failed to focus on FOG. The most prominent effect of rTSMS on PD is the improvement in gait disturbance; hence, evaluation from various aspects of gait function was desirable. Third, no participants dropped out before or after the intervention period, but the dropout rate during the follow-up period (34–36%) was much higher than expected. This may have obscured the statistical difference between the two groups during the follow-up period. Fourth, this study was conducted at a single centre. This may limit the interpretation of the effect of rTSMS. Finally, the 10.28 points of intergroup differences obtained in this study were below the expected 12 points, suggesting that the study was overpowering or that the initial settings might not have been appropriate. This discrepancy may be related to the lack of similar clinical studies published and the characteristics of the controls set up in the present study. We performed sham stimulation in the control group and rTSMS in the test group 2 days a week and rehabilitation 5 days a week in both groups, so the marked rehabilitation effect may have reduced the differences between the groups. As already reported in some rotigotine studies [46, 47], the potential for suppression of intergroup differences should be considered when active effects other than placebo are expected in the control group.
Because of the preliminary nature of this study and its various limitations, the results cannot directly indicate safety and efficacy for clinical application. However, rTSMS is a simple, non-invasive, and safe intervention that might improve the motor symptoms, especially gait disturbance. Further vigorous studies are needed to prove whether rTSMS is an additional treatment option for PD.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 Video File 1 Gait before repetitive trans-spinal magnetic stimulation (rTSMS) in a patients with Parkinson’s disease complicated with marked freezing of gait. (MP4 105941 KB)
Supplementary file3 Video File 2 Gait after rTSMS at 5 Hz delivered in 10 trains of 1-s duration with 10-s inter-train intervals (50 total stimuli delivered). (MTS 56928 KB)
Acknowledgements
We would like to thank the patient who agreed to the video disclosure and all the patients who participated in this study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Abbreviations
- FAB
Frontal Assessment Battery
- FM
Functional mobility
- FOG
Freezing of gait
- PD
Parkinson’s disease
- rTSMS
Repetitive trans-spinal magnetic stimulation
- SCS
Spinal cord stimulation
- TUG
Timed Up and Go
- UPDRS
Unified Parkinson’s Disease Rating Scale
Author Contribution
T.M. supervised all the aspects of the study and wrote and edited the manuscript. T.M. and Y.A. designed the study and interpreted the data. K.T., S.T. and M.K. contributed to the recruitment of patients. Y.A. performed the magnetic stimulation. M.K and M.M. assessed the endpoints. N.S. obtained and aggregated the data. E.M. and Y.K. were involved in the randomisation and data analysis. All authors participated in the critical review of the report.
Funding
This study was supported by Japan Society for the Promotion of Society (JSPS) KAKENHI Grant Numbers 23500639 and 24500636.
Data Availability
Appropriate anonymised data can be made available to qualified investigators on reasonable request. For access, data requestors will need to sign a data access agreement.
Declarations
Ethics Approval and Consent to Participate
We conducted the study in accordance with the International Conference on Harmonisation Guidelines on Good Clinical Practice and the Declaration of Helsinki. The study was approved by the Tokushima National Hospital Ethical Review Board (No. 23–04), and all participants provided written informed consent prior to the initiation of the trial. This trial is registered with the UMIN Clinical Trials Registry (UMIN000014159).
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carlsson A. Biochemical and pharmacological aspects of Parkinsonism. Acta Neurol Scand Suppl. 1972;51:11–42. [PubMed] [Google Scholar]
- 2.Nagatsua T, Sawadab M. L-dopa therapy for Parkinson’s disease: past, present, and future. Parkinsonism Relat Disord. 2009;15(Suppl 1):S3–8. doi: 10.1016/S1353-8020(09)70004-5. [DOI] [PubMed] [Google Scholar]
- 3.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 4.Sprenger F, Poewe W. Management of motor and non-motor symptoms in Parkinson’s disease. CNS Drugs. 2013;27:259–272. doi: 10.1007/s40263-013-0053-2. [DOI] [PubMed] [Google Scholar]
- 5.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 6.Coelho M, Ferreira J, Rosa M, Sampaio C. Treatment options for non-motor symptoms in late-stage Parkinson’s disease. Expert Opin Pharmacother. 2008;9:523–535. doi: 10.1517/14656566.9.4.523. [DOI] [PubMed] [Google Scholar]
- 7.Fabbri M, Coelho M, Abreu D, Guedes LC, Rosa MM, Costa N, Antonini A, Ferreira JJ. Do patients with late-stage Parkinson’s disease still respond to levodopa? Parkinsonism Relat Disord. 2016;26:10–16. doi: 10.1016/j.parkreldis.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 9.Post B, Muslimovic D, van Geloven N, Speelman JD, Schmand B, de Haan RJ, CARPA-study group. Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson’s disease. Mov Disord. 2011;26:449–56. [DOI] [PubMed]
- 10.Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015;11:98–110. doi: 10.1038/nrneurol.2014.252. [DOI] [PubMed] [Google Scholar]
- 11.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 12.Ferraye MU, Debû B, Pollak P. Deep brain stimulation effect on freezing of gait. Mov Disord. 2008;3(S2):489–494. doi: 10.1002/mds.21975. [DOI] [PubMed] [Google Scholar]
- 13.Kumar K, Caraway DL, Rizvi S, Bishop S. Current challenges in spinal cord stimulation. Neuromodulation J Int Neuromodulation Soc. 2014;17(Suppl 1):22–35. doi: 10.1111/ner.12172. [DOI] [PubMed] [Google Scholar]
- 14.Agari T, Date I. Spinal cord stimulation for the treatment of abnormal posture and gait disorder in patients with Parkinson’s disease. Neurol Med Chir (Tokyo) 2012;52:470–474. doi: 10.2176/nmc.52.470. [DOI] [PubMed] [Google Scholar]
- 15.Nishioka K, Nakajima M. Beneficial therapeutic effects of spinal cord stimulation in advanced cases of Parkinson’s disease with intractable chronic pain: a case series. Neuromodulation. 2015;18:751–753. doi: 10.1111/ner.12315. [DOI] [PubMed] [Google Scholar]
- 16.De Andrade EM, Ghilardi MG, Cury RG, Barbosa ER, Fuentes R, Teixeira MJ, Fonoff ET. Spinal cord stimulation for Parkinson’s disease: a systematic review. Neurosurg Rev. 2016;39:27–35. doi: 10.1007/s10143-015-0651-1. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama H, Nukui S, Akamatu M, Hasegawa Y, Nishikido O, Inoue S. Effectiveness of spinal cord stimulation for painful camptocormia with Pisa syndrome in Parkinson’s disease: a case report. BMC Neurol. 2017;17:148. doi: 10.1186/s12883-017-0926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arii Y, Sawada Y, Kawamura K, Miyake S, Taichi Y, Izumi Y, Kuroda Y, Inui T, Kaji R, Mitsui T. Immediate effect of spinal magnetic stimulation on camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1221–1226. doi: 10.1136/jnnp-2014-307651. [DOI] [PubMed] [Google Scholar]
- 19.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahn S, Elton R. Members of the updrs development committee. unified parkinson’s disease rating scale. In: Fahn S, Marsden C, Calne D, Liberman A, editors. Recent developments in Parkinson’s disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–63.
- 21.Fonoff ET, de Lima-Pardini AC, Coelho DB, Monaco BA, Machado B, Pinto de Souza C, Dos Santos Ghilardi MG, Hamani C. Spinal cord stimulation for freezing of gait: from bench to bedside. Front Neurol. 2019;10:905. [DOI] [PMC free article] [PubMed]
- 22.Mitsui T, Arii Y, Tsukamoto A, Taniguchi K, Mabuchi M, Shimizu A, Sumitomo N, Maki YK. Sociability-based fitness approach in Parkinson’s disease: comparison with conventional rehabilitation. Eur J Neurol. 2021;28:1893–1900. doi: 10.1111/ene.14798. [DOI] [PubMed] [Google Scholar]
- 23.Bouça-Machado R, Duarte GS, Patriarca M, Castro Caldas A, Alarcão J, Fernandes RM, Mestre TA, Matias R, Ferreira JJ. Measurement instruments to assess functional mobility in Parkinson’s disease: a systematic review. Mov Disord Clin Pract. 2019;7:129–139. doi: 10.1002/mdc3.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloem BR, Marinus J, Almeida Q, Dibble L, Nieuwboer A, Post B, Ruzicka E, Goetz C, Stebbins G, Martinez-Martin P, Schrag A, Movement Disorders Society Rating Scales Committee. Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: critique and recommendations. Mov Disord. 2016;31:1342–55. [DOI] [PubMed]
- 25.Kaneko T, Muraki T. Development and standardization of hand function test. Bull Allied Med Sci (Kobe) 1990;6:49–54. [Google Scholar]
- 26.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 27.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010;67:64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 28.Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, Hass CJ, Hausdorff JM, Pelosin E, Almeida QJ. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019;18:697–708. doi: 10.1016/S1474-4422(19)30044-4. [DOI] [PubMed] [Google Scholar]
- 29.Verheyden G, Kampshoff CS, Burnett ME, Cashell J, Martinelli L, Nicholas A, Stack EL, Ashburn A. Psychometric properties of 3 functional mobility tests for people with Parkinson disease. Phys Ther. 2014;94:230–239. doi: 10.2522/ptj.20130141. [DOI] [PubMed] [Google Scholar]
- 30.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 31.Fuentes R, Petersson P, Siesser WB, et al. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science. 2009;323:1578–1582. doi: 10.1126/science.1164901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav AP, Fuentes R, Zhang H, Vinholo T, Wang CH, Freire MA, Nicolelis MA. Chronic spinal cord electrical stimulation protects against 6-hydroxydopamine lesions. Sci Rep. 2014;4:3839. doi: 10.1038/srep03839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charles KA, Naudet F, Bouali-Benazzouz R, Landry M, De Deurwaerdère P, Fossat P, Benazzouz A. Alteration of nociceptive integration in the spinal cord of a rat model of Parkinson’s disease. Mov Disord. 2018;33:1010–1015. doi: 10.1002/mds.27377. [DOI] [PubMed] [Google Scholar]
- 34.Shinko A, Agari T, Kameda M, Yasuhara T, Kondo A, Tayra JT, Sato K, Sasaki T, Sasada S, Takeuchi H, Wakamori T, Borlongan CV, Date I. Spinal cord stimulation exerts neuroprotective effects against experimental Parkinson’s disease. PLoS One. 2014;9:e101468. [DOI] [PMC free article] [PubMed]
- 35.Yadav AP, Nicolelis MAL. Electrical stimulation of the dorsal columns of the spinal cord for Parkinson’s disease. Mov Disord. 2017;32:820–832. doi: 10.1002/mds.27033. [DOI] [PubMed] [Google Scholar]
- 36.Samotus O, Parrent A, Jog M. Spinal cord stimulation therapy for gait dysfunction in advanced Parkinson’s disease patients. Mov Disord. 2018;33:783–792. doi: 10.1002/mds.27299. [DOI] [PubMed] [Google Scholar]
- 37.de Lima-Pardini AC, Coelho DB, Souza CP, Souza CO, Ghilardi MGDS, Garcia T, Voos M, Milosevic M, Hamani C, Teixeira LA, Fonoff ET. Effects of spinal cord stimulation on postural control in Parkinson’s disease patients with freezing of gait. Elife. 2018;7:e37727. [DOI] [PMC free article] [PubMed]
- 38.de Souza CP, Dos Santos MGG, Hamani C, Fonoff ET. Spinal cord stimulation for gait dysfunction in Parkinson’s disease: essential questions to discuss. Mov Disord. 2018;33:1828–1829. doi: 10.1002/mds.27508. [DOI] [PubMed] [Google Scholar]
- 39.Pinto de Souza C, Hamani C, Oliveira Souza C, Lopez Contreras WO, Dos Santos Ghilardi MG, Cury RG, Reis Barbosa E, Jacobsen Teixeira M, Talamoni Fonoff E. Spinal cord stimulation improves gait in patients with Parkinson’s disease previously treated with deep brain stimulation. Mov Disord 2017;32:278–82. [DOI] [PubMed]
- 40.Santana MB, Halje P, Simplício H, Richter U, Freire MAM, Petersson P, Fuentes R, Nicolelis MAL. Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson disease. Neuron. 2014;84:716–722. doi: 10.1016/j.neuron.2014.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015;126:1071–107. [DOI] [PMC free article] [PubMed]
- 42.Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Magnetic stimulation over the spinal enlargements. J Neurol Neurosurg Psychiatry. 1989;52:1025–1032. doi: 10.1136/jnnp.52.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krause P, Edrich T, Straube A. Lumbar repetitive magnetic stimulation reduces spastic tone increase of the lower limbs. Spinal Cord. 2004;42:67–72. doi: 10.1038/sj.sc.3101564. [DOI] [PubMed] [Google Scholar]
- 44.Murray LM, Islam MA, Knikou M. Cortical and subcortical contributions to neuroplasticity after repetitive transspinal stimulation in humans. Neural Plast. 2019;2019:4750768. doi: 10.1155/2019/4750768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo YL, Fook-Chong S, Huerto AP, George JM. A randomized, placebo-controlled trial of repetitive spinal magnetic stimulation in lumbosacral spondylotic pain. Pain Med. 2011;12:1041–1045. doi: 10.1111/j.1526-4637.2011.01143.x. [DOI] [PubMed] [Google Scholar]
- 46.Mizuno Y, Nomoto M, Hasegawa K, Hattori N, Kondo T, Murata M, Takeuchi M, Takahashi M, Tomida T; Rotigotine Trial Group. Rotigotine vs ropinirole in advanced stage Parkinson’s disease: a double-blind study. Parkinsonism Relat Disord. 2014;20:1388–93. [DOI] [PubMed]
- 47.Ferrazzoli D, Ortelli P, Riboldazzi G, Maestri R, Frazzitta G. Effectiveness of rotigotine plus intensive and goal-based rehabilitation versus rotigotine alone in “de-novo” parkinsonian subjects: a randomized controlled trial with 18-month follow-up. J Neurol. 2018;265:906–916. doi: 10.1007/s00415-018-8792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file2 Video File 1 Gait before repetitive trans-spinal magnetic stimulation (rTSMS) in a patients with Parkinson’s disease complicated with marked freezing of gait. (MP4 105941 KB)
Supplementary file3 Video File 2 Gait after rTSMS at 5 Hz delivered in 10 trains of 1-s duration with 10-s inter-train intervals (50 total stimuli delivered). (MTS 56928 KB)
Data Availability Statement
Appropriate anonymised data can be made available to qualified investigators on reasonable request. For access, data requestors will need to sign a data access agreement.
Appropriate anonymised data can be made available to qualified investigators on reasonable request. For access, data requestors will need to sign a data access agreement.