Abstract
Deep brain stimulation has been extensively studied as a therapeutic option for treatment-resistant depression (TRD). DBS across different targets is associated with on average 60% response rates in previously refractory chronically depressed patients. However, response rates vary greatly between patients and between studies and often require extensive trial-and-error optimizations of stimulation parameters. Emerging evidence from tractography imaging suggests that targeting combinations of white matter tracts, rather than specific grey matter regions, is necessary for meaningful antidepressant response to DBS. In this article, we review efficacy of various DBS targets for TRD, which networks are involved in their therapeutic effects, and how we can use this information to improve targeting and programing of DBS for individual patients. We will also highlight how to integrate these DBS network findings into developing adaptive stimulation and optimal trial designs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01270-3.
Keywords: Deep brain stimulation, Depression, Treatment-resistant depression, Neurostimulation, Neuromodulation
Introduction
Major depressive disorder (MDD) is one of the most severe and incapacitating psychiatric disorders related to significant occupational, social, and physical impairments. Depression is the leading cause of disability worldwide, affecting more than 300 million people globally, with lifetime prevalence rates of 20.6% in the USA [1, 2]. While there are many treatments currently available for MDD, it is estimated that 10 to 30% of patients are refractory to standard interventions [3, 4]. Novel strategies for treatment refractory depression (TRD) have mostly focused on non-serotonergic drug targets and various focal brain stimulation techniques. In patients who have not responded to any of the available antidepressant treatments including electroconvulsive therapy, deep brain stimulation (DBS) is currently offered only in the context of experimental trials.
The initial exploration of DBS as a possible therapy for patients with TRD was founded on the success of DBS in movement disorders and advanced understanding of the neural circuitry involved in depression. The symptom variability inherent in depressive disorder and putative circuit abnormalities underlying these symptoms are reflected by the variety of targets being considered for DBS in TRD. Selected targets are mainly related to circuits underlying the two primary diagnostic criteria for MDD: negative affect and anhedonia. Targets related to circuits underlying negative affect include the subcallosal cingulate cortex (SCC) and the lateral habenula (LHb). Targets related to anhedonia and reward pathways include the ventral capsule and ventral striatum (vALIC and VC/VS), the medial forebrain bundle (MFB), and the inferior thalamic peduncle (ITP). This review aims to summarize the current efficacy of these DBS targets in TRD with a special focus on their underlying therapeutic networks.
Methods
An electronic search through June 2022 was completed using the following keywords and associated MeSH terms: “deep brain stimulation,” “DBS,” “depression,” “treatment resistant depression,” and “TRD.” Studies included clinically applied chronic deep brain stimulation in patients with TRD. Due to the limited literature available, case reports, open-label studies, and randomized clinical trials were included in this review.
For illustration of the anatomical target locations and network connections, we generated figures depicting the activated target region and white matter tracts on MRI brain slices. White matter activation profile and DBS target was displayed in ICBM 2009b Nlin asymmetric space (0.5 × 0.5 × 0.5 mm3 resolution). Based on the previously reported anatomical location, a 2.5 mm radius ball was generated in the DBS target location [5–9]. Normative group connectome diffusion tractography data from Lead DBS software (www.lead-dbs.org) was then used to generate connectivity profiles from each target [10–12].
Results
After the initial search was screened for non-relevant and duplicate papers, 38 studies including a total of 405 patients were reviewed. The most common target was the SCC (N = 216), followed by the vALIC or VC/VS (N = 85), the MFB (N = 54), the LHb (N = 11), and the ITP (N = 1). In cases when multiple studies are reported using the same cohort, totals are calculated using the study with the largest cohort and longest follow-up. A summary of data collected is presented in Table 1. Foroverview of all reviewed DBS targets see Fig. 1.
Table 1.
Reviewed antidepressant DBS trials
| Target |
Parent study Follow-up |
Year | Study type | Participants | Follow-up (months) | Response rate | Remission rate | Primary measure |
|---|---|---|---|---|---|---|---|---|
| SCC | ||||||||
| Mayberg et al | 2005 | Open label | N = 5 | 6 | 66.0% | 33.0% | HDRS | |
| Lozano et al | 2008 | Open label | N = 20 | 12 | 55.0% | 35.0% | HDRS | |
| Kennedy et al | 2011 | Open label | N = 20 | 42 | 60.0% | 40.0% | HDRS | |
| Aibar-Duran et al | 2021 | Open label | N = 17 | 60 | 58.9% | 35.5% | HDRS | |
| Puigdemont et al | 2015 | Double blind | N = 5 | 6 | N/A | N/A | HDRS | |
| Lozano et al | 2012 | Open label | N = 21 | 12 | 29.0% | N/A | HDRS | |
| Holtzheimer et al | 2012 | Single blind | N = 17 | 24 | 58.0% | 11.0% | HDRS | |
| Holtzheimer et al | 2017 | Double blind | N = 90 | 24 | 49.0% | 26.0% | MADRS | |
| Riva-Posse et al | 2018 | Open label | N = 11 | 12 | 81.8% | 54.5% | HDRS | |
| Eitan et al | 2018 | Double blind | N = 9 | 12 | N/A | N/A | MADRS | |
| Ramasubbu et al | 2017 | Double blind | N = 4 | 9 | 50.0% | N/A | HDRS | |
| Ramasubbu et al | 2020 | Double blind | N = 22 | 12 | 50.0% | 27.0% | HDRS | |
| Conroy et al | 2021 | Double blind | N = 5 | 36 | 20% | 20% | HDRS | |
| Total* | N = 216 | 51.1% | 31.5% | |||||
| LHb | ||||||||
| Sartorius et al | 2010 | Case study | N = 1 | 14 | 100.0% | 100.0% | HDRS | |
| Keining et al | 2013 | Open label | N = 2 | N/A | 100.0% | 100.0% | HDRS | |
| Wang et al | 2021 | Open label | N = 1 | 3 | 100.0% | 0% | HDRS | |
| Zhang et al | 2022 | Open label | N = 7 | 1–12 | 42.9% | 14.3% | HDRS | |
| Total* | N = 11 | 85.7% | 53.6% | |||||
| VC/VS | ||||||||
| Malone et al | 2009 | Open label | N = 15 | 6 | 53.3% | 40.0% | HDRS | |
| Bewernick et al | 2009 | Open label | N = 10 | 12 | 48.6% | 50.0% | HDRS | |
| Bewernick et al | 2012 | Open label | N = 11 | 24 | 48.4% | 45.5% | HDRS | |
| Milett et al | 2014 | Open label | N = 4 | 14 | 75.0% | 25.0% | HDRS | |
| Dougherty et al | 2015 | Double blind | N = 30 | 24 | 23.3% | 23.3% | MADRS | |
| Bergfeld et al | 2016 | Double blind | N = 25 | 13 | 40.0% | 20.0% | HDRS | |
| Van der Wal et al | 2020 | Open label | N = 18 | 24 | 44.4% | 27.8% | HDRS | |
| Total* | N = 85 | 48.0% | 30.8% | |||||
| MFB | ||||||||
| Schlaepfer et al | 2013 | Open label | N = 7 | 3 | 85.7% | 57.1% | MADRS | |
| Bewernick et al | 2017 | Open label | N = 8 | 12 | 75.0% | 50.0% | MADRS | |
| Bewernick et al | 2018 | Open label | N = 21 | 60 | 72.7% | 72.7% | MADRS | |
| Fenoy et al | 2016 | Single blind | N = 6 | 1 week | 50.0% | N/A | MADRS | |
| Fenoy et al | 2018 | Single blind | N = 6 | 12 | 66.0% | 50.0% | MADRS | |
| Sani et al | 2017 | Case study | N = 1 | 6 | 100.0% | 100.0% | CAT-DI | |
| Coenen et al | 2019 | Double blind | N = 16 | 12 | 100.0% | 62.5% | MADRS | |
| Davidson et al | 2020 | Case study | N = 2 | 6 | 0% | 0% | HDRS | |
| Total* | N = 53 | 68.7% | 56.0% | |||||
| ITP | ||||||||
| Jiminez et al | 2005 | Case study | N = 1 | 24 | 100.0% | 100.0% | HDRS | |
| Comparison | ||||||||
| MFB v. VC/VS | Blomstedt et al | 2017 | Case study | N = 1 | 36 | N/A | N/A | HDRS |
| VC/VS | Raymaekers et al | 2017 | Double blind | N = 7 | > 5 | 85.7% | 71.4% | HDRS |
| vs. ITP | N = 7 | > 3 | 57.1% | 14.3% | ||||
*In cases when multiple studies are reported using the same cohort, averages are calculated using the study with the largest cohort and longest follow-up
Fig. 1.
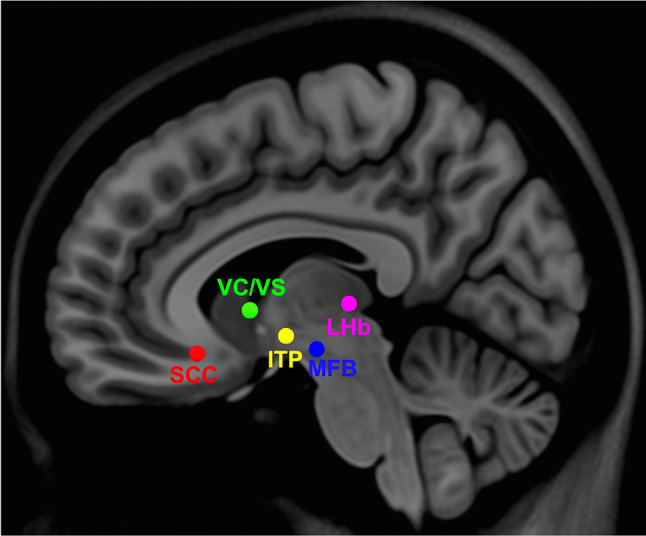
Location of all antidepressant DBS targets
The primary outcome measure in all but one [13] reviewed study was one of the following: HDRS-17, HDRS-24, HDRS-28, and MADRS [14, 15]. Studies using HDRS defined responders as patients achieving a ≥ 50% HDRS score reduction as compared to baseline and remitters were defined as patients achieving an HDRS score < 8. Studies using MADRS defined responders as patients achieving a ≥ 40% MADRS score reduction as compared to baseline and remitters as patients achieving a MADRS score ≤ 10 (exceptions to this noted in Table 1).
Adverse effects of DBS are categorized as definitely, possibly or not related to stimulation or implantation. These adverse events are further grouped into surgery-, device-, or stimulation-related. A detailed overview of adverse effects will be presented per target, while a summary of adverse events is presented in Table 2.
Table 2.
Adverse events
| Target |
Parent study Follow-up |
Year | Number of AE | Number of patients with AE | Serious AE | Suicide Attempts | Suicide |
|---|---|---|---|---|---|---|---|
| SCC | |||||||
| Mayberg et al | 2005 | 40 | NR | 1 | 1 | 0 | |
| Lozano et al | 2008 | N/A | 7 | N/A | 0 | 0 | |
| Kennedy et al | 2011 | N/A | N/A | N/A | 2 | 2 | |
| Puigdemont et al | 2012 | N/A | 5 | N/A | 1 | N/A | |
| Puigdemont et al | 2015 | N/A | 6 | N/A | 1 | N/A | |
| Lozano et al | 2012 | 68 | N/A | 10 | 1 | 1 | |
| Holtzheimer et al | 2012 | 34 | N/A | N/A | 2 | 0 | |
| Holtzheimer et al | 2017 | 299 | N/A | 61 | 4 | 2 | |
| Riva-Posse et al | 2018 | N/A | N/A | N/A | N/A | N/A | |
| Eitan et al | 2018 | 40 | N/A | 1 | 1 | N/A | |
| Ramasubbu et al | 2017 | N/A | 3 | N/A | N/A | N/A | |
| LHb | |||||||
| Sartorius et al | 2010 | N/A | N/A | N/A | N/A | N/A | |
| Keining et al | 2013 | N/A | N/A | N/A | N/A | N/A | |
| PFC | |||||||
| Kopell et al | 2011 | N/A | N/A | 2 | 0 | 0 | |
| Nahas et al | 2010 | 5 | N/A | N/A | 0 | 0 | |
| Williams et al | 2016 | N/A | N/A | 5 | N/A | N/A | |
| VC/VS | |||||||
| Malone et al | 2009 | N/A | 6 | 25 | N/A | N/A | |
| Bewernick et al | 2009 | 48 | N/A | N/A | 1 | 1 | |
| Bewernick et al | 2012 | 59 | N/A | N/A | 1 | 1 | |
| Milett et al | 2014 | 13 | N/A | N/A | 1 | N/A | |
| Dougherty et al | 2015 | 71 | N/A | N/A | 4 | 1 | |
| Bergfeld et al | 2016 | 187 | N/A | N/A | 5 | N/A | |
| MFB | |||||||
| Schlaepfer et al | 2013 | 46 | N/A | 1 | N/A | N/A | |
| Bewernick et al | 2017 | 130 | N/A | 1 | N/A | N/A | |
| Bewernick et al | 2018 | N/A | N/A | N/A | N/A | N/A | |
| Fenoy et al | 2016 | N/A | 6 | N/A | N/A | N/A | |
| Fenoy et al | 2018 | N/A | 6 | N/A | N/A | N/A | |
| Sani et al | 2017 | N/A | 6 | N/A | N/A | N/A | |
| Coenen et al | 2019 | 302 | 54 | 5 | 1 | 0 | |
| ITP | |||||||
| Jiminez et al | 2005 | N/A | N/A | N/A | N/A | N/A | |
| Comparison | |||||||
| MFB v. VC/VS | Blonstedt et al | 2017 | N/A | N/A | N/A | N/A | N/A |
| ITP v. VC/VS | Raymaekers et al | 2017 | 144 | N/A | N.A | 1 | 2 |
Subcallosal Cingulate Cortex
Target Rationale
The subcallosal cingulate cortex (SCC) was the first target studied in the application of DBS for TRD. This region was considered a critical node within a putative mood regulatory circuit, based on a large body of functional imaging literature. The SCC has been linked to the regulation of negative mood states. Activity in this region increases with the provocation of sad mood [16] and decreases in response to antidepressant interventions [16–20]. The SCC is highly interconnected with regions involved in the processing of emotions and motivation, with reciprocal pathways to the medial prefrontal cortex, cingulate cortex, nucleus accumbens, anterior thalamus, and other subcortical regions [6]. The SCC is also functionally connected to antidepressant targets for non-invasive neuromodulation [21]. Ultimately, this target was selected with the goal of interrupting the network underlying negative mood prevalent in depression [22] (Fig.2).
Fig. 2.
SCC stimulation location (left panel) and tractography of connections from the target (right panel)
Studies
The first open-label trial of DBS for TRD conducted by Mayberg et al. demonstrated its safety and efficacy, with a response rate (RS) at 6 months of 66% and remission (RM) rate of 33% [5]. This original cohort (N = 6) was expanded by Lozano and colleagues (N = 20) who showed similar efficacy at 1 year (RS = 55%, RM = 35%) [23], with further improvements at 3-year follow-up (RS = 60%, RM = 40%) [24]. These initial results have been replicated in many additional open-label trials with comparable response and remission rates [22, 25].
In the first randomized, single-blind study of SCC DBS for TRD, Holtzheimer et al. report return of clinical symptoms—including increase in depressive symptoms and suicidal ideations—within approximately 2 weeks after blinded discontinuation of stimulation [26]. Thus, blinded discontinuation was halted after the first three patients. Results from the open-label phase support results from previous studies, demonstrating significant response and remission rates at 2 years (RS: 100%, RM: 92%). In order to further investigate the relapse observed after stimulation discontinuation, Puidgemont et al. studied patients classified as responders to SCC DBS in their previous study [25]. Patients identified as responders at 6 months entered a 6-month double-blinded cross-over period. Results show that 3 out of 5 patients relapsed during cessation of treatment, further indicating a direct therapeutic effect of active SCC DBS [27].
The largest DBS for TRD study to date, the Abbott (formerly St. Jude Medical) sponsored BROADEN trial, consisted of a 6-month double-blinded sham-controlled trial with 230 patients planned but with 90 patients implanted and reported [28]. Open-label stimulation continued following the primary endpoint. Results at the 6-month primary endpoint showed no significant difference between active and sham groups with a low response rate in both groups (20% vs 17%). However, continued observation of the patients showed significant response and remission rates from 12 months (RS: 29%, RM: 14%) to 24 months (RS: 49%, RM: 26%). Nevertheless, the study was halted by the sponsor at the halfway mark (90/230 planned patients) due to low probability to meet the pre-study established primary endpoint. A regression analysis demonstrated that the improvement between 12 and 24 months relative to the first 12 months was attributable to stimulation changes—changes in active contacts and current—which were not permissible under study protocol in the first 12 months. Plausible, but unproven explanations for the delayed but eventually sustained response rates have invoked illness chronicity. Notably, the average length of the current depressive episode for these patients was 12 years, almost double the average of previous published studies, raising the testable hypothesis that the time course of clinically significant antidepressant effects requires consideration of the chronicity of the presenting illness. More supported by available data [28], the precision and consistency of DBS targeting has also been postulated. While there was no significant difference in gross anatomic location of electrode placement among patients, the angle of the lead trajectories was not controlled across surgeons which could mean different passing and/or connected white matter fibers were impacted by ongoing stimulation. Recent studies suggest maximal clinical efficacy of SCC DBS is directly correlated with the specific white matter tracts affected rather than anatomic location of stimulation as defined by traditional gray-white matter landmarks [6].
To define the combination and location of specific white matter tracts mediating the optimal clinical response in SCC DBS for TRD, Riva Posse and colleagues performed a retrospective analysis on their previously implanted cohort [26]. Using individual activation volumes and probabilistic tractography, they revealed that clinical response to SCC DBS was mediated by a direct impact on a combination of four fiber bundles passing through the SCC region. Responders shared a common map of stimulated fibers including bilateral forceps minor, bilateral cingulum bundles, dorsal and anterior midcingulate cortices, and medial branch of uncinated fasciculus. Given these retrospective results, Riva-Posse et al. sought to test the utility of using an individualized tractography map based on the group ‘connectome blueprint’ of past responders to prospectively identify the SCC DBS surgical target in a new cohort (N = 11) [29]. In this open-label study, contacts for chronic stimulation were selected by matching a preoperative deterministic tractography map to a post-operative probabilistic tractography map for each subject. When all patients had bilateral contacts activated that matched the four-bundle blueprint a significant and large response and remission rates was observed at 6 months (RS: 72.7%, RM: 54.5%) and 12 months (RS: 81.9%, RM: 54.5%). These results support the utility of a group probabilistic tractography blueprint for individualized, patient-specific, deterministic tractography targeting with the potential to greatly improve outcomes. A subsequent retrospective analysis of an independent cohort of 19 TRD patients at 6- and 12-month SCC DBS [30] used a similar but not identical, approach. In this group of patients with a lower response rate than the original study (RS: 47.4% vs 81.9%), only activation of one of the four bundles, the cingulum, correlated with response at 6 months, while activation of the forceps minor negatively correlated to response. Another retrospective study of 17 TRD patients (RS: 58.9%, RM: 35.5%) found activation of the forceps minor and uncinate fascicle but not cingulate bundle and no association between activated bundles and response but this study did not apply tractography-based targeting [31].
While in general, studies of SCC DBS for TRD use fixed frequency (130 Hz) and pulse-width (90 μs), three studies were specifically designed to test effects of different stimulation parameters [32–34]. The first study [33] tested different frequencies of stimulation (0–185 Hz) and different pulse widths (0–450 μs) in 4 patients. No effect of frequency was found but stimulation using longer pulse widths (270–450 μs) reduced depression and maximized happy mood, though side effects of anxiety and insomnia were also associated with longer pulse widths. The second study [32] evaluated differences between high (130 Hz) versus low (20 Hz) frequency in a double-blind, randomized 13-month crossover study. While no significant difference between groups was observed at 6 months, depression scores improved more in the high than low frequency group at 12 months. The third study [34] found no difference in benefit between short pulse width (90 μs) and long pulse width (210–450 μs) SCC DBS. Unfortunately, none of these studies modelled the white matter fibers differentially impacted by varying parameter settings.
There are indications that the pathophysiology of depression is asymmetric across hemispheres and left-sided repetitive transcranial magnetic stimulation (rTMS) may be more effective for depression than bilateral rTMS [35]. Two studies report on the effect of unilateral versus bilateral SCC DBS [36, 37]. In one study with 5 patients, 3 of these patients had antidepressant effects with only left unilateral DBS while right unilateral DBS was not effective [36]. The other 2 patients had some benefits with either left or right stimulation. Though this study may suggest stronger antidepressant effects of left SCC DBS, another study in one patient reports remission of depression with right-sided stimulation but not with left or bilateral stimulation [37]. A DTI-analysis in this patient showed that only the effective, right-sided target had connections to both right and left medial OFC suggesting that effective stimulation may still require bilateral circuit engagement.
Adverse Events
A total of 481 adverse events were reported in studies targeting the SCC. The most common SAE associated with surgery were transient infection, seizure, and postoperative pain. The most common device-related adverse events included pain or discomfort around the internal pulse generator (IPG) or extension cables, extension/electrode break, or dislodgement. Stimulation-related adverse events reported most commonly were increased depression or anxiety or sleep disturbances. Stimulation-related SAE were mostly transient or could be resolved by parameter adjustment. Following SCC DBS there have been 12 suicide attempts, and 6 suicides. (Table 2).
Lateral Habenula
Target Rationale
The lateral habenula (LHb) is an epithalamic structure mediating communication between the forebrain and monoaminergic systems in the midbrain and hindbrain [38, 39]. The LHb is implicated in negative mood states in both animal and human research. Animal studies examining a learned-helplessness model of depression have demonstrated that this was associated with marked LHb hypermetabolism [40, 41]. In humans, depletion of tryptophan (a serotonin precursor) results in depressive mood symptoms and increased blood flow to the LHb, supporting the region’s role in the monoamine hypothesis of depression [42]. Furthermore, LHb volume reductions are observed selectively in patients with MDD and not in other psychopathology [43]. Insights from functional studies in animal depression models show the LHb receiving inputs from structures including the globus pallidus (GPi), lateral hypothalamic area (LHA), paraventricular nucleus (PVN) lateral preoptic area (LPO), ventral pallidum (VP), lateral septum (LS), NAc, and diagonal band nuclei (DBN) [44].
Studies
A case study targeting the LHb [45] reports remission of depression in a TRD patient after 12 months of stimulation and at last follow-up. A second report of an additional patient, reports response at an unspecified time [46]. Placebo effects in both patients are excluded by severe relapses with accidental and planned stimulation discontinuation. Relapses after discontinuation in both patients were rapid in onset (1 week) and subsequent remission was gradual (2–3 months) after stimulation was restored. Recently, LHb DBS was investigated in 6 patients with bipolar depression and 1 patient with unipolar depression [47]. Three patients were responders and 1 patient remitted (RS: 42.9%, RM: 14.3%). The clear and well-characterized subcortical LHb connections from optogenetic and functional imaging studies [39] are of limited use for precision targeting of selective LHb white matter connections in human DBS trials which is technically confounded by the small size of this nucleus relative to the large volume of tissue activated by conventional DBS. Therefore, Fig. 3 depicting the LHb-target location does not include activated tracts.
Fig. 3.

LHb stimulation location (no tractography showed due to unclear pathway activation)
Adverse Effects
Transient adverse effects after LHb DBS such as double or blurred vision or dizziness could be reversed by lowering or changing stimulation parameters. During LHb DBS in patients with bipolar depression, one patient experienced a manic episode, one patient developed impulsive behaviors, and one patient suicide ideation. To date, this target has not been examined in a larger sample size.
Ventral Capsule/Ventral Striatum
Different names are used for this target region, i.e., nucleus accumbens (NAc), ventral capsule/ventral striatum (VC/VS), or ventral anterior limb of the internal capsule (vALIC). However, they all share a trajectory through the anterior limb of the internal capsule with the deepest one or two contact points in or bordering the NAc and the remaining contact points in the vALIC, although the exact location in the capsule slightly differs between these targets.
Target Rationale
Ventral capsule/ventral striatum (VC/VS) DBS was first developed for obsessive–compulsive disorder (OCD), given positive capsulotomy outcomes for these patients [48]. DBS for OCD showed significant benefit for OCD symptoms with an associated improvement in mood [49–51]. In addition, VC/VS DBS in OCD patients modulates a circuit connecting the orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), and dorsal anterior cingulate (dACC) with striatum, thalamus, and brainstem [52–54]. This circuitry significantly overlaps with the reward-circuitry assumed to be related to the anhedonia symptoms of MDD. The VC/VS is a central hub in the reward circuitry with dopaminergic inputs from the ventral tegmental area (VTA), and serving as a relay between prefrontal cortex (OFC, vmPFC, ACC), ventral striatum and the amygdala and hippocampus [55, 56] (Fig. 4).
Fig. 4.
VC/VS stimulation location (left panel) and tractography of connections from the target (right panel)
Studies
The first open-label trials for VC/VS stimulation in depression were reported simultaneously across two investigative groups using different patient cohorts. In a multi-center study, Malone and colleagues demonstrated antidepressant effects in 15 patients at a minimum of 6-month follow-up (RS: 53.3%, RM: 40%) [57]. The second, single-site study demonstrated comparable benefits in 10 patients at 1-year (RS: 50%, RM: 30%) and at 2-year follow-ups (RS: 45.5%, RM: 9.1%) [58, 59].
In an open-label trial designed to compare two striatal targets, Millet et al. implanted 4 patients with a single lead that allowed for electrodes to be positioned in the NAc and caudate nucleus [60]. No patient responded after the first 4 months of NAc stimulation or after the second 4 months of caudate stimulation. However, after the final 6 months of NAc stimulation during which stimulation parameters (voltage and contact) were adjusted, response and remission rates of 75% and 25% were observed, respectively. Although these results suggest a more robust response to the NAc than to the caudate, stimulation parameters were not adjusted for the caudate in the manner they were for the NAc, calling for further investigation into this comparison.
Promising open-label findings led to the first randomized double-blind sham-controlled trial of VC/VS DBS for TRD [61]. The Medtronic sponsored RECLAIM trial consisted of a 4-month double-blind on/off block (primary endpoint), followed by an open-label block. Results from the primary endpoint did not demonstrate a significant difference between active and sham groups, resulting in an early termination of the study for futility (N = 30/200). Open-label response and remission rates were lower than previous open-label studies at 1 year (RS: 20%, RM: 13%) and 2 years (RS: 23.3%, RM: 20%). The lack of significant differences between active and control groups may be attributed to insufficient duration of the optimization phase, and an experimental window that was too short. Results from a simultaneous randomized controlled study of vALIC DBS for TRD by Bergfeld et al. appear to confirm the need for a longer optimization phase [9]. Following a 52-week open-label optimization phase, patients entered a 12-week double-blind crossover phase to test for placebo effects. During the crossover phase, response rate was 0% during sham-stimulation and 43.8% during active DBS. Of note, most patients had to be prematurely crossed over due to significantly increased depressive symptoms typically occurring within one day of crossover, raising ethical concerns around discontinuation-trials in DBS for TRD. Continued efficacy 2 years after surgery was reported in the responders [62]. The necessary and sufficient white matter bundles within the vALIC to achieve predictable good outcomes have yet to be defined.
Adverse Effects
A total of 330 adverse events were reported in patients receiving VC/VS DBS. The most frequent surgery-related adverse events were swollen eye, pain at incision site, and dysphagia. Device-related AE included pain or discomfort around the IPG and extension/lead breakage or dislodgement. The most common reversible, stimulation-related AE include increased or worsening depression and hypomania. Following VC/VS DBS there have been 11 suicide attempts and 3 suicides (Table 2).
Medial Forebrain Bundle
Target Rationale
The medial forebrain bundle (MFB) was selected as a DBS-target for TRD based on its key function within the reward circuit [63, 64]. The superolateral branch of the MFB connects the VTA to the OFC and vmPFC via the VC/VS [65]. Notably, the VC/VS target is thought to modulate the MFB in addition to the anterior thalamic radiation (ATR) and other pathways discussed previously. The MFB was selected as a target with the hypothesis that DBS in closer proximity to the VTA, as compared to other reward-circuit targets, would produce significant anti-anhedonia effects [8] (Fig. 5).
Fig. 5.
MFB stimulation location (left panel) and tractography of connections from the target (right panel)
Studies
The first open-label study targeting the MFB in 8 TRD patients showed high response rates from 6 weeks (RS: 85.7%), to 1 year (RS: 75%, RM: 50%) [8, 66]. These patients, in addition to another cohort (N = 21), were followed for a maximum of 5 years and showed significant response and remission rates at 6 months (N = 21, RS: 42.8%, RM: 38.1%), 2 years (N = 17, RS: 64.7%, RM: 47.1%), and 5 years (N = 11, RS: 72.27%, RM: 72.7%) [67]. Furthermore, personality dimensions were not found to differ significantly from baseline at any time point after the onset of DBS.
MFB stimulation has been examined in three case-studies. In the first, Sani et al. [13], demonstrated a general pattern of treatment response in a patient with MFB DBS measured with a computerized adaptive test (CAT-DI). In the second case-study, MFB stimulation was terminated in a patient following sustained blurred vision that could not be attenuated with changes in stimulation parameters. The patient was then re-implanted at the VC/VS and maintained remission over the period of 1 year [68]. In a third case study, two patients with dMRI-guided DBS of the MFB did not reach responder status at any point over the study period of 6 months [69].
Fenoy et al. performed a 1-month single blind study, followed by an open label study (N = 6) [70, 71]. Results do not indicate any significant difference between blinded on/off conditions but in the open-label phase promising response and remission rates were observed at 1 week of MFB DBS (RS: 50%) and at 1 year (RS: 66%, RM: 50%).
In a 12-month randomized clinical trial, 16 patients entered a 2-month double blind control immediately following implantation, followed by 12 months of active stimulation [72]. Results do not demonstrate a significant difference between sham and stimulation groups during the first 2 months but show significant response and remission rates at 1 year (RS: 100%, RM: 50%). Acute antidepressant effects following surgery regardless of stimulation condition could be attributed to either microlesioning effects or to placebo. Again, there has been no delineation so far of the crucial white matter MFB branches and their respective cortical and subcortical targets necessary to achieve early and sustained antidepressant effects and avoid side effects.
Adverse Effects
In patients receiving MFB DBS, 434 adverse events were reported. Common surgery-related adverse events include hemorrhage and infection. The only reported device-related adverse event is contact malfunction in one patient. Frequent stimulation-related adverse events include vision/eye movement disorder and blurred vision—accounting for the majority of listed AEs. There has been one attempted and no completed suicides reported following MFB DBS.
Inferior Thalamic Peduncle
The inferior thalamic peduncle (ITP) connects the dorsomedial thalamus with the orbitofrontal cortex, which both show hypermetabolism in depressed patients [73]. ITP fibers continue rostrally through the ventral limb of the internal capsule, with modulatory connections to the DLPFC, OFC, ventromedial striatum, and intralaminar thalamus [74]. The first case study of ITP-DBS for TRD demonstrated remission at 12 months and last follow-up [75]. In a study comparing VC/VS stimulation to ITP stimulation in 7 TRD patients, Raymaekers et al. [76] fail to demonstrate significant difference between targets. After at least 5 months VC/VS stimulation 6 out of 7 patients were responders, and 5 were in remission (RS: 85%, RM: 71%). After at least 3-month ITP stimulation, 4 out of 7 patients were responders, and 1 was in remission (RS: 57%, RM: 14%). Only 1 out of 7 patients preferred ITP stimulation over VCVS stimulation.
Adverse Effects
In the 8 patients receiving ITP stimulation, 47 adverse events and no suicide attempts or suicides have been reported (Table 2) (Fig. 6).
Fig. 6.
ITP stimulation location (left panel) and tractography of connections from the target (right panel)
Multiple Targets
Serial and combined targeting of multiple stimulation sites has been piloted in several studies.
The VC/VS has been compared to the MFB, ITP, and SCC. In a case study, Blomstedt et al. first targeted the MFB [68]. Although depressive symptoms improved following stimulation, blurred vision could not be attenuated with changes in stimulation parameters and ultimately led to the selection of a new target. Re-implantation at the VC/VS led to remission which has been sustained over a 1-year period.
Raymaekers et al. [76] implanted two sets of bilateral electrodes in seven patients. Each patient was implanted with bilateral electrodes at the VC/VS target and at the ITP target. Following implantation and recovery, stimulation parameters were optimized for the VC/VS target for 5 months. Responders at 5 months entered a 2-week VC/VS on/off crossover. Following this, patients entered an ITP optimization period, followed by a second 6-month crossover phase in which patients received either VC/VS stimulation, ITP stimulation or no stimulation for 2 months each. Results do not demonstrate any significant difference between targets. However, at last follow up, six out of seven patients preferred VC/VS stimulation over ITP stimulation with 66.7% of these responding to simulation. A case study of combined VC/VS and SCC DBS reports remission until 75 weeks follow-up of combined stimulation and differential response-profiles. SCC stimulation reduced “mental noise” and produced calmness and attention and VC/VS stimulation produced energy and motivation [77].
Discussion
Available evidence suggests that DBS for TRD is an effective, safe, and well-tolerated experimental therapeutic option for TRD. Open-label studies of DBS at various targets show comparable response rates of on average 50% in severely treatment resistant patients provided there has been adequate time for initial parameter adjustments. Reported response rates in the first year of SCC DBS in over 200 patients range between 20 and 82%, for VC/VS in 85 patients 23–75%, and for MFB DBS in 53 patients 0–100%. High response rates are reported in studies applying patient-specific targeting of white matter maps that were determined in previous responders. Though substantial relapses after many years of sustained wellness are usually observed during unintended discontinuation, results from placebo-controlled studies have been mixed. These highly variable open-label and placebo-controlled response rates require resolution through the work of future studies for the field to progress. Here, we present the common limitations and possible future directions that we hope will allow for this resolution.
Ruling Out Placebo Response
Though placebo rates are high in medication trials for depression [78], these rates are minimal in the most severely depressed patients that also are eligible for DBS trials [79]. Notably, in the largest double-blind placebo-controlled trial, Broaden, the placebo rate after 6 months was only 17%. Nevertheless, sham surgeries often show significant placebo effects [80] which might have contributed to comparable antidepressant effects of real and sham DBS shortly after surgery. However, no antidepressant placebo response was found during randomized (dis)continuation after an initial 1-year period of active DBS with adequate parameter optimization [81]. Naturalistic placebo discontinuation studies offer additional compelling evidence of the actual antidepressant effects of DBS in various target sites. Notable are relapses after accidental stimulation discontinuation due to unrecognized device malfunctions (lead fracture, IPG dysfunction, or battery depletion), even in the presence of sustained response and remission rates over many years of ongoing active DBS, which make it seem unlikely that the long-term antidepressant effects of DBS can be attributed to chronic placebo effects.
When designing a blinded discontinuation trial is also important to consider that the effects of stimulation discontinuation may differ depending on the antidepressant target. For example, depressive symptoms remained in remission 3 months after SCC DBS discontinuation in 2/5 patients [27] and carryover antidepressant effects of at least 1 week have been observed after transient intraoperative stimulation of the SCC [81] or MFB [71], but symptom relapse within a day was observed after discontinuation of VC/VS DBS [9].
Our review suggests that differences between active and sham groups might not be observable until stimulation parameter optimization has been achieved, and that can take up to 1 year after surgery in some targets [9]. Discontinuation of stimulation is nonetheless generally associated with measurable relapse, raising ethical concerns and the potential for patients’ non-compliance at this critical stage of a clinical trial. When planning a controlled trial, it is therefore important to carefully consider potential contingencies and alternatives, most notably close follow-up and a robust and timely stop and rescue protocol.
Choosing the Outcome for DBS
The HRDS and MADRS are widely used to characterize antidepressant effects of different treatment modalities. However, these scales were designed to globally survey all symptoms of depression while DBS is by definition a circuit-selective intervention likely acting on circuit-specific symptom profiles. In Parkinson’s disease (PD), it is more accepted that DBS selectively improves tremor and rigidity items of the motor Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) rather than freezing, gait, or apathy. However, to date, there is no evidence in DBS for TRD that depression symptom dimensions as measured by the HRDS or MADRS change selectively or at different rates, and alternative measures are under study for example scales for symptom subdimensions or analysis of movements, speech, or facial expression [82–84].
In addition, the HRDS and MADRS are designed to assess severity of depressive symptoms but cannot be used for tracking symptoms once someone is out of episode when it is important to discriminate depression relapse from transient mood instability. For example, in a case study, the effect of MFB DBS measured with the HDRS was masked by the variability in mood and more marked periods of benefit and decline measured with a computerized adaptive test (CAT-DI) that allowed the questions to adapt to the patient’s depressive severity and could be administered multiple times throughout the follow-up period [13]. Since TRD is characterized by a lack of mood variability, successful treatment should restore this variability in patients. Therefore, outcome measures might seek to measure this increase in mood variability. Attempts to monitor moment to moment emotionality in response to DBS are ongoing, including the monitoring of brain signals underlying the slow sustained depression illness change versus fast emotional transients which may be important but different signals [83, 85].
Safety and Adverse Events
The reviewed surgical risks of DBS for TRD are minimal and do not differ significantly from risks of DBS for movement disorders. However, psychiatric adverse events associated with stimulation are not yet fully delineated and appear variable across targets. Insights from limited placebo-controlled studies demonstrate a need for consideration of safety in the case of accidental device shutdown. It appears that symptoms return in the order of weeks when SCC DBS is turned off [26], whereas symptoms return on the order of days when VC/VS DBS is turned off [9]. As such, specific plans should be put into place to care for the patient in the case of accidental device malfunction. Innovation resulting in extended battery life will prove to limit these safety concerns.
Given the population studied, suicide and suicide attempts are to be expected and should be accounted for in developing safety procedures for future studies. Generally, 30% of patients with TRD attempt suicide at least once in their lives [86]. The DBS studies reviewed here demonstrate a suicide attempt rate of 6.7%, suggesting that DBS for TRD does not increase suicide risk.
Optimizing Target Selection
Historically, most targets discussed here have been identified pre-surgically in standard stereotactic anatomical space based on atlases [11, 87]. Atlas-based target selection does not consider the patient-specific variability of white matter fibers in relation to the predefined standard DBS target. Notable exceptions include prospective targeting of a predefined responder map of specific white matter fibers connected to the SCC [29], and the individual tractography identification of the medial forebrain bundle [72] (not visible in standard atlases). For SCC DBS, consistent tractography-based targeting of a site in the SCC region structurally connected to the forceps minor, uncinate fasciculus, cingulum bundle, and frontostriatal fibers was associated with an 88% response rate [88] and bilateral stimulation of all four white matter tracts appears required to achieve a full response [6]. In one study of SCC DBS only activation of the cingulum correlated with response though only half of this trial’s participants were responders and it is unclear to what extent the forceps minor, uncinate fasciculus or subcortical fibers were fully included in the surgical target [30]. Quantitative fiber activation models will help further delineate which stimulation pathways are necessary and sufficient to achieve good clinical outcomes in individual patients. MFB DBS trials have used individual tractography-targeting since their first reports. In this target, tractography is necessary for implantation, and there is no “responder” template. That said, MFB case series and trials have reported high response rates overall. Non-response, however, was reported in a recent case study of two patients despite following the same tractography-based targeting method [69]. For VC/VS DBS, a complex combination of internal capsule fibers to the ventrolateral and medial prefrontal cortex is consistently associated to response in DBS for OCD [89], though no data are available yet using tractography-based targeting to define the necessary nor sufficient tracts for depression. One retrospective group analysis supports improved outcomes with lead placements closer to white matter internal capsule bundles between the prefrontal cortex, thalamus and brainstem [90] but without further specificity.
While tractography-based surgical planning across different DBS targets appears to improve outcomes, it has not directly informed understanding of DBS mechanisms. Tractography has several limitations, including inability to convey information about the direction of current flow through a given bundle, inability to disambiguate cell-specific connections, inability to identify network nodes beyond first-level monosynaptic connections and lack of a uniform methodology to identify the tracts. Finally, implementing tractography based surgical targeting into routine clinical practice may be challenging since it requires high-quality patient-specific diffusion-weighted scans rather than normative connectomic data [72].
Targeting Symptom-Specific Connections
All therapeutic DBS targets reviewed here have comparable effectiveness for treating depression though there are no head-to-head studies to date. A reason behind this comparable effectiveness across targets may be their partially overlapping connections. DBS targets in the VC/VS region structurally and functionally connect to the MFB and partially to the SCC [91], but the SCC has selective fibers that are not directly connected to VC/VS or NAc [92, 93]. It is important to note that NAc or VC/VS stimulation does not affect the SCC monosynaptically as NAc connections go to the thalamus first and then back to frontal cortex via the ALIC. Thus, VC/VS stimulation may be very different from SCC stimulation that involves monosynaptic white matter fibers to part of the NAc and thalamus that do not pass through the ALIC [93]. Nevertheless, SCC DBS modulates activity in the NAc and, conversely, NAc DBS modulates the SCC, while other regions that are influenced via both targets include the brainstem, hypothalamus, amygdala, and OFC [94–96]. VC/VS DBS may thus exert “bottom-up” regulation of a similar limbic-motivational circuit that SCC DBS regulates in an opposite “top-down” fashion. These opposite modulatory entrances into the same circuit appear to result in different but related antidepressant outcomes [97]. For instance, the first manifestation of effective SCC DBS is usually a relief of negative affect and changes in physical awareness [98], in accordance with the primary mood regulatory function of the SCC and its connections to structures related to interception such as the insula and cingulum. On the other hand, patients responding to DBS of the NAc, a region more associated with reward and positive affect, report acutely improved motivation for pleasurable activities [99]. DBS of the vALIC improved mood in patients with primary OCD and was associated with enhanced striatal reward-processing and dopamine release, which may be important mechanisms for the reversal of anhedonia and lack of positive mood in major depression [52, 65, 100, 101]. Tractography studies in healthy individuals have revealed that the MFB connects the ventral tegmental area (VTA) with the OFC and medial and dorsolateral prefrontal cortices via the VC/VS, together constituting a circuit involved in the processing of rewards, motivation, and emotions [65]. Therefore, it is likely that patients within a specific subgroup of symptoms will respond preferentially to different targets. Similarly, comorbid psychiatric disorders may favor the choice of specific targets in depression, i.e., comorbid OCD or significant OC symptoms may favor a VC/VS target, and depressed patients with anorexia may respond to SCC stimulation [102].
Symptom characterization, with concomitant biomarker detection, may be helpful in the decision of target selection in the future but is only theoretical at this time. A single-case report has attempted this [83] and researchers are determining chronic stimulation location and parameters based on the immediately post-operative neurophysiological recordings. It remains to be seen if this biomarker-driven stimulation selection improves the current approach [77].
Optimizing Stimulation Parameters
Only few of the reviewed studies investigated the effect of different stimulation parameters. Low-frequency stimulation does not seem to be effective [32], and the sweet spot is in the high-frequency range (100–150 Hz) [103], which is also supported by animal studies [104, 105]. Nevertheless, future studies may want to investigate the effect of low-frequency stimulation on specific symptoms of depression in parallel to promising findings with this approach in PD [106]. Although longer pulse-widths were beneficial in some patients, this also caused more side effects suggesting a less specific stimulation delivery. Fine-tuning of stimulation parameters may be best approached in an individual patient once the optimal fibers have been identified and selected. However, this fine-tuning may require an immediate readout, and for now, there are not fast behavioral effects with DBS at these targets as there are for tremor in PD.
Closed-Loop Stimulation
The identification of electrophysiological signatures in patients receiving DBS has opened the possibility of using them to optimize stimulation parameters and with recent advances inform stimulation changes by clinicians, and eventually facilitate the design of closed-loop systems. This is already a possibility in epilepsy and is becoming feasible in movement disorders [107]. Closed-loop DBS systems are designed to trigger stimulation onset or parameter adjustment by sensing readouts from intracranial recordings. Closed-loop DBS could be either applied automatically where the system both detects the biomarker and changes the stimulation, or a clinician can decide to adjust the stimulation based on biomarker readouts from the leads (assisted open-loop).
In psychiatric disorders, there are several groups that are utilizing electrophysiology to detect mood signals to select stimulation target and parameters. This work has been facilitated by the National Institutes of Health BRAIN Initiative, seeking for academic and industry collaborations. For closed loop stimulation in psychiatric disorders to become a reality, reliable and accessible biomarkers of depression in TRD patients with DBS first have to be determined. Important as well is the determination of which biomarkers correlate with clinical symptoms in depression, a highly complex disorder that has different clinical manifestations across and within the same patient (i.e., suicidality, anhedonia, negative affect). In psychiatric DBS, there are early reports of changes that occur in brain activity when changes in mood occur. This has been described in intraoperative shifts in mood in direct response to effective stimulation [108]. In chronic stimulation (recording from contacts adjacent to the stimulating electrode), local field potential changes in 1/f slope, a putative correlate of neuronal excitability, recorded weekly at the right SCC-lead during the first 6 months of SCC-DBS discriminated depressed from non-depressed states in three out of the four patients [85].
Closed-loop DBS was recently piloted in a TRD patient [109]. During a 10-day period of intracranial corticolimbic circuitry mapping (recording from SCC, amygdala, VC/VS, OFC, and HPC), amygdala gamma power correlated with high depressive symptom states measured several times daily. With this putative biomarker as target, bilateral leads and a responsive neurostimulator (Neuropace) were implanted in the VC/VS where stimulation was triggered by gamma power increases measured in another set of implanted amygdala leads. This strategy resulted in over 400 daily depressive biomarker detections triggering 6-s stimulations, and acute and sustained remission of depressive symptoms. This promising case report that will hopefully be replicated. While this case-study may suggest the feasibility of closed-loop VC/VS stimulation for TRD based on personalized biomarker-informed stimulation, it does not yet demonstrate effective superiority compared to classic VC/VS DBS or whether stimulation needs to be tailored to biomarkers of daily mood fluctuations or more prolonged depressive states.
A separate group has taken a different approach [110]. DBS-leads were implanted, with the assistance of tractography, in both SCC and VC/VS, as well as 10 temporary stereoEEG depth electrodes across prefrontal and temporal regions. Immediately following the implantation surgery, parameters from stimulation in both electrodes were explored and recordings from the externalized sEEG were gathered for several days in a hospital monitoring unit. Using the information from these recordings, the contacts and stimulation parameters were selected for chronic continuous stimulation (Boston Scientific).
Additional Treatment
None of the reviewed studies systematically investigated antidepressant augmentation strategies for DBS. A case-report suggests augmenting effects of SCC DBS with monoamine oxidase inhibitor but not with other antidepressants [111]. Most trials have kept medications stable during the DBS as withdrawing TRD patients from treatments prior to experimental DBS is an ethical and safety challenge. However, several patients that were withdrawn from unhelpful medication at the time of implant showed similar robust response to DBS [9], suggesting that medication may not be required to achieve or augment DBS response. Future studies might work to reduce medications pre-surgically or early during DBS optimization. This may be easier once there is a target physiological biomarker comparable to for example beta power in PD that can be used to titrate levodopa or stimulation. ECT was safely augmented to SCC DBS in one patient who relapsed after which the depression remained in remission with continued DBS [112]. Regardless of target selection, outcomes in all patients may be enhanced through the addition of rehabilitative and behavioral treatments. This patient population has an average disease duration lasting many years, with a variety of associated secondary problems. Rehabilitation and behavioral therapy have been shown to have an additive effect for patients receiving DBS for OCD [113].
Conclusion
DBS is an effective, safe, and well-tolerated therapeutic option for TRD. Progress in the field may depend on the development of novel outcome measurements capturing the sustained circuit-selective symptom changes observed after DBS, and standardization of randomized placebo-controlled study designs including initial open-label parameter optimization. White matter activation maps in DBS responders across targets will help determining the optimal antidepressant network that can be used for tractography-guided surgery. This precision-stimulation strategy will likely result in faster and higher antidepressant responses that can be maintained or optimized by adjusting the stimulation over time in a closed-loop or assisted-open-loop fashion informed by biomarkers of depressive states.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Depression fact sheet. 2012.
- 2.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. 2018;75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Thase ME, Schwartz TL. Using mechanism of action to choose medications for treatment-resistant depression. J Clin Psychiatry. 2015;76(9):e1147. doi: 10.4088/JCP.14052wc1c. [DOI] [PubMed] [Google Scholar]
- 5.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiat. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Germann J, Mameli M, Elias GJB, Loh A, Taha A, Gouveia FV, et al. Deep brain stimulation of the habenula: systematic review of the literature and clinical trial registries. Front Psychiatry. 2021;12:730931. doi: 10.3389/fpsyt.2021.730931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Notten P, van Laarhoven J, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2016;73(5):456–464. doi: 10.1001/jamapsychiatry.2016.0152. [DOI] [PubMed] [Google Scholar]
- 10.Yeh FC, Tseng WY. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage. 2011;58(1):91–99. doi: 10.1016/j.neuroimage.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Horn A, Li N, Dembek TA, Kappel A, Boulay C, Ewert S, et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage. 2019;184:293–316. doi: 10.1016/j.neuroimage.2018.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn A, Kuhn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–135. doi: 10.1016/j.neuroimage.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Sani S, Busnello J, Kochanski R, Cohen Y, Gibbons RD. High-frequency measurement of depressive severity in a patient treated for severe treatment-resistant depression with deep-brain stimulation. Transl Psychiatry. 2017;7(8):e1207. doi: 10.1038/tp.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 16.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 17.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Investig. 2009;119(4):717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163(4):735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 19.Talbot PS, Cooper SJ. Anterior cingulate and subgenual prefrontal blood flow changes following tryptophan depletion in healthy males. Neuropsychopharmacology. 2006;31(8):1757–1767. doi: 10.1038/sj.npp.1301022. [DOI] [PubMed] [Google Scholar]
- 20.Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci U S A. 2002;99(4):2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqi SH, Schaper F, Horn A, Hsu J, Padmanabhan JL, Brodtmann A, et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav. 2021;5(12):1707–1716. doi: 10.1038/s41562-021-01161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012;116(2):315–322. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- 23.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 25.Puigdemont D, Perez-Egea R, Portella MJ, Molet J, de Diego-Adelino J, Gironell A, et al. Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression. Int J Neuropsychopharmacol. 2012;15(1):121–133. doi: 10.1017/S1461145711001088. [DOI] [PubMed] [Google Scholar]
- 26.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puigdemont D, Portella M, Perez-Egea R, Molet J, Gironell A, de Diego-Adelino J, et al. A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a pilot study of relapse prevention. J Psychiatry Neurosci. 2015;40(4):224–231. doi: 10.1503/jpn.130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839–849. doi: 10.1016/S2215-0366(17)30371-1. [DOI] [PubMed] [Google Scholar]
- 29.Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2018;23(4):843–849. doi: 10.1038/mp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark DL, Johnson KA, Butson CR, Lebel C, Gobbi D, Ramasubbu R, et al. Tract-based analysis of target engagement by subcallosal cingulate deep brain stimulation for treatment resistant depression. Brain Stimul. 2020;13(4):1094–1101. doi: 10.1016/j.brs.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Aibar-Duran JA, Rodriguez Rodriguez R, de Diego Adelino FJ, Portella MJ, Alvarez-Holzapfel MJ, Martin Blanco A, et al. Long-term results of deep brain stimulation for treatment-resistant depression: outcome analysis and correlation with lead position and electrical parameters. Neurosurgery. 2022;90(1):72–80. doi: 10.1227/NEU.0000000000001739. [DOI] [PubMed] [Google Scholar]
- 32.Eitan R, Fontaine D, Benoit M, Giordana C, Darmon N, Israel Z, et al. One year double blind study of high vs low frequency subcallosal cingulate stimulation for depression. J Psychiatr Res. 2018;96:124–134. doi: 10.1016/j.jpsychires.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Ramasubbu R, Anderson S, Haffenden A, Chavda S, Kiss ZH. Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. J Psychiatry Neurosci. 2013;38(5):325–332. doi: 10.1503/jpn.120160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramasubbu R, Clark DL, Golding S, Dobson KS, Mackie A, Haffenden A, et al. Long versus short pulse width subcallosal cingulate stimulation for treatment-resistant depression: a randomised, double-blind, crossover trial. Lancet Psychiatry. 2020;7(1):29–40. doi: 10.1016/S2215-0366(19)30415-8. [DOI] [PubMed] [Google Scholar]
- 35.Aaronson ST, Carpenter LL, Hutton TM, Kraus S, Mina M, Pages K, et al. Comparison of clinical outcomes with left unilateral and sequential bilateral transcranial magnetic stimulation (TMS) treatment of major depressive disorder in a large patient registry. Brain Stimul. 2022. [DOI] [PubMed]
- 36.Susan K. Conroy SM, Mary E. Kelley, Megan M. Filkowski, Ryan M. Trimble, Megan E. Pirtle, Ashley Maher, Sarah Dreyer-Oren, Wilder Doucette, Robert M. Roth, Joshua P. Aronson, David W. Roberts, Ki Sueng Choi, Helen S. Mayberg, Paul E. Holtzheimer. Left versus right subcallosal cingulate deep brain stimulation for treatment-resistant depression. Personalized Med Psychiatry. 2021;25–26.
- 37.Guinjoan SM, Mayberg HS, Costanzo EY, Fahrer RD, Tenca E, Antico J, et al. Asymmetrical contribution of brain structures to treatment-resistant depression as illustrated by effects of right subgenual cingulum stimulation. J Neuropsychiatry Clin Neurosci. 2010;22(3):265–277. doi: 10.1176/jnp.2010.22.3.265. [DOI] [PubMed] [Google Scholar]
- 38.Herkenham M. The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J Comp Neurol. 1979;183(3):487–517. doi: 10.1002/cne.901830304. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Wang H, Hu J, Hu H. Lateral habenula in the pathophysiology of depression. Curr Opin Neurobiol. 2018;48:90–96. doi: 10.1016/j.conb.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Henn FA, Vollmayr B. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29(4–5):799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963(1–2):274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 42.Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10(2):163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- 43.Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein HG. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med. 2010;40(4):557–567. doi: 10.1017/S0033291709990821. [DOI] [PubMed] [Google Scholar]
- 44.Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39(7):1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- 45.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67(2):e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 46.Kiening K, Sartorius A. A new translational target for deep brain stimulation to treat depression. EMBO Mol Med. 2013;5(8):1151–1153. doi: 10.1002/emmm.201302947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Zhang Y, Luo H, Xu X, Yuan T-f, Li D, et al. Bilateral Habenula deep brain stimulation for treatment-resistant depression: clinical findings and electrophysiological features. Translational Psychiatry. 2022;12(1):52. [DOI] [PMC free article] [PubMed]
- 48.Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57(5):510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 49.Goodman WK, Foote KD, Greenberg BD, Ricciuti N, Bauer R, Ward H, et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67(6):535–542. doi: 10.1016/j.biopsych.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuttin BJ, Gabriels L, van Kuyck K, Cosyns P. Electrical stimulation of the anterior limbs of the internal capsules in patients with severe obsessive-compulsive disorder: anecdotal reports. Neurosurg Clin N Am. 2003;14(2):267–274. doi: 10.1016/s1042-3680(02)00117-1. [DOI] [PubMed] [Google Scholar]
- 52.Figee M, Luigjes J, Smolders R, Valencia-Alfonso C-E, van Wingen G, de Kwaasteniet B, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16(4):386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- 53.Haber SN, Heilbronner SR. Translational research in OCD: circuitry and mechanisms. Neuropsychopharmacology. 2013;38(1):252–253. doi: 10.1038/npp.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nanda P, Banks GP, Pathak YJ, Sheth SA. Connectivity-based parcellation of the anterior limb of the internal capsule. Hum Brain Mapp. 2017;38(12):6107–6117. doi: 10.1002/hbm.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nestler EJ. Role of the brain's reward circuitry in depression: transcriptional mechanisms. Int Rev Neurobiol. 2015;124:151–170. doi: 10.1016/bs.irn.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiat. 2009;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bewernick BH, Hurlemann R, Matusch A. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry doi101016jbiopsych09013. 2009;67(2 10.1016/j.biopsych.2009.09.013 SRC - BaiduScholar):110–6. [DOI] [PubMed]
- 59.Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(9):1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Millet B, Jaafari N, Polosan M, Baup N, Giordana B, Haegelen C, et al. Limbic versus cognitive target for deep brain stimulation in treatment-resistant depression: accumbens more promising than caudate. Eur Neuropsychopharmacol. 2014;24(8):1229–1239. doi: 10.1016/j.euroneuro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O'Reardon JP, et al. A Randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiat. 2015;78(4):240–248. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 62.van der Wal JM, Bergfeld IO, Lok A, Mantione M, Figee M, Notten P, et al. Long-term deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression. J Neurol Neurosurg Psychiatry. 2020;91(2):189–195. doi: 10.1136/jnnp-2019-321758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klanker M, Feenstra M, Willuhn I, Denys D. Deep brain stimulation of the medial forebrain bundle elevates striatal dopamine concentration without affecting spontaneous or reward-induced phasic release. Neuroscience. 2017;364:82–92. doi: 10.1016/j.neuroscience.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Schlaepfer TE, Bewernick BH, Kayser S, Hurlemann R, Coenen VA. Deep brain stimulation of the human reward system for major depression–rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303–1314. doi: 10.1038/npp.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coenen VA, Schumacher LV, Kaller C, Schlaepfer TE, Reinacher PC, Egger K, et al. The anatomy of the human medial forebrain bundle: ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. Neuroimage Clin. 2018;18:770–783. doi: 10.1016/j.nicl.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bewernick BH, Kayser S, Gippert SM, Switala C, Coenen VA, Schlaepfer TE. Deep brain stimulation to the medial forebrain bundle for depression- long-term outcomes and a novel data analysis strategy. Brain Stimul. 2017;10(3):664–671. doi: 10.1016/j.brs.2017.01.581. [DOI] [PubMed] [Google Scholar]
- 67.Bewernick BH, Kilian HM, Schmidt K, Reinfeldt RE, Kayser S, Coenen VA, et al. Deep brain stimulation of the supero-lateral branch of the medial forebrain bundle does not lead to changes in personality in patients suffering from severe depression. Psychol Med. 2018;48(16):2684–2692. doi: 10.1017/S0033291718000296. [DOI] [PubMed] [Google Scholar]
- 68.Blomstedt P, Naesstrom M, Bodlund O. Deep brain stimulation in the bed nucleus of the stria terminalis and medial forebrain bundle in a patient with major depressive disorder and anorexia nervosa. Clin Case Rep. 2017;5(5):679–684. doi: 10.1002/ccr3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davidson B, Giacobbe P, Mithani K, Levitt A, Rabin JS, Lipsman N, et al. Lack of clinical response to deep brain stimulation of the medial forebrain bundle in depression. Brain Stimul. 2020;13(5):1268–1270. doi: 10.1016/j.brs.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 70.Fenoy AJ, Schulz P, Selvaraj S, Burrows C, Spiker D, Cao B, et al. Deep brain stimulation of the medial forebrain bundle: Distinctive responses in resistant depression. J Affect Disord. 2016;203:143–151. doi: 10.1016/j.jad.2016.05.064. [DOI] [PubMed] [Google Scholar]
- 71.Fenoy AJ, Schulz PE, Selvaraj S, Burrows CL, Zunta-Soares G, Durkin K, et al. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl Psychiatry. 2018;8(1):111. doi: 10.1038/s41398-018-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coenen VA, Bewernick BH, Kayser S, Kilian H, Bostrom J, Greschus S, et al. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology. 2019;44(7):1224–1232. doi: 10.1038/s41386-019-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Velasco F, Velasco M, Jimenez F, Velasco AL, Salin-Pascual R. Neurobiological background for performing surgical intervention in the inferior thalamic peduncle for treatment of major depression disorders. Neurosurgery. 2005;57(3):439–48; discussion -48. [DOI] [PubMed]
- 74.Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev. 2008;32(3):408–422. doi: 10.1016/j.neubiorev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Jimenez F, Velasco F, Salin-Pascual R, Hernandez JA, Velasco M, Criales JL, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585–93; discussion -93. [DOI] [PubMed]
- 76.Raymaekers S, Luyten L, Bervoets C, Gabriels L, Nuttin B. Deep brain stimulation for treatment-resistant major depressive disorder: a comparison of two targets and long-term follow-up. Transl Psychiatry. 2017;7(10):e1251. doi: 10.1038/tp.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheth S.A. BKR, Metzger B., Allawala A., Pirtle V., Adkinson, J.A. MJ, Mathura R.K., Oswalt D., Tsolaki E., Xiao J., Noecker A., Strutt A.M., Cohn J.F., McIntyre C.C., Mathew S.J., Borton D., Goodman W. & Pouratian N. Deep brain stimulation for depression informed by intracranial recordings. Biol Psychiatry. 2021. [DOI] [PMC free article] [PubMed]
- 78.Jakovljevic M. The placebo-nocebo response: controversies and challenges from clinical and research perspective. Eur Neuropsychopharmacol. 2014;24(3):333–341. doi: 10.1016/j.euroneuro.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Licht RW, Danish University Antidepressant G. Is it possible to evaluate true prophylactic efficacy of antidepressants in severely ill patients with recurrent depression? Lessons from a placebo-controlled trial. The fifth trial of the Danish University Antidepressant Group (DUAG-5). J Affect Disord. 2013;148(2–3):286–90. [DOI] [PubMed]
- 80.Tavel ME. The placebo effect: the good, the bad, and the ugly. Am J Med. 2014;127(6):484–488. doi: 10.1016/j.amjmed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Patricio Riva-Posse ALC, Kathryn Wright, Allison C. Waters, KiSueng Choi, Steven J. Garlow, Paul E. Holtzheimer, Robert E. Gross and Helen S. Mayberg. Rapid antidepressant effects of deep brain stimulation and their relation to surgical protocol. Biol Psychiatry. 2020;88(8):e37-e9. [DOI] [PMC free article] [PubMed]
- 82.Jiang Z, Harati S, Crowell A, Mayberg HS, Nemati S, Clifford GD. Classifying major depressive disorder and response to deep brain stimulation over time by analyzing facial expressions. IEEE Trans Biomed Eng. 2021;68(2):664–672. doi: 10.1109/TBME.2020.3010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scangos KW, Makhoul GS, Sugrue LP, Chang EF, Krystal AD. State-dependent responses to intracranial brain stimulation in a patient with depression. Nat Med. 2021;27(2):229–231. doi: 10.1038/s41591-020-01175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCall MV, Riva-Posse P, Garlow SJ, Mayberg HS, Crowell AL. Analyzing non-verbal behavior throughout recovery in a sample of depressed patients receiving deep brain stimulation. Neurol Psychiatry Brain Res. 2020;37:33–40. doi: 10.1016/j.npbr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Veerakumar A, Tiruvadi V, Howell B, Waters AC, Crowell AL, Voytek B, et al. Field potential 1/f activity in the subcallosal cingulate region as a candidate signal for monitoring deep brain stimulation for treatment-resistant depression. J Neurophysiol. 2019;122(3):1023–1035. doi: 10.1152/jn.00875.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bergfeld IO, Mantione M, Figee M, Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. J Affect Disord. 2018;235:362–367. doi: 10.1016/j.jad.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 87.Nowinski WL, Fang A, Nguyen BT. editor Schaltenbrand-Wahren-Talairach-Tournoux brain atlas registration. Proc SPIE 2431, Medical Imaging: Image Display. 1995.
- 88.Sendi MSE, Waters AC, Tiruvadi V, Riva-Posse P, Crowell A, Isbaine F, et al. Intraoperative neural signals predict rapid antidepressant effects of deep brain stimulation. Transl Psychiatry. 2021;11(1):551. doi: 10.1038/s41398-021-01669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Figee M, Mayberg H. Deep brain stimulation for obsessive-compulsive disorder: why anatomy matters. Biol Psychiatry. 2021;90(10):662–663. doi: 10.1016/j.biopsych.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 90.Liebrand LC, Natarajan SJ, Caan MWA, Schuurman PR, van den Munckhof P, de Kwaasteniet B, et al. Distance to white matter trajectories is associated with treatment response to internal capsule deep brain stimulation in treatment-refractory depression. Neuroimage Clin. 2020;28:102363. doi: 10.1016/j.nicl.2020.102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salgado S, Kaplitt MG. The nucleus accumbens: a comprehensive review. Stereotact Funct Neurosurg. 2015;93(2):75–93. doi: 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- 92.Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65(4):276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Z, Hubbard E, Guo X, Barbosa DAN, Popal AM, Cai C, et al. A connectomic analysis of deep brain stimulation for treatment-resistant depression. Brain Stimul. 2021;14(5):1226–1233. doi: 10.1016/j.brs.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 94.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 95.Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104(4):558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 96.Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, et al. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006;47(5):740–747. [PubMed] [Google Scholar]
- 97.Eggers AE. Treatment of depression with deep brain stimulation works by altering in specific ways the conscious perception of the core symptoms of sadness or anhedonia, not by modulating network circuitry. Med Hypotheses. 2014;83(1):62–64. doi: 10.1016/j.mehy.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 98.Crowell AL, Garlow SJ, Riva-Posse P, Mayberg HS. Characterizing the therapeutic response to deep brain stimulation for treatment-resistant depression: a single center long-term perspective. Front Integr Neurosci. 2015;9:41. doi: 10.3389/fnint.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 100.Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 101.Figee M, de Koning P, Klaassen S, Vulink N, Mantione M, van den Munckhof P, et al. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol Psychiatry. 2014;75(8):647–652. doi: 10.1016/j.biopsych.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 102.Lipsman N, Lam E, Volpini M, Sutandar K, Twose R, Giacobbe P, et al. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry. 2017;4(4):285–294. doi: 10.1016/S2215-0366(17)30076-7. [DOI] [PubMed] [Google Scholar]
- 103.Rudebeck PH, Rich EL, Mayberg HS. From bed to bench side: reverse translation to optimize neuromodulation for mood disorders. Proc Natl Acad Sci USA. 2019. [DOI] [PMC free article] [PubMed]
- 104.Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res. 2010;44(11):683–687. doi: 10.1016/j.jpsychires.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 105.Lim LW, Prickaerts J, Huguet G, Kadar E, Hartung H, Sharp T, et al. Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms. Trans Psychiatry. 2015;5(3):e535-e. [DOI] [PMC free article] [PubMed]
- 106.di Biase L, Fasano A. Low-frequency deep brain stimulation for Parkinson's disease: Great expectation or false hope? Mov Disord. 2016;31(7):962–967. doi: 10.1002/mds.26658. [DOI] [PubMed] [Google Scholar]
- 107.Krauss JK, Lipsman N, Aziz T, Boutet A, Brown P, Chang JW, et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17(2):75–87. doi: 10.1038/s41582-020-00426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smart O, Choi KS, Riva-Posse P, Tiruvadi V, Rajendra J, Waters AC, et al. Initial unilateral exposure to deep brain stimulation in treatment-resistant depression patients alters spectral power in the subcallosal cingulate. Front Comput Neurosci. 2018;12:43. doi: 10.3389/fncom.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scangos KW, Khambhati AN, Daly PM, Makhoul GS, Sugrue LP, Zamanian H, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med. 2021;27(10):1696–1700. doi: 10.1038/s41591-021-01480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Allawala A, Bijanki KR, Goodman W, Cohn JF, Viswanathan A, Yoshor D, et al. A novel framework for network-targeted neuropsychiatric deep brain stimulation. Neurosurgery. 2021;89(2):E116–E121. doi: 10.1093/neuros/nyab112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamani C, Giacobbe P, Diwan M, Balbino ES, Tong J, Bridgman A, et al. Monoamine oxidase inhibitors potentiate the effects of deep brain stimulation. Am J Psychiatry. 2012;169(12):1320–1321. doi: 10.1176/appi.ajp.2012.12060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Puigdemont D, Portella MJ, Perez-Egea R, de Diego-Adelino J, Gironell A, Molet J, et al. Depressive relapse after initial response to subcallosal cingulate gyrus-deep brain stimulation in a patient with a treatment-resistant depression: electroconvulsive therapy as a feasible strategy. Biol Psychiatry. 2009;66(5):e11–e12. doi: 10.1016/j.biopsych.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 113.Mantione M, Nieman DH, Figee M, Denys D. Cognitive-behavioural therapy augments the effects of deep brain stimulation in obsessive-compulsive disorder. Psychol Med. 2014;44(16):3515–3522. doi: 10.1017/S0033291714000956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Disclosure forms provided by the authors are available with the online version of this article.






