Abstract
Background
To estimate the overall situation of Duchenne muscular dystrophy (DMD) screening in newborns in Guangzhou, China.
Method
A total of 62553 newborns including 44268 males and 18285 females were screened for DMD by measuring muscle specific creatine kinase isoform (CK-MM) concentrations using the GSP® Neonatal CK-MM kit based on time-resolved immunofluorescence. We recalled positive cases and recollected dried blood spots (DBS) for retest of CK-MM. The newborns with retest positive result were recalled again for serum creatine kinase (CK) and multiplex ligation-dependent probe amplification (MLPA) test. Whole exon sequencing was performed when MLPA test was negative.
Results
Four males were diagnosed with DMD. The incidence of males was 1/11067. No DMD patient was found in female. There were significant differences of CK-MM concentration between male and female newborns. Among gestational age (GA), birth weight (BW) and age at sampling, linear regression analysis showed that CK-MM concentration was much more closely correlated with GA and age at sampling.
Conclusions
CK-MM concentration is affected by gender, GA, BW and age at sampling. The efficiency of DMD screening might be improved by adjusting a multitier cut-off value according to GA and age at sampling. DMD newborn screening should be male priority.
Keywords: Duchenne muscular dystrophy, Neonatal screening, Creatine kinase, Gestational age, Birth weight, Age at sampling
Highlights
-
•
A total of 62553 newborns including 44268 males and 18285 females were screened for DMD in Guangzhou.
-
•
Four males were diagnosed with DMD. The incidence of males was 1/11067. No DMD patient found in female.
-
•
DMD newborn screening should be male priority.
-
•
The efficiency of DMD screening might be improved by adjusting a multitier cut-off value according to GA and age at sampling.
Duchenne muscular dystrophy; Neonatal screening; Creatine kinase; Gestational age; Birth weight; Age at sampling.
1. Introduction
Duchenne muscular dystrophy (DMD, OMIM 310200) is a common, progressive and lethal X-linked recessive neuromuscular disorder. It predominantly affects males with an incidence of approximately 1/5000 live male births around the world [1]. DMD is caused mostly by out-frame mutations in the DMD gene (OMIM 300377), which encodes the protein called dystrophin, a key element in stabilizing the sarcolemma during muscle contraction. DMD patients present clinical symptoms at any time between birth and age 8 years, including motor delays, abnormal gait, frequent falls and an inability to climb stairs. Duchenne patients usually lose the ambulation by the age of 12 years and die by the third decade of life.
DMD newborn screening has been debatable and not widely available due to lack of effective treatment for decades [2]. However, with the developments of treatments, such as corticosteroids, mutation specific exon skipping drugs, stop-codon read through and genetic treatments, the therapeutic landscape has changed significantly. These developments have rekindled interest in newborn screening for DMD [3, 4, 5].
Over the past 40 years, there have been over 10 DMD newborn screening programs or pilots implemented worldwide, and approximately 1.8 million newborns worldwide have been screened for DMD [6]. Several pilots at early stage used total creatine kinase (CK) as a biomarker for DMD newborn screening [7, 8], with the diagnosis of DMD based on findings of clinical follow-up, muscle biopsy, or direct mutational testing of the DMD gene. Recent pilots focused on measuring the muscle specific creatine kinase isoform (CK-MM), one of the three isoenzyme forms (CK-MM, CK-MB and CK-BB). CK-MM is mainly present in skeletal muscle and is the most specific biomarker of skeletal muscle damage, which can discriminate better between normal, unaffected and Duchenne affected populations [9]. In China, the first DMD newborn screening pilot study by measuring CK-MM in dried blood spots (DBS) for male neonates was conducted at Zhejiang University since 2015 [6]. However, most of the DMD screening pilots focused on screening for males. Some previous studies have showed that although most female carriers are asymptomatic, there are still up to 20% of carrier females who develop some degree of muscle weakness or cardiomyopathy [10]. In view of this, we launched the DMD screening including female newborns for the first time in China aiming to estimate the performance of DMD screening program in Guangzhou.
2. Subjects and methods
2.1. Subjects
The pilot was carried out in two periods:
Period 1: A total including 21506 males and 18285 females were screened for DMD from June to October 2019.
Period 2: Based on the results of phase 1, we adjusted the screening strategy to screen only males, from May to September 2021.
A total of 62553 newborns were screened in the pilot, including 44268 males and 18285 females. As part of routine screening procedure, DBS were collected usually at 48 h to seven days after birth at 129 delivery hospitals in Guangzhou and sent to Guangzhou newborn screening center. The relevant demographic information was collected routinely by electronically, including sex, gestational age (GA), birth weight (BW), and age at sampling. Informed consent was obtained from parents prior to the collection of samples. The pilot study was approved by the Ethics Committee of Guangzhou Women and Children's Medical Center.
2.2. DMD screening program
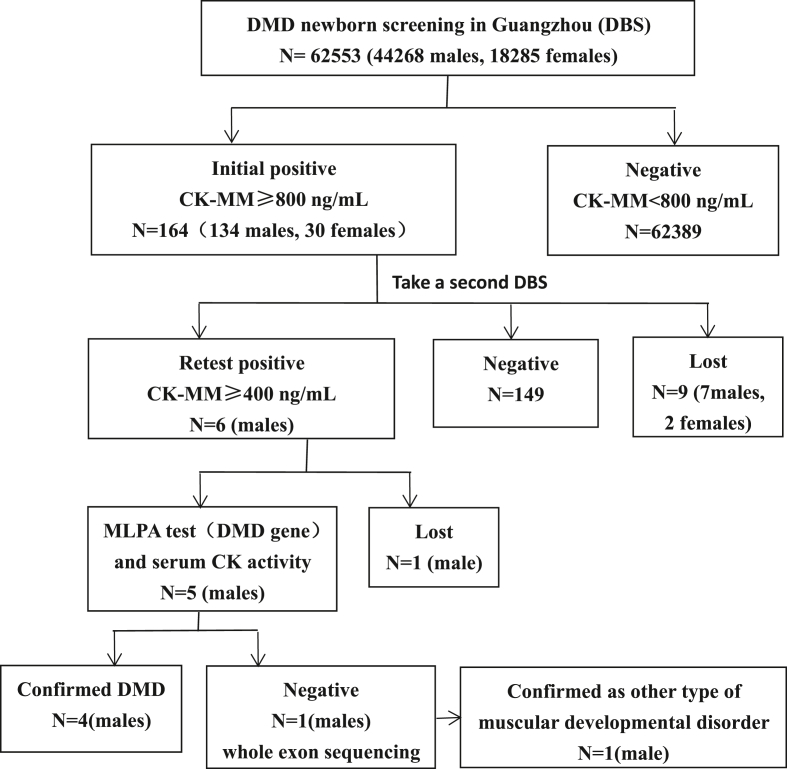
CK-MM concentration in 3.2 mm DBS was measured using the GSP® Neonatal CK-MM kit (PerkinElmer, Finland) which was based on time-resolved immunofluorescence. The analytical performance (accuracy, precision, linear range) of CK-MM was validated before pilot. Cutoff value was established at 99.7th percentile of 8419 samples, corresponding to 800 ng/mL. Newborns with positive screen result (CK-MM≥800 ng/mL) were recalled back to delivery hospitals for recollecting DBS to retest. Then we recalled the newborns with retest positive result (CK-MM ≥ 400 ng/mL) to Guangzhou newborn screening center for further evaluation (Figure 1). DMD was confirmed by multiplex ligation-dependent probe amplification (MLPA) for the DMD gene and increased serum CK activity (normal reference range, 45–390 U/L; Hitachi 7600-020 Automatic Analyzer), which was measured using the creatine kinase-NAC assay kit (Beijing Jiuqiang Biotechnology Company, China) and based on phosphocreatine method. When the MLPA test was negative, whole exon sequencing was performed.
Figure 1.
The flowchart of DMD screening in Guangzhou.
2.3. Analytical performance of CK-MM determined by time-resolved fluorescence method
2.3.1. Precision and accuracy
Take the low, median and high quality control samples provided by the CK-MM kit, each sample was measured for 5 times per day and continuously for 5 days, the intra-assay and inter-assay coefficient of variation (CV) were calculated. At the same time, the deviation of the mean value from the target value was calculated.
2.3.2. Linear range
Take the calibrator provided with the kit, each sample was measured for 3 times, the theoretical value was X, the mean value of the measured value was Y, and the regression equation and r value were calculated.
2.4. Grouping criteria
Using the following designations: preterm birth: <32 weeks (n = 327), 32–36 weeks (n = 2197), 36–37 weeks (n = 2183), full-term birth: 37–42 weeks (n = 57812), post-term birth: ≧42 weeks (n = 34). Low BW: <1000 g (n = 41), 1000–1500 g (n = 227), 1500–2500 g (n = 3924), normal BW: 2500–4000 g (n = 56612), macrosomia: ≧4000 g (n = 1749). The age of Sample collection were designated as follows: 24–48 h (n = 13869), 48–72 h (n = 29103), 72–168 h (n = 18521), and ≧168 h (n = 1060).
2.5. Statistical analysis
Statistical analysis was performed using SPSS ver. 19.0. Single sample Kolmogorov-Smirnov test was used for normality test. The data was visualized with scatter plots and relevant descriptive statistics (sample size, median value and quartile) were calculated. In addition, higher percentiles (99% and 99.7%) were calculated. Incidence rate was described with a 95% confidence interval and compared statistically with the Pearson χ2 test. The nonparametric test between two groups was performed using the Mann-Whitney U test and Kruskal-Wallis test between multiple groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Analytical performance of detection system
The analytical performance (accuracy, precision, linear range) of CK-MM determined by time-resolved fluorescence method was validated. The within-run coefficient of variation (CV), the between-run CV and average bias of CK-MM were <10%. The linear regression equation was Y = 1.0325X − 17.216 (r = 0.999).
3.2. Performance of DMD screening
Among 62553 newborns screened for DMD, 164 cases (0.26%) were screened positive, including 134 males and 30 females. 94.5% (155/164) of the cases were recalled back. Six boys were persistently positive (CK-MM ≥ 400 ng/mL). Among them, four boys were confirmed DMD by MLPA for DMD gene and increased serum CK activity(Table 1). One boy with CK-MM 4580 ng/mL, serum CK 2937 U/L was confirmed as other type of muscular developmental disorder by whole exon sequencing (data not shown). The other male with high serum CK was lost to follow-up due to parents refusal for gene assay. The positive predictive value was 3.2% in males. The total incidence of DMD was 1/15638 (95% confidence interval 0–1/7874). The incidence in males was 1/11067 (95% confidence interval 0–1/5556).
Table 1.
Samples identified as indicating DMD.
| Gender | Initial CK concentration (ng/mL) | Blood serum (U/L) | Gene | Mutation |
|---|---|---|---|---|
| Male-1 | >7040 | 12849 | DMD | Dul ex45, ex54-60 |
| Male-2 | 5130 | 13555 | DMD | Dul ex8-9 |
| Male-3 | >6320 | 11119 | DMD | Del ex48-50 |
| Male-4 | >6320 | 14480 | DMD | Del ex45-50 |
3.3. The distribution of CK-MM concentration according to genders, GA, BW and age at sampling
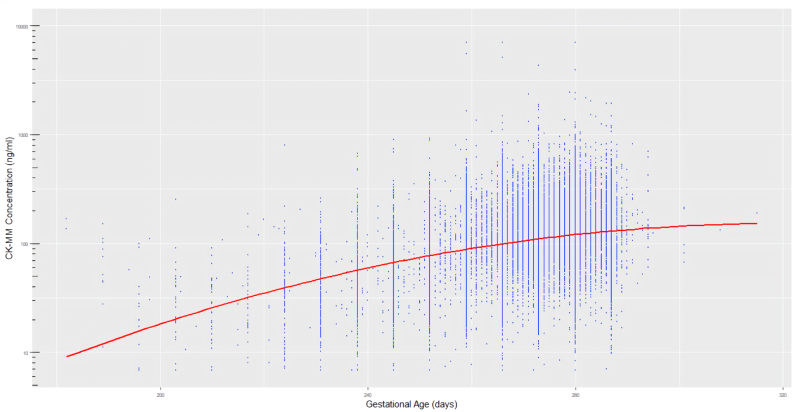
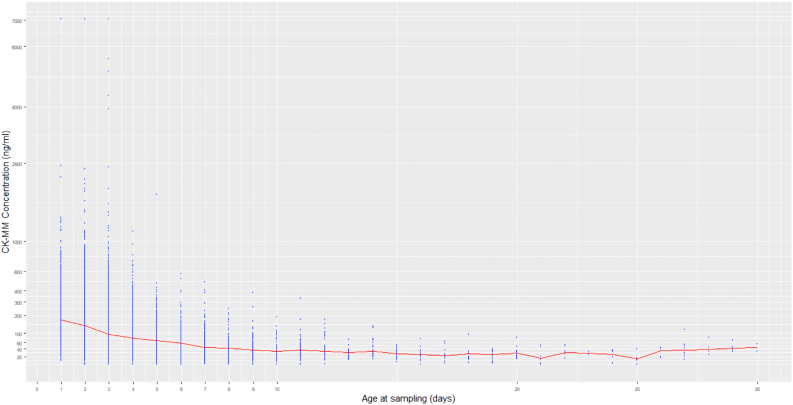
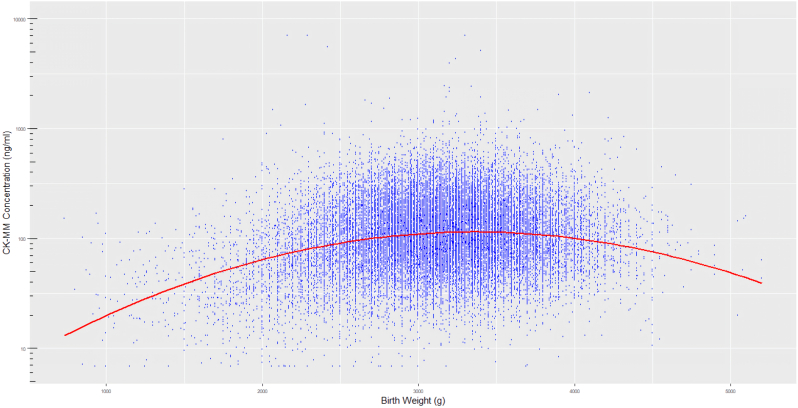
The distribution of CK-MM concentration between male and female subgroups was different (P < 0.001), the median value (Table 2) in male (113.0 ng/mL) was slightly higher than female (98.7 ng/mL). The CK-MM concentrations were statistically correlated with increasing GA, BW and age at sampling (P < 0.001, respectively). The changing relationship between them are shown in Figure 2, Figures 3 and 4. Multiple linear regression analysis was implemented to compare the effects of GA, BW and age at sampling on CK-MM concentration (P = 0.000). The regression equation was Y = −334.4 + 1.98 × GA − 0.02 × BW − 5.2 × sampling time and a stronger correlation was observed between GA, sampling time and CK-MM concentration than between BW and CK-MM concentration. The median of CK-MM concentration was progressively decreased with age at sampling, which was 159.0 ng/mL at 24–48 h, 85.4 ng/mL at 3–7 days, respectively (Table 2).
Table 2.
The distribution of CK-MM concentration in relation to gender, gestational age (GA), birth weight (BW) and age at sampling subgroups.
| Group | N | CK-MM Percentile values (ng/mL) |
|||||
|---|---|---|---|---|---|---|---|
| Median | 25th | 75th | 99th | 99.7th | |||
| Gender | Male | 44268 | 113 | 73.0 | 173 | 544 | 805.8 |
| Female | 18285 | 98.7 | 65.9 | 151 | 469 | 658.3 | |
| Gestational age | Premature | ||||||
| <32 W | 327 | 28.9 | 16.9 | 50.8 | 196.4 | 263.3 | |
| 32–36 W | 2197 | 48.5 | 28.4 | 80.4 | 316 | 687.4 | |
| 36–37 W | 2183 | 77.4 | 50 | 119 | 394.3 | 649.8 | |
| Term 37–42 W | 57812 | 112 | 74.6 | 171 | 529 | 772 | |
| Overdue ≥42 W | 34 | 144 | 104.5 | 208.5 | 675.6 | 694.1 | |
| Birth weight | Low BW | ||||||
| <1000 g | 41 | 34.6 | 21.8 | 81.4 | 161.2 | 166 | |
| 1000–1500 g | 227 | 30.6 | 16.8 | 56 | 196.4 | 232.7 | |
| 1500–2500 g | 3924 | 66.5 | 38 | 111 | 448.8 | 892.3 | |
| Normal BW 2500–4000 g | 56612 | 112 | 73.9 | 170 | 526 | 751.3 | |
| Macrosomia ≥4000 g | 1749 | 98.2 | 66 | 151.5 | 556 | 804.3 | |
| Age at sampling | 24–48 h | 13869 | 159 | 107 | 234 | 669.3 | 993.4 |
| 48–72 h | 29103 | 108 | 73.4 | 161 | 476 | 676.4 | |
| 72–168 h | 18521 | 85.4 | 57.3 | 126 | 388 | 581.3 | |
| ≥168 h | 1060 | 33.1 | 22 | 49.9 | 210.2 | 376.4 | |
The distribution of CK-MM was significantly different between males and females (Mann-Whitney U test, P < 0.001).
Differences in the CK-MM concentrations for each subgroup of gestational age were statistically significant (Kruskal-Wallis test, P < 0.001).
Differences in the CK-MM concentrations for each subgroup of birth weight were statistically significant (Kruskal-Wallis test, P < 0.001).
The differences in the CK-MM concentrations for each subgroup of age at sampling were statistically significant (Kruskal-Wallis test, P < 0.001).
Figure 2.
CK-MM distribution by GA.
Figure 3.
CK-MM distribution by age at sampling.
Figure 4.
CK-MM distribution by BW.
4. Discussion
4.1. The incidence of DMD
The DMD incidence of newborn males was 1/11067 based on the pilot, which is obviously lower than in previous studies reported globally, which ranged from 1/3,802 to 1/6,291 [11]. The difference may be related to two factors. First it is noteworthy that the CK-MM values of the 9 lost to follow-up or refuse to back (7 males, 2 females) ranged from 1070 ng/mL to 2420 ng/mL, there may also be DMD patients among them. Apart from this, one male had a highly suspicion of DMD whose serum CK greater than 10,000 U/L (initial CK-MM concentration 1820 ng/mL), and the diagnosis was not confirmed due to refusal of further examination. So it did not exclude the DMD cases in 10 cases due to loss to follow-up.
In addition, The difference would have to be viewed cautiously based on the sample size, the finished screening sample size is small relatively (44268 males), the increase or decrease of each confirmed case will bring obvious change, so the statistics incidence rate based on current screening results may not be enough to reflect the region's actual incidence of DMD, and further screening is still needed for related statistical analysis.
4.2. The CK-MM concentration of different sex
Whether female should be screened early for DMD has always been controversial. Since Duchenne is an X-linked recessive disease, females are typically asymptomatic carriers of mutations [10]. In more than 1.8 million newborns screened by 10 DMD screening programs worldwide from 1975 to 2011, only about 9% were females [12]. However, some female carriers may exhibit symptoms and have increased CK-MM concentration at birth, leading to a positive screening test [10, 13]. Furthermore there is no precedent for female DMD screening in China, we included female newborns in the pilot to better understand DMD in females.
The results demonstrated that there was significant difference in the concentration distribution of CK-MM between male and female (P < 0.001) in the pilot (Table 2). According to the CK-MM concentration calculated by P99.7, the CK-MM concentration of males and females was 805.8 ng/mL, 658.3 ng/mL respectively. However, we still recommend the same cut-off value for female and male newborns because we did not find any confirmed female cases based on the screening results of 18285 females in the first phase of pilot. Furthermore, we recalled all the females (24 cases) with CK-MM concentration between 660 ng/mL and 800 ng/mL for re-examination of CK, and no positive results was found. The specificity for female cutoffs of 660 ng/mL and 800 ng/mL was 99.7% and 99.8%, respectively, with little difference. Although the results were expected, considering the small amount of specimens at the second phase, we decided to adjust the screening strategy focusing on males only. In the two phases of the pilot, we confirmed two males with DMD respectively, a total of four DMD males were identified. In this study, due to the small sample size, no female is diagnosed with DMD, so large-scale female DMD screening may be still needed. Moreover, according to the wishes of parents, genetic testing may be performed for females who are initially screened positive, which may increase the diagnosis rate of DMD in female.
4.3. Effect of BW, GA and age at sampling on CK-MM concentration
Based on our results the influence of BW, GA and age at sampling on the concentration of CK-MM is evident. The concentration of CK-MM showed a gradual increase with increasing GA (Figure 1), and the finding was consistent with the previous report [9]. It was also found to inversely correlate with the sampling age of the newborn at the same time (Figure 2). There was less correlation between BW and CK-MM value (Figure 3).
A worth considering issue related to neonatal CK detection and age at sampling is the potential contribution of elevated enzymes caused by trauma as the neonate progresses through the birth canal [11, 14]. Therefore, in unaffected newborns, CK-MM may increase at birth and gradually decrease in the following days. In our study the median of CK-MM concentration decrease gradually in the first few days after birth and remained basically constant at approximately 40 ng/mL after 7 days (Figure 2) which was similar with the DMD report in Zhejiang province in China and New York in USA [6, 15]. Therefore, the influence of age at sampling on CK-MM concentration is a factor worth considering, especially in the first few days of birth. A pilot program in Australia was working to identify the optimal time for DMD-NBS, comparing screening at 24–96 h vs. 6–7 days, vs. 6–12 weeks of age [6]. This procedure may limit false-positive findings caused by birth trauma.
However, under the usual neonatal screening time frame, it is not easy to change the initial age at sampling to optimize on biomarker. It is more feasible to establish the cut-off value according to the concentration percentile distribution of DMD at different age at sampling in the existing screening time frame.
4.4. The cut-off value of CK-MM concentration
The CK-MM cut-off value for DMD neonatal screening has typically been based on a fixed value [11, 16]. However, our pilot demonstrated that if only one fixed cut-off value is adopted without considering the influence of GA, BW and age at sampling on the CK-MM concentration, it may lead to an increase in false positive results for newborns with early blood sampling. What is more noteworthy is that for premature and low BW newborns, it may be result in false negative screening [16]. Further linear regression analysis showed that CK-MM concentration was much more closely correlated with GA and age at sampling, with a minimal effect related to BW. Therefore, GA and age at sampling are two influence factors that deserve more attention, if the multitier cut-off value can be applied according to gestational age and age at sampling in DMD screening, the efficiency of DMD screening might be effectively improved.
According to the statistical results in Table 2, it can be found that the subgroup of preterm infants (<32 weeks) has significantly lower CK-MM concentrations, although the subgroup is a small sample size and is a poor representation. Previous studies have showed that some Duchenne affected preterm newborns do not have elevated CK values. Preterm Duchenne affected infants may have not yet sustained enough muscle damage to elevate the CK-MM marker above the cut-off level whereas muscle damage may start in utero [9]. False-negative cases have been reported in the Duchenne pilots of Wales, Germany, Belgium, Cyprus and Manitoba [12, 16, 17], the mean age at time of initial serum/plasma CK and definitive diagnosis was 3.95 and 4.33 years, respectively [16]. In addition, Timonen et al [9] reported a false negative result from a premature infant (gestational age 27 weeks) in a very low premature and very low birth weight (1200 g). The CK-MM concentration value of the first screening was much lower than the cut-off value, and the result of second sample in 35 days was still negative. These reports remind us that when clinical symptoms and manifestations suggest a suspicious DMD, clinicians should re-examine the patient regardless of whether the neonatal screening results are negative or not. The regular pediatric clinical visits are still essential ways to prevent missed diagnosis of DMD. For very premature newborns, second tier screening may be a better feasible option [18]. Since CK-MM may not be elevated right after birth, the optional time for second-tier still needs further research to determine.
4.5. The choice of laboratory screening approach
At present, most programs of DMD screening globally have used 2-step screening and 3-step screening approach [19]. Compared with the 2-step screening approach of direct recall of initial positive samples for DMD gene mutation test [11], we choose a 3-step screening approach for recall of positive samples at the initial screening test and for genetic diagnosis of newborns who were still positive after review.
The current newborn screening network in Guangzhou is composed of Guangzhou newborn screening center, regional maternal and child health centers, and midwifery agencies, which ensures that samples with positive initial screening can be recalled quickly. Among the 164 positive samples recalled, 6 samples were still positive. The number of false positive samples was significantly decreased in the retest. It meant that the number of newborns who needed further genetic sequencing was greatly reduced.
The selected screening approach is more based on the screening framework, the capabilities of the NBS laboratory and on the age at sampling in the region where the screening laboratory is located. In the local screening frame where the laboratory located, if most newborns choose to collect blood at 2–3 days after birth, the false positive results caused by birth canal damage cannot be avoided, and positive newborns can be quickly recalled, the 3-step screening approach might be a better choice. However, if the health care infrastructure is more compatible with 2-step approach [11, 16] or the newborn's sampling time is 5 days or later similar to the model reported in Germany [20], 2-step approach is also an ideal choice.
5. Conclusion
Our pilot included a DMD screening of both male and female newborns for the first time in China. Although the results demonstrated that the CK-MM distribution in male newborns and female newborns has significantly difference, we still recommened a single cut-off value for both sexes when implementing neonatal population based DMD screening. DMD newborn screening should be male priority. CK-MM concentration was found to be associated with GA, BW and age at sampling. Because GA and age at sampling have a better correlation with CK-MM concentration in newborns than BW, the efficiency of DMD screening might be efficiently improved by adjusting the multitier cut-off value according to gestational age and age at sampling. Preterm babies should be tested at a later age or second tier screening since CK-MM may not be elevated right after birth.
The development of new therapies and the application of standardized kits provide a strong argument exists for DMD screening and screening has indeed reduced the diagnostic delay enabling reproductive choice for parents of affected boys and earlier administration of current therapies. However, the treatment is expensive, and few families can afford the financial burden of lifelong medication under current national health care security system. Whether to conduct a wide scale implementation screening for male infants or to move into a nationwide Duchenne screening, there is still a long way to develop.
Declarations
Author contribution statement
Yonglan Huang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xuefang Jia and Xiang Jiang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Moat S.J., Korpimaki T., Furu P., Hakala H., Polari H., Merio L., Makinen P., Weeks I. Characterization of a blood spot creatine kinase skeletal muscle isoform immunoassay for high-throughput newborn screening of duchenne muscular dystrophy. Clin. Chem. 2017;63(4):908–914. doi: 10.1373/clinchem.2016.268425. [DOI] [PubMed] [Google Scholar]
- 2.Pillers D.A. A new day for Duchenne's?: the time has come for newborn screening. Mol. Genet. Metabol. 2014;113(1-2):11–13. doi: 10.1016/j.ymgme.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Cyrus A., Street N., Quary S., Kable J., Kenneson A., Fernhoff P. Clinic-based infant screening for duchenne muscular dystrophy: a feasibility study. PLoS Curr. 2012 doi: 10.1371/4f99c5654147a. e4f99c5654147a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mah J.K. Current and emerging treatment strategies for Duchenne muscular dystrophy. Neuropsychiatric Dis. Treat. 2016;12:1795–1807. doi: 10.2147/NDT.S93873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vita G.L., Vita G. Is it the right time for an infant screening for Duchenne muscular dystrophy? Neurol. Sci. 2020;41(7):1677–1683. doi: 10.1007/s10072-020-04307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ke Q., Zhao Z.Y., Griggs R., Wiley V., Connolly A., Kwon J., Qi M., Sheehan D., Ciafaloni E., Howell R.R., Furu P., Sazani P., Narayana A., Gatheridge M. Newborn screening for Duchenne muscular dystrophy in China: follow-up diagnosis and subsequent treatment. World J Pediatr. 2017;13(3):197–201. doi: 10.1007/s12519-017-0036-3. [DOI] [PubMed] [Google Scholar]
- 7.Orfanos A.P., Naylor E.W. A rapid screening test for Duchenne muscular dystrophy using dried blood specimens. Clin. Chim. Acta. 1984;138(3):267–274. doi: 10.1016/0009-8981(84)90133-5. [DOI] [PubMed] [Google Scholar]
- 8.Bradley D.M., Parsons E.P., Clarke A.J. Experience with screening newborns for Duchenne muscular dystrophy in Wales. BMJ. 1993;306(6874):357–360. doi: 10.1136/bmj.306.6874.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timonen A., Lloyd-Puryear M., Hougaard D.M., Merio L., Makinen P., Laitala V., Polonen T., Skogstrand K., Kennedy A., Airenne S., Polari H., Korpimaki T. Duchenne muscular dystrophy newborn screening: evaluation of a new GSP((R)) neonatal creatine kinase-MM kit in a US and Danish population. Int. J. Neonatal. Screen. 2019;5(3):27. doi: 10.3390/ijns5030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogerwaard E.M., Bakker E., Ippel P.F., Oosterwijk J.C., Majoor-Krakauer D.F., Leschot N.J., Van Essen A.J., Brunner H.G., van der Wouw P.A., Wilde A.A.M., de Visser M. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet. 1999;353(9170):2116–2119. doi: 10.1016/s0140-6736(98)10028-4. [DOI] [PubMed] [Google Scholar]
- 11.Mendell J.R., Shilling C., Leslie N.D., Flanigan K.M., al-Dahhak R., Gastier-Foster J., Kneile K., Dunn D.M., Duval B., Aoyagi A., Hamil C., Mahmoud M., Roush K., Bird L., Rankin C., Lilly H., Street N., Chandrasekar R., Weiss R.B. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann. Neurol. 2012;71(3):304–313. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 12.Gatheridge M.A., Kwon J.M., Mendell J.M., Scheuerbrandt G., Moat S.J., Eyskens F., Rockman-Greenberg C., Drousiotou A., Griggs R.C. Identifying non-duchenne muscular dystrophy-positive and false negative results in prior duchenne muscular dystrophy newborn screening programs: a review. JAMA Neurol. 2016;73(1):111–116. doi: 10.1001/jamaneurol.2015.3537. [DOI] [PubMed] [Google Scholar]
- 13.Kemper A.R., Wake M.A. Duchenne muscular dystrophy: issues in expanding newborn screening. Curr. Opin. Pediatr. 2007;19(6):700–704. doi: 10.1097/MOP.0b013e3282f19f65. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph N., Gross R.T. Creatine phosphokinase activity in serum of newborn infants as an indicator of fetal trauma during birth. Pediatrics. 1966;38(6):1039–1046. [PubMed] [Google Scholar]
- 15.Park S., Maloney B., Caggana M., Tavakoli N.P. Muscle Nerve; 2022. Creatine Kinase-MM Concentration in Dried Blood Spots from Newborns and Implications for Newborn Screening for Duchenne Muscular Dystrophy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moat S.J., Bradley D.M., Salmon R., Clarke A., Hartley L. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK) Eur. J. Hum. Genet. 2013;21(10):1049–1053. doi: 10.1038/ejhg.2012.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheuerbrandt G., Lundin A., Lovgren T., Mortier W. Screening for Duchenne muscular dystrophy: an improved screening test for creatine kinase and its application in an infant screening program. Muscle Nerve. 1986;9(1):11–23. doi: 10.1002/mus.880090103. [DOI] [PubMed] [Google Scholar]
- 18.CLSI . CLSI Guideline NBS03. second ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2019. Newborn screening for preterm, low birth weight, and sick newborns.https://clsi.org/media/3291/nbs03ed2%20sample.pdf,%202022 [Google Scholar]
- 19.Kwon J.M., Abdel-Hamid H.Z., Al-Zaidy S.A., Mendell J.R., Kennedy A., Kinnett K., Cwik V.A., Street N., Bolen J., Day J.W., Connolly A.M. Clinical follow-up for duchenne muscular dystrophy newborn screening: a proposal. Muscle Nerve. 2016;54(2):186–191. doi: 10.1002/mus.25185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plauchu H., Junien C., Maire I., Said R., Gozlan R., Lalouel J.M. Detection of carriers for Duchenne muscular dystrophy. Quality control of creatine kinase assay. Hum. Genet. 1982;61(3):205–209. doi: 10.1007/BF00296443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.