Take Home Message
Silicone DJ stents, and by extension “soft” DJ stents, appear to reduce stent-related symptoms. No definitive conclusion can be drawn because of the lack of quality evidence. A standard for measuring physical stent properties should be developed.
Keywords: Urolithiasis, Ureteral stent, Double-J stent, Material, Stent-related discomfort, Stent-related symptoms, Review
Abstract
Context
Ureteral stents are essential implants that are used on a daily basis. Since their invention, advances in stent design have been directed towards alleviating stent-related symptoms. It remains unclear how the material composition of the stent affects stent-related symptoms.
Objective
To review the literature and define the clinical impact of ureteral stent material on stent-related symptoms.
Evidence acquisition
A literature search of the Embase, MEDLINE (PubMed), and Web of Science databases was conducted on December 17, 2021 to collect articles comparing stent composition materials regarding stent-related symptoms. Thirteen publications met the inclusion criteria, of which only one met the high-quality requirements of the Cochrane Collaboration tool for assessing the risk of bias in randomized trials.
Evidence synthesis
Most trials, including the highest quality trial, seem to support that silicone double-J (DJ) stents reduce stent-related symptoms compared to nonsilicone DJ stents. Regarding physical properties, it seems that “soft” or “flexible” DJ stents reduce stent-related symptoms. However, since there was only one high-quality study with a low risk of bias, it is impossible to draw a definitive conclusion owing to the lack of quality data.
Conclusions
Silicone DJ stents, and by extension “soft” DJ stents, appear to reduce stent-related symptoms compared to nonsilicone polymers and “hard” DJ stents. No definitive conclusion can be drawn owing to a lack of quality evidence. Creating a standard for measuring and reporting physical stent properties should be the first step for further research.
Patient summary
A ureteral stent is a small hollow tube placed inside the ureter to help urine drain from the kidney. We reviewed the literature on the impact of stent material on stent-related symptoms. We found that silicone may reduce stent-related symptoms, but no definitive conclusion can be drawn and further studies are needed.
1. Introduction
Ureteral stents are essential implants that are used on a daily basis. The downside of these widely used implants is that up to 88% of patients experience at least some form of discomfort [1], [2], [3]. Examination of the stents available on the market reveals a wide variety of stent characteristics, with differences in the overall design, material composition, and coating. Several changes in overall stent design have been introduced in attempts to reduce stent-related symptoms, but the double-J (DJ) stent is still the design most commonly used [4], [5], [6], [7], [8].
Advances in stent material composition have focused on improving properties such as flexibility, elasticity, biocompatibility, catheter wall thickness (inner/outer diameter [ID/OD] ratio), and surface properties (e.g. porosity, hydrophilicity, and antibacterial properties) affecting encrustation, bacterial adhesion rates, and the friction coefficient [9], [10], [11]. The first DJ stents introduced to the market were made of silicone, which is considered “soft” and biocompatible [10], [11]. However, at that time, silicone stents showed several limitations. First, the flexibility and lack of hydrophilic guidewires, with silicone causing high interface friction between the stent and the guidewire, resulted in difficult handling [11], [12], [13]. Second, silicone has high susceptibility to compression, which necessitates an unfavorably low ID/OD ratio and results in lower drainage efficacy [11], [12], [13]. Consequently, in the search for better material properties, silicone was replaced by polyurethane in the 1980s [10], [11]. Since then, several efforts have been made by manufacturers to further optimize the stent material composition by modifying the polymers used.
It remains unclear how the chemical and physical properties of stents affect their biocompatibility and tolerability [9], [10], [14]. Our aim here was to review the literature to identify the clinical impact of ureteral stent material on stent-related symptoms.
2. Evidence acquisition
This study was registered in PROSPERO (CRD42022264829). The systematic search was guided by “A systematic approach to searching” [15] in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) checklist [16]. A literature search was conducted by two authors (M.B. and V.D.C) on December 17, 2021, using the Embase, MEDLINE (PubMed), and Web of Science databases.
We determined a clear and focused question using the PICO (Population, Intervention, Compapartor, Outcome) approach: do adult patients receiving a ureteral stent composed of material A, compared to a ureteral stent composed of material B with the same overall stent design, have more or less stent-related symptoms. Then these PICO elements were used to identify appropriate index terms (Emtree and MeSH) and synonyms in the thesaurus of the databases used. Variations in search terms and database-appropriate syntax with parentheses, field codes, and Boolean operators were used to maximize the yield. Searches were restricted to English-language articles on humans. No restriction on time period was applied.
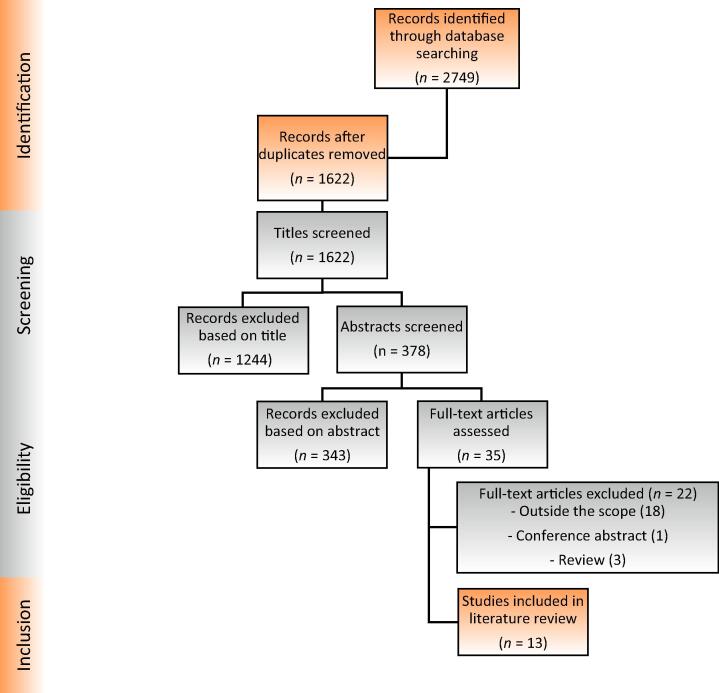
All original articles meeting the PICO approach, both retrospective and prospective, and with the outcome measured in any way as an endpoint (not exclusively the primary endpoint) were included. Case reports and meeting abstracts were not considered eligible. Our search strategy, build, and log are provided in the Supplementary material. Figure 1 shows the PRISMA flowchart. The Embase, MEDLINE (PubMed), and Web of Science search yielded 13 articles eligible for inclusion in the review.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) diagram.
A formal risk-of-bias analysis was conducted for the studies included using the Cochrane Collaboration risk of bias assessment tool (RoB 2) for the randomized studies (7 of the 13 studies; Table 1, Table 2) and the Cochrane Collaboration risk of bias tool for nonrandomized studies for interventions (ROBINS-I) for the nonrandomized prospective studies (3 of the 13 studies; Table 2, Table 3) and the one nonrandomized retrospective study (Table 2, Table 3) [17], [18]. Two of the 13 studies were designed as self-controlled case series studies, for which a risk of bias analysis could not be conducted (Table 2). All the formal assessments are detailed in the Supplementary material.
Table 1.
Risk of bias assessment for the randomized trials using the Cochrane RoB 2 tool
| Study | Bias arising from the randomization process | Bias due to deviations from intended intervention | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported result | Overall |
| Lennon (1995) [9] |  |
 |
 |
 |
 |
 |
| Candela (1997) [19] |  |
 |
 |
 |
 |
 |
| Lee (2005) [20] |  |
 |
 |
 |
 |
 |
| Joshi (2005) [14] |  |
 |
 |
 |
 |
 |
| Mendez-Probst (2012) [21] |  |
 |
 |
 |
 |
 |
| El-Nahas (2018) [22] |  |
 |
 |
 |
 |
 |
| Wiseman (2020) [23] |  |
 |
 |
 |
 |
 |
The symbol  indicates a low risk of bias,
indicates a low risk of bias,  indicates an unclear risk of bias, and
indicates an unclear risk of bias, and  indicates a high risk of bias.
indicates a high risk of bias.
Table 2.
Summary of the studies included and their main characteristicsa
| Study | Stents compared | Hydrogel coating | D (Fr) | Length (cm) | Patients | Inclusion criteria | Exclusion criteria | Symptom evaluation | Stent removal | USSQ | Design | RoB | IF | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smedley (1988) [24] | Silicone Polyurethane |
Unknown Unknown |
NA | NA | 116 | All patients needing placement of a DJ stent | NA | Day of stent removal | Mean D79 (range 1–366) | No | RS | Serious | NA | Less loin discomfort and trigonal irritation with a silicone stent (but not significant). |
| Pryor (1991) [25] | Cook polyurethane Surgitek Silitek Cook C-Flex Van-Tec Soft |
Unknown Unknown No Unknown |
7 | 22–24 | 73 | All patients needing placement of a DJ stent | Bilateral stents, long-term stent | D2 and D6 and 7 d after removal | D6–D30 | No | PCS | Moderate | NA | No evidence of differences in SRS. |
| Lennon (1995) [9] | Cook polyurethane Cook Sof-Flex |
Unknown Yes |
6 | 22–26 | 155 | DJ stent placed for ureteral calculi, SWL, and other miscellaneous endourologic interventions | NA | At day of stent removal | Mean D37 (range 7–182) | No | RCT | High | NA | SRS of renal pain, suprapubic pain, and dysuria were significantly higher in the polyurethane (firm) than in the Sof-Flex (soft) group. No differences in the presence of reflux pain, urgency, frequency, or hematuria. SRS severity was clearly greater with the firm stent. |
| Candela (1997) [19] | Bostonb Percuflex Bostonb Percuflex Plus |
No Yes |
4.8–6 | NA | 60 | Stent for SWL, obstruction, or URS | Bilateral stents. | D7-10 | D7–D10 | No | RCT | High | NA | No evidence of differences in SRS. |
| Lee (2005) [20] | Bard Inlay Cook Endo-Sof Bostonb Contour Applied Medical Vertex Surgitek Classic |
Yes Yes Yes Yes Yes |
6 | 22–30 | 44 (70 included) | All patients undergoing unilateral retrograde ureteral stent placement | Untreated UTI, bladder cancer, additional transurethral procedures, spinal cord injury | D1, D3, D5 and 30 days after removal | NA | Yes | RCT | High | NA | Significantly fewer urinary symptoms with the Bard Inlay stent than the other stents on D3. The Bard Inlay stent had the most significant positive characteristics, while the Vertex and Surgitek Classic stents were associated with more significant negative characteristics. |
| Joshi (2005) [14] | Bostonb Percuflex Plus Bostonb Contour |
Yes Yes |
6 | 24 | 130 | DJ-stent placed after URS and ESWL for stone disease | NCD, pregnancy, bilateral stents, long-term stent, stenting after PCNL | D7, D28, D56 | D28 | Yes | RCT | Some concerns | NA | No evidence of differences in SRS |
| El-Nahas (2006) [26] | Coloplasta silicone Bostonb Percuflex |
Unknown Unknown |
6–14 | 24–26 | 100 | DJ stent placed after endopyelotomy, URS, laparascopic pyeloplasty, or endoureterotomy | SWL, pregnancy, pre-existing LUTS, complicated procedure | Day of stent removal | Mean D56 (range 28–112) | No | PCS | Serious | NA | Significantly more patient discomfort with the Percuflex (“hard”) stent than with the silicone (“soft”) stent. |
| Cadieux (2009) [29] | Bostonb Percuflex Plus Triumph triclosan-eluting stent |
Yes No |
NA. | NA | 8 | Long-term stent for cancer, strictures, or fibrosis | NA. | Day of stent removal | D90 | No | SCCS | NA | Yes | Fewer symptomatic UTI's in patients with Triumph® triclosan eluting stents. |
| Mendez-Probst (2012) [21] | Bostonb Percuflex Plus Triumph triclosan-eluting stent |
Yes No |
NA. | 27 (mean) | 20 | Patients requiring short-term stenting | NA | Day of stent removal | D 7–D15 | No | RCT | High | Yes | Significantly less patient discomfort with Triumph® triclosan eluting stents. |
| Chow (2015) [30] | Cook Resonance Unspecified polymeric stent |
No Unknown |
6 | 20–30 | 42 | Cancer patients with malignant ureteral obstruction | No previous polymeric stent | NA | NA | No | SCCS | NA | No | No evidence for differences on stent-related symptoms. |
| El-Nahas (2018) [22] | Carbothan + hydrogel coating Carbothan + silver sulfadiazine |
Yes No |
6 | 26 | 126 | DJ stent placement after URS lithotripsy | <18 yr | Day of stent removal | Mean 3.1 ± 1.2 wk | Yes | RCT | Some concerns | No | No evidence for differences on stent-related symptoms. |
| Gadzhiev (2020) [27] | Cook Black silicone Rüschc polyurethane |
Unknown Unknown |
6 | 26 | 50 | DJ stent placement for acute renal colic due to ureteral stone | <18 yr, >60 yr, active UTI, urogenital tumor | D1, D14, D28 | D28 | No | PCS | Moderate | Nod | Silicone stents were associated with lower body pain intensity on VASP at 2 wk before and immediately before stent removal vs polyurethane stents. |
| Wiseman (2020) [23] | Coloplast ImaJin Hydro Bostonb Percuflex Plus |
Yes Yes |
6 | 26 | 113 (141 included) | DJ stent placement after fURS for renal stones (5–25mm) | Acute colic pain, UT malformation, urogenital tumor, indwelling DJ stent, untreated UTI | D2, D7, D20 and D35 | D20 | Yes | RCT | Low | Yes | Silicone stents were associated with significantly less patient discomfort and better QoL compared to Percuflex. There was a 25% reduction in USSQ pain score (at D20) in favor of silicone. Urinary symptoms (relevant USSQ domain) were significantly lower in the silicone group at D2, D7, and D20, with the largest difference evident at D20. |
D = diameter; RS = retrospective study; PCS = prospective cohort study; RCT = randomized controlled trial; SCCS = self-controlled case series; RSCCS = retrospective SCCS; USSQ = Ureteral Stent Symptom Questionnaire; RoB = risk of bias; DJ = double J; IF = industry funding; NA = not available; NOS = Newcastle-Ottawa Quality Assessment Scale; SWL = shockwave lithotripsy; URS = ureteroscopy; UT = urinary tract; UTI = UT infection; LUTS = lower urinary tract symptoms; NCD = noncalculus disease; fURS = flexible URS; PCNL = percutaneous nephrolithotomy; SRS = stent-related symptoms; VASP = Visual Analog Scale for Pain; QoL = quality of life.
Porges S.A.
Microvasive, Boston Scientific.
Teleflex.
The first author (Gadzhiev) is a paid consultant for Cook Medical.
Table 3.
Risk of bias assessment for the nonrandomized trials using the Cochrane ROBINS-I tool
| Study | Bias due to confounding | Bias due to selection of participants | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall |
| Smedley (1988) [24] |  |
 |
 |
 |
 |
 |
 |
 |
| Pryor (1991) [25] |  |
 |
 |
 |
 |
 |
 |
 |
| El-Nahas (2006) [26] |  |
 |
 |
 |
 |
 |
 |
 |
| Gadzhiev (2020) [27] |  |
 |
 |
 |
 |
 |
 |
 |
The symbol  indicates a low risk of bias,
indicates a low risk of bias,  indicates a moderate risk of bias, and
indicates a moderate risk of bias, and  indicates a serious risk of bias.
indicates a serious risk of bias.
Our original intention was to perform a pooled data analysis and meta-analysis after collection of all the data from the eligible studies. However, there was a substantial degree of heterogeneity among the studies in terms of both design and outcomes, so the analysis was limited to a narrative synthesis of the results.
3. Evidence synthesis
A total of 13 studies assessing the impact of stent material on stent-related symptoms were included in our review [9], [14], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]. The studies and their main characteristics are summarized in Table 2.
All the stent materials that were compared are listed in Table 2 and Table 4. Table 4 provides information on the composition and commercial name of all the stents compared in the 13 studies. The number of patients included ranged from 8 to 155 per study. All the stents were DJ stents. The material most often used was Percuflex (Table 4). If specified, most of the DJ stents had a hydrogel coating, but the presence of a coating was not always specified by the author or manufacturer. The DJ stent diameter most frequently used was 6 Fr and the average length was 22–26 cm. The stent indwelling time ranged from 7 d to 366 d (Table 2), with high within-study heterogeneity. The time between DJ stent insertion and evaluation of stent-related symptoms also differed highly between studies. The Ureteral Stent Symptom Questionnaire (USSQ) to evaluate symptoms and the impact on quality of life of ureteral stents, developed by Joshi et al in 2003 [30], was used in four of the nine studies published after 2003 [14], [20], [22], [23]. When the USSQ was not used as a symptom evaluation tool, studies used the Visual Analog Scale for Pain (VASP), the overactive bladder (OAB) awareness tool or self-made questionnaires [9], [19], [21], [24], [25], [26], [27], [28], [29]. Seven articles reported on prospective randomized studies (Table 1, Table 2) but only one can be classified as a high-quality study with low risk of bias [23]. Three reports were on prospective cohort studies, two studies were self-controlled case series, and one study was retrospective (Table 2). Of the 13 studies, nine focused on the core composition material of the stent, while four focused on stent coating (Table 2).
Table 4.
Stent materials and commercial namesa
| Material | Class | Chemical composition | Manufacturer and commercial name |
|---|---|---|---|
| Silicone | Silicone | Condensation polymer comprising chains of alternating silicon and oxygen atoms [12] | Cook Black silicone Coloplast ImaJin Hydro Coloplast (Porges S.A.) silicone |
| Silitek | Silicone + polyester | Proprietary modified silicone based polyester block copolymer [10], [11], [12], [31] | Surgitek Silitek |
| C-Flex | Silicone + polyolefin | Silicone-modified styrene/ethylene/butylene thermoplastic block copolymer [12], [31] | Cook C-Flex Van-Tec “Soft” |
| Percuflex | Polyolefin | Proprietary thermoplastic olefinic block copolymer [10], [11], [31] | Boston (Microvasive) – Percuflex – Percuflex Plus (“firm” Percuflex + Hydroplusb) – Contour (“soft” Percuflex + Hydroplusb) |
| Polyurethane (standard) | Polyurethane | Linear polymer of urethane units with a backbone that contains carbamate groups (—NHCO2). The links are produced via chemical reaction between a di-isocyanate and a polyol [11] | Cook polyurethane Rüsch (Teleflex) polyurethane |
| Sof-Flex | Polyurethane | Proprietary modified polyurethane-based polymer with hydrogel [11] | Cook Sof-Flex |
| Tecoflex | Polyurethane | Modified polyurethane-based aliphatic thermoplastic; product of the reaction between methylene-bis(cyclohexyl)-di-isocyanate, poly(tetramethylene ether glycol), and 1,4-butanediol [10], [11], [31] | Surgitek Classic |
| Carbothane | Polyurethane | Proprietary modified polyurethane-based polymer | Amecath Carbothane |
| Pellethane | Polyurethane + fluoropolymer | Proprietary modified polyurethane-based mixture of PTFE and proprietary materials [10] | Bard Inlay (Pellethane with pHreeCoatc) [10] |
| Endo-Sof | Proprietary | Proprietary compound | Cook Endo-Sof |
| Vertex | Proprietary | Proprietary compound | Applied Medical Vertex |
| Resonance | Metal | Nickel‐cobalt‐chromium‐molybdenum alloy [10] | Cook Resonance |
| Triclosan | Antimicrobial | Triclosan | Triumph triclosan-eluting stent |
| Silver sulfadiazine | Antimicrobial | Silver sulfadiazine | Amecath Carbothane with silver sulfadiazine |
PTFE = polytetrafluoroethylene.
Limited to the materials and commercial names for stents used in the studies included in the review.
Proprietary hydrogel by Boston Scientific.
Proprietary hydrogel with pH-stabilizing capabilities.
3.1. Core composition
In 1988, Smedley et al. [24] were the first group to examine the impact of core composition on stent-related symptoms in a comparison of “hard” (polyurethane) versus “soft” (silicone) DJ stents. The authors noted less loin discomfort (24% vs 18%) and less trigonal irritation (29% vs. 18%) in favor of the “soft” silicone stent, although the difference was not significant. Limitations of this study were its heterogeneity in terms of inclusion criteria, stent removal date, and time points for symptom evaluation. There was also no information on the stent manufacturers, eventual coatings, and stent length or width. Lastly, the study was limited by its retrospective nature.
Lennon et al. [9] also compared “hard” (Cook polyurethane) and “soft” (Cook Sof-Flex) DJ stents. Results for the stent-related domains of renal pain (58% vs 38%), suprapubic pain (46% vs 26%), dysuria (40% vs 23%), and continuation of normal life activity (45% vs 67%) were all significantly better among patients with the “soft” stent. In addition, reflux-type pain, daytime frequency, urgency, nocturia, and hematuria were less frequent with the “soft” stent, but the differences were not statistically significant. Although none of the patients were completely free of symptoms, the overall severity of stent-related symptoms was clearly lower in the “soft” stent group. Limitations of this study include unclear inclusion criteria and extreme heterogeneity for the time points for symptom evaluation. In addition, the USSQ was not used and no information about the randomization process or missing outcome data was provided.
Lee et al. [20] evaluated stent-related symptoms for five different 6Fr DJ stents: Bard Inlay, Cook Endo-Sof, Boston Scientific Contour, Applied Medical Vertex and Surgitek Classic. The authors found a significant difference in total USSQ symptom scores on day 3 in favor of the Bard Inlay stent, but the difference was not statistically significant on days 1 and 5. Regarding individual symptoms, significantly fewer patients in the Bard Inlay group reported hematuria, sleep disturbance, a need for analgesia, flank pain, and groin pain. Patients in this group also showed greater patient independence on days 1, 3, and/or 5 in comparison to the other DJ stents examined. The Applied Medical Vertex and Surgitek Classic DJ stents were associated with the most significant symptoms: the Vertex stent causing more groin pain on day 1, more frequency and nocturia on day 3, and necessitated more narcotic use on day 5 in comparison to the other four DJ stents. The Surgitek Classic DJ stent caused more hematuria on days 1 and 3 and more flank pain on day 1. Limitations of this study include the low number of patients (only 44 patients completed the study, to compare five DJ stents), heterogeneous inclusion criteria, and no specification of the stent indwelling time or time points for symptom evaluation. Lastly, no information was given about the randomization process or missing outcome data, as it was noted that only 44 of 70 patients completed the study.
El-Nahas et al. [26] compared a “soft” (Coloplast silicone) DJ stent to a “hard” (Boston Scientific Percuflex) DJ stent. They reported significantly more stent-related symptoms for patients with the “hard” stent (46% vs 75%). Limitations of this study are heterogeneity in inclusion criteria, heterogeneity in stent width, and most notably extreme heterogeneity in the time points for symptom evaluation. Lastly, the USSQ was not used and no information on missing outcome data was provided.
Gadzhiev et al. [27] compared ureteral stent-related symptoms between the Cook Black Silicone and Rüsch polyurethane DJ stents in terms of VASP and OAB awareness tool results at days 1, 14, and 28 (with an indwelling time of 28 d). Silicone DJ stents were associated with significantly lower pain intensity assessed using VASP at days 14 and 28. Limitations of this study were failure to use the USSQ, the nonrandomized design, and the lack of information regarding missing data.
In the most recent and highest-quality study, Wiseman et al. [23] compared the Coloplast ImaJin Hydro silicone DJ stent to the Boston Scientific Percuflex Plus stent after flexible ureteroscopy for renal stones between 5 and 25 mm. The silicone DJ stents were associated with significantly less patient discomfort and better quality of life in comparison to the nonsilicone polymer DJ stent. The authors observed a 25% reduction in USSQ pain score at day 20 and significantly lower urinary symptoms (as for the relative USSQ domain) at days 2, 7, and 20, with the greatest difference at day 20, all in favor of the silicone DJ stent. Limitations of the study are the nonstandardized medical therapy and a notably high dropout rate, although this was methodologically handled correctly.
3.2. Coating
Cadieux et al. [28] investigated the clinical benefit of triclosan-eluting (antimicrobial) stents on urine and stent cultures as a primary aim. Although the authors did not observe a clear benefit in terms of urine and stent cultures, they did note significantly fewer symptomatic infections. Therefore, Mendez-Probst et al. [21] conducted a new study to compare a regular hydrogel-coated DJ stent (Boston Scientific Percuflex Plus) to a Triumph triclosan-eluting DJ stent, with a focus on stent-related symptoms. In general, the results indicated less discomfort during micturition and movement in favor of the triclosan-eluting stent. More specifically, the triclosan-eluting DJ stent was associated with significantly less flank pain during activity, less painful micturition, and less abdominal pain during activity. Limitations of this study were the low number of patients (n = 20), failure to use the USSQ, heterogeneous inclusion criteria, and no information about the randomization process.
Two out of four studies on coating, and three out of nine studies on core composition showed no difference in the impact of DJ stent material on stent-related symptoms [14], [19], [22], [25], [29]. Limitations were the lack of information on DJ stent length and width, low patient numbers, heterogeneous inclusion criteria, very heterogeneous dwell times, lack of USSQ use, and high risk of bias (Table 2 and Supplementary material).
3.3. Discussion
Five out of nine studies on core composition found that the material of a DJ stent has an impact on stent-related symptoms [9], [20], [23], [26], [27]. Of the four studies comparing nonsilicone polymers to silicone, three found that silicone significantly reduces stent-related symptoms [23], [26], [27]. The study of highest quality also favored silicone with a hydrogel coating over a nonsilicone polymer with a hydrogel coating [23]. Of the five studies comparing only nonsilicone polymers, two favored the Bard Inlay and Cook Medical Sof-Flex DJ stents [9], [20].
In general, the results suggest that silicone DJ stents do reduce stent-related symptoms. In addition, since it is stated that the Bard Inlay and Cook Sof-Flex DJ stents are relatively “soft”, it seems that all DJ stents associated with a reduction in stent-related symptoms were “soft” or “flexible” [12], [31]. Multiple problems when trying to answer the review question arose during this systematic review. The main issue is the difficulty in proving an association between chemical or physical stent material properties and clinical impact. First, some manufactures release very little information about stent composition. Table 4 shows that most of the DJ stents were made of proprietary material of undisclosed composition. Second, there is no established “standard” for how physical stent properties are measured, denominated, and used in clinical studies. Taking the most important factors, for example, “stiffness” and “hardness” were used interchangeably but they are not necessarily the same [9], [11], [13], [14], [31], [32]. To measure “hardness”, some studies used the American Society for Testing and Materials D2240 standard, which measures indentation hardness (Shore hardness) with durometer as the unit of measure [9], [11], [14]. The force in grams required to flex the stent coil by 90° is used as a measure of “stiffness” or “flexibility” [9]. Others used Young’s modulus of elasticity, a measure of tensile strength, and converted it to stiffness and hardness, with durometer as the unit of measure [31]. As a result, the measurements reported for “stiffness” and “flexibility” are variable and subject to interpretation [9], [12], [14], [31]. It is also clear that just mentioning “silicone” or “polyurethane” is not sufficient, as physical properties differ between manufacturers using the “same” material, meaning that not every silicone stent is made equally [12]. Of the nine studies evaluating core material, only two examined the physical properties of the DJ stents they used and specified these properties in measurement units [9], [14]. Lastly, it is concerning that even if measurement and denomination standards existed, Hendlin et al. [31] showed that there can be statistically significant variability in durometer results between different lot numbers for stents made by the same manufacturer. If confirmed by other studies, this batch variability increases the complexity in understanding why a particular stent material results in fewer stent-related symptoms.
Stent coatings are another part of the puzzle. The most common coating is hydrogel, consisting of hydrophilic polymers that reduce the friction coefficient, thereby facilitating stent placement and potentially increasing patient comfort [33]. Only one study specifically compared DJ stents with and without a hydrogel with regard to stent-related symptoms and found no significant difference [19]. Other studies researching the impact of DJ stent coating on stent-related symptoms examined the effect of an anti-microbial coating. The findings show that a triclosan coating resulted in significantly less discomfort, while no such benefit was observed for a silver sulfadiazine coating [21], [22], [28]. Nevertheless, since only one study was conducted per coating, no conclusion can be drawn.
Hypotheses on why silicone DJ stents might reduce stent-related symptoms often mention the softness, flexibility, biologic inertness, and low encrustation and bacterial adhesion rates of silicone [10], [11], [12], [13], [34]. It is suspected that higher encrustation and bacterial adhesion rates, related to a longer indwelling time, have a negative impact on stent-related symptoms [13], [35], [36]. Comparing silicone to a nonsilicone polymer, most studies reported less encrustation and bacterial adhesion in favor of silicone [37], [38], [39], [40]. However, the importance of coatings should not be overlooked. When a noncoated silicone DJ stent was compared to a hydrocoated silicone DJ stent, encrustation appeared to be more severe on the noncoated stent [41]. In general, however, the evidence is heterogeneous, with hydrogel coating seeming to both reduce and increase encrustation and biofilm formation [33], [40], [42]. Since a hydrogel coating might influence stent-related symptoms in various ways, subdifferentiation between studies on core composition would be desirable to compare DJ stents without a hydrogel coating (“true” core composition) and DJ stents with a hydrogel coating (combined core composition and coating). However, since more than half of the core composition studies failed to clarify whether or not the stents compared had a hydrogel coating, this differentiation could not be made (Table 2). This makes it even more difficult to draw a conclusion regarding the impact of DJ stent core material on stent-related symptoms.
Studies used in this review varied widely in their methods; the most important variations were in the inclusion and exclusion criteria, the symptom assessments, and the dwell time. First, the inclusion and exclusion criteria differed between some studies that only allowed specific types of stone disease and others that allowed a wide variety of indications. This could possibly have an impact on the results. Second, the method used to assess stent-related symptoms is of utmost importance. Although the USSQ was validated and introduced in 2003, only four of nine studies published after 2003 used this questionnaire for symptom assessment. The USSQ is generally considered a validated and superior tool for assessment of stent-related symptoms [14], [23], [30]. Lastly, very heterogeneous dwell times and different time frames for patient evaluation were noted [20], [23], [27]. The dwell time also varied widely within the studies: the average overall DJ stent indwelling time was a relatively long period of 27 d and the range was 7–366 d (Table 2), while most urologists normally favor a dwell time of 1–2 wk after ureteroscopy. Unfortunately, since the studies we included had very heterogeneous populations, debatable comparability for the intervention comparisons, and no outcome with comparable methodology, it was not possible to conduct any pooled analysis or meta-analysis for these data. Second, there was only one high-quality study with a low risk of bias. It has been suggested that high-quality studies may be superior to meta-analyses based on low-quality data. When there is a conflict between high-quality randomized trials and meta-analyses, readers should analyze the evidence themselves to decide which offers the best-quality evidence [43], [44]. Readers should bear in mind that more than one-third of meta-analyses are later discredited after publication of high-quality randomized controlled trials. Therefore, in cases for which there are many low-quality trials among a limited number of high-quality randomized controlled trials, the latter and not meta-analyses are considered the gold standard in the evaluation of therapies [44], [45].
Here we focused on the material composition of stents. However, other factors such as DJ stent length, diameter, and positioning, as well as use of medical therapy, may also affect stent-related symptoms [35]. Although evidence on this is mixed, DJ stent length and diameter were relatively comparable between studies (Table 2) [35]. Moreover, the importance of pharmacologic treatment of stent-related symptoms is often omitted since use of medication was not or under-reported in all studies. α-Blockers may reduce the morbidity of DJ stents and increase tolerability, while results for the use of anticholinergics are mixed [35], [46], [47], [48]. Mirabegron may have a beneficial effect, but the evidence is based on low-quality studies [49]. A significant improvement in multiple USSQ domains at an early time point can be achieved via pharmacologic intervention [48].
In future research it will be important to establish a standard for measuring and reporting the physical properties of stents to evaluate the impact of stent material composition on stent-related symptoms. If such a standard is established and a reduction in stent-related symptoms is observed, any associations with physical properties could then be identified. Studying the impact of the physical properties of a stent would provide a more feasible framework to draw conclusions and make improvements to implement in clinical practice. An ideal step would be proposal of a validated and standardized measurement unit accepted by both manufacturers and researchers. In addition, uniformity of inclusion and exclusion criteria would facilitate interpretation of study results. These criteria should preferably limit inclusion to DJ stents placed unilaterally and via retrograde access for stone disease in adults. In general, more high-quality, non–industry-sponsored, randomized, prospective, multicenter studies with low risk of bias are needed. Such studies should use the USSQ at well-defined clinically relevant time points (e.g. days 3, 7, 14, and 21). Lastly, knowing that medical therapy can impact on USSQ scores, use of analgesics, nonsteroidal anti-inflammatory drugs, α-blockers, anticholinergics, and mirabegron should be well documented and subsequently analyzed to identify any difference in use between study arms.
4. Conclusions
Silicone DJ stents, and by extension “soft” DJ stents, appear to reduce stent-related symptoms in comparison to nonsilicone polymers and “hard” DJ stents. No definitive conclusion can be drawn owing to the lack of high-quality evidence. A standard for measuring and reporting physical stent properties is paramount to carry out effective comparisons between studies and thus identify the stent modifications needed to reduce patient-reported stent-related symptoms.
Author contributions: Vincent De Coninck had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Boeykens, De Coninck.
Acquisition of data: Boeykens, De Coninck.
Analysis and interpretation of data: Boeykens, De Coninck.
Drafting of the manuscript: Boeykens.
Critical revision of the manuscript for important intellectual content: Keller, Bosio, Wiseman, Contreras, Ventimiglia, Talso, Pietropaolo, Tailly, De Coninck.
Statistical analysis: Boeykens, Keller, De Coninck.
Obtaining funding: None.
Administrative, technical, or material support: Boeykens, De Coninck.
Supervision: De Coninck.
Other: None.
Financial disclosures: Vincent De Coninck certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Vincent De Coninck is a speaker and/or consultant for Axess Vision Technology, BD Bard, Coloplast, and Karl Storz, but has no specific conflicts relevant to this work. Etienne X. Keller is a speaker and/or consultant for Coloplast, Olympus, Boston Scientific, Recordati, Debiopharm, and Alnylam, and has no specific conflicts of interest relevant to this work. Oliver J. Wiseman has a financial interest and/or other relationship with Coloplast, Boston Scientific, and EMS, but has no specific conflicts relevant to this work. The remaining authors have nothing to disclose.
Associate Editor: Silvia Proietti
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.09.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Joshi H.B., Okeke A., Newns N., Keeley F.X., Jr, Timoney A.G. Characterization of urinary symptoms in patients with ureteral stents. Urology. 2002;59:511–516. doi: 10.1016/s0090-4295(01)01644-2. [DOI] [PubMed] [Google Scholar]
- 2.De Coninck V., Keller E.X., Somani B., et al. Complications of ureteroscopy: a complete overview. World J Urol. 2020;38:2147–2166. doi: 10.1007/s00345-019-03012-1. [DOI] [PubMed] [Google Scholar]
- 3.Bosio A., Alessandria E., Dalmasso E., et al. How bothersome double-J ureteral stents are after semirigid and flexible ureteroscopy: a prospective single-institution observational study. World J Urol. 2019;37:201–207. doi: 10.1007/s00345-018-2376-6. [DOI] [PubMed] [Google Scholar]
- 4.Bosio A., Alessandria E., Agosti S.C., et al. Loop-tail stents fail in reducing stent-related symptoms: results of a prospective randomised controlled trial. BJU Int. 2022;129:123–129. doi: 10.1111/bju.15395. [DOI] [PubMed] [Google Scholar]
- 5.Dunn M.D., Portis A.J., Kahn S.A., et al. Clinical effectiveness of new stent design: randomized single-blind comparison of tail and double-pigtail stents. J Endourol. 2000;14:195–202. doi: 10.1089/end.2000.14.195. [DOI] [PubMed] [Google Scholar]
- 6.Park H.K., Paick S.H., Kim H.G., Lho Y.S., Bae S. The impact of ureteral stent type on patient symptoms as determined by the Ureteral Stent Symptom questionnaire: a prospective, randomized, controlled study. J Endourol. 2015;29:367–371. doi: 10.1089/end.2014.0294. [DOI] [PubMed] [Google Scholar]
- 7.Davenport K., Kumar V., Collins J., Melotti R., Timoney A.G., Keeley F.X., Jr. New ureteral stent design does not improve patient quality of life: a randomized, controlled trial. J Urol. 2011;185:175–178. doi: 10.1016/j.juro.2010.08.089. [DOI] [PubMed] [Google Scholar]
- 8.Bosio A., Alessandria E., Agosti S., et al. Pigtail suture stents significantly reduce stent-related symptoms compared to conventional double J stents: a prospective randomized trial. Eur Urol Open Sci. 2021;29:1–9. doi: 10.1016/j.euros.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lennon G.M., Thornhill J.A., Sweeney P.A., Grainger R., McDermott T.E., Butler M.R. ‘Firm’ versus ‘soft’ double pigtail ureteric stents: a randomised blind comparative trial. Eur Urol. 1995;28:1–5. doi: 10.1159/000475010. [DOI] [PubMed] [Google Scholar]
- 10.Liatsikos E., Kallidonis P., Stolzenburg J.U., Karnabatidis D. Ureteral stents: past, present and future. Expert Rev Med Devices. 2009;6:313–324. doi: 10.1586/erd.09.5. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesan N., Shroff S., Jayachandran K., Doble M. Polymers as ureteral stents. J Endourol. 2010;24:191–198. doi: 10.1089/end.2009.0516. [DOI] [PubMed] [Google Scholar]
- 12.Mardis H.K., Kroeger R.M., Morton J.J., Donovan J.M. Comparative evaluation of materials used for internal ureteral stents. J Endourol. 1993;7:105–115. doi: 10.1089/end.1993.7.105. [DOI] [PubMed] [Google Scholar]
- 13.Mosayyebi A., Manes C., Carugo D., Somani B.K. Advances in ureteral stent design and materials. Curr Urol Rep. 2018;19:35. doi: 10.1007/s11934-018-0779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi H.B., Chitale S.V., Nagarajan M., et al. A prospective randomized single-blind comparison of ureteral stents composed of firm and soft polymer. J Urol. 2005;174:2303–2306. doi: 10.1097/01.ju.0000181815.63998.5f. [DOI] [PubMed] [Google Scholar]
- 15.Bramer W.M., de Jonge G.B., Rethlefsen M.L., Mast F., Kleijnen J. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc. 2018;106:531–541. doi: 10.5195/jmla.2018.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Thomas J., Chandler J., et al. Cochrane Collaboration; London, UK: 2021. Cochrane handbook for systematic reviews of interventions. Version 6.2. [Google Scholar]
- 18.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candela J.V., Bellman G.C. Ureteral stents: impact of diameter and composition on patient symptoms. J Endourol. 1997;11:45–47. doi: 10.1089/end.1997.11.45. [DOI] [PubMed] [Google Scholar]
- 20.Lee C., Kuskowski M., Premoli J., Skemp N., Monga M. Randomized evaluation of ureteral stents using validated symptom questionnaire. J Endourol. 2005;19:990–993. doi: 10.1089/end.2005.19.990. [DOI] [PubMed] [Google Scholar]
- 21.Mendez-Probst C.E., Goneau L.W., MacDonald K.W., et al. The use of triclosan eluting stents effectively reduces ureteral stent symptoms: a prospective randomized trial. BJU Int. 2012;110:749–754. doi: 10.1111/j.1464-410X.2011.10903.x. [DOI] [PubMed] [Google Scholar]
- 22.El-Nahas A.R., Lachine M., Elsawy E., Mosbah A., El-Kappany H. A randomized controlled trial comparing antimicrobial (silver sulfadiazine)-coated ureteral stents with non-coated stents. Scand J Urol. 2018;52:76–80. doi: 10.1080/21681805.2017.1376353. [DOI] [PubMed] [Google Scholar]
- 23.Wiseman O., Ventimiglia E., Doizi S., et al. Effects of silicone hydrocoated double loop ureteral stent on symptoms and quality of life in patients undergoing flexible ureteroscopy for kidney stone: a randomized multicenter clinical study. J Urol. 2020;204:769–777. doi: 10.1097/JU.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 24.Smedley F.H., Rimmer J., Taube M., Edwards L. 168 double J (pigtail) ureteric catheter insertions: a retrospective review. Ann R Coll Surg Engl. 1988;70:377–379. [PMC free article] [PubMed] [Google Scholar]
- 25.Pryor J.L., Langley M.J., Jenkins A.D. Comparison of symptom characteristics of indwelling ureteral catheters. J Urol. 1991;145:719–722. doi: 10.1016/s0022-5347(17)38433-1. [DOI] [PubMed] [Google Scholar]
- 26.El-Nahas A.R., El-Assmy A.M., Shoma A.M., Eraky I., El-Kenawy M.R., El-Kappany H.A. Self-retaining ureteral stents: analysis of factors responsible for patients’ discomfort. J Endourol. 2006;20:33–37. doi: 10.1089/end.2006.20.33. [DOI] [PubMed] [Google Scholar]
- 27.Gadzhiev N., Gorelov D., Malkhasyan V., et al. Comparison of silicone versus polyurethane ureteral stents: a prospective controlled study. BMC Urol. 2020;20:10. doi: 10.1186/s12894-020-0577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadieux P.A., Chew B.H., Nott L., et al. Use of triclosan-eluting ureteral stents in patients with long-term stents. J Endourol. 2009;23:1187–1194. doi: 10.1089/end.2008.0437. [DOI] [PubMed] [Google Scholar]
- 29.Chow P.M., Chiang I.N., Chen C.Y., et al. Malignant ureteral obstruction: functional duration of metallic versus polymeric ureteral stents. PLoS One. 2015;10:e0135566. doi: 10.1371/journal.pone.0135566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi H.B., Newns N., Stainthorpe A., MacDonagh R.P., Keeley F.X., Jr, Timoney A.G. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060–1064. doi: 10.1097/01.ju.0000049198.53424.1d. [DOI] [PubMed] [Google Scholar]
- 31.Hendlin K., Dockendorf K., Horn C., Pshon N., Lund B., Monga M. Ureteral stents: coil strength and durometer. Urology. 2006;68:42–45. doi: 10.1016/j.urology.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 32.Thomas R. Indwelling ureteral stents: impact of material and shape on patient comfort. J Endourol. 1993;7:137–140. doi: 10.1089/end.1993.7.137. [DOI] [PubMed] [Google Scholar]
- 33.Chew B.H., Denstedt J.D. Technology insight: novel ureteral stent materials and designs. Nat Clin Pract Urol. 2004;1:44–48. doi: 10.1038/ncpuro0014. [DOI] [PubMed] [Google Scholar]
- 34.Beiko D.T., Knudsen B.E., Denstedt J.D. Advances in ureteral stent design. J Endourol. 2003;17:195–199. doi: 10.1089/089277903765444294. [DOI] [PubMed] [Google Scholar]
- 35.Koprowski C., Kim C., Modi P.K., Elsamra S.E. Ureteral stent-associated pain: a review. J Endourol. 2016;30:744–753. doi: 10.1089/end.2016.0129. [DOI] [PubMed] [Google Scholar]
- 36.Kawahara T., Ito H., Terao H., Yoshida M., Matsuzaki J. Ureteral stent encrustation, incrustation, and coloring: morbidity related to indwelling times. J Endourol. 2012;26:178–182. doi: 10.1089/end.2011.0385. [DOI] [PubMed] [Google Scholar]
- 37.Barghouthy Y., Wiseman O., Ventimiglia E., et al. Silicone-hydrocoated ureteral stents encrustation and biofilm formation after 3-week dwell time: results of a prospective randomized multicenter clinical study. World J Urol. 2021;39:3623–3629. doi: 10.1007/s00345-021-03646-0. [DOI] [PubMed] [Google Scholar]
- 38.Watterson J.D., Cadieux P.A., Stickler D., Reid G., Denstedt J.D. Swarming of Proteus mirabilis over ureteral stents: a comparative assessment. J Endourol. 2003;17:523–527. doi: 10.1089/089277903769013711. [DOI] [PubMed] [Google Scholar]
- 39.Tunney M.M., Keane P.F., Jones D.S., Gorman S.P. Comparative assessment of ureteral stent biomaterial encrustation. Biomaterials. 1996;17:1541–1546. doi: 10.1016/0142-9612(96)89780-8. [DOI] [PubMed] [Google Scholar]
- 40.Desgrandchamps F., Moulinier F., Daudon M., Teillac P., Le Duc A. An in vitro comparison of urease-induced encrustation of JJ stents in human urine. Br J Urol. 1997;79:24–27. doi: 10.1046/j.1464-410x.1997.02775.x. [DOI] [PubMed] [Google Scholar]
- 41.Cormio L., Talja M., Koivusalo A., Mäkisalo H., Wolff H., Ruutu M. Biocompatibility of various indwelling double-J stents. J Urol. 1995;153:494–496. doi: 10.1097/00005392-199502000-00069. [DOI] [PubMed] [Google Scholar]
- 42.Gorman S.P., Tunney M.M., Keane P.F., Van Bladel K., Bley B. Characterization and assessment of a novel poly(ethylene oxide)/polyurethane composite hydrogel (Aquavene) as a ureteral stent biomaterial. J Biomed Mater Res. 1998;39:642–649. doi: 10.1002/(sici)1097-4636(19980315)39:4<642::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 43.Sylvester R.J., Canfield S.E., Lam T.B., et al. Conflict of evidence: resolving discrepancies when findings from randomized controlled trials and meta-analyses disagree. Eur Urol. 2017;71:811–819. doi: 10.1016/j.eururo.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 44.De Coninck V., Antonelli J., Chew B., Patterson J.M., Skolarikos A., Bultitude M. Medical expulsive therapy for urinary stones: future trends and knowledge gaps. Eur Urol. 2019;76:658–666. doi: 10.1016/j.eururo.2019.07.053. [DOI] [PubMed] [Google Scholar]
- 45.LeLorier J., Grégoire G., Benhaddad A., Lapierre J., Derderian F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536–542. doi: 10.1056/NEJM199708213370806. [DOI] [PubMed] [Google Scholar]
- 46.Wang C.J., Huang S.W., Chang C.H. Effects of specific α-1A/1D blocker on lower urinary tract symptoms due to double-J stent: a prospectively randomized study. Urol Res. 2009;37:147–152. doi: 10.1007/s00240-009-0182-8. [DOI] [PubMed] [Google Scholar]
- 47.Lamb A.D., Vowler S.L., Johnston R., Dunn N., Wiseman O.J. Meta-analysis showing the beneficial effect of α-blockers on ureteric stent discomfort. BJU Int. 2011;108:1894–1902. doi: 10.1111/j.1464-410X.2011.10170.x. [DOI] [PubMed] [Google Scholar]
- 48.Betschart P., Zumstein V., Piller A., Schmid H.P., Abt D. Prevention and treatment of symptoms associated with indwelling ureteral stents: a systematic review. Int J Urol. 2017;24:250–259. doi: 10.1111/iju.13311. [DOI] [PubMed] [Google Scholar]
- 49.Van Besien J, Keller EX, Somani B, et al. Mirabegron for the treatment of ureteral stent-related symptoms: a systematic review and meta-analysis. Eur Urol Focus. In press. 10.1016/j.euf.2021.10.002. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.