Abstract
Purpose
To report the efficacy of combination therapy using intravitreal injection of brolucizumab (IVbr) and sub-Tenon's injection of triamcinolone acetonide (STTA) and of monitoring with a laser flare-cell photometer (LFP) in a case of polypoidal choroidal vasculopathy (PCV) with intraocular inflammation (IOI).
Observations
A 72-year-old Japanese woman with PCV had her treatment switched to IVbr due to being refractory to aflibercept. Two weeks after starting IVbr, her visual acuity (VA) declined to 0.40 from 0.10 logarithm of the minimum angle of resolution (logMAR) VA at baseline. In addition, the LFP flare increased to 51.2 photon count/ms (pc/ms) compared with the baseline of 16.1 pc/ms. We diagnosed her with the onset of IOI and immediately started treatment with sub-Tenon's injection of 20 mg triamcinolone acetonide (STTA). Two weeks after receiving STTA, her VA had recovered to 0.15 logMAR, and the LFP flare had decreased to 17.9 pc/ms with dry macula. Eight weeks after the first IVbr treatment, the logMAR VA had improved to −0.18 with achievement of dry macula and stabilization of the LFP flare at 12.2 pc/ms. We administered combined therapy using IVbr and STTA to our patient, and 12 weeks later, the logMAR VA remained at 0.00 with dry macula and 18.1 pc/ms for LFP flare. We continued combination therapy, and after 8 months, her logMAR VA remained at −0.08, and optical coherence tomography showed dry macula, while the LFP flare had stabilized at 16.6 pc/ms.
Conclusions and Importance
Combination therapy of IVbr and STTA stabilized IOI and achieved dry macula. The LFP flare score clearly showed the degree of and changes in inflammation.
Keywords: Brolucizumab, Polypoidal choroidal vasculopathy, Intraocular inflammation, Triamcinolone acetonide, Aflibercept, Age-related macular degeneration
1. Introduction
Exudative age-related macular degeneration (AMD) associated with choroidal neovascularization (CNV) is well known to be responsible for significant visual loss.1, 2, 3 A high proportion of polypoidal choroidal vasculopathy (PCV) is a major characteristic of Asian patients with AMD.4,5 Intravitreal anti-vascular endothelial growth factor (VEGF) drugs, such as ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA, USA) and aflibercept (Eylea, Regeneron, Tarrytown, NY, USA, and Bayer, Berlin, Germany), are evidence-based therapy for exudative AMD.6, 7, 8 Recently, the new anti-VEGF drug brolucizumab (beovu®, Novartis Pharma AG, Basel, Switzerland) was reported to be non-inferior to aflibercept in major clinical trials and became available for medical use in Japan in May 2020. Brolucizumab has been approved for use with a 12-week dosing schedule after 2 monthly loading doses, which can prevent frequent hospital visits for AMD patients.9, 10, 11
Recently, the occurrence of intraocular inflammation (IOI) and retinal vasculitis with or without occlusion after brolucizumab administration has been reported.12, 13, 14, 15, 16, 17, 18, 19, 20 However, the correct diagnosis of the degree of inflammation from an objective perspective is considerably difficult. A laser flare-cell photometer (LFP) can non-invasively evaluate inflammation with numerical information.21 It is important to monitor the degree of inflammation objectively using a laser flare-cell photometer before and after intravitreal injection of brolucizumab (IVbr).
We herein report a patient who developed IOI after IVbr for PCV,a treated with sub-Tenon's injection of triamcinolone acetonide (STTA) and whose condition was monitored by an LFP.
2. Case report
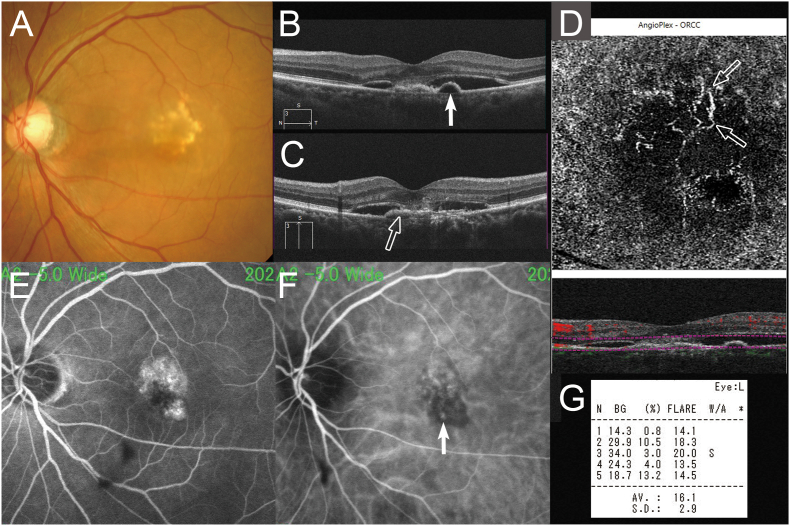
A 72-year-old Japanese woman with polypoidal choroidal vasculopathy was treated with intravitreal aflibercept injections at another clinic. Although she received 12 injections of aflibercept, persistent subretinal fluid was seen on optical coherence tomography (OCT) at 2 weeks after the last injection (Fig. 1).
Fig. 1.
A 72-year-old woman with polypoidal choroidal vasculopathy treated by switching aflibercept to brolucizumab. At baseline, the best-corrected visual acuity (VA) was 0.10 logarithm of the minimum angle of resolution VA (Snellen equivalent: 20/100). (A) A color fundus photograph shows serous retinal detachment (SRD) and lipids. Horizontal (B) optical coherence tomography (OCT) shows SRD and protrusion of the highly reflective retinal pigment epithelium (RPE) line (arrow) corresponding to the polypoidal lesions (arrow) on early-phase indocyanine green (ICG) angiography (F). (C) Vertical OCT shows SRD and the flat elevation of the RPE layer as two separate, highly reflective lines (double-layer sign) (arrow) corresponding to occult choroidal neovascularization on fluorescein angiography (E), which is depicted by OCT angiography as a hyperflow lesion (D). The laser flare-cell photometer shows a value of 16.1 photon count/ms. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
She presented to our hospital with distorted vision in her right eye, the best-corrected visual acuity (VA) of which was 0.10 logarithm of the minimum angle of resolution (logMAR) VA (Snellen equivalent: 20/25). A color fundus photograph showed retinal pigment epithelium (RPE) changes, hard exudates, and serous retinal detachment (SRD (Fig. 1). Fluorescein angiography showed occult choroidal neovascularization (Fig. 1). Indocyanine green angiography showed both regressed polypoidal lesions and a branching vascular network remaining (Fig. 1). Horizontal (e) and vertical (f) OCT images show SRD, a protrusion of the RPE line, and a double-layer sign22 (Fig. 1). We diagnosed this patient as refractory to aflibercept and switched her treatment to brolucizumab with a treat and extend regimen, starting at the prior interval of eight weeks. After the potential risks and benefits were explained fully, we obtained her written informed consent and injected brolucizumab 3.5 mm posterior to the corneal limbus into the vitreous cavity using a 34-gauge needle after topical anesthesia had been applied.
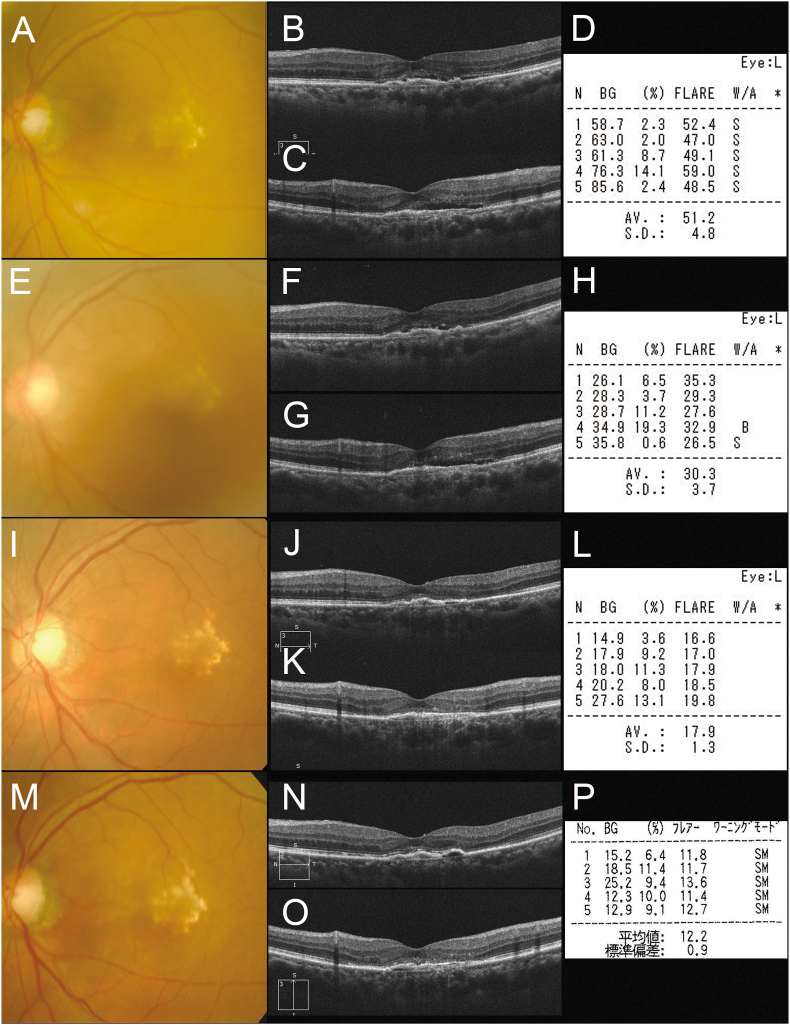
Two weeks after the first injection of brolucizumab, she returned to our hospital due to symptoms of floaters and reduced or blurred vision. Her logMAR VA had decreased to 0.40 (20/50) from 0.10 (20/25) with anterior chamber cells by the slit-lamp examination. The LFP flare had increased from 16.1 photon count/ms (pc/ms) pre-injection to 51.2 pc/ms (Fig. 2). A color fundus photograph and OCT showed the absence of vasculitis and regressed SRD (Fig. 2). We checked the peripheral retina and confirmed no vasculitis. We diagnosed her with IOI and immediately treated her with sub-Tenon's injection of 20 mg triamcinolone acetonide (STTA) and 0.1% fluorometholone eye drops. Three days later, the LFP flare had decreased to 30.3 pc/ms with 0.22 (20/33) logMAR VA (Fig. 2). Over the following 2 weeks, her symptoms improved, and the VA recovered to 0.15 logMAR (20/28). The LFP flare had decreased to 17.9 pc/ms, and OCT showed complete resolved exudation (Fig. 2).
Fig. 2.
(A) Two weeks after the first injection of brolucizumab, the patient's logarithm of the minimum angle of resolution (logMAR) VA had decreased to 0.40 (Snellen equivalent: 20/50) due to intraocular inflammation (IOI). Horizontal (B) and vertical (C) optical coherence tomography showed a decrease in the SRD. (D) The laser flare-cell photometer (LFP) shows an increased value of 51.2 photon count/ms (pc/ms). (E,F,G) Three days after sub-Tenon's injection of 20 mg triamcinolone acetonide (STTA) and 0.1% fluorometholone eye drops, her logMAR VA had slightly improved to 0.22 (20/33) with remnant shallow SRD on OCT. (H) The LFP flare decreased to 30.3 pc/ms. (I,J,K) Two weeks after STTA administration, her logMAR VA had recovered to 0.15 logMAR VA (20/28). Horizontal (J) and vertical (K) OCT show the resolution of SRD. (L) The LFP flare decreased further to 17.9 pc/ms. (M,N,O) Eight weeks after the injection of brolucizumab, the logMAR VA improved to −0.18 (20/13) with dry macula on OCT. (P) The LFP flare is 12.2 pc/ms.
Eight weeks after the first injection of brolucizumab, the logMAR VA improved to −0.18 (20/13) with achievement of dry macula. The LFP flare was 12.2 pc/ms (Fig. 2). We considered this patient's fluid level to be controlled with her IOI reduced by both brolucizumab and subsequent STTA. We thus treated her with combined therapy using IVbr and STTA.
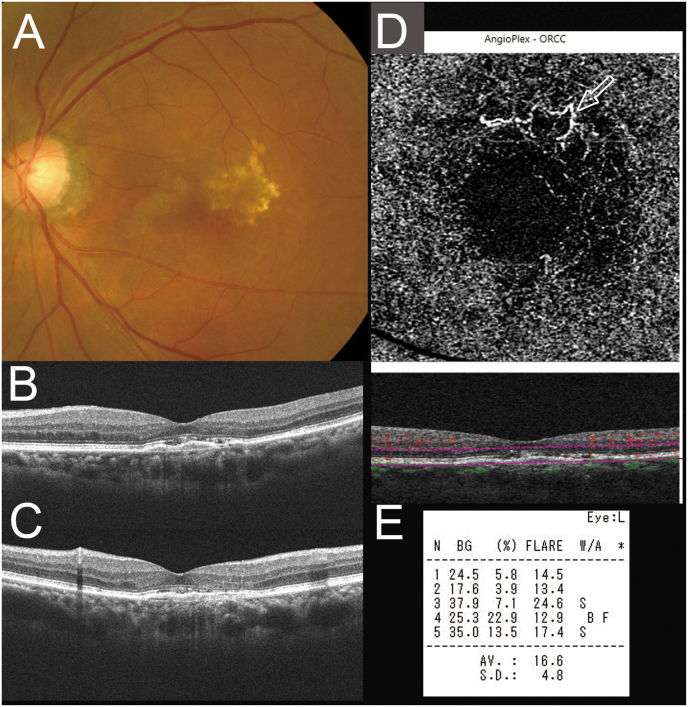
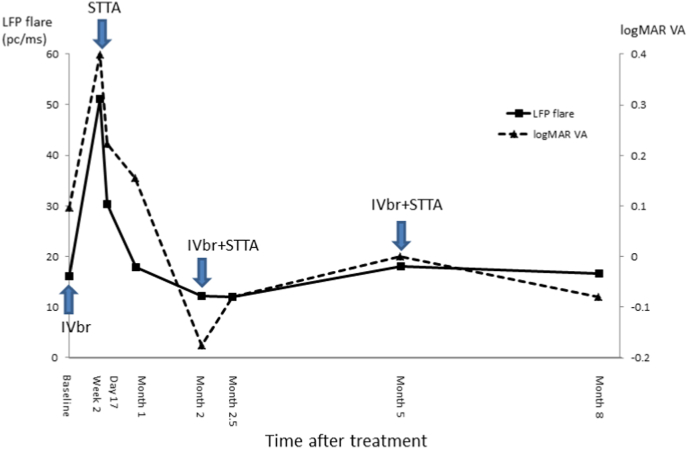
Two weeks after this combination therapy, the LFP flare was 12.0 pc/ms, and she showed no visual disorder and the absence of IOI with −0.08 (20/16) logMAR VA. Twelve weeks after the combination therapy, the logMAR VA was maintained at 0.00 (20/20) with dry macula and 18.1 pc/ms of LFP flare. We continued combination therapy of IVbr and STTA. Eight months after the baseline, her logMAR VA remained at −0.08 (20/16), OCT showed dry macula, and the LFP flare was stabilized at 16.6 pc/ms (Fig. 3). Fig. 4 shows the changes in the LFP flare score and LogMAR VA during follow-up (Fig. 4). Table 1 shows the flare grade during follow-up according to the Standardization of Uveitis Nomenclature (SUN) criteria.23
Fig. 3.
(A) Eight months after the baseline, her logarithm of the minimum angle of resolution VA was maintained at −0.08 (20/16). Horizontal (B) and vertical (C) optical coherence tomography (OCT) show dry macula. (D) OCT angiography shows a remaining hyperflow lesion. (P) The LFP flare is 16.6 photon count/ms.
Fig. 4.
The graph shows the changes in the laser flare-cell photometer (LFP) flare and logarithm of the minimum angle of resolution visual acuity (logMAR VA) during follow-up. IOI occurred two weeks after intravitreal brolucizumab injection (IVbr) (Week 2) with an increase in the LFP flare (51.2 photon count [pc]/ms) and decline in the logMAR VA (0.40). After the first sub-Tenon's injection of 20 mg triamcinolone acetonide (STTA), the LFP flare decreased and the logMAR VA improved to respective values of 30.3 pc/ms and 0.22 at Day 17, 17.9 pc/ms and 0.15 at Month 1, and 12.2 pc/ms and −0.18 at Month 2. Combination therapy of IVbr and STTA was performed at Months 2 and 5. The LFP flare and logMAR VA were stabilized at 12.0 pc/ms and −0.08 at Month 2.5, 18.1 pc/ms and 0.00 at Month 5, and 16.6 pc/ms and −0.18 at Month 8, respectively. LFP = laser flare-cell photometer; pc/ms = photon count/ms; logMAR VA = logarithm of the minimum angle of resolution visual acuity; IVbr = intravitreal brolucizumab injection; STTA = sub-Tenon's injection of 20 mg triamcinolone acetonide.
Table 1.
The LFP flare score and the flare grade during follow-up.
| Time after treatment | LFP flare score (pc/ms) | SUN |
Corresponding figures | ||
|---|---|---|---|---|---|
| cell | flare | vitreous | |||
| Baseline | 16.1 | 0 | 0 | 0 | Fig. 1 |
| Week 2 | 51.2 | 2+ | 1+ | 1+ | Fig. 2 A to D |
| Day 17 | 30.3 | 1+ | 1+ | 1+ | Fig. 2 E to H |
| Month 1 | 17.9 | 0 | 0 | 0 | Fig. 2 I to L |
| Month 2 | 12.2 | 0 | 0 | 0 | Fig. 2 M to P |
| Month 2.5 | 12.0 | 0 | 0 | 0 | NA |
| Month 5 | 18.1 | 0 | 0 | 0 | NA |
| Month 8 | 16.6 | 0 | 0 | 0 | Fig. 3 |
LFP = laser flare-cell photometer; pc/ms = photon count/ms; SUN = the Standardization of Uveitis Nomenclature; NA = not applicable.
3. Discussion
The current case demonstrated that combination therapy of IVbr and STTA stabilized IOI and induced a dry macula. The LFP flare score clearly showed the degree of and changes in inflammation.
Brolucizumab is a low-molecular-weight agent (26 kDa) that has been approved for use with a 12-week dosing schedule after 3 monthly loading doses. A high percentage of PCV is a major characteristic of Japanese patients with AMD.4,5 The HAWK study subanalysis for PCV patients reported that eyes treated with brolucizumab had a probability of receiving an injection every 12 weeks for dosing after loading through weeks 48 and 96 of 76% and 68%, respectively.11 According to the short-term results of PCV patients treated with brolucizumab, complete regression of polypoidal lesions was achieved in 15 of 19 eyes (78.9%) and 11 of 14 eyes (78.6%), which is superior to the results in the PLANET study (38.9% and 44.8%).24, 25, 26 Therefore, switching to brolucizumab appears reasonable for the treatment of PCV patients refractory to previous regimens of ranibizumab or aflibercept. In the current case, the residual SRD regressed within 2 weeks after the first injection, and dry macula was achieved at month 7, with improvement of the VA to 1.2 (20/16) from 0.8 (20/25).
IOI is well known to potentially occur after IVbr.12, 13, 14, 15, 16, 17, 18, 19, 20 Although IOI has been reported to occur in 4.6% of patients after injections in the HAWKS and HARRIER studies, it tends to be seen more frequency in Japanese patients by approximately 15% to 20%.15,18,23 We should be alert for the occurrence of IOI and promptly diagnose early-phase IOI should it develop after the use of brolucizumab. However, it is difficult to determine the inflammation level objectively. In the current case, we were able to monitor the degree of and change in the inflammation and demonstrate the stabilization of IOI using an LFP. Expert opinions have emphasized the need for the early diagnosis, prompt and timely intervention, and intensive treatment of IOI in order to minimize the risk of progression of these events.16 It has also been reported that most cases of IOI occur within six months of starting IVbr after the first four injections.20 Therefore, it is important to monitor the flare cells using an LFP or the flare grade according to the SUN criteria, to perform examinations particularly carefully using widefield imaging during the first six months and to encourage patients to report any symptoms promptly, especially floaters.
We were able to examine the current case after 2 weeks, at which point we confirmed an increase in the LFP flare to a high value of 51.2 pc/ms and diagnosed IOI in the early phase. The occurrence of IOI may be associated with type III or IV hypersensitivity reaction and anti-drug antibodies (ADAs).14 Treatment with corticosteroids in the early phase has reportedly been proposed for patients with IOI after IVbr.17,19 In the current case, we were able to perform early intensive treatment with STTA due to the prompt recognition of IOI showing an LFP value of 51.2 pc/ms. We were also able to monitor the efficacy of STTA based on the reduction in the LFP value to 30.3 pc/ms after 3 days and 17.9 pc/ms after 2 weeks from STTA. Furthermore, using combination therapy of IVbr and STTA as second- and third-line therapy may help stabilize IOI with LFP values near the baseline. It goes without saying that it is important to perform informed consent carefully if a second injection of IVbr is to be administered, even in conjunction with STTA to mitigate inflammation as was done in this case, as it is against the recommendations on the drug label to continue treating with brolucizumab if inflammation has occurred. Combination therapy of IVbr and STTA may be particularly useful in cases with high values of LFP, as this treatment may prevent the progression of inflammation events and reduction in VA, if a physician and patient determine together that this off-label treatment approach is the best option for a patient's condition.
The current case report is limited by its retrospective nature and small sample size. A large, randomized, prospective study with longer follow-up in multiple centers is needed to confirm the current results.
4. Conclusions
LFP flare was useful for easily diagnosing IOI, and its score clearly showed the degree and changes in inflammation over time. Combination therapy of IVbr and STTA was able to stabilize IOI and prevent its recurrence. Combination therapy of IVbr and STTA appears reasonable and useful according to the LFP flare scores in patients with AMD for long-term follow up.
5. Patient consent
After the potential risks and benefits were explained in detail, this patient provided her written informed consent to undergo the treatment and be included in a retrospective chart review with permission for the publication of all images. This case report does not contain any personal information that could identify the patient.
Acknowledgement and Disclosures
The authors have no proprietary interest in any aspect of this study and received no government funding for this report.
References
- 1.Ferris F.L., 3rd, Fine S.L., Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 2.Macular Photocoagulation Study Group Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109(9):1220–1231. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 3.Gass J.D. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol. 1994;118(3):285–298. [PubMed] [Google Scholar]
- 4.Maruko I., Iida T., Saito M., Nagayama D., Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144(1):15–22. doi: 10.1016/j.ajo.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 5.Saito M., Iida T., Saito K., et al. Long-term characteristics of exudative age-related macular degeneration in Japanese patients. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0261320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfeld P.J., Brown D.M., Heier J.S., et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 7.Brown D.M., Kaiser P.K., Michels M., et al. ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 8.Heier J.S., Brown D.M., Chong V., et al. VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Dugel P.U., Koh A., Ogura Y., et al. HAWK and HARRIER study investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Dugel P.U., Singh R.P., Koh A., et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89–99. doi: 10.1016/j.ophtha.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Ogura Y., Jaffe G.J., Cheung C.M.G., et al. Efficacy and safety of brolucizumab versus aflibercept in eyes with polypoidal choroidal vasculopathy in Japanese participants of HAWK. Br J Ophthalmol. 2022;106(7):994–999. doi: 10.1136/bjophthalmol-2021-319090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haug S.J., Hien D.L., Uludag G., et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18 doi: 10.1016/j.ajoc.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumal C.R., Spaide R.F., Vajzovic L., et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345–1359. doi: 10.1016/j.ophtha.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A., Kumar N., Parachuri N., et al. Brolucizumab-related retinal vasculitis: emerging disconnect between clinical trials and real world. Eye. 2020;35(5):1292–1294. doi: 10.1038/s41433-020-01227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruko I., Okada A.A., Iida T., et al. Japan AMD Research Consortium Brolucizumab-related intraocular inflammation in Japanese patients with age-related macular degeneration: a short-term multicenter study. Graefes Arch Clin Exp Ophthalmol. 2021;259(9):2857–2859. doi: 10.1007/s00417-021-05136-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumal C.R., Bodaghi B., Singer M., et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5(6):519–527. doi: 10.1016/j.oret.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Shigemoto Y., Sakurada Y., Fukuda Y., Matsubara M., Parikh R., Kashiwagi K. The combination therapy of subtenon triamcinolone acetonide injection and intravitreal brolucizumab for brolucizumab-related intraocular inflammation. Medicine (Baltim) 2021;100(42) doi: 10.1097/MD.0000000000027580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukai R., Matsumoto H., Akiyama H. Risk factors for emerging intraocular inflammation after intravitreal brolucizumab injection for age-related macular degeneration. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0259879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurup S.K., Tabbaa T., Echegaray J.J., Oliver A.L. Intraocular inflammation secondary to intravitreal brolucizumab treated successfully with Sub-Tenon triamcinolone: a case report. Am J Ophthalmol Case Rep. 2022;25 doi: 10.1016/j.ajoc.2022.101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M., Albini T.A., Seres A., et al. Clinical characteristics and outcomes of eyes with intraocular inflammation after brolucizumab: post hoc analysis of HAWK and HARRIER. Ophthalmol Retina. 2022;6(2):97–108. doi: 10.1016/j.oret.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Sawa M. Laser flare-cell photometer: principle and significance in clinical and basic ophthalmology. Jpn J Ophthalmol. 2017;61(1):21–42. doi: 10.1007/s10384-016-0488-3. [DOI] [PubMed] [Google Scholar]
- 22.Saito M., Iida T., Nagayama D. Cross-sectional and en face optical coherence tomographic features of polypoidal choroidal vasculopathy. Retina. 2008;28(3):459–464. doi: 10.1097/IAE.0b013e318156db60. [DOI] [PubMed] [Google Scholar]
- 23.Jabs D.A., Nussenblatt R.B., Rosenbaum J.T. Standardization of uveitis nomenclature(SUN) working Group:Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto H., Hoshino J., Mukai R., Nakamura K., Akiyama H. Short-term outcomes of intravitreal brolucizumab for treatment-naive neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci Rep. 2021;11(1):6759. doi: 10.1038/s41598-021-86014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda Y., Sakurada Y., Matsubara M., et al. Comparison of outcomes between 3 monthly brolucizumab and aflibercept injections for polypoidal choroidal vasculopathy. Biomedicines. 2021;9(9):1164. doi: 10.3390/biomedicines9091164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee W.K., Iida T., Ogura Y., et al. PLANET investigators. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136(7):786–793. doi: 10.1001/jamaophthalmol.2018.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]