Abstract
Broilers are frequently exposed to various immunological stresses, which lead to intestinal damage, weakened immunity, and even growth retardation. Lutein, as a kind of carotenoid, possesses antioxidant and immunomodulatory functions. Therefore, this study was conducted to investigate the effects of lutein on jejunal mucosal barrier function and inflammatory responses of yellow-feather broilers challenged with lipopolysaccharide (LPS). A total of two hundred eight-eight 1-day-old yellow-feather broilers were randomly allocated to 3 groups with 8 replicate cages containing 12 birds each. Birds were fed broken-rice-soybean basal diet containing 0, 20 and 40 mg/kg lutein (CON, LU20 and LU40) for 26 d. On days 21, 23, and 25 of the trial, broilers were intraperitoneally injected with LPS (1 mg/kg body weight). The results showed that, compared with CON group, LU40 supplementations significantly increased the average daily gain (ADG) of broilers at 1 to 21 and 22 to 26 d of age (P < 0.05), significantly decreased the ratio of feed to gain (F/G) of broilers at 22 to 26 d of age (P < 0.05). LU20 and LU40 supplementations increased goblet cell density in jejunum of broilers under LPS challenge, and LU20 supplementation elevated the villus area (P < 0.05). Scanning electron microscopy of jejunal mucosa revealed significant villi damage, while transmission electron microscopy demonstrated severe enterocyte damage and loss of cellular integrity in CON group. In particular, mitochondria were morphologically altered, appearing irregular or swollen. Apical junctional complexes between adjacent enterocytes were obviously shorter and saccular in CON group. LU20 and LU40 supplementations increased the mRNA expressions of Occludin, Claudin-1, and ZO-1 in the jejunal mucosa of broilers under LPS challenge (P < 0.05), restrained TLR4/MyD88/NF-κB pathway activation in the jejunal mucosa, decreased the mRNA expressions of IL-1β and IL-6, and strengthened the mRNA expressions of IL-4 and IL-10 (P < 0.05). Meanwhile, the protein expressions of p38 and JNK in LU40 group were lower than CON group (P < 0.05). It can be concluded that 40 mg/kg lutein supplementation improved LPS-induced jejunal mucosal barrier function and tamed inflammation of yellow-feather broilers.
Key words: lutein, lipopolysaccharide, mucosal barrier, inflammatory response, broiler
INTRODUCTION
Many factors such as vaccination, drugs, density and temperature can affect the immunologic status of birds and trigger immune responses directly or indirectly in the large-scale farming (Mashaly et al., 2004; Liu et al., 2015b; Wang et al., 2021a). Under stress, chickens may be vulnerable to suffer from enteric diseases, which will affect their growth performance (Yang et al., 2011; Liu et al., 2015a). Intestine is the primary site of digestion and nutrient absorption and maintaining intestinal integrity is essential for human and animal health. The mechanical barrier is an important part of intestinal mucosal barrier, includes intestinal mucosal epithelial cells and the intercellular tight junctions. The epithelial cells absorb nutrients and prevents proinflammatory molecules (pathogens, toxins, and antigens) from the luminal environment into the mucosal tissues or circulatory system (Noda et al., 2012). These processes are regulated by tight junction (TJ) and adherence junction (AJ) (Oda and Takeichi, 2011). Disruption of the intestinal barrier results in luminal inflammatory molecules to penetrate into the lamina propria, leading to persistent inflammation and tissue destruction (Fries et al., 2013; Suzuki, 2013). Lipopolysaccharide (LPS), which is an essential component of the cell wall of gram-negative bacteria and an effective activator of the innate immune responses, can lead to the inflammatory mediator production(Wang and Quinn, 2010) . Our previous study has shown that intraperitoneal injection of LPS can cause intestinal villi atrophy, intestinal microflora change, intestinal inflammation and oxidative damage (Gao et al., 2018a). Hence, we established an immunological stress model of broilers (1 mg/kg LPS challenge was performed intraperitoneally at 21, 23, and 25 d of age), demonstrated that LPS injection induced significant growth performance degradation and intestinal inflammatory responses in broilers (Gao et al., 2018b). Therefore, some safe and effective nutritional additives to modulate the immune function are of great significance to protect chicken from immunological stress. Lutein belongs to the carotenoid, which widely distributed in vegetables, fruits and flowers (Britton, 2008). Lutein product applied in poultry production mainly comes from marigold petals. Studies confirmed that lutein is not only a coloring agent for poultry products but also contributes to decreasing parameters of systemic inflammation in growing chicks (Koutsos et al., 2006; Wang et al., 2017). Our early studies have showed that lutein can regulate the immune response of hens and broilers, and the main site of its effect is jejunum (Gao et al., 2012, 2016). However, the protective effect of lutein on intestines under inflammatory conditions has not been elaborated. In order to further investigate the possible regulatory mechanisms of lutein on intestinal inflammation, LPS was used to establish an immunologic stress model to explore the protective effect of lutein on the intestinal mucosal barrier in broiler chickens.
MATERIALS AND METHODS
Experimental Design, Animals and Diets
The animal study in the present experimental was reviewed and approved by Animal Care Committee, Fujian Agriculture and Forestry University (Fuzhou, Fujian, China, approval ID 202003016). A total of 288 1-day-old Chinese indigenous yellow-feather broilers were randomly allocated to 3 groups with 8 replicate cages containing 12 birds each. Birds in the 3 groups were fed a broken rice-soybean meal basal diet with 0, 20, and 40 mg/kg lutein (CON, LU20, and LU40) for 26 d. Previous studies have shown that the establishment of an LPS stress model at 21, 23, and 25 d of age could induce a significant decrease in production performance and intestinal inflammatory response of broilers (Gao et al., 2018). The dosage of lutein was selected corresponding to previous researches (Meriwether et al., 2010; Shanmugasundaram and Selvaraj, 2011). At 21, 23, and 25 d of age, the LPS (Escherichia coli serotype O55.B5, Sigma Chemical, St Louis, MO) was dissolved in 9 g/L sterile saline solution at 01 mg/mL, so that injection of 1 mL/kg body weight of solution would achieve the desired dosage. The doses and routes of LPS administration referred to the previous studies (Zhang et al., 2010; Li et al., 2015). The birds had ad libitum access to feed and water. The temperature of chicken coop was set at 33°C at the age of 1 to 4 d and then reduced by 3°C per week to a final temperature of around 24°C. Broken rice-soybean basal diet was formulated according to Chinese Feeding Standard of Chicken (NY/T 33-2004). Composition and nutrient levels of the basal diet are presented in Table 1. Lutein (purity ≥ 2%) used in the experiment was purchased from Juyuan Biochemical Col., Ltd. (Zhaoqing, Guangdong, China).
Table 1.
Ingredient composition and nutrient levels of the basal diets (%, as-fed basis).
| Items | 1 to 21 d | 22 to 26 d |
|---|---|---|
| Ingredients | ||
| Broken rice | 61.86 | 68.00 |
| Soybean meal | 32.55 | 25.27 |
| Expanded soybean | 1.00 | 2.58 |
| Limestone | 1.21 | 1.15 |
| Calcium hydrophosphate | 1.87 | 1.58 |
| DL-Methionine | 0.21 | 0.12 |
| Premix1 | 1.00 | 1.00 |
| Salt | 0.30 | 0.30 |
| Total | 100.00 | 100.00 |
| Calculated nutrient levels2 | ||
| Apparent metabolisable energy (MJ/kg) | 12.12 | 12.54 |
| Crude protein | 21.00 | 19.00 |
| Calcium | 1.00 | 0.90 |
| Total phosphorus | 0.73 | 0.67 |
| Available phosphorus | 0.45 | 0.40 |
| Lysine | 1.13 | 1.00 |
| Methionine + cysteine | 0.85 | 0.72 |
Premix provided per kilogram of diet: 8,000 IU of vitamin A, 2,000 IU of vitamin D3, 20 IU of vitamin E, 1 mg of vitamin K3, 2.6 mg of thiamin, 5.4 mg of riboflavin, 6 mg of vitamin B6, 0.02 mg of vitamin B12, 40 mg of niacin, 0.8 mg of folic acid, 0.2 mg of biotin, 1200 mg of choline, and 20 mg of pantothenic acid, 60 mg of Zn, 80 mg of Mn, 80 mg of Fe, 8 mg of Cu, 0.35 mg of I, and 0.15 mg of Se.
Nutrient levels were analyzed values.
Sample Collection
At 26 d, 8 broilers of moderate weight (one bird from each replication) were randomly picked from each treatment. Then, birds were individually weighed, euthanized by cervical dislocation and collected for jejunal tissue samples. A 2-cm-length of the middle part jejunum was collected, and flushed gently with 4°C phosphate-buffered saline (PBS) to remove the contents. Then the samples were thereafter placed in 4% paraformaldehyde for morphology measurement. Another 2-cm-length of the middle part jejunum was rinsed with 4°C PBS and then cut into thin strips (2 mm × 2 mm). The tissue was fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer 7.4 at 4°C. Subsequent processing was conducted using a microwave processor. For scanning electron microscopy (SEM), specimens were post-fixed in osmium acid, dehydrated in ethanol, and critical point dried using a K850 point dryer (Quorum Corporation, United Kingdom), sputter coated with gold using a MC1000 sputter coated (Hitachi Corporation, Japan) prior to viewing with a SU8010 SEM (Hitachi Corporation, Japan). For transmission electron microscope (TEM), tissue was post-fixed, dehydrated, embedded, and polymerized in resin. Ultrathin sections were stained with 2% uranyl acetate and 3% lead citrate and examined in a HT7800 (Hitachi Corporation, Japan) microscope operating at 80 kV. The remaining jejunal sections were subsequently opened longitudinally, and the contents were flushed with ice-cold phosphate-buffered saline. Mucosa of each sample was collected utilizing a sterile glass microscope slide, rapidly stored in liquid nitrogen, and then frozen at −80°C for further analysis.
Jejunal Histomorphology
The harvested jejunal segments were dehydrated, cleared, and embedded in paraffin after a 24-h fixation in buffered formalin. They were then cut into serial sections at 5-mm depth for subsequent staining with hematoxylin and eosin stain. The villus width (VW) and villus area (VA) were determined using Biological Microscope Eclipse Ci-L (Nikon Corporation, Japan). Goblet cell density (GCD) were counted and expressed as per 100 epithelial cells. The numbers of goblet cell of 5 villi from 1 jejunal cross section per bird were counted for statistical analysis.
Jejunal Ultrastructure
Using ImageJ software program (1.51 V)(Schneider, et al., 2012), two observers took all SEM and TEM measurements independently. SEM images were employed to observe changes on conditions of the villi. The degree of villi damage was graded using a scale developed by Gomide et al. (2004). TEM images were used assess the morphology of epithelial cells, in particular enterocytes and their content features, such as cytoplasmic organelles (mainly mitochondria), microvilli and the apical junctional complex (AJC). To evaluate changes in enterocyte AJC morphology, the length of AJC between 2 adjacent enterocytes and their degree of separation was determined. The length of AJC was taken from the apical membrane, where the tight junction (TJ) began and included not only TJ and adherens junction (AJ), but also desmosome (DS) (Schneider et al., 2012).
ELISA
Interleukin-1β (IL-1β), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), and interferon-γ (IFN-γ) in the jejunal mucosa were determined using chicken-specific ELISA kits purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd (Shanghai, China) according to the manufacturer's instructions. Total protein in jejunal mucosa was measured by assay kits A045-4 from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer's instructions. The results were normalized against total protein concentration in each sample for intersample comparison.
Gene Expression Analysis
TRIzol reagent (Beijing, China) was used to extract the total RNA from the jejunal mucosa in accordance with the manufacturer's instructions. Then, quantity and quality of total RNA were assessed using a Nanodrop 2000 (Thermo Fisher Scientific, Wilmington, NC). The integrity of RNA was verified by eletrophoretic analysis. Reverse transcription was run with the PrimeScript RT reagent Kit (Promega Biotechnology Co., Ltd Beijing, China). The resulting cDNA products were stored at −20°C.
The mRNA expression of selected genes, including Occludin, Claudin-1, Zonula occludens-1 (ZO-1), Toll-like receptor 4 (TLR4), Myeloid differentiation protein 88 (MyD88), Nuclear factor-κB (NF-κB p65), IL-1β, IL-4, IL-6, IL-10, and IFN-γ were evaluated by the real-time quantitative PCR. The primers of these genes and the house-keeping gene (β-actin) were synthesized by Sangon Biotech Co., Ltd (Shanghai, China) and the sequences are listed in Table 2. The real-time quantitative PCR assay was carried out in the CFX ConnectTM Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) using SYBR Premix Ex Taq kits (Promega Biotechnology Co., Ltd Beijing, China). The reaction volume was 20 μL, containing 10 μL SYBR Premix Ex Taq II (2 ×), 0.4 μL of each primer, 1.5 μL cDNA, and 7.7 μL sterilized double-distilled water. The reaction conditions were used as follows: 95°C for 5 min; 40 cycles at 95°C for 10 s, 60°C for 10 s, 72°C for 10 s, and 95°C for 5 s; and 4°C for 5 s. The cycle threshold values were normalized to the expression level of β-actin. Relative mRNA expression levels of selected genes were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001), and CON group was used as a reference.
Table 2.
Primer sequences.
| Gene | Primer Sequence 5′-3′ | GenBank | Product Length/bp |
|---|---|---|---|
| β-Actin | F:5ʹ- CAAAAGCCAACAGAGAGAAGAT -3ʹ | NM_205518.1 | 138 |
| R:5ʹ- CATCACCAGAGTCCATCACAAT-3ʹ | |||
| Occludin | F:5ʹ- AGTTCGACACCGACCTGAAG -3ʹ | NM_205128.1 | 124 |
| R:5ʹ- TCCTGGTATTGAGGGCTGTC -3ʹ | |||
| Claudin-1 | F:5ʹ- TATGGCAACAGAGTGGCTCG -3ʹ | NM_001013611.2 | 171 |
| R:5ʹ- GCTGGGTGGGTAGGATGTTT -3ʹ | |||
| ZO-1 | F:5ʹ- AAGTGTTTCGGGTTGTGGAC -3ʹ | XM_413773.4 | 160 |
| R:5ʹ- GCTGTCTTTGGAAGCGTGTA -3ʹ | |||
| MyD88 | F:5ʹ- GGGATGTCTTGCCAGGAACG -3ʹ | NM_001030962.4 | 186 |
| R:5ʹ- TGCACTTGACCGGAATCAGC -3ʹ | |||
| TLR4 | F:5ʹ- TTCGGTTGGTGGACCTGAATCTTG -3ʹ | NM_001030693.1 | 114 |
| R:5ʹ- ACAGCTTCTCAGCAGGCAATTCC -3ʹ | |||
| NF-κB | F:5ʹ- CCACAACACAATGCGCTCTG -3ʹ | NM_205129.1 | 112 |
| R:5ʹ- AACTCAGCGGCGTCGATG -3ʹ | |||
| IL-1β | F:5ʹ- GGAGCAGGGACTTTGCTGAC -3ʹ | NM_204524.1 | 130 |
| R:5ʹ- AAGGACTGTGAGCGGGTGTA -3ʹ | |||
| IL-4 | F:5ʹ- TCTTCCTCAACATGCGTCAG -3ʹ | NM_001007079.1 | 127 |
| R:5ʹ- TGGTGGAAGAAGGTACGTAGG -3ʹ | |||
| IL-6 | F:5ʹ- AAATCCCTCCTCGCCAATCT -3ʹ | NM_204628.1 | 106 |
| R:5ʹ- CCCTCACGGTCTTCTCCATAAA -3ʹ | |||
| IL-10 | F:5ʹ- CAGACCAGCACCAGTCATCA -3ʹ | NM_001004414.2 | 163 |
| R:5ʹ- TCCCGTTCTCATCCATCTTCTC -3ʹ | |||
| IFN-γ | F:5ʹ- CTTCCTGATGGCGTGAAGA -3ʹ | NM_205149.1 | 127 |
| R:5ʹ- GAGGATCCACCAGCTTCTGT -3ʹ |
Statistical Analysis
The data were analyzed using one-way analysis of variance (ANOVA) and Duncan's multiple range test for multiple comparisons using SPSS version 20.0 (SPSS Inc., Chicago, IL). Results are presented as means ± standard error. Statistical significance was considered if P < 0.05 in all analyses.
RESULTS
Growth Performance
The effects of lutein supplementation on the growth performance of broilers is shown in Supplementary Table S1 (Wang et al., 2021b). Compared with CON group, dietary supplemented with LU40 significantly increased the ADG of broilers at 1 to 21 and 1 to 26 d of age (P < 0.05), significantly decreased the F/G of broilers at 22 to 26 d of age (P < 0.05).
Jejunal Histomorphology
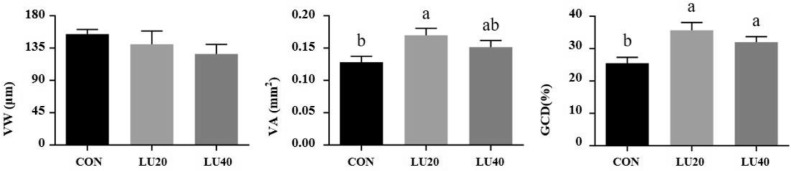
As shown in Figure 1, dietary supplemented with LU20 or LU40 increased GCD (P < 0.05), and LU20 supplementation increased the VA compared with CON group (P < 0.05). However, there was no significant difference in VW among 3 groups (P > 0.05).
Figure 1.
Effects of lutein on jejunal morphology of LPS-challenged yellow-feather broilers. CON group, basal diet; LU20 group, basal diet added with 20 mg/kg lutein; LU40 group, basal diet with added 40 mg/kg lutein. VW, villus width; VA, villus area; GCD, goblet cell density. The values with different superscript letters are different (P < 0.05). Error bars stand for the standard error of mean.
Jejunal Ultrastructure
Villi alterations
As shown in Figure 2, SEM examinations demonstrate three different degrees of epithelium loss in jejunal villi. Dietary supplementation with lutein gradually reduced villi damage compared with the CON group, as described below:
Degree 0: no apparent the shed epithelium (Normal villi);
Degree 1: small areas of apical epithelium shedding (Figure 2, LU40 group);
Degree 2: small areas of epithelium loss, associated with atrophy villi (Figure 2, LU20 group);
Degree 3: lack of epithelium in the entire villus, and exposure of connective tissue, associated with atrophy villi (Figure 2, CON group).
Figure 2.
Scanning electron micrographs of jejunal ultrastructural feature. White arrow, the villi damage. CON group, basal diet; LU20 group, basal diet added with 20 mg/kg lutein; LU40 group, basal diet added with 40 mg/kg lutein. Scale bar = 500 μm.
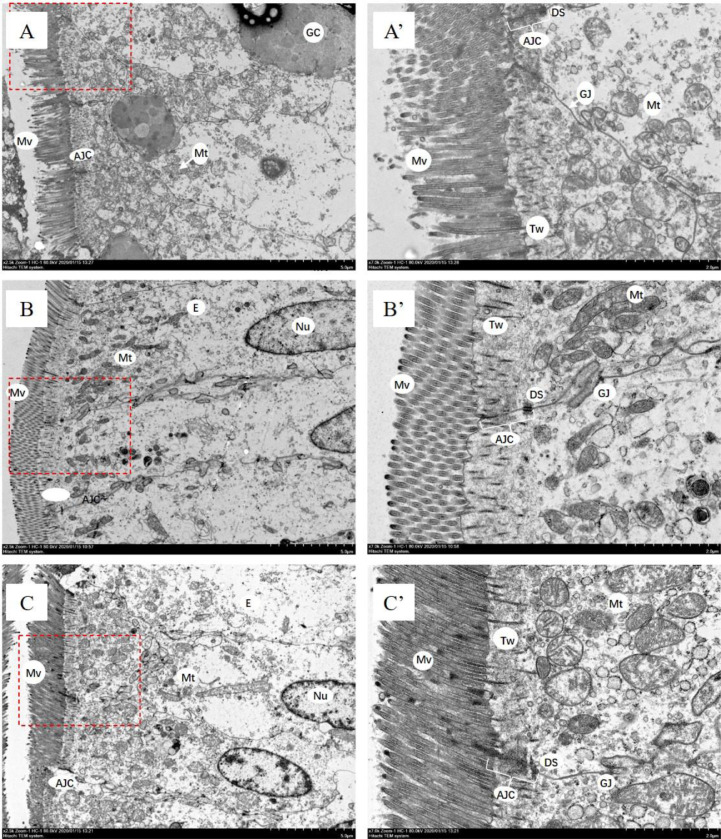
Mitochondria, microvilli and apical junctional complex changes
As shown in Figure 3, enterocytes’ substructures (microvilli) and cytosolic organelles (mitochondria), as well as the AJC of adjacent enterocytes were observed by SEM. In LU20 and LU40 groups, mitochondria were round or oval and more abundant, with little structural damage. However, mitochondria were swollen, damaged and the cristae was indistinct in CON group. Additionally, the microvilli were broken and sparse in CON group. Compared with LU20 and LU40 groups, the length of AJC was shorter in CON group and dilatations within the AJC were evident in the AJ and DS regions.
Figure 3.
Transmission electron micrographs of jejunal epithelial cells. Abbreviations: AJ, adherens junction; AJC, apical junctional complex; DS, desmosome; E, enterocyte; GC, goblet cells; GJ, gap junctions; Tw, terminal web; Mt, mitochondria; Mv, microvilli; Nu, nucleus; TJ, tight junction. The AJC between neighboring cells is composed of TJ followed by AJ and DS. A & A’ from CON group, basal diet; B & B’ from LU20 group, basal diet added with 20 mg/kg lutein; C & C’ from LU40 group, basal diet added with 40 mg/kg lutein. A’ B’, and C’ are the enlarged picture in the red dashed box of A, B, and C, respectively. Scale bar are 5 μm in image A, B or C and 2 μm in image A’, B’ or C’.
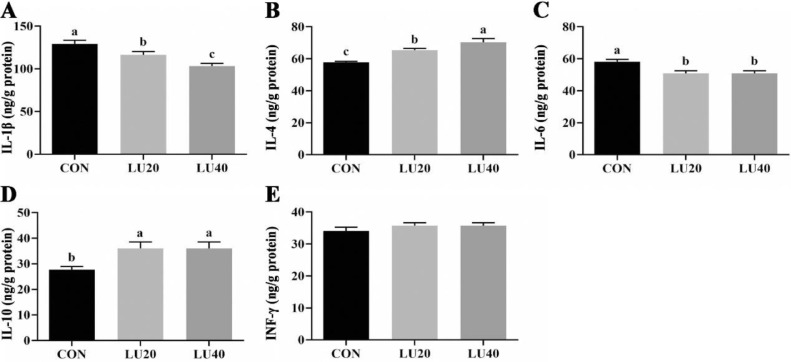
Jejunal Mucosa Inflammatory Cytokines
As shown in Figure 4, diet supplemented with LU20 and LU40 decreased the concentrations of IL-1β and IL-6 in the jejunal mucosa of LPS-challenged broilers (P < 0.05), but increased the concentrations of IL-4 and IL-10 compared with CON group (P < 0.05). Meanwhile, LU40 supplementation reduced the concentrations of IL-1β, but elevated the concentration of IL-4 compared with LU20 group (P < 0.05). No significant differences were observed in the concentration of INF-γ among 3 groups (P > 0.05).
Figure 4.
Effects of lutein on inflammatory cytokines in jejunal mucosa of LPS-challenged in yellow-feather broilers. CON group, basal diet; LU20 group, basal diet added with 20 mg/kg lutein; LU40 group, basal diet added with 40 mg/kg lutein. The values with different superscript letters are different (P < 0.05). Error bars stand for the standard error of mean.
Gene Expression of Tight Junction Proteins in Jejunal Mucosa
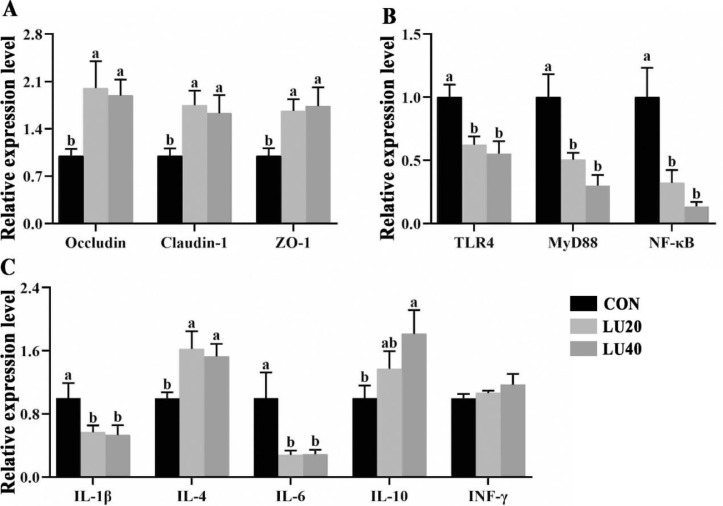
As shown in Figure 5A, dietary supplemented with LU20 and LU40 increased the mRNA expression of Occludin, Claudin-1 and ZO-1 in the jejunal mucosa of LPS-challenged broilers compared with CON group (P < 0.05). No significant differences of Occludin, Claudin-1, and ZO-1 mRNA expression were observed between LU20 and LU40 groups.
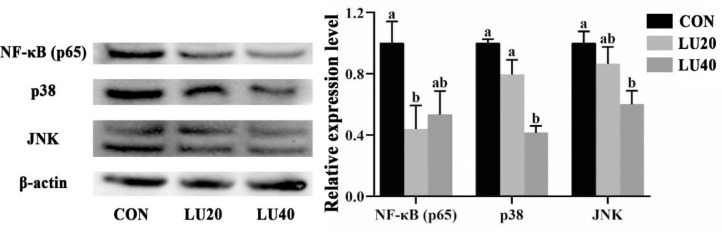
Figure 6.
Effects of lutein on protein expression of NF-κB p65, p38 and JNK in jejunal mucosa of LPS-challenged yellow-feather broilers. CON group, basal diet; LU20 group, basal diet added with 20 mg/kg lutein; LU40 group, basal diet added with 40 mg/kg lutein. The values with different superscript letters are different (P < 0.05). Error bars stand for the standard error of mean.
Figure 5.
Effects of lutein on mRNA expression of tight junction protein and TLR4 pathway in jejunal mucosal of LPS-challenged yellow-feather broilers. CON group, basal diet; LU20 group, basal diet added with 20 mg/kg lutein; LU40 group, basal diet added with 40 mg/kg lutein. The values with different superscript letters are different (P < 0.05). Error bars stand for the standard error of mean.
Relative mRNA Expression of NF-κB Pathway-Related Genes in Jejunal Mucosa
As shown in Figure 5B, C. Compared to CON group, dietary supplemented with LU20 and LU40 downregulated the mRNA expressions of TLR4, MyD88, NF-κB, IL-1β, and IL-6 (P < 0.05), but upregulated the mRNA expression of IL-4 (P < 0.05). The mRNA expression of IL-10 in LU40 group was higher than CON group (P < 0.05). No differences were observed in the mRNA expression of INF-γ among 3 groups (P > 0.05).
Protein Expression of NF-κB (p65), p38, and JNK in Jejunal Mucosa
As shown in Figure 5, compared with the CON group, dietary LU40 supplementation reduced protein expression levels of p38 and JNK in the jejunal mucosa of LPS-challenged broilers (P < 0.05). Meanwhile, the protein expression levels of NF-κB (p65) in LU20 group was lower than CON group (P < 0.05).
DISCUSSION
The intestinal mucosal barrier has the ability to digest and absorb nutrients, which are the first line of defense against most external pathogens (France and Turner, 2017). By observing the changes in villi, intestinal epithelial cells and tight junction between adjacent cells, we identified potential regulatory structures associated with damaged or healthy intestinal mucosa. Tongue-shaped villi and zigzag pattern increase digesta retention time in the intestinal tract, and promote nutrient absorption by the host (Yamauchi and Isshiki, 1991). Similarly, microvilli was formed the brush border structure (Mooseker, 1985), which increase the surface area of cells and improve the ability to absorb nutrients and receive stimuli (Crawley et al., 2014). The results showed that with the increase of lutein in the diet, the villus damage in LU40 group gradually decreased, but there was no significant difference in villus area compared with the CON group. We also found that the microvilli in the CON group were broken and disordered, while the microvilli in the LU40 group were dense and regular, which was consistent with the above results of villi injury. Our finding indicated that dietary 40 mg/kg lutein supplementation could repair LPS-induced villi damage.
Intestinal epithelial cells are a single layer of cells lining the lumen that permit the translocation of nutrients, electrolytes, and water from the lumen into the circulation, while prevent the passage of harmful intraluminal entities (Blikslager et al., 2007; Broer, 2008; Groschwitz and Hogan, 2009). Goblet cells are specialized intestinal epithelial cells, which are distributed between monolayer columnar cells and can secrete biologically active products to protect against bacterial invasion (Zhang and Wu, 2020). In addition, goblet cells are essential for maintaining the integrity of intestinal epithelium and mucosal barrier (Gadaleta et al., 2011; McCauley and Guasch, 2015; Yu et al., 2020). In the present study, 20 and 40 mg/kg lutein supplementation could alleviate the decrease of goblet cells induced by LPS. Enterocyte mitochondria are involved in regulating numerous aspects of cellular activity, including the regulation of gut functions such as intestinal barrier integrity and mucosal immune responses (Kang and Pervaiz, 2012; Clark and Mach, 2017). Maintenance of the intestinal epithelial barrier is an energy-dependent process, and mitochondrial dysfunction leads to decreased energy production, resulting in the loss of intestinal epithelial barrier integrity, apoptosis and bacterial invasion (Picard et al., 2013; Brito et al., 2019). The results showed that dietary 40 mg/kg lutein supplementation inhibited LPS-induced mitochondria swollen, irregular, vacuolated or cristae damaged. It is speculated that 40 mg/kg lutein may improve the barrier injury of intestinal epithelial cells.
There is growing evidence that altered AJC structure and increased in intestinal permeability play a pathogenic role in inflammatory bowel diseases (Camilleri et al., 2012). Intestinal epithelium through the formation of protein-protein networks that link adjacent cells and seal the intercellular space (Farquhar and Palade, 1963), which coordinate tissue morphogenesis and homeostasis (Baum and Georgiou, 2011; Citi et al., 2012; Zihni et al., 2014). Tight junctions are the key component of the mucosal barrier and play an important role in regulating intestinal permeability (Quiros and Nusrat, 2014). The expressions of tight junction proteins in healthy intestinal tissue were significantly higher than that in inflammatory mucosa (Gassler et al., 2001). Previous researches have demonstrated that LPS challenge destroyed the jejunal tight junction and decreased the expression of Occludin and ZO-1 in the intestinal mucosa (Wang et al., 2014; Zhu et al., 2015). In the current study, we found that the AJC (in the AJ and DS parts) was longer and less saccular in 40 mg/kg lutein group compared with CON group. Moreover, dietary 20 and 40 mg/kg lutein supplementation reversed the downregulations of the mRNA expressions of Occludin, Claudin-1, and ZO-1 induced by LPS challenge. Taken together, the above data revealed that 40 mg/kg lutein supplementation may regulate barrier function by regulating tight junctions.
Impairment of the intestinal barrier is usually accompanied by inflammation, while intestinal inflammation is largely driven through upregulated TLR4/NF-κB signaling pathway (Bruewer et al., 2006). Activation of TLR4 initiates an innate immune response and subsequent inflammation in intestinal inflammatory diseases (Boone and Ma, 2003; Le Mandat Schultz, et al., 2007). TLR4 recognizes LPS and then induces activation of NF-κB through MyD88 pathway, then promoting the release of proinflammatory cytokines such as IL-1β and IL-6, thus affect the inflammatory responses (Chow et al., 1999; Al-Sadi et al., 2008). Previous researches have showed that lutein can affect inflammation responses, which suppresses NF-κB activation (Kijlstra et al., 2012). In endotoxin-induced uveitis in rat models, lutein has been shown to ameliorate the disease through a reduction in NF-κB signal transduction and the downstream proinflammatory cytokines expression (Jin et al., 2006). Previous studies have shown that dietary lutein could reverse LPS-induced upregulation of proinflammatory cytokines expression and alleviate inflammatory (Meriwether et al., 2010; Selvaraj et al., 2010). In the current study, we found that lutein CON group increased the levels of IL-1β and IL-6, and decrease the levels of IL-4 and IL-10. We speculated that inflammatory response has occurred in broilers, and hope to explore the possible regulatory mechanism of lutein on the intestinal mucosal barrier by detecting the expression of TLR4/MyD88/NF-κB signaling pathway and downstream inflammatory cytokines. We also found that lutein could attenuate the activation of TLR4/MyD88/NF-κB pathway by LPS, inhibit the expression of IL-1β and IL-6, whereas enhance the expression of IL-4 and IL-10. Moreover, the best effect was found in the 40 mg/kg lutein group. Previous studies indicated that lutein and zeaxanthin had anti-inflammatory effects, inhibiting the protein expressions of JNK and NF-κB (p65), thereby alleviating LPS-induced inflammation (Chao, et al., 2015). Similarly, we found that 20 and 40 mg/kg lutein supplementation decreased the protein expression of NF-κB (p65) and MAPK (p38/JNK), respectively. This is consistent with the findings found on other carotenoids (β-carotene and β-cryptoxanthin) (Liu et al., 2016; Yang et al., 2021). Therefore, we inferred that dietary 40 mg/kg lutein inhibited LPS-induced inflammatory reaction by suppressing the TLR4/MyD88 pathway.
CONCLUSION
Lutein improves LPS-induced intestinal barrier function and tames the inflammation by decreasing intestinal epithelial cells destruction, strengthening tight junction, and inhibiting TLR4/MyD88 signaling pathway. Overall, 40 mg/kg lutein is the optimal dosage for broilers under LPS challenge.
Acknowlegments
This study was supported by National Natural Science Foundation of China (31802079) and Poultry Industry System in Fujian Province of China (KKE19013A).
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.102191.
Appendix. Supplementary materials
References
- Al-Sadi R., Ye D., Dokladny K., Ma T.Y. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B., Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikslager A.T., Moeser A.J., Gookin J.L., Jones S.L., Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 2007;87:545–564. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- Boone D.L., Ma A. Connecting the dots from Toll-like receptors to innate immune cells and inflammatory bowel disease. J Clin. Invest. 2003;111:1284–1286. doi: 10.1172/JCI18545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito C., Cabanes D., Mesquita F.Sarmento, Sousa S. Mechanisms protecting host cells against bacterial pore-forming toxins. Cell. Mol. Life Sci. 2019;76:1319–1339. doi: 10.1007/s00018-018-2992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G. In: Carotenoids. Britton G., Liaaen-Jensen S., Pfander H., editors. Birkhäuser Basel; 2008. Functions of Intact Carotenoids; pp. 189–212. Pages. eds. [Google Scholar]
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Bruewer M., Samarin S., Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann. Ny Acad. Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Madsen K., Spiller R., Greenwood-Van Meerveld B., Verne G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao S.C., Vagaggini T., Nien C.W., Huang S.C., Lin H.Y. Effects of Lutein and Zeaxanthin on LPS-Induced Secretion of IL-8 by Uveal Melanocytes and Relevant Signal Pathways. J. Ophthalmol. 2015;2015 doi: 10.1155/2015/152854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J.C., Young D.W., Golenbock D.T., Christ W.J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Citi S., Pulimeno P., Paschoud S. Cingulin, paracingulin, and PLEKHA7: signaling and cytoskeletal adaptors at the apical junctional complex. Barriers Chann. Formed Tight Junction Proteins I. 2012;1257:125–132. doi: 10.1111/j.1749-6632.2012.06506.x. [DOI] [PubMed] [Google Scholar]
- Clark A., Mach N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017:8. doi: 10.3389/fphys.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley S.W., Mooseker M.S., Tyska M.J. Shaping the intestinal brush border. J. Cell Biol. 2014;207:441–451. doi: 10.1083/jcb.201407015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M.G., Palade G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France M.M., Turner J.R. The mucosal barrier at a glance. J. Cell Sci. 2017;130:307–314. doi: 10.1242/jcs.193482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W., Belvedere A., Vetrano S. Sealing the broken barrier in IBD: intestinal permeability, epithelial cells and junctions. Curr. Drug. Targets. 2013;14:1460–1470. doi: 10.2174/1389450111314120011. [DOI] [PubMed] [Google Scholar]
- Gadaleta R.M., van Erpecum K.J., Oldenburg B., Willemsen E.C., Renooij W., Murzilli S., Klomp L.W., Siersema P.D., Schipper M.E., Danese S., Penna G., Laverny G., Adorini L., Moschetta A., van Mil S.W. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- Gao Y.Y., Chen L.Z., Zhang J., Fan Q., Xu L.H., Huang Y.Q., Wang C.K. Effects of lipopolysaccharide (LPS) challenge on antioxidant capability of broiler chickens. Indian J. Anim. Sci. 2018;88:1070–1077. [Google Scholar]
- Gao Y.Y., Chen L.Z., Zhang J. Effects of lipopolysaccharide (LPS) challenge on antioxidant capability of broiler chickens. Indian J. Anim. Sci. 2018;88:1070–1077. [Google Scholar]
- Gao Y.Y., Jin L., Ji J., Sun B.L., Xu L.H., Wang Q.X., Wang C.K., Bi Y.Z. Xanthophyll supplementation reduced inflammatory mediators and apoptosis in hens and chicks. J. Anim. Sci. 2016;94:2014–2023. doi: 10.2527/jas.2015-9628. [DOI] [PubMed] [Google Scholar]
- Gao Y.Y., Jin L., Peng H., Xu L.H., Wang Q.X., Ji J., Wang C.K., Bi Y.Z. Xanthophylls increased HDLC level and nuclear factor PPARgamma, RXRgamma and RARalpha expression in hens and chicks. J. Anim. Physiol. Anim. Nutr. (Berl.). 2018;102:e279–e287. doi: 10.1111/jpn.12739. [DOI] [PubMed] [Google Scholar]
- Gao Y.Y., Xie Q.M., Jin L., Sun B.L., Ji J., Chen F., Ma J.Y., Bi Y.Z. Supplementation of xanthophylls decreased proinflammatory and increased anti-inflammatory cytokines in hens and chicks. Br. J. Nutr. 2012;108:1746–1755. doi: 10.1017/S0007114512000025. [DOI] [PubMed] [Google Scholar]
- Gassler N., Rohr C., Schneider A., Kartenbeck J., Bach A., Obermüller N., Otto H.F., Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G216–G228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- Gomide M.H., Sterzo E.V., Macari M., Boleli I.C. Use of scanning electron microscopy for the evaluation of intestinal epithelium integrity. Revista Brasileira De Zootecnia-Braz. J. Anim. Sci. 2004;33:1500–1505. [Google Scholar]
- Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X.H., Ohgami K., Shiratori K., Suzuki Y., Hirano T., Koyama Y., Yoshida K., Ilieva I., Iseki K., Ohno S. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Invest. Ophthalmol. Vis. Sci. 2006;47:2562–2568. doi: 10.1167/iovs.05-1429. [DOI] [PubMed] [Google Scholar]
- Kang J., Pervaiz S. Mitochondria: redox metabolism and dysfunction. Biochem. Res. Int. 2012;2012 doi: 10.1155/2012/896751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijlstra A., Tian Y., Kelly E.R., Berendschot T.T.J.M. Lutein: more than just a filter for blue light. Prog. Retin. Eye Res. 2012;31:303–315. doi: 10.1016/j.preteyeres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Koutsos E.A., Garcia Lopez J.C., Klasing K.C. Carotenoids from in ovo or dietary sources blunt systemic indices of the inflammatory response in growing chicks (Gallus gallus domesticus) J. Nutr. 2006;136:1027–1031. doi: 10.1093/jn/136.4.1027. [DOI] [PubMed] [Google Scholar]
- Le Mandat Schultz A., Bonnard A., Barreau F., Aigrain Y., Pierre-Louis C., Berrebi D., Peuchmaur M. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PLoS One. 2007;2:e1102. doi: 10.1371/journal.pone.0001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang H., Chen Y.P., Yang M.X., Zhang L.L., Lu Z.X., Zhou Y.M., Wang T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult. Sci. 2015;94:1504–1511. doi: 10.3382/ps/pev124. [DOI] [PubMed] [Google Scholar]
- Liu L., Qin D.K., Wang X.F., Feng Y., Yang X.J., Yao J.H. Effect of immune stress on growth performance and energy metabolism in broiler chickens. Food Agr. Immunol. 2015;26:194–203. [Google Scholar]
- Liu L., Shen J., Zhao C., Wang X., Yao J., Gong Y., Yang X. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int. J. Biol. Macromol. 2015;72:624–632. doi: 10.1016/j.ijbiomac.2014.08.057. [DOI] [PubMed] [Google Scholar]
- Liu X.R., Wang Y.Y., Dan X.G., Kumar A., Ye T.Z., Yu Y.Y., Yang L.G. Anti-inflammatory potential of beta-cryptoxanthin against LPS-induced inflammation in mouse Sertoli cells. Reprod. Toxicol. 2016;60:148–155. doi: 10.1016/j.reprotox.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mashaly M.M., Hendricks G.L., 3rd, Kalama M.A., Gehad A.E., Abbas A.O., Patterson P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- McCauley H.A., Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015;21:492–503. doi: 10.1016/j.molmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Meriwether L.S., Humphrey B.D., Peterson D.G., Klasing K.C., Koutsos E.A. Lutein exposure, in ovo or in the diet, reduces parameters of inflammation in the liver and spleen laying-type chicks (Gallus gallus domesticus) J. Anim. Physiol. Anim. Nutr. (Berl.) 2010;94:e115–e122. doi: 10.1111/j.1439-0396.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- Mooseker M.S. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu. Rev. Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- Noda S., Tanabe S., Suzuki T. Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. J. Agric. Food Chem. 2012;60:4628–4633. doi: 10.1021/jf300382h. [DOI] [PubMed] [Google Scholar]
- Oda H., Takeichi M. Evolution Structural and functional diversity of cadherin at the adherens junction. J. Cell Biol. 2011;193:1137–1146. doi: 10.1083/jcb.201008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M., Shirihai O.S., Gentil B.J., Burelle Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R393–R406. doi: 10.1152/ajpregu.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros M., Nusrat A. RhoGTPases, actomyosin signaling and regulation of the epithelial. Apical Junct. Complex. Semin. Cell Dev. Biol. 2014;36:194–203. doi: 10.1016/j.semcdb.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R.K., Shanmugasundaram R., Klasing K.C. Effects of dietary lutein and PUFA on PPAR and RXR isomer expression in chickens during an inflammatory response. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010;157:198–203. doi: 10.1016/j.cbpa.2010.06.172. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram R., Selvaraj R.K. Lutein supplementation alters inflammatory cytokine production and antioxidant status in F-line turkeys. Poult. Sci. 2011;90:971–976. doi: 10.3382/ps.2010-01150. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.Y., Tong Y.X., Min Y., Wang C.K., Gao Y.Y. Effects of different levels of lutein on growth performance, jejunum morphology and cecal microorganisms of lipopolysaccharide-stimulated broilers. Chin. J. Anim. Nutr. 2021;33:5569–5580. [Google Scholar]
- Wang Q., Wang X.F., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang L., Li J., Cong J., Gao F., Zhou G. Effects of dietary marigold extract supplementation on growth performance, pigmentation, antioxidant capacity and meat quality in broiler chickens. Asian-Australas J. Anim. Sci. 2017;30:71–77. doi: 10.5713/ajas.16.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Quinn P.J. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog. Lipid. Res. 2010;49:97–107. doi: 10.1016/j.plipres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Wang X., Shen J., Li S., Zhi L., Yang X., Yao J. Sulfated Astragalus polysaccharide regulates the inflammatory reaction in LPS-infected broiler chicks. Int. J. Biol. Macromol. 2014;69:146–150. doi: 10.1016/j.ijbiomac.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Isshiki Y. Scanning electron microscopic observations on the intestinal villi in growing White Leghorn and broiler chickens from 1 to 30 days of age. Br. Poult. Sci. 1991;32:67–78. doi: 10.1080/00071669108417328. [DOI] [PubMed] [Google Scholar]
- Yang X.J., Li W.L., Feng Y., Yao J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011;90:2740–2746. doi: 10.3382/ps.2011-01591. [DOI] [PubMed] [Google Scholar]
- Yang Y., Li R., Hui J., Li L., Zheng X. beta-Carotene attenuates LPS-induced rat intestinal inflammation via modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK signaling pathways. J. Food Biochem. 2021;45:e13544. doi: 10.1111/jfbc.13544. [DOI] [PubMed] [Google Scholar]
- Yu T.X., Chung H.K., Xiao L., Piao J.J., Lan S., Jaladanki S.K., Turner D.J., Raufman J.P., Gorospe M., Wang J.Y. Long noncoding RNA H19 impairs the intestinal barrier by suppressing autophagy and lowering Paneth and goblet cell function. Cell Mol. Gastroenterol. Hepatol. 2020;9:611–625. doi: 10.1016/j.jcmgh.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wu C. The relationship between intestinal goblet cells and the immune response. Biosci. Rep. 2020;40 doi: 10.1042/BSR20201471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhong X., Zhou Y., Wang G., Du H., Wang T. Dietary RRR-alpha-tocopherol succinate attenuates lipopolysaccharide-induced inflammatory cytokines secretion in broiler chicks. Br. J. Nutr. 2010;104:1796–1805. doi: 10.1017/S0007114510002801. [DOI] [PubMed] [Google Scholar]
- Zhu C., Wu Y., Jiang Z., Zheng C., Wang L., Yang X., Ma X., Gao K., Hu Y. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 2015;28:288–294. doi: 10.1016/j.intimp.2015.04.054. [DOI] [PubMed] [Google Scholar]
- Zihni C., Balda M.S., Matter K. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J. Cell Sci. 2014;127:3401–3413. doi: 10.1242/jcs.145029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.