Abstract
Esophageal cancer is a significant health burden in the United States and worldwide and is the 8th leading cause of cancer-related death. Over 90% of esophageal cancers are squamous cell cancers (ESCC). Despite the development of new therapies, the overall 5-year survival rate remains lower than 20%. Recent clinical trials of immunotherapy approaches in ESCC have shown that blocking PD-1/PD-L1 interactions can reduce tumor burden and increase survival, but this only occurs in a fraction of patients. This emphasizes the need for additional therapeutic options to improve overall response rates, duration of response, and overall survival. Glucocorticoid-induced TNFR-related protein (GITR) stimulation has emerged as a promising immunotherapy target, as its stimulation appears to promote tumor regression. In this study, we evaluated the consequences of GITR agonistic stimulation with the DTA-1 antibody (anti-GITR agonist) on esophageal squamous cell carcinoma (ESCC) progression. Increased expression of GITR was observed in esophageal tumors from ESCC patients in comparison to normal adjacent tissue and in a mouse model of ESCC. 100% of mice treated with 4-NQO/IgG control antibody developed invasive squamous cell carcinoma. Less advanced esophageal tumors were seen in mice treated with 4-NQO/anti-GITR agonist compared to 4-NQO/IgG treatment. 4-NQO/anti-GITR agonist-treated mice demonstrated a significant increase in mucosal CTL/Treg ratios as well as decreased gene expression profiles of pathways related to esophageal squamous cell carcinogenesis. Thus, GITR agonism merits further study as a treatment strategy for ESCC patients.
In this study, we found that GITR agonistic stimulation has an anti-tumor effect in a mouse model of ESCC. Anti-GITR treatment response was associated with an increase in CTL/Treg ratio and the decrease of known drivers of esophageal carcinogenesis.
Graphical Abstract
Introduction
Esophageal cancer (EC) is a significant burden in the U.S. and throughout the world and is the 8th most common cause of cancer-related death worldwide with an overall 5 year survival rate of less than 20% (1). There are two main histological subtypes of EC: esophageal squamous cell carcinoma (ESCC), which accounts for over 90% of EC cases worldwide, and esophageal adenocarcinoma (EAC), which is the most prevalent in the United States and other western countries (1). ESCCs arise within the stratified squamous epithelial cells that normally line the esophagus (1). Several studies have provided evidence that chronic inflammation is strongly associated with the pathogenesis of EC. Irritating factors, such as alcohol and tobacco use, produce chronic epithelial irritation, leading to inflammation and predisposing to esophageal squamous cell cancer (ESCC) (2,3).
Despite extensive characterization of the genomic landscape of ESCC (4), effective targeted therapies are still urgently needed to improve ESCC patient outcomes, as the 5-year survival rate remains poor. Accumulating evidence from clinical trials has suggested that immune checkpoint modulation as an anti-cancer therapeutic strategy may improve response and ultimately survival. Immunotherapies have been extensively explored in esophageal and gastroesophageal junction (GEJ) cancer but with very limited success. In recent years, the use of immunotherapies targeting programmed death 1 (PD-1), programmed death ligand 1 (PD-L1) and cytotoxic T-lymphocyte–associated 4 (CTLA-4) has improved survival for patients with several cancers, including melanoma and non-small cell lung cancer (5). The potential role of immunotherapy in EC is emerging but only limited information is available. Recently, clinical trials looking at PD-1/PD-L1 inhibition in ESCC patients have shown increased overall survival, increased progression-free survival, and higher overall response rates compared with current standards of therapy (6–10). Despite these encouraging results, tumor regression only occurs in a small fraction of patients, particularly those with higher biomarker expression of PD-L1 (11). Although these results involving checkpoint inhibitors are exciting and encouraging, strategies to improve overall response rates, duration of response, and overall survival in the ESCC patient population are needed.
It has been suggested that modulation of additional immune checkpoints can regulate the effectiveness of immunosurveillance in human cancers. For this reason, there has been increasing interest to explore the potential role of additional immune checkpoint therapies. One emerging promising strategy involves the agonistic stimulation of Glucocorticoid-induced TNFR-related protein (GITR). GITR is a co-stimulatory immune modulating receptor expressed on various immune cell subsets, with particularly high expression on regulatory T cells (12,13). The co-stimulatory effects of an anti-GITR agonistic monoclonal antibody have been shown on T cells in vitro and in vivo (13). Furthermore, potent anti-tumor efficacy of this anti-GITR agonistic antibody has been shown in multiple mouse tumor models (14–16). Most interestingly, these mouse studies have demonstrated that GITR agonistic stimulation suppresses tumor growth not only in immunogenic tumors, but also in poorly immunogenic tumors, putting GITR agonists in a unique position compared to other immune checkpoint inhibitors.
A recent study characterizing the immune landscape of human ESCC found an overall increase in T cells in ESCC tissue compared to normal adjacent tissue, further supporting that GITR may be a good immunotherapeutic target in human ESCC (17). The ESCC T cell compartment was further reported to contain an increase in Tregs as well as a decrease in active CTLs (17). These changes in the T cell landscape in ESCC suggest that ESCC could be a good candidate for GITR agonism.
In this study, we evaluated the consequences of GITR agonistic stimulation on ESCC progression in a mouse model of ESCC.
Materials and methods
4-NQO mouse treatment
8 Weeks-old c57BL/6 mice were treated with the carcinogen 4-nitroquinoline 1-oxide (4-NQO) as previously described (18). Briefly, 100 μg/ml 4-NQO was administered in the drinking water for 16 weeks followed by access to normal drinking water for 12 weeks. 100% Incidence of invasive ESCC has been reported (18). Control mice were treated with drinking water containing 2% propylene glycol as a vehicle control. Gross examination of mice was performed prior to their processing for histology. Tissue was fixed in zinc formalin (Sigma-Aldrich, St-Louis, MO) for 4 days, embedded in paraffin, and 4-μm sections were applied to charged plus slides. Hematoxylin and eosin staining was performed to evaluate histology.
Immunohistochemistry and tissue microarray
Immunohistochemical staining was performed as previously described (19). Briefly, heat antigen retrieval (2100 Antigen Retriever, Electron Microscopy Sciences, Hatfield, PA) was performed on paraffin-embedded esophageal sections, slides were blocked and incubated with the following antibodies: 1:50 rabbit anti-GITR (human: Cell Signaling #68014, mouse: Cell Signaling #37472), 1:500 rabbit anti-SOX2 (Cell Signaling #14962). Species-specific biotinylated secondary antibodies were used, and chromogenic detection was performed as previously described (19). Tissue microarray of human biopsies from esophageal squamous cell cancer (n = 93) and normal adjacent tissue (n = 79) was obtained from US Biomax, Inc (Derwood, MD, HEso-Squ172Sur-02). Staining intensity was scored on a scale from 0 to 3 (0 = none, 1 = weak or mild, 2 = moderate, 3 = intense) in a blinded fashion by two investigators. Images were captured on a Nikon Eclipse Ci-E microscope with a Nikon DS-Ri2 camera and NIS Elements software.
Protein analyses
Mouse esophagi were harvested and the esophageal mucosa was mechanically separated from whole tissue. Tissue was homogenized in triton lysis buffer (1% Triton X-100, 50mM Tris-HCl pH 7.5, 100mM NaCl, 5mM EDTA, 40mM β-glycerophosphate, 5% glycerol, 50mM NaF) containing protease (Pierce, Rockford, IL) and phosphatase inhibitors (Sigma-Aldrich) to extract proteins. Pierce BCA protein assay was used to determine protein concentration (Thermo Fisher Scientific). The Quantibody Q4000 multiplex ELISA testing service of RayBiotech Inc. (Norcross, GA) was used to determine the concentration of 200 biomarkers.
In vivo agonistic GITR antibody treatment
At the end of 4-NQO or propylene glycol treatments, mice were injected intraperitoneally with 10 µg of the agonistic GITR antibody DTA-1 (BioXCell, West Hanover, NH, #BE0063) or rat IgG2b isotype control. Mice were treated every Tuesday and Friday for 2.5 weeks and were harvested the day after the last injection.
Flow cytometry
For flow cytometry experiments, mice were sacrificed after 28 weeks of treatment. Spleen, blood and esophagus were harvested. Esophageal epithelia were separated mechanically from whole esophageal tissue, and both tissue fractions were minced. Digestion of esophageal epithelia was performed in 0.05% Trypsin-EDTA (Thermo Fisher Scientific) for 2 min at 37°C and the remaining esophageal tissue was digested in collagenase V (Sigma-Aldrich) for 15 min at 37°C. Cell suspensions were filtered through 70 μm nylon filters. Blood was obtained via cardiac puncture and whole spleen was harvested and filtered through 70 μm nylon filters. Red blood cells were lysed with ACK lysis buffer (Thermo Fisher Scientific). Spleen suspensions were filtered for a second time through 70 µm nylon filters. Up to 1 million cells were stained per sample. Live/dead cell stain was performed using eBioscienceTM Fixable Viability Dye eFluorTM 506 (Thermo Fisher Scientific) as stated by the manufacturer’s instructions. A 30 min cell surface staining was performed for the indicated antibodies. A Foxp3 fixation/permeabilization kit (BioLegend, San Diego, CA) was used to perform intracellular staining according to manufacturer’s instructions. Monocytes were phenotyped as CD45+CD115+, macrophages as CD45+F4/80+CD11b+, neutrophils as CD45+CD11b+Ly6G+, eosinophils as CD45+SiglecF+, mast cells as CD45+CD117+, B cells as CD45+CD11b‐Ly6G‐CD19+CD3‐, dendritic cells as CD45+CD11c+, natural killer cells as CD45+,CD49b+,NK1.1+, T cells as CD45+CD11b‐Ly6G‐CD19‐CD3+, regulatory T cells as CD45+CD11b‐Ly6G‐CD19‐CD3+CD4+CD8‐Foxp3+, T-helper cells as CD45+CD11b‐Ly6G‐CD19‐CD3+CD4+,CD8‐, and cytotoxic T lymphocytes as CD45+CD11b‐Ly6G‐CD19‐CD3+CD8+,CD4‐. A BD FACSymphony A5 (BD Biosciences, San Jose, CA) was used to perform flow cytometry analyses and data was analyzed using FlowJo software (BD Biosciences).
RNA sequencing
Mouse esophagi were harvested and the esophageal mucosa was mechanically separated from whole esophagus. RNA was extracted from both tissue fractions using the RNeasy Mini kit (Qiagen). cDNA libraries were generated using the Tru-Seq Total RNA-seq library prep (Illumina, San Diego, CA) following the manufacturer’s instructions. Sequencing was performed using Illumina HiSeq 4000 (Northwestern University Nu-Seq Core Facility). DNA read quality was evaluated using FastQC, and DNA reads were then cleaned by trimming adapters, filtering rRNA, and filtering poor-quality reads. STAR was used to align the cleaned reads to the Mus musculus genome (mm10) (20). The number of reads per gene were calculated by htseq-count (21) along with a mm10 gene annotation file obtained from University of California Santa Cruz (genome.ucsc.edu). Count normalization and differential expression of genes were calculated using DESeq2 (22). Genes were considered significantly differentially expressed with an FDR-adjusted P-value less than 0.05 and a log2 fold change of > 0.9 or < ‐0.9. Gene ontology (GO) analysis was performed using the Metascape online platform (metascape.org) (23). RNA-seq was deposited in Gene Expression Omnibus (GEO # GSE202545) and can be accessed at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE202545.
Antibodies for flow cytometry
The table below lists the antibodies for flow cytometry experiments.
| Antibody | Clone | Fluorophore | Company | Catalog number |
|---|---|---|---|---|
| CD115 | T38-320 | BV421 | BD Biosciences | 743638 |
| CD117 | 2B8 | BV711 | BD Biosciences | 563160 |
| CD11b | M1/70 | BUV737 | BD Biosciences | 564443 |
| CD11c | HL3 | BUV395 | BD Biosciences | 564080 |
| CD170 | E50-2440 | PE-CF594 | BD Biosciences | 562757 |
| CD19 | 1D3 | APC-Cy7 | BD Biosciences | 557655 |
| CD31 | 390 | AF488 | BD Biosciences | 563607 |
| CD3e | 500A2 | AF700 | BD Biosciences | 557984 |
| CD4 | GK1.5 | BUV496 | BD Biosciences | 564667 |
| CD44 | 1M7 | BV786 | BD Biosciences | 563736 |
| CD45.2 | 104 | BV650 | BD Biosciences | 740490 |
| CD49b | HMa2 | BV605 | BD Biosciences | 740363 |
| CD8a | 53-6.7 | BB700 | BD Biosciences | 566409 |
| F4/80 | T45-2342 | A647 | BD Biosciences | 565854 |
| FoxP3 | FJK-16s | PE | eBioscience | 12-5773-80 |
| Live/Dead | N/A | 506e | eBioscience | 65-0866-14 |
| Ly6G | 1A8 | PE-Cy7 | BD Biosciences | 560601 |
| NK 1.1 | PK136 | BB630 | BD Biosciences | 624294 |
Statistical analyses
Results are expressed as mean ± standard deviation unless otherwise indicated, with statistical significance of differences performed on normalized values between experimental conditions established at 95%. Two-tailed Student’s t-test was used to indicate statistical difference between pair-wise comparisons. One-way analysis of variance (ANOVA) was used to analyze the statistical difference between groups followed by Tukey’s post-hoc test to compare preselected groups of means. All statistics were performed using GraphPad Prism version 8.4.1 (GraphPad Software, San Diego, CA).
Results
Increased GITR expression is seen in ESCC human patients and in a mouse model of ESCC
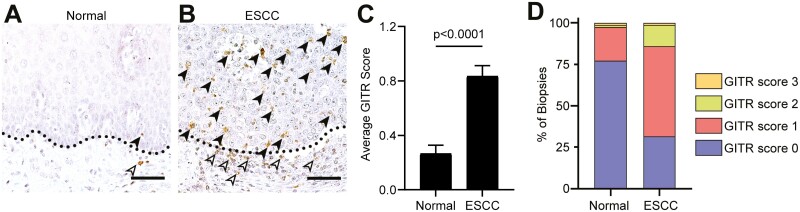
Increased expression of the co-stimulatory immune checkpoint protein GITR has been reported in many solid human cancers (24). We first evaluated GITR expression levels in esophageal tumor biopsies from ESCC patients. As shown in Figure 1, ESCC patients have elevated GITR expression levels on leukocytes (Figure 1B) compared to normal tumor-adjacent areas (Figure 1A). GITR expression was observed on leukocytes located both within the tumor and in the surrounding stroma. No or very low staining was observed on esophageal epithelial tumor cells. GITR staining intensity was scored on a scale from 0 to 3 for all ESCC biopsies and normal adjacent areas. Average scores showed increased GITR expression in ESCC tumors compared to normal adjacent areas (Figure 1C). Furthermore, the distribution of scores among ESCC specimens revealed an increased percentage of patients with a higher intensity for GITR staining in ESCC tumors compared to normal adjacent normal areas, where most patients had no GITR staining (Figure 1D). We observed no correlations with the stage of the disease (data not shown).
Figure 1.
Staining for GITR showed increased GITR expression in ESCC subjects. (A, B) IHC for GITR in esophageal tumors of ESCC subjects (B) and normal adjacent tissue from these subjects (A). Dotted black line indicates barrier between epithelial cells and stroma. Black arrowheads indicate intraepithelial leukocytes, and white arrowheads indicate stromal leukocytes. (C) Bar plot showing scoring for GITR positive leukocytes in esophageal tumors from ESCC subjects or normal adjacent tissue from these subjects. (D) 100% stacked bar graph showing distribution of scores for GITR positive leukocytes. n = 79 matched samples. Bar graphs represent mean ± SEM. Statistics were calculated by Mann–Whitney non-parametric t-test. Scale bars = 50 µm.
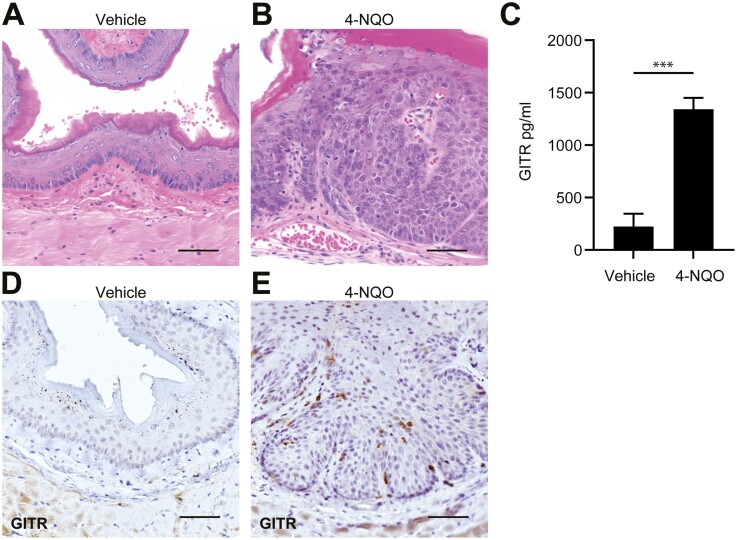
Many aspects of human ESCC can be mimicked in mice by treatment with 4-nitroquinoline 1-oxide (4-NQO), a tobacco surrogate causing DNA and protein adducts (18). Administration of 4-NQO in the drinking water for 4 months, followed by access to normal water for 3 months, leads to a 100% incidence of invasive squamous cell carcinoma in mice (Figure 2B) (18). Mice were treated with 4-NQO (Figure 2B) or propylene glycol (vehicle) (Figure 2A) according to the previously established protocol (18) and GITR protein expression levels were determined by multiplex ELISA. As shown in Figure 2C, we observed increased GITR expression in mice treated with 4-NQO compared to vehicle-treated mice. To confirm this finding, immunohistochemistry for GITR was performed in mice treated with 4-NQO or propylene glycol. Increased GITR staining was seen in leukocytes of 4-NQO-treated mice (Figure 2E) compared to vehicle-treated mice (Figure 2D).
Figure 2.
Mice treated with 4-NQO have increased GITR expression levels. (A, B) Mice were treated with propylene glycol (vehicle) (A) or 4-NQO (B) to induce invasive ESCC. (C) ELISA shows increased GITR expression levels in mice treated with 4-NQO compared to vehicle. n = 3 mice per group. P = 0.0003 calculated by two-tailed Student’s t-test. Bar graph represents mean ± SD. (D, E) IHC for GITR in esophageal sections of mice treated with vehicle (D) or 4-NQO (E). n = 8 mice per group. Scale bars = 50 µm.
GITR agonistic stimulation slows the progression of esophageal tumors in mice
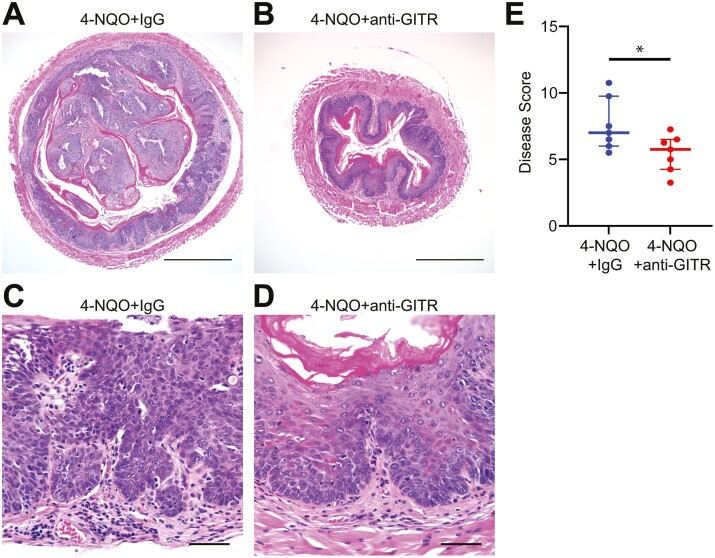
Administration of a GITR agonist antibody results in modulation of GITR-expressing cells and can cause anti-tumor effects in vivo (13). To determine the consequences of GITR agonistic stimulation on esophageal squamous cell carcinogenesis, mice were administered 4-NQO drinking water followed by normal water for a total of 6 months and then treated with the GITR agonistic antibody DTA-1 twice a week for 2.5 weeks. We observed no significant differences in weight between 4-NQO/IgG treated mice and 4-NQO/anti-GITR agonist-treated mice (data not shown). Mice treated with 4-NQO/anti-GITR agonist had less advanced esophageal tumors (Figure 3B and D) compared to mice treated with 4-NQO/IgG (Figure 3A and C). This was reflected by significantly decreased histological scores in 4-NQO/anti-GITR agonist-treated mice compared to 4-NQO/IgG treated mice (Figure 3E).
Figure 3.
GITR agonistic stimulation decreases esophageal squamous cell cancer progression. Mice treated with 4-NQO to induce ESCC received IgG antibody (A, C) or anti-GITR agonist antibody (B, D) for 2.5 weeks and were processed for hematoxylin/eosin staining. (E) Histological scoring of H&E staining for 4-NQO treated mice that received IgG or anti-GITR agonist antibody. n = 14. Scale bars for A, B = 500 µm. Scale bars for C, D = 50 µm. P = 0.0388 calculated by two-tailed Student’s t-test. Graph shows median with interquartile range.
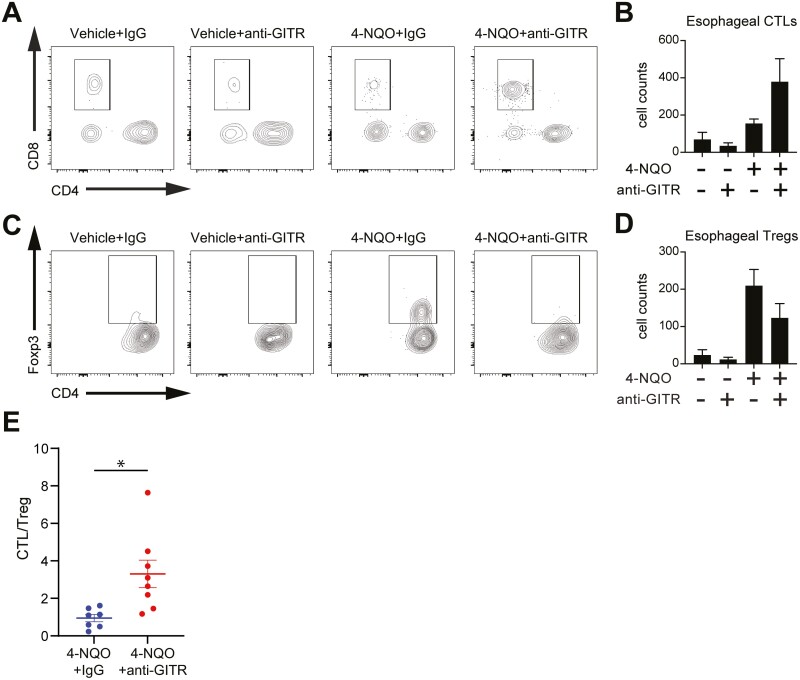
Engagement of GITR with an anti-GITR agonist leads to an increase in the ratio between cytotoxic T cells and regulatory T cells in mouse esophagus during esophageal carcinogenesis
GITR agonistic antibody treatment in various mouse models of cancer has been associated with functional modulation and reduction of intra-tumoral regulatory T cells (Tregs) as well as enhancement of anti-tumor CD8+ T cell functions (14–16). Based on these characteristics of GITR activity, we developed a flow cytometry panel to determine the composition of immune cells in the esophagus of 4-NQO mice treated with IgG or an anti-GITR agonist. We analyzed the live, CD45+ fraction of esophageal cells for T cells (CD3+), regulatory T cells (CD3+ CD4+ Forkhead Box P3 (FoxP3) +), cytotoxic T lymphocytes (CTLs) (CD3+, CD8+), helper T cells (CD3+, CD4+), B cells (CD19+), macrophages (F4/80+), dendritic cells (CD11c+), NK cells (CD49b+ Natural killer (NK) 1.1+), eosinophils (CD11b+ Sialic acid binding immunoglobulin-like lectin (Siglec)-F+), and neutrophils (CD11b+ Lymphocyte antigen 6 complex locus G (Ly6G)hi). As shown in Figure 4, we also observed an enrichment of CTLs (Figure 4A and B) and a decreased presence of Tregs (Figure 4C and D) in the esophagus of 4-NQO/anti-GITR-agonist-treated mice compared to 4-NQO/IgG controls, but these changes in individual cell types did not reach statistical significance. However, studies have shown that the ratio between CTL and Tregs in tumors is more predictive for overall cancer survival than the measurement in changes for a single immune cell type alone (25). Furthermore, favorable outcomes are correlated with higher ratios of CTL/Treg cells in ESCC (26). Thus, we calculated the ratio between CTL and Treg for each mouse and found a significant increase in the CTL/Treg ratio in the esophageal mucosa of 4-NQO/anti-GITR agonist-treated mice compared to 4-NQO/IgG controls (Figure 4E).
Figure 4.
GITR agonistic stimulation shows an increase in CTL to Treg ratio in mice treated with 4-NQO. (A, C) Representative flow plots of CTLs (A) and Tregs (C) found in esophageal tissue from 4-NQO or vehicle control mice treated with IgG or GITR antibody. (B, D) Total cell counts of CTLs (B) and Tregs (D) found in esophageal tissue from 4-NQO or vehicle control mice treated with IgG or anti-GITR agonist antibody. n = 22. (E) CTL/Treg ratio in esophageal tissue. P = 0.0120 calculated by two-tailed Student’s t-test. All graphs represent mean ± SEM. n = 15.
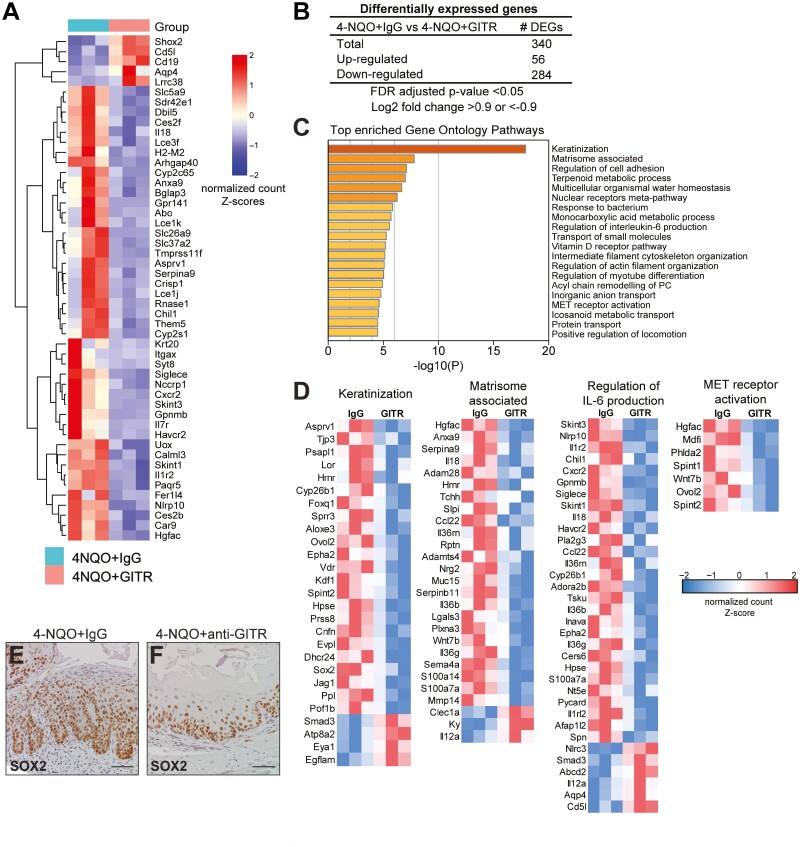
GITR agonistic stimulation leads to changes in gene expression related to esophageal carcinogenesis
To identify potential targets of GITR agonistic stimulation in ESCC, we analyzed changes in gene expression between 4-NQO challenged mice treated with IgG or anti-GITR agonist antibody by performing bulk RNA sequencing. To compare changes between 4-NQO/IgG control mice and 4-NQO/anti-GITR agonist, differential gene expression was determined. The top 50 most significant genes are shown in Figure 5A. Overall, 340 genes were significantly differentially regulated (false-discovery rate–adjusted P value < 0.05, |log2 fold change| ≥ 0.9). 56 of these genes were significantly up-regulated and 284 genes were down-regulated (Figure 5B). To identify the functional roles of these differentially regulated genes, GO pathway enrichment analysis was performed. The top 20 enriched pathways are shown in Figure 5C, and the genes responsible for these pathways, along with the directionality of their expression changes, are shown in Figure 5D. Keratinization was the most enriched pathway, and most genes involved in this pathway were found to decrease following GITR agonism, including genes of the cornified cell envelope (Lor, Hrnr, Cnfn). This suggests a decrease in differentiation in 4-NQO mice treated with GITR agonism compared to IgG controls. Genes associated with the matrisome or the extracellular matrix were decreased in expression, suggesting decreased remodeling of the extracellular matrix following GITR agonism. These included genes involved in cytoskeletal processing and remodeling (Plxna3, Adam28), cell migration (S100a14, Mmp14), and tumor adhesion to the extracellular matrix (Lgals3). Regulation of IL-6 production was also down-regulated, due to decreased expression of genes positively associated with IL-6 production (Nlrp10, Il1rl2, Adora2b) and increased expression of genes negatively associated with IL-6 production (Aqp4, Nlrc3). Furthermore, MET receptor activation was down-regulated, as shown by decreased expression of Hgfac, an activator of HGF/MET signaling, and Wnt7b, a gene target downstream of MET receptor activation. Further supporting the anti-tumor effect of GITR agonism, we observed that Sox2 was among the DEGs significantly down-regulated in 4-NQO/anti-GITR agonist mice compared to 4-NQO/IgG. Sox2 is a transcription factor with established oncogenic function in esophageal squamous cell carcinogenesis (27). Immunostaining for SOX2 confirmed that SOX2 expression was decreased in esophageal epithelial cells of 4-NQO/anti-GITR mice (Figure 5F) compared to 4-NQO/IgG controls (Figure 5E). Altogether, this suggests that GITR agonism leads to downregulation in pathways related to tumor/microenvironmental crosstalk and carcinogenesis.
Figure 5.
Transcriptomic analysis identifies pathways differentially regulated by GITR agonistic stimulation in mice treated with 4-NQO. (A) RNA sequencing was performed from mouse esophageal tissue treated with 4-NQO, with IgG or anti-GITR. Heat map shows the log2 fold change for all DEGs identified from RNA-seq. n = 3 mice per group. (B) DEGs from 4-NQO/anti-GITR mice compared to controls are shown. (C) The enrichment of functional pathways was identified by Gene Ontology using DEGs from 4-NQO/anti-GITR mice compared to controls, and the top 20 pathways are shown. (D) Heatmaps show normalized expression values of genes responsible for the enrichment of selected pathways. n = 3 mice per group. (E, F) Representative immunohistochemistry for SOX2 is shown in mouse esophageal sections from 4-NQO/ anti-GITR mice (F) compared to 4-NQO/IgG mice (E).
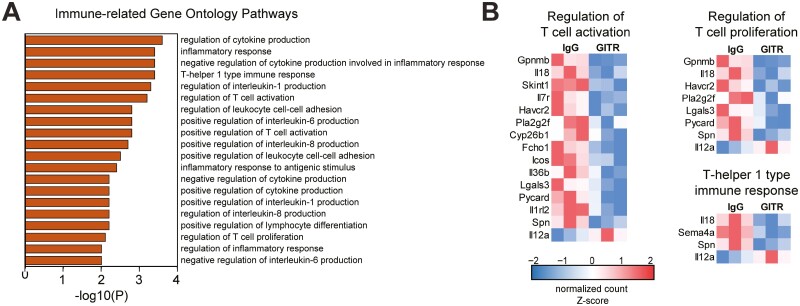
Although most of the top enriched processes indicated that GITR agonism plays a role in esophageal homeostasis and carcinogenesis, studies have shown that it is most recognized to directly impact immune cells (14–16). Importantly, among the GO pathways enriched in 4-NQO mice treated with GITR agonism (Figure 6A), we found the enrichment of pathways involved in immune regulation. As shown in Figure 6B, genes involved in immunosuppressive T cell responses, such as Gpnmb, Il18, and Havcr2 were decreased in expression following GITR agonism, which may be indicative of the activation of anti-tumor immune responses. Overall, the modulation of pathways by RNA sequencing suggests that GITR agonism regulates processes involved in esophageal homeostasis, carcinogenesis, and T cell responses.
Figure 6.
Gene Ontology analysis of transcriptomic data identifies immune-related functionally enriched pathways following GITR agonistic stimulation in 4-NQO mice. (A) Immune-related functionally enriched pathways were identified from GO analysis of 4-NQO/anti-GITR mice compared to controls. (B) Differentially expressed genes involved in T cell activation and proliferation were identified. n = 3 mice per group.
Discussion
Improved clinical response and patient survival have been observed in patients with many types of tumors treated with immunotherapy through the re-activation of an anti-tumor immune response. Effects of immunotherapy on outcomes for ESCC patients are only recently emerging compared to other tumor types, but clinical trials show preliminary promise for these therapies. PD-1 blockade in the post-surgical adjuvant setting has improved disease-free survival following resection of EC tumors (6). Additionally, PD-1 blockade has improved overall survival for patients with advanced or metastatic EC when used as a first-line therapy in combination with chemotherapy (7,8) and when used as a second-line therapy (9,10). These trials have resulted in dramatic changes to the standard of care currently being implemented in the clinic (28). However, still only a fraction of ESCC patients see these benefits of reduced tumor burden and increased survival, and thus additional therapeutic strategies are urgently needed (11). In this study, we observed increased GITR expression in tumor specimens from ESCC patients, adding ESCC to the category of solid tumors with high levels of GITR and suggesting that ESCC might be a good candidate for anti-GITR therapy (29). Based on this finding, we evaluated the anti-tumor effect of the agonistic stimulation of the co-stimulatory immune checkpoint GITR in the progression of ESCC. Although the anti-tumor effects of the GITR agonist antibody DTA-1 have been reported in various pre-clinical models of cancer (14–16), this is the first study to explore the consequences of GITR agonist stimulation on progression of esophageal squamous cell carcinoma.
Using an established mouse model of ESCC, we found that GITR agonistic stimulation led to a significant decrease in histopathological scoring in the esophagus, indicating that progression of esophageal tumors was significantly slowed following GITR agonistic stimulation. In many mouse models of cancer, the therapeutic benefit of anti-GITR agonist DTA-1 treatment has been attributed to GITR’s ability to inhibit Treg function and to promote CD8+ and CD4+ effector T cell function (14–16). Notably, anti-GITR agonism was shown to reduce the presence of Tregs at tumor sites, which was attributed both to decreased ability of Tregs to infiltrate tumors as well as to the loss of Foxp3 expression in resident intra-tumoral Tregs (15). Targeting GITR has also been shown to expand the population of CD8+ T cells and prolong their survival in the tissue (30). Our flow cytometry analyses similarly demonstrated a decrease in Tregs and an increase in CTLs in mouse esophageal fractions following anti-GITR agonism, but these changes did not reach significance. Because the function of Tregs is to interact with and suppress effector T cells, including CTLs, we considered that the balance of these two populations was more critical than their absolute numbers (25). Indeed, following anti-GITR agonism, we observed a significant increase in CTL/Treg ratio, indicating a shift from an immunosuppressive to an immunostimulatory environment following GITR stimulation. Increased CTL/Treg ratios indicate an enhanced capacity to mount an effective immune response and have been suggested to be predictive of anti-tumor activity in tumor models (25,26).
Further supporting the anti-tumor effect of GITR agonistic stimulation in our mouse model of ESCC, RNA-seq analyses revealed changes in known drivers of esophageal squamous cell carcinogenesis following anti-GITR agonism. Surprisingly, the top enriched pathways were related to tumor-stromal interactions and epithelial biology, such as keratinization and matrisomal protein expression, rather than the regulation of immune cells, which were the cell types most expected to be affected following GITR modulation. This likely reflects the fact that esophageal samples used for bulk RNA sequencing mostly consisted of epithelial or tumor cells, with immune cells only constituting a small subset of the entire bulk sample. Potentially these changes in tumor-stromal interactions resulted due to modulation of GITR on immune cells, which resulted in subsequent anti-tumor immune activity. Although it is unlikely given the very low or lack of GITR expression on tumor cells (Figure 1), we cannot completely rule out that these pathway changes may reflect a direct role of GITR agonism on esophageal tumor cells, as GITR expression on skin keratinocytes has previously been demonstrated to protect against UVB-induced apoptosis (31).
The observation that most genes associated to keratinization were decreased following GITR agonism of 4-NQO mice is unexpected, considering that loss of epithelial character is associated with cancer pathogenesis, and thus evidence of keratinization would be expected to increase along with anti-tumor effects. However, it is important to consider the biology of ESCC tumors and those generated by 4-NQO treatment. Keratinization has been observed as a feature of well-differentiated squamous cell carcinomas, including ESCCs. These lesions are typically found during early stages of carcinogenesis and can include structures such as keratin pearls (32). Furthermore, the 4-NQO model has been shown to drive the formation of well-differentiated squamous cell carcinomas, including formation of keratin pearls (33). Thus, the decreased enrichment of genes related to keratinization following GITR agonism suggests a reduction in the presence of these well-differentiated squamous cell carcinoma lesions, which correlates with the lower histological scoring we observed in 4-NQO/anti-GITR agonist-treated mice.
Extracellular matrix remodeling is a critical process during cancer progression, and expression of extracellular matrix proteins is altered in ESCC samples (34). We found decreased expression of genes related to the extracellular matrix (matrisome) following GITR agonism, which may correlate with anti-tumor activity. For example, one of the most significantly down-regulated genes we identified, Anxa9, has been shown to play a role in proliferation and migration of gastric cancer cells (35), and is a feature of well-differentiated squamous cell carcinomas (36). Additional studies would be needed to determine the contributions of such changed genes to anti-tumor effects following GITR agonism in ESCC.
Our transcriptomics data also show the enrichment of genes associated with Met signaling. Met is a receptor tyrosine kinase expressed on epithelial cells that acts as a receptor for hepatocyte growth factor (HGF). HGF/Met signaling can enhance growth, invasion, and metastasis of cancer cells in many cancer types (37). Interestingly, HGF/Met signaling plays an important role in promoting esophageal tumor progression (37,38). Levels of HGF and Met correlate with increased tumor stage and worse survival in human ESCC (37,39), and HGF can promote invasion of esophageal epithelial cells (38). We saw decreased mRNA expression levels of the HGF activator Hgfac in 4-NQO mice treated with anti-GITR agonism, indicating that paracrine signaling in the tumor microenvironment may be impacted by GITR modulation. Down-regulated expression of genes related to interleukin (IL)-6 signaling, another crucial contributor to ESCC pathogenesis (40), were observed following anti-GITR treatment as well. IL-6 is a proinflammatory cytokine that commonly activates the signal transducers and activators of transcription (STAT) 3 pathway; interestingly, IL-6/STAT3 has been shown to collaborate with SOX2 during esophageal carcinogenesis (27). Thus, many different pathways involved in esophageal carcinogenesis and tumor progression may be impacted following agonistic stimulation of GITR.
Gene expression changes in known drivers of ESCC development were identified following GITR agonism. One such driver, SOX2, is a transcription factor that regulates homeostatic stem cell self-renewal and drives cancer progression by promoting survival, invasion, migration, and proliferation of cancer cells in a variety of tumors (41). Gain-of-function mutations in SOX2 have been observed in human ESCC precursor lesions and tumors (42), and SOX2 is also frequently amplified in human ESCC (43). In inflamed murine esophagus, SOX2 has further been found to play an oncogenic role (27,44). We observed a decrease in Sox2 mRNA expression in 4-NQO/anti-GITR mice compared to 4-NQO/IgG controls, indicating that GITR agonism may regulate drivers of ESCC pathology. In this study, we have not established whether SOX2 is a direct target following anti-GITR agonism. However, we believe that the decrease in SOX2 at both the mRNA and protein level reflects an overall shift in the balance of esophageal stem cell proliferation and differentiation following anti-GITR agonism, but further studies would be needed to establish a mechanistic role for SOX2 downstream of GITR agonism.
The mechanism through which GITR agonism exerts effects on tumor cells is widely considered to be through modulation of immune cell responses. In addition to processes related to tumor biology, our transcriptomic dataset also shows enrichment of processes involved in immune cell signaling and inflammation following GITR agonism, such as regulation of cytokine production, inflammatory response, and regulation of T cell activation. Because GITR is most understood for its role on T cells, we looked deeper at the changes in genes related to T cell activation and proliferation. Of these, Gpnmb, a transmembrane glycoprotein that can promote tumor cell migration and invasion (45), is the most significantly changed gene following GITR agonism in 4-NQO mice. Overexpression of Gpnmb has been previously found in squamous cell carcinomas and was predictive of poor prognosis (46). Downregulation of Gpnmb has also been shown to inhibit tumor growth and suppress CD8+ T cell exhaustion in a model of hepatocellular carcinoma (47). Furthermore, we found that genes including Havcr2 and Il18 are also decreased following GITR agonism. Havcr2 encodes for a T cell checkpoint inhibitor which has been shown to restrict anti-tumor immunity in a CD8+ cell-dependent manner (48). IL-18 has also been shown to promote immunosuppression and diminished anti-tumor T cell responses (49). Thus, decreased expression of these genes following GITR agonism may indicate an increase in CTL activation, suggesting a mechanism for subsequent anti-tumor effects in murine ESCC. It is important to note that although we did identify enrichment of immune and inflammatory pathways from our RNA sequencing data, the top enriched pathways were related to tumor cell biology and tumor-stromal interactions, reflective of the high proportion of tumor cells found within our samples over immune cells. Additional studies to identify specific changes in T cell subsets following GITR agonism in ESCC are therefore an important next step, which can be addressed using techniques to isolate T cells from esophageal tissue or more sophisticated next-generation sequencing strategies such as single cell RNA sequencing.
Despite the encouraging pre-clinical results we have found using GITR agonistic stimulation to treat ESCC, there is uncertainty about the utility of GITR agonism as a monotherapy in the clinic. A recent phase 1 clinical trial of GITR agonism to treat advanced cancer found that despite reduction in intra-tumoral Tregs and increased CTL/Treg ratios, anti-tumor responses were not sustained or long-lasting (50). This was thought to occur due to persistent exhaustion and insufficient activation of CTLs (50). Pre-clinical studies indicate that this limitation may be overcome by combining GITR agonistic stimulation with PD-1 blockade, where GITR may enhance intra-tumoral CTL numbers and PD-1 can promote CTL re-activation (50). The synergy between anti-PD-1 and anti-GITR was reported to reject different types of established murine tumors (50,51). Interestingly, in recent years, the safety and clinical benefit of PD-1/PD-L1 blockade either alone or combined with chemotherapy or chemoradiotherapy has been shown in ESCC patients (28). However, despite very encouraging results, tumor regression only occurs in a small fraction of patients (11). Therefore, expanding immunotherapy-based treatment beyond PD-1/PD-L1 is a clear unmet clinical need in ESCC. Thus, a combination therapy approach using anti-GITR and anti-PD-1 could be extremely beneficial to improve clinical response and overall survival rates in ESCC patients.
In sum, we show that GITR agonistic stimulation leads to an anti-tumor response in ESCC, with an increase in CTL/Treg ratio. Thus, GITR agonist could serve as an effective treatment strategy for ESCC patients.
Supplementary material
Supplementary data are available at Carcinogenesis online.
Supplementary Figures 1 and 2 can be found at https://doi.org/10.1093/carcin/bgac064.
Acknowledgements
Conflict of Interest Statement: K.N.W., L.E.T., C.M.A., P.A.H., M-P.T.: None declared. V.M.V.: Stock ownership at Johnson & Johnson, received Takeda Research Funding and is advisory at Astra Zeneca.
Contributor Information
Kelsey Nicole Wiles, Department of Medicine, Gastroenterology and Hepatology Division, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Lia Elyse Tsikretsis, Department of Medicine, Gastroenterology and Hepatology Division, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Cara Maria Alioto, Department of Medicine, Gastroenterology and Hepatology Division, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Pedro Antonio Hermida de Viveiros, Department of Medicine, Hematology and Oncology Division, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Victoria Meucci Villaflor, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Marie-Pier Tétreault, Department of Medicine, Gastroenterology and Hepatology Division, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Funding
Robert H. Lurie Cancer Center Translational Bridge Program Award (to V.M.V. and M-P.T.), Horyn Foundation (to V.M.V), Northwestern Feinberg School of Medicine Start-up funds (to M-P.T.), National Institute of Health (NCI CCSG P30 CA060553 to the Northwestern University Pathology Core, Flow cytometry Facility and NU-Seq Core Facility).
References
- 1. Rustgi, A.K., et al. (2014) Esophageal carcinoma. N. Engl. J. Med., 371, 2499–2509. [DOI] [PubMed] [Google Scholar]
- 2. Enzinger, P.C., et al. (2003) Esophageal cancer. N. Engl. J. Med., 349, 2241–2252. [DOI] [PubMed] [Google Scholar]
- 3. Liu, X., et al. (2017) Genetic alterations as esophageal tissues from squamous dysplasia to carcinoma. Gastroenterology, 153, 166–177. [DOI] [PubMed] [Google Scholar]
- 4. Reichenbach, Z.W., et al. (2019) Clinical and translational advances in esophageal squamous cell carcinoma. Adv. Cancer Res., 144, 95–135. [DOI] [PubMed] [Google Scholar]
- 5. Zappasodi, R., et al. (2018) Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell, 33, 581–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly, R.J., et al. (2021) Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med., 384, 1191–1203. [DOI] [PubMed] [Google Scholar]
- 7. Janjigian, Y.Y., et al. (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet, 398, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun, J.M., et al. (2021) Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet, 398, 759–771. [DOI] [PubMed] [Google Scholar]
- 9. Kato, K., et al. (2019) Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol., 20, 1506–1517. [DOI] [PubMed] [Google Scholar]
- 10. Kojima, T., et al. (2020) Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol., 38, 4138–4148. [DOI] [PubMed] [Google Scholar]
- 11. Leone, A.G., et al. (2022) Efficacy and activity of PD-1 blockade in patients with advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis with focus on the value of PD-L1 combined positive score. ESMO Open, 7, 100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nocentini, G., et al. (1997) A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. U.S.A., 94, 6216–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimizu, J., et al. (2002) Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol., 3, 135–142. [DOI] [PubMed] [Google Scholar]
- 14. Ko, K., et al. (2005) Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med., 202, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen, A.D., et al. (2010) Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One, 5, e10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coe, D., et al. (2010) Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol. Immunother., 59, 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng, Y., et al. (2020) Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat. Commun., 11, 6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang, X.H., et al. (2004) Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin. Cancer Res., 10, 301–313. [DOI] [PubMed] [Google Scholar]
- 19. Yang, Y., et al. (2007) Kruppel-like factor 5 activates MEK/ERK signaling via EGFR in primary squamous epithelial cells. FASEB J., 21, 543–550. [DOI] [PubMed] [Google Scholar]
- 20. Dobin, A., et al. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anders, S., et al. (2015) HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics, 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Love, M.I., et al. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol., 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou, Y., et al. (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun., 10, 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ronchetti, S., et al. (2015) Glucocorticoid-induced tumour necrosis factor receptor-related protein: a key marker of functional regulatory T cells. J Immunol Res, 2015, 171520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katz, S.C., et al. (2013) Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann. Surg. Oncol., 20, 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hatogai, K., et al. (2016) Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget, 7, 47252–47264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu, K., et al. (2013) Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell, 12, 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thuss-Patience, P., et al. (2022) Immunotherapy in squamous cell cancer of the esophagus. Curr. Oncol., 29, 2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vence, L., et al. (2019) Characterization and comparison of GITR expression in solid tumors. Clin. Cancer Res., 25, 6501–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snell, L.M., et al. (2010) CD8 T cell-intrinsic GITR is required for T cell clonal expansion and mouse survival following severe influenza infection. J. Immunol., 185, 7223–7234. [DOI] [PubMed] [Google Scholar]
- 31. Wang, J., et al. (2005) Glucocorticoid-induced tumor necrosis factor receptor is a p21Cip1/WAF1 transcriptional target conferring resistance of keratinocytes to UV light-induced apoptosis. J. Biol. Chem., 280, 37725–37731. [DOI] [PubMed] [Google Scholar]
- 32. Baba, E.R., et al. (2021) Keratin pearls in magnifying endoscopy of superficial esophageal squamous cell carcinoma. Gastrointest. Endosc., 94, 424–425. [DOI] [PubMed] [Google Scholar]
- 33. Hawkins, B.L., et al. (1994) 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck, 16, 424–432. [DOI] [PubMed] [Google Scholar]
- 34. Senthebane, D.A., et al. (2018) The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int. J. Mol. Sci. 19, 2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou, Y., et al. (2021) High expression of Annexin A9 promotes cell proliferation and migration in gastric cancer via the TGF-beta signaling pathway. J. Environ. Pathol. Toxicol. Oncol., 40, 87–94. [DOI] [PubMed] [Google Scholar]
- 36. Salom, C., et al. (2019) Frequent alteration of Annexin A9 and A10 in HPV-negative head and neck squamous cell carcinomas: correlation with the histopathological differentiation grade. J Clin Med, 8, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ozawa, Y., et al. (2015) c-Met in esophageal squamous cell carcinoma: an independent prognostic factor and potential therapeutic target. BMC Cancer, 15, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grugan, K.D., et al. (2010) Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc. Natl. Acad. Sci. U.S.A., 107, 11026–11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ren, Y., et al. (2005) Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin. Cancer Res., 11, 6190–6197. [DOI] [PubMed] [Google Scholar]
- 40. Karakasheva, T.A., et al. (2018) IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res., 78, 4957–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Novak, D., et al. (2020) SOX2 in development and cancer biology. Semin. Cancer Biol., 67, 74–82. [DOI] [PubMed] [Google Scholar]
- 42. Liu, X., et al. (2017) Genetic alterations in esophageal tissues from squamous dysplasia to carcinoma. Gastroenterology, 153, 166–177. [DOI] [PubMed] [Google Scholar]
- 43. Cancer Genome Atlas Research, N, et al. (2017) Integrated genomic characterization of oesophageal carcinoma. Nature, 541, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu, Z., et al. (2021) Reprogramming of the esophageal squamous carcinoma epigenome by SOX2 promotes ADAR1 dependence. Nat. Genet., 53, 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou, L.T., et al. (2012) Gpnmb/osteoactivin, an attractive target in cancer immunotherapy. Neoplasma, 59, 1–5. [DOI] [PubMed] [Google Scholar]
- 46. Li, H., et al. (2019) High expression of GPNMB predicts poor prognosis in head and neck squamous cell carcinoma. Histol. Histopathol., 34, 803–810. [DOI] [PubMed] [Google Scholar]
- 47. Sakano, Y., et al. (2022) Tumor endothelial cell-induced CD8(+) T-cell exhaustion via GPNMB in hepatocellular carcinoma. Cancer Sci., 113, 1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dixon, K.O., et al. (2021) TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature, 595, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakamura, K., et al. (2018) Dysregulated IL-18 is a key driver of immunosuppression and a possible therapeutic target in the multiple myeloma microenvironment. Cancer Cell, 33, 648.e5 e5. [DOI] [PubMed] [Google Scholar]
- 50. Zappasodi, R., et al. (2019) Rational design of anti-GITR-based combination immunotherapy. Nat. Med., 25, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lu, L., et al. (2014) Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J. Transl. Med., 12, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.