Abstract
Chemoresistance is a huge clinical challenge in the treatment of advanced colorectal cancer (CRC). Non-coding RNAs (ncRNAs) and messenger RNA (mRNA) are involved in CRC chemoresistance. However, the profiles of long ncRNAs (lncRNAs), microRNAs (miRNAs), mRNAs and competing endogenous RNA (ceRNA) networks in CRC chemoresistance are still largely unknown. Here, we compared the gene expression profiles in chemosensitive (HCT8) and chemoresistant [HCT8/5-fluorouracil (5-Fu) and HCT8/cisplatin (DDP)] cell lines by whole-transcriptome sequencing. The common differentially expressed RNAs in two drug-resistant cells were selected to construct lncRNA–miRNA–mRNA networks. The ceRNA network closely related to chemoresistance was further established based on the widely accepted drug resistance-associated genes enriched in three signaling pathways involved in chemoresistance. In total 52 lncRNA–miRNA–mRNA pathways were screened out, among which EPHA2 and LINC02418 were identified as hub genes; thus, LINC02418/miR-372-3p/EPHA2 were further selected and proved to affect the 5-Fu and DDP resistance of CRC. Mechanistically, LINC02418 upregulated EPHA2 by functioning as a ‘sponge’ of miR-372-3p to modulate the chemoresistance of CRC. Collectively, our study uncovered the underlying mechanism of LINC02418/miR-372-3p/EPHA2 in 5-Fu and DDP resistance of CRC, which may provide potential therapeutic targets for improving the chemosensitivity of CRC.
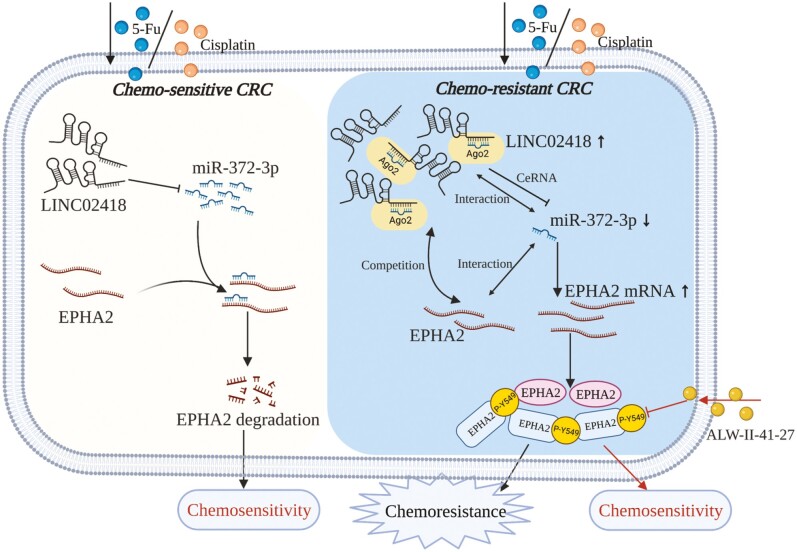
Chemoresistance is the main causes for the failure of chemotherapy in colorectal cancer (CRC). LINC02418 was markedly upregulated in chemoresistant cell lines, and regulated EPHA2 expression through functioning as a ‘sponge’ of miR-372-3p to modulate chemoresistance of CRC.
Graphical Abstract
Introduction
Colorectal cancer (CRC), a common gastrointestinal malignant tumor that occurs in the colon, ranks third in terms of incidence and is the second leading cause of cancer-related mortality around the world (1). Chemotherapy is an optimal method for treating advanced or inoperable CRC patients, and the basic and classic drugs commonly used to treatment of CRC include 5-fluorouracil (5-Fu) and cisplatin (DDP). However, some patients are primarily resistant to 5-Fu- or DDP-based chemotherapy, while some will acquire resistance after a period of treatment, resulting in a limited therapeutic effect and low 5-year survival rate (2–4). Thus, it is urgent to reveal the molecular mechanisms underlying the chemoresistance in CRC to identify more effective therapeutic targets and improve the efficacy of chemotherapy.
Long non-coding RNA (lncRNA) is a type of RNA molecule that measures >200 nucleotides in length with a low protein-coding potential (5,6). With the development of high-throughput sequencing and bioinformatics technologies, a variety of lncRNAs have been explored and revealed (7–9). Recently, accumulating evidence has shown that lncRNAs are usually differentially expressed (DE) in various cancers and function as oncogenes or tumor suppressor genes to affect biological processes of cancers, such as cell proliferation, migration, metastasis and apoptosis (10–12). Moreover, lncRNAs have been proved to play an important role in tumor chemoresistance (13–15). For example, Chen et al. demonstrated that Forkhead box D1 could bind with the promoter of lncRNA CYTOR and then activate its transcription to induce the epithelial–mesenchymal transition and chemoresistance of oral squamous cell cancer (16). Yang et al. reported that the lncRNA SLC7A11-AS1 was overexpressed in gemcitabine-resistant pancreatic ductal adenocarcinoma cell lines, which can block the ubiquitination of NRF2, a key regulator of SCFβ-TRCP-mediated antioxidant defense, to eliminate reactive oxygen species and promote cancer cell stemness and chemoresistance (17). In addition, mesenchymal stem cells were found to be associated with the development of drug resistance through fatty acid oxidation. Mechanically, mesenchymal stem cells could secrete TGF-β1 to upregulate the lncRNA MACC1-AS1, resulting in fatty acid oxidation-dependent stemness and chemoresistance (18). Recently, mounting evidence has demonstrated that lncRNAs can serve as sponges of microRNAs (miRNAs) via competitive endogenous RNA (ceRNAs) to regulate the expression of target genes, then modulate the chemoresistance of cancers (19–21). Although a minor fraction of lncRNAs have been reported to be involved in the chemoresistance of CRC, the expressions and roles of most lncRNAs in CRC harboring both 5-Fu and DDP chemoresistance remain unclear, and lncRNA-associated ceRNA networks related to CRC chemoresistance have not been constructed, thus requiring further investigation. Therefore, identifying the key regulator of lncRNA and its ceRNA network related to chemoresistance is particularly important for improving the prognosis of CRC.
In the current study, we identified DE lncRNAs, miRNAs and messenger RNAs (mRNAs) in CRC chemosensitive cells (HCT8) and two different chemoresistant cell lines (HCT8/5-Fu and HCT8/DDP) via whole-transcriptome sequencing. Then, lncRNA–miRNA–mRNA networks were predicted and constructed based on the common DE RNAs in two drug-resistant cells, and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed for mRNAs regulated by ceRNA networks. We screened and then found that LINC02418/miR-372-3p/EPHA2 may act as a key regulator network of 5-Fu and DDP resistance of CRC. It has been reported that LINC02418 participates in CRC tumorigenesis (22) and EPHA2 contributes to the chemoresistance of cancers (23,24). However, the effects of LINC02418/miR-372-3p/EPHA2 on 5-Fu and DDP resistance in CRC are unknown. In the study, LINC02418 and EPHA2 were significantly upregulated, while miR-372-3p was downregulated in chemoresistant cell lines, and all of these trends were related to CRC chemoresistance. Mechanically, LINC02418 functions as a sponge of miR-372-3p to upregulate EPHA2 expression. Therefore, this study reveals that LINC02418 exerts oncogenic potential and may be a new therapeutic target and a promising marker to predict chemoresistance in CRC.
Materials and methods
Cell culture
A normal colon epithelial cell line (CCD-18Co) was purchased from the American Type Culture Collection (Manassas, VA), HCT8, HCT116 and SW480 CRC cell lines and 293T cells were purchased from the China Center for Type Culture Collection (Wuhan, China) with short tandem repeat DNA profiling analysis, and cultured for fewer than 6 months after resuscitation. Chemoresistant CRC cell lines (HCT8/5-Fu and HCT8/DDP) were derived from the parental cell line HCT8 by continuous exposure to drugs (25). CCD-18Co and 293T cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, MD) containing 10% fetal bovine serum (Gibco, MD), HCT8, HCT116, SW480, HCT8/5-Fu and HCT8/DDP cells were maintained in RPMI-1640 (Gibco, MD) supplemented with 10% fetal bovine serum (Gibco, MD) and 1% penicillin/streptomycin (Gibco, MD). Additionally, HCT8/5-Fu and HCT8/DDP cells were cultured with 5 µg/ml 5-Fu (Sigma–Aldrich, MO) and 1 µg/ml DDP (Sigma–Aldrich, MO to maintain drug resistance, respectively.
RNA extraction and quality control
Total RNAs were extracted using TRIzol reagent (Life Technologies, CA) according to the manufacturer’s protocol. The quantity of total RNA samples was assessed according to the description in our previous study (25).
Library preparation and RNA-sequencing
Total RNA (5 µg) of the per sample was used as input material for the preparation of strand-specific complementary DNA (cDNA). Strand-specific cDNA libraries were constructed as follows: ribosomal RNA depletion was first performed using the Ribo-Zero Magnetic kit (Epicentre, CA) and then was fragmented into 300 bp fragments with fragmentation buffer. Secondly, random hexamer primers were used to synthesize first-strand cDNA, and dUTPs were used to instead dTTPs when synthesizing second-strand cDNA. Double-stranded cDNA (ds cDNA) were separated from the second-strand reaction mix using AMPure XP beads, and a single ‘A’ nucleotide was added to the 3ʹ ends of blunt fragments. Lastly, the ends of the ds cDNA were ligated with multiple indexing adapters. Libraries were selected for cDNA target fragments of 300–400 bp, then were enriched by using Phusion DNA polymerase (NEB) for 15 cycles of PCR. After being quantified with a TBS-380 Fluorometer (Turner Biosystem, CA), the libraries were sequenced on Hiseq 2000 platform (2 × 150 bp paired-end reads). For small RNA, the sequencing libraries were constructed using the Truseq™ Small RNA sample prep kit (Illumina, CA) following the manufacturer’s introduction. Briefly, small RNA was ligated with sequencing adapters and cDNA was synthesized and amplified with 12 PCR cycles to produce libraries, and the products were then purified with 6% Novex TBE PAGE gel (Thermo Fisher Scientific, MA). The quality of the library was assessed with the 2100 bioanalyzer (Agilent Technologies, CA) and single-end sequencing was performed on the Hiseq 2000 platform. Each group had three replicates, and the whole-transcriptome sequencing was performed by Shanghai Majorbio Bio-Pharm Biotechnology Co., Ltd. (Shanghai, China).
Differential expression analysis
The sequencing raw data were filtered some reads with adapters and low quality to obtain clean reads. The HISAT2 software (http://ccb.jhu.edu/software/hisat2/index.shtml) was used to compare the filtered reads to the reference genome. The expression levels of mRNAs and lncRNAs were normalized using the fragments per kilobase of exon per million mapped reads (FRKM) method, and the expression levels of miRNAs were determined by transcript per million. In addition, the DESeq software was used to identify DE lncRNAs, miRNAs and mRNAs between CRC chemosensitive and chemoresistant cell lines with |log2FoldChange| >1 and P < 0.05. Moreover, the common DE lncRNAs, miRNAs and mRNAs were selected between the two drug resistance groups to find the lncRNAs, miRNAs and mRNAs simultaneously associated with 5-Fu and DDP resistance in CRC.
Construction of lncRNA–miRNA–mRNA network
A lncRNA–miRNA–mRNA regulatory network was constructed based on common DE lncRNA, miRNA and mRNA according to the ceRNA hypothesis. First, the miRNA–mRNA and miRNA–lncRNA pairs were predicted using miRanda and TargetScan software, mRNA and lncRNA in the ceRNA network generally had a positive correlation, while the expression levels of miRNAs were negatively correlated with those of mRNAs and lncRNAs. The pairs with Pearson’s correlation coefficients >0.7 were selected for further analysis. Second, two scoring methods were used to evaluate the regulatory function of miRNA on a competitive lncRNA–mRNA pairs, as follows: (i) regulate the similarity score, comparing the similarity of the expression correlation between miRNA–lncRNA and miRNA–mRNA pairs; and (ii) sensitivity correlation score, where the average value of lncRNA–miRNA–mRNA correlation was used as the sensitivity correlation between lncRNAs and mRNAs. Finally, lncRNA–miRNA–mRNA ceRNA networks (sensitivity correlation score >0.3) were visualized using the Cytoscape version 3.6 software (Supplementary Figure 1, available at Carcinogenesis Online).
Functional enrichment analysis
GO analysis was used to investigate the possible functions of the mRNAs in the lncRNA–miRNA–mRNA ceRNA network. The results of GO enrichment analysis were classified into three categories, which were biological process, molecular function and cell component, and the top 10 significantly enriched GO terms in each category were displayed. KEGG pathway analysis was used to determine the biological pathway of the mRNAs in the ceRNA network, and P < 0.05 was considered to be statistically significant.
Quantitative real-time PCR
First, TRIzol reagent (Thermo Fisher Scientific, MA) was used to extract total RNAs from HCT8, HCT8/5-Fu and HCT8/DDP cell lines. Then, the ReverTra Ace qPCR RT kit (TOYOBO, Osaka, Japan) was used to synthesize cDNA following the manufacturer’s instructions. Subsequently, quantitative PCR was performed using iTaq™ Universal SYBR Green Supermix (Bio-Rad, CA). The primer sequences of DE lncRNA, miRNA and mRNA in the study were synthesized by Sangon Biotech (Shanghai, China), and are shown in Supplementary Table 1, available at Carcinogenesis Online. GAPDH (for lncRNA and mRNA) and U6 (for miRNA) were regarded as the internal controls, and the relative expression levels of lncRNAs, miRNAs and mRNAs were calculated with the 2−ΔΔCt method.
Western blotting
Proteins from cells were lysed using RIPA lysis buffer supplemented with phosphatase inhibitor (Beyotime Biotechnology, Shanghai, China), and its concentrations were quantified with a bicinchoninic acid protein assay kit (Biosharp, Shanghai, China). Protein samples (20 µg) were separated on 8.75% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, then transferred to a polyvinylidene difluoride membrane (Bio-Rad, CA) and blocked using 5% skim milk at room temperature for 1 h, then incubated overnight with primary antibodies for EPHA2 (1:1000, Cell Signaling Technology, 6997T), phosphor-EPHA2-Y549 (1:1000, ABclonal, AP0818) and β-actin (1:10 000, ABclonal, AC026) at 4°C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. Finally, the expression of EPHA2 protein was visualized by enhanced chemiluminescence reagents (Bio-Rad, CA) and its relative level was determined by densitometric analysis using the ImageJ software.
Cell transfection
Chemoresistant CRC cells were transiently transfected with (i) small interfering RNAs (siRNAs) for LINC02418 (si-LINC02418) and the corresponding negative control (si-NC) (GenePharma, Shanghai, China) or (ii) miR-372-3p mimics/inhibitors and corresponding negative control (NC mimics/inhibitors) (GenePharma, Shanghai, China). Cell transfection was performed using Lipofectamine 2000 (Invitrogen, CA) following the manufacturer’s protocol. In addition, cells were treated with serially diluted EPHA2 inhibitor (ALW-II-41-27) and its negative control dimethyl sulfoxide to explore the biological functions of EPHA2. The related siRNAs and miRNA mimic/inhibitor sequences are listed in Supplementary Table 2, available at Carcinogenesis Online.
MTT assay
To evaluate the effects of LINC02418, miR-372-3p and EPHA2 on chemosensitivity, the transfected HCT8/5-Fu and HCT8/DDP cells for 48 h were trypsinized and reseeded in 96-well plates (5000 cells/well), then combined with different concentrations of 5-Fu or DDP for 48 h to assess cell viability. Additionally, 5-Fu or DDP with the same concentration was supplemented into wells and cultured for 12, 24 or 36 h to assess the cell proliferation ability. The OD490 value was detected after adding the MTT reagent (Sigma–Aldrich, MO) for 4 h at 37°C.
Flow cytometry assay
Flow cytometry was used to measure cell apoptosis and cell cycle. For the apoptosis assay, the transfected HCT8/5-Fu and HCT8/DDP cells were harvested and washed with pre-cold phosphate-buffered saline, and about 1–5 × 105 cells were resuspended in 500 µl of binding buffer with 5 µl of Annexin V-FITC and 10 µl of PI staining solution and then incubated for 15 min at room temperature. For cell cycle analysis, the transfected cells were collected and fixed with pre-cold 70% ethanol at 4°C overnight. Then the cells were washed with pre-cold phosphate-buffered saline, PI staining solution and RNase solution were added to each sample, and the cells were resuspended and incubated for 30 min at 37°C water bath and 4°C for 30 min, protected from light. The cell apoptosis rate and cycle distribution were detected by the Accuri C6 flow cytometer (BD Biosciences, NJ) flow cytometer, and the data were analyzed with the FlowJo software (Tree Star, Ashland, OR).
Luciferase reporter assay
The sequence of wild-type (WT) LINC02418 and EPHA2 and the sequence of mutant-type (MUT) LINC02418 and EPHA2 were inserted into the pmirGLO reporter vector (GenePharma, Shanghai, China). Then, the WT (MUT) 3ʹ-UTR of LINC02418 vector or WT (MUT) EPHA2 vector and control mimics or miR-372-3p mimics were cotransfected into 293T cells with Lipofectamine 2000 (Invitrogen, CA). Luciferase activity was measured with the Dual-Luciferase Reporter Assay System (Promega, WI) according to the manufacturer’s protocol, and Renilla luciferase was regarded as the control reporter for normalization.
Statistical analysis
SPSS 25.0 statistical software (SPSS, Chicago) and GraphPad Prism 5 Software (GraphPad, SanDiego, CA) were used for data analysis and graph representations, respectively. The experimental data were expressed as mean ± standard deviation (mean ± SD). The independent-sample t-test was used for the comparison of means between the two groups, P < 0.05 was considered statistically significant. The DE lncRNA, miRNA and mRNA were considered to be statistically significant with |log2FoldChange| >1 and P < 0.05.
Results
Expression profiles of lncRNAs, miRNAs and mRNAs
In our previous study, we confirmed that CRC chemoresistant cell lines (HCT8/5-Fu, HCT8/DDP) were more resistant to drugs than parental cells HCT8 (25), which laid the foundation for whole-transcriptome sequencing to obtain the profiles of lncRNAs, miRNAs and mRNAs in the parental cell lines and two drug-resistant cell lines. Compared with parental cells, DE lncRNAs, miRNAs and mRNAs were screened out in drug-resistant cells with the criteria of |log2FoldChange| >1 and P < 0.05.
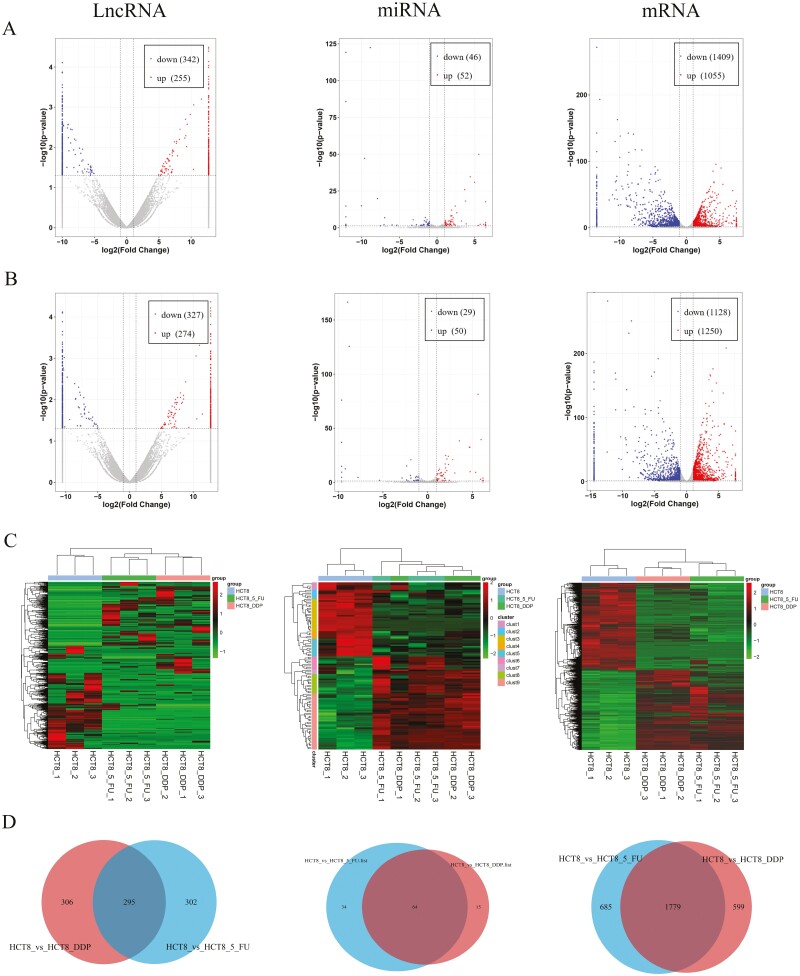
A volcano plot and heatmap were used to display the expressions of mRNAs and ncRNAs. The results showed that there were 597 lncRNAs (255 upregulation and 342 downregulation), 98 miRNAs (52 upregulation and 46 downregulation) and 2464 mRNAs (1055 upregulation and 1409 downregulation) DE in HCT8/5-Fu cells; as well as 601 lncRNAs (274 upregulation and 327 downregulation), 79 miRNAs (50 upregulation and 29 downregulation) and 2378 mRNAs (1250 upregulation and 1128 downregulation) DE in HCT8/DDP cells compared with the chemosensitive group, respectively (Figure 1A and B). In addition, distinguishable lncRNA, miRNA and mRNA expression profiles among samples were revealed by hierarchical clustering analysis (Figure 1C). In order to find the same regulating RNAs involved in both 5-Fu and DDP resistance, Venn diagram analysis was used to screen out common DE lncRNAs, miRNAs and mRNAs in the two chemoresistant cell lines. This analysis showed that 295 lncRNAs, 64 miRNAs and 1779 mRNAs were common DE in two comparisons (Figure 1D and Supplementary Table 3, available at Carcinogenesis Online).
Figure 1.
Expression profiles of lncRNAs, miRNAs and mRNAs. The volcano plot of DE lncRNAs, miRNAs and mRNAs in (A) HCT8/5-Fu cell lines and (B) HCT8/DDP cell lines. (C) Cluster analysis of DE lncRNAs, miRNAs and mRNAs. (D) Venn diagram displayed DE and overlapping lncRNAs, miRNAs and mRNAs between HCT8/5-Fu and HCT8/DDP cell lines.
GO and KEGG pathway analysis in the ceRNA network
To further explore the same mechanisms in different chemoresistance of CRC, we selected the common DE lncRNAs, miRNAs and mRNAs in HCT8/5-Fu and HCT8/DDP cells to construct lncRNA–miRNA–mRNA ceRNA regulatory networks based on the filter conditions described previously. Finally, there were 1844 lncRNA–miRNA–mRNA relationship pairs (Supplementary Figure 2A, available at Carcinogenesis Online).
Due to the numerous relationship pairs, GO enrichment and KEGG pathway analyses were performed on mRNAs in the lncRNA–miRNA–mRNA network to analyze functions. As shown in Supplementary Figure 2B, available at Carcinogenesis Online, GO analysis showed that these DE-mRNAs are mainly related to anatomical structure morphogenesis (biological process), plasma membrane (cell component) and transmembrane receptor protein tyrosine kinase activity (molecular function). KEGG pathway analysis revealed that there were 20 pathways enriched in these DE-mRNAs involved in the lncRNA–miRNA–mRNA networks. Of them, the mitogen-activated protein kinase (MAPK) signaling pathway was the most enriched (Supplementary Figure 2C, available at Carcinogenesis Online).
Construction of chemoresistance-related ceRNA networks of CRC
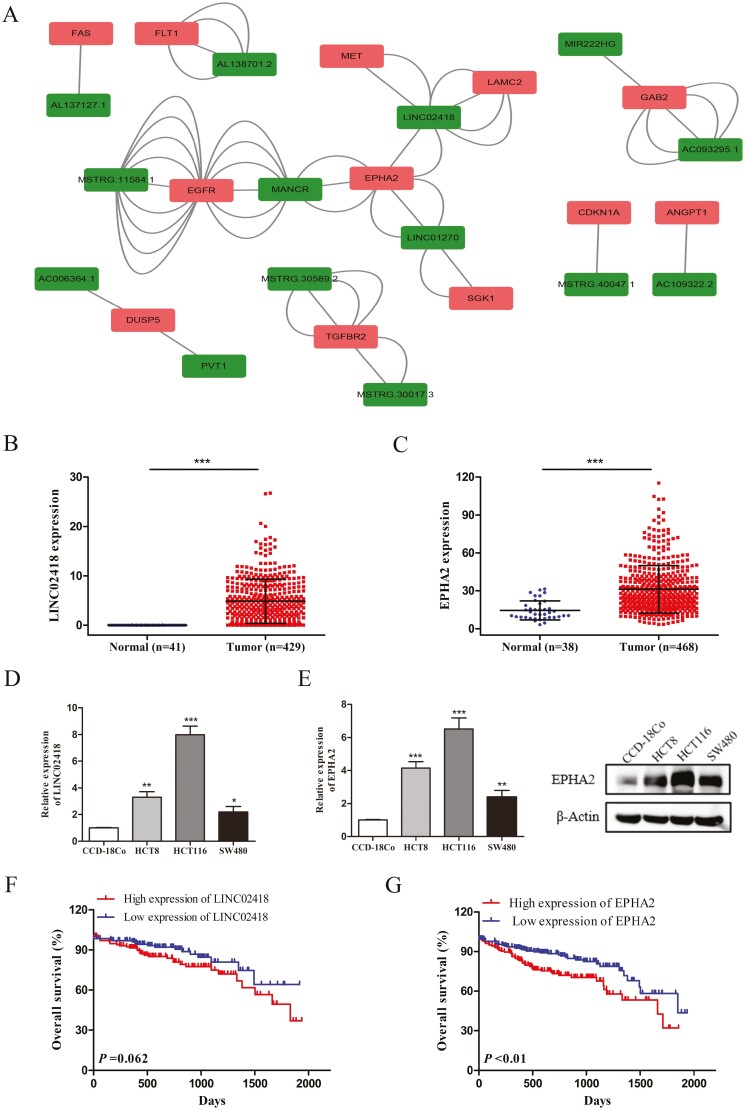
In the above ceRNA network, the relational pairs of upregulated lncRNAs attached our attention (Supplementary Figure 3A, available at Carcinogenesis Online). To fully explore the core regulators related to chemoresistance, we screened out six pathways related to drug resistance from 20 pathways enriched in the network, including the MAPK signaling pathway, TNF signaling pathway, focal adhesion, HIF-1 signaling pathway, PI3K–Akt signaling pathway and Ras signaling pathway, among which the MAPK, PI3K–Akt and Ras signaling pathways were the most enriched. Then, we further filtered the key DE-mRNAs closely related to drug resistance from the genes enriched in the above three pathways according to the literature. Based on the screening results, 52 lncRNA–miRNA–mRNA networks were selected from above 626 relational pairs, including 14 lncRNAs, 19 miRNAs and 12 mRNAs (Figure 2A). These networks were regarded to be related to chemoresistance, among which EPHA2 and LINC02418 were identified as the hub genes (Supplementary Figure 3B, available at Carcinogenesis Online). Moreover, the LINC02418 and EPHA2 expression data downloaded from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/) showed that EPHA2 and LINC02418 were upregulated in human CRC tissues compared with normal tissues (Figure 2B and C). Next, the expressions of LINC02418 and EPHA2 were examined on CRC cell lines compared with normal colon epithelial cells and increased EPHA2 and LINC02418 expression levels were observed (Figure 2D and E). In addition, it was observed from TCGA data that the overall survival rate in the LINC02418 high expression group was shorter than that in the low expression group, but the difference was not statistically significant (P = 0.062, Figure 2F). Furthermore, Kaplan–Meier survival analysis showed that patients with high EPHA2 expression had a significantly poorer overall survival than those with low EPHA2 expression (P < 0.01, Figure 2G). Collectively, these results suggested that high EPHA2 and LINC02418 expression may indicate a poor prognosis among CRC patients. In this study, there were two relationship pairs found between EPHA2 and LINC02418, including LINC02418/miR-372-3p/EPHA2 and LINC02418/miR-33a-3p/EPHA2 (Supplementary Figure 3C, available at Carcinogenesis Online). The results of RNA-sequencing showed that miR-33a-3p and miR-372-3p were significantly downregulated in HCT8/5-Fu and HCT8/DDP cells, but the expression of miR-372-3p was particularly lower (Supplementary Table 4, available at Carcinogenesis Online). Additionally, as there is less research on miR-372-3p, miR-372-3p caught our attention. Therefore, we focused on the LINC02418/miR-372-3p/EPHA2 relationship pair to explore its roles in mediating CRC chemoresistance.
Figure 2.
The clinical significance of key genes in chemoresistant related lncRNA–miRNA–mRNA regulatory networks. (A) CeRNA networks are based on the genes related to chemoresistance. The expression of LINC02418 (B) and EPHA2 (C) in CRC tissues and normal tissues from TCGA database. The expression of LINC02418 (D) and EPHA2 (E) in CRC cell lines was detected by qRT-PCR or Western blotting. Data were shown as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001. Kaplan-Meier survival analysis revealed that CRC patients with high LINC02418 (F) and EPHA2 (G) expression showed poorer overall survival compared to those with low expression.
LINC02418 is overexpressed in chemoresistant CRC cells and LINC02418 inhibition promotes chemosensitivity
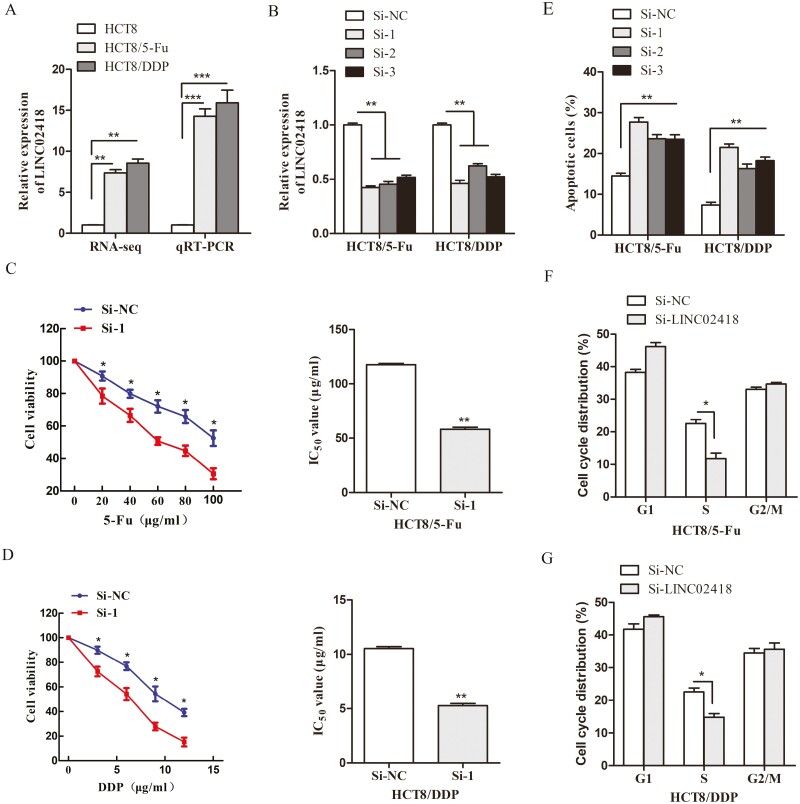
The results of RNA-sequencing showed that LINC02418 was significantly upregulated in HCT8/5-Fu and HCT8/DDP cells. In addition, quantitative real-time PCR (qRT-PCR) also showed that the expression level of LINC02418 in chemoresistant CRC cells was significantly increased compared with that in parental cells (Figure 3A). To explore the function of LINC02418 in CRC chemoresistance in vitro, three siRNAs were constructed and transfected into HCT8/5-Fu and HCT8/DDP cells, and the knockdown efficiency was evaluated by qRT-PCR, indicating si-LINC02418-1 with the highest inhibition effect to be used for further assays (Figure 3B). MTT assays were performed to measure the sensitivity of HCT8/5-Fu and HCT8/DDP cells to chemotherapeutic drugs, and the results indicated that the survival rate of cells transfected with si-LINC02418 was significantly lower than that of si-NC cells when combined with the same concentration. In addition, the IC50 values were remarkably decreased in cells with LINC02418 silencing (Figure 3C and D). Furthermore, depletion of LINC02418 in the two chemoresistant cell lines induced significant apoptosis and led to a decrease in the S phase of the cell cycle according to flow cytometry (Figure 3E–G). The same results were also detected in chemoresistant cells transfected with si-LINC02418-2 and si-LINC02418-3 (Supplementary Figure 4, available at Carcinogenesis Online). Collaboratively, these results indicated that silencing of LINC02418 significantly enhances the chemosensitivity of HCT8/5-Fu and HCT8/DDP cells.
Figure 3.
LINC02418 is upregulated in CRC chemoresistant cell lines and responsible for the chemoresistance of CRC. (A) LINC02418 was upregulated in HCT8/5-Fu and HCT8/DDP cells detected and validated by RNA-sequencing and qRT-PCR, respectively. (B) The expression of LINC02418 was knocked down by three different siRNAs in two chemoresistant cell lines. Cell viability and IC50 values of HCT8/5-Fu (C) and HCT8/DDP cells (D) with LINC02418 knockdown were assessed by MTT assay. (E) Knockdown LINC02418 in chemoresistant cells increased cell apoptosis rates. (E) LINC02418 knockdown led to a decrease in the S phase in HCT8/5-Fu (F) and HCT8/DDP cells (G). Data are shown as mean ± SD, and the results represent three independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001.
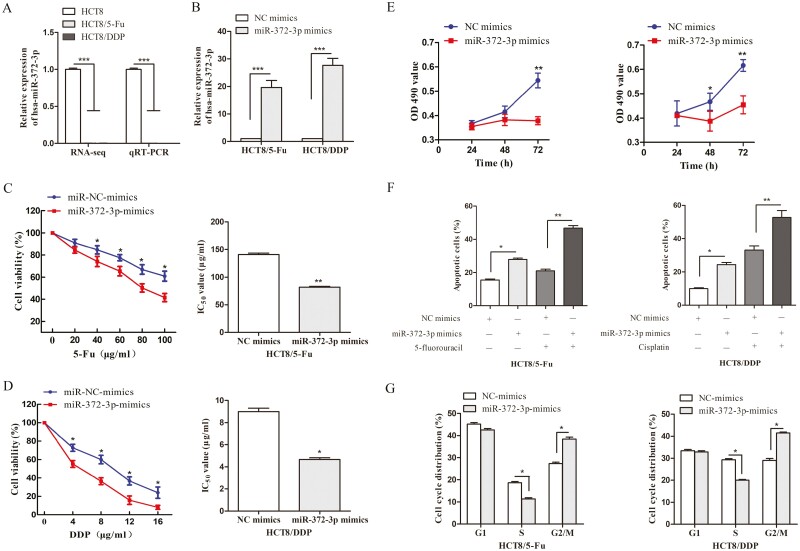
miR-372-3p is downregulated in chemoresistant CRC cells and overexpressing miR-372-3p increased chemosensitivity
The expression level of miR-372 in the CRC samples derived from TCGA database and CRC cell lines was analyzed. The expression of miR-372 was significantly upregulated in CRC tissues and cells (Supplementary Figure 5A and B, available at Carcinogenesis Online), and a positive correlation was found between high miR-372 expression and reduced overall survival rates using Kaplan–Meier analysis (P = 0.043, Supplementary Figure 5C, available at Carcinogenesis Online). In the study, the results of RNA-sequencing showed that miR-372-3p was significantly downregulated in HCT8/5-Fu and HCT8/DDP cells, and we subsequently confirmed that miR-372-3p was lowly expressed in chemoresistant cells compared with HCT8 cells by qRT-PCR (Figure 4A). Among the ceRNAs we selected above, miR-372-3p was predicted to have potential interaction sites with LINC02418, and highly expressed LINC02418 could act as a ‘sponge’ to adsorb miR-372-3p, which may be the reason for the low expression of miR-372-3p in chemoresistant cell lines. To further explore the functions of miR-372-3p, we overexpressed miR-372-3p in two chemoresistant cell lines using transfection of miR-372-3p mimics, then validated our findings with qRT-PCR (Figure 4B). Compared with cells transfected with NC mimic, it was obvious that overexpression of miR-372-3p enhanced the sensitivity of HCT8/5-Fu cells to 5-Fu and HCT8/DDP cells to DDP with reduced IC50 values (Figure 4C and D). In addition, cell proliferation, apoptosis and cell cycle were examined after transfection with NC mimics and miR-372-3p mimics, and the results showed that miR-372-3p upregulation could inhibit the proliferation of chemoresistant CRC cells compared with the NC mimics group (Figure 4E). The cell apoptosis ratios were increased following transfection with miR-372-3p mimics, in a manner more obvious than that seen when 5-Fu or DDP was added (Figure 4F). Additionally, compared with the NC mimics transfection group, the numbers of HCT8/5-Fu and HCT8/DDP cells transfected with miR-372-3p mimics were decreased in the S phase, and arrested in the G2/M phase (Figure 4G). Thus, these results indicated that the functions of overexpressing miR-372-3p are similar to that of silencing LINC02418, which strongly supports the idea that miR-372-3p plays important roles in the regulation of CRC chemoresistance.
Figure 4.
MiR-372-3p is downregulated in CRC chemoresistant cell lines and responsible for the chemoresistance of CRC. (A) MiR-372-3p was lowly expressed in HCT8/5-Fu and HCT8/DDP cells detected and validated by RNA-sequencing and qRT-PCR, respectively. (B) The expression of miR-372-3p was detected when transfected with miR-372-3p mimics or NC mimics in two chemoresistant cell lines. Cell viability and IC50 values of HCT8/5-Fu (C) and HCT8/DDP cells (D) transfected with miR-372-3p and NC mimics were detected. (E) The proliferation capacities were inhibited in miR-372-3p overexpressing chemoresistant cells. (F) Flow cytometry assays suggested that the percentage of apoptotic chemoresistant cells was increased by miR-372-3p overexpression. (G) Overexpressed miR-372-3p led to the changes in cell cycle distribution in chemoresistant cells. Data are shown as mean ± SD, and the results represent three independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001.
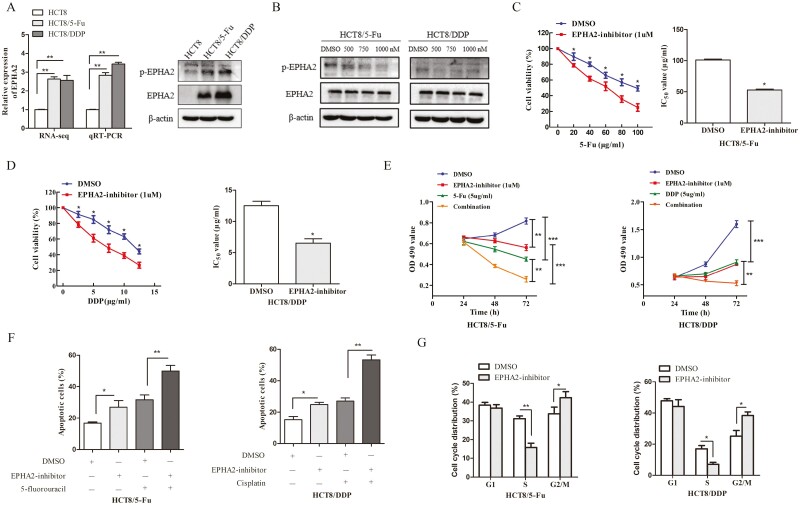
EPHA2 is upregulated in chemoresistant CRC cells and responsible for chemoresistance in CRC
In this study, EPHA2 was identified as a hub gene in the constructed ceRNA network. RNA-sequencing results showed that EPHA2 was upregulated in HCT8/5-Fu and HCT8/DDP cells, and we analyzed the expression and activity of EPHA2 and found EPHA2 and its phosphorylation levels were highly expressed in chemoresistant cells compared with HCT8 cells (Figure 5A). To assess whether EPHA2 is related to CRC chemoresistance, ALW-II-41-27, a novel EPHA2 receptor tyrosine kinase inhibitor, was used to block the EPHA2 function of chemoresistant cells, and a strong reduction in phosphorylation of EPHA2 was found after treatment with different concentrations of EPHA2 inhibitor (Figure 5B). Then, MTT assay results showed that the sensitivity of chemoresistant cells to chemotherapeutic drugs was enhanced and the value of IC50 was decreased following treatment with 1 µM of ALW-II-41-27 (Figure 5C and D). Additionally, the proliferation of drug-resistant cells was significantly inhibited, and apoptosis was promoted, results which were made even more obvious with the combination of ALW-II-41-27 and 5-Fu or DDP (Figure 5E and F). The cell cycle distributions were also altered, the drug-resistant cells were reduced in the S phase, and blocked in the G2/M phase when inhibited the activity of EPHA2 (Figure 5G). Collectively, inhibition of EPHA2 activity could increase the sensitivity of drug-resistant cells, indicating that EPHA2, like LINC02418 and miR-372-3p, is involved in regulating the chemoresistance of CRC.
Figure 5.
EPHA2 is upregulated in CRC chemoresistant cell lines and related to the chemoresistance of CRC. (A) EPHA2 was highly expressed in HCT8/5-Fu and HCT8/DDP cells detected and validated by RNA-sequencing and qRT-PCR and western blotting, respectively. (B) The activity of EPHA2 was inhibited when treated with ALW-II-41-27 for 72 h. Cell viability and IC50 values of HCT8/5-Fu (C) and HCT8/DDP cells (D) treated with 1 µM of ALW-II-41-27 and dimethyl sulfoxide (DMSO) for 72 h were assessed by MTT assay. (E) The proliferation capacities, (F) Apoptotic rates and (G) distributions of the cell cycle were assessed in HCT8/5-Fu and HCT8/DDP cells treated with either DMSO or 1 µM of ALW-II-41-27 for 72 h. Data are shown as mean ± SD, and the results represent three independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001.
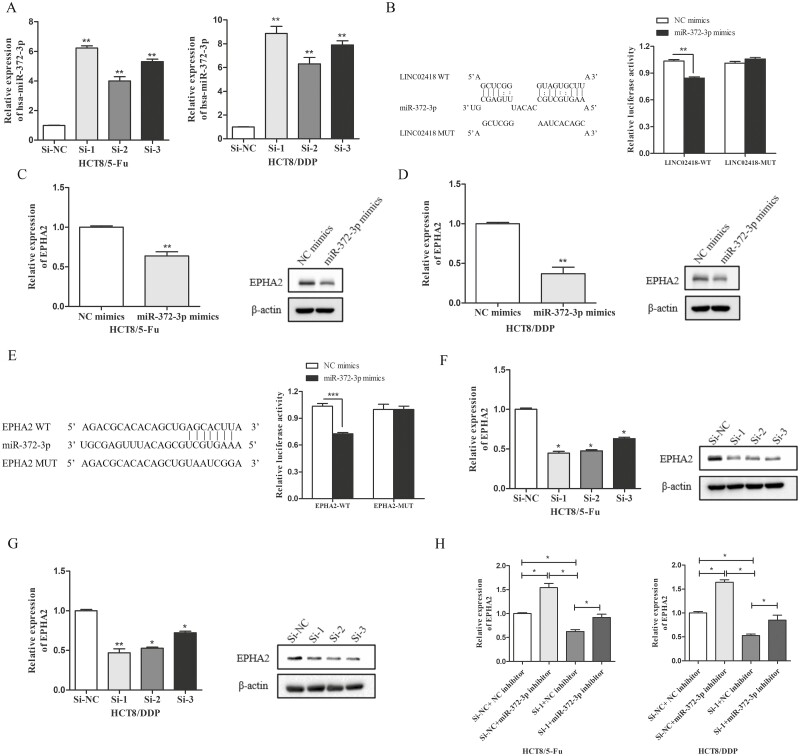
LINC02418 affects EPHA2 expression by acting as a ceRNA interacting with miR-372-3p
It is well known that lncRNAs in the cytoplasm can act as ceRNAs to indirectly regulate downstream gene expression by competing for shared miRNAs (26). To investigate whether LINC02418 regulates CRC chemoresistance through the ceRNA mechanism, we first used lncLocator to predict the location of LINC02418 and found that it was mostly located in the cytoplasm of cells (Supplementary Table 5, available at Carcinogenesis Online). Then, we measured the expression changes of miR-372-3p when LINC02418 was downregulated, finding that the expression of miR-372-3p was significantly enhanced in LINC02418 knockdown cells (Figure 6A), indicating LINC02418 may influence the deregulation of miR-372-3p. Furthermore, luciferase reporter plasmids containing WT LINC02418 and MUT LINC02418 were constructed and transfected into 293T cells along with miR-372-3p mimics or NC mimics. The luciferase activity of LINC02418-WT was reduced by cotransfection with miR-372-3p mimics, while overexpression of miR-372-3p did not influence the luciferase activity of vectors containing LINC02418-MUT (Figure 6B), indicating that LINC02418 acts as a molecular sponge for miR-372-3p. In addition, EPHA2 was predicted to be a potential target of miR-372-3p, and we also found that overexpression of miR-372-3p reduced the expression of EPHA2 in two chemoresistant cell lines (Figure 6C and D), suggesting that EPHA2 is regulated by miR-372-3p. Then luciferase reporter assays confirmed the direct binding of miR-372-3p and EPHA2. We found that the overexpression of miR-372-3p decreased the luciferase activity driven by the WT 3ʹ-UTRs of EPHA2 rather than the mutant 3ʹ-UTRs of EPHA2 (Figure 6E). The expression of EPHA2 after transfection with LINC02418 or cotransfection of LINC02418 and miR-372-3p were detected to determine whether LINC02418 regulated EPHA2 in CRC chemoresistant cells, and whether or not this regulation depends on miR-372-3p. We found that EPHA2 mRNA and protein levels decreased with LINC02418 knockdown (Figure 6F and G). Meanwhile, we also observed that LINC02418 knockdown inhibited the expression of EPHA2, but this inhibition was abolished by simultaneous miR-372-3p knockdown (Figure 6H) in both HCT8/5-Fu and HCT8/DDP cells. Taken together, these results indicate that LINC02418 may function as a ceRNA to promote EPHA2 expression by sponging miR-372-3p in CRC chemoresistance.
Figure 6.
LINC02418 functions as a molecular sponge for miR-372-3p to regulate EPHA2. (A) The expression levels of miR-372-3p in HCT8/5-Fu and HCT8/DDP cells following knockdown of LINC02418 were evaluated by qRT-PCR. (B) The potential interaction sites between miR-372-3p and LINC02418 were predicted with bioinformatics analysis. Dual-luciferase reporter assay was applied to demonstrate the combination between miR-372-3p and LINC02418 in 293T cells. The expression levels of EPHA2 in HCT8/5-Fu (C) and HCT8/DDP cells (D) transfected with miR-372-3p mimics or NC mimics were evaluated by qRT-PCR and western blotting. (E) Schematic diagram of putative base pairing between miR-372-3p and EPHA2. Luciferase activity was measured in 293T cells cotransfected with pmirGLO-EPHA2-WT or pmirGLO-EPHA2-MUT and miR-372-3p mimics. The mRNA and protein levels of EPHA2 in HCT8/5-Fu (F) and HCT8/DDP cells (G) transfected with si-LINC02418 were detected by qRT-PCR and western blotting assay. (H) EPHA2 mRNA levels in HCT8/5-Fu and HCT8/DDP cells transfected with si-NC + miR-NC inhibitor, si-LINC02418 + miR-NC inhibitor, si-NC + miR-372-3p inhibitor and si-LINC02418 + miR-372-3p inhibitor were detected by qRT-PCR. Data are shown as mean ± SD, and the results represent three independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
As one of the most common malignant tumors of the digestive tract in humans, CRC is well known for its high morbidity and mortality rates (27). Although fluorouracil and platinum chemotherapy drugs are widely used to treat CRC, many patients develop drug resistance and carry a poor prognosis. Understanding the specific molecular mechanism involved in CRC chemoresistance is urgently needed to improve survival (28,29). In this study, we constructed a chemoresistance-related lncRNA-associated ceRNA network of CRC and confirmed the critical role of this regulatory network in 5-Fu and DDP resistance in CRC cells. We found that LINC02418 promoted CRC chemoresistance by upregulating EPHA2 through competitively sponging miR-372-3p. In addition, TCGA data indicated that LINC02418 and EPHA2 as well as miR-372 expression were significantly upregulated in CRC patients, and their high expression correlated with a worse prognosis, consistent with findings of previous studies on the prognostic significance of LINC02418 and EPHA2 and miR-372 in CRC patients (30–32).
Previously, some studies have demonstrated that lncRNAs are involved in 5-Fu or DDP chemoresistance of CRC. For example, Zhu et al. indicated that the lncRNA NEAT1 could affect cancer cell stem to regulate 5-Fu resistance of CRC. Mechanistically, NEAT1 increased histone acetylation levels via affecting chromatin remodeling, resulting in increased acetylation levels of ALDH1 and c-Myc and enhanced the stemness of CRC cells (33). In addition, Han et al. found that knockdown of lncRNA SNHG14 or higher expressed miR-186 suppressed cell autophagy, thereby inhibiting the cell proliferation and promoting cell apoptosis of DDP-resistant CRC cell line, while overexpression of the autophagy-related gene ATG14 could significantly recover their effects, demonstrating that SNHG14/miR-186/ATG14 could affect the DDP resistance of CRC (34). However, most studies have only focused on 5-Fu or DDP resistance and specific lncRNAs. To the best of our knowledge, we are the first to identify novel lncRNAs, miRNAs and mRNAs that were both dysregulated in chemoresistant HCT8/5-Fu and HCT8/DDP cells using whole-transcriptome sequencing.
Recent studies have uncovered a new mechanism of lncRNAs as ceRNAs, that is, lncRNAs functioned as ceRNAs to sponge miRNAs, then further affected the expression of mRNAs, thereby regulating carcinogenesis (10–12). At present, many studies have shown that lncRNAs play important roles in the chemoresistance of cancers (13–15). However, the mechanism research of lncRNA and CRC chemoresistance requires further exploration. Therefore, it is significant to identify the roles and mechanisms of lncRNAs as ceRNAs in the drug resistance of CRC. Based on the ceRNA hypothesis and bioinformatics approaches, we constructed chemoresistance-related ceRNA networks and identified LINC02418 and EPHA2 as the hub genes in the regulatory work.
LINC02418, an oncogenic lncRNA, has been reported to be upregulated in CRC, mechanistically, LINC02418 can physically bind to miR-1273g-3p and then upregulate MELK expression to affect tumorigenesis (22). In addition, LINC02418 was also found to be upregulated in both tissues and cells of non-small cell lung cancer (NSCLC), and it could regulate SEC61G expression via interacting with miR-4677-3p to accelerate NSCLC progression (35). There is little knowledge regarding the role and regulatory mechanism of LINC02418 in the chemoresistance of CRC. In this study, we found that it was highly expressed in HCT8/5-Fu and HCT8/DDP cells, and we subsequently focused our research on the regulation of CRC progression and chemoresistance by LINC02418. Loss-of-function assays indicated that LINC02418 knockdown increased the chemosensitivity, affected cell cycle progression and induced apoptosis of CRC chemoresistant cells, indicating that LINC02418 not only affects chemoresistance, but is also important for CRC progression. The other hub gene, EPHA2, a key member of the erythropoietin-producing hepatocellular (Eph) receptor family, is abundantly expressed in several cancers, including CRC (36), gastric cancer (37) and lung cancer (38). It was reported that highly expressed EPHA2 closely relates to poor progression-free survival and an increased progression rate, which could be regarded as a predictive biomarker of resistance (39). However, whether EPHA2 is related to acquired resistance to 5-Fu and DDP in CRC remains unknown. In this investigation, EPHA2 was upregulated, and our functional studies indicated that inhibiting the activity of EPHA2 could increase chemosensitivity, promote cell apoptosis, inhibit cell proliferation and alter the cell cycle distribution of drug-resistant cells. To our surprise, the results showed that LINC02418 positively regulates EPHA2. Bioinformatics prediction was performed to clarify the underlying mechanism between LINC02418 and EPHA2. Here, miR-372-3p was identified as a target of LINC02418 and a novel regulator of EPHA2 through bioinformatics prediction. At present, only one study has indicated that miR-372-3p is dramatically increased in CRC tissues and may be regarded as an independent prognostic factor for recurrence-free survival (RFS) and disease-specific survival (DSS) in CRC patients, and its overexpression could promote tumor progression (32). In addition, miR-372-3p was recently reported to be downregulated in osteosarcoma, regulated by the lncRNA HULC to modulate HMGB1 expression, and ultimately promoted osteosarcoma progression (40). In this study, we observed that miR-372 was overexpressed in CRC cell lines, but lowly expressed in CRC chemoresistant cells, and overexpression of miR-372-3p could also enhance chemosensitivity and induce apoptosis, and inhibit cell proliferation and cell cycle progression of CRC chemoresistant cells. Then, we found that silencing of LINC02418 upregulated miR-372-3p expression in two chemoresistant cell lines, while luciferase reporter assay demonstrated that LINC02418 acted as a molecular sponge for miR-372-3p by directly binding to complementary sequences, which explained the reason for the low expression of miR-372 in CRC chemoresistant cells. Thus, we speculate that LINC02418 affects CRC chemoresistance via downregulating miR-372-3p expression. It is known that miRNAs can regulate gene expression by directly binding to the 3ʹ-UTR of target mRNAs and leading to mRNA degradation or translation inhibition (41). EPHA2 was identified as a direct target of miR-372-3p, and further luciferase reporter assay revealed that miR-372-3p could directly bind to EPHA2 and inhibit its expression. Furthermore, we observed that the reduced expression of EPHA2 by LINC02418 knockdown in HCT8/5-Fu and HCT8/DDP cells was restored by simultaneous transfection of miR-372-3p inhibitors. Together, these results indicate that LINC02418 may affect the chemosensitivity of CRC by regulating the expression of EPHA2 as a ceRNA for miR-372-3p.
In summary, this study screened lncRNA, miRNA and mRNA profiles in CRC chemosensitive and chemoresistant cell lines through the whole-transcriptome sequencing. To find RNAs that regulate 5-Fu and DDP resistance, the common DE lncRNAs, miRNAs and mRNAs in HCT8/5-Fu and HCT8/DDP cells were screened out to construct the ceRNA regulatory network. GO and KEGG pathway analyses were used to find the potential functions of DE-mRNAs regulated by lncRNA–miRNA pairs. A total of 52 lncRNA–miRNA–mRNA pathways closely related to chemoresistance were further constructed, including 14 lncRNAs, 19 miRNAs and 12 mRNAs, among which LINC02418/miR-372-3p/EPHA2 was further selected. Collectively, this study demonstrated that LINC02418 and EPHA2 are upregulated and miR-372-3p is downregulated in HCT8/5-Fu and HCT8/DDP cells, and these trends are responsible for the 5-Fu and DDP resistance of CRC. Mechanistically, LINC02418 functioned as an oncogenic lncRNA by acting as a ceRNA to sponge miR-372-3p and subsequently enhanced EPHA2 expression. Our results indicate that targeting the LINC02418/miR-372-3p/EPHA2 axis might be a new therapeutic strategy to overcome chemoresistance in CRC.
Supplementary Material
Acknowledgements
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- 5-Fu
5-fluorouracil
- cDNA
complementary DNA
- CRC
colorectal cancer
- DE
differentially expressed
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- qRT-PCR
quantitative real-time PCR.
Contributor Information
Fei Yao, Institute of Infection, Immunology and Tumor Microenvironment, School of Medicine, Wuhan University of Science and Technology, Wuhan 430065, China; College of Medical Science, China Three Gorges University, Yichang 443002, China.
Xiaoying Huang, Institute of Infection, Immunology and Tumor Microenvironment, School of Medicine, Wuhan University of Science and Technology, Wuhan 430065, China.
Zhufu Xie, Institute of Infection, Immunology and Tumor Microenvironment, School of Medicine, Wuhan University of Science and Technology, Wuhan 430065, China.
Jie Chen, Tianyou Hospital, Wuhan University of Science and Technology, Wuhan 430064, China.
Ling Zhang, Institute of Infection, Immunology and Tumor Microenvironment, School of Medicine, Wuhan University of Science and Technology, Wuhan 430065, China.
Qiang Wang, Institute of Infection, Immunology and Tumor Microenvironment, School of Medicine, Wuhan University of Science and Technology, Wuhan 430065, China.
Hui Long, Tianyou Hospital, Wuhan University of Science and Technology, Wuhan 430064, China.
Jue Jiang, Institute of Infection, Immunology and Tumor Microenvironment, School of Medicine, Wuhan University of Science and Technology, Wuhan 430065, China.
Qingming Wu, Institute of Infection, Immunology and Tumor Microenvironment, School of Medicine, Wuhan University of Science and Technology, Wuhan 430065, China; Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Wuhan University of Science and Technology, Wuhan 430065, China.
Funding
This study was supported by Health Commission of Hubei Province Scientific Research Project (WJ2019M256).
Authors’ Contributions
Q.W. and J.J. conceived and supervised the study. Q.W., J.J. and F.Y. wrote the manuscript. F.Y. performed most of the experiments and statistical analysis. L.Z., J.C., Q.W. and H.L. analyzed the RNA-sequencing data. X.H. and Z.X. provided assistance for cell culture. All authors contributed to the final manuscript.
References
- 1. Siegel, R.L., et al. (2020) Cancer statistics, 2020. CA Cancer J. Clin., 70, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Kuipers, E.J., et al. (2015) Colorectal cancer. Nat. Rev. Dis. Primers, 1, 15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao, P., et al. (2018) Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study. BMC Cancer, 18, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu, Q., et al. (2019) Survival benefit of adjuvant chemotherapy for patients with poorly differentiated stage IIA colon cancer. J. Cancer, 10, 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dragomir, M.P., et al. (2020) Non-coding RNAs in GI cancers: from cancer hallmarks to clinical utility. Gut, 69, 748–763. [DOI] [PubMed] [Google Scholar]
- 6. Kopp, F.. et al. (2018) Functional classification and experimental dissection of long noncoding RNAs. Cell, 172, 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu, X.J., et al. (2019) RP11-51O6.1 sponges miR-206 to accelerate colorectal cancer carcinogenesis and metastasis through upregulating YAP1. Carcinogenesis, 42, 984–994. [DOI] [PubMed] [Google Scholar]
- 8. Sharma, E., et al. (2016) Global mapping of human RNA-RNA interactions. Mol. Cell, 62, 618–626. [DOI] [PubMed] [Google Scholar]
- 9. Bridges, M.C., et al. (2021) LNCcation: lncRNA localization and function. J. Cell Biol., 220, e202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anastasiadou, E., et al. (2018) Non-coding RNA networks in cancer. Nat. Rev. Cancer, 18, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou, B., et al. (2021) Translation of noncoding RNAs and cancer. Cancer Lett., 497, 89–99. [DOI] [PubMed] [Google Scholar]
- 12. Slack, F.J., et al. (2019) The role of non-coding RNAs in oncology. Cell, 179, 1033–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei, L., et al. (2020) Noncoding RNAs in gastric cancer: implications for drug resistance. Mol. Cancer, 19, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren, J., et al. (2018) Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics, 8, 3932–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiong, G., et al. (2017) The underlying mechanisms of non-coding RNAs in the chemoresistance of pancreatic cancer. Cancer Lett., 397, 94–102. [DOI] [PubMed] [Google Scholar]
- 16. Chen, S., et al. (2021) Forkhead box D1 promotes EMT and chemoresistance by upregulating lncRNA CYTOR in oral squamous cell carcinoma. Cancer Lett., 503, 43–53. [DOI] [PubMed] [Google Scholar]
- 17. Yang, Q., et al. (2020) lncRNA SLC7A11-AS1 promotes chemoresistance by blocking SCF(β-TRCP)-mediated degradation of NRF2 in pancreatic cancer. Mol. Ther. Nucleic Acids, 19, 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He, W., et al. (2019) MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene, 38, 4637–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang, Z., et al. (2019) Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J. Exp. Clin. Cancer Res., 38, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang, H., et al. (2020) The roles of ceRNAs-mediated autophagy in cancer chemoresistance and metastasis. Cancers, 12, 2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu, K.P., et al. (2019) Analyzing the interactions of mRNAs and ncRNAs to predict competing endogenous RNA networks in osteosarcoma chemo-resistance. Mol. Ther., 27, 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao, Y., et al. (2019) Long noncoding RNA LINC02418 regulates MELK expression by acting as a ceRNA and may serve as a diagnostic marker for colorectal cancer. Cell Death Dis., 10, 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fan, J., et al. (2018) Chemoresistance transmission via exosome-mediated EphA2 transfer in pancreatic cancer. Theranostics, 8, 5986–5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moyano-Galceran, L., et al. (2020) Adaptive RSK-EphA2-GPRC5A signaling switch triggers chemotherapy resistance in ovarian cancer. EMBO Mol. Med., 12, e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao, F., et al. (2021) Identification of circular RNAs associated with chemoresistance in colorectal cancer. Front. Genet., 12, 696948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Statello, L., et al. (2021) Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol., 22, 96–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dekker, E.et al. (2019) Colorectal cancer. Lancet, 394, 1467–1480. [DOI] [PubMed] [Google Scholar]
- 28. Zhang, S.et al. (2019) Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res., 38, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang, X., et al. (2016) Macrophages induce resistance to 5-fluorouracil chemotherapy in colorectal cancer through the release of putrescine. Cancer Lett., 381, 305–313. [DOI] [PubMed] [Google Scholar]
- 30. Tian, J., et al. (2020) LINC02418 promotes colon cancer progression by suppressing apoptosis via interaction with miR-34b-5p/BCL2 axis. Cancer Cell. Int., 20, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Robertis, M., et al. (2017) Dysregulation of EGFR pathway in EphA2 cell subpopulation significantly associates with poor prognosis in colorectal cancer. Clin. Cancer Res., 23, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peng, H., et al. (2019) MiR-372-3p promotes tumor progression by targeting LATS2 in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci., 23, 8332–8344. [DOI] [PubMed] [Google Scholar]
- 33. Zhu, Y., et al. (2020) LncRNA NEAT1 remodels chromatin to promote the 5-Fu resistance by maintaining colorectal cancer stemness. Cell Death Dis., 11, 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han, Y., et al. (2020) SNHG14 stimulates cell autophagy to facilitate cisplatin resistance of colorectal cancer by regulating miR-186/ATG14 axis. Biomed. Pharmacother., 121, 109580. [DOI] [PubMed] [Google Scholar]
- 35. Han, B. (2019) LncRNA LINC02418 regulates proliferation and apoptosis of non-small cell lung cancer cells by regulating miR-4677-3p/SEC61G. Eur. Rev. Med. Pharmacol. Sci., 23, 10354–10362. [DOI] [PubMed] [Google Scholar]
- 36. Dunne, P.D.et al. (2016) EphA2 expression is a key driver of migration and invasion and a poor prognostic marker in colorectal cancer. Clin. Cancer Res., 22, 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee, P.C.et al. (2020) C1GALT1 is associated with poor survival and promotes soluble Ephrin A1-mediated cell migration through activation of EPHA2 in gastric cancer. Oncogene, 39, 2724–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan, Y.C., et al. (2019) EPHA2 mutations with oncogenic characteristics in squamous cell lung cancer and malignant pleural mesothelioma. Oncogenesis, 8, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martini, G.et al. (2019) EPHA2 is a predictive biomarker of resistance and a potential therapeutic target for improving antiepidermal growth factor receptor therapy in colorectal cancer. Mol. Cancer Ther., 18, 845–855. [DOI] [PubMed] [Google Scholar]
- 40. Li, Y., et al. (2020) LncRNA HULC induces the progression of osteosarcoma by regulating the miR-372-3p/HMGB1 signalling axis. Mol. Med., 26, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Syeda, Z.A.et al. (2020) Regulatory mechanism of MicroRNA expression in cancer. Int. J. Mol. Sci., 21, 1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.