Perspective
The ongoing COVID-19 pandemic has affected millions of people worldwide. One interesting observation, revealed by a few recent studies [1–3], is that SARS-CoV-2 infection can apparently cause an Alzheimer disease (AD)-like neuropathological phenotype as well as clinical “brain fog”. This raises an interesting question: “where and how might the molecular pathways underlying SARS-CoV-2 infection and AD converge?”
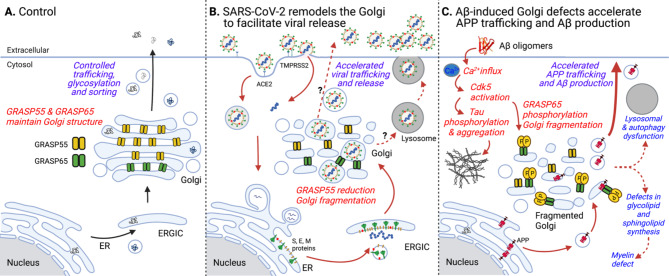
SARS-CoV-2 is an enveloped virus whose replication, assembly and release rely on the secretory pathway of the host cell. Three non-structural proteins (Nsp3, 4, 6) remodel the endoplasmic reticulum (ER) to form double-membrane vesicles (DMVs) that facilitate viral RNA replication. Virions are assembled and bud into the lumen of the ER-Golgi intermediate compartment (ERGIC) and Golgi where the spike protein is glycosylated and cleaved by furin. The Golgi is a key cellular structure for membrane trafficking, processing, and sorting. To perform these functions, it needs to form a multilayer stacked structure by two Golgi stacking proteins, GRASP55 and GRASP65 (Fig. 1A). Depletion of GRASPs leads to Golgi fragmentation (GF), which accelerates trafficking but causes missorting of lysosomal enzymes, alters protein glycosylation and secretion, and affects other cellular activities such as cell attachment, migration, and growth [4]. SARS-CoV-2 infection triggers GF, which accelerates viral trafficking and release [5] (Fig. 1B). In addition, the spike-ACE2 interaction is enhanced by heparan sulfate [6] whose synthesis is increased by GRASP depletion [7]. In a recent study [2], treatment with serum from SARS-CoV-2-infected hamsters causes a SARS-CoV-2 like phenotype in naive hamsters, indicating that SARS-CoV-2 infection triggers the secretion of unknown molecules into the serum.
Fig. 1.
Golgi defects accelerate SARS-CoV-2 virus maturation in COVID-19 and Aβ production in AD. (A) In healthy cells, Golgi structure formation serves as a quality control mechanism for protein glycosylation and sorting. When Golgi cisternae are fully stacked, which is mediated by Golgi structural proteins including GRASP55 and GRASP65, vesicles can only form and fuse at the rims. This slows down trafficking, but enforces accurate glycosylation and sorting [4]. (B) In COVID-19, SARS-CoV-2 infection causes Golgi fragmentation possibly by downregulating GRASP55 expression, which may accelerate virus maturation and release. (C) In AD, Aβ oligomer accumulation leads to the influx of Ca2+ ions; increased cytosolic Ca2+ activates calpain to cleave p35 to p25. p25 then activates cdk5 for GRASP65 phosphorylation. GRASP65 phosphorylation results in Golgi fragmentation, which, in turn, accelerates APP trafficking and increases Aβ production [9]. In addition, Golgi defects also cause autophagy and lysosomal dysfunction [4] and impact the synthesis of glycolipids [4, 7], major components of the myelin sheath.
GF occurs widely in brain samples of AD patients and can be induced by excessive activation of neurons [8]. Many AD pathologies, including abnormal protein sorting and glycosylation, impaired lysosomal/autophagosomal degradation, increased production of the toxic Aβ peptide, as well as tau phosphorylation and aggregation, can be related to Golgi defects. We have discovered that Aβ oligomer accumulation triggers GF by activating cyclin-dependent protein kinase 5 (cdk5), which, in turn, phosphorylates GRASP65 and causes GF [9]. Subsequently, GF accelerates amyloid precursor protein (APP) trafficking and increases Aβ production (Fig. 1C). Conversely, rescue of the Golgi structure by expressing a phosphorylation-deficient mutant of GRASP65 reduces Aβ production by increasing non-amyloidogenic α-cleavage of APP [9]. Given that cdk5 is a major kinase for tau phosphorylation, this observation links enhanced APP amyloidogenic cleavage with tau phosphorylation.
GRASP55 also regulates the secretion and aggregation of cytoplasmic neurotoxic proteins through autophagy. Under growth conditions, GRASP55 is modified by a post-translational modification that attaches O-linked N-acetylglucosamine (O-GlcNAc) moieties to serine and threonine residues of nucleocytoplasmic proteins (O-GlcNAcylation) and functions as a Golgi stacking protein. Glucose deprivation reduces GRASP55 O-GlcNAcylation, allowing it to relocate from the Golgi to the interface between autophagosomes and lysosomes, where it functions as a membrane tether to facilitate autophagosome-lysosome fusion [4]. This increases unconventional secretion of cytosolic neurotoxic proteins such as tau and mutant huntingtin and reduces their aggregation [10]. Therefore, molecular and cellular dysfunctions related to GF may extend beyond the Golgi per se, resulting in dysfunction of distal compartments of the secretory pathway including autophagosomes and lysosomes, and impact a variety of cellular activities.
The similarity between COVID-19 and AD in causing GF indicates that the Golgi may be a candidate subcellular locus where the two diseases converge. Both the SARS-CoV-2 spike protein and APP are type I membrane proteins that travel through the secretory pathway. In the Golgi, they are both modified by N- and O-glycosylation and processed by proteases, both of which are impacted by GF. It is possible that SARS-CoV-2 infection may cause GF and subsequently accelerate APP trafficking and processing, thus leading to AD neuropathology [1].
“Brain fog” is a general term used to describe cognitive impairment observed under different circumstances including SARS-CoV-2 infection. SARS-CoV-2 infects all neuronal types [1], and SARS-CoV-2 infection-caused GF may also enhance the secretion of cytokines such as TNFα and IL-1β. While the mechanism of brain fog is currently unknown, one possible explanation is that SARS-CoV-2 infection causes a defect in myelination [3]. The Golgi is the cellular “factory” for sphingolipid and glycolipid synthesis. GF leads to a substantial elevation in the levels of monosialotetrahexosylganglioside (GM1) with concomitant reduction of globotriaosylceramide (Gb3) [4]. While both GM1 and Gb3 play important roles in myelination and function, GM1 has also been shown to enhance Aβ aggregation and toxicity.
In summary, the mechanisms by which SARS-CoV-2 infection impacts brain cells at the molecular and cellular levels are important issues to elucidate, and the appearance of GF may serve as a key starting point. Perhaps it is time to look more deeply into Golgi abnormalities, which may shed light on the connection between AD pathology and clinical dementia. Elucidation of how Aβ fibrils and oligomers cause defects in trafficking and sorting of many proteins in a variety of brain cell types will be required to make sense of the abnormalities in glycosylation, lysosomal function, and autophagy that define the molecular cell pathology of AD. Speculation about the potential role for GF in neurodegeneration and SARS-CoV-2 infection may be more than just an academic exercise. It is conceivable that manipulation of GRASPs and other Golgi proteins may provide novel potential therapeutic opportunities for a host of currently untreatable major human diseases. This Perspective raises the possibility that GRASPs per se, or GRASP-mimetic molecules or drugs, might be useful in the treatment or prevention of neurodegenerative or other diseases. Efforts at testing this possibility in various cellular and animal models of human diseases are currently underway.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Dr. Jianchao Zhang and other members of the Wang, Ehrlich-Gandy, and Tortorella labs for stimulating discussions. Y.W. is supported by NIH/NIGMS (R35GM130331) and the Fast Forward Protein Folding Disease Initiative of the University of Michigan. S.G. is supported by RF1AG058469, RF1AG059319, U01AG046170, R01AG061894, and P30 AG066514 (to Mary Sano).
Declarations
Conflict of interest
Y.W. is a founder of Camillo, Inc., a biotechnology company focusing on the potential use of GRASPs and other Golgi structural proteins as treatments for neurodegenerative diseases.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanzhuang Wang and Sam Gandy have contributed equally.
Contributor Information
Yanzhuang Wang, Email: yzwang@umich.edu.
Sam Gandy, Email: samuel.gandy@mssm.edu.
References
- 1.Shen WB, Logue J, Yang P, Baracco L, Elahi M, Reece EA, Wang B, Li L, Blanchard TG, Han Z, et al: SARS-CoV-2 invades cognitive centers of the brain and induces Alzheimer’s-like neuropathology. bioRxiv: the preprint server for biology 2022.
- 2.Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H, McArthur NG, Moeller R, Uhl S, Omer AD, et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022;185:1052–64 e1012. doi: 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Castaneda A, Lu P, Geraghty AC, Song E, Lee MH, Wood J, O’Dea MR, Dutton S, Shamardani K, Nwangwu K, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–68 e2416. doi: 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Wang Y. Nonredundant Roles of GRASP55 and GRASP65 in the Golgi Apparatus and Beyond. Trends Biochem Sci. 2020;45:1065–79. doi: 10.1016/j.tibs.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Kennedy AA, Xing L, Bui S, Reid W, Joppich J, Ahat E, Rose M, Tang Q, Tai AW, Wang Y: SARS-CoV-2 triggers Golgi fragmentation via downregulation of GRASP55 to facilitate viral trafficking. bioRxiv: the preprint server for biology 2022, https://doi.org/10.1101/2022.03.04.483074.
- 6.Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, Narayanan A, Majowicz SA, Kwong EM, McVicar RN, et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell. 2020;183:1043–57 e1015. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahat E, Song Y, Xia K, Reid W, Li J, Bui S, Zhang F, Linhardt RJ, Wang Y. GRASP depletion-mediated Golgi fragmentation impairs glycosaminoglycan synthesis, sulfation, and secretion. Cell Mol Life Sci. 2022;79:199. doi: 10.1007/s00018-022-04223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thayer DA, Jan YN, Jan LY. Increased neuronal activity fragments the Golgi complex. Proc Natl Acad Sci U S A. 2013;110:1482–7. doi: 10.1073/pnas.1220978110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi G, Chi Y, Huang Z, Wang Y. Abeta-induced Golgi fragmentation in Alzheimer’s disease enhances Abeta production. Proc Natl Acad Sci U S A. 2014;111:E1230–9. doi: 10.1073/pnas.1320192111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahat E, Bui S, Zhang J, da Veiga Leprevost F, Sharkey L, Reid W, Nesvizhskii AI, Paulson HL, Wang Y. GRASP55 regulates the unconventional secretion and aggregation of mutant huntingtin. J Biol Chem 2022:102219. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.