Abstract
Background
Total thyroidectomy (TT) and subtotal thyroidectomy (ST) are worldwide treatment options for multinodular non‐toxic goitre in adults. Near TT, defined as a postoperative thyroid remnant less than 1 mL, is supposed to be a similarly effective but safer option than TT. ST has been shown to be marginally safer than TT, but it may leave an undetected thyroid cancer in place.
Objectives
The objective was to assess the effects of total or near‐total thyroidectomy compared to subtotal thyroidectomy for multinodular non‐toxic goitre.
Search methods
We searched the Cochrane Library, MEDLINE, PubMed, EMBASE, as well as the ICTRP Search Portal and ClinicalTrials.gov. The date of the last search was 18 June 2015 for all databases. No language restrictions were applied.
Selection criteria
Two review authors independently scanned the abstract, title or both sections of every record retrieved to identify randomised controlled trials (RCTs) on thyroidectomy for multinodular non‐toxic goitre for further assessment.
Data collection and analysis
Two review authors independently extracted data, assessed studies for risk of bias and evaluated overall study quality utilising the GRADE instrument. We calculated the odds ratio (OR) and corresponding 95% confidence interval (CI) for dichotomous outcomes. A random‐effects model was used for pooling data.
Main results
We examined 1430 records, scrutinized 14 full‐text publications and included four RCTs. Altogether 1305 participants entered the four trials, 543 participants were randomised to TT and 762 participants to ST. A total of 98% and 97% of participants finished the trials in the TT and ST groups, respectively. Two trials had a duration of follow‐up between 12 and 39 months and two trials a follow‐up of 5 and 10 years, respectively. Risk of bias across studies was mainly unknown for selection, performance and detection bias. Attrition bias was generally low and reporting bias high for some outcomes. In the short‐term postoperative period no deaths were reported for both TT and ST groups. However, longer‐term data on all‐cause mortality were not reported (1284 participants; 4 trials; moderate quality evidence). Goiter recurrence was lower in the TT group compared to ST. Goiters recurred in 0.2% (1/425) of the TT group compared to 8.4% (53/632) of the ST group (OR 0.05 (95% CI 0.01 to 0.21); P < 0.0001; 1057 participants; 3 trials; moderate quality evidence). Re‐intervention due to goitre recurrence was lower in the TT group compared to ST. Re‐intervention was necessary in 0.5% (1/191) of TT patients compared to 0.8% (3/379)of ST patients (OR 0.66 (95% CI 0.07 to 6.38); P = 0.72; 570 participants; 1 trial; low quality evidence). The incidence of permanent recurrent laryngeal nerve palsy was lower for ST compared with TT. Permanent recurrent laryngeal nerve palsy occurred in 0.8% (6/741) of ST patients compared to 0.7% (4/543) of TT patients (OR 1.28, (95% CI 0.38 to 4.36); P = 0.69; 1275 participants; 4 trials; low quality evidence). The incidence of permanent hypoparathyroidism was lower for ST compared with TT. Permanent hypoparathyroidism occurred in 0.1% (1/741) of ST patients compared to 0.6% (3/543) of TT patients (OR 3.09 (95% CI 0.45 to 21.36); P = 0.25; 1275 participants: 4 trials; low quality evidence). The incidence of thyroid cancer was lower for ST compared with TT. Thyroid cancer occurred in 6.1% (41/669) of ST patients compared to 7.3% (34/465)of TT patients (OR 1.32 (95% CI 0.81 to 2.15); P = 0.27; 1134 participants; 3 trials; low quality evidence). No data on health‐related quality of life or socioeconomic effects were reported in the included studies.
Authors' conclusions
The body of evidence on TT compared with ST is limited. Goiter recurrence is reduced following TT. The effects on other key outcomes such as re‐interventions due to goitre recurrence, adverse events and thyroid cancer incidence are uncertain. New long‐term RCTs with additional data such as surgeons level of experience, treatment volume of surgical centres and details on techniques used are needed.
Plain language summary
Total or near‐total thyroidectomy versus subtotal thyroidectomy for multinodular non‐toxic goitre in adults
Review question
What are the effects of total or near‐total thyroidectomy compared with subtotal thyroidectomy for multinodular non‐toxic goitre in adults?
Background
Multinodular goitre refers to a generalised enlarged thyroid gland with recognisable nodules within it. The thyroid gland consists of two connected lobes. People affected by goitre often present with a non‐symmetrical enlargement of the thyroid gland with a visible swelling in the anterior aspect of the neck. One or more nodules can be recognised. The most frequent cause of multinodular goitre is iodine deficiency. Non‐toxic goitre means that the nodules do not secret thyroid hormones in an uncontrolled way. Total thyroidectomy is an operation that involves the surgical removal of the whole thyroid gland. Near‐total thyroidectomy is an operation that involves the surgical removal of both thyroid lobes except for a small amount of thyroid tissue (on one or both sides less than 1.0 mL). Subtotal thyroidectomy leaves 3 g to 5 g on the less affected side of the thyroid gland.
After thyroidectomy one of the most important complications is recurrent nerve palsy because this nerve might be traumatised during the surgery. A wide spectrum of complications to the voice, swallowing mechanisms, or both, can occur. A temporary or permanent voice change can result. If the goitre reappears (goitre recurrence) some time after thyroidectomy, another surgical intervention might be necessary. This surgery is more complicated than the initial surgery because of scar tissue making it difficult to identify nerves and other important tissues. There is also the possibility that subtotal thyroidectomy, which is thought to be somewhat safer than total thyroidectomy, may leave an undetected thyroid cancer in place.
Study characteristics
We included four randomised controlled trials with a total of 1305 participants. A total of 543 participants were randomised to total or near‐total thyroidectomy and 762 participants to subtotal thyroidectomy. Two trials had a duration of follow‐up between 12 and 39 months and two trials a follow‐up of 5 and 10 years, respectively. Most participants were women and the average age was around 50 years.
Key results
In the short‐term period after surgery no deaths were reported for both total thyroidectomy and subtotal thyroidectomy groups, however longer‐term data on all‐cause mortality were not reported. Goitre recurrence was lower for total thyroidectomy compared to subtotal thyroidectomy: the risk for goitre recurrence was 84 per 1000 trial participants for subtotal thyroidectomy and 5 per 1000 participants (with a possible range of 1 to 19) for total thyroidectomy. There was no clear benefit or harm of either surgical technique for re‐operations because of goitre recurrence, side effects like permanent recurrent laryngeal nerve palsy or development of thyroid cancer. No data on health‐related quality of life or socioeconomic effects were reported in the included trials.
Quality of the evidence
The overall quality was low to moderate, mainly because of the small number of studies and participants as well as low rates of events which makes distinction between harms and benefits of the two surgical techniques difficult.
Currentness of evidence
This evidence is up to date as of June 2015.
Summary of findings
Summary of findings for the main comparison. Total or near‐total thyroidectomy compared with subtotal thyroidectomy for multinodular non‐toxic goitre in adults.
| Total or near‐total thyroidectomy compared with subtotal thyroidectomy for multinodular non‐toxic goitre in adults | ||||||
|
Patient: adults with multinodular non‐toxic goitre Settings: tertiary referral centre Intervention: (near) total thyroidectomy Comparison: subtotal thyroidectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Subtotal thyroidectomy | (Near)total thyroidectomy | |||||
|
All‐cause mortality Follow‐up: 1 to 10 years |
See comment | See comment | See comment | 1284 (4) | a) ⊕⊕⊕⊝ moderatea | No postoperative hospital deaths or deaths within the first 30 days occurred after total or subtotal thyroidectomy |
| a)Goitre recurrence b)Re‐intervention due to goitre recurrence Follow‐up: a) 3 to 10 years, b) 5 years |
a)84 per 1000 b) 8 per 1000 |
a) 5 per 1000 (1 to 19) b) 5 per 1000 (1 to 48) |
a) OR 0.05 (0.01 to 0.21) b) OR 0.66 (0.07 to 6.38) |
a) 1057 (3) b) 570 (1) |
a) ⊕⊕⊕⊝
moderateb b) ⊕⊕⊝⊝ lowc |
‐ |
|
Adverse events: a) Permanent recurrent laryngeal nerve palsy b) Permanent hypoparathyroidism Follow‐up: a) 1 to 10 years, b) 1 to 10 years |
a) 8 per 1000 b) 1 per 1000 |
a) 10 per 1000 (3 to 34) b) 4 per 1000 (1 to 28) |
a) OR 1.28 (0.38 to 4.36) b) OR 3.09 (0.45 to 21.36) |
a) 1275 (4) b) 1275 (4) |
a) ⊕⊕⊝⊝ lowd b) ⊕⊕⊝⊝ lowd |
‐ |
|
Thyroid cancer incidence Follow‐up: 1 to 5 years |
61 per 1000 | 79 per 1000 (50 to 123) | OR 1.32 (0.81 to 2.15) | 1134 (3) | ⊕⊕⊝⊝ lowd |
‐ |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | Outcome not investigated |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Outcome not investigated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Assumed risk: mean control group risk across studies
aDowngraded by one level because of high risk of outcome reporting bias (no trial investigated all‐cause mortality over longer periods of follow‐up) bDowngraded by one level because of small number of events and unknown risk of detection bias cDowngraded by two levels because of high risk of outcome reporting bias (3 of 4 trials did not report re‐interventions due to goitre recurrence), imprecision and one study only with small number of participants and low event rates dDowngraded by two levels because of imprecision and low event rates
Background
Multinodular non‐toxic goitre is the most common endocrine condition, consisting of a nodular overgrowth of the thyroid gland which is not associated with either malignancy or hyperthyroidism. This can be treated conservatively, but in the vast majority of cases it needs surgical intervention. The indications for surgical treatment of multinodular non‐toxic goitre are:
large size goitre or increase in size despite treatment with thyroid‐stimulating hormone (TSH);
compression symptoms (i.e. dyspnoea or dysphagia);
suspected or proven malignant changes; and
cosmetic reasons.
The optimal surgical procedure for multinodular non‐toxic goitre is still debated. Surgeons may decide to remove the entire thyroid gland in a single operation (total thyroidectomy) or to limit the resection to only part of the thyroid tissue (subtotal thyroidectomy), in order to remove only the diseased part and thus minimise the risk of recurrent nerve palsy and hypocalcaemia, which are more frequently associated with total thyroidectomy. Total thyroidectomy has the advantage of one‐stage removal of incidental thyroid cancer and a lower risk of developing recurrent goitre. In addition, in total thyroidectomy there is no consensus on the safety and cost‐effectiveness of the use of the clamp and tie technique versus the use of a diathermy (i.e. electrically induced heat) system for vessel ligation and division (i.e. LigaSure or Ultracision) (Cirocchi 2010).
Developments in surgical technique including systematic identification of recurrent laryngeal nerve palsy, and intraoperative neuro‐monitoring have led to a reduction in the incidence of recurrent nerve lesions and post‐surgical hypoparathyroidism (Donatini 2012).
Although this topic has been the subject of several studies (Foster 1978; Perzik 1976), the question of which of the two surgical procedures (i.e. total or subtotal thyroidectomy) should be performed remains unanswered.
Description of the condition
The term multinodular goitre describes an enlarged, diffusely heterogeneous thyroid gland. Initial findings may include diffuse enlargement, but asymmetrical nodularity of the mass often develops. Multinodular goitre refers to a generalised enlarged thyroid gland with recognisable nodules within it. Patients affected by this condition often present with a non‐symmetrical enlargement of the thyroid gland with a visible swelling in the anterior aspect of the neck and a heterogeneously rough surface on palpation. One or more nodules can be recognised. The most frequent cause of multinodular goitre is iodine deficiency.
Description of the intervention
Total thyroidectomy is an operation that involves the surgical removal of the whole thyroid gland, with the preservation of the parathyroid glands. A small transverse incision is performed in the inferior portion of the neck at the level of one of the natural creases, 2 to 3 cm above the sternal notch. The platysma is divided and the skin flaps are elevated superiorly and inferiorly. The midline is divided on avascular plane and the strap muscles are retracted laterally and the thyroid is retracted medially. Using blunt dissection the thyroid is dissected from the surrounding structures and then a ligation of the inferior and superior pole vessels is performed. The inferior laryngeal nerve is visualized and preserved as well as parathyroids. Finally it is possible to perform the lobectomy. The same technique is used for both lobes. After total thyroidectomy patients usually take a prescribed oral synthetic thyroid hormone to prevent hypothyroidism.
Near‐total thyroidectomy is an operation that involves the surgical removal of both lobes except for a small amount of thyroid tissue (i.e. less than 1.0 mL) on one or both sides in the vicinity of the recurrent laryngeal nerve entry point and the superior parathyroid gland. This operation follows the same technique as total thyroidectomy.
Subtotal thyroidectomy, including thyroid lobectomy and isthmusectomy, is defined as a lobectomy with contralateral subtotal resection leaving 3 g to 5 g of normal remnant tissue on the less affected side following the same technique as described above (Barczynski 2010). Thyroid hormonal release is maintained.
The Dunhill procedure consists of unilateral extracapsular total thyroidectomy and contralateral subtotal thyroid lobe resection leaving a thyroid stump of approximately 2 g of normal remnant tissue.
Adverse effects of the interventions
The most frequent complications of total thyroidectomy and subtotal thyroidectomy are transient or permanent hypocalcaemia and recurrent nerve palsy. Hypocalcaemia is caused by the unintentional excision of the parathyroid glands or by devascularization of the parathyroid glands during the surgical manoeuvres. Rates of postprocedural hypocalcaemia are approximately 5%, resolving in 80% of cases within approximately 12 months. Transient or permanent recurrent nerve palsy occurs due to a traumatised inferior laryngeal nerve. A wide spectrum of injuries to the voice, swallowing mechanisms, or both, can occur because of the mixed fibres contained within the nerve. A temporary or permanent voice change can result, which can be extremely distressing to the patient. Recurrent nerve palsy, when unilateral, presents with a one‐sided paralysed vocal cord causing voice change. Bilateral nerve palsy can seriously jeopardise the breathing mechanism and often requires the establishment of surgical airways.
The incidence of recurrent goitre after bilateral subtotal thyroidectomy is elevated and varies between 1.2% at a maximum follow‐up of 55 months (Ozbas 2005), to 50% at a maximum follow‐up of 180 months (Ríos 2005). After a secondary intervention for recurrent goitre, the incidence of nerve lesions and hypoparathyroidism is higher due to the difficulty in identifying, within scar tissue, the inferior laryngeal nerves and the parathyroid glands (Lefevre 2007).
How the intervention might work
Although total thyroidectomy allows a more easy follow‐up of patients and avoids relapses of disease, a good proportion of surgeons prefer a more conservative intervention because multinodular nontoxic goitre is a benign disease. Thus the eventual complications of total thyroidectomy may not be justified and the conservative approach may preserve endogenous thyroid function (Yang 2009). Sub‐total thyroidectomy could avoid bilateral inferior laryngeal nerve injury but some relapses of disease are possible over time.
Why it is important to do this review
Agarwal 2008 performed a systematic review to evaluate total thyroidectomy as a surgical procedure for benign multinodular goitre. Total thyroidectomy appeared to be a safe option in the hands of expert surgeons. Near‐total thyroidectomy was shown to be a similarly effective but safer option than total thyroidectomy. However, although subtotal thyroidectomy was marginally safer than total thyroidectomy, it may leave undetected thyroid cancers in place. The review authors concluded that total thyroidectomy should be the procedure of choice for the surgical management of benign multinodular goitre (Agarwal 2008).
In their recent review on surgical treatment of endemic goitre Dralle 2011 provide several arguments in support of the use of total thyroidectomy as the treatment of choice:
non‐toxic goitres involve the entire gland;
the development of sophisticated technologies has reduced the intraoperative complications associated with total thyroidectomy;
in many cases (33%) patients receiving subtotal thyroidectomy required completion because of detection of incidental cancer;
ultrasonography often reveals recurrent goitres post subtotal thyroidectomies; and
hormone replacement therapy is not expensive and is generally well tolerated by patients.
However, Dralle 2011 points out several factors that need to be considered when planning surgical treatment:
the treatment of iodine insufficiency does not necessarily reverse a nodular goitre and the behaviour of the nodules over time is difficult to predict;
to gain a high level of expertise surgeons need to 'super‐specialise' which is often not sustainable worldwide; and
hormone‐replacement therapy is not viable everywhere in the world.
Due to the high prevalence of multinodular non‐toxic goitre, this review aims to assess the effects of total versus subtotal thyroidectomy. Although total thyroidectomy appears to be effective, safe and convenient, clinical evidence supporting its use is still lacking.
Objectives
The primary objective was to assess the effects of total or near‐total thyroidectomy compared to subtotal thyroidectomy for multinodular non‐toxic goitre.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
Adult (older than 18 years) participants with non‐toxic multinodular goitre were considered for inclusion.
Diagnostic criteria
The diagnostic criteria were palpable thyroid nodules or multiple solid or mixed lesions found on thyroid ultrasonography in an euthyroid person.
Types of interventions
Intervention
-
Total thyroidectomy
Near‐total thyroidectomy (unilateral or bilateral thyroid remnant < 1.0 ml) was also regarded a total thyroidectomy.
Comparator
-
Subtotal thyroidectomy (defined as the removal of part of the gland, including thyroid lobectomy and isthmusectomy, allowing maintenance of thyroid hormone release)
Dunhill procedure (hemithyroidectomy plus subtotal resection: unilateral thyroid remnant of 2 ml to 4 ml) is also regarded a subtotal thyroidectomy.
Types of outcome measures
Primary outcomes
The primary outcomes include:
All‐cause mortality.
Goitre recurrence and re‐intervention due to goitre recurrence.
Adverse events.
Secondary outcomes
The secondary outcomes include:
Thyroid cancer incidence.
Health‐related quality of life.
Socioeconomic effects.
Method and timing of outcome measurement
All‐cause mortality: defined as any postoperative death and measured 30 days postoperatively.
Goitre recurrence: defined as the presence of nodular involvement or an enlargement of the residual thyroid remnant.
Re‐intervention due to goitre recurrence: defined as completion thyroidectomy for goitre recurrence.
-
Adverse events included the following.
Postoperative bleeding: defined as any postoperative bleeding; minor bleeding defined as development of small, superficial wound haematoma or bruising not requiring intervention; or major bleeding defined as bleeding requiring intervention. All bleeding outcomes were measured 30 days postoperatively.
Red cell transfusions: defined as any red cell transfused after thyroidectomy and measured 30 days postoperatively.
Permanent or transient recurrent laryngeal nerve (RLN) palsy: transient was defined as RLN palsy during indirect laryngoscopy performed by a throat specialist and measured 30 days postoperatively; permanent RLN palsy: was defined as a vocal cord paresis for more than 12 months after the operation.
Permanent or transient hypoparathyroidism: this could be defined by hypocalcaemia (i.e. a total serum calcium level less than 2.0 mmol/L in either asymptomatic or symptomatic patients), perioral or fingertip paraesthesia or numbness, tetany, or a newly positive Chvostek sign. These outcomes were measured 12, 24, 48, and 72 hours postoperatively. Persistent hypocalcaemia for more than six months after the operation was regarded as permanent hypoparathyroidism.
Thyroid cancer incidence: defined as an unsuspected cancer identified incidentally on pathologic examination of thyroid tissue postoperatively.
Health‐related quality of life: measured by a validated instrument such as the Medical Outcomes Study Short Form Survey (SF‐36) and measured 30 days postoperatively.
Socioeconomic effects: defined as socioeconomic costs of complications and measured at 6 and 12 months after surgery.
'Summary of findings' table
We present a 'Summary of findings' table' reporting the following outcomes based on priority:
All‐cause mortality.
Goitre recurrence and re‐intervention due to goitre recurrence.
Adverse events (especially recurrent laryngeal nerve injury).
Thyroid cancer incidence.
Health‐related quality of life.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date:
-
Cochrane Library (18 June 2015):
Cochrane Central Register of Controlled Trials (CENTRAL, issue 5, May 2015)
Database of Abstracts of Reviews of Effects (DARE, issue 2, April 2015)
Health Technology Assessment (HTA, issue 2, April 2015)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) (1946 to Present, 18 June 2015).
PubMed (only subsets not available on Ovid, 18 June 2015)
EMBASE (1974 to 2015 Week 24, 18 June 2015)
ClinicalTrials.gov. (18 June 2015)
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Protal (http://apps.who.int/trialsearch/), which is a meta‐register of trials with links to several trial registers,
Australian New Zealand Clinical Trials Registry (15 June 2015).
Chinese Clinical Trial Registry (15 June 2015).
ClinicalTrials.gov (15 June 2015).
EU Clinical Trials Register (EU‐CTR, 4 May 2015).
ISRCTN (15 June 2015).
The Netherlands National Trial Register (15 June 2015).
Brazilian Clinical Trials Registry (ReBec, 26 May 2015).
Clinical Trials Registry ‐ India (26 May 2015).
Clinical Research Information Service ‐ Republic of Korea (26 May 2015).
Cuban Public Registry of Clinical Trials (26 May 2015).
German Clinical Trials Register (1 June 2015).
Iranian Registry of Clinical Trials (26 May 2015).
Japan Primary Registries Network (26 May 2015).
Pan African Clinical Trial Registry (26 May 2015).
Sri Lanka Clinical Trials Registry (1 June 2015).
Thai Clinical Trials Register (TCTR, 26 May 2015).
Detailed search strategies are reported in Appendix 1. We utilized a MEDLINE via Ovid SP email alert service for identification of newly published studies using the search strategy detailed in Appendix 1. If we identified new studies for inclusion we would have evaluated these and incorporated the findings in our review before submission of the final review draft (Beller 2013).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials, systematic reviews, meta‐analyses, review articles and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors (RC, GD) independently scanned the abstract, title or both sections of every record retrieved to identify studies for further assessment. A third party (AS) resolved any differences in opinion. If resolving disagreement was not possible, we planned to add the article to those 'awaiting assessment' and would have contacted study authors for clarification. We present a PRISMA (i.e. preferred reporting items for systematic reviews and meta‐analyses) flow‐chart to document study selection (Liberati 2009).
Data extraction and management
For the studies that fulfilled the inclusion criteria, two review authors (RC, GDR) independently extracted key participant and intervention characteristics. We report data on efficacy outcomes and adverse events using standard data extraction templates supplied by the Cochrane Metabolic and Endocrine Disorders (CMED) Group. We resolved any disagreements by discussion, or if required, by consulting a third review author (ST). For full details see Characteristics of included studies; additional Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6;Appendix 7; Appendix 8; Appendix 9; Appendix 10.
1. Overview of study populations.
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible [N] | Randomised [N] | Safety [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] | Follow‐up time | |
| (1) Barczynski 2010 | I: total thyroidectomy | b | 3133 | 200 | 200 | ‐ | 191 | 95 | 5 years |
| C1: Dunhill procedure | 200 | 200 | ‐ | 189 | 94 | ||||
| C2: bilateral subtotal thyroidectomy | 200 | 200 | ‐ | 190 | 95 | ||||
| total: | 600 | 600 | ‐ | 570 | 95 | ||||
| (2) Giles 2004 | I: total or near total thyroidectomy | ‐ | ‐ | 109 | 109 | ‐ | 109 | 100 | 1‐3 years |
| C: subtotal thyroidectomy | 109 | 109 | ‐ | 109 | 100 | ||||
| total: | 218 | 218 | ‐ | 218 | 100 | ||||
| (3) Pappalardo 1998 | I: total or near total thyroidectomy | ‐ | ‐ | 69 | 69 | ‐ | 69 | 100 | 10 years |
| C: bilateral subtotal thyroidectomy | 72 | 72 | ‐ | 72 | 100 | ||||
| total: | 141 | 141 | ‐ | 141 | 100 | ||||
| (4) Yang 2009 | I: total or near total thyroidectomy | ‐ | ‐ | 165 | 165 | ‐ | 165 | 100 | I: median follow‐up 36 months |
| C: subtotal thyroidectomy | 181 | 181 | ‐ | 181 | 100 | C: median follow‐up 39 months | |||
| total: | 346 | 346 | ‐ | 346 | 100 | ||||
| Grand total | All interventions | 543 | 534 | 98 | |||||
| All comparators | 762 | 741 | 97 | ||||||
| All interventions and comparators | 1305 | 1275 | 98 | ||||||
aAccording to power calculation in study publication or report bQuote from publication: "The sample size was estimated based on the principle of detecting a 5% difference in the prevalence of recurrent goiter with a 90% probability at P < 0.05"; comment: no sample size provided
"‐" denotes not reported
C: comparator; I: intervention; ITT: intention‐to‐treat
We provide information about potentially‐relevant ongoing studies including trial identifier in the table Characteristics of ongoing studies and in Appendix 5 'Matrix of study endpoints (publication and trial documents)'. We tried to find the protocol for each included study, either in databases of ongoing trials, in publications of study designs, or both, and report these data in Appendix 5.
We sent an email request to authors of included studies to enquire whether they were willing to answer questions regarding their trials. Appendix 11 reports the results of this survey. Thereafter if required, we sought relevant missing information on the trial from the study authors of the article.
Dealing with duplicate publications and companion papers
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised the yield of information by collating all available data. In case of doubt the publication reporting the longest follow‐up associated with our primary or secondary outcomes obtained priority.
Assessment of risk of bias in included studies
Two review authors (ST, RC) independently assessed the risk of bias of each included study. We resolved disagreements by consensus, or with consultation of a third party (MB).
We used Cochrane's 'Risk of bias' assessment tool (Higgins 2011a; Higgins 2011b), and evaluated the following criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias.
We judged the above 'Risk of bias' criteria as either 'low', 'high', or 'unclear' risk and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We present a risk of bias graph and a risk of bias summary. We assessed the impact of individual bias domains on study results at endpoint and study levels.
For blinding of participants and personnel (performance bias), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data) we intended to evaluate risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We considered the implications of missing outcome data from individual participants.
We assessed outcome reporting bias by integrating the results of the Appendix 5 'Matrix of study endpoints (publication and trial documents)' with those of the Appendix 6 'High risk of outcome reporting bias according to ORBIT classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting (reporting bias).
We defined the following outcomes as subjective outcomes.
Adverse events.
Health‐related quality of life.
We defined the following outcomes as objective outcomes.
All‐cause mortality.
Goitre recurrence and re‐intervention due to goitre recurrence.
Thyroid cancer incidence.
Socioeconomic effects.
Measures of treatment effect
We expressed dichotomous data as odds ratios (ORs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MD) with 95% CIs.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
If feasible, we obtained relevant missing data from trial authors. We evaluated important numerical data such as the number of screened, eligible, and randomised participants; as well as intention‐to‐treat (ITT), as‐treated and per‐protocol populations. We investigated attrition rates including drop‐outs, losses to follow‐up and withdrawals. We critically appraised issues of missing data and imputation methods (e.g. last observation carried forward).
Where standard deviations for outcomes were not reported and we did not receive information from study authors, we planned to impute these values by assuming the standard deviation of the missing outcome to be the average of the standard deviations from those studies where this information was reported. We planned to investigate the impact of imputation on pooled effect estimates by means of sensitivity analysis.
Assessment of heterogeneity
We did not pool data for meta‐analysis in the event of substantial clinical, methodological or statistical heterogeneity. We assessed heterogeneity by visual inspection of the forest plots and by calculating the Chi² test. In view of the low power of this test an α of 0.1 was considered to be statistically significant. We also examined heterogeneity using the I² statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). An I² statistic of 75% or more indicates a high degree of heterogeneity.
Had we found heterogeneity, we would have attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
We expected the following characteristics to introduce clinical heterogeneity.
Age.
Preoperative thyroid‐stimulating hormone (TSH) levels.
Preoperative thyroid volume (assessed by ultrasound).
Surgeon's experience.
Assessment of reporting biases
Because there were fewer than 10 studies investigating a particular outcome, we did not construct a funnel plot to assess small‐study effects (Sterne 2011).
Data synthesis
Even if there was good evidence for homogeneous effects across studies, we utilized a random‐effects model because such models are more appropriate in medical decision making contexts, especially when there are rare events (Ades 2005; DerSimonian 1986; Fleiss 1991; Shuster 2007). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects (Higgins 2009). In addition, we performed statistical analyses according to the statistical guidelines described in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Quality of evidence
We assessed the overall quality of the body of evidence supporting each outcome using the 'Grading of Recommendations Assessment, Development and Evaluation (GRADE)' criteria which takes into account issues related to internal (e.g. risk of bias, inconsistency, imprecision, and publication bias) and external validity (e.g. directness of results). Two review authors (ST, RC) independently rated the quality for each outcome. We reported a summary of the GRADE analysis in a 'Summary of findings' table. This table provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences, for each relevant comparison of alternative management strategies, numbers of participants, and studies addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). We reported results for the outcomes described in the Types of outcome measures section. If meta‐analysis was not possible, we reported results in a narrative 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses with investigation of interactions:
Treatment volume: low volume (defined as less than 50 thyroid operations per year) versus high volume (defined as 50 or more thyroid operations per year).
Residents in general surgery versus surgeons.
Participants aged less than 75 years versus 75 years or older.
Participants with a body mass index less than 35 kg/m² versus body mass index greater than 40 kg/m².
Thyroidectomy with tie and clamp versus Ultracision or Ligasure.
However, there were not enough data to carry out these analyses.
Sensitivity analysis
We intended to perform sensitivity analyses in order to explore the influence of the following factors on effect size.
Published studies.
Taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section.
Very long or large studies to establish the extent to which they dominate the results.
Studies using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry versus other), or country.
However, there were too few studies to do so.
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (RR, OR, etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of studies, see the 'Characteristics of included studies', 'Characteristics of excluded studies, and 'Characteristics of ongoing studies' sections.
Results of the search
Our comprehensive literature searches identified a total of 1430 records. There were 1173 records after de‐duplication. From these, 14 full‐text papers were identified for further examination. We excluded the other publications on the basis of titles or abstracts because inclusion criteria were not met or because the studies were not relevant to the review objectives. See Figure 1 for the amended PRISMA study flow diagram. After screening the full‐text of the selected publications, four studies (four publications) met the inclusion criteria. All studies were published in English.
1.
Study flow diagram.
Included studies
A detailed description of the characteristics of the included studies is presented elsewhere (see Characteristics of included studies and appendices). The following is a succinct overview:
Source of data
Using the literature search strategy 1430 publications were identified: 504 in MEDLINE (via OVID), 57 in PubMed (subsets not available on OVID), 106 in the Cochrane Library, 734 in EMBASE, 13 in the WHO ICTRP and 16 in ClinicalTrials.gov.
Comparisons
One study compared total to subtotal thyroidectomy (Pappalardo 1998). Two studies compared total or near‐total thyroidectomy to subtotal thyroidectomy (Giles 2004; Yang 2009), and one study compared total thyroidectomy to the Dunhill procedure or subtotal thyroidectomy (Barczynski 2010).
Overview of study populations
A total of 1305 participants were included in the four trials, 543 participants were randomised to total or near‐total thyroidectomy and 762 to subtotal thyroidectomy. A total of 534 (98%) participants finished the study in the total or near‐total thyroidectomy group compared to 741 (97%) participants in the subtotal thyroidectomy group. The individual sample size of randomised participants ranged from 141 (Pappalardo 1998) to 600 (Barczynski 2010)
Study design
Studies were randomised controlled trials. All four trials utilized a parallel group superiority design. One trial was multicentric (Yang 2009). In terms of blinding, none of the trials was double‐blinded for participants and personnel. No information about blinding of outcome assessors was available. Studies were performed between the years 1975 and 2006. The median duration of follow‐up ranged from 3 to 14.5 years (Pappalardo 1998). No trials were terminated early.
Settings
All studies were conducted in academic hospitals. No study was performed in an outpatient setting.
Participants
The participating population consisted of the following: all trials included participants from economically developed countries, which recruited participants from China, Italy, Poland, Turkey and the USA. Surgical procedures were most commonly performed in women. The mean (standard deviation) age of participants in the trials ranged from 46.5 (14.1) to 50.3 (12.5) years in participants undergoing total thyroidectomy compared to 45.7 (15.6) to 48.2 (15.4) years in participants undergoing subtotal thyroidectomy. Body mass index (BMI) was not reported. Criteria for entry into the individual studies are described in the Characteristics of included studies tables. Major exclusion criteria were Grave's disease, Plummer disease, thyroiditis, hyperfunctioning single adenoma or cancer.
Diagnosis
Multinodular non‐toxic goitre was diagnosed with ultrasonographic examinations and thyroid function tests. One trial did not report diagnostic procedures (Pappalardo 1998).
Interventions
Total thyroidectomy or near‐total thyroidectomy was compared to subtotal thyroidectomy or the Dunhill procedure or partial thyroidectomy.
Outcomes
Only Barczynski 2010 explicitly stated primary and secondary endpoints in the publication. The defined primary outcomes were recurrent goitre and the need for a redo surgery.
Excluded studies
Ten studies had to be excluded after careful evaluation of the full publication ‐ see Figure 1. The main reasons for exclusion were: not a randomised controlled trial, Grave's disease and wrong intervention or control group (for details see Characteristics of excluded studies).
Risk of bias in included studies
For details on risk of bias of included studies see Characteristics of included studies. For an overview of review authors' judgments about each risk of bias item for individual studies and across all studies see Figure 2 and Figure 3. We investigated performance bias, detection bias and attrition bias separately for objective and subjective outcome measures.
2.
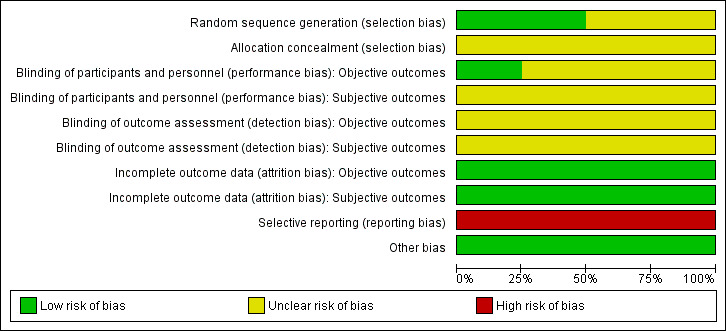
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
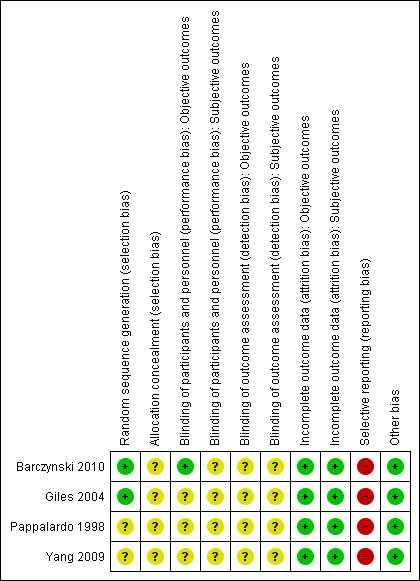
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies utilized adequate methods for random sequence generation and were rated as low risk of bias ( Barczynski 2010; Giles 2004). None of the studies described the method of allocation concealment.
Blinding
Only one study explicitly reported that blinding of the participants and personnel was undertaken (Barczynski 2010). However the methods used to achieve blinding were not described (Barczynski 2010). No study provided information about blinding of outcome assessors.
Incomplete outcome data
The numbers of participants discontinuing the trial were described in two publications (Barczynski 2010; Pappalardo 1998) which had losses to follow‐up of 30 and no participants, respectively. No publication mentioned the use of an intention‐to‐treat analysis. Two studies did not clearly report losses to follow‐up (Giles 2004; Yang 2009). Detailed descriptions of participants' withdrawals or reasons underpinning them were not provided in all of the included studies. No trial had attrition rates that would have a probable impact on the effect estimates.
Selective reporting
According to the Outcome Reporting Bias In Trials (ORBIT) classification all included studies had a high risk of bias for certain outcomes (for details see Appendix 6).
Other potential sources of bias
We did not detect any other additional risk of bias.
Effects of interventions
See: Table 1
Baseline characteristics
For details of baseline characteristics, see Appendix 3 and Appendix 4.
We investigated the effects of total and near‐total thyroidectomy versus subtotal thyroidectomy and evaluated the following outcomes:
Primary outcomes
All‐cause mortality
No postoperative hospital deaths or deaths within the first 30 days occurred after total or subtotal thyroidectomy (Analysis 1.1). However, all‐cause mortality was not measured over longer periods of follow‐up in any of the included trials indicating a high risk of reporting bias (moderate quality evidence, see Appendix 6).
1.1. Analysis.
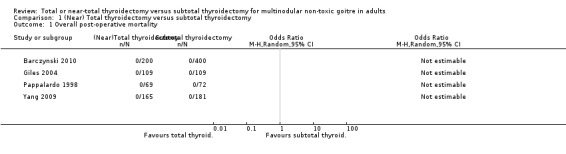
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 1 Overall post‐operative mortality.
Goitre recurrence and re‐intervention due to goitre recurrence.
Goitre recurrence was lower in the total thyroidectomy group (OR 0.05 (95% CI 0.01 to 0.21); P < 0.0001; 1057 participants; 3 trials; moderate quality evidence; Analysis 1.2).
1.2. Analysis.
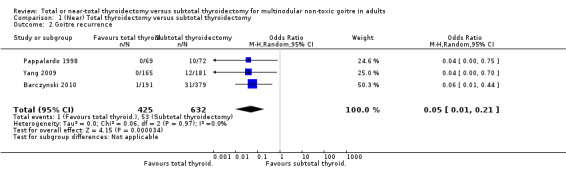
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 2 Goitre recurrence.
Re‐intervention due to goitre recurrence, which was only mentioned in one included trial, was lower in the total thyroidectomy group than in the subtotal thyroidectomy group (OR 0.66 (95% CI 0.07 to 6.38); P = 0.72; 570 participants; 1 trial; low quality evidence; Analysis 1.3). There was a high risk of outcome reporting bias for three of four trials (Giles 2004; Pappalardo 1998; Yang 2009).
1.3. Analysis.
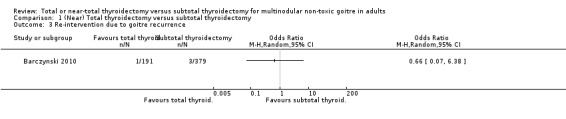
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 3 Re‐intervention due to goitre recurrence.
Adverse events
The total number of adverse events were lower in the subtotal thyroidectomy group (OR 1.85 (95% CI 1.17 to 2.92); P = 0.009; 1267 participants; 4 trials; Analysis 1.4).
1.4. Analysis.
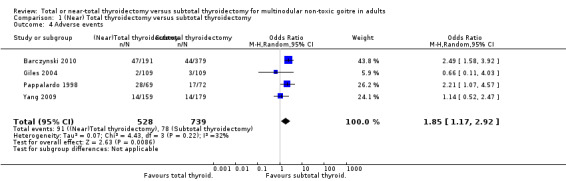
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 4 Adverse events.
Postoperative bleeding and red cell transfusions
These outcomes were not evaluated in any of the analysed trials.
Permanent recurrent laryngeal nerve (RLN) palsy
The incidence of permanent RLN palsy was lower in the subtotal thyroidectomy group (OR 1.28 (95% CI 0.38 to 4.36); P = 0.69; 1275 participants; 4 trials; low quality evidence; Analysis 1.5).
1.5. Analysis.
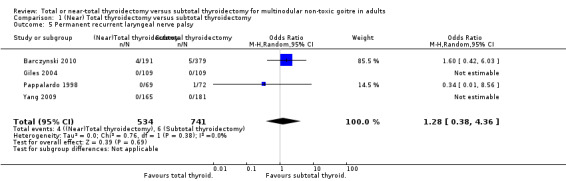
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 5 Permanent recurrent laryngeal nerve palsy.
Transient RLNpalsy
The incidence of transient RLN palsy was lower in the subtotal thyroidectomy group (OR 1.62 (95% CI 0.94 to 2.78); P = 0.08; 1275 participants; 4 trials; Analysis 1.6).
1.6. Analysis.
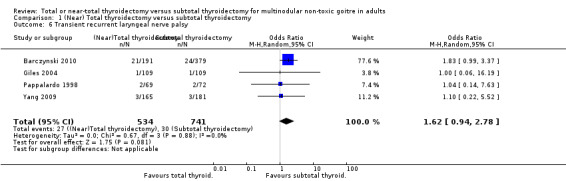
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 6 Transient recurrent laryngeal nerve palsy.
Permanent hypoparathyroidism
The incidence of permanent hypoparathyroidism was lower in the subtotal thyroidectomy group (OR 3.09 (95% CI 0.45 to 21.36); P = 0.25; 1272 participants; 4 trials; low quality evidence; Analysis 1.7).
1.7. Analysis.
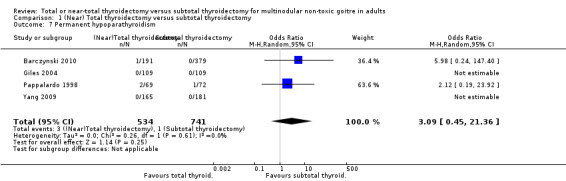
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 7 Permanent hypoparathyroidism.
Transient hypoparathyroidism
The incidence of transient hypoparathyroidism was lower in the subtotal thyroidectomy group (OR 2.47 (95% CI 1.57 to 3.88); P < 0.0001; 1275 participants; 4 trials; Analysis 1.8).
1.8. Analysis.
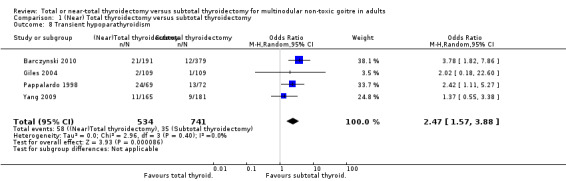
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 8 Transient hypoparathyroidism.
Secondary outcomes
Thyroid cancer incidence
The incidence of thyroid cancer was less in the subtotal thyroidectomy group (OR 1.32 (95% CI 0.81 to 2.15); P = 0.27; 1134 participants; 3 trials; low quality evidence; Analysis 1.9).
1.9. Analysis.
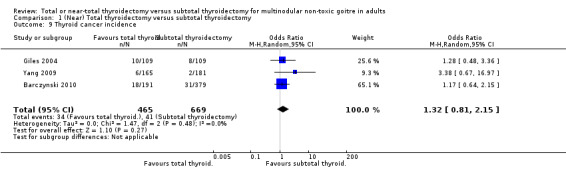
Comparison 1 (Near) Total thyroidectomy versus subtotal thyroidectomy, Outcome 9 Thyroid cancer incidence.
Health‐related quality of life
Health‐related quality of life was not measured in any trial.
Socioeconomic effects
Socioeconomic effects were not evaluated in any trial.
Subgroup analyses
We did not perform subgroups analyses because there were not enough studies to estimate effects in various subgroups.
Sensitivity analyses
We performed sensitivity analyses by examining each outcome using fixed‐effect and random‐effects models. There were no substantial differences in the results between models. Similarly, we examined how varying the effect size statistic using odds ratio or relative risk influenced study outcomes. We also found no substantial differences between effect size measures.
Assessment of reporting bias
We did not construct funnel plots due to limited number of studies per outcome.
Discussion
Summary of main results
A systematic review of the literature identified four RCTs comparing total or near‐total thyroidectomy to subtotal thyroidectomy for the surgical management of non‐toxic multinodular goitre (Barczynski 2010; Giles 2004; Pappalardo 1998; Yang 2009). The four trials showed some heterogeneity in surgical approaches. For the purpose of this review participants undergoing total and near‐total thyroidectomy were summarised in the intervention group and participants undergoing subtotal, partial or Dunhill thyroidectomy were classified in the comparator group.
Our key outcome measures health‐related quality of life and socioeconomic effects were not adequately addressed in the included trials. Also, postoperative bleeding and the need for transfusion were not reported. There were no deaths in the short‐term postoperative period up to 30 days after surgery. Goiter recurrence was higher for subtotal thyroidectomy. Thyroid cancer incidence and the most important adverse effects (permanent recurrent laryngeal nerve palsy and permanent hypoparathyroidism) did not differ substantially when comparing intervention with control groups. Two of the trials had only a short follow‐up period, which must be taken into consideration especially regarding goitre recurrence rates and potential surgical re‐interventions (Giles 2004; Yang 2009).
Overall completeness and applicability of evidence
The four included trials reported on most of our primary outcomes. Only one trial provided data on re‐interventions due to goitre recurrence (Barczynski 2010). As a caveat, there was some heterogeneity regarding the surgical techniques used by the different study authors: near‐total, subtotal, partial and Dunhill thyroidectomies can be subjectively interpreted and are operator dependent.
Quality of the evidence
In the postoperative period no deaths were observed. However, information on all‐cause mortality for longer follow‐up periods is missing which was a reason to downgrade the quality of the evidence to moderate in the Table 1. The data on goitre recurrence were of moderate quality due to the small number of events and an unknown risk of detection bias. The body of evidence supporting all other key outcomes including re‐intervention due to goitre recurrence, main adverse events and thyroid cancer incidence was low quality, mainly because of high risk of outcome reporting bias and serious imprecision (i.e. very sparse data).
Potential biases in the review process
Despite extensive search efforts we might have overlooked unpublished data especially regarding the Chinese literature. Information such as the experience of the surgeon performing the thyroidectomy would have been essential for the interpretation of the results but was not available.
Agreements and disagreements with other studies or reviews
Recently, a systematic review assessing the use of total thyroidectomy (including near‐total thyroidectomy) compared to subtotal thyroidectomy for the surgical management of multinodular goitre was reported (Cao 2014). The authors included a mixture of seven observational and controlled clinical studies, comprising 2192 participants. Total thyroidectomy was associated with lower nodule recurrence rates (OR 0.13 (95% CI 0.07 to 0.22); P < 0.001; 1927 participants; 6 trials) and higher transient hypoparathyroidism rates (OR 2.33 (95% CI 1.72 to 3.17); P < 0.0001; 2145 participants; 7 trials) which was comparable with our results. The OR for permanent RLN palsy was 0.81 (95% CI 0.24 to 2.74); P = 0.74; 2145 participants; 7 trials) and the OR for permanent hypothyroidism was 2.94 (95% CI 0.48 to 18.11); P = 0.24; 2145 participants; 7 trials). These results were compared to our own results.
Authors' conclusions
Implications for practice.
The body of evidence on (near) total thyroidectomy compared with subtotal thyroidectomy is limited. Moderate quality evidence indicates that there are no substantial differences between these surgical techniques on postoperative deaths up to 30 days after surgery. Goiter recurrence is reduced following (near) total thyroidectomy which has to be interpreted with caution due to unknown risk of detection bias and the small number of events overall. The other findings of our review could only partially address our objectives, mainly because information on re‐interventions due to goitre recurrence, adverse events and thyroid cancer incidence was limited.
Implications for research.
Longer follow‐up periods were only provided in two trials which limits the body of evidence especially for the incidence of goitre recurrence. None of the considered trials described data on all‐cause mortality over longer follow‐up periods, health‐related quality of life or socioeconomic effects of the two interventions. Subgroup analyses would have been vital for the completeness of this review but data were not reported: the treatment volume of the surgical centres, participants age, participants body mass index, the technique used to divide vessels and the surgeons level of experience may play an important role in postoperative outcomes. New trials reporting on these aspects are required.
Notes
We have based parts of the background, the methods section, appendices, additional tables and figures 1 to 3 of this review on a standard template established by the CMED Group.
Acknowledgements
The review was substantially edited by the Coordinating Editor of the CMED Group, Bernd Richter. Gudrun Paletta, Assistant managing Editor of the CMED Group, assisted us during the review and revision of manuscript. The TCMED Group's Trials Search Coordinator, Maria‐Inti Metzendorf developed and performed the search strategy.
Appendices
Appendix 1. Search strategies
| Cochrane Library (Wiley) |
| 1. [mh ^"Goiter, Nodular"] 2. [mh ^"Thyroid Nodule"] 3. ((goiter* or goitre*) near/7 (nodul* or multinodul* or multi nodul* or "nontoxic" or "non toxic")):ti,ab,kw 4. {or #1‐#3} 5. [mh ^"Thyroidectomy"] 6. thyroidectom*:ti,ab,kw 7. surg*:ti,ab,kw 8. {or #5‐#7} 9. #4 and #8 |
| MEDLINE (Ovid SP) |
| 1. Goiter, Nodular/ 2. Thyroid Nodule/ 3. ((goiter* or goitre*) adj6 (nodul* or multinodul* or multi nodul* or nontoxic or non toxic)).tw. 4. or/1‐3 5. Thyroidectomy/ 6. thyroidectom*.tw. 7. surg*.tw. 8. or/5‐7 9. 4 and 8 [10‐19: Cochrane Handbook 2008 RCT filter ‐ sensitivity maximizing version, without "drug therapy.fs."] 10. randomized controlled trial.pt. 11. controlled clinical trial.pt. 12. randomi?ed.ab. 13. placebo.ab. 14. randomly.ab. 15. trial.ab. 16. groups.ab. 17. or/10‐16 18. exp animals/ not humans/ 19. 17 not 18 20. 9 and 19 |
| PubMed Search Strategy |
| (only subsets not available on Ovid SP) #1 ((goiter*[tiab] OR goitre*[tiab] OR thyroid*[tiab]) AND (nodul*[tiab] OR multinodul*[tiab] OR multi nodul*[tiab] OR nontoxic[tiab] OR non toxic[tiab])) #2 thyroidectom*[tiab] OR surg*[tiab] #3 #1 AND #2 #4 random*[tiab] OR trial[tiab] OR groups[tiab] #5 #3 AND #4 #6 #5 NOT medline[sb] NOT pmcbook |
| EMBASE (Ovid SP) |
| 1. Nodular goiter/ 2. Thyroid nodule/ 3. ((goiter* or goitre*) adj6 (nodul* or multinodul* or multi nodul* or nontoxic or non toxic)).tw. 4. or/1‐3 5. Thyroidectomy/ 6. Subtotal thyroidectomy/ 7. thyroidectom*.tw. 8. surg*.tw. 9. or/5‐8 10. 4 and 9 [11: Wong et al. 2006 "sound treatment studies" filter – SDSSGS version] 11. random*.tw. or clinical trial*.mp. or exp treatment outcome/ 12. 10 and 11 13. limit 12 to embase |
| ICTRP Search Portal |
|
Standard search: goit* AND nodul* AND thyroidectom* OR goit* AND nodul* AND surg* OR goit* AND multinodul* AND thyroidectom* OR goit* AND multinodul* AND surg* OR goit* AND nontoxic AND thyroidectom* OR goit* AND nontoxic AND surg* OR goit* AND non toxic AND thyroidectom* OR goit* AND non toxic AND surg* |
| ClinicalTrials.gov |
|
Advanced Search: Conditions: (goiter OR goitre OR goiters OR goitres) AND (multinodular OR nodular OR multinodules OR nodules OR nontoxic OR “non toxic”) Interventions: thyroidectomy OR thyroidectomies OR surgery OR surgical OR surgeries Age Group: Adult + Senior |
Appendix 2. Description of interventions
| Intervention(s) | Comparator(s) | |
| Barczynski 2010 | Total thyroidectomy (extracapsular thyroidectomy) | C1: Dunhill procedure |
| C2: bilateral subtotal thyroidectomy | ||
| Giles 2004 | Total or near‐total thyroidectomy (extracapsular thyroidectomy) | Subtotal thyroidectomy (the total amount of remnant thyroid tissue was intended to be ≥ 5 g) |
| Pappalardo 1998 | Total thyroidectomy (extracapsular thyroidectomy) | Subtotal thyroidectomy ( leaving 3‐5 g on the less affected side) |
| Yang 2009 | Total or near‐total thyroidectomy (extracapsular‐ thyroidectomy or ‐small remnant of thyroid tissue < 1 mg) | Subtotal thyroidectomy |
| Dunhill procedure: unilateral extracapsular total thyroidectomy and contralateral subtotal thyroid lobe resection | ||
Appendix 3. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Duration of follow‐up | Participating population | Study period [year to year] | Country | Setting | Duration of MNG | |
| Barczynski 2010 | I: total thyroidectomy | 5 years | Bilateral nontoxic MNG | 2000 to 2003 | Poland | Department of endocrine surgery, tertiary referral centre | ‐ |
| C1: Dunhill procedure | |||||||
| C2: bilateral subtotal thyroidectomy | |||||||
| Giles 2004 | I: (near) total thyroidectomy | 1‐3 years | Multinodular euthyroid goitre with or without a dominant nodule | 2003 to 2005 | Turkey | Departments of general surgery and endocrinology, tertiary referral centre | ‐ |
| C: subtotal thyroidectomy | |||||||
| Pappalardo 1998 | I: total thyroidectomy | 10 years | Unilateral and bilateral multinodular euthyroid goitre | 1975 to 1985 | Italy | Surgical clinic & department of internal medicine, tertiary referral centre | ‐ |
| C: subtotal thyroidectomy | |||||||
| Yang 2009 | I: (near) total thyroidectomy | Median follow‐up 36 months | Bilateral nontoxic MNG | 2003 to 2006 | China | Departments of general surgery, clinical and molecular pharmacology and pathology, tertiary referral centre | ‐ |
| C: subtotal thyroidectomy | Median follow‐up 39 months | ||||||
| "‐" denotes not reported Dunhill procedure: unilateral extracapsular total thyroidectomy and contralateral subtotal thyroid lobe resection C: comparator; I: intervention; MNG: multinodular nontoxic goitre | |||||||
Appendix 4. Baseline characteristics (II)
| Intervention(s) and comparator(s) | Sex [female %] | Age [mean/range years (SD), or as reported] | Thyroid volume [mean ml (SD)] | Comedications/Cointerventions | Comorbidities | |
| Barczynski 2010 | I: total thyroidectomy | 91 | 46.5(14.1) | 76.6 (38.9) | All participants, irrespective of the individual group assignment, received postoperative levothyroxine treatment at a dose adjusted to the serum TSH concentration to keep it within the lowest two‐thirds of the reference range (0.3–2.5 mU/L) | ‐ |
| C1: Dunhill procedure | 89 | 47.2 (15.6) | 77.8 (39.5) | |||
| C2: bilateral subtotal thyroidectomy | 91 | 48.2 (15.4) | 78.9 (40.1) | |||
| Giles 2004 | I: (near) total thyroidectomy | 86 | 50.3 (12.5) | ‐ | Suppressive doses of thyroid hormone for some participants (e.g. patients with noninvasive microcarcinoma without radioiodine ablation) | ‐ |
| C: subtotal thyroidectomy | 84 | 45.7 (12.1) | ||||
| Pappalardo 1998 | I: total thyroidectomy | 71 | Mean 48 (25‐67) | ‐ | All patients were discharged taking L‐thyroxine 1.5–2.25 µg/kg body weight daily; dose of L‐thyroxine was subsequently adjusted depending on the concentration of circulating TSH; higher doses of L‐thyroxine were prescribed for patients who had subtotal resections (TSH < 0.8 mU/L) and lower doses to patients who had total thyroidectomy and to older and postmenopausal patients with the risk of iatrogenic osteoporosis | ‐ |
| C: subtotal thyroidectomy | 71 | Median 50 (27‐70) | ||||
| Yang 2009 | I: (near) total thyroidectomy | 78 | 22‐72 | ‐ | Thyroxine substitution was prescribed to those with insufficient thyroid function | ‐ |
| C: subtotal thyroidectomy | 77 | |||||
| "‐" denotes not reported Dunhill procedure: unilateral extracapsular total thyroidectomy and contralateral subtotal thyroid lobe resection C: comparator; I: intervention; SD: standard deviation; TSH: thyroid‐stimulating hormone | ||||||
Appendix 5. Matrix of study endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a |
Study results posted in trial register [Yes/No] |
Publications specified in trial register [Yes/No] |
Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c | |
| Barczynski 2010 |
Source: NCT00946894 Primary outcome measure(s): prevalence of recurrent goiter and need for redo surgery (time frame: at 12, 24, 36, 48 and 60 months after surgery) |
No | Yes |
Primary outcome measure(s): prevalence of recurrent goiter and need for redo surgery |
Primary outcome measure(s): prevalence of recurrent goiter and need for redo surgery |
|
Secondary outcome measure(s): postoperative morbidity rate (hypoparathyroidism and recurrent laryngeal nerve injury ‐ time frame: at 3, 6, 9, 12, 24, 36, 48 and 60 months after surgery) |
Secondary outcome measure(s): postoperative morbidity rate (hypoparathyroidism and recurrent laryngeal nerve injury) |
Secondary outcome measure(s): postoperative morbidity rate (hypoparathyroidism and recurrent laryngeal nerve injury |
|||
|
Other outcome measure(s): ‐ |
Other outcome measure(s): serum TSH; serum calcium level; operative time; haemorrhage |
Other outcome measure(s): ‐ |
|||
| Giles 2004 | N/T |
Primary outcome measure(s): ‐ |
Primary outcome measure(s): ‐ |
||
|
Secondary outcome measure(s): ‐ |
Secondary outcome measure(s): ‐ |
||||
|
Other outcome measure(s): postoperative complications (permanent/temporary hypoparathyroidism and permanent/temporary vocal cord paralysis); thyrotropin; serum thyroglobulin; incidence of thyroid cancer, difference in the rate of thyroid cancer requiring radioactive iodine ablation and completion thyroidectomy; operation for benign goitre |
Other outcome measure(s): complication rates (permanent/temporary hypoparathyroidism and permanent/temporary vocal cord paralysis); incidence of thyroid cancer requiring radioactive iodine ablation and completion thyroidectomy |
||||
| Pappalardo 1998 | N/T |
Primary outcome measure(s): ‐ |
Primary outcome measure(s): ‐ |
||
|
Secondary outcome measure(s): ‐ |
Secondary outcome measure(s): ‐ |
||||
|
Other outcome measure(s): recurrence rate after subtotal resection; serum calcium concentrations; measurement of parathyroid hormone; triiodothyronine (T3), thyroxine (T4) and TSH concentrations; postoperative stay in hospital; rate of injuries to the recurrent nerve; hypothyroidism; reoperations in the subtotal group the rate of injuries to the recurrent nerve, hypoparathyroidism, and recurrences were the only variables evaluated |
Other outcome measure(s): temporary or permanent palsy of the recurrent laryngeal nerve; temporary or permanent hypoparathyroidism; recurrence of the goitre; incidence of iatrogenic injuries after completion thyroidectomy |
||||
| Yang 2009 | N/T |
Primary outcome measure(s): ‐ |
Primary outcome measure(s): ‐ |
||
|
Secondary outcome measure(s): ‐ |
Secondary outcome measure(s): ‐ |
||||
|
Other outcome measure(s): serum concentrations of thyroid hormone parameters, such as free triiodothyronine (FT3), free thyroxine (FT4), and supersensitive thyroid‐stimulating hormone (TSH); vocal cord mobility, transient/permanent recurrent laryngeal nerve palsy; serum calcium/hypoparathyroidism; recurrence rate |
Other outcome measure(s): incidences of postoperative complications and recurrence rate; thyroid cancer; transient recurrent laryngeal nerve paralysis; injury to superior laryngeal nerve; transient hypocalcaemia symptoms; permanent hypoparathyroidism; recurrence |
||||
| ‐ denotes not reported aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers) bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary study) cOther outcome measures refer to all outcomes not specified as primary or secondary outcome measures EMA: European Medicines Agency; FDA: Food and Drug Administration (US); N/T: no trial document available; TSH: thyroid‐stimulating hormone | |||||
Appendix 6. High risk of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| Barczynski 2010 | All‐cause mortality | x | |||
| Giles 2004 | All‐cause mortality | x | |||
| Re‐intervention due to goitre recurrence | x | ||||
| Pappalardo 1998 | All‐cause mortality | x | |||
| Re‐intervention due to goitre recurrence | x | ||||
| Yang 2009 | All‐cause mortality | x | |||
| Re‐intervention due to goitre recurrence | x | ||||
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but only reports that result was not significant
(Classification 'A', table 2, Kirkham 2010)
bClear that outcome was measured and analysed; trial report states that outcome was analysed but no results reported
(Classification 'D', table 2, Kirkham 2010)
cClear that outcome was measured; clear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results
(Classification 'E', table 2, Kirkham 2010)
dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results
(Classification 'G', table 2, Kirkham 2010) N/A: not applicable; ORBIT: Outcome Reporting Bias In Trials | |||||
Appendix 7. Definition of endpoint measurement
| Goitre recurrence | Re‐intervention due to goitre recurrence | Transient recurrent laryngeal nerve palsy | Transient hypoparathyroidism | Health‐related quality of life | Severe/serious adverse events | |
| Barczynski 2010 | "Presence of hypoechoic or hyperechoic nodular pattern at least 5 mm in diameter, identification of perinodular hypoechogenic or hyperechogenic halo, and presence of an anechoic lesion with a reinforced posterior wall" | Completion thyroidectomy; presence of a 3 cm or larger nodule; result of FNA suggestive of an increased risk for malignancy; and presence of compressive symptoms | Vocal cord paresis for ≤ 12 months after the operation; indirect laryngoscopy by a throat specialist was mandatory before surgery and on day 2 postoperatively; in patients with RLN paresis, additional examinations were scheduled at 2 weeks and 1, 2, 4, 6, and 12 months after surgery, or until the vocal cord function recovered | Hypocalcaemia for ≤ 6 months after the operation; patients were monitored for postoperative biochemical hypocalcaemia at 12, 24, 48, and 72 h (during hospitalization and after discharge on morning outpatient visits), with hypocalcaemia being defined as a total serum calcium level less than 2.0 mmol/L in either asymptomatic or symptomatic patients (perioral or fingertip paraesthesia or numbness, tetany, a newly positive Chvostek sign) | N/I | Vocal cord paresis for more than 12 months after the operation was regarded as permanent palsy. Persistent hypocalcaemia for more than 6 months after the operation was regarded as permanent hypoparathyroidism |
| Giles 2004 | N/I | N/I | Not explained | Not explained | N/I | Not explained |
| Pappalardo 1998 | Goitre recurrence was diagnosed "when physical examination or follow‐up ultrasound scan showed nodular involvement or an enlargement of the residual thyroid remnant" | Completion thyroidectomy | "A palsy of the vocal cord diagnosed by an otolaryngologist that lasted less than six months postoperatively" | "A fall in the serum calcium concentration (less than 2.1 mmol/L) corrected according to the serum albumin concentration" | N/I | "Permanent injury to the recurrent nerve was considered as palsy of the vocal cord diagnosed by an otolaryngologist that lasted for more than six months postoperatively" "Permanent hypoparathyroidism as the need for oral vitamin D and calcium six months after the operation" |
| Yang 2009 | "The recurrence was defined as de novo nodules more than 3 mm in remnant thyroid" | N/I | "Those who displayed weak mobility for less than two months after surgery" | "If patients required an oral or venous calcium supplement for less than 2 months" | N/I | "Those who displayed weak mobility within 2 months after surgery were considered to have permanent RLN injury" "Permanent hypoparathyroidism if the patients required an oral or venous calcium supplement and vitamin D for more than 2 months with undetectable plasma parathyroid hormone levels" |
| FNA: fine needle aspiration: N/I: not investigated; RLN: recurrent laryngeal nerve | ||||||
Appendix 8. Adverse events (I)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Deaths [N] | Deaths [%] | All adverse events [N] | All adverse events [%] | Severe/serious adverse events [N] | Severe/serious adverse events [%] | |
| Barczynski 2010 | I: total thyroidectomy | 191 | 0 | 0 | 47 | 24.6 | 4 | 2.1 |
| C1: Dunhill procedure | 189 | 0 | 0 | 28 | 14.8 | 4 | 2.1 | |
| C2: bilateral subtotal thyroidectomy | 190 | 0 | 0 | 16 | 8.4 | 5 | 2.6 | |
| all: | 570 | 0 | 0 | 91 | 16 | 13 | 2.3 | |
| Giles 2004 | I: (near) total thyroidectomy | 109 | 0 | 0 | 28 | 27 | 0 | 0 |
| C: subtotal thyroidectomy | 119 | 0 | 0 | 12 | 11 | 0 | 0 | |
| all: | 218 | 0 | 0 | 40 | 18.3 | 0 | 0 | |
| Pappalardo 1998 | I: total thyroidectomy | 69 | 0 | 0 | 28 | 40.6 | 2 | 2.9 |
| C: subtotal thyroidectomy | 72 | 0 | 0 | 17 | 23.6 | 2 | 2.7 | |
| all: | 141 | 0 | 0 | 45 | 31.9 | 4 | 2.8 | |
| Yang 2009 | I: (near) total thyroidectomy | 165 | 0 | 0 | 14 | 8.8 | ‐ | ‐ |
| C: subtotal thyroidectomy | 181 | 0 | 0 | 12 | 6.7 | 2 | 1.1 | |
| all: | 346 | 0 | 0 | 26 | 7.5 | N/A | N/A | |
| "‐" denotes not reported Dunhill procedure: unilateral extracapsular total thyroidectomy and contralateral subtotal thyroid lobe resection C: comparator; I: intervention; N/A: not applicable | ||||||||
Appendix 9. Adverse events (II)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Participants discontinuing study due to adverse events [N] | Participants discontinuing study due to adverse events [%] | Rehospitalisation [N] | Rehospitalisation [%] | Out‐patient treatment [N] | Out‐patient treatment [%] | |
| Barczynski 2010 | I: total thyroidectomy | 191 | 0 | 0 | 0 | 0 | 0 | 0 |
| C1: Dunhill procedure | 189 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C2: bilateral subtotal thyroidectomy | 190 | 0 | 0 | 0 | 0 | 0 | 0 | |
| all: | 570 | 0 | 0 | 0 | 0 | 0 | 0 | |
|
Giles 2004 |
I: (near) total thyroidectomy | 109 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: subtotal thyroidectomy | 109 | 0 | 0 | 4 | 3.7 | 0 | 0 | |
| all: | 218 | 0 | 0 | 4 | 3.7 | 0 | 0 | |
| Pappalardo 1998 | I: total thyroidectomy | 69 | 0 | 0 | 0 | 0 | 0 | |
| C: subtotal thyroidectomy | 72 | 0 | 0 | 0 | 0 | 0 | 0 | |
| all: | 141 | 0 | 0 | 0 | 0 | 0 | 0 | |
|
Yang 2009 |
I: (near) total thyroidectomy | 165 | 6 | 3.6 | 0 | 0 | 0 | 0 |
| C: subtotal thyroidectomy | 181 | 2 | 1.1 | 2 | 1.1 | 0 | 0 | |
| all: | 346 | 8 | 2.3 | 2 | 0.6 | 0 | 0 | |
| "‐" denotes not reported Dunhill procedure: unilateral extracapsular total thyroidectomy and contralateral subtotal thyroid lobe resection C: comparator; I: intervention | ||||||||
Appendix 10. Adverse events (III)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Permanent recurrent laryngeal nerve palsy [N] (%) | Transient recurrent laryngeal nerve palsy [N] (%) | Permanent hypoparathyroidism [N] (%) | Transient hypoparathyroidism [N] (%) | |
| Barczynski 2010 | I: total thyroidectomy | 191 | 4 (2.1) | 21 (11) | 1 (0.5) | 21 (11) |
| C1: Dunhill procedure | 189 | 3 (1.6) | 16 (8.5) | 0 (0) | 8 (4.2) | |
| C2: bilateral subtotal thyroidectomy | 190 | 2 (1.1) | 8 (4.2) | 0 (0) | 4 (2.1) | |
| all: | 570 | 9 (1.6) | 45 (7.9) | 1 (0.2) | 33 (5.8) | |
|
Giles 2004 |
I: (near) total thyroidectomy | 109 | 0 (0) | 1 (0.9) | 0 (0) | 2 (1.8) |
| C: subtotal thyroidectomy | 109 | 0 (0) | 1 (0.9) | 0 (0) | 1 (0.9) | |
| all: | 218 | 0 (0) | 2 (0.9) | 0 (0) | 3 (1.4) | |
| Pappalardo 1998 | I: total thyroidectomy | 69 | 0 (0) | 2 (2.9) | 2 (2.9) | 24 (34.8) |
| C: subtotal thyroidectomy | 72 | 1 (1.4) | 2 (2.8) | 1 (1.4) | 13 (18.1) | |
| all: | 141 | 1(1.4) | 4 (2.8) | 3 (2.1) | 37 (26.2) | |
| Yang 2009 | I: (near) total thyroidectomy | 165 | 0 (0) | 3 (1.9) | 0 (0) | 11 (6.9) |
| C: subtotal thyroidectomy | 181 | 0 (0) | 3 (1.7) | 0 (0) | 9 (5) | |
| all: | 346 | 0 (0) | 6 (1.8) | 0 (0) | 20 (5.9) | |
| "‐" denotes not reported Dunhill procedure: unilateral extracapsular total thyroidectomy and contralateral subtotal thyroid lobe resection C: comparator; I: intervention | ||||||
Appendix 11. Survey of authors providing information on included trials
| Study author contacted | Study author replied | Study author asked for additional information | Study author provided data | |
| Barczynski 2010 | Yes | Yes | Yes | Yes |
| Giles 2004 | Yes | No | N/A | N/A |
| Pappalardo 1998 | Yes | Yes | Yes | No |
| Yang 2009 | Yes | No | N/A | N/A |
| N/A: not applicable | ||||
Data and analyses
Comparison 1. (Near) Total thyroidectomy versus subtotal thyroidectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall post‐operative mortality | 4 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Goitre recurrence | 3 | 1057 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.21] |
| 3 Re‐intervention due to goitre recurrence | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 4 | 1267 | Odds Ratio (M‐H, Random, 95% CI) | 1.85 [1.17, 2.92] |
| 5 Permanent recurrent laryngeal nerve palsy | 4 | 1275 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.38, 4.36] |
| 6 Transient recurrent laryngeal nerve palsy | 4 | 1275 | Odds Ratio (M‐H, Random, 95% CI) | 1.62 [0.94, 2.78] |
| 7 Permanent hypoparathyroidism | 4 | 1275 | Odds Ratio (M‐H, Random, 95% CI) | 3.09 [0.45, 21.36] |
| 8 Transient hypoparathyroidism | 4 | 1275 | Odds Ratio (M‐H, Random, 95% CI) | 2.47 [1.57, 3.88] |
| 9 Thyroid cancer incidence | 3 | 1134 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.81, 2.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barczynski 2010.
| Methods | Parallel RCT, superiority design | |
| Participants |
Inclusion criteria: bilateral non‐toxic MNG with the posterior aspects of both thyroid lobes appearing normal on ultrasound of the neck Exclusion criteria: MNG involving the posterior aspect/s of thyroid lobe/s, suspicion of thyroid cancer, previous thyroid surgery, thyroiditis, subclinical or clinically overt hypothyroidism or hyperthyroidism, pregnancy or lactation, age < 18 years or > 65 years, American Society of Anesthesiology (ASA) grade 4, and a participant’s inability to comply with the follow‐up protocol Diagnostic criteria: high‐resolution doppler ultrasound of the neck. Fine needle aspiration of the thyroid. Serum free T3, free T4 and thyroid‐stimulating hormone (TSH); thyroid peroxidase antibodies levels |
|
| Interventions |
Number of study centres: 1 Treatment before study: not reported |
|
| Outcomes |
Outcomes reported in abstract of publication: Primary outcome: prevalence of recurrent goitre and need for redo surgery Secondary outcome: postoperative morbidity rate (hypoparathyroidism and recurrent laryngeal nerve injury) |
|
| Study details |
Run‐in period: none Study terminated before regular end (for benefit / because of adverse events): no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "The aim of the present study was to evaluate results of various thyroid resection modes (Total Thyroidectomy versus Dunhill Operation versus Bilateral Subtotal Thyroidectomy), with special emphasis put on the recurrence rate and morbidity rate, in a 5‐year follow‐up" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Randomization was performed by computer‐generated permuted block sequencing" Comment: unclear block size |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication: "Patients allocated by opening sealed envelopes in the operating theatre" Comment: questionable whether envelopes were opaque and sequentially numbered |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote from publication: "All participants were blinded to treatment assignment for the duration of the study" Comment: no information about personnel blinding provided but we judge that the objective outcomes of this review are not likely to be influenced by lack of blinding of participants or personnel |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: unclear whether adverse events were measured by study personnel (potential risk of bias) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: no information |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no information |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: during follow‐up a total of 30 participants were lost to follow‐up, the reasons are not provided in the publication but the number of participants lost to follow‐up is balanced among treatment arms; it is unlikely that the low attrition rate affected outcome measures |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: during follow‐up a total of 30 participants were lost to follow‐up, the reasons are not provided in the publication but the number of participants lost to follow‐up is balanced among treatment arms; it is unlikely that the low attrition rate affected outcome measures |
| Selective reporting (reporting bias) | High risk | Comment: high risk of bias for outcome measure all‐cause mortality according to the ORBIT classification (see Appendix 6) |
| Other bias | Low risk | Comment: none detected |
Giles 2004.
| Methods | Parallel RCT, superiority design | |
| Participants |
Inclusion criteria: nontoxic MNG Exclusion criteria: no history of hyperthyroidism, radiation exposure, familial thyroid cancer, preoperative or perioperative suspicion of cancer Diagnostic criteria: ultrasonography examination (multinodular hyperplasia of the thyroid gland) |
|
| Interventions |
Number of study centres: 1 Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: transient recurrent laryngeal nerve paralysis, transient hypocalcaemia symptoms, permanent recurrent laryngeal nerve paralysis, permanent hypoparathyroidism, papillary cancer, goitre recurrence, reoperation or radioactive iodine ablation for goitre recurrence | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit / because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim of study | Quote from publication: "To investigate the impact of total thyroidectomy on the rate of completion thyroidectomy for incidentally found thyroid cancer in euthyroid multinodular goiter" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were selected according to the number on the random table for 2 different extensions of surgical procedures" |
| Allocation concealment (selection bias) | Unclear risk | Comment: information about allocation concealment not provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: information about blinding of personnel and participants not provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: information about blinding of personnel and participants not provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: information about blinding of outcome assessment not provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: information about blinding of outcome assessment not provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: no drop‐outs |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: high risk of bias for outcome measures all‐cause mortality and re‐intervention due to goitre recurrence according to the ORBIT classification (see Appendix 6) |
| Other bias | Low risk | Comment: none detected |
Pappalardo 1998.
| Methods | Parallel RCT, superiority design | |
| Participants |
Inclusion criteria: unilateral and bilateral multinodular euthyroid goitres Exclusion criteria: graves' disease, Plummer disease, thyroiditis, hyperfunctioning single adenoma or cancer Diagnostic criteria: not reported |
|
| Interventions |
Number of study centres: 1 Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: temporary or permanent palsy of the recurrent laryngeal nerve, temporary or permanent hypoparathyroidism, recurrence of the goitre, and the incidence of iatrogenic injuries after completion thyroidectomy | |
| Study details |
Run‐in period: none Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the outcome after total and subtotal thyroidectomy for the treatment of single and multinodular goitres in two comparable groups of patients" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The patients were randomly assigned to have total thyroidectomy or subtotal thyroidectomy" Comment: the procedure to perform randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: information about allocation concealment not provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: information about blinding of participants and personnel not provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: information about blinding of participants and personnel not provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: information about blinding of outcome assessment not provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: information about blinding of participants and personnel not provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: no missing outcome data, all participants completed the follow‐up |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no missing outcome data, all participants completed the follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: high risk of bias for outcome measures all‐cause mortality and re‐intervention due to goitre recurrence according to the ORBIT classification (see Appendix 6) |
| Other bias | Low risk | Comment: none detected |
Yang 2009.
| Methods | Parallel RCT, superiority design | |
| Participants |
Inclusion criteria: bilateral multinodular goitre Exclusion criteria: no history of thyroidectomy Diagnostic criteria: ultrasonography examination (a bilateral multinodular goitre) |
|
| Interventions |
Number of study centres: 1 Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: transient recurrent laryngeal nerve paralysis, transient hypocalcaemia symptoms, permanent hypoparathyroidism, goitre recurrence | |
| Study details | Run‐in period: none Study terminated before regular end (for benefit / because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal | |
| Stated aim of study | "To evaluate the feasibility and safety of total and near‐total bilateral thyroidectomy for the treatment of bilateral multinodular goiter." | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "... patients with a diagnosis of bilateral multinodular goiter were randomly divided into two groups .." Comment: the procedure to perform randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: information about allocation concealment not provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: information about blinding of participants and personnel not provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: information about blinding of participants and personnel not provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: information about blinding of outcome assessment not provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: information about blinding of outcome assessment not provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: no drop‐outs |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: high risk of bias for outcome measures all‐cause mortality and re‐intervention due to goitre recurrence according to the ORBIT classification (see Appendix 6) |
| Other bias | Low risk | Comment: none detected |
ASA: American Society of Anesthesiology; MNG: multinodular nontoxic goitre; ORBIT: Outcome Reporting Bias In Trials; RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barczynski 2012 | In this RCT bilateral subtotal thyroidectomy is compared with total thyroidectomy for the surgical management of Grave's disease |
| Chi 2005 | In this RCT bilateral subtotal thyroidectomy is compared with hemithyroidectomy plus subtotal resection (Dunhill procedure) for the surgical management of Grave's disease |
| Erbil 2006 | In this RCT total thyroidectomy is compared with near‐total thyroidectomy for the surgical management of multinodular goitre |
| Rayes 2013 | In this RCT bilateral subtotal thyroidectomy is compared with hemithyroidectomy plus subtotal resection (Dunhill procedure) for the surgical management of bilateral multinodular goitre |
| Rouwen 2011 | A systematic review on surgical approach for treating multinodular goitre: total thyroidectomy/near‐ total thyroidectomy with subtotal thyroidectomy/bilateral subtotal thyroidectomy/Dunhill procedure |
| Sancho 2012 | In this RCT hemithyroidectomy is compared with hemithyroidectomy plus subtotal resection (Dunhill procedure) for the surgical management of asymmetrical multinodular goitre |
| Tezelman 2009 | A retrospective cohort study comparing subtotal versus near‐total or total thyroidectomy in the treatment of patients with multinodular goitre |
| Unalp 2009 | In this RCT total thyroidectomy is compared with near‐total thyroidectomy for the surgical management of multinodular goitre |
| Vaiman 2008 | A retrospective cohort study comparing subtotal versus near‐total thyroidectomy in the treatment of patients with multinodular goitre |
| Witte 2000 | In this RCT total thyroidectomy is compared with subtotal resection for the surgical management of Grave's disease |
RCT: randomised controlled trial
Differences between protocol and review
None
Contributions of authors
Roberto Cirocchi (RC) protocol draft, search strategy development, trial selection, acquiring trial reports, data extraction, data analysis, data interpretation, write discussion/author conclusion and future update draft.
Stefano Trastulli (STr): protocol draft, quality trials analysis and risk of bias.
Justus Randolph (JR): statistical consultation, data analysis, data interpretation and future update of draft.
Salvatore Guarino (SG): protocol draft and write the discussion.
Giorgio Di Rocco (GDR): trial selection, acquiring trial reports, data extraction, data analysis.
Alberto Arezzo (AR): trial selection, acquiring trial reports, data extraction, data analysis, data interpretation, write discussion/author conclusion and future update draft.
Vito D'Andrea (VDA): revised draft.
Alberto Santoro (AS): revised draft.
Marcin Barczyñski (MB): protocol draft, write the discussion/author conclusion, revised draft and future update draft.
Nicola Avenia (NA): protocol draft, acquiring trial reports. write the discussion/author conclusion, revised draft and future update draft
Declarations of interest
RC: none known.
STr: none known.
JR: none known.
SG: none known.
GDR: none known.
AR: none known.
VDA: none known.
AS: none known.
ARe: none known.
MB: was the principal investigator of Barczynski 2010. MB did not extract data from his own study. The Coordinating Editor of the CMED Group extracted these data, and checked the interpretation against the study registration details (NCT00946894).
NA: none known.
New
References
References to studies included in this review
Barczynski 2010 {published data only}
- Barczyński M, Konturek A, Hubalewska‐Dydejczyk A, Gołkowski F, Cichoń S, Nowak W. Five‐year follow‐up of a randomized clinical trial of total thyroidectomy versus Dunhill operation versus bilateral subtotal thyroidectomy for multinodular nontoxic goiter. World Journal of Surgery 2010;34:1203–13. [DOI] [PubMed] [Google Scholar]
Giles 2004 {published data only}
- Giles Y, Boztepe H, Terzioglu T, Tezelman S. The advantage of total thyroidectomy to avoid reoperation for incidental thyroid cancer in multinodular goiter. Archives of Surgery 2004;139:179‐82. [DOI] [PubMed] [Google Scholar]
Pappalardo 1998 {published data only}
- Pappalardo G, Guadalaxara A, Frattaroli FM, Illomei G, Falaschi P. Total compared with subtotal thyroidectomy in benign nodular disease: personal series and review of published reports. European Journal of Surgery 1998;164:501‐6. [DOI] [PubMed] [Google Scholar]
Yang 2009 {published data only}
- Yang W, Shao T, Ding J, Jin X, Li Q, Chu PG, et al. The feasibility of total or near‐total bilateral thyroidectomy for the treatment of bilateral multinodular goiter. Journal of Investigative Surgery 2009;22:195‐200. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barczynski 2012 {published data only}
- Barczyński M, Konturek A, Hubalewska‐Dydejczyk A, Gołkowski F, Nowak W. Randomized clinical trial of bilateral subtotal thyroidectomy versus total thyroidectomy for Graves' disease with a 5‐year follow‐up. British Journal of Surgery 2012;99:515‐22. [DOI] [PubMed] [Google Scholar]
Chi 2005 {published data only}
- Chi SY, Hsei KC, Sheen‐Chen SM, Chou FF. A prospective randomized comparison of bilateral subtotal thyroidectomy versus unilateral total and contralateral subtotal thyroidectomy for graves' disease. World Journal of Surgery 2005;29:160‐3. [DOI] [PubMed] [Google Scholar]
Erbil 2006 {published data only}
- Erbil Y, Barbaros U, Salmaslioğlu A, Yanik BT, Bozbora A, Ozarmağan S. The advantage of near‐total thyroidectomy to avoid postoperative hypoparathyroidism in benign multinodular goiter. Langenbeck's Archives of Surgery 2006;391:567‐73. [DOI] [PubMed] [Google Scholar]
Rayes 2013 {published data only}
- Rayes N, Steinmüller T, Schröder S, Klötzler A, Bertram H, Denecke T, et al. Bilateral subtotal thyroidectomy versus hemithyroidectomy plus subtotal resection (Dunhill procedure) for benign goiter: long‐term results of a prospective randomized study. World Journal of Surgery 2013;37:84‐90. [DOI] [PubMed] [Google Scholar]
Rouwen 2011 {published data only}
- Rouwen KW, Fest J. The best surgical approach for treating multinodular goiter. A systematic review. Erasmus Journal of Medicine 2011;2:24‐6. [Google Scholar]
Sancho 2012 {published data only}
- Sancho JJ, Prieto R, Dueñas JP, Ribera C, Ripollés J, Larrad A, et al. A randomized trial of hemithyroidectomy versus Dunhill for the surgical management of asymmetrical multinodular goiter. Annals of Surgery 2012;256:846‐51. [DOI] [PubMed] [Google Scholar]
Tezelman 2009 {published data only}
- Tezelman S, Borucu I, Senyurek Giles Y, Tunca F, Terzioglu T. The change in surgical practice from subtotal to near‐total or total thyroidectomy in the treatment of patients with benign multinodular goiter. World Journal of Surgery 2009;33:400‐5. [DOI] [PubMed] [Google Scholar]
Unalp 2009 {published data only}
- Unalp HR, Erbil Y, Akguner T, Kamer E, Derici H, Issever H. Does near total thyroidectomy offer advantage over total thyroidectomy in terms of postoperative hypocalcemia?. International Journal of Surgery 2009;9:120‐5. [DOI] [PubMed] [Google Scholar]
Vaiman 2008 {published data only}
- Vaiman M, Nagibin A, Hagag P, Buyankin A, Olevson J, Shlamkovich N. Subtotal and near total versus total thyroidectomy for the management of multinodular goiter. World Journal of Surgery 2008;32:1546‐51. [DOI] [PubMed] [Google Scholar]
Witte 2000 {published data only}
- Witte J, Goretzki PE, Dotzenrath C, Simon D, Felis P, Neubauer M, et al. Surgery for Graves' disease: total versus subtotal thyroidectomy‐results of a prospective randomized trial. World Journal of Surgery 2000;24:1303‐11. [DOI] [PubMed] [Google Scholar]
Additional references
Ades 2005
- Ades AE, Lu G, Higgins JP. The interpretation of random‐effects meta‐analysis in decision models. Medical Decision Making 2005;25:646‐54. [DOI] [PubMed] [Google Scholar]
Agarwal 2008
- Agarwal G, Aggarwal V. Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence‐based review. World Journal of Surgery 2008;32:1313‐24. [DOI] [PubMed] [Google Scholar]
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2014
- Cao H, Han J, Zhang D, Yu Z, Wang M, Jiao Z. Meta‐analysis of total thyroidectomy for multinodular goiter. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:625‐31. [DOI] [PubMed] [Google Scholar]
Cirocchi 2010
- Cirocchi R, D'Ajello F, Trastulli S, Santoro A, Rocco G, Vendettuoli D, et al. Meta‐analysis of thyroidectomy with ultrasonic dissector versus conventional clamp and tie. World Journal of Surgical Oncology 2010;8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Donatini 2012
- Donatini G, Carnaille B, Dionigi G. Increased detection of non‐recurrent inferior laryngeal nerve (NRLN) during thyroid surgery using systematic intraoperative neuromonitoring (IONM). World Journal of Surgery 2013;37:91‐3. [DOI] [PubMed] [Google Scholar]
Dralle 2011
- Dralle H, Lorenz K, Machens A. State of the art: surgery for endemic goiter ‐ a plea for individualizing the extent of resection instead of heading for routine total thyroidectomy. Langenbecks Archives of Surgery 2011;396:1137‐43. [DOI] [PubMed] [Google Scholar]
Fleiss 1991
- Fleiss JL, Gross AJ. Meta‐analysis in epidemiology, with special reference to studies of association between exposure to environmental tobacco smoke and lung cancer: a critique. Journal of Clinical Epidemiology 1991;44:127‐39. [DOI] [PubMed] [Google Scholar]
Foster 1978
- Foster RS Jr. Morbidity and mortality after thyroidectomy. Surgery, Gynecology & Obstetrics 1978;146:423‐9. [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172:137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011c
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Lefevre 2007
- Lefevre JH, Tresallet C, Leenhardt L, Jublanc C, Chigot JP, Menegaux F. Reoperative surgery for thyroid disease. Langenbecks Archives of Surgery 2007;392:685‐91. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozbas 2005
- Ozbas S, Kocak S, Aydintug S, Cakmak A, Demirkiran MA, Wishart GC. Comparison of the complications of subtotal, near total and total thyroidectomy in the surgical management of multinodular goitre. Endocrine Journal 2005;52:199‐205. [DOI] [PubMed] [Google Scholar]
Perzik 1976
- Perzik S. The place of total thyroidectomy in the management of 909 patients with thyroid disease. American Journal of Surgery 1976;132:480‐3. [DOI] [PubMed] [Google Scholar]
Ríos 2005
- Ríos A, Rodríguez JM, Balsalobre MD, Torregrosa NM, Tebar FJ, Parrilla P. Results of surgery for toxic multinodular goiter. Surgery Today 2005;35:901‐6. [DOI] [PubMed] [Google Scholar]
Shuster 2007
- Shuster JJ, Jones LS, Salmon DA. Fixed vs random effects meta‐analysis in rare event studies: the rosiglitazone link with myocardial infarction and cardiac death. Statistics in Medicine 2007;26:4375‐85. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]


