Abstract
Background
To evaluate the potential intracranial efficacy of immunotherapy among patients with breast cancer brain metastases (BrM), we analyzed the immunohistochemical expression of programmed death-ligand 1 (PD-L1), a predictive biomarker of response to immunotherapy.
Methods
In this single-center retrospective cohort study, consecutive patients with breast cancer BrM (immunotherapy naïve) who underwent surgery for BrM at Sunnybrook Health Sciences Center between July 1999 and June 2013 were identified. PD-L1 expression by immunohistochemistry (IHC) was assessed on BrM samples in triplicate; PD-L1 positive status was defined as PD-L1 expression ≥1% on tumor-infiltrating cells as a percentage of tumor area using the Ventana SP142 antibody. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2) status was determined using 2018 ASCO/CAP guidelines.
Results
The median patient age at the time of BrM diagnosis was 52 (range 32–85). PD-L1 expression using the SP42 antibody was identified in 9 out of 59 (15.3%) breast cancer BrM. The frequency of PD-L1 positive BrM by subtype is as follows: TNBC (n = 3/12, 25.0%), HER2+/HR- (n = 3/14, 21.4%), HR+/HER2- (n = 2/18, 11.1%), and HER2+/HR+ (n = 1/14, 7.1%). 24-month brain-specific progression-free survival was 66.7% (95% CI 37.9%–100%) among patients with PD-L1 positive BrM versus 42% (95% CI 26.6%–67.3%) among those with PD-L1 negative BrM (log-rank P-value .142).
Conclusions
One in 7 patients in our cohort had PD-L1 positive BrM; this proportion was highest (25%) among those with TNBC. Intracranial efficacy of immunotherapy warrants further study, particularly among patients with treatment-naïve metastatic TNBC, for whom extracranial efficacy has already been established.
Keywords: breast cancer, brain metastases, PD-L1
Key points.
1- One in 7 patients with metastatic breast cancer in our cohort had PD-L1 positive brain metastases; this proportion was highest (25%) among those with triple negative breast cancer.
2- Efficacy of immunotherapy for breast cancer BrM warrants evaluation in clinical trials.
Importance of the Study.
Immunotherapy has potential intracranial efficacy among patients with metastatic breast cancer, though patients with breast cancer brain metastases were largely underrepresented in relevant clinical trials. Furthermore, the expression of PD-L1 (a predictive biomarker of response to immunotherapy) in breast cancer brain metastases is poorly understood. In this single-center retrospective cohort study of 59 breast cancer patients who underwent surgery for intracranial metastatic disease between July 1999 and June 2013, PD-L1 was expressed in 9 out 59 (15.3%) brain metastases; immunohistochemical PD-L1 expression using the Ventana SP142 antibody was defined as ≥1% expression on tumor-infiltrating cells as a percentage of tumor area, in accordance with the IMpassion130 and Impassion131 clinical trials. This proportion was highest (25%) among those with triple-negative breast cancer (TNBC) brain metastases. Intracranial efficacy of immunotherapy warrants further study, particularly among patients with treatment-naïve metastatic TNBC, for whom extracranial efficacy has already been established.
Brain metastases (BrM) are a major cause of morbidity and mortality in women with breast cancer. Women with metastatic HER2-positive (HER2+) and triple-negative breast cancer (TNBC) have a particularly high propensity to develop intracranial disease compared to women with hormone receptor-positive, HER2-negative (HR+/HER2−) breast cancer.1–3
Immunotherapy has the potential for intracranial efficacy among patients with breast cancer BrM since intracranial response has been observed in patients with metastatic melanoma and non-small cell lung cancer.4,5 Unfortunately, patients with breast cancer BrM were underrepresented in relevant clinical trials evaluating the efficacy of immunotherapy. Furthermore, the expression of the programmed death-ligand 1 (PD-L1), a predictive biomarker of response to immunotherapy in the brain is poorly understood. To evaluate the possible role of immunotherapy among patients with breast cancer BrM, we assessed the expression of PD-L1 in a retrospective cohort of breast cancer BrM at our institution.
Methods
Study Sample
A retrospective cohort study of consecutive patients with breast cancer BrM who underwent surgery for BrM at Sunnybrook Health Sciences Center between July 1999 and June 2013 were identified through the Anatomic Pathology departmental database. Clinical variables and features of the BrM as well as the primary tumor were retrieved from the patient’s electronic records. We received research ethics board approval from the Sunnybrook Research Institute to conduct this study.
Immunohistochemistry
A tissue microarray using 1um cores was obtained. PD-L1 expression by immunohistochemistry (IHC) was assessed on BrM samples in triplicate; PD-L1 positive status was defined as PD-L1 expression ≥1% on tumor-infiltrating cells as a percentage of tumor area using Ventana SP142 antibody. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2) status was determined using 2018 ASCO/CAP guidelines. Cases were considered to have concordant PD-L1 expression when both tumor samples (primary and BrM) were positive or negative, according to a cutoff value of 1%.
Statistical Analysis
Data were analyzed using the SPSS software. We used χ 2 tests to explore the relationship between 2 groups for categorical data. Brain-specific progression-free survival (bsPFS) was assessed as the duration of months from the time of BrM surgery to the time of BrM progression or death. Overall Survival (OS) was assessed as the duration of months from the time of BrM surgery to the time of death due to any cause. BsPFS and OS were estimated by using the Kaplan–Meier method and compared across groups using the log-rank test. For all analyses, statistical significance was defined as a P-value of <.05.
Results
This study included 59 breast cancer patients who underwent surgical resection of BrM (Table 1). The median patient age at the time of BrM diagnosis was 52 (range 32–85). Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor (HER2) status was available for BrM cases in 58 out of 59 patients as follows: TNBC (n = 12, 20.3%), HER2+/ HR- (n = 14, 23.7%), HER2+/HR+ (n = 14, 23.7%), HR+/HER2- (n = 18, 30.5%). The median time to development of BrM from first diagnosis of breast cancer was 36 months (range 0– 410 months). The majority of patients (n = 37, 62.7%) had a solitary BrM. The median size of BrM was 2.9 cm (range 0.3 cm to 6.2 cm) with the most common location being the cerebellum, followed by frontal lobe (n = 25, 42.4% and n = 15, 25.4%, respectively). Most patients (n = 51, 86.4%) had neurologic symptoms when they presented with metastatic disease. The most common sites of extracranial metastases included bone (n = 22, 37.3%), lung (n = 19, 32.2%), liver (n = 13, 22%), lymph nodes (n = 11, 18.6%), and chest wall (n = 2, 3.4%). After surgical excision of BrM, 88.1% of patients received adjuvant stereotactic radiosurgery (SRS) or whole brain radiotherapy (WBRT). 39% of patients received at least 1 line of systemic therapy for metastatic disease prior to the development of BrM; administered systemic therapies are outlined in Table 1. None of these patients received immunotherapy-based treatments.
Table 1.
Patient and Tumor Characteristics
| Characteristic | N = 59 |
|---|---|
| Age at BrM diagnosis | |
| Median | 52 |
| Range | 32–85 |
| BrM subtype | |
| Triple negative | 12 (20.3%) |
| HER2+/HR- | 14 (23.7%) |
| HER2+/HR+ | 14 (23.7%) |
| HR+/HER2- | 18 (30.5%) |
| Unknown | 1 (1.7%) |
| Number of BrM | |
| One | 37 (62.7%) |
| More than 1 | 21 (35.6%) |
| Unknown | 1 (1.7%) |
| BrM size (cm) | |
| Median | 2.9 |
| Range | 0.3–6.2 |
| BrM location | |
| Frontal | 15 (25.4%) |
| Parietal | 13 (22%) |
| Temporal | 3 (5.1%) |
| Cerebellar | 25 (42.4%) |
| Occipital | 2 (3.4%) |
| Unknown | 1 (1.7%) |
| BrM grade | |
| 1 | 16 (27.1%) |
| 2 | 22 (37.3%) |
| 3 | 15 (25.4%) |
| Unknown | 6 (10.2%) |
| BrM symptoms | |
| Yes | 51 (86.4%) |
| No | 7 (11.9%) |
| Unknown | 1 (1.7%) |
| Other sites of metastases | |
| Lung | 19 (32.2%) |
| Liver | 13 (22%) |
| Lymph node | 11 (18.6%) |
| Bone | 22 (37.3%) |
| Chest wall | 2 (3.4%) |
| Other | 4 (6.8%) |
| Radiotherapy for BrM | |
| Yes | 52 (88.1%) |
| No | 3 (5.1%) |
| Unknown | 4 (6.8%) |
| Systemic therapy for metastatic disease prior to BrM | |
| Chemotherapy | 9 (15.3%) |
| Herceptin-based treatment | 10 (16.9%) |
| Endocrine therapy | 4 (6.8%) |
| Unknown | 36 (61%) |
BrM, brain metastases.
PD-L1 status was available for all 59 cases. PD-L1 expression using the SP142 antibody was identified in 9 out of 59 (15.3%) breast cancer BrM irrespective of subtype. As shown in Table 2, patients with TNBC BrM were most likely to have PD-L1+ BrM (n = 3/12, 25%), followed by those with HER2+/HR− (n = 3/14, 21.4%), HR+/HER2− (n = 2/18, 11.1%) and HER2+/HR+ (n = 1/14, 7.1%) disease.
Table 2.
PD-L1 expression in Brain Metastases as Stratified by Clinicopathological Features
| Characteristic | Patients | Number (%) of PD-L1+ cases | P |
|---|---|---|---|
| Overall | 59 | 9 (15.3) | |
| BrM subtype | .53 | ||
| Triple negative | 12 | 3 (25) | |
| HER2+/HR− | 14 | 3 (21.4) | |
| HR+/HER2− | 18 | 2 (11.1) | |
| HER2+/HR+ | 14 | 1 (7.1) | |
| Unknown | 1 | 0 (0) | |
| Age at BrM (years) | .25 | ||
| <52 | 28 | 6 (21.4) | |
| ≥52 | 29 | 3 (10.3) | |
| Unknown | 2 | 0 (0) | |
| BrM location | .82 | ||
| Frontal | 15 | 2 (13.3) | |
| Parietal | 13 | 3 (23.1) | |
| Temporal | 3 | 0 (0) | |
| Cerebellar | 25 | 4 (16) | |
| Occipital | 2 | 0 (0) | |
| Unknown | 1 | 0 (0) | |
| BrM size (cm) | .77 | ||
| < 2.9 | 24 | 4 (16.7) | |
| ≥ 2.9 | 29 | 4 (13.8) | |
| Unknown | 6 | 1 (16.7) | |
| BrM grade | .86 | ||
| 1 | 16 | 3 (18.8) | |
| 2 | 22 | 3 (13.6) | |
| 3 | 15 | 3 (20) | |
| Unknown | 6 | 0 (0) |
Unknown cases were excluded from association studies.
BrM, brain metastases.
Table 2 shows expression of PD-L1 in BrM as stratified by BrM subtype, age, location, size, and grade; however, sample size was too small to adequately power statistical analyses for associations between PD-L1 status and aforementioned clinicopathological features including tumor subtype, age, location, size, and grade.
ER, PR, and HER2 status were available for corresponding primary breast cancer cases in 33 out of 59 patients; tissue from extracranial metastatic sites was not available (Table 3). The distribution of primary breast cancer subtypes included the following frequencies: TNBC (n = 6, 10.2%), HER2+/HR− (n = 11, 18.6%), HER2+/HR+ (n = 4, 6.8%), and HR+/HER2− (n = 12, 20.3%) (Table 3). There was a discordance in the expression of ER and/or PR and/or HER2 in the primary breast cancer versus BrM in 7 of 33 cases (21.2%) for which matched local tissue subtype was known; ER and/or PR status was discordant in 6 of 33 (18.2%) cases and HER2 status was discordant in 1 of 33 (3%) cases. PD-L1 status was only available for 10 matched primary breast cancers. Discordant PD-L1 expression was observed in 3 of 10 cases; 2 cases demonstrated positive expression in the primary breast cancer and negative expression in the corresponding BrM, while 1 case demonstrated negative expression in the primary breast cancer and positive expression in the corresponding BrM.
Table 3.
Patient and Tumor Characteristics in Corresponding Primary Breast Cancer Cases
| Characteristic | N = 59 |
|---|---|
| Primary breast cancer subtype | |
| Triple negative | 6 (10.2%) |
| HER2+/HR− | 11(18.6%) |
| HER2+/HR+ | 4 (6.8%) |
| HR+/HER2− | 12 (20.3%) |
| Unknown | 26 (44.1%) |
| Primary breast cancer stage | |
| 1 | 12 (20.3%) |
| 2 | 14 (23.7%) |
| 3 | 8 (13.6%) |
| 4 | 1 (1.7%) |
| Unknown | 24 (40.7%) |
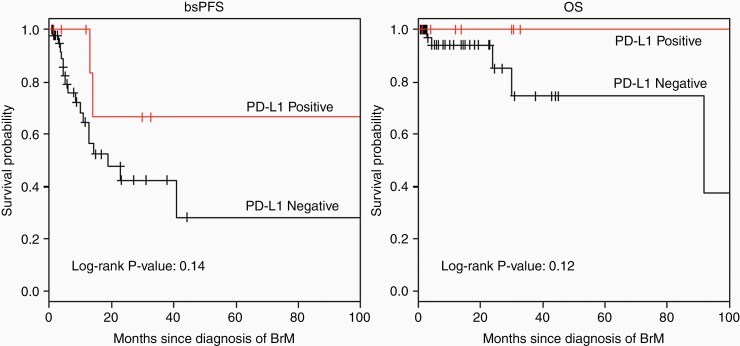
Brain-specific progression-free survival (bsPFS) and overall survival (OS), measured from time of BrM diagnosis, were assessed in 57 of the 59 patients. The median follow-up for BrM events was 16.1 months. Median bsPFS was 22.7 months and median OS could not be assessed due to a small number of reported deaths in this cohort. At 24 months, bsPFS was 47.5% (95% CI 32.9%–68.7%). When stratified by PD-L1 status, 24-month bsPFS was 66.7% (95% CI 37.9%–100%) among patients with PD-L1 positive BrM versus 42% (95% CI 26.6%–67.3%) among those with PD-L1 negative BrM (log-rank P-value .142), as shown in Figure 1.
Figure 1.
Kaplan Meier survival curve (A) bsPFS and (B) OS in BrM stratified by PD-L1 status. bsPFS, brain-specific progression-free survival; OS, overall survival.
Discussion
In this single-center, retrospective cohort study, we analyzed PD-L1 expression using the SP142 antibody in a cohort of 59 surgically resected breast cancer BrM. One in 7 patients had PD-L1 positive BrM; this proportion was highest (25%) among those with TNBC who are most likely to benefit from immunotherapy.
In large, randomized trials for patients with treatment-naive metastatic TNBC,6,7 approximately 40%–45% of patients had PD-L1 positive tumors as assessed by the SP142 antibody.6,8–10 Concordance in PD-L1 status between the primary and metastatic site was not reported in these trials; furthermore, PD-L1 status in the few patients with BrM was unknown. In our study, the expression of PD-L1 in BrM cannot be directly compared to expression levels in the aforementioned trials, given that patients in our cohort were heavily pretreated for metastatic disease. However, the proportion of patients with PD-L1 positive triple negative BrM (25%) was lower than that reported in other studies using the same SP142 antibody in other metastatic sites, such as the lymph nodes (51.1% PD-L1 positive), lung (68.8% PD-L1 positive), and soft tissues (65.2% PD-L1 positive); conversely, expression of PD-L1 in BrM was slightly higher than in the liver (17.4% PD-L1 positive) and bone (16.7% PD-L1 positive).11 Concordance in PD-L1 expression between primary breast tumors and BrM in TNBC specifically was not possible in this study due to small sample sizes, but PD-L1 expression has previously been reported to be higher in the primary tumor compared to metastatic sites (63.7% vs. 42.2%, P < .0001).11
In a recent study of PD-L1 expression in 223 BrM from different solid tumors including 111 paired primary tumors using the 22C3 antibody (with 1% membranous staining defining positive PD-L1 status), PD-L1 expression was positive in 23.6% of BrM and 29.0% of primary tumors with 75.5% concordance.12 In the subset of patients with breast cancer BrM (n = 31), PD-L1 expression was positive in 13% of cases. Similar expression of PD-L1 in surgically resected BrM from patients with metastatic non-small cell lung cancer (n = 7/32, 21.9% PD-L1 positive)13 and metastatic TNBC (n = 6/16, 37.5% PD-L1 positive)14 using the SP142 antibody was also demonstrated. In another study of 84 breast cancer BrM cases, PD-L1 was positive in 53% of cases, but the clinical relevance of this data is limited given the use of a nonstandard PD-L1 antibody.15 It is also noted that Sabatier et al. analyzed mRNA expression of PD-L1 in 45 breast cancer cell lines and 5454 breast cancers but in contrast to our study, a validated predictive biomarker of response to immunotherapy was not assessed.16
Our study is limited by the single-center, retrospective design, and relatively small sample size. Among 59 breast cancer BrM cases, only 10 had matched primary tissue available for analysis and tissue from other metastatic sites was not available. In addition, we used only 1 of 2 validated measures of PD-L1 expression in breast cancer. Subsequent assessment of PD-L1 expression using the combined positive score (CPS) via 22C3 antibody testing, which predicts benefit of first-line pembrolizumab among patients with metastatic TNBC, would be of value. Finally, although we explored the prognostic significance of PD-L1 expression among patients with breast cancer BrM, our power was limited by small sample size and low event rates. Hence, our results are hypothesis-generating and only generalizable to patients requiring surgery for intracranial metastatic disease. Although not statistically significant, there was a trend for longer bsPFS and OS among patients with PD-L1 positive BrM compared to those with PD-L1 negative intracranial disease. Hence, further exploration of the immune microenvironment in PD-L1 positive versus PD-L1 negative BrM is warranted.
To the best of our knowledge, this is the largest study of breast cancer BrM evaluating PD-L1 expression using a validated predictive biomarker of response to immunotherapy. The fact that 25% of triple-negative BrM were PD-L1 positive suggests that a proportion of patients with metastatic TNBC have the potential to derive intracranial benefit from immunotherapy. This calls for immunotherapy-based trials in this patient population. Beyond checkpoint inhibitors, expanding the characterization of the tumor microenvironment may lead to the identification of novel therapeutic targets for breast cancer BrM. For example, Griguolo et al. used 2 multiplex immunofluorescence panels to identify M2 microglia/macrophage polarization as a potential target in HER2-negative BrM and PD-1/PD-L1 interaction in HER2+ BrM.17 In addition, a comparison of the immune landscape between primary tumors and BrM would be of value to better understand brain-specific drivers of immunosuppression.18 Unfortunately, noninvasive methods to determine biomarker expression in BrM are not currently available and a priori prediction of intracranial response to immunotherapy may therefore remain a challenge. Hence, enrollment of patients with treated breast cancer BrM (as opposed to those with active/untreated disease) into immunotherapy clinical trials may be favored.
Contributor Information
Rania Chehade, Department of Medical Oncology, Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Maleeha A Qazi, Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Marguerite Ennis, Applied Statistician, Markham; ON, Canada.
Arjun Sahgal, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada; Department of Radiation Oncology, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Sunit Das, Division of Neurosurgery, St. Michael’s Hospital, Toronto, Ontario, Canada.
Sharon Nofech-Mozes, Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada.
Katarzyna J Jerzak, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada; Division of Medical Oncology, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Funding
This study was not funded.
Author Contributions
Generation and Drafting of Manuscript: R.C., M. A.Q., and K.J.J. Conception and Design of Experiment: K.J.J. Data Collection: R.C., M. A.Q. Data Analysis and Interpretation: R.C., M. A.Q., M.E., A.S., S.D., S. NM., and K.J.J. Manuscript Revision and Final Approval: R.C., M. A.Q., M.E., A.S., S.D., S. NM., and K.J.J.
Competing Interests
KJJ has been a speaker/advisor board/consultant for Amgen, Apo Biologix, AstraZeneca, Eli Lilly, Esai, Exact Sciences, Gilead Sciences, Knight Therapeutics, Novartis, Pfizer, Purdue Pharma, Roche, Seagen and Merck. KJJ received research funding unrelated to this study from: AstraZeneca and Eli Lilly. SD is an advisor/consultant for AbbVie, Xpan Medical, Synaptive, Omniscient. SD is also a board member in Subcortical Surgery Group and provided past educational seminars: AbbVie, Subcortical Surgery Group, Congress of Neurological Surgeons, American Association of Neurological Surgeons, Society for NeuroOncology. SD received research grants from Alkerme, Medicenna and received travel accommodations/expenses from Subcortical Surgery Group, Congress of Neurological Surgeons, American Association of Neurological Surgeons, Society for NeuroOncology, Integra. AS is an advisor/consultant for AbbVie, Merck, Roche, Varian (Medical Advisory Group), Elekta (Gamma Knife Icon), BrainLAB, and VieCure (Medical Advisory Board). AS is a board member in International Stereotactic Radiosurgery Society (ISRS) and is a co-chair in AO Spine Knowledge Forum Tumor. AS also provided educational seminars in Elekta AB, Accuray Inc., Varian (CNS Teaching Faculty), BrainLAB, Medtronic Kyphon; received research grant from Elekta AB; received travel accommodations/expenses: Elekta, Varian, BrainLAB. AS also belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia.
References
- 1. Aversa C, Rossi V, Geuna E, et al. . Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23(5):623–628. [DOI] [PubMed] [Google Scholar]
- 2. Kuksis M, Gao Y, Tran W, et al. . The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 2021;23(6):894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komorowski AS, Warner E, MacKay HJ, et al. . Incidence of brain metastases in nonmetastatic and metastatic breast cancer: is there a role for screening? Clin Breast Cancer. 2020;20(1):e54–e64. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg SB, Gettinger SN, Mahajan A, et al. . Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortinovis D, Abbate M, Bidoli P, Capici S, Canova S. Targeted therapies and immunotherapy in non-small-cell lung cancer. Ecancermedicalscience. 2016;10:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmid P, Adams S, Rugo HS, et al. . Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. [DOI] [PubMed] [Google Scholar]
- 7. Cortes J, Cescon DW, Rugo HS, et al. . Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. [DOI] [PubMed] [Google Scholar]
- 8. Schmid P, Rugo HS, Adams S, et al. . Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. [DOI] [PubMed] [Google Scholar]
- 9. Helwick C. IMpassion131: No Benefit for Atezolizumab Plus Paclitaxel in Triple-Negative Breast Cancer. 2020; https://ascopost.com/issues/october-10-2020/impassion131-no-benefit-for-atezolizumab-plus-paclitaxel-in-triple-negative-breast-cancer/, Accessed; December 14, 2020. [Google Scholar]
- 10. Huang RSP, Haberberger J, Severson E, et al. . A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. Mod Pathol. 2021;34(2):252–263. [DOI] [PubMed] [Google Scholar]
- 11. Rozenblit M, Huang R, Danziger N, et al. . Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J ImmunoTher Cancer. 2020;8(2):e001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camy F, Karpathiou G, Dumollard JM, et al. . Brain metastasis PD-L1 and CD8 expression is dependent on primary tumor type and its PD-L1 and CD8 status. J ImmunoTher Cancer. 2020;8(2):e000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takamori S, Toyokawa G, Okamoto I, et al. . Clinical significance of PD-L1 expression in brain metastases from non-small cell lung cancer. Anticancer Res. 2018;38(1):553–557. [DOI] [PubMed] [Google Scholar]
- 14. Huang RSP, Haberberger J, McGregor K, et al. . Clinicopathologic and genomic landscape of breast carcinoma brain metastases. Oncologist. 2021;26(10):835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duchnowska R, Pęksa R, Radecka B, et al. . Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res. 2016;18(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sabatier R, Finetti P, Mamessier E, et al. . Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6(7):5449–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griguolo G, Tosi A, Dieci MV, et al. . A comprehensive profiling of the immune microenvironment of breast cancer brain metastases. Neuro Oncol. 2022:noac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noh MG, Kim SS, Kim YJ, et al. . Evolution of the tumor microenvironment toward immune-suppressive seclusion during brain metastasis of breast cancer: implications for targeted therapy. Cancers (Basel). 2021;13(19):4895. [DOI] [PMC free article] [PubMed] [Google Scholar]