Abstract
Background:
Many risk factors have been described for dislocation following total hip arthroplasty (THA), yet a patient-specific risk assessment tool remains elusive. The purpose of this study was to develop a high-dimensional, patient-specific risk-stratification nomogram that allows dynamic risk modification based on operative decisions.
Methods:
In this study, 29,349 THAs, including 21,978 primary and 7371 revision cases, performed between 1998 and 2018 were evaluated. During a mean 6-year follow-up, 1521 THAs were followed by a dislocation. Patients were characterized, through individual-chart review, according to non-modifiable factors (demographics, indication for THA, spine disease, prior spine surgery, and neurologic disease) and modifiable operative decisions (operative approach, femoral head diameter, and type of acetabular liner [standard, elevated, constrained, or dual-mobility]). Multivariable regression models and nomograms were developed with dislocation as a binary outcome at 1 year and 5 years postoperatively.
Results:
Dislocation risk, based on patient-specific comorbidities and operative decisions, was wide-ranging—from 0.3% to 13% at 1 year and from 0.4% to 19% at 5 years after primary THA, and from 2% to 32% at 1 year and from 3% to 42% at 5 years after revision THA. In the primary-THA group, the direct anterior approach (hazard ratio [HR] = 0.27) and lateral approach (HR = 0.58) decreased the dislocation risk compared with the posterior approach. After adjusting for the approach in that group, the combination of a ≥36-mm-diameter femoral head and an elevated liner yielded the largest decrease in dislocation risk (HR = 0.28), followed by dual-mobility constructs (HR = 0.48). In the patients who underwent revision THA, the adjusted risk of dislocation was most markedly decreased by the use of a dual-mobility construct (HR = 0.40), followed by a ≥36-mm femoral head and an elevated liner (HR = 0.88). The adjusted risk of dislocation after revision THA was decreased by acetabular revision (HR = 0.58), irrespective of whether other components were revised.
Conclusions:
Our patient-specific dislocation risk calculator, which was strengthened by our use of a robust multivariable model that accounted for comorbidities associated with instability, demonstrated wide-ranging patient-specific risks based on comorbidity profiles. The resultant nomograms can be used as a screening tool to identify patients at high risk for dislocation following THA and to individualize operative decisions for evidence-based risk mitigation.
Level of Evidence:
Prognostic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Instability remains one of the most common and challenging problems following total hip arthroplasty (THA)1,2. Indeed, recent data from the American Joint Replacement Registry indicate that it is one of the most frequent indications for revision THA, and institutional registry data demonstrate that it accounts for the largest percentage of repeat revision THAs3,4. Myriad non-modifiable patient characteristics have been identified as risk factors for dislocation, including age, sex, body mass index (BMI), THA indication, spine disease, spine surgery, and neurologic disease5–8. Accordingly, surgeons have focused substantial attention on high-risk groups with attempts to attenuate instability risk with their operative approach as well as on implant choices such as larger-diameter femoral heads and various acetabular liner configurations including elevated, face-changing, constrained, and more recently dual-mobility constructs7,9,10.
Although substantial literature has evaluated the impact of individual characteristics, achieving patient-specific risk prediction has remained elusive due to small data sets or, more commonly, cohorts with insufficient characterization1,7. An ideal risk prediction model would evaluate patients based on a wide spectrum of covariates while remaining parsimonious by including only causal factors with strong associations. Perhaps more importantly, a risk prediction clinical tool should be adaptive to decisions within the control of the surgeon.
The purpose of this study was to develop a high-dimensional, patient-specific risk prediction model for dislocation following THA that allows for dynamic risk modification based on operative decisions. The goal of the model was to create a usable clinical nomogram that could calculate individual risk for any patient. The nomograms presented in this article could further serve as the basis for a user-friendly electronic calculator of patient-specific dislocation risk.
Materials and Methods
Following institutional review board approval, 29,349 THAs, including 21,978 primary and 7371 revision cases, performed at a single institution from 1998 to 2018 were evaluated with a mean of 6 years of follow-up. Patients were characterized using a prospectively-collected total joint registry with augmentation to determine specific “instability comorbidities” of interest (i.e., neurologic disease, spine disease, and spine surgery) using diagnosis/procedure codes and natural language processing (NLP)-assisted chart review of the medical record with individual manual review of all diagnoses. Ultimately, this enabled determination of patient profiles with non-modifiable preoperative variables and modifiable intraoperative variables. Included variables are detailed in Table I, and a complete list of codes and NLP terms is provided in the Appendix. All patients were assumed to be at risk for dislocation after THA and were followed until dislocation, the last follow-up, or death. Cox regression analysis determined hazard ratios (HRs) for variables associated with differential dislocation risk.
TABLE I.
Characterization of Preoperative Non-Modifiable Patient Variables and Intraoperative Modifiable Variables
| Variables | Characterization |
|---|---|
| Preoperative non-modifiable | |
| Demographics | Age, sex, body mass index |
| Surgery type | Primary or revision |
| Indication | |
| Primary THA | Osteoarthritis, osteonecrosis, inflammatory, posttraumatic/nonunion, other |
| Revision THA | Dislocation, aseptic loosening, wear/osteolysis, periprosthetic fracture/nonunion, periprosthetic joint infection |
| Neurologic disease | Parkinson disease, dementia, alcoholism, fibromyalgia, related conditions |
| Spine disease | Special subcategory designation of diseases causing spine stiffening or biological fusion as “major” disease (e.g., ankylosing spondylitis). See Appendix for all included conditions. |
| Spine surgery | Special subcategory designation of fusion procedures as “major” surgery. See Appendix for all included procedures. |
| Intraoperative modifiable | |
| Approach | Posterior, lateral, direct anterior, trochanteric osteotomy |
| Femoral head diameter | ≥36 mm or ≤32 mm |
| Acetabular liner | Standard (i.e., flat or neutral), elevated (i.e., face-changing, lipped, or lateralized), constrained, dual-mobility |
| Revised component(s) | Acetabular component, femoral component, both components |
A patient-specific dislocation risk calculator was created with nomograms from multivariable modeling such that the individual risk for a patient with any combination of non-modifiable factors could be calculated and would determine a differential risk based on modifiable operative decisions. These nomograms were built separately for primary and revision THA cohorts for both 1-year and 5-year time points. Discrimination was assessed using the concordance statistic (c-statistic) for the Cox models11. Calibration was assessed by comparing observed and expected events in deciles of predicted risk using goodness-of-fit tests, which included standardized incidence ratios (SIRs)12. All HRs reported in the Results section are significant, with confidence intervals and p values reported in the tables.
Patient Characteristics and Operative Management
Baseline characteristics for the overall cohort as well as the primary and revision subgroups are summarized in Table II. Overall, the mean patient age was 65 years (range, 18 to 100 years), the mean BMI was 30 kg/m2 (range, 13 to 82 kg/m2), and 52% of the patients were female. The mean follow-up was 5.5 years (range, 2 to 21 years). A history of minor spine disease, major spine disease, minor spine surgery, and major spine surgery was identified for 37%, 7%, 4%, and 1% of the patients, respectively. Neurologic disease was present in 19% of the patients (Table II).
TABLE II.
Patient Characteristics and Operative Management*
| Total (N = 29,349) | Primary THA (N = 21,978) | Revision THA (N = 7371) | |
|---|---|---|---|
| Age (yr) | |||
| Mean (SD) | 64.7 (13.5) | 64.2 (13.5) | 66.2 (13.3) |
| Median | 66.0 | 66.0 | 68.0 |
| Range | 18.0, 100.0 | 18.0, 100.0 | 18.0, 98.0 |
| Sex (n [%]) | |||
| Female | 15,331 (52.2%) | 11,423 (52.0%) | 3908 (53.0%) |
| Male | 14,018 (47.8%) | 10,555 (48.0%) | 3463 (47.0%) |
| BMI (kg/m2) | |||
| Mean (SD) | 29.8 (6.4) | 29.9 (6.4) | 29.4 (6.5) |
| Median | 28.9 | 29.0 | 28.4 |
| Range | 13.0, 81.5 | 13.0, 79.3 | 14.5, 81.5 |
| BMI category (n [%]) | |||
| <18.0 kg/m2 | 283 (1.0%) | 193 (0.9%) | 90 (1.2%) |
| 18.0-24.9 kg/m2 | 6394 (21.8%) | 4580 (20.8%) | 1814 (24.6%) |
| 25.0-29.9 kg/m2 | 10,162 (34.6%) | 7644 (34.8%) | 2518 (34.2%) |
| 30.0-34.9 kg/m2 | 7267 (24.8%) | 5522 (25.1%) | 1745 (23.7%) |
| 35.0-39.9 kg/m2 | 3211 (10.9%) | 2485 (11.3%) | 726 (9.9%) |
| ≥40.0 kg/m2 | 2021 (6.9%) | 1551 (7.1%) | 470 (6.4%) |
| Follow-up (yr) | |||
| Mean (SD) | 5.5 (5.0) | 5.5 (5.0) | 5.6 (5.1) |
| Median | 4.6 | 4.6 | 4.7 |
| Range | 0.0, 21.0 | 0.0, 21.0 | 0.0, 20.8 |
| Spine disease (n [%]) | |||
| None | 16,253 (55.4%) | 11,598 (52.8%) | 4655 (63.2%) |
| Minor | 10,958 (37.3%) | 8726 (39.7%) | 2232 (30.3%) |
| Major† | 2138 (7.3%) | 1654 (7.5%) | 484 (6.6%) |
| Prior spine surgery (n [%]) | |||
| None | 27,841 (94.9%) | 20,770 (94.5%) | 7071 (95.9%) |
| Minor | 1172 (4.0%) | 949 (4.3%) | 223 (3.0%) |
| Major‡ | 336 (1.1%) | 259 (1.2%) | 77 (1.0%) |
| Neurologic disease (n [%]) | |||
| No | 23,833 (81.2%) | 17,654 (80.3%) | 6179 (83.8%) |
| Yes | 5516 (18.8%) | 4324 (19.7%) | 1192 (16.2%) |
| Indication for revision THA (n [%]) | |||
| Dislocation | 920 (12.5%) | 0 (%) | 920 (12.5%) |
| Loosening | 3711 (50.3%) | 0 (%) | 3711 (50.3%) |
| Wear/osteolysis | 949 (12.9%) | 0 (%) | 949 (12.9%) |
| Fracture/nonunion | 701 (9.5%) | 0 (%) | 701 (9.5%) |
| Infection | 1090 (14.8%) | 0 (%) | 1090 (14.8%) |
| Indication for primary THA (n [%]) | |||
| Osteoarthritis | 24,529 (83.6%) | 19,517 (88.8%) | 5012 (68.0%) |
| Osteonecrosis | 1770 (6.0%) | 1172 (5.3%) | 598 (8.1%) |
| Acute fracture or nonunion | 1649 (5.6%) | 559 (2.5%) | 1090 (14.8%) |
| Inflammatory arthritis | 674 (2.3%) | 232 (1.1%) | 442 (6.0%) |
| Other | 724 (2.5%) | 498 (2.3%) | 226 (3.1%) |
| Operative approach (n [%]) | |||
| Lateral | 11,200 (38.2%) | 7762 (35.3%) | 3438 (46.6%) |
| Posterior | 13,824 (47.1%) | 11,068 (50.4%) | 2756 (37.4%) |
| Direct anterior | 2921 (10.0%) | 2895 (13.2%) | 26 (0.4%) |
| Trochanteric osteotomy | 1404 (4.8%) | 253 (1.2%) | 1151 (15.6%) |
| Femoral head diameter (n [%]) | |||
| ≤32 mm | 15,213 (52.4%) | 10,954 (49.8%) | 4259 (60.2%) |
| ≥36 mm | 13,842 (47.6%) | 11,024 (50.2%) | 2818 (39.8%) |
| Acetabular liner configuration (n [%]) | |||
| Standard | 24,801 (84.5%) | 20,125 (91.6%) | 4676 (63.4%) |
| Elevated | 2339 (8.0%) | 1486 (6.8%) | 853 (11.6%) |
| Dual mobility | 814 (2.8%) | 367 (1.7%) | 447 (6.1%) |
| Constrained | 710 (2.4%) | 0 (0.0%) | 710 (9.6%) |
| Unknown | 685 (2.3%) | 0 (0.0%) | 685 (9.3%) |
| Acetabular cup revision (n [%]) | |||
| Yes | 4418 (59.9%) | — | 4418 (59.9%) |
| No | 2953 (40.1%) | — | 2953 (40.1%) |
SD = standard deviation, BMI = body mass index, and THA = total hip arthroplasty.
Diseases resulting in stiffness or biological fusion.
Operative spine fusion.
The indications for the primary THAs were osteoarthritis (89%), osteonecrosis (5%), posttraumatic (3%), inflammatory (1%), and other (2%, with the 3 most common diagnoses being dysplasia, skeletal dyscrasia, and neoplasm). The revision THAs were performed for aseptic loosening (50%), periprosthetic joint infection (15%), wear/osteolysis (13%), dislocation (13%), and periprosthetic fracture/nonunion (10%). The acetabular component was revised in 60% of the revision cases (Table II).
Fifty percent of the primary THAs were performed with a posterior approach; 35%, with a lateral approach; 13%, with a direct anterior approach; and 1%, with trochanteric osteotomy. A ≥36-mm-diameter femoral head was utilized in 50% and a ≤32-mm head, in 50%. A standard flat liner was used in 92%; an elevated/face changing liner, in 7%; and a dual-mobility construct, in 2%. In the revision-THA group, 47% of the operations were performed with a lateral approach; 37%, with a posterior approach; 16%, with trochanteric osteotomy; and 0.4%, with a direct anterior approach. A ≤32-mm-diameter femoral head was utilized in 60% and a ≥36-mm head, in 40%. A standard flat liner was used in 70%; an elevated/face changing liner, in 13%; a constrained liner, in 11%; and a dual-mobility construct, in 7% (Table II).
Source of Funding
This study was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grants R01AR73147 and P30AR76312.
Results
Among the 29,349 THAs, 1521 (2.9% of the primary THAs, 12.1% of the revisions, and 5.2% overall) were associated with a postoperative dislocation. The results of the univariable and multivariable analyses of dislocation risk associated with patient factors are summarized in Table III. The following factors were significantly associated with an increased dislocation risk after primary THAs: trochanteric osteotomy (HR = 1.77), “other” as the indication for THA versus osteoarthritis (HR = 1.71), major spine disease (HR = 1.52), and neurologic disease (HR = 1.52). The following factors were associated with a decreased dislocation risk after primary THA: a direct anterior approach or a lateral approach versus a posterior approach (HR = 0.27 and 0.58, respectively), an elevated liner (HR = 0.63), a ≥36-mm femoral head (HR = 0.69), male sex (HR = 0.73), and increasing age (HR = 0.92 per 10-year increment) (Table III).
TABLE III.
Univariable and Multivariable Analysis of Dislocation Risk Associated with Patient Factors by Procedure*
| Patient Factor | Primary THA | Revision THA | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted P Value | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted P Value | |
| Age, per 10 years | 0.91 (0.86-0.97) | 0.92 (0.86-0.98) | 0.006 | 0.97 (0.92-1.02) | 1.00 (0.94-1.06) | 0.984 |
| Sex (ref. = female) | ||||||
| Male | 0.67 (0.57-0.79) | 0.73 (0.61-0.87) | <0.001 | 0.83 (0.72-0.95) | 0.80 (0.68-0.95) | 0.009 |
| BMI (ref. = normal) | ||||||
| <18.0 kg/m2 | 1.42 (0.72-2.78) | 1.22 (0.58-2.59) | 0.606 | 1.43 (0.85-2.42) | 1.36 (0.76-2.44) | 0.297 |
| 25.0-29.9 kg/m2 | 0.95 (0.77-1.17) | 1.07 (0.86-1.32) | 0.553 | 1.03 (0.87-1.22) | 1.03 (0.85-1.25) | 0.771 |
| 30.0-34.4 kg/m2 | 0.77 (0.61-0.97) | 0.88 (0.68-1.12) | 0.289 | 0.96 (0.79-1.16) | 0.94 (0.75-1.78) | 0.601 |
| 35.0-39.9 kg/m2 | 0.83 (0.61-1.12) | 0.93 (0.68-1.27) | 0.642 | 1.26 (0.99-1.60) | 1.37 (1.04-1.79) | 0.023 |
| ≥40.0 kg/m2 | 1.13 (0.82-1.56) | 1.22 (0.87-1.71) | 0.241 | 0.99 (0.73-1.35) | 0.92 (0.66-1.29) | 0.645 |
| Neurologic disease (ref. = no) | ||||||
| Yes | 1.57 (1.31-1.87) | 1.52 (1.26-1.84) | <0.001 | 1.46 (1.24-1.72) | 1.33 (1.10-1.62) | 0.004 |
| Spine disease (ref. = none) | ||||||
| Major† | 1.55 (1.17-2.05) | 1.52 (1.13-2.05) | 0.006 | 1.32 (1.02-1.71) | 1.39 (1.05-1.85) | 0.022 |
| Minor | 1.10 (0.93-1.30) | 1.18 (0.98-1.43) | 0.084 | 1.08 (0.93-1.25) | 1.06 (0.89-1.27) | 0.523 |
| Prior spine surgery (ref. = none) | ||||||
| Major‡ | 1.52 (0.81-2.84) | 1.21 (0.63-2.31) | 0.571 | 0.78 (0.35-1.74) | 0.61 (0.27-1.38) | 0.236 |
| Minor | 1.39 (0.99-1.96) | 1.28 (0.90-1.81) | 0.170 | 0.79 (0.51-1.23) | 0.62 (0.38-1.02) | 0.058 |
| Indication for primary THA (ref. = osteoarthritis) | ||||||
| Osteonecrosis | 1.50 (1.11-2.04) | 1.35 (0.99-1.83) | 0.055 | 1.29 (1.03-1.62) | 1.31 (1.01-1.69) | 0.041 |
| Acute fracture or nonunion | 165 (1.06-2.59) | 1.78 (1.12-2.82) | 0.015 | 1.08 (0.89-1.30) | 1.11 (0.89-1.37) | 0.355 |
| Inflammatory arthritis | 1.00 (0.47-2.12) | 0.86 (0.41-1.83) | 0.702 | 1.26 (0.97-1.62) | 1.19 (0.88-1.62) | 0.244 |
| Other | 1.94 (1.32-2.86) | 1.71 (1.15-2.54) | 0.008 | 1.32 (0.92-1.88) | 1.36 (0.87-2.12) | 0.175 |
| Indication for revision THA (ref. = aseptic loosening) | ||||||
| Dislocation | — | — | — | 2.09 (1.75-2.50) | 1.89 (1.49-2.40) | <0.001 |
| Wear/osteolysis | — | — | — | 1.12 (0.90-1.38) | 0.91 (0.72-1.15) | 0.438 |
| Fracture/nonunion | — | — | — | 1.43 (1.14-1.79) | 1.38 (1.05-1.81) | 0.019 |
| Infection | — | — | — | 1.30 (1.06-1.59) | 1.69 (1.35-2.12) | <0.001 |
| Operative approach (ref. = posterior) | ||||||
| Lateral | 0.62 (0.52-0.74) | 0.58 (0.48-0.70) | <0.001 | 0.89 (0.77-1.02) | 0.95 (0.81-1.11) | 0.515 |
| Direct anterior | 0.26 (0.16-0.43) | 0.27 (0.16-0.44) | <0.001 | 0.47 (0.07-3.38) | 0.37 (0.06-2.39) | 0.295 |
| Trochanteric osteotomy | 1.92 (1.22-3.01) | 1.77 (1.11-2.82) | 0.016 | 0.52 (0.41-0.66) | 0.56 (0.43-0.74) | <0.001 |
| Acetabular liner configuration (ref. = standard) | ||||||
| Elevated | 0.74 (0.54-1.02) | 0.63 (0.45-0.88) | 0.006 | 0.80 (0.64-1.00) | 0.74 (0.58-0.93) | 0.010 |
| Dual mobility | 1.08 (0.48-2.42) | 0.59 (0.26-1.33) | 0.205 | 0.59 (0.38-0.91) | 0.44 (0.28-0.69) | <0.001 |
| Constrained | — | — | 1.28 (1.05-1.58) | 0.67 (0.52-0.87) | 0.003 | |
| Femoral head diameter (ref. = ≤32 mm) | ||||||
| ≥36 mm | 0.70 (0.59-0.83) | 0.69 (0.57-0.85) | <0.001 | 0.84 (0.73-0.97) | 0.82 (0.70-0.97) | 0.023 |
| Acetabular revision (ref. = no) | ||||||
| Yes | — | — | — | 0.60 (0.53-0.68) | 0.60 (0.51-0.70) | <0.001 |
HR = hazard ratio, CI = confidence interval, THA = total hip arthroplasty, and BMI = body mass index.
Diseases resulting in stiffness or biological fusion.
Operative spine fusion.
The following factors were associated with an increased dislocation risk after revision THA: instability of a previous THA, infection, or periprosthetic fracture as the indication for revision compared with aseptic loosening (HR = 1.89, 1.69, and 1.38, respectively), major spine disease (HR = 1.39), a BMI of 35.0 to 39.9 kg/m2 (HR = 1.37), neurologic disease (HR = 1.33), and osteonecrosis as the underlying diagnosis (HR = 1.31). The following factors were associated with a decreased dislocation risk after revision THA: a dual-mobility construct (HR = 0.44), trochanteric osteotomy (HR = 0.56), constrained acetabular liner (HR = 0.67), elevated liner (HR = 0.74), male sex (HR = 0.80), and ≥36-mm femoral head (HR = 0.82) (Table III). Furthermore, acetabular component revision decreased the risk of dislocation, with the decrease similar in association with both isolated acetabular revision (HR = 0.58) and acetabular revision in combination with femoral revision (HR = 0.59) (Table IV). Acetabular revision remained protective across all indications for revision.
TABLE IV.
Dislocation Risk After Revision THA Based on Which Component(s) Were Revised*
| Cup Not Revised (N = 2953) | Cup Revised but Not Stem (N = 2232) | Cup and Stem Revised (N = 2186) | |
|---|---|---|---|
| Univariable | |||
| Hazard ratio (95% CI) | Reference | 0.62 (0.52-0.73) | 0.58 (0.49-0.69) |
| Wald p value | Reference | <0.001 | <0.0001 |
| Multivariable* | |||
| Hazard ratio (95% CI) | Reference | 0.58 (0.48-0.70) | 0.59 (0.49-0.71) |
| Wald p value | Reference | <0.001 | <0.0001 |
Adjusted for age, sex, body mass index, neurologic disease, spine disease, previous spine procedure, underlying diagnosis, indication for revision, liner configuration, operative approach, and head diameter.
THA = total hip arthroplasty, and CI = confidence interval.
The multivariable models demonstrated calibration SIR values of 0.96 to 1.02, consistent with “excellent” calibration. C-statistic discrimination ranged from 0.63 to 0.65, consistent with “good” discrimination.
Nomogram Risk Calculator
Nomograms of individual-patient dislocation risk were created from Cox proportional hazard models (Figs. 1-A through 1-D). Each patient factor was calibrated to be worth a certain number of points. The total number of points was calculated to obtain the projected risk of dislocation at 1 year and 5 years after primary or revision THA. Revision THA nomograms include acetabular revision as a modifiable operative factor. The final data input line in the nomograms was approach/head diameter/liner. The combination of these 3 factors yields the greatest differential in possible point total, underscoring the power surgeons have to modify risk.
Figs. 1-A through 1-D.
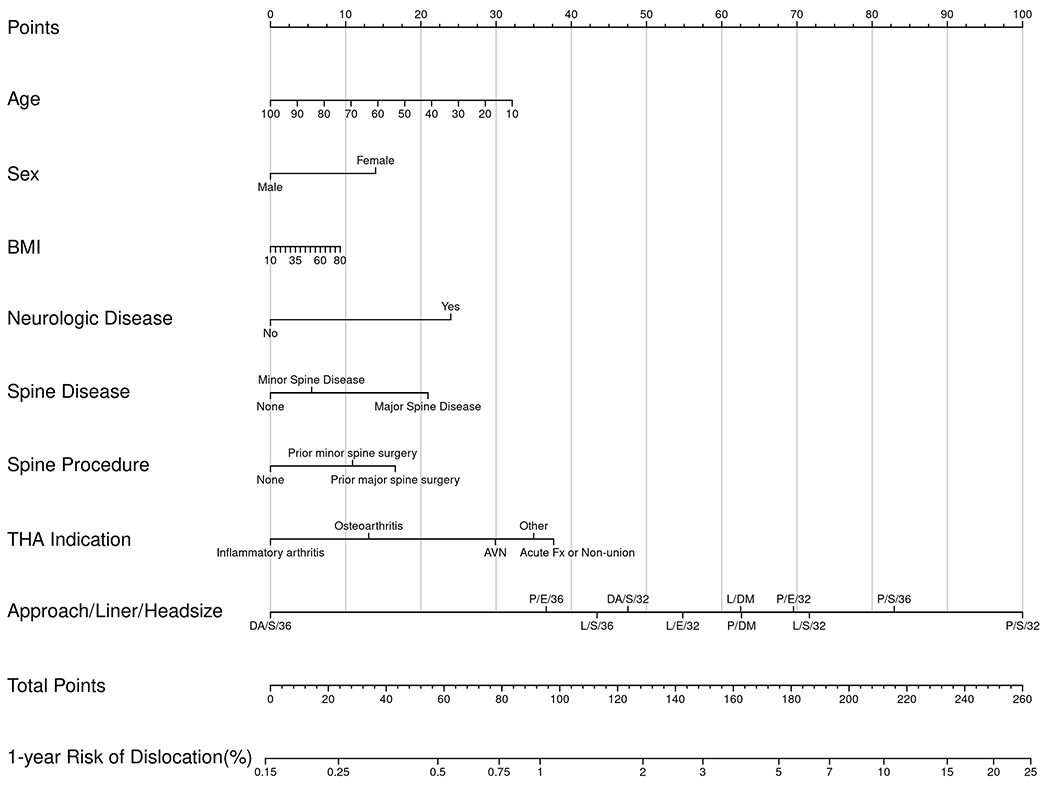
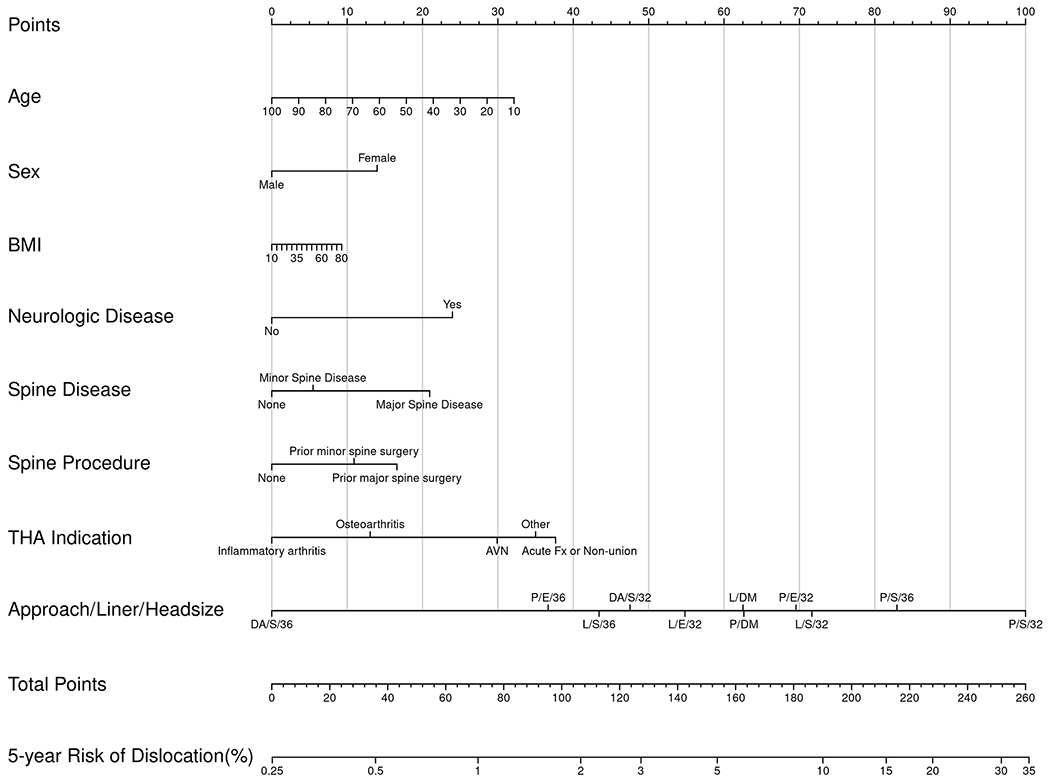
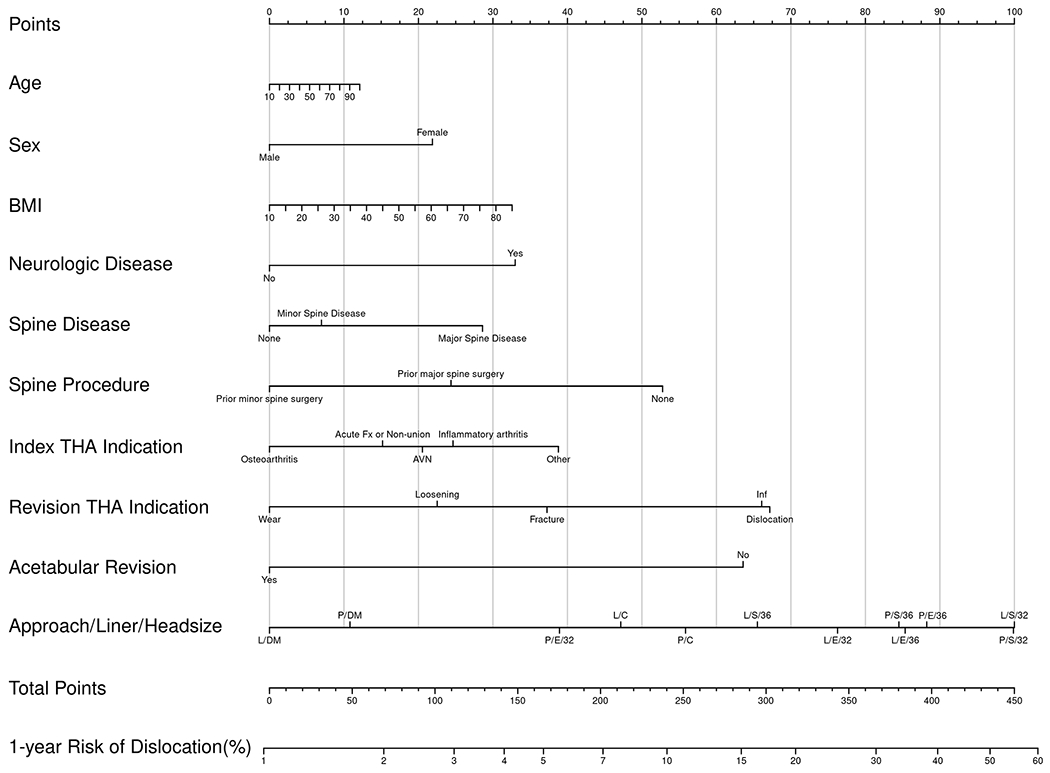
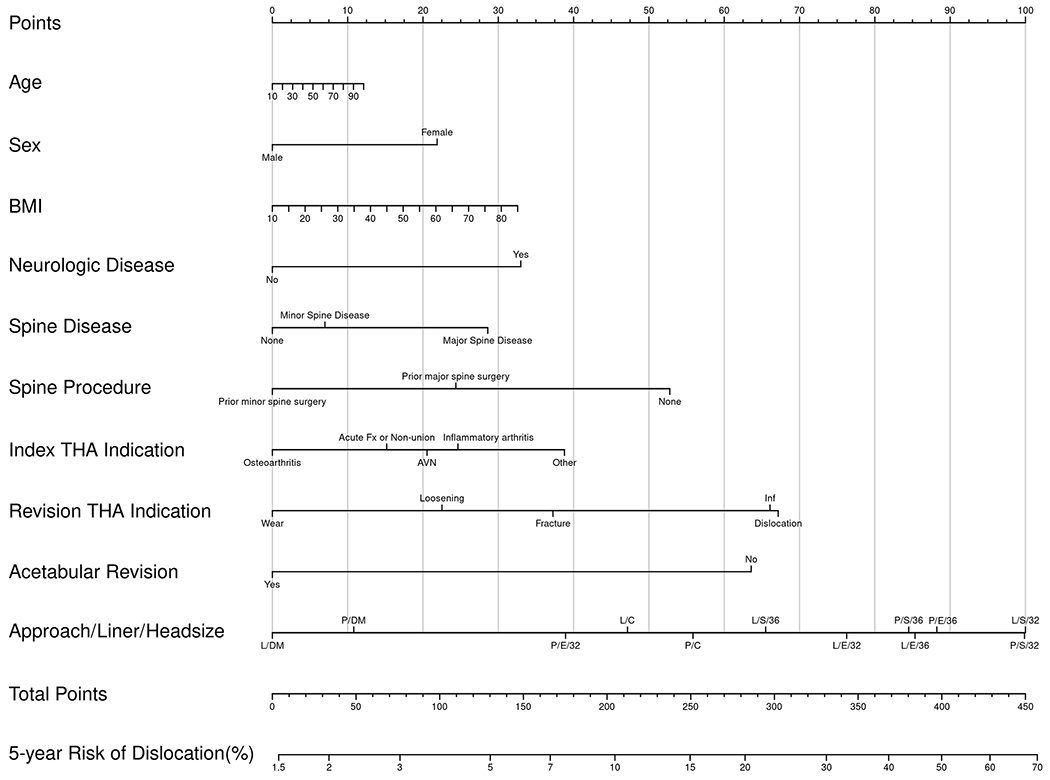
Patient-specific dislocation-absolute-risk nomograms for primary THA at 1 year (Fig. 1-A) and 5 years (Fig. 1-B) and for revision THA at 1 year (Fig. 1-C) and 5 years (Fig. 1-D). Risk is calculated by selecting the patient factor and drawing a vertical line up to the top row “Points” to calculate the number of points for that factor. The sum of the points for all factors is applied to the row “Total Points,” and a vertical line is draw downward to obtain the result for absolute risk. AVN = osteonecrosis, Fx = fracture, and Inf = infection. DA = direct anterior, P = posterior, and L = lateral approach. S = standard liner, E = elevated liner, and DM = dual-mobility construct.
To understand the range of risk associated with non-modifiable patient factors as well as the impact of modifiable operative decisions, a series of patient scenarios were created to define the upper and lower boundaries of the nomogram using best and worst possible patient scenarios (Tables V and VI). The nomograms demonstrated that patient-specific dislocation risk was wide-ranging, from 0.3% to 13% at 1 year and 0.4% to 19% at 5 years after primary THA, and 2% to 32% at 1 year and 3% to 42% at 5 years after revision THA. In the primary-THA group, after adjusting for approach, the combination of a ≥36-mm femoral head and an elevated liner yielded the largest decrease in risk (HR = 0.28), followed by a dual-mobility construct (HR = 0.48). In the revision-THA group, the adjusted risk of dislocation was most markedly decreased by use of a dual-mobility construct (HR = 0.40), followed by a ≥36-mm femoral head and an elevated liner (HR = 0.88).
TABLE V.
Dislocation Risk After Primary THA Based on Non-Modifiable Patient Factors and Modifiable Operative Decisions*
| Direct Anterior Approach | Lateral Approach | Posterior Approach | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥36-mm Head | ≤32-mm Head | Dual Mobil. | ≥36-mm Head | ≤32-mm Head | Dual Mobil. | ≥36-mm Head | ≤32-mm Head | Dual Mobil. | |||||||
| Stand. Liner | Elev. Liner | Stand. Liner | Elev. Liner | Stand. Liner | Elev. Liner | Stand. Liner | Elev. Liner | Stand. Liner | Elev. Liner | Stand. Liner | Elev. Liner | ||||
| No. of THAs | 2159 | 0 | 726 | 2 | 8 | 2971 | 40 | 4161 | 523 | 66 | 5645 | 132 | 4237 | 773 | 280 |
| HR for dislocation risk | 0.13 | — | 0.35 | — | — | 0.33 | — | 0.57 | 0.41 | 0.48 | 0.71 | 0.28 | 1.00 (ref.) | 0.55 | 0.48 |
| Absolute risk at 1 year (%) | |||||||||||||||
| Worst host† | 1.8 | — | 4.7 | — | — | 4.4 | — | 7.5 | 5.5 | 6.3 | 9.3 | 3.8 | 12.8 | 7.2 | 6.3 |
| Best host‡ | 0.3 | — | 0.7 | — | — | 0.6 | — | 1.1 | 0.8 | 0.9 | 1.4 | 0.6 | 2.0 | 1.1 | 0.9 |
| Absolute risk at 5 years (%) | |||||||||||||||
| Worst host† | 2.8 | — | 7.2 | — | — | 6.7 | — | 11.4 | 8.3 | 9.6 | 14.0 | 5.9 | 19.1 | 11.0 | 9.6 |
| Best host‡ | 0.4 | — | 1.1 | — | — | 1.0 | — | 1.7 | 1.2 | 1.4 | 2.2 | 0.9 | 3.0 | 1.7 | 1.4 |
HR = hazard ratio (reference set to 1.00 individually for highest-risk group). Dashes represent combinations with an insufficient sample size to calculate reliable HRs or absolute risks.
Worst possible host: female, age = 65 years, body mass index = 35 kg/m2, osteonecrosis indication for THA, neurologic disease present, major spine disease present, and prior major spine surgery.
Best possible host: male, age = 75 years, body mass index = 25 kg/m2, osteoarthritis indication for THA, neurologic disease absent, spine disease absent, and no prior spine surgery.
TABLE VI.
Dislocation Risk After Revision THA Based on Non-Modifiable Patient Factors and Modifiable Operative Decisions*
| Lateral Approach | Posterior Approach | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥36-mm Head | ≤32-mm Head | Dual Mobil. | Constrained | ≥36-mm Head | ≤32-mm Head | Dual Mobil. | Constrained | |||||
| Stand. Liner | Elev. Liner | Stand. Liner | Elev. Liner | Stand. Liner | Elev. Liner | Stand. Liner | Elev. Liner | |||||
| No. of THAs | 909 | 73 | 1123 | 261 | 177 | 398 | 926 | 116 | 777 | 216 | 200 | 220 |
| HR for dislocation risk | 0.72 | 0.87 | 1.00 | 0.80 | 0.38 | 0.60 | 0.86 | 0.89 | 1.00 (ref) | 0.55 | 0.42 | 0.65 |
| Absolute risk at 1 year (%) | ||||||||||||
| Worst host† | 23.9 | 28.2 | 31.7 | 26.2 | 13.5 | 20.5 | 28.0 | 28.9 | 31.7 | 19.1 | 14.9 | 22.0 |
| Best host‡ | 4.5 | 5.4 | 6.2 | 5.0 | 2.4 | 3.8 | 5.4 | 5.6 | 6.2 | 3.5 | 2.7 | 4.1 |
| Absolute risk at 5 years (%) | ||||||||||||
| Worst host† | 31.9 | 37.2 | 41.5 | 34.7 | 18.5 | 27.5 | 37.0 | 38.1 | 41.5 | 25.7 | 20.3 | 29.6 |
| Best host‡ | 6.3 | 7.6 | 8.7 | 7.0 | 3.4 | 5.3 | 7.5 | 7.8 | 8.7 | 4.9 | 3.8 | 5.8 |
HR = hazard ratio (reference set to 1.00 individually for highest-risk group). Dashes represent combinations with an insufficient sample size to calculate reliable HRs or absolute risks.
Worst possible host: female, age = 75 years, body mass index = 35 kg/m2, underlying diagnosis of osteonecrosis, dislocation indication for revision THA, neurologic disease present, major spine disease present, prior major spine surgery, and acetabular component not revised.
Best possible host: male, age = 65 years, body mass index = 25 kg/m2, underlying diagnosis of osteoarthritis, loosening indication for revision THA, neurologic disease absent, spine disease absent, no prior spine surgery, and acetabular component revised.
Case Example
A sixty-nine-year-old woman with a BMI of 36 kg/m2 underwent THA for osteoarthritis and had a positive history of neurologic disease and major spine disease and surgery. Her absolute risk of dislocation at 1 year ranges from 1.2% to 8.4%, based on choices within the control of the surgeon. Using the highest risk as a reference, but maintaining the posterior approach, the 1-year risk decreases from 8.4% to 6.1% with use of a ≥36-mm head and decreases to 2.5% with use of a ≥36-mm head and an elevated liner. In contrast, use of a dual-mobility construct in this patient reduces the risk to 4.1% (Fig. 2-A). The risk for this patient with the use of a lateral or direct anterior approach is shown in Figure 2-A.
Figs. 2-A and 2-B.
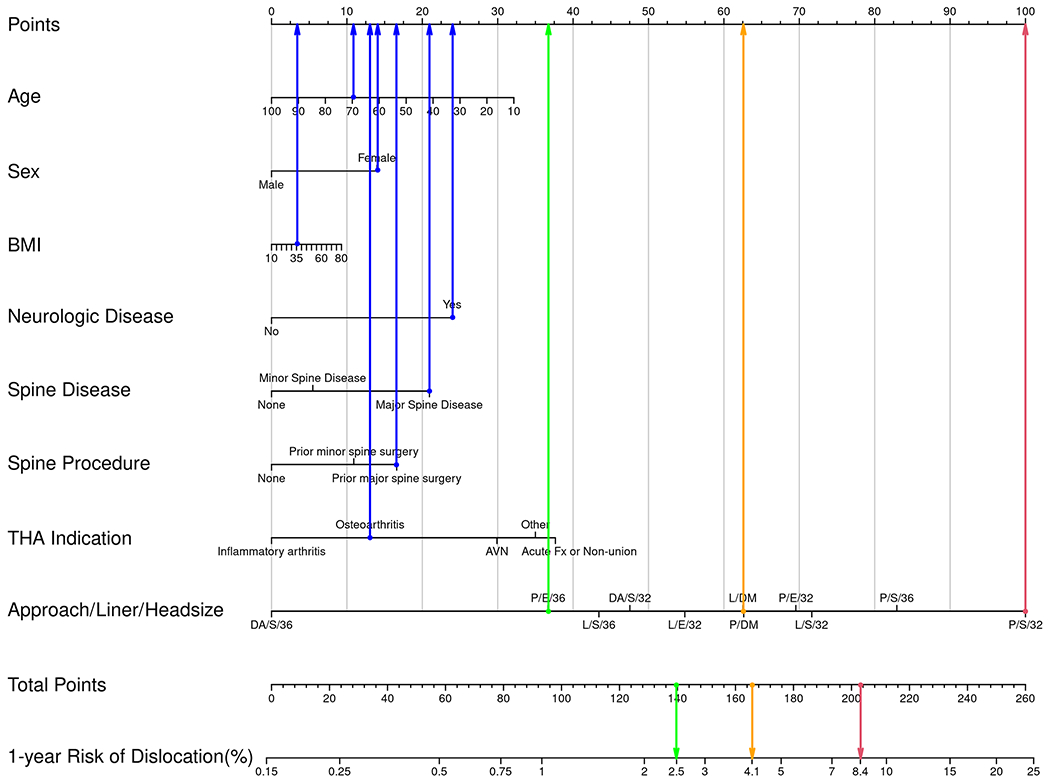
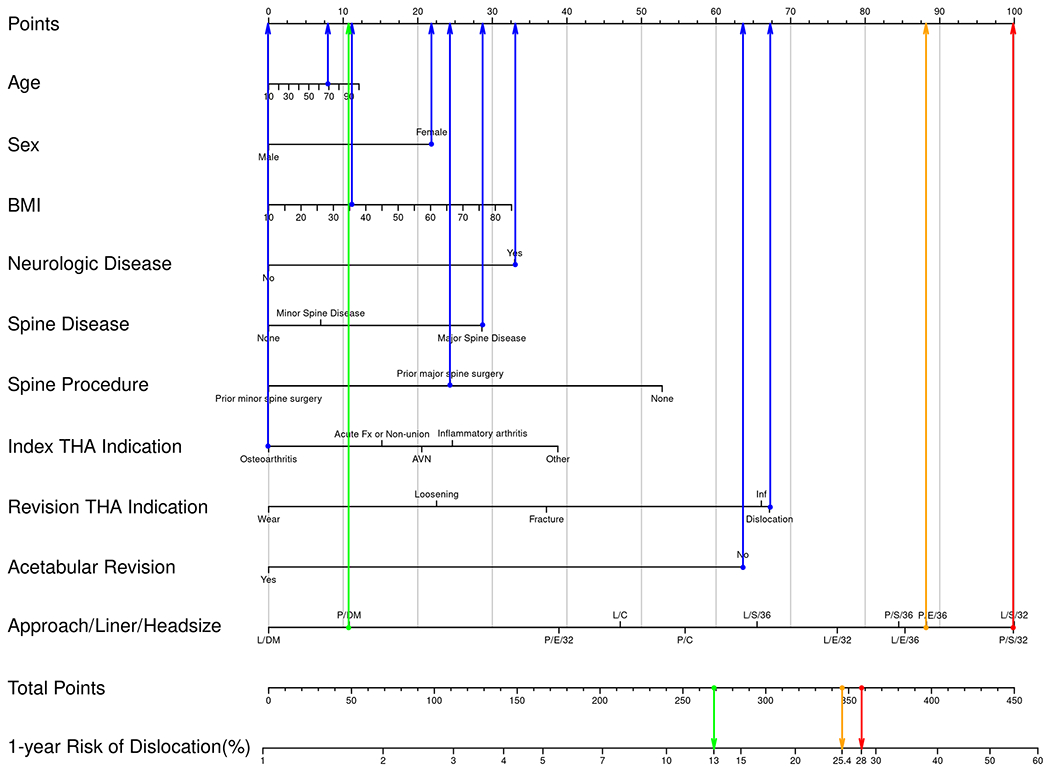
Examples of how to use the nomogram for hypothetical patients and various risk estimates in the primary (Fig. 2-A) and revision (Fig. 2-B) settings. In these examples, blue dots are assigned to the non-modifiable patient factors. Blue arrows then point upward to indicate the number of points assigned to that calibrated data point. The row indicating “Approach/Liner/Head size” has a possible data point for all combinations included in this study. Three example combinations have been chosen for illustration, all with a posterior approach. The 3 Approach/Liner/Head size choices are (1) posterior/elevated/36 mm, (2) posterior/dual-mobility, and (3) posterior/standard/32 mm. For illustration, the combination portending the highest risk is assigned a red dot and arrow pointing upward to indicate the number of points assigned to that calibrated data point. There is also a corresponding red dot shown for the row indicating “Total Points” and a red arrow pointing downward to show the 1-year risk of dislocation for this specific patient. The exact same method is applied for the combination with intermediate risk (yellow dots and arrows) and the combination with lowest risk (green dots and arrows). Note that in the primary setting, the lowest risk (green) comes from use of an elevated liner and 36-mm head, whereas in the revision setting, the lowest risk (green) is associated with a dual-mobility construct. AVN = osteonecrosis, Fx = fracture, and Inf = infection. DA = direct anterior, P = posterior, and L = lateral approach. S = standard liner, E = elevated liner, and DM = dual-mobility construct.
Presuming that the same patient presented for revision THA due to instability, her absolute risk of dislocation at 1 year ranges from 11.8% to 28%. Using the highest risk as a reference, but maintaining the posterior approach, the 1-year risk decreases from 28% to 24.6% with use of a ≥36-mm head and decreases to 25.4% with use of a ≥36-mm head and an elevated liner. In contrast, use of a dual-mobility construct in this situation decreases the risk to 13% (Fig. 2-B). An indication to revise the acetabular component in this patient (or if the surgeon chooses to revise it to optimize stability) results in an additional 40% decrease in the relative risk of dislocation for any combination of approach/liner/head diameter.
Discussion
Dislocation remains one of the most frequent complications and reasons for revision following THA. Ultimately, individual-patient risk is a complex amalgamation of non-modifiable characteristics and modifiable operative decisions. This study leveraged a large cohort of patients that was meticulously characterized across a broad range of important dislocation-risk comorbidities to derive risk prediction nomograms that are patient-specific and responsive to operative decisions. Surgeons can use these prediction tools to forecast 1-year and 5-year probabilities of dislocation and determine the impact of implant factors and operative approach on dislocation risk mitigation.
It should be emphasized that this study involved thorough characterization of patients beyond traditional demographic and operative factors by including other “instability comorbidities”—namely, spine disease, spine surgery, and neurologic disease. We further characterized spine disease and spine surgery according to whether they induced either a biological or operative fusion to account for the stiff spine that has become increasingly recognized as a factor in instability. These data were all individually and manually validated. All included factors were highly influential in the multivariable model. The aforementioned diagnoses were then used to supplement data from our total joint registry, which already tracks patient demographic, operative, and complication data with a >98% capture rate.
The baseline risk of dislocation was shown to be highly variable based on non-modifiable comorbidities and risk factors, with all factors included in the final model impacting the risk of dislocation. This fact is most clearly demonstrated by comparing the “worst case” and “best case” scenarios in Tables V and VI. This underscores the complex nature of THA stability and highlights the importance of comprehensively assessing comorbid status to accurately classify patients. Nevertheless, an encouraging message that can be derived from the present work is that surgeons can have a considerable influence on outcomes through their operative decisions. Indeed, operative covariates were the most impactful nomogram variables by a wide margin. Operative approach demonstrated a marked influence on dislocation risk, consistent with previous literature7,9. For surgeons who perform some combination of approaches in practice, this may afford an opportunity to more selectively use one approach versus another. However, for the many surgeons who default to a specific approach, the data provide highly actionable information as well. For example, a surgeon who predominantly uses a posterior approach can see in Table V that changing from a 32-mm to a 36-mm femoral head decreases the risk of dislocation by 30%, and adding an elevated liner further decreases the risk by 70%. Similarly, if it is not possible to upsize to a 36-mm femoral head, an isolated change to an elevated liner decreases the risk by 45%. The relationship between head size and dislocation protection was similar across all 3 operative approaches. We also noted in the revision THA population that acetabular revision markedly decreased the risk of dislocation (by 40%) if performed in isolation or concomitantly with femoral revision. This relationship was consistent across all indications for revision. Revising the acetabular component yields intuitive advantages for mitigating dislocation risk. First, it affords an opportunity to improve component position. Second, it usually leads to a larger acetabular component, which may enable use of a larger-diameter head or more diverse liner options. We also noted that performance of a trochanteric osteotomy in revision THA decreased dislocation risk by 44% (Table III). This was not included in the nomograms as proximal femoral osteotomies are typically undertaken out of necessity and thus would not be considered a modifiable operative decision. Nevertheless, the protective effect is interesting to note and may relate to maintenance of proximal soft tissues, ability to tension the abductors, and protected weight-bearing and bracing postoperatively.
It should be noted that absolute risk is important to consider in addition to relative risk. The overall impact of a mitigation strategy is quite different for a patient with a baseline 1-year dislocation risk of 2% versus one with a 16% risk. In this example, making an operative decision that reduces the relative risk by 50% changes the absolute risk from 2% to 1% for the hypothetical first patient compared with changing it from 16% to 8% for the second. This fact emphasizes the importance of determining patient-specific baseline risk, which this study shows is highly variable and subject to many demographic and comorbid influences.
Another increasingly common question among surgeons is when to use a dual-mobility construct. Several studies have demonstrated a decreased risk of dislocation with these implants10,13. However, dual mobility comes with increased cost and potential unique complications such as intraprosthetic dislocation, malseating, and corrosion at the additional interface14–16. Therefore, until more data become available, judicious use is likely warranted. In aggregate, dual-mobility constructs decreased the risk of dislocation by roughly 50% in the primary-THA cohort, but importantly this was not superior to the use of a larger head and an elevated liner. However, use of dual-mobility constructs in the primary setting was relatively rare in this cohort (n = 367; 1.7%), and trends toward increased use may alter these data in future studies. Furthermore, there was wide discrepancy between the risk modification in the univariable analysis (HR = 1.08) and that in the multivariable analysis (HR = 0.59), suggesting that these implants were used in complex cases. In contrast, dual-mobility constructs were the most influential risk mitigator in revision THA (Table VI), with an HR of 0.40 after adjustment for surgical approach. Therefore, the data are more supportive of dual-mobility use in revision cases, with benefits attenuated in the primary setting compared with those of other implant-related risk mitigation strategies.
Some may be surprised by the high prevalence of dislocation in this study. However, these rates are consistent with previous studies from our institution that not only tracked patients clinically at routine intervals but also incorporated telephone and mail communications to document any complications that had been managed at other facilities. Thus, these rates of dislocation, although higher than those in many reports, probably are more representative of the true complication burden, which further underscores the importance of risk mitigation. Indeed, a recent study from the Danish Hip Arthroplasty Register documented a 3.5% “true” prevalence of dislocation within 2 years after THA17, which is even higher than what we are reporting in this paper. The authors17 concluded that most studies to date have systematically underreported dislocation rates as most dislocations are treated in emergency departments without surgery.
This study must be interpreted in light of potential limitations. First, although the nomograms were derived using robust, manually validated clinical data on patient characteristics, they are not comprehensive. Notably, implant position was not considered given the constraints in a cohort this large. Second, dislocation is a relatively rare event with a multifactorial etiology. That combination, for any clinical prediction problem, makes model discrimination and calibration difficult. Our model had only “good” discrimination, which is not unexpected given the aforementioned challenges inherent to modeling problems such as dislocation. However, we did achieve “excellent” calibration, which is a testament to model fine-tuning. Third, these results represent a single-center experience and have not been externally validated. This will be a critical step to broader acceptance and generalizability. Fourth, “elevated liners” included face-changing, lateralized, and lipped liners but sample size precluded evaluating these liner types individually.
To our knowledge, this study is the first to yield a patient-specific dislocation risk calculator that incorporates important comorbidities affecting instability risk. The resultant nomograms are responsive to implant and operative approach decisions, and thus can be used as a screening tool to identify and individualize recommendations and treatment for patients undergoing THA. This is especially important given the wide range of individual-patient risk identified in this study and the degree of risk mitigation portended by various operative strategies. Nomograms derived from this work will serve as the underlying foundation for a digital clinical tool to calculate patient risk in a streamlined fashion.
Supplementary Material
Footnotes
Investigation performed at the Mayo Clinic, Rochester, Minnesota
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/XXXXXXX).
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/XXXXXXX).
References
- 1.Berry DJ, von Knoch M, Schleck CD, Harmsen WS. The cumulative long-term risk of dislocation after primary Charnley total hip arthroplasty. J Bone Joint Surg Am. 2004. Jan;86(1):9–14. [DOI] [PubMed] [Google Scholar]
- 2.Norambuena GA, Wyles CC, Van Demark RE 3rd, Trousdale RT. Effect of dislocation timing following primary total hip arthroplasty on the risk of redislocation and revision. Hip Int. 2019. Sep;29(5):489–95. [DOI] [PubMed] [Google Scholar]
- 3.American Joint Replacement Registry Annual Report. 2021. Accessed 2022 Mar 14. https://www.aaos.org/registries/publications/ajrr-annual-report/
- 4.Goldman AH, Sierra RJ, Trousdale RT, Lewallen DG, Berry DJ, Abdel MP. The Lawrence D. Dorr Surgical Techniques & Technologies Award: Why Are Contemporary Revision Total Hip Arthroplasties Failing? An Analysis of 2500 Cases. J Arthroplasty. 2019. Jul;34(7S):S11–6. [DOI] [PubMed] [Google Scholar]
- 5.Buckland AJ, Puvanesarajah V, Vigdorchik J, Schwarzkopf R, Jain A, Klineberg EO, Hart RA, Callaghan JJ, Hassanzadeh H. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J. 2017. May;99-B(5):585–91. [DOI] [PubMed] [Google Scholar]
- 6.Esposito CI, Carroll KM, Sculco PK, Padgett DE, Jerabek SA, Mayman DJ. Total Hip Arthroplasty Patients With Fixed Spinopelvic Alignment Are at Higher Risk of Hip Dislocation. J Arthroplasty. 2018. May;33(5):1449–54. [DOI] [PubMed] [Google Scholar]
- 7.Goel A, Lau EC, Ong KL, Berry DJ, Malkani AL. Dislocation rates following primary total hip arthroplasty have plateaued in the Medicare population. J Arthroplasty. 2015. May;30(5):743–6. [DOI] [PubMed] [Google Scholar]
- 8.Heckmann N, McKnight B, Stefl M, Trasolini NA, Ike H, Dorr LD. Late Dislocation Following Total Hip Arthroplasty: Spinopelvic Imbalance as a Causative Factor. J Bone Joint Surg Am. 2018. Nov 7;100(21):1845–53. [DOI] [PubMed] [Google Scholar]
- 9.Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005. Nov;87(11):2456–63. [DOI] [PubMed] [Google Scholar]
- 10.Hartzler MA, Abdel MP, Sculco PK, Taunton MJ, Pagnano MW, Hanssen AD. Otto Aufranc Award: Dual-mobility Constructs in Revision THA Reduced Dislocation, Rerevision, and Reoperation Compared With Large Femoral Heads. Clin Orthop Relat Res. 2018. Feb;476(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996. Feb 28;15(4):361–87. [DOI] [PubMed] [Google Scholar]
- 12.Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016. Aug;25(4):1692–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigdorchik JM, D’Apuzzo MR, Markel DC, Malkani AL, Raterman S, Sharpe KP, Cornell CN, Westrich GH. Lack of early dislocation following total hip arthroplasty with a new dual mobility acetabular design. Hip Int. 2015. Jan-Feb;25(1):34–8. [DOI] [PubMed] [Google Scholar]
- 14.Elbuluk AM, Slover J, Anoushiravani AA, Schwarzkopf R, Eftekhary N, Vigdorchik JM. The cost-effectiveness of dual mobility in a spinal deformity population with high risk of dislocation: a computer-based model. Bone Joint J. 2018. Oct;100-B(10):1297–302. [DOI] [PubMed] [Google Scholar]
- 15.Kolz JM, Wyles CC, Van Citters DW, Chapman RM, Trousdale RT, Berry DJ. In Vivo Corrosion of Modular Dual-Mobility Implants: A Retrieval Study. J Arthroplasty. 2020. Nov;35(11):3326–9. [DOI] [PubMed] [Google Scholar]
- 16.Romero J, Wach A, Silberberg S, Chiu YF, Westrich G, Wright TM, Padgett DE. 2020 Otto Aufranc Award: Malseating of modular dual mobility liners. Bone Joint J. 2020. Jul;102-B(7_Supple_B)(Supple_B):20–6. [DOI] [PubMed] [Google Scholar]
- 17.Hermansen LL, Viberg B, Hansen L, Overgaard S. “True” Cumulative Incidence of and Risk Factors for Hip Dislocation within 2 Years After Primary Total Hip Arthroplasty Due to Osteoarthritis: A Nationwide Population-Based Study from the Danish Hip Arthroplasty Register. J Bone Joint Surg Am. 2021. Feb 17;103(4):295–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


