Abstract
Introduction and Objective:
In 2018, the U.S. Food and Drug Administration approved the da Vinci single-port (SP) system, in which four instruments are still utilized, but enter through a single-site access trocar. Herein, we report the largest case series for SP robot-assisted radical prostatectomy (RARP) to date. Our primary aim is to analyze the perioperative and short-term outcomes of this procedure. Our secondary aim is an assessment of the learning curve with this new platform.
Methods:
A total of 157 patients underwent SP RARP by two surgeons who have completed >3000 multiport robotic surgeries collectively. Institutional Review Board-approved prospectively collected data were used. Basic demographic preoperative variables and perioperative outcomes were analyzed.
Results:
Median patient age and prostate-specific antigen was 63 years and 6.3 ng/mL before treatment (interquartile range [IQR] 4.7–8.2 ng/mL). Average prostate weight was 47 g. The median operating time was 195 minutes (IQR 165–221.25 minutes) with a median estimated blood loss of 100 mL (IQR 100–200 mL). Surgeon 1's operating time stabilized around case #56, and Surgeon 2 around case #26. Surgeon 2 used the transperitoneal approach for the first 7 cases. There were no intraoperative complications. There were six total postoperative complications (3.8%) and four (2.5%) were Clavien–Dindo scale ≥IIIa. One hundred ten patients went home same day, 45 stayed 1 night at the hospital, with only 2 patients requiring stay in the hospital for more than 1 night (70%, 29%, and 1% respectively). With the median follow-up period of 9 months, rates of biochemical recurrence, pad-free, and potency preservation were 8.3%, 82.5%, and 64.4%, respectively.
Conclusions:
This case series confirms the safety and efficacy of SP RARP with acceptable short-term outcomes. There is a significant learning curve for this new modality. Shorter hospital stay appears to be an early benefit of the SP platform.
Keywords: prostate cancer, robotic surgery, single-port, case review
Introduction
Robot-assisted radical prostatectomy (RARP) has been a standard of care in the management of prostate cancer since the approval of the da Vinci surgical robotic platform by the U.S. Food and Drug Administration (USFDA) in 2001.1 With articulating instruments, RARP greatly reduced technical difficulties associated with laparoscopy, and thereby reduced blood loss and postoperative recovery. Patients were also attracted to the procedure because of the prospect of minimizing postoperative surgery-related complications and surgical scars; in 2014, RARP accounted for 90% of radical prostatectomies nationwide.2
In 2018, the USFDA approved the da Vinci single-port (SP) system, in which four instruments are still utilized, but enter through a single-site access trocar, allowing for one instead of four surgical scars. This new platform includes similar surgical elements such as articulation of instruments and high-definition three-dimensional observation of the original multiport system. However, despite such similarity and camera now including two points of articulation, their fixed positions reduce the dimensions of the surgical field and limit the extent of movement.3
Previous case studies have investigated the safety and efficacy of the SP-RARP in a small sample size.4,5 Herein, we report the largest case series for SP-RARP to date. Our primary aim is to analyze the perioperative and short-term outcomes of this procedure. Our secondary aim is to assess the learning curve of this new platform for surgeons who have extensive experience with the multiport system.
Methods
Surgical procedure
One 2.5 cm incision is made either horizontally just below the umbilicus or vertically 2 cm below the umbilicus. Then, subcutaneous tissues are separated from the rectus fascia to the level of arcuate line (∼3 cm below umbilicus). After incising the fascia, the belly of the rectus muscle is divided and the preperitoneal space is finger dissected to the level of the pubic bone. SP da Vinci is docked and the preperitoneal space is further developed. If necessary, an 8 mm assistant port is placed at either the left or right lower quadrant. The rest of the procedure is similar to the multiport RARP.
Since July 2019, a total of 157 patients underwent SP RARP by two surgeons who have completed >3000 multiport robotic surgeries collectively. For one surgeon, first seven cases were done through a transperitoneal approach. The rest of the cases were completed using a preperitoneal approach.
Data collection
Prospective data collection was approved by the respective Institutional Review Board. Variables included basic demographics (height, weight, and body mass index [BMI]), prostate-specific antigen (PSA) levels before surgery, clinical stage, Gleason scores, and quality of life (scores from the American Urological Association symptom score and sexual health inventory for men [SHIM]). Operative outcome measures collected included console time, estimated blood loss (EBL), and complications. Post-RARP variables analyzed were hospital stay, pathology, Clavien–Dindo scores when relevant, and status biochemical progression, continence, and potency. Continence was defined as being pad-free and potency as being able to engage in sexual intercourse with or without phosphodiesterase (PDE)-5 inhibitors in at least 50% of the attempts.
Results
Patient characteristics
The preoperative patient characteristics are shown in Table 1. All patients with localized prostate cancer who opted against extended pelvic lymph node dissection (PLND) were deemed candidates for SP-RARP. However, men with BMI >35 and prostate volume >100 g were excluded. Of the 157 patients, 138 were Caucasian-White and 8 were African American. Median age and PSA were 63 and 6.3 ng/mL. Overwhelming majority of the cohort had cT1c disease (154, 98%) and 122 had grade group 1 or 2. There were 22 patients with Gleason score ≥8 (grade group 4 or 5).
Table 1.
Preoperative Patients' Characteristics
| Characteristics | Median (IQR) |
|---|---|
| Sample size (n) | 157 |
| Age (years) | 63 (59–68) |
| BMI (kg/m2) | 27.8 (25.8–29.6) |
| PSA (ng/mL) | 6.3 (4.7–8.2) |
| AUAss | 7 (3–14) |
| SHIM | 19 (12–24) |
| Count (%) | |
|---|---|
| Race | |
| Caucasian | 138 (87.9) |
| African American | 8 (5.1) |
| Others | 11 (7.0) |
| Clinical staging | |
| T1c | 154 (98.1) |
| T2a | 3 (1.9) |
| Clinical Gleason score | |
| 3 + 3 (GG1) | 39 (24.8) |
| 3 + 4 (GG2) | 83 (52.9) |
| 4 + 3 (GG3) | 13 (8.3) |
| ≥8 (GG4 or GG5) | 22 (14.0) |
| Pathologic Gleason score | |
| 6 | 17 (10.8) |
| 7 | 127 (80.9) |
| 8 | 7 (4.5) |
| 9 | 6 (3.8) |
| Pathologic stage | |
| T2 | 119 (75.8) |
| T3a | 33 (21.0) |
| T3b | 5 (3.2) |
AUAss = American Urological Association symptom score; BMI = body mass index; GG = Grade Group; IQR = interquartile range; PSA = prostate-specific antigen; SHIM = sexual health inventory for men.
Perioperative outcomes
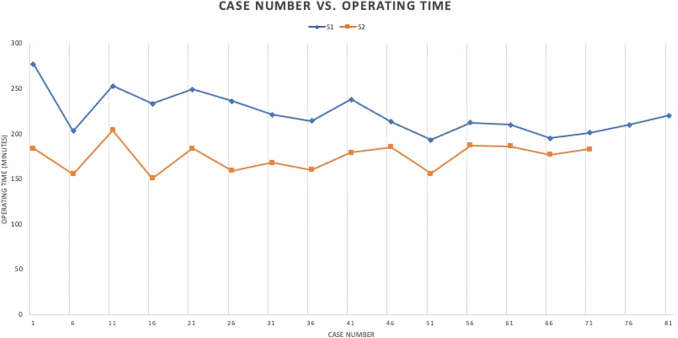
There were no cases that required a conversion to open surgery, or any intraoperative complications that required blood transfusions. Figure 1 shows the skin-to-skin operative times of the two surgeons. Surgeon 1's operative time stabilized around case #56, and Surgeon 2 around case #26. Surgeon 2 used the transperitoneal approach for the first seven cases. The overall median operative time and anesthesia time were 195 minutes (interquartile range [IQR] 165–221.25 minutes) and 222 minutes (IQR 190–245 minutes), respectively, with a median EBL of 100 mL (IQR 100–200 mL). The average prostate volume was 47 g.
FIG. 1.
Learning curve (skin-to-skin operative time) of two surgeons. For surgeon 1, operative time plateaued at approximately case #56. For surgeon 2, operative time stabilized around case #26. Color images are available online.
Postoperative outcomes
There were six total postoperative complications (3.8%) shown in Table 2. Four (2.5%) were major complications as determined by the Clavien–Dindo scale (IIIa or higher), which included two patients with bladder neck contracture (BNC), small anterior urine leak, and urinary retention after Foley removal. The first men with BNC were treated with a single treatment of dilation, whereas the second underwent transurethral incision of BNC. Both patients are continent and remain free of any further complications.
Table 2.
Postoperative Complications
| Grade | Count |
|---|---|
| I | 1 |
| II | 1 |
| III-a | 2 |
| III-b | 2 |
| IV | 0 |
Postoperative minor complications included one each of lymphocele and deep vein thrombosis. Total of 110 (70%) patients went home the same day, 45 (29%) stayed 1 night at the hospital, and only 2 (1%) patients required stay in the hospital of 2 days. Regarding PLND, 72 men opted for the procedure. Average lymph node yield was 5.9 (IQR 3–7) and five had lymph node metastasis. Oncologically, positive surgical margin (PSM) rate was 28% for the entire series (Table 3). When stratified by stage, PSM rate was 26%, 36%, and 40% for pT2, pT3a, and pT3b, respectively.
Table 3.
Postoperative Pathology
| Pathologic stage | Positive margin rate (%) |
|---|---|
| T2 | 31 (26) |
| T3a | 12 (36) |
| T3b | 2 (40) |
| Total | 45 (29) |
Short-term outcome of our SP-RAPR series is shown in Table 4. Thirty-five patients had more than 12 months of follow-up. With a median follow-up of 9 months, the overall biochemical recurrence rate was 8.3%. Of the 13 men who recurred biochemically, eight had a high-risk disease. Overall continence rate (pad-free) was 82.5% and potency preservation rate was 64.4% in men who had preoperative SHIM score >20.
Table 4.
Short-Term Outcomes
| Median follow-up | 9 Months, % |
|---|---|
| BCR | 8.3 |
| Continence (pad-free) | 82.5 |
| Potency | 64.4 |
BCR = biochemical recurrence rate.
Discussion
In this study, we describe our initial preperitoneal RARP experience with the da Vinci SP platform. In this series, experience from two surgeons with more than 3000 multiport robotic cases were analyzed. The results demonstrated that preperitoneal SP-RARP is feasible and safe. However, the learning curve for the SP platform was substantial. The results of our series should be carefully considered for surgeons considering the implementation of preperitoneal SP-RARP.
To the best of our knowledge, this is the largest case series to date analyzing the outcomes of SP-RARP. Consistent with previous smaller studies, SP-RARP appears to be a safe and valuable modality for treatment of prostate cancer.4,6 We report acceptable postoperative complications, with majority of patients returning home same day. Before the adoption of the SP technology, no patients in our practice were sent home on the same day of the surgery. The new SP system offers reduced number of surgical incisions, improved cosmesis, and shorter length of stay as potential advantages.
When adopting a new technology, the change must improve outcome and benefit patients. In this regard, we chose to take the preperitoneal route in performing RARP. Such approach theoretically reduces the risk of bowel complications. Although the preperitoneal RARP can be performed with the multiport system,7 the surgical route is more easily developed with the SP system. In addition, re-engineering of the robotic arms in the SP system prevents the collision of arms that can be seen with the multiport system. In short, the preperitoneal approach for RARP may be more feasible with the SP platform,
Despite these potential benefits, the SP platform is associated with a significant learning curve. Such learning curve is attributable to few specific differences between the SP and multiport platform. First, SP instruments do not have an endowrist. Instead, the articulation is at the elbow. Thus, the SP instrumentation is essentially a compromise between straight laparoscopic instruments and the endowristed multiport da Vinci instruments. Second, SP instruments are not as rigid as multiport instruments. As a result, blunt dissection is not proficient. Third, true SP-RARP does not have an assistant port.
Accordingly, parts of the procedure that is facilitated by an assistant takes significantly longer time. Such surgical steps include controlling the vascular pedicles and dissecting neurovascular bundles. To circumvent this shortcoming, we cauterize significantly more. Our short-term results suggest that the effect of such technical modification on continence and potency is not dramatically different than that of our historical results. With a median follow-up of 9 months, the pad-free rate was 82.5% and potency preservation rate 64.4%. Long-term data are necessary to further assess the outcome of SP-RARP.
To shorten the learning curve for preperitoneal SP-RARP, we recommend the following strategy. First, because the engineering limitation between the SP and multiport da Vinci is significant, the surgeon should initially complete SP-RARP through the more familiar transperitoneal approach. Indeed, the major difference in terms of learning curve between the two surgeons in this study is that one completed the first seven cases transperitoneally. Second, in developing the preperitoneal space, microperforation of the peritoneum can occur.
To avoid this, entry into the preperitoneal space should be below the arcuate line. If peritoneal microperforation occurs as evident by the frequent collapse of the working space, the procedure should be converted to transperitoneal approach by widely opening the peritoneum. Third, an assistant port should be placed at either right or left lower quadrant. This assistant port can be removed as the surgeon's learning curve flattens. Nevertheless, the presence of the assistant port does not affect the surgical outcome. Collectively, when implementing the preperitoneal SP-RARP, we recommend a careful planning to optimize the outcome.
Lessons described herein should shorten the learning curve for surgeons considering the preperitoneal SP-RARP. Nevertheless, this study is based on the experience of only two surgeons. Therefore, the applicability of the findings and recommendations may be limited. Future studies investigating long-term oncologic and functional results such as return of sexual function and incontinence rates will be critical to understanding the long-term benefits of SP RARP.
Conclusion
This case series analysis confirms the safety and efficacy of SP-RARP with acceptable short-term outcomes. Satisfactory operative time, complication rates, and shorter hospital stays were achieved. There is a significant learning curve for this modality caused by the reduced surgical field and engineering limitations of the instruments. Further studies should investigate the long-term outcomes after SP-RARP.
Abbreviations Used
- AUAss
American Urological Association symptom score
- BCR
biochemical recurrence rate
- BMI
body mass index
- BNC
bladder neck contracture
- EBL
estimated blood loss
- IQR
interquartile range
- PLND
pelvic lymph node dissection
- PSA
prostate-specific antigen
- PSM
positive surgical margin
- RARP
robot-assisted radical prostatectomy
- SHIM
sexual health inventory for men
- SP
single-port
- USFDA
U.S. Food and Drug Administration
Authors' Contributions
Data collection, data analysis, article writing, and revision by J.E.K. Data collection, article writing, and revision by A.K. and B.L. Review data analysis, review, and editing of article by E.A.S., T.L.J., and S.G. Data collection, review data analysis, review, and editing of article by M.M.K. Supervision of the entire project by I.Y.K.
Author Disclosure Statement
All authors have no significant conflict of interest with the content of this study. IYK and MK are ad hoc consultant to Intuitive Surgical, Inc.
Funding Information
This study is supported in part by grants from the National Cancer Institute (P30CA072720) and generous support from the Marion and Norman Tanzman Charitable Foundation and Mr. Malcolm Wernik.
References
- 1. Dobbs RW, Magnan BP, Abhyankar N, et al. Cost effectiveness and robot-assisted urologic surgery: Does it make dollars and sense? Minerva Urol Nefrol 2017;69(4):313–323; doi: 10.23736/S0393-2249.16.02866-6. [DOI] [PubMed] [Google Scholar]
- 2. Arenas-Gallo C, Shoag JE, Hu JC. Optimizing surgical techniques in robot-assisted radical prostatectomy. Urol Clin North Am 2021;48(1):1–9; doi: 10.1016/j.ucl.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 3. Checcucci E, De Cillis S, Pecoraro A, et al. Single-port robot-assisted radical prostatectomy: A systematic review and pooled analysis of the preliminary experiences. BJU Int 2020;126(1):55–64; doi: 10.1111/bju.15069. [DOI] [PubMed] [Google Scholar]
- 4. Lai A, Dobbs RW, Talamini S, et al. Single port robotic radical prostatectomy: A systematic review. Transl Androl Urol 2020;9(2):898–905; doi: 10.21037/tau.2019.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khalil M, Cranwell A, Ouyang J, et al. Single-port robot assisted concomitant hemi-nephrectomy, ureterectomy and radical prostatectomy using the da Vinci SP platform. Urol Case Rep 2021;36:101550; doi: 10.1016/j.eucr.2020.101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Covas Moschovas M, Bhat S, Onol F, et al. Early outcomes of single-port robot-assisted radical prostatectomy: Lessons learned from the learning-curve experience. BJU Int 2021;127(1):114–121; doi: 10.1111/bju.15158. [DOI] [PubMed] [Google Scholar]
- 7. Joseph JV, Rosenbaum R, Madeb R, et al. Robotic extraperitoneal radical prostatectomy: An alternative approach. J Urol 2006;175(3 Pt 1):945–950; discussion 951; doi: 10.1016/S0022-5347(05)00340-X. [DOI] [PubMed] [Google Scholar]