Abstract
The endocannabinoid system (ECS) has been found at the blood–brain barrier (BBB), as Cannabinoid receptors were characterized in human brain microvascular endothelial cells and astrocytes. In several in vitro and in vivo studies, cannabinoids decreased BBB permeability and enhanced membrane integrity, which may be achieved through endothelial tight junctions and other mechanisms. These permeability regulation effects of cannabinoids suggested that the ECS may protect the brain by enhancing barrier integrity. Related questions about cannabinoid–drug interaction and drug distribution across the BBB are also raised. Specifically, can cannabinoids significantly reduce drug bioavailability to the brain? More in-depth and systematic investigations are needed to characterize and quantify these effects of cannabinoids on brain microvasculature physiopathology. Therefore, this review summarizes literatures from different disciplines to promote more research on assessing the therapeutic benefits and risks of using cannabinoids to protect BBB from dysfunctions or breakdown, and to avoid consequent brain damages due to inflammation, neurodegenerations, hemorrhage, ischemia, or other causes.
Keywords: blood–brain barrier, human brain microvascular cells, stroke, ischemia, permeability, drug interaction
Introduction
At the interface between the central nervous system (CNS) and peripheral circulation, the blood–brain barrier (BBB) not only protects the CNS from hostile chemicals and pathogens, but also transports and regulates essential nutrients, signaling molecules and immune factors. Along the complex brain capillary network, the BBB is constituted by the neurovascular unit (NVU) of microvascular endothelial cells, pericytes, and astrocytes, as well as associated microglia and neurons. NVU is the essential cellular building block that contributes to the stability and functions of vascular BBB, as a critical interface of substance exchanges and communications across CNS, cardiovascular, and immune systems. Studying the molecular mechanisms of NVU cells can reveal potential or novel therapeutic strategies.
Cannabinoid receptors have been found in brain microvascular cells and astrocytes of the NVU.1 The endocannabinoid system at the BBB (BBB ECS) may have important functions, such as to maintain barrier integrity and regulate the transportation of important molecules under both physiological or pathological conditions.1 Several recent reviews implied the significances of the BBB ECS.2,3 The structures and functions of BBB ECS can be further characterized, as the recent advancements of cannabinoid research brought more insightful mechanisms, such as structure–activity relationship of cannabinoids, and more powerful methodologies, such as specific imaging probes and crystallography and cryoelectron microscopy that revealed high-resolution structures of cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2).4,5
This review integrates current publications and understandings of the BBB ECS, focusing on the cellular functions and molecular mechanisms of NVU, to discuss potential avenues of translating these mechanistic findings from in vitro and in vivo models to successful clinical applications.
The BBB
Blood vessels that supply the CNS have unique structures and mechanisms to control the movement of ions, molecules, and even cells between peripheral circulation and the cerebral environment.6 This interface, traditionally called the BBB, is essential in maintaining CNS homeostasis, which protects the CNS against infections, toxins, and the continually changing environment in the bloodstream.7
Most transportations of molecules across the BBB cells are strictly protected and regulated by protein transporters, and many are ATP-binding cassette transporters. For example, efflux pumps such as p-glycoprotein are highly expressed on the brain endothelial cells, actively recognizing many drug molecules, and transporting them back to the blood vessel without entering the brain through transcellular mechanisms. The paracellular movement of fluid and molecules across the BBB is restricted by adherens and tight junction (TJ) complexes between the cerebral endothelial cells.8,9
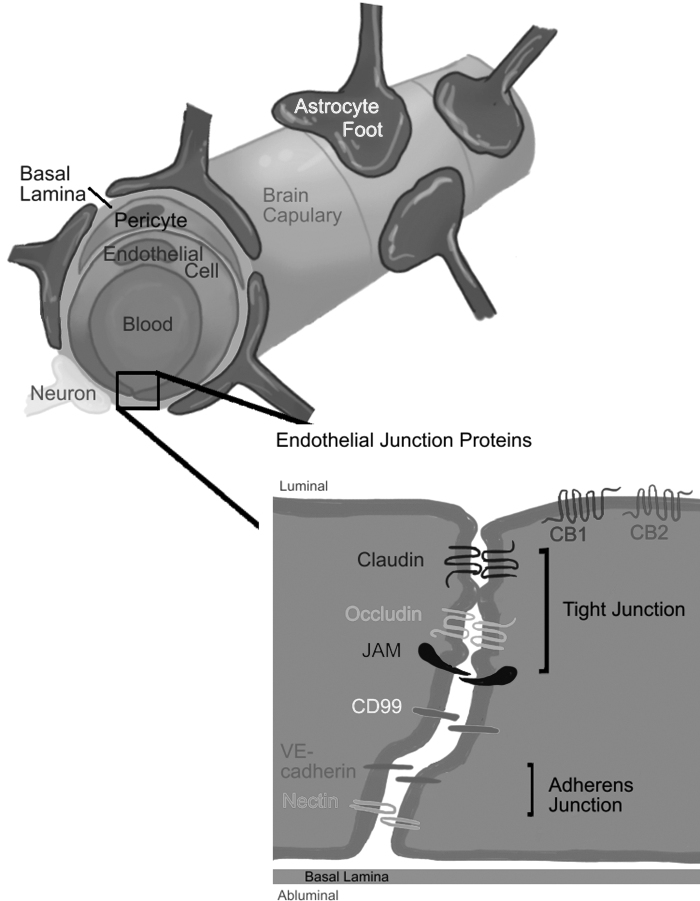
The NVU contributes to barrier integrity by comprehensive structures and interactions among endothelial cells, pericytes, and the end feet of surrounding astrocytes and neurons. Interactions between astrocytes and endothelial cells enhance the formation and development of the TJ complex.10 Pericytes regulate survival, migration, differentiation, and vascular branching of the endothelial cells.11 The interactions and coordination among these cells are important for the development and maintenance of the BBB integrity (Fig. 1).9
FIG. 1.
NVU are comprised of endothelial cells, pericytes, and astrocytes, and associated neurons and microglia. Adherens junctions (VE-cadherin and nectin) and tight junctions (claudin, occludin, JAM), and another transmembrane protein CD99 connected to the actin cytoskeleton are located at the border of the brain microvascular endothelial cells of the BBB (as illustrated). Association of these transmembrane junctions and cytoplasmic proteins result in the high resistance across the BBB. We hypothesize that some of these tight junction molecules may be regulated through the endocannabinoid system. BBB, blood–brain barrier; JAM, junction adhesion molecule; NVU, neurovascular units; VE-cadherin, vascular endothelial cadherin.
Endothelial Junctions at the BBB
Endothelial junctions, including adherens junctions (AJs) and TJs, are responsible for the highly restrictive and selective molecular permeation through BBB paracellular route.12–14 The BBB is characterized by high expression levels of integral TJ proteins and low expression level of AJs,15–18 but stable AJs are still needed for the existence of TJs. For example, vascular endothelial cadherin (VE-cadherin) causes phosphorylation of the transcription factor FoxO1, allowing it to activate the expression of TJ protein claudin-5.19 AJs comprise a cluster of cadherins and associated molecules, including p120-catenin, β-catenin, and α-catenin.20 AJ mediate endothelial cell–cell adhesion through homotypic interactions between VE-cadherins.21 They also provide a link between transmembrane proteins and the actin cytoskeleton.16–18
TJs consist of integral membrane proteins, such as occludin and claudin, together with the cytoplasmic accessory proteins, such as zonula occludin ZO-1 and ZO-2.9,22 Endothelial TJ proteins interact with the same proteins of adjacent endothelial cells to control paracellular diffusion of solutes and ions, and limit the free movement of lipids and proteins from the apical and basolateral cell surfaces, thus, contributing to the polarity of the BBB.23
BBB Dysfunctions or Breakdown and the Endothelial Junctions
BBB dysfunctions can be observed in neurological disorders or brain injuries, such as epilepsy, multiple sclerosis, Alzheimer's disease, hemorrhagic or ischemic stroke, and ischemia by various causes. Pathological conditions of BBB can undermine its function of protection, selective transportation, and clearance, thus can be the direct or indirect cause for these diseases.
BBB dysfunction could be complete barrier breakdown or subtle barrier impairments without manifesting end-organ damage.7 Due to the constitution of the BBB, it is imperative to understand that BBB dysfunction involves myriad signaling cascades that affect the different components during a pathological disorder. BBB dysfunction can also be termed acute or chronic, considering the molecular mechanisms and clinical presentation during a pathological condition.24 Acute BBB dysfunction results in cytotoxic and vasogenic edema formation, ionic imbalance that may promote seizures and inflammation. Chronic effects of BBB dysfunction are directed toward epilepsy or neuronal damages such as altered synaptic connectivity.25,26
BBB dysfunctions are often related to alterations in BBB structures and properties such as redistribution of TJs or leukocyte adhesion molecules.9 In vivo and in vitro studies have indicated that endothelial junction disruption is central for the BBB breakdown. Inflammation induced by TNF-α could alter cerebral endothelial permeability in cultured human brain endothelial cells.27 TNF-α and IL-6 downregulated ZO-1 expression and occludin/ZO-1 association, which correlates with ZO-1 phosphorylation tyrosine and threonine sites.28
In animal models, ischemia/reperfusion stimulates actin polymerization in brain endothelial cells through phosphorylation of myosin light chain (MLC) and Rho-associated protein kinase-enhanced postischemia MLC phosphorylation that lead to the formation of F-actin-enriched stress fibers that increase cellular tension.29 These cytoskeletal alterations induce redistribution of junctional transmembrane proteins to the cytosol, loosening the paracellular pathway. These reports confirmed that the changing permeability of endothelial cells in the brain can be caused by redistribution of TJ that interacts between the actin cytoskeleton and other scaffolding proteins.30
The ECS
The identification of psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) from cannabis31 further led to the discovery of cannabinoid receptors and the ECS in the 1990s.
The ECS comprises cannabinoid receptor proteins, endocannabinoid (eCB) ligands, such as N-arachidonoylethanolamine (AEA, also called anandamide) and 2-arachidonylglycerol (2-AG), and their synthesis and degradation enzymes.32,33 AEA is synthesized in vivo through hydrolysis by phospholipase D from a membrane phospholipid precursor, N-arachidonoyl phosphatidylethanolamine. Another important eCB in the brain, 2-AG, is synthesized mainly by diacylglycerol lipase from diacylglycerol. AEA is degraded mainly by fatty acid amide hydrolase (FAAH), and 2-AG by monoacylglycerol lipase (MAGL).34 FAAH is a membrane-bound homodimer, and MAGL a 303-amino-acid protein (∼33 kDa), both belong to the enzyme family of serine hydrolase.
MAGL has no identifiable transmembrane domain and is associated with cytosolic and particulate compartments.35,36 These enzymes of biosynthesis and degradation may regulate ECS signaling and tone throughout the human body.37 How these enzymes influence homeostasis and functions under pathological conditions are still an important subject of ongoing basic and clinical research.
The cannabinoid receptor family has at least two G protein-coupled receptors: CB1 and CB2.38,39 The CB1 receptor was discovered in the CNS and is particularly abundant in specific brain areas such as basal ganglia, cerebellum, and hippocampus.38 It is also expressed in human retina, peripheral neurons, testis, sperm cells, colonic tissues, adipocytes, and other organs such as the adrenal gland, heart, lungs, prostate, uterus, and ovary.40–42 The CB2 receptor is abundantly expressed on immune cells, macrophages, monocytes, CD4+ and CD8+ T cells, and B cells.43
CB1 and CB2 are expressed in the brain on presynaptic and postsynaptic neuronal terminals.44 In the brain, CB1 is also found in hippocampal astrocytes.45 CB2 receptors are expressed at low levels in the brain under physiological conditions. However, their expression is upregulated in pathological conditions, such as traumatic brain injury (TBI), neurological diseases, stroke, or ischemia, caused by cardiovascular or pulmonary failures.46,47
The expressions of CB1 and CB2 in microglia change depending on the phenotype and activation profile.48 Healthy microglia cells usually do not express CB2 receptors.49 Studies on most primary cell cultures have demonstrated that CB2 expression are typically activated during the preparation of the cultures, hence detecting trace amounts of CB2 mRNA.50,51 At the BBB, both CB1 and CB2 are expressed in the human brain microvascular endothelial cells (HBMECs).1,52,53 CB1 and CB2 were also found in astrocytes.1 Their in vivo expression level and distribution in other NVU cells have not been published to our knowledge. Their mechanisms of regulation and coordination need to be better characterized for a complete picture of BBB ECS, so that related functions can be further revealed.
BBB Permeability and the ECS
The presence of ECS on the cerebral microvascular endothelia provides the basis to hypothesize that ECS may influence and regulate BBB permeability.14,16,54 A few studies have reported effects of cannabinoids on the BBB, but more systematic investigations focusing on the BBB ECS are still needed.
Table 1 summarized the cannabinoid effects on the BBB, from published in vitro and in vivo studies, and clinical trials. In 2008, Lu et al. reported that Δ9-THC could prevent the downregulation of ZO-1, claudin-5, and junction adhesion molecule 1 in HBMEC in vitro model.1 In mice models, activating CB2 receptor suppressed inflammation caused by TBI, prevented BBB damage, and attenuated expression increase of intercellular adhesion molecule 1 (ICAM-1). ICAM-1 promotes immune cell adherence to the endothelium and the transmigration caused by injury and inflammation.55–58 CB2 selective agonist JWH133 was shown to extenuate the increasing expression of ICAM-1, thus protect the BBB integrity.57 When another CB2 receptor agonist, JWH015, was injected to a rat model 20 min before transient spinal cord ischemia–reperfusion injury, occludin, and ZO-1 expression was upregulated at the blood–spinal cord barrier.59
Table 1.
Cannabinoid Effects on the Blood–Brain Barrier by in vitro and in vivo Models and a Published Clinical Trial
| Cannabinoids | Models | Effects and mechanism |
|---|---|---|
| Nonselective agonist, CP 55940; selective CB1 ACEA vs. inverse agonist AM251 | BBB coculture model of HBMEC and human astrocyte | Inhibition of HIV-1 Gp120-induced calcium influx mediated by substance P to decrease permeability of HBMEC and preventing downregulation of ZO-1, claudin-5, and JAM-1 in HBMEC.1 |
| CBD | BBB cellular coculture model of HBMEC and human astrocyte | Preventing increase in permeability caused by 4 h OGD; most effective when administered before the OGD.58 |
| Endocannabinoids of anandamide, oleoylethanolamide, PEA | BBB Cellular coculture model of HBMEC and human astrocyte | OEA, PEA decreased the OGD-induced increase in permeability during reperfusion.15 |
| Selective CB2 agonist JWH133 vs. selective CB2 antagonist SR144528 | Rat model of SAH | Reducing leukocyte infiltration improved neuro score, reduced water contents, and increased the ZO-1 expression, when SAH decreased.59 |
| Selective CB2 agonist JWH015 | Rat model of transient spinal cord ischemia | Downregulation of the expression of ICAM-1, upregulation of the expression of TJ proteins to decrease the permeability of BBB.57 |
| Agonist WIN55,212-2 | Mouse model of virus induced multiple sclerosis | Suppression of ICAM-1 and VCAM-1 in brain endothelium, together with a reduction in perivascular CD4+ T lymphocyte infiltrates and microglial responses.53 |
| Selective CB2 agonists (0-1966 and JWH133) vs. a selective CB2 antagonist | Wild-type C57BL/6 vs. CB2 knockout mice of CCI and craniotomy | Attenuation of TNF-α protein, ICAM-1 mRNA was increased at 6 h, and at 1 to 2 days after CCI, reduced in mice treated with a CB2 agonist, and increased in CB2 knockout mice with CCI.55 |
| Selective CB2 agonist, 0-1966 | C57BL/6 mice model of CCI | Decrease in permeability shown by reduction in NaF uptake and number of degenerating neurons. Prolonged reduction in macrophage/microglia cell counts.56 |
| Selective CB2 agonist AM1241 | Rat MCAo | Pretreatment with AM1241 significantly reduced brain infarction and neurological deficits.60 |
| Endocannabinoid 2-AG | Mice models of closed head injury | Decreased BBB permeability and inhibited the acute expression of the main proinflammatory cytokines: TNF-a, IL-1B, and IL-6.61 |
| PEA, an endocannabinoid, with Luteolin | Ischemic stroke patients; rat models | Significant improvement in neurological status, impairment of cognitive abilities, the degree of spasticity, pain, and independence in daily living.62 |
2-AG, 2-arachidonylglycerol; BBB, blood–brain barrier; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; CBD, cannabidiol; CCI, controlled cortical impact; HBMEC, human brain microvascular endothelial cells; ICAM-1, intercellular adhesion molecule 1; JAM-1, junction adhesion molecule 1; MCAo, model of middle cerebral artery occlusion; NaF, sodium fluorescein; OGD, oxygen and glucose deprivation; PEA, palmitoylethanolamide; SAH, subarachnoid hemorrhage; TJ, tight junction.
These findings indicated that cannabinoids can regulate BBB permeability through junction protein complexes, and CB2 selective agonist could prevent TJ breakdown induced by ischemia–reperfusion injury.59 To evaluate the barrier protection potentials of cannabinoids, as well as their impacts on the brain bioavailability of CNS drugs, these BBB regulation effects on BBB permeability need to be further characterized and quantified. Then, the questions about cannabinoid–drug interactions at the BBB also can be addressed, as both medical and recreational cannabinoid-based products become increasingly prevalent.
Discussions: ECS as Therapeutic Target to Protect BBB Integrity
Physiological processes regulated by the ECS include homeostasis, energy balance, gastrointestinal motility, musculoskeletal development, cardiovascular regulation, fertility, immune functions, and CNS functions (such as synaptic plasticity, mood, memory, analgesia, pain transmission, movement, and food intake).60 As generally recognized, the ECS regulates through two molecular signaling pathways: neurotransmitters from neurons and cytokines from the immune cells.61 Clinically, cannabinoids have been approved as important therapeutic agents for epilepsy, vomiting, pain, and other potential CNS applications. If ECS directly regulates physical BBB permeability and protects its barrier functions, this can be another promising therapeutic paradigm of cannabinoid-based therapy for brain injuries, strokes, epilepsy, and neurodegenerative diseases.
In vitro studies using cocultures of HBMEC and astrocytes demonstrated cannabidiol's (CBDs) protective effects.62 Besides CB1 and CB2, it was suggested that these effects of cannabinoids may also exert through other targets such as PPARγ (peroxisome proliferator-activated receptor gamma) and 5-HT1A receptors, which are also expressed in brain endothelial cells.62 Studies using animal models have shown that enhancing eCB tone offers therapeutic benefits, by either adding exogenous cannabinoids or using FAAH inhibitors that prolong the eCB half-life.63,64 The effect of 2-AG for TBI recovery was demonstrated in mouse models. An increased level of 2-AG was observed in mouse models in the ipsilateral brain 1 and 24 h after TBI. In the same experiment, administration of additional 2-AG also resulted in reduced inflammation and edema, and improved clinical recovery through CB1-mediated mechanisms.63 In another in vitro model of ischemic stroke, rats exposed to 20-min oxygen and glucose deprivation had reduced brain hippocampal injury when treated with 2-AG.64
Before middle cerebral artery occlusion, pretreatment of mice with CB2 agonist AM1241 significantly reduced brain infarction and neurological deficits, while delayed treatment with AM1241 offered no protective benefit.65 In a rat model of subarachnoid hemorrhage, CB2 selective agonist JWH133 reduced leukocyte infiltration of the BBB, improved neuro score, and reduced edema, while ZO-1 expression also increased.66 These preclinical findings point to the putative BBB protection effect through ECS under pathological conditions.
Several clinical studies have reported promising results about cannabinoids' therapeutic potential of neuroprotection from TBI or stroke. As early as 1989, a nonpsychotropic cannabinoid HU210 demonstrated neuroprotective effects in animal models, but the latter did not find significant improvements through a clinical trial that enrolled 846 patients.67 eCB palmitoylethanolamide was tested on stroke rehabilitation patients in combination with luteolin, and reported improvements on spasticity and cognitive impairment.68 Also, oral mucosal spray of THC/CBD is under investigation for poststroke spasticity conditions.69 These studies focused on the neuroprotective mechanisms of cannabinoids, which may be related to the BBB ECS. Although these clinical studies focused on clinical endpoints of stroke without monitoring BBB, BBB integrity and function could be an important outcome indicator to demonstrate the clinical benefits of cannabinoids.
Also, osmotic substances, such as mannitol or hypertonic sodium chloride solutions, are used to reduce the cerebral edema and intracranial pressure during brain injury treatments. The intact BBB ensures osmotic gradient between the brain and the blood. These life-saving therapeutics are administered intravenously. As the BBB integrity is critical to withdraw water from the intra- and extracellular brain compartment to the endovascular compartment, compromised or leaky BBB is a frequent complication, leading to cytotoxic, ionic, or vasogenic cerebral edema in overlapping phases.70 Multiple intervention targets to protect the BBB have been proposed such as vascular endothelial growth factor, aquaporins, or ion channels.71–73 Direct protection of the BBB through ECS may be a promising and new therapeutic strategy to support osmotic treatment.
Challenges and Opportunities
Translational research and drug development to protect BBB are challenging, partially because of the BBB differences between human and animal models. Also, the BBB is a complex system between the CNS and the vascular networks, and the immune system; therefore, result interpretation from in vivo models and clinical data can be challenging. In addition, due to the ubiquitous and complex nature of the ECS throughout the body, modifying ECS balance may result in multidirectional or unpredictable clinical effects. To harvest the beneficial effects without interfering other ECS-containing organs, smart drug delivery and targeting strategies are important. The in vivo spatial distribution and regulation of human BBB ECS should be characterized and quantified to understand their physiology and pathology.
Nevertheless, translational and clinical research has been exciting to reveal the complex ECS as “endocannabinoidome,” which provides more comprehensive view that involves more endogenous cannabinoid ligands and targeting receptors, such as GPR55 and PPARs.74 With recent methodology advancements, characterizing and investigating the whole BBB ECS is becoming feasible. Investigations focusing on NVU are foundational and essential, and should be continued to reveal ECS mechanisms in whole.
Conclusion
Pioneering studies have confirmed the presence of ECS at the BBB. Endogenous, botanical, or synthetic cannabinoids have been found to enhance BBB barrier integrity by in vivo and in vitro models. Published evidence suggested that ECS may protect and regulate the BBB integrity, probably through modifying TJ protein complexes and other mechanisms. Future human clinical trials with proper treatment strategies and BBB assessments are needed to confirm these benefits. Continuing investigations at molecular and cellular mechanisms of BBB ECS are essential to provide practical guidance on cannabinoid selection, drug delivery strategies, dosing regimen design, sex-based difference monitoring, and other details, to develop and optimize pharmacotherapy protocols through clinical trials. We believe BBB ECS research can unlock the therapeutics potentials of cannabinoids for neurodegeneration, brain injuries, strokes, and ischemia caused by cardiorespiratory arrests or other diseases in the near future.
Acknowledgments
The authors thank Drs. Marcel Musteata, Jeffery Voigt, Timothy LaRocca, Mathew Philips, Alejandro Adam, and Hava Avraham for their continuous collaborations and critical review on this article. Figure 1 was illustrated by Emilia Johnson-Viola, a talented student.
Abbreviations Used
- Δ9-THC
Δ9-tetrahydrocannabinol
- 2-AG
2-arachidonylglycerol
- AEA
N-arachidonoylethanolamine
- AJs
adherens junctions
- BBB
blood–brain barrier
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CBD
cannabidiol
- CCI
controlled cortical impact
- CNS
central nervous system
- eCBs
endocannabinoids
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydrolase
- FoxO1
fork head box factor 1
- HBMEC
human brain microvascular endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- JAM-1
junction adhesion molecule 1
- MAGL
monoacylglycerol lipase
- MCAo
model of middle cerebral artery occlusion
- MLC
myosin light chain
- NaF
sodium fluorescein
- NVU
neurovascular unit
- OGD
oxygen and glucose deprivation
- PEA
palmitoylethanolamide
- PPARγ
peroxisome proliferator-activated receptor gamma
- SAH
subarachnoid hemorrhage
- TBI
traumatic brain injury
- TJs
tight junctions
- VE-cadherin
vascular endothelial cadherins
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Kofi Hagan thanks ACPHS for providing Graduate Research Assistantship for this project.
Cite this article as: Hagan K, Varelas P, Zheng HA (2022) Endocannabinoid system of the blood-brain barrier: current understandings and therapeutic potentials, Cannabis and Cannabinoid Research 7:5, 561–568, DOI: 10.1089/can.2021.0101.
References
- 1. Lu T-S, Avraham HK, Seng S, et al. Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells. J Immunol. 2008;181:6406–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vendel E, De Lange ECM. Functions of the CB1 and CB2 receptors in neuroprotection at the level of the blood-brain barrier. Neuromolecular Med. 2014;16:620–642. [DOI] [PubMed] [Google Scholar]
- 3. Kho DT, Glass M, Graham ES. Is the cannabinoid CB2 receptor a major regulator of the neuroinflammatory axis of the neurovascular unit in humans? Adv Pharmacol. 2017;80:367–396. [DOI] [PubMed] [Google Scholar]
- 4. Hua T, Vemuri K, Pu M, et al. Crystal structure of the human cannabinoid article crystal structure of the human cannabinoid receptor CB 1. Cell. 2016;167:750–762.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li X, Hua T, Vemuri K, et al. Crystal structure of the human cannabinoid article crystal structure of the human cannabinoid receptor CB2. Cell. 2019;176:459–467.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ribatti D, Nico B, Crivellato E, et al. Development of the blood-brain barrier: a historical point of view. Anat Rec B New Anat. 2006;289:3–8. [DOI] [PubMed] [Google Scholar]
- 7. Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. [DOI] [PubMed] [Google Scholar]
- 8. Wong AD, Ye M, Levy AF, et al. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987;7:3293–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lai CH, Kuo KH. The critical component to establish in vitro BBB model: pericyte. Brain Res Rev. 2005;50:258–265. [DOI] [PubMed] [Google Scholar]
- 12. Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hind WH, Tufarelli C, Neophytou M, et al. Endocannabinoids modulate human blood-brain barrier permeability in vitro. Br J Pharmacol. 2015;172:3015–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golech SA, McCarron RM, Chen Y, et al. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Mol Brain Res. 2004;132:87–92. [DOI] [PubMed] [Google Scholar]
- 17. Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–1237. [DOI] [PubMed] [Google Scholar]
- 18. Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist's view. Brain Res Rev. 2003;42:221–242. [DOI] [PubMed] [Google Scholar]
- 19. Taddei A, Giampietro C, Conti A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. [DOI] [PubMed] [Google Scholar]
- 20. Adams CL, Chen YT, Smith SJ, et al. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shigetomi K, Ono Y, Inai T, et al. Adherens junctions influence tight junction formation via changes in membrane lipid composition. J Cell Biol. 2018;217:2373–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. [DOI] [PubMed] [Google Scholar]
- 24. Schoknecht K, Shalev H. Blood-brain barrier dysfunction in brain diseases: clinical experience. Epilepsia. 2012;53(Suppl 6):7–13. [DOI] [PubMed] [Google Scholar]
- 25. Seiffert E, Dreier JP, Ivens S, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tomkins O, Friedman O, Ivens S, et al. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25:367–377. [DOI] [PubMed] [Google Scholar]
- 27. Ni Y, Teng T, Li R, et al. TNFα alters occludin and cerebral endothelial permeability: role of p38MAPK. PLoS One. 2017;12:e0170346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rochfort KD, Cummins PM. Cytokine-mediated dysregulation of zonula occludens-1 properties in human brain microvascular endothelium. Microvasc Res. 2015;100:48–53. [DOI] [PubMed] [Google Scholar]
- 29. Shi Y, Zhang L, Pu H, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun. 2016;7:e0170346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang X, Andjelkovic A V., Zhu L, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163–164:144–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of Hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 32. Devane WA, Hanus L, Breuer A, et al. Constituent receptor. Lancet. 1992;1558:19–22. [Google Scholar]
- 33. Sugiura T, Kondo S, Sukagawa A, et al. 2-arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. [DOI] [PubMed] [Google Scholar]
- 34. Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. [DOI] [PubMed] [Google Scholar]
- 35. Bachur NR, Udenfriend S. Microsomal synthesis of fatty acid amides. J Biol Chem. 1966;241:1308–1313. [PubMed] [Google Scholar]
- 36. Long JZ, Li W, Booker L, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schurman LD, Lichtman AH. Endocannabinoids: a promising impact for traumatic brain injury. Front Pharmacol. 2017;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devane WA, Dysarz FA, Johnson MR, et al. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 39. Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol. 1995;35:607–634. [DOI] [PubMed] [Google Scholar]
- 40. Gerard CM, Mollereau C, Vassart G, et al. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Straiker A, Stella N, Piomelli D, et al. Cannabinoid CB1 receptors and ligands in vertebrate retina: localization and function of an endogenous signaling system. Proc Natl Acad Sci U S A. 1999;96:14565–14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. [DOI] [PubMed] [Google Scholar]
- 43. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. [DOI] [PubMed] [Google Scholar]
- 44. Katona I, Sperlágh B, Sík A, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. [DOI] [PubMed] [Google Scholar]
- 46. Galiègue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. [DOI] [PubMed] [Google Scholar]
- 47. Kolb B, Saber H, Fadel H, et al. The endocannabinoid system and stroke: a focused review. Brain Circ. 2019;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carlisle SJ, Marciano-Cabral F, Staab A, et al. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2:69–82. [DOI] [PubMed] [Google Scholar]
- 50. Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol. 2003;139:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walter L, Franklin A, Witting A, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Helms HC, Abbott NJ, Burek M, et al. In vitro models of the blood-brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab. 2015;36:862–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nuñez-Lumbreras M de los Á, Castañeda-Cabral JL, Valle-Dorado MG, et al. Drug-resistant temporal lobe epilepsy alters the expression and functional coupling to Gαi/o proteins of CB1 and CB2 receptors in the microvasculature of the human brain. Front Behav Neurosci. 2021;14:611780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maccarrone M, Fiori A, Bari M, et al. Regulation by cannabinoid receptors of anandamide transport across the blood-brain barrier and through other endothelial cells. Thromb Haemost. 2006;95:117–127. [PubMed] [Google Scholar]
- 55. Mestre L, Docagne F, Correa F, et al. A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulating adhesion molecules. Mol Cell Neurosci. 2009;40:258–266. [DOI] [PubMed] [Google Scholar]
- 56. Dowie MJ, Grimsey NL, Hoffman T, et al. Cannabinoid receptor CB2 is expressed on vascular cells, but not astroglial cells in the post-mortem human Huntington's disease brain. J Chem Neuroanat. 2014;59–60:62–71. [DOI] [PubMed] [Google Scholar]
- 57. Amenta PS, Jallo JI, Tuma RF, et al. Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J Neuroinflammation. 2014;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Amenta PS, Jallo JI, Tuma RF, et al. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res. 2012;90:2293–2305. [DOI] [PubMed] [Google Scholar]
- 59. Yang MC, Zhang HZ, Wang Z, et al. The molecular mechanism and effect of cannabinoid-2 receptor agonist on the blood-spinal cord barrier permeability induced by ischemia-reperfusion injury. Brain Res. 2016;1636:81–92. [DOI] [PubMed] [Google Scholar]
- 60. Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. [DOI] [PubMed] [Google Scholar]
- 61. Maccarrone M. Metabolism of the endocannabinoid anandamide: open questions after 25 years. Front Mol Neurosci. 2017;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hind WH, England TJ, O'Sullivan SE. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br J Pharmacol. 2016;173:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Panikashvili D, Shein NA, Mechoulam R, et al. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22:257–264. [DOI] [PubMed] [Google Scholar]
- 64. Nagayama T, Sinor AD, Simon RP, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu SJ, Reiner D, Shen H, et al. Time-dependent protection of CB2 receptor agonist in stroke. PLoS One. 2015;10:e0132487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fujii M, Sherchan P, Soejima Y, et al. Cannabinoid receptor type 2 agonist attenuates apoptosis by activation of phosphorylated CREB-Bcl-2 pathway after subarachnoid hemorrhage in rats. Exp Neurol. 2014;261:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maas AIR, Murray G, Henney H, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5:38–45. [DOI] [PubMed] [Google Scholar]
- 68. Caltagirone C, Cisari C, Schievano C, et al. Co-ultramicronized palmitoylethanolamide/luteolin in the treatment of cerebral ischemia: from rodent to man. Transl Stroke Res. 2016;7:54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marinelli L, Balestrino M, Mori L, et al. A randomised controlled cross-over double-blind pilot study protocol on THC:CBD oromucosal spray efficacy as an add-on therapy for post-stroke spasticity. BMJ Open. 2017;7:e016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab. 2016;36:513–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. King ZA, Sheth KN, Kimberly WT, et al. Profile of intravenous glyburide for the prevention of cerebral edema following large hemispheric infarction: evidence to date. Drug Des Devel Ther. 2018;12:2539–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Robert SM, Reeves BC, Alper SL, et al. New drugs on the horizon for cerebral edema: what's in the clinical development pipeline? Expert Opin Investig Drugs. 2020;29:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roth P, Regli L, Tonder M, et al. Tumor-associated edema in brain cancer patients: pathogenesis and management. Expert Rev Anticancer Ther. 2013;13:1319–1325. [DOI] [PubMed] [Google Scholar]
- 74. Piscitelli F, Carta G, Bisogno T, et al. Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutr Metab. 2011;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]