Abstract
Introduction:
Phytocannabinoids have emerged as a potential alternative treatment option for individuals experiencing persistent pain. However, evidence-based research regarding their clinical utility in both males and females remains incomplete. In addition, it is unknown whether combining readily available cannabinoids with opioids has a synergistic or subadditive effect on pain modulation. To begin to fill this knowledge gap, we investigated the antinociceptive effects of the phytocannabinoid, CBD, either alone or in combination with opioids in male and female C57BL/6J mice.
Results:
Using the formalin test, our results show that CBD (10 mg/kg, i.p.) treatment evoked antinociception in phase I, but not in phase II, of the formalin test in male mice. However, in female mice, CBD showed no significant antinociceptive effect. In addition, a direct sex comparison showed that CBD evoked a significant increase in nociceptive behaviors in female versus male mice during phase I of the formalin test. Furthermore, we show that CBD (10 mg/kg, i.p.) in combination with low-dose morphine (1 mg/kg, i.p.) was ineffective at eliciting a synergistic antinociceptive response in both male and female mice. Lastly, consistent with previous literature, we showed that females treated with a relatively higher dose of morphine (10 mg/kg, i.p.) displayed a significant increase in the variability of nociceptive behaviors compared to morphine-treated male mice.
Conclusion:
Overall, our results suggest that CBD treatment may have beneficial antinociceptive effects during the acute phase of persistent pain, but these effects are more beneficial to males than females. We provide further pre-clinical support that treatments geared toward reducing nociceptive behaviors differentially affect males and females.
Keywords: cannabinoids, formalin assay, CBD, male and female mice, pain
Introduction
Currently, opioids are considered the gold standard for pain treatment.1 However, the side effects, including sedation, respiratory depression, tolerance, and abuse liability, reduce the effectiveness of opioids.1,2 In addition, it has been demonstrated in pre-clinical studies that opioids are more effective in males than in females, with females requiring higher doses of opioids for a comparable therapeutic effect.3–8 This has the potential to lead to an increased risk of negative side effects. Due to the ongoing opioid epidemic, there is a need to identify opioid alternatives for pain management that take into account sex-specific outcomes.
There is mixed evidence that activation of the endocannabinoid system is effective in reducing pain, which is based on clinical studies testing the effectiveness of Cannabis sativa and its main active ingredient, Δ9-THC.9–13 Unfortunately, the use of Δ9-THC comes with cognitive risks, organ-specific toxicity, and high abuse potential making it an ineffective substitute for opioids.14 Despite this, C. sativa contains numerous other cannabinoids that are potentially effective in treating pain due to their binding affinity to receptors within the endocannabinoid system as well as the binding affinity to nociceptive receptors.15–17
In pre-clinical models, it has been shown that CBD reduces pain alone or in combination with other cannabinoids.18–20 In addition, in clinical and pre-clinical research, it has been shown that CBD has low abuse potential.21,22 This has led to a growing market of pure CBD available for over-the-counter use, but evidence-based research regarding the clinical utility of pure CBD remains incomplete. In addition, it is unknown whether combining readily available cannabinoids with opioids will have synergistic effects on pain modulation, thus enabling CBD to act as a potential opioid limiting therapeutic in both males and females.
To fill this knowledge gap and assess the effects of CBD on pain, we used the formalin test. The formalin test is a widely used model of persistent pain that evokes two distinct phases of nociceptive behavior, with an initial acute phase (phase I) followed by an inflammatory phase (phase II).23 Phase I occurs 0–15 min after formalin injection into the rodent paw with pain behaviors associated predominantly with chemical stimulation of C fiber nociceptors,24 which are involved in slow, poorly localized pain perception.25 Phase II occurs 15–60 min postformalin injection and is associated with peripheral inflammatory processes produced by inflammatory mediators released following tissue injury.23
Here, using the formalin test, we assessed the antinociceptive potential of pure CBD both alone and in combination with morphine in male and female mice.
Methods
Animals
All experiments were performed in accordance with procedures approved by the Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee. Mice used in this study included male and female C57BL/6 wild-type mice age matched (10–12 weeks; Jackson Laboratory, Bar Harbor, ME). All mice were group housed on a 12-h light/dark cycle with ad libitum food and water.
Drugs
(−)-morphine sulfate pentahydrate was provided by the National Institute on Drug Abuse Drug Supply Program. CBD was purchased from Cayman Chemical (Ann Arbor, MI; Cat. # 90080). CBD-d3 (HPLC standard) was purchased from Sigma Aldrich (St. Louis, MO). Formalin solution was prepared from 37% formaldehyde stock solution (Cat. # F79; Thermo Fisher Scientific, Waltham, MA).
Formalin testing
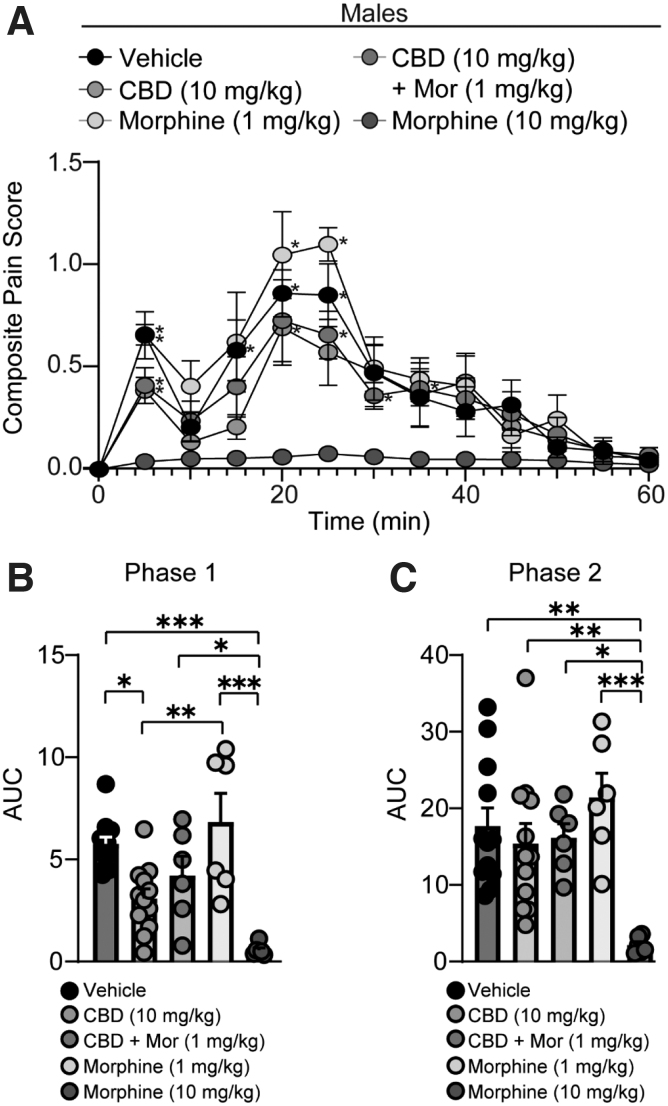
Mice received injections of vehicle (DMSO, Tween 80, saline [1:1:18], i.p.), CBD (10 mg/kg, i.p.), morphine (1 mg/kg, i.p.) (low-dose morphine), or morphine (10 mg/kg, i.p.) (high-dose morphine) 1 h before tests by an experimenter blinded to treatment. High-dose morphine (10 mg/kg) was used as a positive control due to its antinociceptive effects in both male and female mice (Figs. 1 and 2), while low-dose morphine (1 mg/kg) was used due to the lack of antinociceptive effects observed in the formalin assay in both male and female mice (Figs. 1 and 2). Mice were randomly assigned to groups.
FIG. 1.
CBD reduces nociceptive behaviors in male mice, but in combination with low-dose morphine, CBD does not evoke synergistic antinociceptive effects. (A) Summary of the time course corresponding to the composite pain score from 0 to 60 min after formalin injection (F(48,444)=1.424, p=0.0374; two-way repeated-measures ANOVA with Tukey's post-test) (Vehicle: n=12; CBD: n=12; CBD + Mor: n=6; Mor [1 mg/kg]: n=6; Mor [10 mg/kg]: n=6). Asterisks denote significant difference from high-dose morphine (10 mg/kg). (B) Summary graph showing the AUC during phase I of the formalin assay (F(4,37)=11.78, p<0.0001; one-way ANOVA with Tukey's post-test). (C) Summary graph showing the AUC during phase II of the formalin assay (F(4,37)=5.999, p=0.0008; one-way ANOVA with Tukey's post-test). *p<0.05, **p<0.01, ***p<0.001. ANOVA, analysis of variance; AUC, area under the curve.
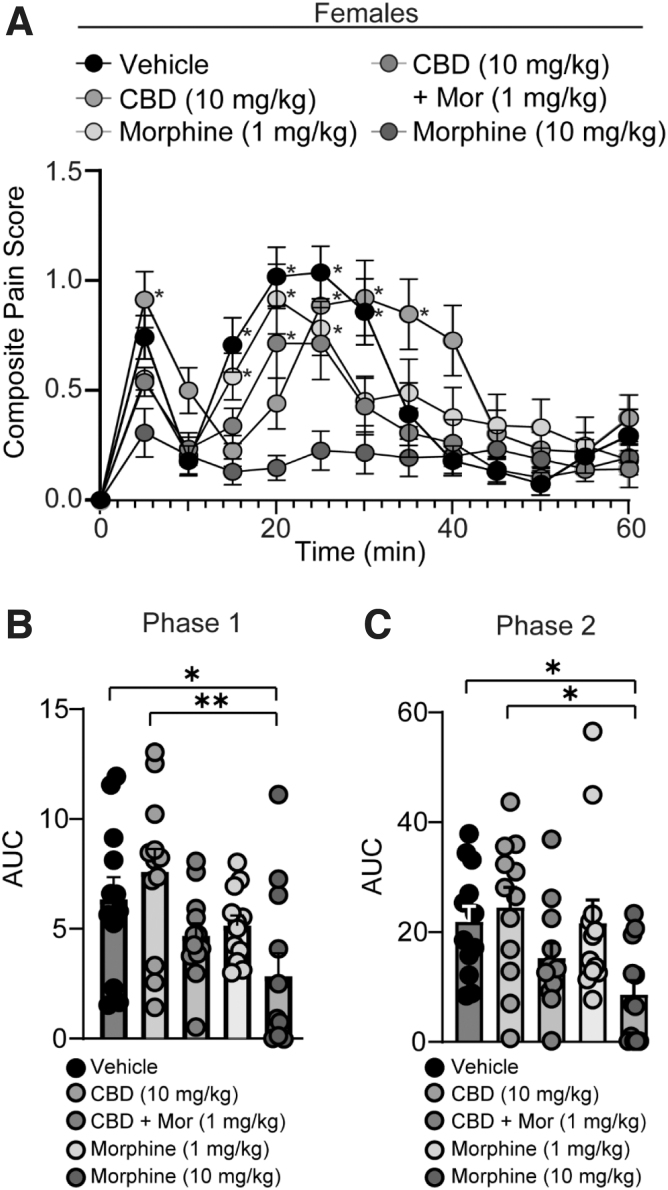
FIG. 2.
CBD alone or in combination with low-dose morphine does not reduce pain during phase I or phase II of the formalin test in female mice. (A) Summary of the time course corresponding to the composite pain score from 0 to 60 min after formalin injection (F(48,660)=4.181, p<0.0001; two-way repeated-measures ANOVA with Tukey's post-test) (Vehicle: n=12; CBD: n=12; CBD + Mor: n=12; Mor [1 mg/kg]: n=12; Mor [10 mg/kg]: n=12). Asterisks denote significant difference from high-dose morphine (10 mg/kg). (B) Summary graph showing the AUC during phase I of the formalin assay (F(4,55)=4.236, p=0.0046; one-way ANOVA with Tukey's post-test). (C) Summary graph showing the AUC during phase II of the formalin assay (F(4,55)=3.820, p=0.0082; one-way ANOVA with Tukey's post-test). *p<0.05, **p<0.01.
Mice were acclimated to a plexiglass observation chamber (in cm: 13×13×13) on a transparent table for 20 min. After the 20-min acclimation period, 2.5% formalin solution (10 μL) was injected to the plantar surface of the right hind paw. Mice were immediately placed back into the plexiglass observation chamber. Mouse behavior was recorded for 60 min using a GoPro camera (San Mateo, CA), which was placed underneath the observation chamber.
Four behavioral categories were observed by a trained observer (blinded to condition), including (1) no behavior, (2) little or no weight placed on the injected paw, (3) the injected paw is raised, or (4) licking, shaking, biting, or rapid lifting of the injected paw. These behaviors were assessed during twelve 5-min (i.e., 300 sec) bins.
The time that the animal spent displaying each behavior was recorded and a weighted composite score was calculated based on the following formula for each 5-min bin, as previously published26–28: , where a refers to the time the animal spent placing little or no weight on the injected paw, b refers to the time the animal spent raising the injected paw, c refers to the time the animal spent licking, shaking, biting, or rapid lifting of the injected paw, and d refers to the total time (i.e., 300 sec). The weighted composite score was selected based on the severity of pain associated with c.
Based on this formula, the maximum composite score would equal 2, signifying a maximum pain state. The area under the curve was calculated for the acute phase (phase I; 0–15 min) and the inflammatory phase (phase II; 15–60 min) using GraphPad Prism (9.1.2).
Analysis of plasma drug levels
Two hours following CBD (10 mg/kg, i.p.) treatment, mice were anesthetized with isoflurane and tail blood was collected in a microtube coated with EDTA-K2 (Cat. # 041-TOM-14C; Milian Dutscher Group). Whole blood was spun at 4°C–2000 RPM for 10 min at which point serum (supernatant) was pipetted off and placed in sterile tubes stored at −80°C until analysis.
Plasma concentrations of CBD were determined using mass spectrometry. Standard curves were constructed by plotting the ratio of the analyte peak area to internal standard peak area versus analyte concentration. The standard working solution (4 μL) and internal standard (4 μL) were spiked into control plasma (10 μL), and after vortexing, acetonitrile/H2O/formic acid (90/10/0.1) (22 μL) was added to extract the analytes from plasma. Proteins were precipitated by vortexing with subsequent centrifugation at 8765 g for 10 min at 4°C. The supernatant was taken and loaded to the high-performance liquid chromatography/mass spectrometry/mass spectrometry (HPLC/MS/MS) system, with final concentrations of 0.025 ng/mL to 1000 ng/mL for CBD.
Treated plasma was processed the same way as standards: after spiking internal standards (4 μL) into plasma (10 μL), samples were vortexed and acetonitrile/H2O/formic acid (90/10/0.1) (26 μL) was added for extraction. The calculated concentrations from the standard curves were multiplied by 4 to reflect the in vivo levels of CBD in plasma.
CBD in plasma was analyzed using a Sciex QTRAP 6500+ mass spectrometer coupled with a Sciex EXion HPLC separation system. A 1.7 μm Acquity UPLC BEH C18 analytical column (2.1×100 mm; Waters, Ireland) was used to separate CBD with other isomers as well as impurities. The gradient elution was conducted using a flow rate of 0.4 mL/min with the following conditions: initial at 70% mobile phase B (acetonitrile) and 30% mobile phase A (0.1% formic acid in water), followed by a linear gradient to 90% mobile phase B in 1 min, and kept at 90% mobile phase B for three additional minutes to flush the column before back to initial conditions to equilibrate the column.
The Sciex QTrap 6500+ mass spectrometer was equipped with an electrospray ionization probe operated in positive active mode. The decluster potential was 70 V for CBD; the entrance potential was 10 V, the collision energy was 33 V, and the collision cell exit potential was 12 V for CBD while the curtain gas was 35 L/h, and the collision gas (CAD) was medium. The ion spray voltage was 5500 V, the temperature was 550°C, gas 1 was 15 L/h, and gas 2 was 15 L/h.
The multiple reaction monitoring mode was used to analyze and quantify CBD as well as CBD-d3, with the transitions of m/z 315>193 for CBD and 318>196 for CBD-d3. All peaks were integrated and quantified by Sciex OS 1.5 software.
Statistical analysis
All results are shown as mean±SEM. Each experimental group consisted of at least six mice. No data points were excluded. Statistical significance was assessed in GraphPad Prism software (9.1.2) using a one-way analysis of variance (ANOVA), two-way ANOVA, or a two-way repeated-measures ANOVA with Tukey's correction for multiple comparisons to identify differences as specified. F-values for two-way ANOVA statistical comparisons represent interactions between variables unless otherwise stated. Two-tail tests were performed for all studies.
Results
Male and female mice respond differently to CBD administration during phase I of the formalin test
We investigated how male and female mice responded to CBD treatment (10 mg/kg, i.p.) during phase I and phase II of the formalin test. The CBD dose of 10 mg/kg was selected based on our observation that systemic injections of CBD (10 mg/kg, i.p.) in female mice produced blood plasma concentrations that were within the range observed in humans after smoking CBD-containing cigarettes (152.2±15.5 ng/mL, 483.99±49.28 nM; Table 1).29 In addition, the CBD blood plasma concentrations observed were within the range shown to reduce pain and decrease depressive-like behaviors in rats and male mice, respectively (Table 1).30,31
Table 1.
Plasma Concentrations of CBD in Females at 2 h After i.p. Injection
| Plasma level | ||
|---|---|---|
| Drug | Dose (mg/kg) | Steady state (ng/mL) |
| CBD (n=8) | 10 | 152.2±15.5 |
Mean±SEM.
Furthermore, it has been previously shown that this dose of CBD is effective at reducing mechanical allodynia in female neuropathic mice,32,33 and is below the plasma concentrations known to produce adverse effects in mammals.34,35 Using this dose, we found that CBD was effective at reducing nociceptive behaviors in male mice during phase I (vehicle- vs. CBD-treated male mice: p=0.0105, Tukey's post-test), but not phase II (vehicle- vs. CBD-treated male mice: p=0.9429, Tukey's post-test), of the formalin test (Fig. 1A–C).
In contrast, we found that CBD was ineffective at preventing nociceptive behaviors in female mice during both phase I (vehicle- vs. CBD-treated female mice: p=0.8473, Tukey's post-test) and phase II (vehicle- vs. CBD-treated female mice: p=0.9802, Tukey's post-test) of the formalin test (Fig. 2A–C). These data demonstrate that males and females respond differently to CBD treatment.
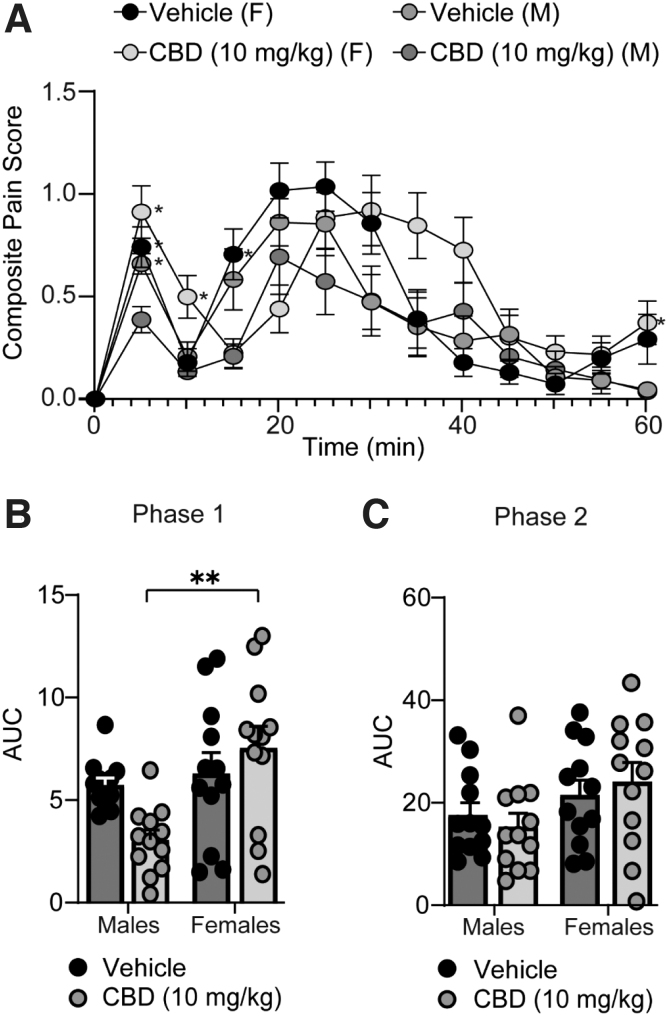
Based on this finding, we next performed analyses to directly compare sex differences between males and females treated with CBD using the data shown in Figures 1 and 2. First, we found that there were no significant sex differences in nocifensive behaviors between vehicle control groups during phase I (p=0.9553, Tukey's post-test) (Fig. 3A, B) or II of the formalin test (p=0.7849, Tukey's post-test) (Fig. 3A, C), suggesting that male and female mice express similar nociceptive responses to formalin injection.
FIG. 3.
CBD differentially alters nociception during phase I, but not phase II, of the formalin test in male versus female mice. (A) Summary of the time course corresponding to the composite pain score from 0 to 60 min after formalin injection (F(36,528)=2.905, p<0.0001; two-way repeated-measures ANOVA with Tukey's post-test) (Vehicle [male (M)]: n=12; CBD [M]: n=12; Vehicle [female (F)]: n=12; CBD [F]: n=12). Asterisks denote significant difference from males treated with CBD (10 mg/kg). (B) Summary graph showing the AUC during phase I of the formalin assay (F(1,44)=6.284, p=0.0160; two-way ANOVA with Tukey's post-test). (C) Summary graph showing the AUC during phase II of the formalin assay (F(1,44)=0.6902, p=0.4106; two-way ANOVA with Tukey's post-test). Male/female vehicle and CBD group data were the same data shown in Figures 1 and 2, but reanalyzed to directly investigate sex differences using a two-way ANOVA. *p<0.05, **p<0.01.
We next performed a pairwise comparison to statistically compare the effects of CBD treatment between male and female mice. We observed that CBD-treated female mice displayed a significant increase in nociceptive behaviors compared to CBD-treated male mice during phase I of the formalin test (p=0.0011, Tukey's post-test) (Fig. 3B). In contrast, during phase II of the formalin test, no sex differences were observed between CBD-treated mice (p=0.1651, Tukey's post-test) (Fig. 3C).
CBD in combination with low-dose morphine has no effect on formalin-induced nociception in male or female mice
To investigate whether a synergistic antinociceptive effect exists when coadministering CBD (10 mg/kg) with low-dose morphine (1 mg/kg), we measured nocifensive behaviors in male and female mice using the formalin test.
In male mice, we found that CBD (10 mg/kg, i.p.) in combination with low-dose morphine (1 mg/kg, i.p.), which does not evoke antinociception in the formalin assay (Figs. 1 and 2), did not further reduce formalin-induced nociception (Fig. 1A–C). In contrast, morphine, at the higher dose of 10 mg/kg, abolished formalin-induced nociception during both phase I and phase II of the formalin test (Fig. 1A–C).
In female mice, we found that CBD in combination with low-dose morphine (1 mg/kg, i.p.) did not reduce nociceptive behaviors during either phase I or phase II of the formalin test (Fig. 2A–C). However, we did observe that high-dose morphine (10 mg/kg, i.p.) significantly reduced formalin-induced nociception during phase I and phase II of the formalin test in female mice (Fig. 2A–C).
Lastly, we found that, in comparison with females, males displayed significantly less variability in their antinociceptive response to high-dose morphine (10 mg/kg) treatment during both phases of the formalin test (Phase I: F(11,5)=159.4, p<0.0001; Phase II: F(11,5)=70.01, p=0.0002; F test to compare variances).
Discussion
In this study, first, we found that CBD was effective at reducing nociceptive behaviors in male mice, but only during phase I of the formalin test. Second, we observed that CBD treatment did not reduce nociceptive behaviors in female mice during phase I or II of the formalin test. Third, we found that CBD-treated females showed a significant increase in nociceptive behaviors compared to CBD-treated male mice, but only during phase I of the formalin test. Fourth, we found that CBD (10 mg/kg) in combination with low-dose morphine (1 mg/kg) was ineffective at producing synergistic antinociception responses in both male and female mice.
Lastly, we showed that female mice responded to the high-dose morphine (10 mg/kg, i.p.) treatment with a significantly greater variability than male mice during both phases of the formalin test.
CBD effects on nociception in the formalin test
Here, we show that CBD, in male mice, is effective at attenuating acute pain (i.e., phase I) in the formalin test. Our results partly align with another study that demonstrated that CBD (5 and 50 mg/kg, i.p.) did not reduce nociceptive behaviors in the formalin test in male rats.36 The reason that we observed a significant antinociceptive effect in CBD-treated male mice during phase I of the formalin assay may be due to the species used (rat vs. mouse) or the dose of CBD, as evidence suggests that the dose/effect curve of CBD in relation to behavioral effects follows a U shape rather than a sigmoidal curve.37
An important feature of the formalin test is that it includes two distinct nociceptive phases. The first phase, which represents acute pain, begins immediately after injection, likely through formalin-induced C fiber activation.23,24 The second phase of the formalin test is mediated by inflammatory factors, including substance P, bradykinin, histamine, serotonin, and prostaglandins,38 which activate, in part, transient receptor potential vanilloid (TRPV) and transient receptor potential subfamily A (TRPA) channels directly or indirectly through the activation of downstream signaling pathways.25,39
Importantly, the representative acute and inflammatory pain states of the formalin test do not actually represent disease-specific acute or inflammatory pain. Clinically, acute pain is mediated by nociceptor activation following exposure to a mechanical, thermal, or chemical stimulus, while inflammatory pain is mediated by the release of endogenous signaling molecules (i.e., inflammatory soup) that sensitize primary afferent nerve fibers.25
Although the formalin test encompasses many similar underlying mechanisms of acute and inflammatory pain as those manifested clinically (e.g., activation of nociceptive C fibers during the acute phase and peripheral inflammatory processes during phase II23), the results may not translate to the human condition due to the engagement of highly plastic molecules and circuits that are unique to each individual's response to a given pain condition. Even in animal models of disease, antinociceptive responses will vary to treatments that target the endocannabinoid system.40
For example, a systematic review and meta-analysis of modulators of the endocannabinoid system found variability in treatment outcomes based on the pain model implemented with the largest attenuation of pain-associated behavior reported in models of burn injury and the smallest significant attenuation reported in models of inflammation.40 Furthermore, it was found that CBD significantly attenuated nocifensive behaviors in neuropathic pain models with mixed results in inflammatory pain models.40 These findings suggest that treatment options targeting the endocannabinoid system may be more effective for some forms of pain rather than others.
Another interesting aspect to our findings was that attenuation of the first phase of the formalin test in males was not sufficient to prevent the transition to the second phase of persistent pain. Given that persistent pain is a consequence of the initial acute response to a nociceptive stimulus, it would be expected that attenuating the nociceptive response to phase I of the formalin test would mitigate the nociceptive response to phase II.
It has been previously shown that local anesthesia at the site of the formalin injection or spinal cord anesthesia given during the first phase is sufficient to prevent or reduce the second nociceptive phase of the formalin test.41–43 Future studies will need to address the mechanisms mediating CBD-induced reduction in phase I of the formalin test to help understand the lack of effect as acute pain transitions to persistent pain.
Despite our findings that show a partial effect of CBD treatment on persistent pain, other clinical and pre-clinical studies have found CBD to be a promising antinociceptive agent for the reduction of inflammatory and neuropathic pain.17,19,30,44–48 Therefore, it is plausible that the formalin test initiates a cascade of nociceptive-related signals that require the simultaneous activation and/or inhibition of multiple receptor targets. Because of this, CBD in combination with other drugs may be required to reduce persistent tonic pain.
CBD as an opioid sparing therapeutic
In addition to investigating the effects of CBD alone, we investigated whether CBD in combination with low-dose morphine had synergistic effects on reversing formalin-induced nociceptive behaviors. Evidence supports the opioid-sparing potential of CBD, but these observations are not consistent across multiple pre-clinical pain models.49,50
Our results show that CBD in combination with low-dose morphine produces neither synergistic nor subadditive effects on formalin-induced nociception. This observed lack of effect is unlikely due to metabolic drug/drug interactions as CBD is metabolized by cytochrome P450 enzymes, while morphine is metabolized exclusively by glucuronidation via UGT2B7.51,52 In addition, CBD has a long half-life.31,53 Therefore, we expect that the physiological effects of CBD were active throughout the entirety of our experiments, which is supported by our observed plasma CBD concentrations (Table 1).
Sex differences following CBD treatment in the formalin test
We identified a significant difference between male and female mice treated with CBD during phase I, but not phase II, of the formalin assay, suggesting that CBD (10 mg/kg) differentially alters the pain response in male versus female mice during formalin-induced acute pain.
Importantly, we did not perform a dose response to find an optimal dose of CBD for both males and females undergoing the formalin test. Instead, our intention was to investigate a dose of CBD that is known to produce (1) blood plasma concentrations that are similar to those observed in humans 2 h after CBD administration54,55 and (2) antinociceptive behaviors in arthritic male rodents and neuropathic female rodents.30,32,33 Therefore, these investigations highlight that one dose may not be optimal for all individuals or all pain states as many clinical and pre-clinical studies have shown that females respond differently compared to males following pain treatment.56–61
In line with this, we observed a significant sex difference between male and female responses to high-dose morphine (10 mg/kg), with females displaying greater variability compared to males. This is consistent with other studies showing that females require a higher dose of morphine to reach analgesic effects that are equivalent to males.3–7,62
We cannot definitively conclude from our investigation that CBD is ineffective for females in a model of persistent pain as sex-specific differences may be attributed to the dosing schedule, pain model used, dose administered, and/or localization of the injection site. For example, in models of neuropathic pain, it has been shown that CBD (10 mg/kg, i.p.) was effective at reducing paclitaxel-induced cold and mechanical allodynia in female mice.32
However, CBD was not administered acutely, as in our study, but rather CBD was repeatedly administered once a day for 14 consecutive days before paclitaxel injections.32 Yet, in a similar dosing schedule through which naive rodents received repeated CBD treatment, it was observed that CBD (10 mg/kg, i.p.) administered twice a day for four days was only effective at increasing tail withdrawal latencies in males, with no effect observed in females.63 These results suggest that the antinociceptive effects of CBD (10 mg/kg, i.p.) are dependent upon the dosing schedule or pain state.
Interestingly, in contrast to the sex differences observed in our study, others have found comparable outcomes between males and females following CBD treatment. For example, following acute CBD treatment (10 or 30 mg/kg, i.p.), in naive rodents, a previous report found no change in response latency to paw pressure or tail withdrawal tests in both male and female rodents.64 In another study implementing a model of Parkinson's-induced myofascial pain, the authors discovered that localized injection of CBD into the masseter muscle (the site of formalin injection) evoked antinociceptive behaviors in both phases of the formalin test as well as increased mechanical allodynia in both male and female rodents.65
Taking these previous findings into account, our observations that an acute systemic injection of CBD (10 mg/kg) was more effective at reducing nociception in phase I of the formalin test in males rather than in females may not hold true when varying the dosing schedule, dose used, or placement of injection (i.e., localized to the site of injury versus systemic).
In addition to only using one dose of CBD in our assessments, another limitation to our study is that we did not monitor the estrus phase of the female mice, which may explain the variability observed following treatment with either CBD or morphine. It is known that the estrus phase is accompanied by shifts in hormones, which regulate receptor expression/function,66–72 drug metabolism,73–75 and neuroimmune function,76–78 all factors that contribute to a pain response.79–81 Therefore, future studies addressing the mechanisms of CBD action and how the estrus cycle may influence CBD's effects are required.
Despite these sex differences in response to pain treatment, we observed that both males and females displayed comparable nocifensive behaviors to formalin injection, as demonstrated by a lack of significant difference between vehicle-treated male and female mice. Our results are consistent with studies in mice, showing that male nociceptive responses to formalin injection during phase I and II of the formalin test were no different than female mice across all stages of the estrus cycle.82
Conclusion
In conclusion, we interpret our results to indicate that CBD (10 mg/kg) in combination with low-dose morphine (1 mg/kg) is ineffective at reducing nociception in the formalin test and that CBD may have sex-specific effects on nociceptive severity. Importantly, our results, in combination with other published findings, suggest that CBD has the potential to be an effective antinociceptive therapeutic for some, but not all, forms of pain.
In addition, caution should be implemented in self-medicating with CBD, as CBD when combined with morphine has shown to evoke subadditive effects in distinct forms of pain.49 This subadditive behavioral phenotype is supported by a pharmacological study, which showed that CBD decreased in vitro binding of morphine to opioid receptors, which would reduce morphine-induced opioid receptor activation and likely diminish morphine-induced analgesia.83,84 Moreover, given its metabolism by CYP3A4 and CYP2C19, self-medication may interfere with the metabolism of many prescription drugs.85
Given the inconsistent effects of CBD on reducing pain across multiple pain models, it is clear that future work, in addition to the important contributions already made,86 is required to understand the mechanisms by which CBD evokes antinociceptive responses in both males and females. This will help clinicians effectively implement CBD-based modalities for pain management.
Acknowledgments
The authors would like to acknowledge the Pennsylvania-approved Medicine Medical Marijuana Academic Clinical Research Center at Penn State for discussions and insight on this project and the Mass Spectrometry and Proteomics Core Facility at Penn State College of Medicine for performing analysis of CBD concentrations in mouse plasma. They also thank the anonymous reviewer for the time and helpful comments, which greatly improved their article.
Abbreviations Used
- Δ9-THC
delta-9-tetrahydrocannabinol
- ANOVA
analysis of variance
- AUC
area under the curve
- CBD
cannabidiol
- HPLC/MS/MS
high-performance liquid chromatography/mass spectrometry/mass spectrometry
- TRPV
transient receptor potential vanilloid channel
Authors’ Contributions
D.E.S., W.M.R.-K., K.E.V., and N.M.G. designed the experiments. D.E.S., D.P.M., and D.S. performed the experiments. D.E.S., D.P.M., W.M.R.-K., D.S., K.E.V., and N.M.G. performed the analyses. D.E.S., W.M.R.-K., K.E.V., D.S., and N.M.G. wrote the article.
Author Disclosure Statement
K.E.V. and the Penn State College of Medicine are the recipients of research support from PA Options for Wellness (a state-approved medical marijuana clinical registrant). The funding source had no involvement in the following: study design, data collection, analysis and interpretation; writing of the report; or the decision to submit the article for publication. All other authors have no conflicts of interest to report.
Funding Information
This project is supported by the NARSAD Young Investigator Award (27364; NG) and by the Pennsylvania Department of Health using Tobacco CURE Funds (NG). The authors have no financial or nonfinancial competing interests to declare. K.E.V. and the Penn State College of Medicine are recipients of research support from PA Options for Wellness, a Pennsylvania-approved medical marijuana clinical registrant.
Cite this article as: Sepulveda DE, Morris DP, Raup-Konsavage WM, Sun D, Vrana KE, Graziane NM (2022) Evaluating the antinociceptive efficacy of cannabidiol alone or in combination with morphine using the formalin test in male and female mice, Cannabis and Cannabinoid Research 7:5, 648–657, DOI: 10.1089/can.2021.0108.
References
- 1. Ochiai W, Kaneta M, Nagae M, et al. Mice with neuropathic pain exhibit morphine tolerance due to a decrease in the morphine concentration in the brain. Eur J Pharm Sci. 2016;92:298–304. [DOI] [PubMed] [Google Scholar]
- 2. Kulkarni SK, Ninan I. Inhibition of morphine tolerance and dependence by Withania somnifera in mice. J Ethnopharmacol. 1997;57:213–217. [DOI] [PubMed] [Google Scholar]
- 3. Dawson-Basoa MB, Gintzler AR. 17-β-Estradiol and progesterone modulate an intrinsic opioid analgesic system. Brain Res. 1993;601:241–245. [DOI] [PubMed] [Google Scholar]
- 4. Craft RM, Ulibarri C, Leitl MD, et al. Dose- and time-dependent estradiol modulation of morphine antinociception in adult female rats. Eur J Pain. 2008;12:472–479. [DOI] [PubMed] [Google Scholar]
- 5. Loyd DR, Wang X, Murphy AZ. Sex differences in μ-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28:14007–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mogil JS, Chesler EJ, Wilson SG, et al. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291:R300–R306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hussain AM, Khan FA, Ahmed A, et al. Effect of gender on pain perception and analgesic consumption in laparoscopic cholecystectomy: an observational study. J Anaesthesiol Clin Pharmacol. 2013;29:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X, Vigil JM, Stith SS, et al. The effectiveness of self-directed medical cannabis treatment for pain. Complement Ther Med. 2019;46:123–130. [DOI] [PubMed] [Google Scholar]
- 10. van de Donk T, Niesters M, Kowal MA, et al. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lötsch J, Weyer-Menkhoff I, Tegeder I. Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur J Pain 2018;22:471–484. [DOI] [PubMed] [Google Scholar]
- 12. Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3:CD012182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Häuser W, Petzke F, Fitzcharles MA. Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management—an overview of systematic reviews. Eur J Pain. 2018;22:455–470. [DOI] [PubMed] [Google Scholar]
- 14. Nader DA, Sanchez ZM. Effects of regular cannabis use on neurocognition, brain structure, and function: a systematic review of findings in adults. Am J Drug Alcohol Abuse. 2018;44:4–18. [DOI] [PubMed] [Google Scholar]
- 15. Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: A complex picture. In: Phytocannabinoids: unraveling the complex chemistry and pharmacology of Cannabis sativa. Kinghorn AD, Falk H, Gibbons S, et al., eds. Springer International Publishing: New York, NY, 2017, Chapter 4, pp. 103–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Britch SC, Babalonis S, Walsh SL. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology. 2021;238:9–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Comelli F, Giagnoni G, Bettoni I, et al. Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain: mechanisms involved. Phytother Res. 2008;22:1017–1024. [DOI] [PubMed] [Google Scholar]
- 18. Mammana S, Cavalli E, Gugliandolo A, et al. Could the combination of two non-psychotropic cannabinoids counteract neuroinflammation? Effectiveness of cannabidiol associated with cannabigerol. Medicina (Kaunas) 2019;55:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Philpott HT, O'Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158:2442–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160:136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schoedel KA, Szeto I, Setnik B, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav. 2018;88:162–171. [DOI] [PubMed] [Google Scholar]
- 22. Viudez-Martínez A, García-Gutiérrez MS, Medrano-Relinque J, et al. Cannabidiol does not display drug abuse potential in mice behavior. Acta Pharmacol Sin. 2019;40:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tjølsen A, Berge OG, Hunskaar S, et al. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. [DOI] [PubMed] [Google Scholar]
- 24. Heapy CG. Afferent C-fiber and A-delta activity in models of inflamation. Br J Pharmacol. 1987;90:164P. [Google Scholar]
- 25. Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. [DOI] [PubMed] [Google Scholar]
- 27. Watson GS, Sufka KJ, Coderre TJ. Optimal scoring strategies and weights for the formalin test in rats. Pain. 1997;70:53–58. [DOI] [PubMed] [Google Scholar]
- 28. Henderson-Redmond AN, Nealon CM, Davis BJ, et al. c-Jun N terminal kinase signaling pathways mediate cannabinoid tolerance in an agonist-specific manner. Neuropharmacology. 2020;164:107847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Millar SA, Stone NL, Yates AS, et al. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu C, Chang T, Du Y, et al. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ Toxicol Pharmacol. 2019;70:103202. [DOI] [PubMed] [Google Scholar]
- 32. Ward SJ, Ramirez MD, Neelakantan H, et al. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth Analg. 2011;113:947–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ward SJ, McAllister SD, Kawamura R, et al. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 2014;171:636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mejia S, Duerr FM, Griffenhagen G, et al. Evaluation of the effect of cannabidiol on naturally occurring osteoarthritis-associated pain: a pilot study in dogs. J Am Anim Hosp Assoc. 2021;57:81–90. [DOI] [PubMed] [Google Scholar]
- 35. Taylor L, Gidal B, Blakey G, et al. A Phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finn DP, Beckett SR, Roe CH, et al. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci. 2004;19:678–686. [DOI] [PubMed] [Google Scholar]
- 37. Nedelescu H, Wagner GE, De Ness GL, et al. Cannabidiol produces distinct U-shaped dose-response effects on cocaine conditioned place preference and associated recruitment of prelimbic neurons in male rats. Biol Psychiatry Glob Open Sci. 2021. DOI: 10.1016/j.bpsgos.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shibata M, Ohkubo T, Takahashi H, et al. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. [DOI] [PubMed] [Google Scholar]
- 39. McNamara CR, Mandel-Brehm J, Bautista DM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soliman N, Haroutounian S, Hohmann AG, et al. Systematic review and meta-analysis of cannabinoids, cannabis-based medicines, and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. Pain. 2021;162:S26–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neurosci Lett. 1987;83:207–211. [DOI] [PubMed] [Google Scholar]
- 42. Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987;30:349–360. [DOI] [PubMed] [Google Scholar]
- 43. Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990;535:155–158. [DOI] [PubMed] [Google Scholar]
- 44. Costa B, Giagnoni G, Franke C, et al. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Costa B, Colleoni M, Conti S, et al. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:294–299. [DOI] [PubMed] [Google Scholar]
- 46. Costa B, Trovato AE, Comelli F, et al. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83. [DOI] [PubMed] [Google Scholar]
- 47. Wade DT, Robson P, House H, et al. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21–29. [DOI] [PubMed] [Google Scholar]
- 48. Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neelakantan H, Tallarida RJ, Reichenbach ZW, et al. Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behav Pharmacol. 2015;26:304–314. [DOI] [PubMed] [Google Scholar]
- 50. Rodríguez-Muñoz M, Onetti Y, Cortés-Montero E, et al. Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor. Mol Brain. 2018;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology. 2012;219:859–873. [DOI] [PubMed] [Google Scholar]
- 54. Ohlsson A, Lindgren J-E, Andersson S, et al. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom. 1986;13:77–83. [DOI] [PubMed] [Google Scholar]
- 55. Contin M, Mohamed S, Santucci M, et al. Cannabidiol in pharmacoresistant epilepsy: clinical pharmacokinetic data from an expanded access program. Front Pharmacol. 2021;12:637801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pieretti S, Di Giannuario A, Di Giovannandrea R, et al. Gender differences in pain and its relief. Ann Ist Super Sanita. 2016;52:184–189. [DOI] [PubMed] [Google Scholar]
- 57. Calderone KL. The influence of gender on the frequency of pain and sedative medication administered to postoperative patients. Sex Roles. 1990;23:713–725. [Google Scholar]
- 58. Leresche L. Defining gender disparities in pain management. Clin Orthop Relat Res. 2011;469:1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pieh C, Altmeppen J, Neumeier S, et al. Gender differences in outcomes of a multimodal pain management program. Pain. 2012;153:197–202. [DOI] [PubMed] [Google Scholar]
- 60. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chanda ML, Mogil JS. Sex differences in the effects of amiloride on formalin test nociception in mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R335–R342. [DOI] [PubMed] [Google Scholar]
- 62. Doyle HH, Eidson LN, Sinkiewicz DM, et al. Sex differences in microglia activity within the periaqueductal gray of the rat: a potential mechanism driving the dimorphic effects of morphine. J Neurosci. 2017;37:3202–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Greene NZ, Wiley JL, Yu Z, et al. Cannabidiol modulation of antinociceptive tolerance to Δ(9)-tetrahydrocannabinol. Psychopharmacology. 2018;235:3289–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Britch SC, Wiley JL, Yu Z, et al. Cannabidiol-Δ(9)-tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend. 2017;175:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vivanco-Estela AN, Dos-Santos-Pereira M, Guimaraes FS, et al. Cannabidiol has therapeutic potential for myofascial pain in female and male parkinsonian rats. Neuropharmacology. 2021;196:108700. [DOI] [PubMed] [Google Scholar]
- 66. Kumar S, Singh O, Singh U, et al. Transient receptor potential vanilloid 1–6 (Trpv1–Trpv6) gene expression in the mouse brain during estrous cycle. Brain Res. 2018;1701:161–170. [DOI] [PubMed] [Google Scholar]
- 67. Payrits M, Sághy É, Csekő K, et al. Estradiol sensitizes the transient receptor potential vanilloid 1 receptor in pain responses. Endocrinology. 2017;158:3249–3258. [DOI] [PubMed] [Google Scholar]
- 68. Uchida Y, Izumizaki M. Effect of menstrual cycle and female hormones on TRP and TREK channels in modifying thermosensitivity and physiological functions in women. J Therm Biol. 2021;100:103029. [DOI] [PubMed] [Google Scholar]
- 69. Riebe CJN, Hill MN, Lee TTY, et al. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci. 2011;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bradshaw HB, Rimmerman N, Krey JF, et al. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol. 2006;291:R349–R358. [DOI] [PubMed] [Google Scholar]
- 72. Liu NJ, Storman EM, Gintzler AR. Pharmacological modulation of endogenous opioid activity to attenuate neuropathic pain in rats. J Pain. 2019;20:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Terner JM, Lomas LM, Picker MJ. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005;6:372–383. [DOI] [PubMed] [Google Scholar]
- 74. Kashuba AD, Nafziger AN. Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clin Pharmacokinet. 1998;34:203–218. [DOI] [PubMed] [Google Scholar]
- 75. Mitchell SC, Smith RL, Waring RH. The menstrual cycle and drug metabolism. Curr Drug Metab. 2009;10:499–507. [DOI] [PubMed] [Google Scholar]
- 76. Arakawa K, Arakawa H, Hueston CM, et al. Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology. 2014;100:162–177. [DOI] [PubMed] [Google Scholar]
- 77. Osborne BF, Turano A, Schwarz JM. Sex differences in the neuroimmune system. Curr Opin Behav Sci. 2018;23:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gregus AM, Levine IS, Eddinger KA, et al. Sex differences in neuroimmune and glial mechanisms of pain. Pain. 2021;160:2186–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vincent K, Stagg CJ, Warnaby CE, et al. “Luteal Analgesia”: progesterone dissociates pain intensity and unpleasantness by influencing emotion regulation networks. Front Endocrinol. 2018;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jarahi M, Sheibani V, Safakhah HA, et al. Effects of progesterone on neuropathic pain responses in an experimental animal model for peripheral neuropathy in the rat: a behavioral and electrophysiological study. Neuroscience. 2014;256:403–411. [DOI] [PubMed] [Google Scholar]
- 81. Blanton HL, Barnes RC, McHann MC, et al. Sex differences and the endocannabinoid system in pain. Pharmacol Biochem Behav. 2021;202:173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim SJ, Calejesan AA, Li P, et al. Sex differences in late behavioral response to subcutaneous formalin injection in mice. Brain Res. 1999;829:185–189. [DOI] [PubMed] [Google Scholar]
- 83. Vaysse PJ, Gardner EL, Zukin RS. Modulation of rat brain opioid receptors by cannabinoids. J Pharmacol Exp Ther. 1987;241:534–539. [PubMed] [Google Scholar]
- 84. Raehal KM, Schmid CL, Groer CE, et al. Functional selectivity at the μ-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev. 2011;63:1001–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kocis PT, Vrana KE. Delta-9-tetrahydrocannabinol and cannabidiol drug-drug interactions. Med Cannabis Cannabinoid. 2020;3:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mlost J, Bryk M, Starowicz K. Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci. 2020;21:8870. [DOI] [PMC free article] [PubMed] [Google Scholar]