Abstract
Introduction:
Opioid use disorder (OUD) is a major public health crisis worldwide. Patients with OUD inevitably experience withdrawal symptoms when they attempt to taper down on their current opioid use, abstain completely from opioids, or attempt to transition to certain medications for opioid use disorder. Acute opioid withdrawal can be debilitating and include a range of symptoms such as anxiety, pain, insomnia, and gastrointestinal symptoms. Whereas acute opioid withdrawal only lasts for 1–2 weeks, protracted withdrawal symptoms can persist for months after the cessation of opioids. Insufficient management of opioid withdrawal often leads to devastating results including treatment failure, relapse, and overdose. Thus, there is a critical need for cost-effective, nonopioid medications, with minimal side effects to help in the medical management of opioid withdrawal syndrome. We discuss the potential consideration of cannabidiol (CBD), a nonintoxicating component of the cannabis plant, as an adjunctive treatment in managing the opioid withdrawal syndrome.
Materials and Methods:
A review of the literature was performed using keywords related to CBD and opioid withdrawal syndrome in PubMed and Google Scholar. A total of 144 abstracts were identified, and 41 articles were selected where CBD had been evaluated in clinical studies relevant to opioid withdrawal.
Results:
CBD has been reported to have several therapeutic properties including anxiolytic, antidepressant, anti-inflammatory, anti-emetic, analgesic, as well as reduction of cue-induced craving for opioids, all of which are highly relevant to opioid withdrawal syndrome. In addition, CBD has been shown in several clinical trials to be a well-tolerated with no significant adverse effects, even when co-administered with a potent opioid agonist.
Conclusions:
Growing evidence suggests that CBD could potentially be added to the standard opioid detoxification regimen to mitigate acute or protracted opioid withdrawal-related symptoms. However, most existing findings are either based on preclinical studies and/or small clinical trials. Well-designed, prospective, randomized-controlled studies evaluating the effect of CBD on managing opioid withdrawal symptoms are warranted.
Keywords: CBD, opioid use disorder, detoxification, opioid withdrawal syndrome, withdrawal management, nonopioid adjunctive treatment
Introduction
Opioid use disorder (OUD) is a major public health crisis. In 2018, an estimated two million people aged 12 or older met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for OUD in the United States,1 and opioids contributed to the majority of ∼93,000 drug overdose deaths in 2020.2 Withdrawal management plays an essential role in the treatment continuum for patients with OUD. Whether attempting to taper down their current opioid use, abstain from opioid use, or transitioning to medications for opioid use disorder (MOUD), they will invariably experience opioid withdrawal syndrome. Insufficient management of opioid withdrawal often leads to treatment failure, relapse, or overdose. The ongoing opioid epidemic emphasizes the urgent need for cost-effective nonopioid medications with minimal side effects to help manage opioid withdrawal symptoms. Emerging evidence suggests that cannabidiol (CBD) may be a promising candidate as an adjunctive treatment for managing opioid withdrawal. This review examined clinical studies where CBD had been evaluated for its impact on symptoms relevant to opioid withdrawal.
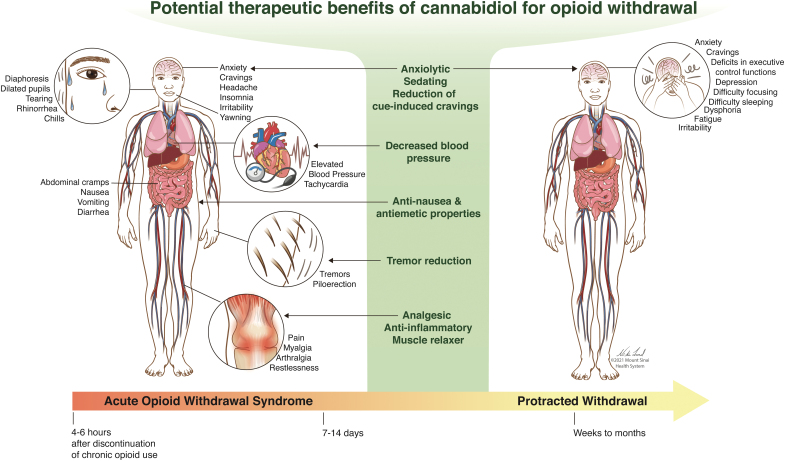
When an opioid-dependent patient undergoes medically supervised withdrawal management, commonly known as detoxification, they may experience arthralgias, myalgias, anxiety, vomiting, diarrhea, insomnia, cravings, sweats, and chills3–5 (Fig. 1). Depending on the type of opioid used, the onset, peak, and duration of withdrawal can vary. Withdrawal from heroin often starts 4–6 h after discontinuation, with symptoms peaking at 24–48 h, and a duration of 7–14 days. Withdrawal from longer acting opioids starts at 24–48 h after discontinuation and symptoms can persist for weeks.6 After acute opioid withdrawal, some symptoms can last for weeks or months and is often referred to as protracted withdrawal.7,8 Symptoms of protracted withdrawal can include anxiety, depression, difficulty sleeping, fatigue, dysphoria, irritability, difficulty focusing, and deficits in executive control functions.8–10 Protracted opioid withdrawal is destabilizing for individuals with OUD and could significantly hamper recovery (e.g., maintain employment, etc.). Experiencing negative withdrawal symptoms can be frightening.11 Individuals with OUD commonly cite avoidance of withdrawal as the primary overwhelming barrier to seeking treatment,12 and those with more intense symptoms of withdrawal and anxiety are more likely to drop out of detoxification prematurely.13 Health care providers should not underestimate the extreme amount of anxiety, fear, aversion, and pain that accompanies this process, even if it is not an acute life-threatening experience. Insufficient management of these withdrawal symptoms may prompt relapse and lead to detoxification failure, preventing the patient from receiving further addiction treatment.14
FIG. 1.
The diagram illustrates common symptoms that present during acute and protracted opioid withdrawal periods. The potential therapeutic benefits of CBD relevant for these symptoms are highlighted in the middle. CBD, cannabidiol. Reproduced with permission from © Mount Sinai Health System. Color images are available online.
Although MOUD is the gold standard for treating OUD, detoxification continues to be part of the OUD treatment continuum.15 Patients may choose detoxification over methadone or buprenorphine maintenance for multiple reasons including medication costs, lack of access to MOUD, negative beliefs,16 a wider cultural stigma toward opioid agonists,17 or desire to transition to an opioid antagonist such as naltrexone.18 Patients with chronic pain may also require opioid detoxification when tapering from long-term use of prescription pain medication. In addition, OUD patients on long-term methadone or suboxone maintenance may need supportive treatment for withdrawal symptoms when tapering medication dosage or attempting to come off of long-term methadone or buprenorphine maintenance.
In the United States, opioid withdrawal is commonly managed by administering cross-tolerant opioid agonists of the mu-opioid receptor (MOR) such as methadone or buprenorphine in tapered doses. Unfortunately, this clinical paradigm is limited by the prescribing restrictions on opioid agonists, making the service less accessible.14 In other countries like Russia where access to these medications are restricted for geopolitical or legal reasons,19 managing opioid withdrawal becomes even more challenging. Even when methadone or buprenorphine is utilized, nonopioid medications are often needed in managing breakthrough withdrawal symptoms during opioid tapering, such as insomnia, anxiety, and pain. Nonopioid medications become especially important in circumstances when patients choose not to pursue opioid agonist treatment or they desire to transition to an opioid antagonist treatment. The a2-adrenergic agonist Lofexidine, an analog of clonidine, is a newly Food and Drug Administration (FDA)-approved nonopioid option for withdrawal management.20 However, its efficacy is compromised by insufficiently addressing withdrawal-induced symptoms including body aches, arthralgia, myalgia, and headaches. In addition, Lofexidine is associated with some undesirable side effects including QT prolongation, hypotension, orthostasis, and bradycardia.11 Furthermore, the use of Lofexidine is also limited by its high cost.21 Most importantly, a2-adrenergic agonists do not help alleviate drug cravings during the withdrawal process, which often lead to detoxification failure and relapse. Other adjunctive medications, often termed “comfort medications” include nonsteroidal anti-inflammatory drugs (NSAIDS) or acetaminophen for pain, loperamide for diarrhea, ondansetron for nausea, and trazodone for insomnia are sometimes required to treat signs and symptoms of withdrawal. CBD has the potential of replacing one or several of these medications, thereby simplifying the withdrawal management regimen, and decreasing the risk of drug interactions and side effects.
The endocannabinoid system has been identified as an emerging biological target for addiction, in particular craving, a significant relapse predictor.22 The endocannabinoid system has close interactions with other neurotransmitter systems involved in substance use disorders. Type 1 cannabinoid (CB1) receptors regulate dopamine neurotransmission and are colocalized with MORs in many brain areas, including the nucleus accumbens and dorsal striatum23 that modulate reward, goal-directed behavior, and habit formation.24 CBD is a nonintoxicating cannabinoid that is a negative allosteric modulator of CB1 receptors25 and allosterically modulates the MOR.26 Emerging evidence from preclinical and clinical studies points to CBD having potential in relieving opioid withdrawal-related symptoms. For example, CBD reduces symptoms of morphine withdrawal in rodents27–29 and has potential pharmacological benefits in the treatment of craving, pain, anxiety, and nausea,30 which are common withdrawal complaints among OUD patients. In a randomized control study, Hurd et al. showed that compared with placebo, CBD reduced heroin cue-induced cravings and anxiety in abstinent OUD individuals.31 Although there have been anecdotal reports that CBD is beneficial for opioid withdrawal,32 and one case report showing the benefit of CBD for acute opioid withdrawal,33 there have been no randomized-controlled trials to investigate the role of CBD for managing opioid withdrawal syndrome in humans. However, emerging evidence indicates the beneficial role CBD may play in alleviating opioid withdrawal symptoms (Table 1).
Table 1.
Clinical Research Studying the Effect of Cannabidiol on Opioid Withdrawal Related Symptomatology
| Study | Population | CBD products | Outcome measures | CBD effect |
|---|---|---|---|---|
| Anxiety, restlessness, and irritability | ||||
| Crippa et al. (2004)43 | 10 healthy volunteers | Single dose of 400 mg oral CBD 99.9% pure powder (THC-Pharm, Frankfurt, Germany) dissolved in corn oil or placebo |
VAMS Regional cerebral blood flow was measured with 99mTc-ECD SPECT |
Decrease in anxiety Increase in sedation |
| Fusar-Poli et al. (2009)48 | 15 healthy male volunteers | Single dose of either 10 mg THC capsule 99.6% pure, 600 mg CBD capsule 99.9% pure (THC-Pharm, Frankfurt, Germany) or placebo | Brain BOLD and skin conductance in response to fearful faces. VAMS | Decrease in anxiety |
| Bhattacharyya et al. (2010)41 | 15 healthy men | Single dose of either 10 mg THC capsule 99.6% pure, 600 mg CBD capsule 99.9% pure (THC-Pharm, Frankfurt, Germany and STI Pharmaceuticals Ltd) or placebo | Brain BOLD and skin conductance response while participants performed a verbal memory task, viewing fearful faces, a response inhibition task (Go No-Go) and a visual and auditory stimulation task. VAMS |
Decrease in anxiety |
| Martin-Santos et al. (2012)52 | 16 healthy men | Single dose of either 10 mg THC capsule 99.6% pure, 600 mg CBD capsule 99.9% pure (THC-Pharm, Frankfurt, Germany and STI Pharmaceuticals Ltd) or placebo | PANSS VAMS STAI-S |
CBD had no difference to placebo |
| Das et al. (2013)49 | 48 healthy volunteers | Single dose of 32 mg CBD (STI pharmaceuticals, United Kingdom) in 0.08 mg ethanol vehicle vaporized using a Volcano Medic vaporizer (Storz & Bickel, Tuttlingen, Germany) or placebo | Skin conductance and shock expectancy measures | CBD enhanced consolidation of extinction learning |
| Arndt and de Wit (2017)50 | 38 healthy volunteers | Single dose of 300, 600, and 900 mg oral solution (300 mg/mL solution; Insys Therapeutics) or placebo vehicle | Heart rate Blood pressure POMS VAS for Drug Effects Questionnaire Emotional Stroop Rating of positivity and negativity to affective pictures The DEIT ABT Measure of social acceptance and ostracism (Cyberball task) |
Minimal behavioral and subjective effects |
| Zuardi et al. (2017)46 | 60 healthy volunteers | Single dose of 100, 300, and 900 mg oral capsule (99.6% pure CBD powder dissolved in corn oil; BSPG-Pharm, United Kingdom), clonazepam, or placebo | VAMS | Decrease in anxiety with 300 mg dose, but not 100 or 900 mg. |
| Linares et al. (2019)44 | 57 healthy volunteers | Single dose 150, 300, and 600 mg oral capsules 99.9% pure CBD (STI-Pharm, United Kingdom) dissolved in corn oil or placebo | VAMS | 300 mg CBD decreased anxiety |
| Bergamaschi et al. (2011)40 | 24 patients with social anxiety disorder | Single dose of 600 mg oral capsule (∼99.9% pure CBD dissolved in corn oil supplied by STI-Pharm, United Kingdom and THC-Pharm, Germany), or placebo | VAMS SSPS-N |
Decrease in anxiety |
| Crippa et al. (2011)42 | 10 patients with social anxiety disorder | Single dose of 400 mg oral capsule (99.9% pure CBD from THC-Pharm, Germany dissolved in corn oil) or placebo | SPECT imaging VAMS |
Decrease in anxiety |
| Hundal et al. (2018)51 | 32 participants with high paranoid traits | Single-dose 600 mg oral capsules (Synthetic CBD capsules (100 mg) from GW Pharmaceuticals, United Kingdom) or placebo | The University of Wales Mood Adjective Checklist Beck's Anxiety Inventory | No evidence of any benefit of CBD in anxiety or persecutory ideation |
| Masataka (2019)45 | 37 teenagers with social anxiety disorder and avoidant personality disorder | 4 weeks of daily doses of 300 mg oral CBD (RSHO-X Hemp Oil that did not contain other cannabinoids or terpenes from HempMeds) or placebo | Fear of Negative Evaluation Questionnaire Liebowitz Social Anxiety Scale |
Decrease in anxiety |
| Appiah-Kusi et al. (2020)39 | 26 healthy controls and 32 clinically high risk for psychosis | Daily dose of 600 mg daily for 1 week (STI Pharmaceuticals, United Kingdom) or placebo | SSPS-N TSST Blood cortisol level |
CBD group had an intermediate response to anxiety, experience of public speaking stress and change in cortisol associated with experimental stress exposure |
| Pain, arthralgias, and myalgias | ||||
| Sexton et al. (2016)55 | 1429 medical cannabis users | Various cannabis products | Self-reports on Pain Anxiety Depression Headache/migraine Nausea Muscle spasticity |
Survey respondents reported an average of 86% reduction in symptoms as a result of cannabis use |
| Wade et al. (2003)56 | 24 patients with intractable neurogenic pain symptoms | Daily use of pump-action sublingual spray that delivered whole-plant extracts of 2.5 mg THC and/or CBD with each actuation (GW Pharmaceuticals, United Kingdom) or placebo for 2 weeks each | VAS for pain NRS for spasticity |
Decreased pain, decreased spasticity |
| Notcutt et al. (2004)65 | 34 chronic pain patients | 2.5 mg of THC, 2.5 mg CBD, 2.5 mg THC +2.5 mg CBD whole plant extracts (GW Pharmaceuticals, United Kingdom) or placebo in aerosol or pump action sprays | BDI General Health Questionnaire 28 VAS for pain Sleep duration (hours), and quality of sleep (good, fair, poor) |
CBD monoproducts had little benefit |
| Cuñetti et al. (2018)62 | 7 kidney transplant patients with chronic pain | Increasing twice a day dose of 50–150 mg/day whole plant extract CBD Charlotte's Web, in oral solution from Stanley Brothers Social Enterprises (Colorado Springs, CO) orally for 3 weeks | Self-report pain scores | Two patients had total pain improvement, four had partial improvement, and one had no change |
| van de Donk et al. (2019)66 | 20 chronic pain patients with fibromyalgia | Single vapor inhalations of four strains of cannabis -22.4-mg THC and <1-mg CBD -13.4-mg THC and 17.8-mg CBD -18.4-mg CBD and <1-mg THC -Placebo All products from Bedrocan International BV (Veendam, the Netherlands) |
VAS for pain | There was a small analgesic response after single inhalation of the 13.4-mg THC and 17.8-mg CBD cannabis strain |
| Gulbransen et al. (2020)63 | 400 patients with noncancer chronic pain | Self-titrated daily dose 100 mg CBD/mL oil in 25 mL bottles taken orally (Tilray CBD100, Tilray, Nanaimo, Canada) | EQ-5D-5L questionnaire EQ VAS |
Improvements in pain/discomfort and anxiety/depression |
| Xu et al. (2020)64 | 29 patients with peripheral neuropathy | Up to four times a day application of CBD topical cream containing 250 mg/3 fl. oz (Theramu Relieve CBD compound cream Bakersfield, CA) or placebo | Neuropathic Pain Scale | Decreased pain |
| Capano et al. (2020)61 | 131 chronic pain patients | Daily dose of two 15 mg, CBD-rich hemp-derived soft gels (contained 15.7 mg CBD, 0.5 mg THC, 0.3 mg CBDV, 0.9 mg CBDA, 0.8 mg CBC, and >1% botanical terpene blend from Ananda Professional) for 8 weeks | PDI-4 PEG Scale assessing Pain Intensity and Interference PSQI Prescribed opioid dose |
Decreased pain, improved sleep quality, 53% of patients decreased or eliminated their prescribed opioid use |
| Craving | ||||
| Hurd et al. (2019)31 | 42 drug-abstinent individuals with heroin use disorder | Daily dose of 400 or 800 mg (100 mg/mL; Epidiolex, GW Pharmaceuticals, United Kingdom), or placebo for 3 consecutive days | VAS for Craving and Anxiety | Decrease in cue-induced craving and anxiety |
| Abdominal cramps, nausea, vomiting and diarrhea | ||||
| Grimison et al. (2020)77 | 81 patients with chemotherapy-induced nausea and vomiting | 1–4 self-titrated capsules of oral THC 2.5 mg/CBD 2.5 mg cannabis extract (TN-TC11M Tilray, Canada) three times daily, from days −1 to 5, or placebo | Self-reported experience of nausea and vomiting, use of rescue medications, and dose of study medication. Functional Living Index-Emesis | THC:CBD improved nausea and vomiting |
| Insomnia | ||||
| Linares et al. (2018)73 | 27 healthy volunteers | 300 mg CBD capsule (99.9% purity without THC from STI-Pharm, Brentwood, United Kingdom) or placebo |
Polysomnography VAMS State-Trait Anxiety Inventory Epworth Sleepiness Scale PSQI Psychomotor Vigilance Test |
No interference with sleep cycle or no change in cognitive or subjective measures |
| Carlini and Cunha (1981)70 | 15 individuals with insomnia | Once a week dose of either CBD 40, 80, 160 mg (crystalline CBD supplied by the National Institutes of Health), placebo, and 5 mg nitrazepam | 10-point questionnaire on sleep, sleep quality, dream recall, and reawakening | 160 mg dose of CBD increased amount of sleep. All doses of CBD resulted in less dream recall. |
| Fleury-Teixeira et al. (2019)72 | 18 patients with autism spectrum disorder | CBD-enriched Cannabis extract with a CBD to THC ratio of 75:1 capsules containing 25 or 50 mg CBD and 0.34 or 0.68 mg THC (CBDRx) for 6–9 months | Symptoms of autism spectrum disorder reported by parents/caregivers | Improvement in sleep problems |
| Barchel et al. (2019)71 | 53 children with autism spectrum disorder | Daily dose of cannabinoid oil solution containing CBD 16 mg/kg with maximal daily dose 600 mg, and for THC 0.8 mg/kg with maximal daily dose of 40 mg (Tikun Olam, Inc., Israel) | Specialists assessed participants for four autism spectrum disorder comorbidities (hyperactivity symptoms, sleep problems, self-injury and, anxiety) at baseline. Then biweekly telephone interviews with parents |
For 21 children with difficulty sleeping, sleep problems improved in 71.4% and worsened in 4.7% |
| Shannon et al. (2019)47 | 72 adults total (n=42 with anxiety and n=25 with poor sleep) | Daily dose of 25,50, or 75 mg CBD oral capsule (CV Sciences, Inc.) | PSQI Hamilton Anxiety Rating Scale |
Improvements in anxiety and sleep quality |
| Tremors | ||||
| de Faria et al. (2020)89 | 24 patients with Parkinson's disease | Single dose crossover 300 mg 99.9% pure CBD powder (BSPG-Pharm, United Kingdom) dissolved in corn oil and packaged in gelatin capsules or placebo | VAMS Tremors measured by an accelerometer |
Decrease in tremor amplitude Decrease in anxiety |
| Muscle spasms | ||||
| Wade et al. (2004)86 | 160 patients with multiple sclerosis | THC:CBD 1:1 oromucosal spray (Sativex) or placebo for 6 weeks, then optional THC:CBD 1:1 oromucosal spray for 4 additional weeks (GW Pharma, United Kingdom) | VAS for spasticity | Improvement in spasticity |
| Wade et al. (2006)85 | 137 patients with multiple sclerosis | THC:CBD 1:1 oromucosal spray (Sativex) or placebo for 10 weeks (GW Pharma, United Kingdom) | VAS for spasticity, diary scores of main symptoms | Improvement in spasticity |
| Collin et al. (2007)80 | 189 patients with multiple sclerosis | THC:CBD 1:1 oromucosal spray (Sativex) or placebo for 6 weeks (GW Pharma, United Kingdom) | NRS for spasticity | Improvement in spasticity |
| Collin et al. (2010)81 | 337 patients with multiple sclerosis | THC:CBD 1:1 oromucosal spray (Sativex) or placebo for 15 weeks (GW Pharma, United Kingdom) | NRS for spasticity | Improvement in spasticity |
| Novotna et al. (2011)84 | 572 patients with multiple sclerosis | THC:CBD 1:1 oromucosal spray (Sativex) or placebo for 19 weeks (GW Pharma, United Kingdom) | NRS for spasticity | Improvement in spasticity |
| Notcutt et al. (2012)83 | 36 patients with multiple sclerosis | THC:CBD 1:1 oromucosal spray (Sativex) or placebo for 6 weeks (GW Pharma, United Kingdom) | Time to Treatment Failure, Carer Global Impression of Change, Subject Global Impression of Change | Improvement in spasticity |
| Tachycardia and hypertension | ||||
| Jadoon et al. (2017)88 | 9 healthy male volunteers | Single dose of 600 mg Epidiolex (GW Pharmaceuticals, United Kingdom) or placebo | Finometer and laser Doppler flowmetry for cardiovascular monitoring | Decreased blood pressure Increased heart rate |
| Sultan et al. (2020)87 | 26 healthy male volunteers | Daily dose of 600 mg CBD or placebo for 7 days Phivida Neutrafuels (Phivida Organics) | Finometer and brachial site pulse wave analysis for cardiovascular monitoring | Decrease in blood pressure |
| Depression | ||||
| Beale et al. (2018)94 | 20 cannabis users | 50 mg CBD oral capsules (99.5% pure crystalline herbal origin CBD in Miglyol 812 and Softisan 378 from Trigal Pharma Ltd. and BioSynthesis Pharma Group Ltd.) Twice daily dose of two capsules×10 weeks |
Structural MRI scan | CBD may be therapeutic for clinical disorders characterized by hippocampal pathology including depression |
| Solowij et al. (2018)95 | 20 cannabis users | 50 mg oral capsules (99.5% pure crystalline herbal origin CBD in Miglyol 812 and Softisan 378 from Trigal Pharma Ltd. and BioSynthesis Pharma Group Ltd.) Twice daily dose of two capsules×10 weeks |
BDI | Decreased depressive symptoms |
| Fatigue | ||||
| Rosenberg et al. (2017)97 | 48 childhood epilepsy patients | Caregiver titrated daily dose of 99% CBD extract 100 mg/mL in sesame seed oil solution taken orally (GW Pharmaceuticals, United Kingdom) | Caregiver reported Quality Of Life for Childhood Epilepsy | Improvement in energy/fatigue |
| Mathur et al. (2020)96 | 371 patients with autoimmune hepatitis (n=93 respondents reported current or past use of CBD) | Nonspecified CBD products | Self-report on CBD and autoimmune hepatitis-related questions | 38% of CBD users reported fatigue as their reason for CBD use and the majority of these users reported a significant improvement in fatigue |
ABT, Attentional Bias Task; BDI, Beck Depression Inventory; BOLD, blood oxygenation level dependent; CB1, cannabinoid; CBC, cannabichrome; CBD, cannabidiol; CBDA, cannabidiolic acid; CBDV, cannabidivarin; DEIT, Dynamic Emotion Identification Task; EQ VAS, EuroQol Visual Analogue Scales; MRI, magnetic resonance imaging; NRS, Numerical Rating Scale; PANSS, Positive and Negative Psychotic Syndrome Scale; PDI-4, Pain Disability Index; PEG, Pain, Enjoyment, General Activity; POMS, Profile of Mood States; PSQI, Pittsburgh Sleep Quality Index; SPECT, Single Photon Emission Computed Tomography; SSPS-N, Self-Statements during Public Speaking Scale; STAI-S, Spielberger State Anxiety Inventory; THC, Tetrahydrocannabinol; TSST, Trier Social Stress Test; VAMS, Visual Analogue Mood Scale; VAS, Visual Analogue Scale.
What has not really been considered is whether CBD could be beneficial as an adjunct to the standard opioid detoxification regimen. It has been shown that CBD does not lead to negative effects even if coadministered with a potent opioid agonist34,35 and it has a favorable safety profile.36 So, could CBD help to alleviate the common withdrawal symptoms that prevent patients from seeking/continuing treatments and decrease premature discharges of patients undergoing detoxification? The sections hereunder provide an overview of the specific role that CBD might play, or not, for common opioid withdrawal symptoms.
Methods and Materials
A review of the literature was performed by searching PubMed and Google Scholar for studies published from inception until April 2021. A combination of the following keywords was used: Cannabidiol, CBD, opioid use disorder, opioid withdrawal syndrome, withdrawal management, and all acute and protracted opioid withdrawal related symptoms (anxiety, pain, insomnia, nausea, etc.…). Studies were included if they conducted a clinical trial where a CBD containing product had been evaluated for its effectiveness on symptoms relevant to opioid withdrawal.
Results
A total of 144 articles were identified from the database search and 41 were reviewed in-depth based on their relevance to the symptoms of interest. The results are summarized in Table 1 and presented hereunder with regard to the specific symptoms relevant to opioid withdrawal.
Anxiety, restlessness, and irritability
Anxiety is a common symptom of opioid withdrawal that increases the odds of relapse, even when treated with MOUD.37 The anxiolytic effect of CBD has been the most studied symptom relevant to common opioid withdrawal-related conditions.38 Emerging evidence from preclinical and clinical studies supports CBD as beneficial in reducing anxiety.39–49 Highly relevant to OUD are the findings from the placebo-controlled CBD clinical trial that demonstrated its effect to reduce cue-induced anxiety in abstinent OUD individuals.31 Moreover, this clinical study showed that CBD reduced physiological measures of stress and anxiety including decreased salivary cortisol and heart rate levels that were induced by the salient drug cues.31 Only a few studies suggest that CBD does not help or might even worsen anxiety, but this has mainly been observed in healthy subjects.50–52 However, in patients with generalized anxiety disorder and seasonal affective disorder, there is consensus that CBD acutely reduces anxiety-related symptoms while exhibiting minimal adverse effects compared with other anxiolytic medications.53
Pain, arthralgias, and myalgias
Pain is an often-underappreciated symptom of opioid withdrawal. Patients in the midst of opioid withdrawal experience hyperalgesia. Several studies suggest that CBD may be an effective analgesic.54–56 Indeed, there is a growing body of evidence suggesting that CBD might serve as an analgesic owing to its anti-inflammatory property. A review by Gazendam et al. highlights several animal studies demonstrating that CBD reduces pain and inflammation in conditions like osteoarthritis and neuropathic pain.57 Specifically, CBD exerts such actions through promoting T cell attrition, inhibition of proinflammatory cytokine release, and anti-inflammatory cytokine production.58–60 CBD's anti-inflammatory and immune modulatory effects have been documented in in vivo animal models, in vitro methods, and human clinical studies.58–60 In a prospective cohort study of 131 chronic pain patients, 97 patients completed an 8-week course of 15 mg CBD soft gels. Fifty-three percent of the patients were able to decrease or eliminate their prescription opioid use.61 In a study of kidney transplant patients with chronic pain, CBD was given at an increasing dose over 3 weeks. Six of seven patients had partial or total pain improvement.62 In an audit of 400 patients with chronic pain, a self-titrated dose of prescribed CBD decreased both pain and anxiety.63 In another study, pain was improved in 29 patients with peripheral neuropathy by applying CBD topical oil up to four times a day for 4 weeks.64 However, other research involving CBD for pain showed little or no benefit.65,66 Further clinical studies are warranted to determine whether CBD is effective in decreasing pain, in particular, withdrawal-induced pain.
Craving
Craving is a core component of OUD and associated with failure of detoxification/treatments or relapse. Preclinical animal evidence strongly suggested that CBD reduces heroin-seeking behavior after a drug-free period that was specifically triggered by a prior drug-associated cue in animals with a history of heroin self-administration.67 Moreover, this effect persisted weeks after the last CBD dose. As mentioned previously, in the double-blind randomized placebo-controlled trial by Hurd et al., oral administration of 400 or 800 mg of CBD reduced drug cue-induced craving in heroin-abstinent individuals.31 Furthermore, the craving reduction effect of CBD was persistent for 1 week after CBD administration consistent with the preclinical animal research. These studies highlight a specific effect of CBD to reduce craving (clinical trial) and drug-seeking behavior (animal model) associated with drug-related cue. This is particularly important given that environmental cues are strong triggers for craving and ultimately relapse during the opioid withdrawal period.
Insomnia
Opioid withdrawal induces a hyperadrenergic state that may lead to insomnia, a frequent complaint often leading to relapse. The evidence of cannabinoids for the treatment of insomnia is conflicting.68 However, somnolence is one of the most commonly reported side effect of CBD.69 In two clinical studies of adults with poor sleep, CBD was found to improve sleep in self-reports.47,70 In addition, in two studies of CBD in children with autism spectrum disorder, CBD was observed to improve their autistic symptoms including sleep problems.71,72 However, in a double-blind crossover study, 27 healthy participants were randomized to 300 mg of CBD or placebo and then received 8 h of polysomnography recording to evaluate their sleep–wake cycle. This study found CBD did not interfere with the sleep cycle and did not change subjective measures of sleep, compared with placebo.73
Abdominal cramps, nausea, vomiting, and diarrhea
Opioids decrease gut motility by activating MORs in the gastrointestinal tract. During withdrawal, these sensitized opioid receptors contribute to symptoms such as nausea, vomiting, abdominal cramps, and diarrhea. Treating these symptoms is important to avoid complications like dehydration.14 There is a clear involvement of the endocannabinoid system in gastrointestinal function. CBD has been reported to alleviate gastrointestinal symptoms of withdrawal based on studies demonstrating that CBD can suppress vomiting in animals through activation of somatodendritic 5-HT(1A) autoreceptors in the dorsal raphe nucleus.74 This anti-nausea property is most likely related to CBD acting as an agonist at the 5HT1A receptor.74–76 In a study of 81 patients with chemotherapy-induced nausea and vomiting, patients self-titrated capsules containing a combination of CBD and THC (1:1) or placebo. Compared with placebo, THC:CBD was observed to be associated with less nausea and vomiting.77 Although some cannabinoid products are approved by the FDA for the treatment of chemotherapy-induced nausea and vomiting, there are no clinical trials on the evaluation of CBD alone for nausea in humans. Using CBD alone is of interest for treatment for nausea because of its nonintoxicating effects, but there may be a biphasic anti-emetic effects of CBD based on some preclinical evidence where 5 mg/kg suppressed vomiting, but 40 mg/kg potentiated vomiting.78
Diarrhea has also been identified as a potential side effect of high-dose CBD.69,79 However, this side effect might potentially be leveraged to alleviate the constipation complaint by methadone- or buprenorphine-treated OUD patients in withdrawal management, indicating a potential benefit for adding CBD to the regimen. Caution nevertheless is warranted as there is a possibility that CBD may potentially worsen diarrhea in patients going through acute opioid withdrawal.
Muscle spasms
Muscle spasms are a common and distressing symptom of opioid withdrawal that can lead to relapse if not alleviated. Historically, cannabinoids have been used to alleviate muscular ailments, but most research has focused on THC, with very limited information available on CBD in this regard. The only studies utilizing CBD for muscle spasms involve the oromucosal spray containing THC and CBD in a 1:1 fixed ratio, which has generally been shown to be a useful treatment option for patients with multiple sclerosis-related spasticity.80–86
Tachycardia and hypertension
Opioid withdrawal results in an increase of noradrenaline, precipitating sympathetic over-activity such as tachycardia and hypertension. Information on the effects of CBD on the cardiovascular system is very limited. There is some evidence that CBD may increase heart rate in cannabis-naive individuals and CBD may decrease blood pressure, especially under stress-induced conditions.87,88 Orthostatic hypotension has also been noted as a potential adverse effect of CBD administration.69
Other acute withdrawal symptoms
Information on the effects of CBD on other opioid withdrawal symptoms, such as tremors, dilated pupils, tearing, rhinorrhea, diaphoresis, chills, and piloerection, is limited. There is only one randomized, double-blinded, placebo-controlled, crossover clinical trial that investigated the effects of CBD and placebo on patients with Parkinson's disease when anxiety and tremors were induced by a simulated public speaking test. The study demonstrated that CBD administration attenuates public speaking-induced anxiety and decreases tremor amplitude.89 Although cannabis is known to cause pupillary dilation,90 there is insufficient evidence to support CBD having any affect, whether positive or negative, on pupil dilation or tearing. In addition, there is no sufficient evidence to support CBD having any effect, whether positive or negative, on rhinorrhea, diaphoresis, chills, or piloerection.
Protracted withdrawal symptoms
Protracted withdrawal symptoms can persist 12–36 months after opioid detoxification. During this period, the relapse rate remains high (i.e., 72–88%).91 Upon the completion of detoxification, most OUD patients do not receive MOUD,92 thereby increasing their risk of experiencing protracted withdrawal symptoms. As mentioned, CBD could be beneficial in alleviating many symptoms of protracted opioid withdrawal including anxiety, cravings, and insomnia (Fig. 1). Other protracted withdrawal symptoms that may also be alleviated by CBD include depression and fatigue. Although preclinical evidence exists supporting CBD as an antidepressant, clinical evidence is scarce.93 In two clinical trials where chronic cannabis users were given 200 mg CBD orally daily for 10 weeks, both studies showed improvement in depressive symptoms.94,95 Finally, although there are no randomized control trials, there have been studies reporting CBD as beneficial for improving fatigue associated with other disease states such as autoimmune hepatitis and childhood epilepsy.96,97 If CBD has such properties in OUD patients, then it may have the potential to alleviate lingering withdrawal symptoms that could reduce post-detox relapse rates.
Conclusions
Growing evidence suggests that CBD may have the potential to reduce anxiety, pain, and insomnia with also some signals for reducing craving, nausea, vomiting, muscle spasms, and blood pressure. These clinical symptoms are commonly observed in OUD patients undergoing withdrawal, indicating that CBD could potentially be added to the standard opioid detoxification regimen to mitigate acute withdrawal-related symptoms as well as protracted withdrawal symptoms. However, most of these observations are either based on preclinical studies and/or small clinical trials, and a number of the withdrawal symptoms studied to date make up a small percentage of the outcomes currently investigated for CBD. Another concern is the wide variety of CBD formulations and dosages used in published studies. In addition, the bioavailability of each CBD product is unclear owing to the lack of pharmacokinetic data. These limitations make comparisons of effectiveness of different CBD formulations difficult. Nevertheless, this is a rapidly evolving field with new formulations and innovative delivery systems emerging. At this time, however, the only FDA-approved CBD product is Epidiolex, which is used for rare childhood seizure disorders.98 Given the unreliability of commercially available CBD products that are not FDA regulated, the use of Epidiolex may be the most reasonable to consider off-label for opioid withdrawal syndrome.
In summary, CBD has a good safety profile, is well tolerated with opioid agonists, and reduces key withdrawal symptoms. Accumulating data provide the foundation for future studies using randomized placebo-control designs to investigate CBD as a potential adjunctive treatment in managing opioid withdrawal in clinical settings. Easing withdrawal symptoms with CBD could improve clinical outcomes by keeping patients engaged in treatment, facilitating smoother transition to MOUD like buprenorphine or extended-release naltrexone, and helping with tapering of opioid agonist treatment or opioid analgesics.
Acknowledgement
The authors thank Ni-Ka Ford for help with developing Figure 1.
Abbreviations Used
- ABT
Attentional Bias Task
- BDI
Beck Depression Inventory
- BOLD
blood oxygenation level-dependent
- CB1
Type 1 cannabinoid
- CBC
cannabichrome
- CBD
Cannabidiol
- CBDA
cannabidiolic acid
- CBDV
cannabidivarin
- DEIT
Dynamic Emotion Identification Task
- EQ VAS
EuroQol Visual Analogue Scales
- FDA
Food and Drug Administration
- MOR
mu opioid receptor
- MOUD
medications for opioid use disorder
- MRI
magnetic resonance imaging
- NRS
Numerical Rating Scale
- OUD
Opioid use disorder
- PANSS
Positive and Negative Psychotic Syndrome Scale
- PDI-4
Pain Disability Index
- PEG
Pain, Enjoyment, General Activity
- POMS
Profile of Mood States
- PSQI
Pittsburgh Sleep Quality Index
- SPECT
Single Photon Emission Computed Tomography
- SSPS-N
Self-Statements during Public Speaking Scale
- STAI-S
Spielberger State Anxiety Inventory
- THC
Tetrahydrocannabinol
- TSST
Trier Social Stress Test
- VAMS
Visual Analogue Mood Scale
- VAS
Visual Analogue Scale
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by funds from the National Institutes of Health/NIDA DA048613 and DA050323.
Cite this article as: Kudrich C, Hurd YL, Salsitz E, Wang AL (2022) Adjunctive management of opioid withdrawal with the nonopioid medication cannabidiol, Cannabis and Cannabinoid Research 7:5, 569–581, DOI: 10.1089/can.2021.0089.
References
- 1. Substance Abuse and Mental Health Services Administration (SAMHSA). Key substance use and mental health indicators in the United States: results from the 2018 national survey on drug use and health. HHS publication no PEP19-5068 2019;NSDUH Series H-54. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf Accessed August 4, 2019.
- 2. Knopf A. CDC: 93,000 deaths from drug overdoses in the US in 2020. Alcohol Drug Abuse Wkly. 2021;33:4–6. [Google Scholar]
- 3. Swift RM, Stout RL. The relationship between craving, anxiety, and other symptoms in opioid withdrawal. J Subst Abuse. 1992;4:19–26. [DOI] [PubMed] [Google Scholar]
- 4. Vernon MK, Reinders S, Mannix S, et al. Psychometric evaluation of the 10-item Short Opiate Withdrawal Scale-Gossop (SOWS-Gossop) in patients undergoing opioid detoxification. Addict Behav. 2016;60:109–116. [DOI] [PubMed] [Google Scholar]
- 5. Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 2003;35:253–259. [DOI] [PubMed] [Google Scholar]
- 6. O'Connor PG, Fiellin DA. Pharmacologic treatment of heroin-dependent patients. Ann Intern Med. 2000;133:40–54. [DOI] [PubMed] [Google Scholar]
- 7. Satel SL, Kosten TR, Schuckit MA, et al. Should protracted withdrawal from drugs be included in DSM-IV? Am J Psychiatry. 1993;150:695–704. [DOI] [PubMed] [Google Scholar]
- 8. Center for Substance Abuse Treatment. Protracted withdrawal. Subst Abuse Treat Advis. 2010;9:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Fu L-p, Bi G-h, Zou Z-t, et al. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438:322–326. [DOI] [PubMed] [Google Scholar]
- 10. Prosser J, London ED, Galynker II. Sustained attention in patients receiving and abstinent following methadone maintenance treatment for opiate dependence: performance and neuroimaging results. Drug Alcohol Depend. 2009;104:228–240. [DOI] [PubMed] [Google Scholar]
- 11. Pergolizzi JV Jr., Annabi H, Gharibo C, et al. The role of lofexidine in management of opioid withdrawal. Pain Ther. 2019;8:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiss RD, Potter JS, Griffin ML, et al. Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. J Subst Abuse Treat. 2014;47:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nikolaou K, Kapoukranidou D, Ndungu S, et al. Severity of withdrawal symptoms, plasma oxytocin levels, and treatment outcome in heroin users undergoing acute withdrawal. J Psychoact Drugs. 2017;49:233–241. [DOI] [PubMed] [Google Scholar]
- 14. Kosten TR, Baxter LE. Review article: effective management of opioid withdrawal symptoms: a gateway to opioid dependence treatment. Am J Addict. 2019;28:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunn KE, Huhn AS, Strain EC. Differential adoption of opioid agonist treatments in detoxification and outpatient settings. J Subst Abuse Treat. 2019;107:24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370:2063–2066. [DOI] [PubMed] [Google Scholar]
- 17. Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction in opioid treatment programs. Substance Abuse and Mental Health Services Administration: Rockville (MD), 2005. [PubMed] [Google Scholar]
- 18. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Idrisov B, Murphy SM, Morrill T, et al. Implementation of methadone therapy for opioid use disorder in Russia—a modeled cost-effectiveness analysis. Subst Abuse Treat Prev Policy. 2017;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doughty B, Morgenson D, Brooks T. Lofexidine: a newly FDA-approved, nonopioid treatment for opioid withdrawal. Ann Pharmacother. 2019;53:746–753. [DOI] [PubMed] [Google Scholar]
- 21. Juurlink DN. Lofexidine for opioid withdrawal: small effects at an exorbitant price. J Addict Med. 2019;13:167–168. [DOI] [PubMed] [Google Scholar]
- 22. Spanagel R. Cannabinoids and the endocannabinoid system in reward processing and addiction: from mechanisms to interventions. Dialogues Clin Neurosci. 2020;22:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodríguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in μ-opioid receptor patches of the rat caudate putamen nucleus. J Neurosci. 2001;21:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belin D, Jonkman S, Dickinson A, et al. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. [DOI] [PubMed] [Google Scholar]
- 25. Laprairie R, Bagher A, Kelly M, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kathmann M, Flau K, Redmer A, et al. Cannabidiol is an allosteric modulator at mu-and delta-opioid receptors. Naunyn-Schmiedeberg's Arch Pharmacol. 2006;372:354–361. [DOI] [PubMed] [Google Scholar]
- 27. Bhargava HN. Effect of some cannabinoids on naloxone-precipitated abstinence in morphine-dependent mice. Psychopharmacology (Berl). 1976;49:267–270. [DOI] [PubMed] [Google Scholar]
- 28. Hine B, Torrelio M, Gershon S. Interactions between cannabidiol and Δ9-THC during abstinence in morphine-dependent rats. Life Sci. 1975;17:851–857. [DOI] [PubMed] [Google Scholar]
- 29. Hine B, Torrelio M, Gershon S. Differential effect of cannabinol and cannabidiol on THC-induced responses during abstinence in morphine-dependent rats. Res Commun Chem Pathol Pharmacol. 1975;12:185–188. [PubMed] [Google Scholar]
- 30. Pisanti S, Malfitano AM, Ciaglia E, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150. [DOI] [PubMed] [Google Scholar]
- 31. Hurd Y, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176:911–922. [DOI] [PubMed] [Google Scholar]
- 32. Leas EC, Hendrickson EM, Nobles AL, et al. Self-reported cannabidiol (CBD) use for conditions with proven therapies. JAMA Netw Open. 2020;3:e2020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaw C, Marcu J. Case report: cannabidiol in the management of acute opioid withdrawal. Am J Endocannabinoid Med. 2021;3:6–11. [Google Scholar]
- 34. Hurd Y, Yoon M, Manini AF, et al. Early phase in the development of cannabidiol as a treatment for addiction: opioid relapse takes initial center stage. Neurotherapeutics. 2015;12:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manini AF, Yiannoulos G, Bergamaschi MM, et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med. 2015;9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization. Cannabidiol (CBD) critical review report. : WHO: Geneva, Switzerland, 2018. [Google Scholar]
- 37. Moradinazar M, Farnia V, Alikhani M, et al. The effects of anxiety on relapse of patients with opioid use disorders under methadone maintenance treatment: control of the confounding variables. J Subst Use. 2020;25:34–39. [Google Scholar]
- 38. Wright M, Di Ciano P, Brands B. Use of cannabidiol for the treatment of anxiety: a short synthesis of pre-clinical and clinical evidence. Cannabis Cannabinoid Res. 2020;5:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Appiah-Kusi E, Petros N, Wilson R, et al. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology. 2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crippa JA, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121–130. [DOI] [PubMed] [Google Scholar]
- 43. Crippa JA, Zuardi AW, Garrido GE, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29:417–426. [DOI] [PubMed] [Google Scholar]
- 44. Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Br J Psychiatry. 2019;41:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front Psychol. 2019;10:2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zuardi AW, Rodrigues NP, Silva AL, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. 2017;8:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18–041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch General Psychiatry. 2009;66:95–105. [DOI] [PubMed] [Google Scholar]
- 49. Das RK, Kamboj SK, Ramadas M, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology. 2013;226:781–792. [DOI] [PubMed] [Google Scholar]
- 50. Arndt DL, de Wit H. Cannabidiol does not dampen responses to emotional stimuli in healthy adults. Cannabis Cannabinoid Res. 2017;2:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hundal H, Lister R, Evans N, et al. The effects of cannabidiol on persecutory ideation and anxiety in a high trait paranoid group. J Psychopharmacol. 2018;32:276–282. [DOI] [PubMed] [Google Scholar]
- 52. Martin-Santos R, a Crippa J, Batalla A, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18:4966–4979. [DOI] [PubMed] [Google Scholar]
- 53. Skelley JW, Deas CM, Curren Z, et al. Use of cannabidiol in anxiety and anxiety-related disorders. J Am Pharm Assoc (2003). 2020;60:253–261. [DOI] [PubMed] [Google Scholar]
- 54. Argueta DA, Ventura CM, Kiven S, et al. A balanced approach for cannabidiol use in chronic pain. Front Pharmacol. 2020;11:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sexton M, Cuttler C, Finnell JS, et al. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wade DT, Robson P, House H, et al. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21–29. [DOI] [PubMed] [Google Scholar]
- 57. Gazendam A, Nucci N, Gouveia K, et al. Cannabinoids in the management of acute pain: a systematic review and meta-analysis. Cannabis Cannabinoid Res. 2020;5:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bergamaschi MM, Queiroz RH, Zuardi AW, et al. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–249. [DOI] [PubMed] [Google Scholar]
- 59. Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown JD, Winterstein AG. Potential adverse drug events and drug-drug interactions with medical and consumer cannabidiol (CBD) use. J Clin Med. 2019;8:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Capano A, Weaver R, Burkman E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. Postgrad Med. 2020;132:56–61. [DOI] [PubMed] [Google Scholar]
- 62. Cuñetti L, Manzo L, Peyraube R, et al. Chronic pain treatment with cannabidiol in kidney transplant patients in Uruguay. Transplant Proc. 2018;50:461–464. [DOI] [PubMed] [Google Scholar]
- 63. Gulbransen G, Xu W, Arroll B. Cannabidiol prescription in clinical practice: an audit on the first 400 patients in New Zealand. BJGP Open. 2020;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu DH, Cullen BD, Tang M, et al. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol. 2020;21:390–402. [DOI] [PubMed] [Google Scholar]
- 65. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’ studies. Anaesthesia. 2004;59:440–452. [DOI] [PubMed] [Google Scholar]
- 66. van de Donk T, Niesters M, Kowal MA, et al. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ren Y, Whittard J, Higuera-Matas A, et al. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009;29:14764–14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bhagavan C, Kung S, Doppen M, et al. Cannabinoids in the treatment of insomnia disorder: a systematic review and meta-analysis. CNS Drugs. 2020;34:1217–1228. [DOI] [PubMed] [Google Scholar]
- 69. Calderon B, Sayre T. Cannabidiol use in older adults. US Pharm. 2020;45:34–38. [Google Scholar]
- 70. Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21:417S–427S. [DOI] [PubMed] [Google Scholar]
- 71. Barchel D, Stolar O, De-Haan T, et al. Oral cannabidiol use in children with autism spectrum disorder to treat related symptoms and co-morbidities. Front Pharmacol. 2019;9:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fleury-Teixeira P, Caixeta FV, Ramires da Silva LC, et al. Effects of CBD-enriched Cannabis sativa extract on autism spectrum disorder symptoms: an observational study of 18 participants undergoing compassionate use. Front Neurol. 2019;10:1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Linares IMP, Guimaraes FS, Eckeli A, et al. No acute effects of cannabidiol on the sleep-wake cycle of healthy subjects: a randomized, double-blind, placebo-controlled, crossover study. Front Pharmacol. 2018;9:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rock EM, Bolognini D, Limebeer CL, et al. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br J Pharmacol. 2012;165:2620–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wickham RJ. Revisiting the physiology of nausea and vomiting-challenging the paradigm. Support Care Cancer. 2020;28:13–21. [DOI] [PubMed] [Google Scholar]
- 76. Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. [DOI] [PubMed] [Google Scholar]
- 77. Grimison P, Mersiades A, Kirby A, et al. Oral THC: CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Ann Oncol. 2020;31:1553–1560. [DOI] [PubMed] [Google Scholar]
- 78. Kwiatkowska M, Parker LA, Burton P, et al. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology. 2004;174:254–259. [DOI] [PubMed] [Google Scholar]
- 79. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–1096. [DOI] [PubMed] [Google Scholar]
- 80. Collin C, Davies P, Mutiboko I, et al. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14:290–296. [DOI] [PubMed] [Google Scholar]
- 81. Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451–459. [DOI] [PubMed] [Google Scholar]
- 82. Keating GM. Delta-9-tetrahydrocannabinol/cannabidiol oromucosal spray (Sativex(®)): a review in multiple sclerosis-related spasticity. Drugs. 2017;77:563–574. [DOI] [PubMed] [Google Scholar]
- 83. Notcutt W, Langford R, Davies P, et al. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex®(nabiximols). Mult Scler J. 2012;18:219–228. [DOI] [PubMed] [Google Scholar]
- 84. Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols*(Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18:1122–1131. [DOI] [PubMed] [Google Scholar]
- 85. Wade DT, Makela P, House H, et al. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler J. 2006;12:639–645. [DOI] [PubMed] [Google Scholar]
- 86. Wade DT, Makela P, Robson P, et al. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler J. 2004;10:434–441. [DOI] [PubMed] [Google Scholar]
- 87. Sultan SR, O'Sullivan SE, England TJ. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: a randomised controlled trial. Br J Clin Pharmacol. 2020;86:1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jadoon KA, Tan GD, O'Sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI insight. 2017;2:e93760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Faria SM, de Morais Fabrício D, Tumas V, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson's disease. J Psychopharmacol. 2020;34:189–196. [DOI] [PubMed] [Google Scholar]
- 90. Newmeyer MN, Swortwood MJ, Taylor ME, et al. Evaluation of divided attention psychophysical task performance and effects on pupil sizes following smoked, vaporized and oral cannabis administration. J Appl Toxicol. 2017;37:922–932. [DOI] [PubMed] [Google Scholar]
- 91. Chalana H, Kundal T, Gupta V, et al. Predictors of relapse after inpatient opioid detoxification during 1-year follow-up. J Addict. 2016;2016:7620860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat. 2013;45:302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. García-Gutiérrez MS, Navarrete F, Gasparyan A, et al. Cannabidiol: a potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules. 2020;10:1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Beale C, Broyd SJ, Chye Y, et al. Prolonged cannabidiol treatment effects on hippocampal subfield volumes in current cannabis users. Cannabis Cannabinoid Res. 2018;3:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Solowij N, Broyd SJ, Beale C, et al. Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open-label clinical trial. Cannabis Cannabinoid Res. 2018;3:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mathur K, Vuppalanchi V, Gelow K, et al. Cannabidiol (CBD) consumption and perceived impact on extrahepatic symptoms in patients with autoimmune hepatitis. Dig Dis Sci. 2020;65:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rosenberg EC, Louik J, Conway E, et al. Quality of life in childhood epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia. 2017;58:e96–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wise J. FDA approves its first cannabis based medicine, BMJ 2018;361. 10.1136/bmj.k2827 [DOI] [PubMed]