Abstract
Importance:
Owing to its anti-inflammatory properties and antiviral “in vitro” effect against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), cannabidiol (CBD) has been proposed as a potential treatment for coronavirus disease 2019 (COVID-19).
Objective:
To investigate the safety and efficacy of CBD for treating patients with mild to moderate COVID-19.
Design:
Randomized, parallel-group, double-blind, placebo-controlled clinical trial conducted between July 7 and October 16, 2020, in two sites in Brazil.
Setting:
Patients were recruited in an emergency room.
Participants:
Block randomized patients (1:1 allocation ratio—by a researcher not directly involved in data collection) with mild and moderate COVID-19 living in Ribeirão Preto, Brazil, seeking medical consultation, and those who voluntarily agreed to participate in the study.
Interventions:
Patients received 300 mg of CBD or placebo added to standard symptomatic care during 14 days.
Main Outcome and Measure:
The primary outcome was reduction or prevention of the deterioration in clinical status from mild/moderate to severe/critical measured with the COVID-19 Scale or the natural course of the resolution of typical clinical symptoms. Primary study outcome was assessed on days 14, 21, and 28 after enrollment.
Results:
A total of 321 patients were recruited and assessed for eligibility, and 105 were randomly allocated either in CBD (n=49) or in placebo (n=42) group. Ninety-one participants were included in the analysis of efficacy. There were no baseline between-group differences regarding disease severity (χ2=0.025, p=0.988) and median time to symptom resolution (12 days [95% confidence interval, CI, 6.5–17.5] in the CBD group, 9 days [95% CI, 4.8–13.2] in the placebo group [χ2=1.6, p=0.205 by log-rank test]). By day 28, 83.3% in the CBD group and 90.2% in the placebo group had resolved symptoms. There were no between-group differences on secondary measures. CBD was well tolerated, producing mostly mild and transient side effects (e.g., somnolence, fatigue, changes in appetite, lethargy, nausea, diarrhea, and fever), with no significant differences between CBD and placebo treatment groups.
Conclusions and Relevance:
Daily administration of 300 mg CBD for 14 days failed to alter the clinical evolution of COVID-19. Further trials should explore the therapeutic effect of CBD in patients with severe COVID-19, possibly trying higher doses than the used in our study. Trial Registration: ClinicalTrials.gov identifier NCT04467918 (date of registration: July 13, 2020).
Keywords: SARS-CoV-2, COVID-19, cannabidiol, clinical trial, infectious diseases, internal medicine
Introduction
The new coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the death of >33 million people worldwide as of this writing, and so far, no treatment has gained regulatory approval. Since the beginning of the pandemic, several editorials, reviews, and pre-clinical studies have suggested that cannabidiol (CBD), a nonpsychotomimetic phytocannabinoid, could have potential beneficial effects in the course of COVID-19.1–6
CBD has putative anti-inflammatory properties7–14 and may attenuate the cytokine storm by reducing the levels of cytokines (such as interleukin-6, IL-6; tumor necrosis factor-α, TNF-α; and interferon-γ) and symptoms of acute respiratory distress syndrome in a mouse model, frequent clinical conditions observed in patients with severe COVID-19.7,8 CBD reduced brain levels of cytokines (such as TNF-α9 and IL-1β10) and microglia activation,10–12 and decreased the levels of proinflammatory cytokines (IL-5, IL-6, and IL-13) in pre-clinical models of lung inflammation13 and allergic asthma.14 Moreover, CBD has potential antiviral properties.2,4,5,15 Recently, in vitro and in silico analysis suggested that in VERO cells, CBD reduces intracellular expression of the spike protein S of the SARS-CoV-2.15
CBD showed anxiolytic and antidepressant effects in pre-clinical16–18 and clinical19,20 studies. It could also improve burnout syndrome symptoms and other mental health outcomes in health care workers treating COVID-19 patients.21
Considering CBD's potential therapeutic properties and its safety profile, we conducted a single-site clinical trial assessing the putative efficacy of 300 mg/day CBD administered during 2 weeks in patients diagnosed with mild to moderate COVID-19 in the city of Ribeirão Preto-Brazil.
Methods
Design
This study was designed as a two-site, randomized, parallel-group, double-blind, placebo-controlled clinical trial of oral CBD 300 mg/daily added to standard clinical care during 14 days to prevent or reduce the clinical deterioration of patients diagnosed with COVID-19 (ClinicalTrials.gov identifier NCT04467918). Patients with mild and moderate forms of COVID-19 were recruited in a public-affiliated emergency room of Ribeirão Preto County and in the Emergency Care Unit or Ribeirão Preto Medical School University Hospital. Participants were randomized by block randomization with a 1:1 allocation ratio with 16 blocks formed by sex, age (<OR> 60 years), disease severity (mild or moderate), and comorbidity (controlled diabetes and/or hypertension). A researcher not directly involved in data collection performed the allocation of the patients in each group. The study adhered to the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline, and the protocol is available in the eMethods (Supplementary Data).
Participants
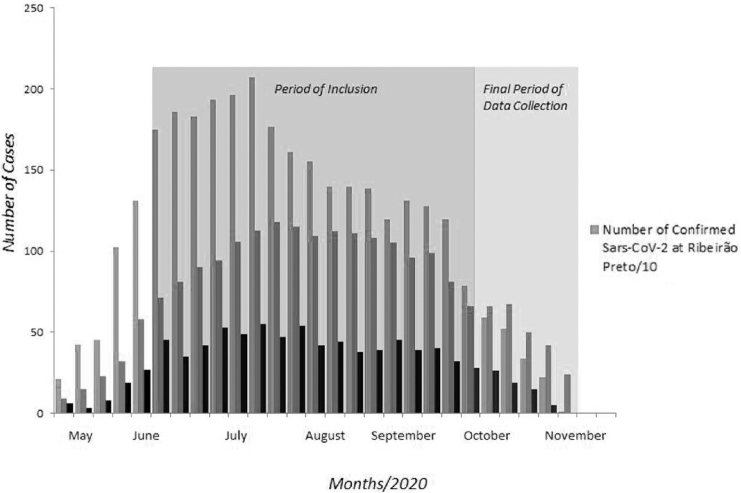
Participants were enrolled between July 7 and October 16, 2020. This period coincided with the start, peak, plateau, and initial reduction curve of the first wave of COVID-19 cases in Ribeirão Preto, São Paulo, Brazil (eFig. 1 in the Supplementary Data). Figure 1 summarizes the period of enrollment and data collection in this study.
FIG. 1.
Period with the start, peak, plateau, and initial reduction curve of the first wave of COVID-19 cases in Ribeirão Preto, São Paulo, Brazil. COVID-19, coronavirus disease 2019.
We recruited and assessed for eligibility 321 patients. Patients were recruited after receiving the clinical diagnosis of COVID-19 and performing a swab collection of material from the upper respiratory tract for posterior detection of the presence of SARS-CoV-2 using the reverse transcription (RT) followed by the quantitative polymerase chain reaction (PCR). A total of 105 patients who tested positive for SARS-CoV-2 and met the inclusion criteria signed the informed consent and were randomly allocated to one of the groups to receive therapeutic interventions. Participants were male or female adults (aged ≥18 years old) diagnosed with mild and moderate forms of COVID-19. Women of child-bearing age were asked if they were sexually abstinent or using approved contraceptive methods. The severity of the COVID-19 symptoms was classified based on the 5th edition of the Chinese manual for COVID-19 management.22 The mild form included patients with nonsevere symptomatic symptoms but without clinical manifestations of pneumonia. The moderate form included patients with fever, cough, secretion production, and other respiratory or nonspecific symptoms without severe pneumonia manifestation, defined by SaO2/SpO2 <94% in an airy room or a PaO2/FiO2 of 300 or below. Exclusion criteria included patients who did not want or could not fulfill the necessary home isolation for at least 14 days, current use of any medication with potential interactions with CBD (such as chloroquine, clobazam, warfarin, or valproic acid) or other experimental drugs used in the management of COVID-19 symptoms (ivermectin, lopinavir, ritonavir, and azithromycin among others) a history of undesirable reactions to CBD or other cannabinoids, patients with severe forms of COVID-19 (on screening, inclusion, or initial visit), patients with unstable chronic diseases (uncontrolled diabetes types 1/2, uncontrolled hypertension, lung, hematological, and liver diseases, chronic kidney disease in advanced stage, metabolic disorders, and immunosuppression), history of substance-related disorders, smoking record in the last 3 years, cannabis recreational use in the last 3 months, inability to cooperate because of cognitive impairment or other mental state, inability to use oral medication, or pregnancy during the study (extended to male participants who had a pregnant partner during the trial). Eligible patients were stratified according to sex, age, disease severity (mild or moderate), and presence of selected comorbidities (controlled diabetes and/or hypertension).
Procedure
All patients were managed according to the standard care recommended by the Brazilian Ministry of Health practical guidelines for diagnosis and treatment for mild and moderate cases of COVID-19 (https://portalarquivos.saude.gov.br/images/pdf/2020/April/18/Dirursos-Covid19.pdf). Pharmacological measures included the following: “Prescription of drugs for COVID-19 general symptom control, if there is no contraindication, with the possibility of intercalating antipyretic drugs in cases of difficult control of fever. Oral antipyretic: 1st option: Paracetamol/Acetaminophen 500–1000 mg/dose (maximum 3 mg/day); 2nd option: Dipyrone 500–1000 mg VO (maximum dose in adults 4 grams).” Clinical measures included the following: “Home isolation for 14 days from the date of symptoms onset; review every 48 hours, preferably by phone, providing face-to-face assistance, if necessary; maintain rest, a balanced diet and a good supply of fluids; isolation of home contacts for 14 days.”
The active arm received clinical and pharmacological measures plus oral CBD (99.6% purity; PurMed Global™, Delray Beach, FL) dissolved in medium-chain triglyceride oil (150 mg/mL concentration). Participants received 300 mg CBD/daily (1 mL or 150 mg per dose, twice a day) for 2 weeks. As there are no chronic studies of CBD on viral infections, the dose was chosen based on the minimum safe range observed in previous studies that detected an acute anxiolytic effect.23 The duration of 14-day treatment was chosen based on the observation that SARS-CoV-2 symptoms may appear (or increase in severity level) up to 14 days after exposure to the virus, as recommended by the World Health Organization.24 CBD vials were weighed before delivery to the participant and at the end of the trial to check for treatment compliance. Patients in the placebo group received clinical and pharmacological measures plus 1 mL twice a day of vehicle for 14 days using a dosing device/syringe indistinguishable from the CBD medication. Patients, nursing staff, laboratory technicians, physicians who carried out the assessments, researchers, and statisticians were all blind to the treatment allocation.
Swab collection (from the oropharynx, to minimize discomfort) and blood samples were obtained by a nurse visiting the patient's home on the screening period (day 3 to day 1) and on days 1, 2, 3, 4, 5, 7, 10, 14, 21, and 28 of the clinical segment. On these occasions, nurses also evaluated weight, vital signs (blood pressure, heart rate, and body temperature), pulse oximetry, treatment adherence, and a smell test to evaluate anosmia/hyposmia associated with COVID-19. This test, adapted from the Peanut Butter Smell Test,25 measured the distance (in centimeters) necessary for the patient to start smelling or perceiving the scent of peanut butter placed in a small cup inside a tube. Each patient daily (immediately before lunch and dinner) measured the axillary temperature in case of suspected fever.
Participants were also assessed remotely daily (between days 1 and 14; and on days 21 and 28) by psychiatrists who evaluated the clinical and emotional symptoms, and possible side effects of the treatments. A modified version of the UKU side-effect rating scale of the Scandinavian Society of Psychopharmacology, highlighting the most common adverse effects of CBD, the “CBD Adverse Effects Scale” (CARE Scale), evaluated treatment safety. Validated scales to measure anxiety and depression symptoms (see Outcomes) we also used. On the 14th day, patients did a chest computed tomography (CT; full description of the method given in the eMethods in the Supplementary Data), and on the 28th day they received a complete clinical evaluation at a private medical unit. If not tolerated, CBD use was suspended. In case of a clinical picture worsening, physicians referred the patients to the State health system according to the official guidelines. In case of hospitalization, CBD or placebo treatment was interrupted.
Outcomes
Our primary outcome was the proportion of patients with clinical deterioration, (classified as mild, moderate, or severe) from randomization to the 28-day follow-up period. The COVID-19 severity classification used the following criteria (adapted from Long et al.26 and Dong et al.27):
Mild cases (mild clinical symptoms and no chest CT imaging showing pneumonia);
Moderate cases (fever and/or any respiratory symptom plus chest CT imaging showing pneumonia);
Severe cases (dyspnea and/or severe clinical symptoms that require immediate medical assistance, and/or oxygen saturation <93% at rest plus chest CT imaging showing viral pneumonia).
The secondary outcome was the time from randomization to complete resolution of symptoms within the 28-day follow-up period. Improvement of clinical symptoms was defined as “interruption of fever with an axillary temperature of 37.8°C (100°F) or below, normalization of SpO2 (>94% in an airy room), and disappearance of COVID-19 symptoms (e.g., cough, nasal congestion, pain throat, shortness of breath, chest pain, chills, myalgia).” Moreover, the COVID-19 Clinical Symptoms (COV2-CS) scale evaluated the severity of symptoms. This scale contains 22 COVID-19 symptoms scored on a 3- or 4-point scale according to the codification dictionary Medical Dictionary for Regulatory Activities using the terminology of the National Cancer Institute Common Terminology Criteria for Adverse Events.
Additional secondary outcomes were the clinical conditions as assessed by (1) emotional symptoms scales; (2) laboratory parameters, including proinflammatory cytokines and C-reactive protein plasma levels; (3) smell test; (4) viral load; (5) CBD plasm level; and (6) occurrence of side effects. Anxiety and depressive symptoms were measured with the validated Brazilian versions of the Generalized Anxiety Disorder Questionnaire-7 (GAD-7)28 and the Patient Health Questionnaire-9 (PHQ-9),29 respectively (full description of the scales given in the eMethods in the Supplementary Data). Blood samples were collected at baseline and at days 7, 14, 21, and 28 to assess plasma levels of proinflammatory cytokines (IL-6 and TNF-α), C-reactive protein, CBD, viral load, and general clinical measures (full description of the methods given in the eMethods in the Supplementary Data).
Ethics
The trial protocol was submitted and approved by both the institutional and national review boards, namely Ribeirão Preto Medical School University Hospital and the National Council on Research Ethics (CONEP; CAAE No. 33841120.0.0000.5440). The trial was conducted in accordance with the Declaration of Helsinki, the Good Clinical Practice guidelines, and local regulatory requirements. Before enrollment and allocation to the study arms, informed consent was obtained from all participants. An independent Data Safety Monitoring Committee was engaged to periodically review the safety of the entire clinical program and selected cases, including test abnormalities.
Statistical analysis
As there are no previous studies about the effects of CBD on COVID-19 symptoms, the sample size was calculated by estimating a level of significance of 0.05, statistical power of 0.85, and effect size (Cohen's f) of 0.10, resulting in a sample of 90 volunteers. Collected data were stored in the RedCap platform and then exported to the Statistical Package for the Social Sciences (SPSS) v.26.0 for analysis. Patients were analyzed according to the treatment they received in the as-treated population (sensitivity analysis). We compared the sample clinical and demographic data using Student's t test for continuous data and the chi-square test for nominal data. Data from the rating scales were analyzed with a repeated-measures analysis of variance with time, group, and time×group interaction factors. Tests of within-subjects contrasts with a significant time×group interaction were used to assess differences between groups in each measure concerning the baseline. In cases where sphericity conditions were not met, the Huynh–Feldt epsilon corrected the degrees of freedom of the repeated factor. The UKU/CARE scale was analyzed using Fisher's exact test. The time from randomization to undetectable RT-PCR in the oropharynx swab collection and the complete resolution of symptoms were assessed by a Kaplan–Meier plot and compared with a log-rank test. The significance level was set at p<0.05.
Results
Patients
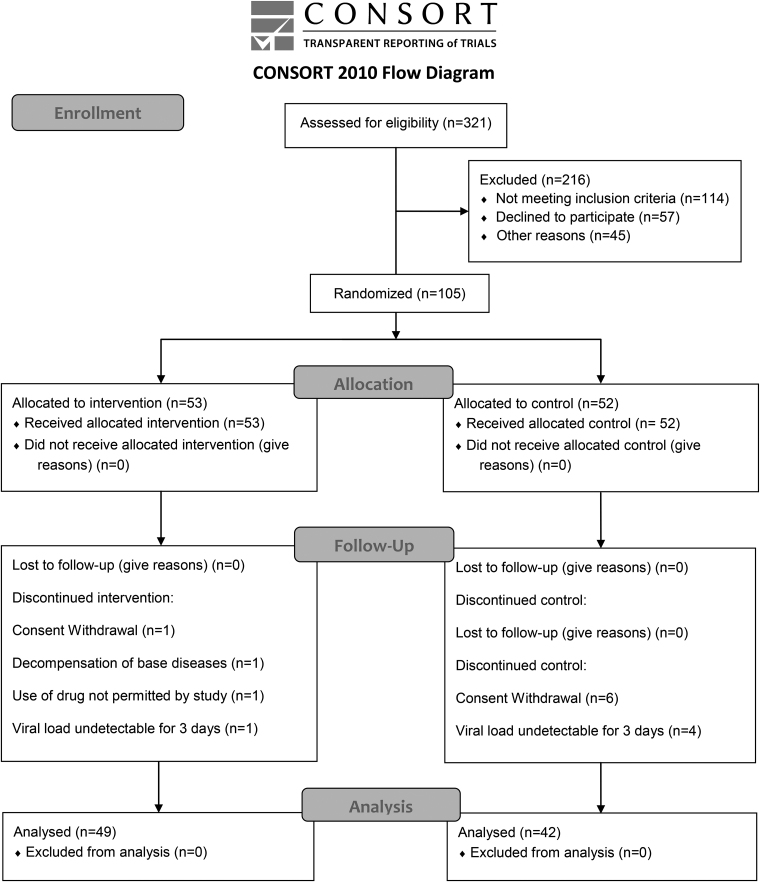
A total of 105 patients were randomized and 14 were discontinued during follow-up. The number of patients and their reasons for discontinuation were as follows: one patient owing to inclusion criteria failure (decompensated diabetes on the first day of data collection—CBD group); five because of negative viral load in the first three time-points after inclusion (four in placebo group and one in CBD group); seven for consent withdrawal (six in placebo group and one in CBD group); and one owing to noncompliance with the protocol (started using other medications such as hydroxychloroquine, azithromycin, and ivermectin, without the recommendation of the study medical team). Three patients in the CBD group had to be hospitalized during the trial because of complications from SARS-CoV2 infection: one owing to serious venous thrombosis and two because of saturation alterations All these patients were monitored and no deaths occurred. Therefore, the data of 91 patients were included in the final analysis, 49 being randomly assigned to CBD and 42 to the placebo group (Fig. 2).
FIG. 2.
CANDIDATE study flow diagram. CANDIDATE, Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms.
There were no significant differences between groups in the demographic and baseline clinical characteristics (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients at Baseline
| Characteristic | Cannabidiol (N=49) | Placebo (N=42) | p |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 38.7 (11.0) | 40.9 (10.9) | 0.85 |
| Sex, n (%) | |||
| Female | 33 (67.3) | 27 (64.3) | 0.76 |
| Male | 16 (32.7) | 15 (35.7) | |
| Body mass index | |||
| Mean (SD) | 28.4 (6.1) | 27.5 (5.2) | 0.93 |
| Occupation, n (%) | |||
| Physician | 6 (12.2) | 3 (7.1) | 0.54 |
| Nurse | 11 (22.4) | 13 (31.0) | |
| Others | 32 (65.3) | 26 (61.9) | |
| Living situation, n (%) | |||
| Lives alone | 8 (16.3) | 3 (7.1) | 0.37 |
| Lives with partner and/or children | 34 (69.4) | 35 (83.3) | |
| Lives with parents | 7 (14.2) | 4 (9.5) | |
| Comorbidities | |||
| Hypertension | 2 (4.1) | 2 (4.9) | 0.69 |
| Diabetes | 1 (2.0) | 1 (2.4) | |
| Obesity/dyslipidemia | 2 (4.1) | 4 (9.8) | |
| Thyroid diseases | 2 (4.1) | 1 (2.4) | |
| Asthma | 2 (4.1) | 1 (2.4) | |
| Allergic diseases | 0 (0.0) | 3 (7.3) | |
| Chronic neurological disease | 1 (2.0) | 1 (2.4) | |
| Neoplasia | 1 (2.0) | 0 (0.0) | |
| Others chronic diseases | 6 (12.2) | 3 (7.3) | |
| Medication, n (%) | 18 (37.5) | 12 (29.3) | 0.41 |
| Signs and symptoms, n (%) | |||
| Hyposmia | 39 (79.6) | 32 (76.2) | 0.70 |
| Myalgia | 34 (69.4) | 19 (45.2) | 0.14 |
| Fatigue | 30 (61.2) | 22 (52.4) | 0.68 |
| Headache | 30 (61.2) | 21 (50.0) | 0.30 |
| Cough | 27 (55.1) | 19 (45.2) | 0.63 |
| Anorexia | 26 (53.1) | 22 (52.4) | 0.99 |
| Malaise | 26 (53.1) | 20 (47.7) | 0.61 |
| Coryza | 19 (38.8) | 13 (31.0) | 0.44 |
| Sore throat | 16 (30.7) | 14 (29.4) | 0.45 |
| Fever (chills) | 9 (18.3) | 11 (26.1) | 0.45 |
| Dyspnea | 9 (18.3) | 3 (7.1) | 0.25 |
| Chest pain | 9 (18.3) | 2 (4.8) | 0.13 |
| Nausea | 7 (14.3) | 8 (19.1) | 0.64 |
| Diarrhea | 7 (14.3) | 9 (21.4) | 0.45 |
| Current smoking, n (%) | 2 (4.1) | 3 (7.1) | 0.52 |
| Alcohol abuse, n (%) | 12 (24.4) | 10 (23.8) | 0.85 |
| Mean arterial pressure | |||
| Median (interquartile range) | 99.8 (14.9) | 97.3 (18.5) | 0.93 |
| Heart rate | |||
| Median (interquartile range) | 86.0 (19.75) | 82.0 (17.5) | 0.70 |
| Oximetry | |||
| Median (interquartile range) | 99.8 (14.8) | 97.3 (18.5) | 0.25 |
SD, standard deviation.
Most patients were women (67.3% in the CBD, 64.30% in the placebo group), with a median age of 38.7 (11.0) years in the CBD and 40.9 (107.9) years in the placebo group.
Primary outcome
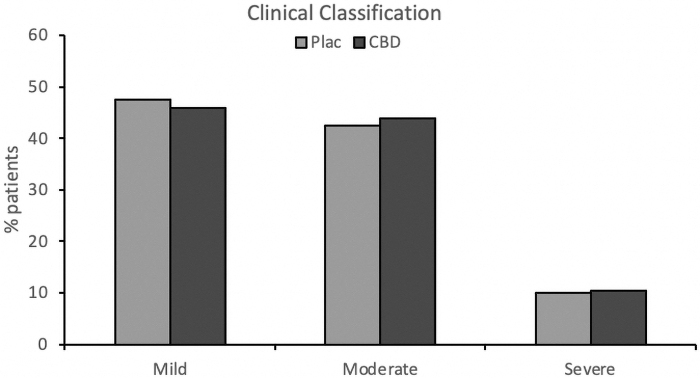
There were no significant differences between the groups regarding the percentage of patients classified as mild, moderate, or severe cases (χ2=0.025, p=0.988) between randomization and day 28 (Fig. 3).
FIG. 3.
Percentage of COVID-19 patients classified as mild, moderate, or severe cases between randomization and day 28 in the CBD and placebo groups. CBD, cannabidiol.
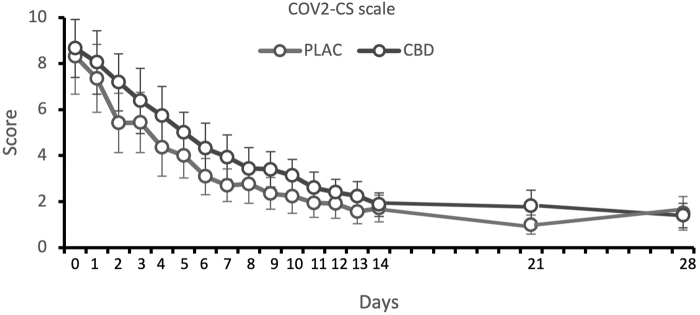
The mean scores of the COV2-CS scale showed a significant positive correlation with clinical classification (Spearman's rho=0.32, p=0.004). Symptoms severity significantly decreased along the study [time factor, F(4.92,379.18)=72.66, p<0.001], but there was no significant group effect [F(1,77)=3.03, p=0.09] or time×group interaction [F(4.92,379.18)=0.78, p=0.56] (Fig. 4).
FIG. 4.
The mean scores of the COV2-CS scale between randomization and day 28 in the CBD and placebo groups. COV2-CS, COVID-19 Clinical Symptoms.
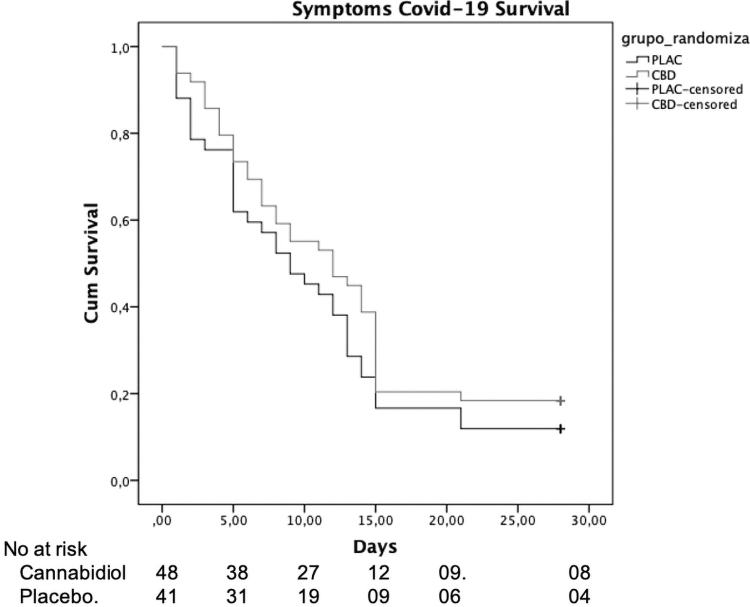
There were no significant between-group differences regarding the time from randomization to the complete resolution of typical COVID-19 symptoms (χ2=1.6, p=0.205 by log-rank test). The median time to resolution of symptoms was 12 days (95% confidence interval [CI], 6.5–17.5) in the CBD compared with 9 days (95% CI, 4.8–13.2) in the placebo group (Fig. 5).
FIG. 5.
The symptoms severity along the study between randomization and day 28 in the CBD and placebo groups.
Secondary outcomes
Emotional symptoms
Anxiety and depression symptoms decreased along the study [GAD-7, F(5.49,477.31)=20.24, p<0.001; PHQ-9, F(5.64,490.36)=26.55, p=<0.001], but there was no group [GAD-7, F(1,87)=0.71, p=0.79; PHQ-9, F(1,87)=2.34, p=0.13] or time×group interaction [GAD-7, F(5.49,477.31)=0.41, p=0.81; PHQ-9, F(5.64,490.36)=0.97, p=0.44] effect (eFigs. 2 and 3 in the Supplementary Data).
Smell test
The distance where volunteers could smell the peanut butter cup increased along the study [time factor, F(84,435.32)=69.81, p<0.001], but there was no group [F(1,90)=2.15, p=0.15] or time×group interaction [F(5.49,477.31)=0.81, p=0.54] effect (eFig. 4 in the Supplementary Data).
Viral load
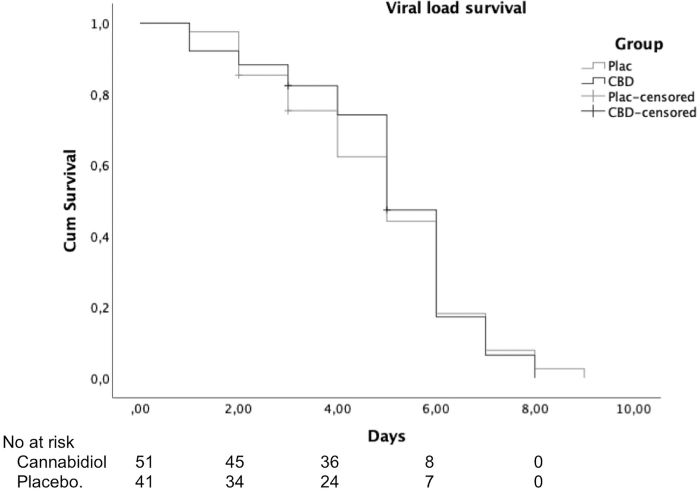
There were no significant differences between groups on viral load (log rank [Mantel–Cox]-χ2=0.027, p=0.869) (Fig. 6).
FIG. 6.
Time to complete resolution of typical COVID-19 symptoms along the study between randomization and day 28 in the CBD and placebo groups.
Cytokines
TNF-α and IL-6 plasma levels did not differ between groups. TNF-α decreased on day 14 in both groups (placebo: χ2=21.37, df=2, p<0.001; CBD: χ2=19.44, df=2, p<0.001). There was no time effect regarding IL-6 (eFig. 5 in the Supplementary Data). C-reactive protein levels showed that patients had mild to moderate infection severity (CBD, median: baseline, 3.88; day 28, 1.31; placebo, median: baseline 4.83; day 28, 2.25) (based on Osório et al.,29 Fu et al.,30 and Jimeno et al.31) that decreased along the study (placebo, χ2=59.23, df=2, p<0.001; CBD: χ2=39.10, df=2, p<0.001).
CBD plasma levels
There was a significant time effect (χ2=185.23, N=46, df=8, p<0.001, Friedman test). Compared with day 2 (when plasma was first collected), CBD levels were significantly higher up to day 14, and lower on days 21 and 28 (Z values ranging from 3.22 to 5.39 ng/mL, p≤0.001, Dunn test) (eFig. 6 in the Supplementary Data).
Safety
Adverse events recorded with the UKU/CARE scale are given in Table 2. Both interventions were well tolerated and the most common (>10%) adverse events in both arms were somnolence, fatigue, decreased appetite, lethargy, weight loss, nausea, diarrhea, increased appetite, and fever. No serious adverse events were observed during the trial.
Table 2.
Adverse Events by Treatment Arm
| Adverse events | No. of patients that refereed adverse events during the study (%) |
|
|
|---|---|---|---|
| Cannabidiol (n=49) | Placebo (n=42) | Chi-square test p | |
| Somnolence | 38 (77.6) | 33 (78.6) | 0.933 |
| Fatigue | 38 (77.6) | 33 (78.6) | 0.933 |
| Decreased appetite | 38 (77.6) | 32 (76.2) | 0.860 |
| Lethargy | 25 (51.0) | 15 (35.7) | 0.142 |
| Weight loss | 24 (49.0) | 22 (52.4) | 0.761 |
| Nausea | 23 (46.9) | 16 (38.1) | 0.409 |
| Diarrhea | 21 (42.9) | 20 (47.6) | 0.392 |
| Increased appetite | 17 (34.7) | 10 (23.8) | 0.255 |
| Fever | 11 (22.5) | 15 (45.7) | 0.167 |
| Weight gain | 10 (20.4) | 8 (19.1) | 0.865 |
| Vomiting | 6 (12.3) | 4 (9.5) | 0.675 |
| Headache | 4 (8.2) | 3 (7.1) | 0.852 |
| Abdominal pain | 4 (8.2) | 2 (4.8) | 0.512 |
| Rash | 3 (6.2) | 0 (0.0) | 0.103 |
| Bitter mouth | 3 (6.2) | 4 (9.5) | 0.547 |
There was no group×time effect in weight, blood pressure, heart rate, body temperature, pulse oximetry, and general blood parameters (p<0.05). As with C-reactive protein levels, neutrophils/lymphocytes levels showed that patients had mild to moderate infection severity (CBD, median: baseline, 1.25; day 28, 1.84; placebo, median: baseline 1.45; day 28, 1.57) (based on Osório et al.,29 Fu et al.,30 and Jimeno et al.31).
Discussion
Daily administration of 300 mg CBD for 14 days to patients with a recent diagnosis of COVID-19 with mild or moderate severity was safe but did not alter the clinical evolution in the first 28 days of follow-up. The results do not confirm the suggestions that CBD could have a therapeutic effect on COVID-19 owing to its anti-inflammatory (especially concerning cytokines)1–3,5–14 and antiviral2,4,5,15 effects. However, this result should be considered with caution because the patients had mild or moderate forms of COVID-19, with low inflammation levels.23,30,31 Besides, a uniform dose of 300 mg/day was tested, and the dose-dependent effects of CBD are well known.32 Thus, it is possible that greater inflammation levels are necessary to respond to CBD, or that higher CBD doses are needed to observe anti-inflammatory and antiviral effects. Moreover, CBD did not show anxiolytic or antidepressive effects, which also does not confirm previous literature showing anxiolytic properties of this dose.5,16–21 However, as in the case of inflammation, patients did not have high anxiety and depression levels at baseline (mean GAD-7 and PHQ-9 scores <10). In addition, all the patients received significant support, with repeated visits of nurses at the residence and remote daily monitoring by the physician.
To the best of our knowledge, this is the first trial assessing the effects of CBD in COVID-19 patients, and the largest assessing inflammatory measures in a clinical sample with an infectious disease. Limitations of the trial include its short follow-up duration, single-intervention dose, two-center design, and the inclusion of only patients with mild and moderate forms of the infection.
Conclusion
Daily administration of 300 mg CBD for 14 days failed to alter the clinical evolution of COVID-19. Further trials with patients with different severity levels of COVID-19 and CBD doses are necessary to confirm the absence of effects of CBD in the clinical course of COVID-19 observed in our study. Finally, considering the anti-inflammatory, neuroprotective, and safe profile of CBD, future double-blind trials assessing whether this compound could act as an effective preventive agent for chronic post-COVID-19 syndrome symptoms are necessary and suitable. Such a study is underway by our research group.
Supplementary Material
Acknowledgments
The authors thank PurMed Global who kindly donated CBD. The authors also thank Salomão e Zoppi Serviços Médicos e Participações S/A (São Paulo, Brazil) and Laboratório Chromatox/Dasa for dosing plasma levels of CBD at no cost. The authors finally thank the additional contributions from the members of the data and safety monitoring board for their commitment and responsiveness: Prof. Dr. Antonio Carlos dos Santos, MD, PhD (Department of Medical Imaging, Haematology, and Clinical Oncology, FMRP, University of São Paulo, Brazil); Prof. Dr. Fábio Carmona, MD, PhD (Department of Pediatrics, FMRP, University of São Paulo, Brazil.); Prof. Dr. Osvaldo Takayanagui, MD, PhD (Department of Health Sciences, FMRP, University of São Paulo, Brazil). None of these individuals received compensation for their role in the study.
Abbreviations Used
- CANDIDATE
Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms
- CARE
CBD Adverse Effects
- CBD
cannabidiol
- CI
confidence interval
- COV2-CS
COVID-19 Clinical Symptoms
- COVID-19
coronavirus disease 2019
- CT
computed tomography
- GAD-7
Generalized Anxiety Disorder Questionnaire-7
- IL
interleukin
- PHQ-9
Patient Health Questionnaire-9
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- TNF-α
tumor necrosis factor-α
Contributor Information
Collaborators: for the Cannabidiol for COVID-19 Patients (CANDIDATE) Trial Investigators
Authors' Contributions
J.A.S.C., A.W.Z., F.S.G., F.L.O., A.C.C., S.R.L., and J.E.C.H. designed the study, and J.A.S.C. and R.G.S. wrote the report. J.A.S.C., A.W.Z., F.S.G., F.L.O., A.C.C., J.C.P., and J.E.C.H. coordinated the study, and A.W.Z., F.S.G., and A.C.C. analyzed the data. R.R.F., K.C.M.C., D.S.S., I.P.-.D.-S., F.F.S., and A.C.C. designed, performed, and analyzed the cytokines plasma levels. All authors critically revised the report or contributed important intellectual content.
Author Disclosure Statement
J.A.S.C. is a member of the International Advisory Board of the Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE)—National Health and Medical Research Council (NHMRC). J.A.S.C. and J.E.C.H. have received travel support to attend scientific meetings and personal consultation fees from BSPG-Pharm. J.A.S.C., J.E.C.H., F.K., F.S.G., A.W.Z., and R.M. are coinventors of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023,” Def. US number Reg. 62193296; July 29, 2015; INPI on August 19, 2015 (BR1120150164927; R.M., A.W.Z., F.K., J.E.C.H. F.S.G., J.A.S.C., A. Breuer). Universidade de São Paulo (USP) has licensed this patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1) and has an agreement with Prati-Donaduzzi to “develop a pharmaceutical product containing synthetic CBD and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson's disease, and anxiety disorders.” J.A.S.C., J.E.C.H., F.S.G., A.C.C., and A.W.Z. are coinventors of the patent “Cannabinoid-containing oral pharmaceutical composition, method for preparing and using same,” INPI on September 16, 2016 (BR 112018005423-2). The other authors declare that they have no conflicts of interest.
Funding Information
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and by the Instituto Nacional de Ciência e Tecnologia Translational em Medicina (INCT-TM; CNPq/FAPESP; 465458/2014-9; 2014/50891-1). J.A.S.C. received a grant from the University Global Partnership Network (UGPN)—Global Priorities in Cannabinoid Research Excellence Program. J.A.S.C., J.E.C.H., F.L.O., S.R.L., A.C.C., and A.W.Z. are recipients of CNPq research fellowships. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Supplementary Material
Cite this article as: Crippa JAS, Pacheco JC, Zuardi AW, Guimarães FS, Campos AC, Osório FL, Loureiro SR, dos Santos RG, Souza JDS, Ushirohira JM, Ferreira RR, Mancini Costa KC, Scomparin DS, Scarante FF, Pires-Dos-Santos I, Mechoulam R, Kapczinski F, Fonseca BAL, Esposito DLA, Passos ADC, Dal Fabbro AL, Bellissimo-Rodrigues F, Arruda E, Scarpelini S, Andraus MH, Nather Junior JC, Wada DT, Koenigkam-Santos M, Santos AC, Busatto Filho G, Hallak JEC; for the Cannabidiol for COVID-19 Patients (CANDIDATE) Trial Investigators (2022) Cannabidiol for COVID-19 patients with mild to moderate symptoms (CANDIDATE study): a randomized, double-blind, placebo-controlled clinical trial, Cannabis and Cannabinoid Research 7:5, 658–669, DOI: 10.1089/can.2021.0093.
References
- 1. El Biali M, Broers B, Besson M, et al. Cannabinoids and COVID-19. Med Cannabis Cannabinoids. 2020;3:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrareddy SN, Mohan M. SARS-CoV2 induced respiratory distress: can cannabinoids be added to anti-viral therapies to reduce lung inflammation? Brain Behav Immun. 2020;87:120–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Esposito G, Pesce M, Seguella L, et al. The potential of cannabidiol in the COVID-19 pandemic. Br J Pharmacol. 2020;177:4967–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mabou Tagne A, Pacchetti B, Sodergren M, et al. Cannabidiol for viral diseases: hype or hope? Cannabis Cannabinoid Res. 2020;5:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malinowska B, Baranowska-Kuczko M, Kicman A, et al. Opportunities, challenges and pitfalls of using cannabidiol as an adjuvant drug in COVID-19. Int J Mol Sci. 2021;22:1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suryavanshi SV, Kovalchuk I, Kovalchuk O. Cannabinoids as key regulators of inflammasome signaling: a current perspective. Front Immunol. 2021;11:613613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khodadadi H, Salles ÉL, Jarrahi A, et al. Cannabidiol modulates cytokine storm in acute respiratory distress syndrome induced by simulated viral infection using synthetic RNA. Cannabis Cannabinoid Res. 2020;5:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salles ÉL, Khodadadi H, Jarrahi A, et al. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J Cell Mol Med. 2019;843:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campos AC, Brant F, Miranda AS, et al. Cannabidiol increases survival and promotes rescue of cognitive function in a murine model of cerebral malaria. Neuroscience. 2015;289:166–180. [DOI] [PubMed] [Google Scholar]
- 10. Mecha M, Feliú A, Iñigo PM, et al. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013;59:141–150. [DOI] [PubMed] [Google Scholar]
- 11. Dos-Santos-Pereira M, Guimarães FS, Del Bel E, et al. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia. 2020;68:561–573. [DOI] [PubMed] [Google Scholar]
- 12. Sonego AB, Prado DS, Vale GT, et al. Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors. Brain Behav Immun. 2018;74:241–251. [DOI] [PubMed] [Google Scholar]
- 13. Anil SM, Shalev N, Vinayaka AC, et al. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci Rep. 2021;11:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vuolo F, Abreu SC, Michels M, et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur J Pharmacol. 2019;843:251–259. [DOI] [PubMed] [Google Scholar]
- 15. Raj V, Park JG, Cho KH, et al. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int J Biol Macromol. 2021;168:474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zanelati TV, Biojone C, Moreira FA, et al. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol. 2010;159:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campos AC, Ferreira FR, Guimarães FS. Cannabidiol blocks long-lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. J Psychiatr Res. 2012;46:1501–1510. [DOI] [PubMed] [Google Scholar]
- 18. Campos AC, Ortega Z, Palazuelos J, et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int J Neuropsychopharmacol. 2013;16:1407–1419. [DOI] [PubMed] [Google Scholar]
- 19. Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front Psychol. 2019;10:2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pacheco JC, Souza JDS, Hallak JEC, et al. Cannabidiol as a treatment for mental health outcomes among health care workers during the coronavirus disease pandemic. J Clin Psychopharmacol. 2021;41:327–329. [DOI] [PubMed] [Google Scholar]
- 22. China NHC. New Coronavirus Pneumonia Prevention and Control Protocol. 7th ed. National Health Commission of the People's Republic of China; 2020. https://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf (accessed March 5, 2020).
- 23. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. WHO R&D blueprint: novel coronavirus: COVID-19 therapeutic trial synopsis. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf (accessed June 28, 2020).
- 25. Stamps JJ, Bartoshuk LM, Heilman KM. A brief olfactory test for Alzheimer's disease. J Neurol Sci. 2013;333:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long L, Zeng X, Zhang X, et al. Short-term outcomes of COVID-19 and risk factors for progression. Eur Respir J. 2020;55:2000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dong D, Tang Z, Wang S, et al. The role of imaging in the detection and management of COVID-19: a review. IEEE Rev Biomed Eng. 2021;14:16–29. [DOI] [PubMed] [Google Scholar]
- 28. Moreno A L, DeSousa DA, Souza AMFLP, et al. Factor structure, reliability, and item parameters of the Brazilian-Portuguese version of the GAD-7 questionnaire. Temas Psicol. 2016;24:367–376. [Google Scholar]
- 29. Osório FL Vilela Mendes A, Crippa JA, et al. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Perspect Psychiatr Care. 2009;45:216–227. [DOI] [PubMed] [Google Scholar]
- 30. Fu J, Kong J, Wang W, et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb Res. 2020;192:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jimeno S, Ventura PS, Castellano JM, et al. Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur J Clin Invest. 2021;51:e13404. [DOI] [PubMed] [Google Scholar]
- 32. Crippa JA, Guimarães FS, Campos AC, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.