Abstract
Human immunodeficiency virus type 1 (HIV-1) infection results in impaired immune function that can be measured by changes in immunophenotypically defined lymphocyte subsets and other in vitro functional assays. These in vitro assays may also serve as early indicators of efficacy when new therapeutic strategies for HIV-1 infection are being evaluated. However, the use of in vitro assays of immune function in multicenter clinical trials has been hindered by their need to be performed on fresh specimens. We assessed the feasibility of using cryopreserved peripheral blood mononuclear cells (PBMC) for lymphocyte immunophenotyping and for lymphocyte proliferation at nine laboratories. In HIV-1-infected patients with moderate CD4+ lymphocyte loss, the procedures of density gradient isolation, cryopreservation, and thawing of PBMC resulted in significant loss of CD19+ B cells but no measurable loss of total T cells or CD4+ or CD8+ T cells. No significant changes were seen in CD28− CD95+ lymphocytes after cell isolation and cryopreservation. However, small decreases in HLA-DR+ CD38+ lymphocytes and of CD45RA+ CD62L+ were observed within both the CD4+ and CD8+ subsets. Fewer than 10% of those specimens that showed positive PBMC proliferative responses to mitogens or microbial antigens lost their responsiveness after cryopreservation. These results support the feasibility of cryopreserving PBMC for immunophenotyping and functional testing in multicenter AIDS clinical trials. However, small changes in selected lymphocyte subsets that may occur after PBMC isolation and cryopreservation will need to be assessed and considered in the design of each clinical trial.
Therapeutic approaches that reduce the rate of human immunodeficiency virus type 1 (HIV-1) replication result in fewer clinical events and prolong the survival of infected individuals (10, 22). In addition to an increase in CD4 T cells, immune function as measured by in vitro assays also improves significantly in response to antiretroviral treatments. Following initiation of highly active antiretroviral therapy, functionally relevant lymphocyte subsets in blood begin to return to normal levels. In addition, in vitro proliferation and cytokine secretion by peripheral blood mononuclear cells (PBMC) also show improvement (4, 14, 16, 24). As a result, in vitro measurements of immune function are being explored as a substitute for clinical endpoints in therapeutic trials (21). Furthermore, quantitating immune function through the use of these assays will provide a better definition of the immune reconstitution that occurs during antiretroviral therapy and may advance our understanding of AIDS pathogenesis.
The in vitro assays currently used to measure immune function are technically complex, prone to variability, and usually performed in real time on fresh specimens. The problems of performing these assays efficiently and reproducibly are compounded in multicenter clinical trials. In these clinical trials, specimens are collected over many months and at multiple locations, where, often, the technical ability to perform these assays may not exist. The precision and accuracy of complex immunologic assays could be greatly improved if specimens obtained at multiple sites could be analyzed in a single, highly skilled laboratory. Within-patient variability might also be reduced if multiple specimens obtained over time could be analyzed simultaneously in the same assay. Moreover, in studies of opportunistic infections where few clinical endpoints are found, retrospective analysis of cryopreserved specimens from selected subjects in a case-controlled fashion would provide a more efficient use of laboratory resources.
Previous studies have indicated that the immunophenotypic characteristics of the major lymphocyte subsets are retained in cryopreserved PBMC (13, 18, 27). The expression of certain functionally related molecules of particular relevance in HIV-1 infection, such as CD38, is also relatively stable following cryopreservation (23). Likewise, the potential for PBMC to be induced to proliferate, secrete cytokines, or exhibit antigen-specific or nonspecific lytic activity can be preserved in stored, frozen PBMC (7, 11, 12, 18, 27, 30). However, these studies were always performed in individual laboratories, often using specimens from normal or non-HIV-infected donors.
In the present study, we assessed the feasibility of isolating and cryopreserving PBMC from HIV-1-infected individuals for assays of immune function at nine clinical sites. Using a uniform, simplified cryopreservation technique, the proliferative capacity of PBMC was largely preserved. However, small but statistically significant changes were detected in the sizes of some immunophenotypically defined lymphocyte subsets after isolation and cryopreservation. These results provide evidence that cryopreservation of PBMC may be appropriate for some retrospective analyses of immune function in multicenter AIDS clinical trials.
MATERIALS AND METHODS
Sites and protocols.
Nine laboratories participated in this study. Each site was serving as an Immunology Advanced Technology Laboratory in support of clinical trials for the Adult AIDS Clinical Trials Group (ACTG), Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID). Sites performed the specified immunologic assays using consensus methods established by the ACTG (http://aactg.s-3.immeth.htm). Prior to performing this study, all sites had demonstrated proficiency in performing flow cytometric assays by analyzing common specimens that had been shipped to each site. Proficiency in lymphocyte proliferation assays was confirmed by demonstrating the ability to detect positive responses to mitogens and microbial antigens in HIV-infected donors.
Clinical specimens.
Each site obtained blood specimens after informed consent from three HIV-1-infected donors whose CD4 T-cell count was expected to be 200 to 400 cells/μl based on the medical history. Donors were selected irrespective of treatment; however, most were receiving conventional antiretroviral medications. Specimens were drawn directly into evacuated blood collection tubes containing EDTA or heparin and delivered directly to the laboratories for processing or analysis. Within 6 h of drawing, a complete blood count was performed on each specimen to determine the CD4 T-cell count.
Isolation of PBMC.
PBMC were isolated from heparinized blood by routine density gradient centrifugation over Ficoll-diatrizoate within 6 h of drawing. Contaminating red blood cells (RBCs) were lysed, and PBMC were washed and counted. Viability was estimated by trypan blue dye exclusion. Freshly isolated PBMC were divided into two aliquots. One aliquot (fresh Ficoll-isolated PBMC) was used in lymphocyte proliferation assays and immunophenotyped as described below on the same day that the specimen was drawn. The other aliquot of cells was cryopreserved as described below.
Cryopreservation and thawing of PBMC.
Density gradient-isolated PBMC were resuspended in ice-cold fetal bovine serum with 10% dimethyl sulfoxide (DMSO) at 107 cells/ml. Aliquots of cell suspension (0.5 to 1.0 ml) were transferred to cryovials that had been chilled to −20°C. Immediately after the cell suspension was placed in cryovials, specimens were transferred to a precooled (4°C) Nalgene Cryo 1C freezing container (Nalge Nunc International, Rochester, N.Y.) and placed in a −70°C freezer overnight. This simplified method of controlled-rate freezing lowers specimen temperature by approximately 1°C per h (28, 29). Frozen specimens were transferred to a liquid nitrogen freezer within 24 h. Specimens were maintained in liquid nitrogen for 6 to 14 days before being thawed and assayed.
Thawing and recovery of cells were performed similarly to the method described previously (7). Frozen specimens were thawed in a 37°C water bath with continuous agitation until completely melted and then placed on ice for 2 min. Each 1 ml of thawed cell suspension was slowly diluted with RPMI 1640 medium supplemented with 20% human AB serum and 25 mM HEPES buffer at room temperature. To accomplish this slow dilution, 50, 100, 200, 400, and 800 μl of supplemented medium were added sequentially at 1-min intervals with agitation. Five minutes after the last addition of medium, the total volume was brought to 10 ml with supplemented medium, centrifuged, and washed a second time with 10 ml of medium. These cells (frozen/thawed PBMC) were then assessed for viability by trypan blue dye exclusion, counted, and resuspended in the medium required for lymphocyte immunophenotyping or proliferation assays.
Lymphocyte immunophenotyping.
Immunophenotyping was performed on three specimen types from each donors: (i) fresh whole blood (unfractionated, EDTA-anticoagulated blood analyzed within 12 h of drawing), (ii) fresh Ficoll-isolated PBMC, and (iii) frozen/thawed PBMC (as described above). The antibody panels used are shown in Table 1. Analysis with panel 1 identified the major T- and B-lymphocyte subsets and was performed according to each laboratory's standard protocol that complied with the NIAID guideline (6). The source of fluorochrome-conjugated antibodies for panel 1 varied between laboratories.
TABLE 1.
Antibody combinations used for lymphocyte immunophenotypic analysis
| Panel | Antigen identified by
fluorochrome-conjugated antibodya
|
||
|---|---|---|---|
| FITC | PE | CyChrome | |
| 1 | CD45 | CD14 | |
| CD4 | CD3 | ||
| CD8 | CD3 | ||
| CD19 | CD3 | ||
| 2 | CD45RA | CD62L | CD4 |
| CD28 | CD95 | CD4 | |
| HLA-DR | CD38 | CD4 | |
| CD45RA | CD62L | CD8 | |
| CD28 | CD95 | CD8 | |
| HLA-DR | CD38 | CD8 | |
FITC, fluorescein isothiocyanate; PE, phycoerythrin; CyChrome, PE-cyanine 5 tandem dye (PharMingen).
Immunophenotyping with panel 2 was performed according to ACTG guidelines (http://aactg.s-3.immeth.htm) using the following antibodies: CD4 (RPA-T4), CD8 (RPA-T8), CD45RA (HI100), CD62L (DREG56), CD95 (DX2), CD28 (CD28.2.1), CD38 (HIT2), and HLA-DR (G46-6), all from Pharmingen, Inc., San Diego, Calif. This panel identified subsets of CD4+ and CD8+ T cells known to change after HIV infection (5, 8, 25) and return to more normal levels in response to antiretroviral therapy (2, 9, 14). Antibodies were incubated with EDTA-anticoagulated, fresh whole blood, with fresh Ficoll-isolated PBMC, or with frozen/thawed PBMC for 20 min at room temperature. RBCs were lysed in fresh, whole-blood specimens using a commercial lysing reagent (FACS Lyse; Becton Dickinson, San Jose, Calif., or ImmunoPrep Reagent System, Beckman Coulter, Miami, Fla.). Whole-blood and PBMC specimens were washed once and resuspended in phosphate-buffered saline–2% paraformaldehyde. Stained, fixed specimens were routinely analyzed on a flow cytometer. For specimens stained using panel 2, expression of CD28 and CD95, CD45RA and CD62L, or CD38 and HLA-DR was determined on CD4+ and CD8+ subsets. This was accomplished by first gating on CD4bright or CD8bright + dim and then analyzing for fluorescence using the antibody pairs described. A minimum of 5,000 lymphocytes were analyzed for each antibody combination.
Lymphocyte proliferation assays.
The ability of lymphocytes to proliferate in vitro in response to mitogens or microbial antigens was assessed in both fresh Ficoll-isolated and frozen/thawed PBMC. To standardize the assay as much as possible across laboratories, a single lot of 96-well, round-bottomed plates sufficient for the entire study was prepared by one laboratory. Each well contained the appropriate amount of mitogen or antigen in a volume of 100 μl of complete medium (RPMI 1640 supplemented with glutamine [25 mM], penicillin [200 U/ml], streptomycin [200 μg/ml], and 10% heat-inactivated human AB serum) to yield the following final concentrations after PBMC were added: phytohemagglutinin (PHA; Sigma, St. Louis, Mo.), 5 μg/ml; pokeweed mitogen (PWM; Sigma) 5 μg/ml; tetanus toxoid (Wyeth-Lederle, St. Davids, Pa.), 1:25 dilution; and Candida antigen (Greer Laboratories, Lenoir, N.C.), 10 μg/ml. Plates were sealed, frozen, shipped to each laboratory, and stored at −70°C until used. In addition, all laboratories used the same lot of human AB serum for supplementing medium. On the day of the assay, 100,000 viable PBMC in 100-μl aliquots of complete medium were plated in the 96-well plates prepared earlier. Each culture contained a final volume of 200 μl, and each mitogen or antigen was tested in quadruplicate cultures. Quadruplicate control cultures contained the same cell population but were not supplemented with mitogen or antigen. After incubation for 6 days at 37°C with 5% CO2 and a 95% humidified atmosphere, each well was pulse-labeled with 1 μCi (25 μl) of [3H]thymidine in supplemented RPMI 1640 without serum. After 6 h, cells were harvested on glass fiber filters and analyzed for incorporation of radioactivity into DNA by standard scintillography. A stimulation index (SI) for each antigen was calculated by dividing the median counts per minute (cpm) in stimulated cultures by the median cpm in control cultures. For PHA and PWM, an SI ≥5 was considered a positive response. For tetanus toxoid and Candida antigen, an SI ≥3 was considered a positive response (31).
Study design and statistical analysis.
Differences in immunophenotyping results (lymphocyte subset percentages) were compared (i) between fresh whole blood and fresh Ficoll-isolated PBMC, (ii) between fresh Ficoll-isolated PBMC and frozen/thawed PBMC, and (iii) between fresh whole blood and frozen/thawed PBMC. Since we made three comparisons, a Bonferroni-type correction was made to maintain an overall type I error rate of 5%. Therefore, P values of <0.0167 were considered significant. First, the association between percentage values in fresh whole blood and those measured in either fresh Ficoll-isolated PBMC or frozen/thawed PBMC was studied by using the Pearson product-moment correlation coefficient, and the 98% confidence bounds were determined by using the Fisher r to z transformation (32). Next, simple linear regression analysis was used to explore the degree of equivalence of immunophenotyping results in pairwise comparisons of the three specimen types described above. To assess whether a bias might exist in one specimen type, we determined whether the regression lines were significantly different from a line of equivalence where the y intercept = 0 and the slope = 1. Finally, the Wilcoxon signed-rank test was used to study whether or not the pairwise within-subject differences in subset percentages among all three specimen types were non-zero (17).
The primary endpoint for the lymphocyte proliferation assay portion of the study was to compare the number of positive proliferative responses in fresh Ficoll-isolated PBMC with the number of positive responses in frozen/thawed PBMC. We estimated the proportion of lost responses, given an initial positive response, along with a 95% confidence interval, given an initial response using exact estimation methods (1, 20). We also tested the association between SIs observed in assays performed with fresh Ficoll-isolated PBMC and frozen/thawed PBMC using the Pearson product-moment correlation coefficient, the square of which estimates the variability explained by the linear relationship between the two variables. The null hypothesis that the correlation equaled zero was tested by using Student's t test.
RESULTS
Specimen donors and PBMC viability.
Specimens for analysis were obtained from 27 HIV-1-infected donors at the nine different laboratory sites (three donors per site). The median CD4 T-cell count for these donors was 299 cells/μl (25th and 75th percentiles, 251 and 401, respectively). Despite specifying a target CD4 T-cell count of 200 to 400 cells/μl for these specimens, four donors had counts outside the target range (two were >400 cells/μl, and two were <200 cells/μl). However, specimens from all donors were used in the analyses.
The median percent viability of fresh Ficoll-isolated PBMC after isolation was 98% (25th and 75th percentiles, 97 and 100, respectively). Only one fresh Ficoll-isolated specimen had a viability of ≤85%. Median percent viability after freeze/thawing was 95% (25th and 75th percentiles, 89 and 97, respectively). Only four frozen/thawed specimens had a viability of ≤85%.
Changes in major lymphocyte subsets after Ficoll isolation and freeze/thaw processing.
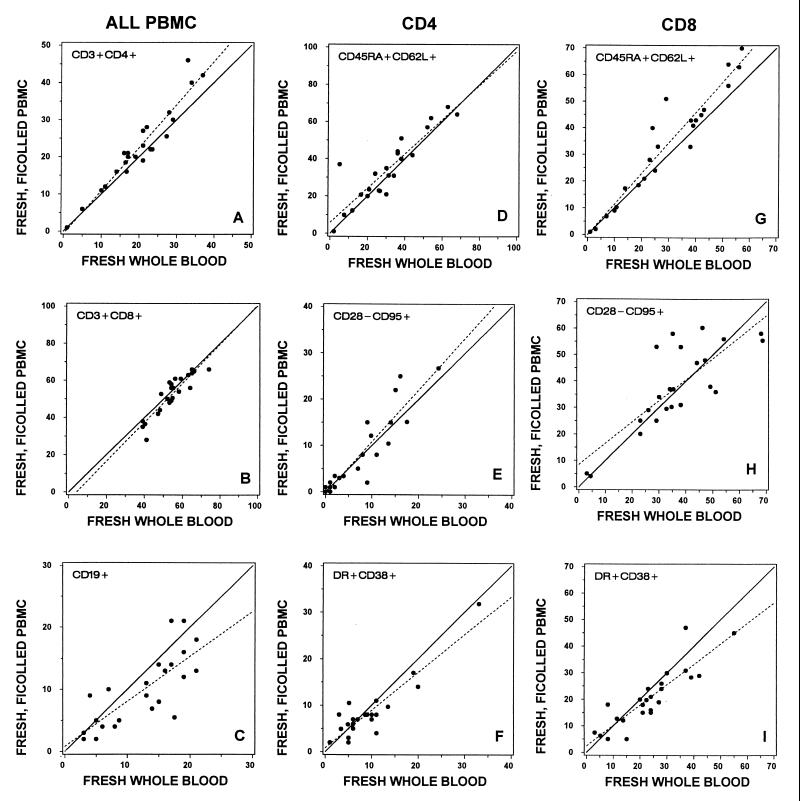
To determine how Ficoll isolation of PBMC and the freeze/thaw processing would affect the major lymphocyte subsets, we measured the percent T and B cells and the percent CD4+ and CD8+ T cells in fresh whole blood, fresh Ficoll-isolated PBMC, and frozen/thawed PBMC. The subset percentages in fresh whole blood correlated significantly with those observed in fresh Ficoll-isolated PBMC and in freeze/thawed PBMC. However, when compared with fresh whole blood, the major lymphocyte subset percentages correlated more strongly with fresh Ficoll-isolated PBMC than with frozen/thawed PBMC (Table 2).
TABLE 2.
Strength of correlation between immunophenotyping results in fresh whole blood versus fresh Ficoll-isolated PBMC and fresh whole blood versus frozen/thawed PBMC
| Subset | Correlation
coefficienta (98% confidence limits) for fresh
whole blood vs:
|
|
|---|---|---|
| Fresh whole blood vs fresh Ficoll-isolated PBMC | Fresh whole blood vs frozen/thawed PBMC | |
| All PBMC | ||
| CD3+ | 0.92 (0.80, 0.98) | 0.88 (0.69, 0.96) |
| CD3+ CD4+ | 0.96 (0.88, 0.98) | 0.71 (0.38, 0.88) |
| CD3+ CD8+ | 0.90 (0.75, 0.97) | 0.70 (0.36, 0.87) |
| CD19+ | 0.78 (0.46, 0.92) | 0.83 (0.57, 0.94) |
| CD4+ cells | ||
| CD45RA+ CD62L+ | 0.90 (0.72, 0.96) | 0.83 (0.60, 0.93) |
| CD28− CD95+ | 0.92 (0.77, 0.97) | 0.80 (0.53, 0.92) |
| CD38+ DR+ | 0.91 (0.76, 0.97) | 0.76 (0.45, 0.90) |
| CD8+ cells | ||
| CD45RA+ CD62L+ | 0.96 (0.88, 0.99) | 0.74 (0.44, 0.90) |
| CD28− CD95+ | 0.80 (0.53, 0.93) | 0.67 (0.32, 0.86) |
| CD38+ DR+ | 0.88 (0.69, 0.96) | 0.60 (0.20, 0.83) |
All correlations were significant (P < 0.001).
To determine whether lymphocyte isolation or freezing and thawing resulted in a subset bias, we used linear regression analysis to generate the best fit line plotting the percentages of the major lymphocyte subsets in fresh whole blood versus fresh Ficoll-isolated PBMC (Fig. 1) and fresh whole blood versus frozen/thawed PBMC (Fig. 2). If percentage results were equivalent for the specimen types compared, the regression line should have a slope of 1 and a y intercept of 0. As shown in Fig. 1 and 2, neither the slope of the regression lines nor the y intercept deviated significantly from the line of equivalence for B cells or for CD4+ or CD8+ T cells.
FIG. 1.
Lymphocytes from 27 HIV-1-infected donors were immunophenotyped by nine laboratories (three donors per laboratory). Specimens were analyzed using a whole-blood lysis technique (fresh whole blood) and after density gradient isolation of PBMC (fresh Ficoll-isolated PBMC). (A to C) Percentage of total lymphocytes representing (A) CD4+ and (B) CD8+ T-cell or (C) CD19+ B-cell subset. (D to F). Percentage of CD4+ lymphocytes bearing indicated surface antigens. (G to I) Percentage of CD8+ lymphocytes bearing the indicated surface antigens. In all comparisons between fresh whole blood and Ficoll-isolated PBMC, there was a significant correlation (P < 0.001). Correlation coefficients are shown in Table 2. Regression lines (dashed) generated from the plotted data points were not significantly different from the line of equivalence (solid), where the slope is 1 and the y intercept is 0 (P > 0.017).
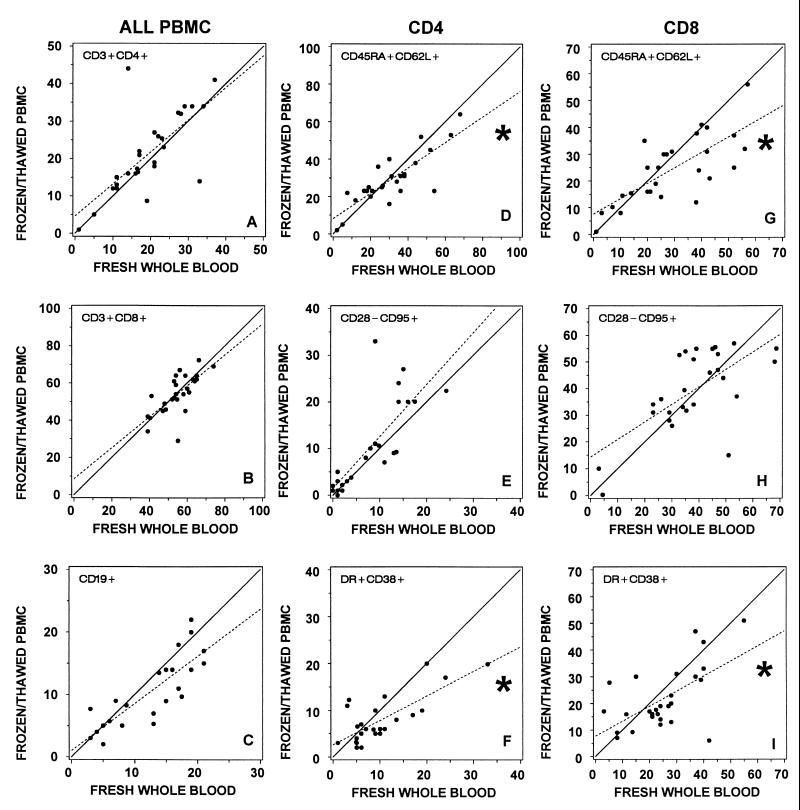
FIG. 2.
Lymphocytes from 27 HIV-1-infected donors were immunophenotyped by nine laboratories (three donors per laboratory). Specimens were analyzed using a whole-blood lysis technique (fresh whole blood) and after cryopreservation and thawing of isolated PBMC (frozen/thawed PBMC). (A to C) Percentage of total lymphocytes representing (A) CD4+ and (B) CD8+ T-cell or (C) CD19+ B-cell subset. (D to F) Percentage of CD4+ lymphocytes bearing indicated surface antigens. (G to I) Percentage of CD8+ lymphocytes bearing the indicated surface antigens. In all comparisons between fresh whole blood and frozen/thawed PBMC, there was a significant correlation (P < 0.001). Correlation coefficients are shown in Table 2. Regression lines (dashed) were generated from the plotted data points. ∗, slope of the regression line significantly different from the line of equivalence (solid), where the slope is 1 and the y intercept is 0 (P < 0.017).
We also compared the absolute percentage values among specimen types by determining whether the pairwise differences (e.g., fresh whole blood minus fresh Ficoll-isolated PBMC) varied significantly from zero (Table 3). The percentage of CD3+ T cells did not change significantly after either processing procedure. However, the percentage of B cells was significantly lower in fresh Ficoll-isolated PBMC and in freeze/thawed PBMC than in fresh whole blood. These changes were evidenced by the decreases in median percent CD19+ lymphocytes (from 14 to 9%) for both fresh Ficoll-isolated and frozen/thawed PBMC compared with fresh whole blood and were confirmed by the Wilcoxon signed-rank test (Table 3). While the slope of the regression lines for CD19+ lymphocytes was less than 1, the deviation was not significant. The percentage of CD8+ T cells was not significantly affected by either procedure (Table 3 and Fig. 1B and 2B). However, within the CD4+ T-cell subset, there was a small but significant increase in the percentage of these cells in Ficoll-isolated PBMC but not freeze/thawed PBMC compared with fresh whole blood (Table 3). This small increase in the percentage of CD4+ T cells might have represented a relative increase due to the loss of B cells during the Ficoll isolation procedure. However, no significant change was observed in the CD3+4+ subset when frozen/thawed cells were compared with fresh whole blood or fresh Ficoll-isolated PBMC, and the median CD3+ CD4+ percentages were similar in all three specimen types (Table 3).
TABLE 3.
Changes in PBMC immunophenotype during density gradient isolation and cryopreservationa
| Subset | (A) Fresh
whole blood
|
(B) Fresh Ficoll-isolated
PBMC
|
(C) Frozen/thawed PBMC
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Median % | 25th, 75thb | Median % | 25th, 75th | A vs B | Median % | 25th, 75th | B vs C | A vs C | |
| All PBMC | |||||||||
| CD3+ | 76 | 71, 82 | 76 | 73, 83 | 79 | 74, 83 | |||
| CD19+ | 14 | 6, 18 | 9 | 5, 14 | 9 | 6, 15 | |||
| CD3+ CD4+ | 19 | 14, 27 | 21 | 16, 28 | 19 | 14, 32 | |||
| CD3+ CD8+ | 55 | 49, 61 | 55 | 46, 61 | 54 | 46, 62 | |||
| CD4+ cells | |||||||||
| CD45RA+ CD62L+ | 30 | 19, 38 | 32 | 21, 44 | 25 | 23, 36 | ▾ | ▾ | |
| CD28− CD95+ | 8 | 1, 14 | 3 | 1, 15 | 8 | 2, 20 | |||
| CD38+ DR+ | 9 | 5, 11 | 8 | 5, 10 | 6 | 5, 10 | ▾ | ▾ | |
| CD8+ cells | |||||||||
| CD45RA+ CD62L+ | 26 | 19, 42 | 33 | 17, 47 | 25 | 15, 32 | ▾ | ▾ | |
| CD28− CD95+ | 38 | 29, 47 | 37 | 29, 53 | 39 | 31, 53 | |||
| CD38+ DR+ | 24 | 15, 37 | 19 | 14, 27 | 18 | 14, 30 | ▾ | ||
We investigated the differences in percentages between values obtained from fresh whole blood and fresh Ficoll-isolated PBMC, between fresh Ficoll-isolated PBMC and frozen/thawed PBMC, and between fresh whole blood and frozen/thawed PBMC. Arrows indicate that the Wilcoxon signed-rank test detected a within-subject change in percentage values that was significantly different from 0. Arrowheads indicate that the slope of the regression line plotting values using the indicated specimen types was significantly different from 1. Differences were considered significant when P was <0.017. From 23 to 27 samples were tested for each lymphocyte subset measured.
Values indicated the 25th and 75th percentiles.
Changes in selected T-cell subsets after Ficoll isolation and freeze/thaw processing.
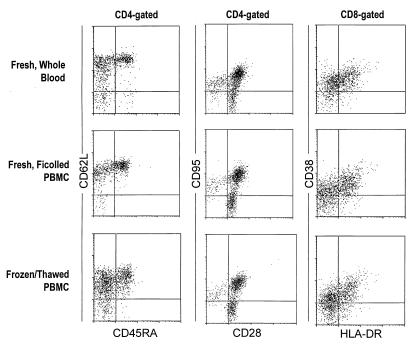
We also assessed the changes that occurred in three functionally relevant CD4+ and CD8+ lymphocyte subsets during Ficoll isolation and cryopreservation using the analyses described above. We quantitated the percentage of CD45RA+ CD62L+, CD28− CD95+, and HLA-DR+ CD38+ cells in either CD4+ or CD8+ lymphocytes. These lymphocyte subsets have been shown to be altered after HIV infection and to recover following initiation of antiretroviral therapies (2, 5, 8, 9, 25). Typical staining patterns observed for CD45RA/CD62L/CD4, CD28/CD95/CD4, and HLA-DR/CD38/CD8 are illustrated in Fig. 3. Neither Ficoll isolation nor cryopreservation caused obvious changes in the staining intensity for CD45RA, CD95, CD28, CD38, or HLA-DR. However, the staining intensity for CD62L appeared substantially lower in frozen/thawed PBMC than in the other two specimen types. As observed with the major lymphocyte subsets, the size of the functionally relevant subsets in fresh Ficoll-isolated PBMC and in frozen/thawed PBMC correlated significantly with values observed in fresh whole blood (Fig. 1 and 2 and Table 2). The correlation with values observed in fresh whole blood was stronger for fresh Ficoll-isolated PBMC than for frozen/thawed PBMC (Table 2). This can also be seen in the slight increase in scatter of the plotted points around the regression line (Fig. 1 and 2).
FIG. 3.
Typical appearance of fluorescence intensity histograms for leukocyte antigens assessed on CD4+ or CD8+ gated lymphocytes. Specimens analyzed were fresh whole blood samples, fresh Ficoll-isolated PBMC, and frozen/thawed PBMC.
By regression analysis, equivalent results for the functionally related subsets were obtained when fresh whole blood was compared with fresh Ficoll-isolated PBMC (Fig. 1). However, when fresh whole blood was compared with frozen/thawed PBMC, the slopes of the regression lines were significantly less than 1 for the CD45RA+ CD62L+ subsets and for the CD38+ HLA-DR+ subsets on both CD4+ and CD8+ lymphocytes (Fig. 2). Similar results were observed in the slopes of the regression lines comparing fresh Ficoll-isolated PBMC with frozen/thawed PBMC (Table 3). These results suggests that the size of these functionally relevant subsets may be reduced after cryopreservation and recovery. The CD28− CD95+ subsets showed no significant change.
We also assessed whether cell processing resulted in a bias in these functionally related subsets by comparing the median differences in subset size for each specimen type. We confirmed these observations with the Wilcoxon signed-rank test results based on within-subject changes (Table 3). Neither Ficoll isolation of PBMC nor cryopreservation caused significant changes in the CD28− CD95+ subset of CD4+ or CD8+ lymphocytes (Table 3). However, cell processing and cryopreservation caused decreases in the percentage of CD38+ HLA-DR+ cells. The median CD38+ HLA-DR+ fractions declined 3 percentage points and 6 percentage points on CD4+ and CD8+ lymphocytes, respectively (Table 3). The decline in the CD4+ subset was statistically significant. Although not statistically significant, the median percentage of lymphocytes expressing CD45RA+ CD62L+ also declined slightly during isolation and cryopreservation in both CD4+ and CD8+ lymphocytes (−5 and −1 percentage points, respectively) (Table 3). However, somewhat larger declines in the CD45RA+ CD62L+ subset occurred solely after cryopreservation and thawing in both the CD4+ and CD8+ subsets (−7 and −8 percentage points, respectively) (Table 3). This decrease was significant in the CD8+ lymphocyte subset. Overall, the results from linear regression analysis and comparison of median and within-subject differences in percentages showed similar trends (Table 3).
Changes in lymphocyte proliferative responses after freeze/thaw processing.
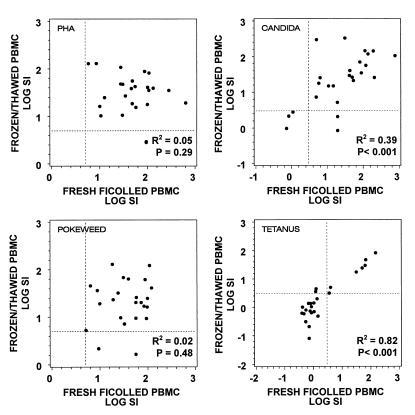
We next determined the effect that cryopreservation would have on the ability of lymphocytes to proliferate in response to mitogens or microbial antigens. Proliferation assays were performed by each laboratory with fresh Ficoll-isolated PBMC and repeated with frozen/thawed PBMC from the same blood specimen after 6 to 14 days of cryopreservation. In assays performed with microbial antigens, the magnitude of each donor's proliferative responses (SI values) using frozen/thawed PBMC correlated significantly with those performed using fresh Ficoll-isolated PBMC. However, there was no apparent correlation of SI between fresh and frozen specimens when cells were stimulated with PHA or PWM (Fig. 4).
FIG. 4.
Lymphocyte proliferation assays were performed by nine laboratories on a total of 27 samples from HIV-1-infected donors using freshly isolated PBMC (fresh Ficoll-isolated PBMC) or PBMC that had been cryopreserved for 6 to 14 days (frozen/thawed PBMC). Data are plotted as the log10 of the SI (median cpm in stimulated cultures/median cpm in control cultures) for each mitogen or microbial antigen tested. Broken lines indicate the cutoff value for a positive response. The squared correlations and significance between SI obtained with fresh Ficoll-isolated PBMC and frozen/thawed PBMC (testing whether correlations were significantly different from zero) are indicated for each antigen or mitogen.
We also compared the proportion of specimens that produced positive proliferative responses before and after the freeze/thawing procedure using the criteria for positive responses described earlier (31). As shown in Table 4, positive proliferative responses to both mitogens and microbial antigens were usually retained following cryopreservation. The probability that a positive response would be lost after cryopreservation was less than 10% for all assays (Table 5). Surprisingly, the predicted incidence of lost responses was no greater for microbial antigens than for mitogens (Table 4). Of note, two donors who were both analyzed in the same laboratory accounted for five of the six responses that were lost after cryopreservation (two PHA, one PWM, and two Candida responses). The frozen/thawed PBMC of two of the three donors whose proliferative responses were lost after cryopreservation had a viability of less than 85%.
TABLE 4.
Comparison of fresh Ficoll-isolated PBMC and frozen/thawed PBMC in proliferative response
| Mitogen or antigen | Response by fresh Ficoll-treated PBMC | Response by frozen/thawed PBMC [no. (%) of 27
samples]
|
No. of samples missing | |
|---|---|---|---|---|
| No | Yes | |||
| PHA | No | 0 (0) | 0 (0) | 0 |
| Yes | 2 (8) | 23 (92) | 2 | |
| Total | 2 | 23 | 2 | |
| PWM | No | 0 (0) | 0 (0) | 0 |
| Yes | 2 (8) | 22 (92) | 3 | |
| Total | 2 | 22 | 3 | |
| Candida antigen | No | 3 (12) | 0 (0) | 1 |
| Yes | 2 (8) | 20 (80) | 1 | |
| Total | 5 | 20 | 2 | |
| Tetanus toxoid | No | 16 (64) | 2 (8) | 2 |
| Yes | 0 (0) | 7 (28) | 0 | |
| Total | 16 | 9 | 2 | |
TABLE 5.
Probability that a positive PBMC proliferative response would be lost after specimen freezing and thawing
| Mitogen or antigen | Probability of lost response | 95% confidence interval | na |
|---|---|---|---|
| PHA | 0.08 | 0.01–0.26 | 25 |
| PWM | 0.08 | 0.01–0.27 | 24 |
| Tetanus toxoid | 0.00 | 0.00–0.41 | 7 |
| Candida antigen | 0.09 | 0.01–0.29 | 22 |
Number of positive proliferative responses observed in fresh Ficoll-isolated PBMC.
DISCUSSION
This study assessed the feasibility of isolating and cryopreserving PBMC from HIV-1-infected donors at multiple clinical sites for future use in immunophenotyping and in assays of immune function. The ability to retrospectively analyze specimens obtained during AIDS clinical trials for immune function has the potential to improve assay precision and accuracy, reduce within-patient variability, and allow selective study of subjects with specific outcomes, providing a more efficient use of laboratory resources.
Using a simplified method of cell isolation and cryopreservation, all nine laboratories successfully froze and recovered specimens without a substantial loss in cell viability. We assessed the effect of PBMC isolation and cryopreservation on the sizes of immunophenotypically defined lymphocyte subsets using two statistical methods: comparison of within-subject differences in percentages (which, based on the sample size and variability, had the power to detect differences in median percentages of ±3 to ±9 percentage points) and linear regression analysis. While observed changes were not always statistically significant by both methods of analysis, they did indicate similar trends.
Interestingly, a considerable disruption in the relative sizes of the major lymphocyte subsets resulted from density gradient isolation of PBMC from blood, where a significant proportion of B cells were lost. Previous reports have shown B cells to increase (26), decrease (15), or remain constant (3, 19) after density gradient centrifugation of normal human blood. However, most earlier studies did not use CD45/CD14 lymphocyte gating which might have resulted in excluding some B cells from their lymphocyte scatter gate, making small changes difficult to detect. In addition to B-cell loss, we found that the processing associated primarily with cryopreservation may result in small losses of those CD4+ and CD8+ lymphocytes bearing HLA-DR and CD38 and those bearing CD45RA and CD62L. These findings were somewhat surprising, since they suggest that both activated and naïve, nonactivated T cells are subject to loss during cell isolation and cryopreservation. The loss of CD38+ HLA-DR+ cells probably represents a true loss of this subset, since staining intensity for these antigens was preserved after freezing. However, the apparent loss of CD45RA+ 62L+ cells may have resulted in part from a loss of CD62L staining intensity after freezing. Although these cell losses were sometimes statistically significant, the magnitude of change in the median percent was small and may not hold biological significance in the context of a clinical trial. Nevertheless, the potential for the selective loss of lymphocytes after cryopreservation will need to be assessed for the subpopulations measured and considered in the design of each clinical trial.
The magnitude of proliferative responses to tetanus toxoid and Candida antigen showed good correlation between assays performed with fresh Ficoll-isolated PBMC and frozen/thawed PBMC. In proliferation assays performed with PHA and PWM, the magnitude of proliferative responses between fresh and frozen specimens did not correlate. This absence of correlation was likely due to fact that the 6-day assays were optimized for microbial antigen and not for mitogens. Nevertheless, more than 90% of all specimens that showed a positive response to mitogens or microbial antigens retained positive responses after freezing and thawing. These results concur with previous reports that proliferative responses could be preserved in previously frozen specimens (12, 27).
Five of the six proliferative responses that were lost after freezing occurred in samples from only 2 of the 27 donors, both of which were processed and analyzed in the same laboratory. Specimens from both of these donors became unresponsive to a mitogen as well as to a microbial antigen after freezing. This observation suggests that the loss of PBMC proliferation after cryopreservation may be donor specific or laboratory specific. In addition, frozen/thawed PBMC from two of the three donors in which proliferative responses were lost after cryopreservation had a viability of <85%. Although definitive conclusions cannot be made, this study suggests that assessing the viability of frozen specimens and the responses to mitogens such as PHA or PWM may serve as appropriate controls in assays that measure responses to microbial antigens.
Because this study was performed at nine different laboratories, it represents a better estimation of the proficiency with which specimens could be cryopreserved in a multisite clinical trial than a similar study performed in a single laboratory. An important advantage of archiving specimens during clinical trials is the potential to utilize more-skilled laboratories for performing a particular assay on all clinical trial specimens. A highly skilled laboratory may, in fact, exceed the concordance with fresh specimens that we observed in this study. The suitability of cryopreserved specimens for each immunophenotypic and functional assay will need to be verified prior to their implementation in a clinical trial. However, this study confirms that freezing PBMC for future immunophenotypic and immune function testing is a feasible approach in AIDS clinical trials.
ACKNOWLEDGMENTS
This work was supported in part by the Adult AIDS Clinical Trials Group of the NIAID, grant AI-38858.
REFERENCES
- 1.Agresti A, Coull B A. “Approximate” is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–126. [Google Scholar]
- 2.Angel J B, Kumar A, Parato K, Filion L G, Diaz-Mitoma F, Daftarian P, Pham B, Sun E, Leonard J M, Cameron D W. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus sanquinavir. J Infect Dis. 1998;177:898–904. doi: 10.1086/515244. [DOI] [PubMed] [Google Scholar]
- 3.Ashmore L M, Shopp G M, Edwards B S. Lymphocyte subset analysis by flow cytometry. Comparison of three different staining techniques and effects of blood storage. J Immunol Methods. 1989;118:209–215. doi: 10.1016/0022-1759(89)90008-2. [DOI] [PubMed] [Google Scholar]
- 4.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 5.Brinchmann J E, Dobloug J H, Heger B H, Haaheim L L, Sannes M, Egeland T. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–738. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 6.Calvelli T, Denny T N, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–715. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 7.El-Daher N, Nichols J E, Roberts N J., Jr Analysis of human antiviral cytotoxic T-lymphocyte responses for vaccine trials using cryopreserved mononuclear leukocytes: demonstration of feasibility with influenza virus-specific responses. Clin Diagn Lab Immunol. 1994;1:487–492. doi: 10.1128/cdli.1.5.487-492.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giorgi J V, Liu Z, Hultin L E, Cumberland W G, Hennessey K, Detels R. Elevated levels of CD38+CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of followup. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 9.Giorgi J V, Majchrowicz M A, Johnson T D, Hultin P, Matud J, Detels R. Immunologic effects of combined protease inhibitor and reverse transcriptase inhibitor therapy in previously treated chronic HIV-1 infection. AIDS. 1998;12:1833–1844. doi: 10.1097/00002030-199814000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 11.Huang X-L, Fan Z, Liebmann J, Rinaldo C. Detection of human immunodeficiency virus type 1-specific memory cytotoxic T lymphocytes in freshly donated and frozen-thawed peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 1995;2:678–684. doi: 10.1128/cdli.2.6.678-684.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes G B, Paulic Z M, Shu S, Fairchild R L, Barna B P. Can sensitized lymphocytes retain reactivity to inner ear antigens after retrieval from frozen storage? Laryngoscope. 1997;107:878–882. doi: 10.1097/00005537-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Islam D, Lindberg A A, Christensson B. Peripheral blood cell preparation influences the level of expression of leukocyte cell surface markers as assessed with quantitative multicolor flow cytometry. Cytometry. 1995;22:128–134. doi: 10.1002/cyto.990220208. [DOI] [PubMed] [Google Scholar]
- 14.Kelleher A D, Carr A, Zaunders J, Cooper D A. Alterations in the immune response of human immunodeficiency virus (HIV)-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J Infect Dis. 1996;173:321–329. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 15.Kutvirt S G, Lewis S L, Simon T L. Lymphocyte phenotypes in infants are altered by separation of blood on density gradients. Br J Biomed Sci. 1993;50:321–328. [PubMed] [Google Scholar]
- 16.Lederman M M, Connick E, Landay A, Kuritzkes D R, Spritzler J, St. Clair M, Kotzin B L, Fox L, Chiozzi M H, Leonard J M, Rousseau F, Wade M, Roe J D, Martinez A, Kessler H. Immunological responses associated with 12 weeks of combined antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann E L. Nonparametrics: statistical methods based on ranks. San Francisco, Calif: Holden-Day, Inc.; 1975. [Google Scholar]
- 18.Letellier C, Rameliarison L, Fizet D, Ferrer A M, Vezon G. The influence of cryopreservation on activity and surface markers of lymphokine-activated killer cells. Vox Sang. 1991;61:90–95. doi: 10.1111/j.1423-0410.1991.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 19.Macey M G, Hyam C J, Newland A C. Enumeration of lymphocyte subpopulations: a comparative study of whole blood and gradient centrifugation methods. Med Lab Sci. 1988;45:187–191. [PubMed] [Google Scholar]
- 20.Mehta C, Patel N. Proc-StatXact for SAS users: statistical software for exact nonparametric inference. Cambridge, Mass: CYTEL Software Corporation; 1997. [Google Scholar]
- 21.Mildvan D, Landay A, De Gruttola V, Machado S G, Kagan J. An approach to the validation of markers for use in AIDS clinical trials. Clin Infect Dis. 1997;24:764–774. doi: 10.1093/clinids/24.5.764. [DOI] [PubMed] [Google Scholar]
- 22.Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 23.Perfetto S P, Malone J D, Hawkes C, McCrary G, August B, Zhou S, Garner R, Dolan M J, Brown A E. CD38 expression on cryopreserved CD8+ T cells predicts HIV disease progression. Cytometry. 1998;33:133–137. doi: 10.1002/(sici)1097-0320(19981001)33:2<133::aid-cyto7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 24.Powderly W G, Landay A, Lederman M M. Recovery of the immune system with antiretroviral therapy: the end of opportunism? J Am Med Assoc. 1998;280:72–77. doi: 10.1001/jama.280.1.72. [DOI] [PubMed] [Google Scholar]
- 25.Roederer M, Dubs J G, Anderson M T, Raju P A, Herzenberg L A, Herzenberg L A. CD8 naïve T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romeu M A, Mestre M, Gonzalez L, Valls A, Verdaguer J, Corominas M, Bas J, Massip E, Buendia E. Lymphocyte immunophenotyping by flow cytometry in normal adults. Comparison of fresh whole blood lysis technique, Ficoll-Paque separation and cryopreservation. J Immunol Methods. 1992;154:7–10. doi: 10.1016/0022-1759(92)90206-9. [DOI] [PubMed] [Google Scholar]
- 27.Sobota V, Bubenik J, Indrova M, Vlk V, Jakoubkova J. Use of cryopreserved lymphocytes for assessment of the immunological effects of interferon therapy in renal cell carcinoma patients. J Immunol Methods. 1997;203:1–10. doi: 10.1016/s0022-1759(97)00020-3. [DOI] [PubMed] [Google Scholar]
- 28.Venkataraman M. Effects of cryopreservation on immune responses. VI. An inexpensive method for freezing human peripheral blood mononuclear cells. J Clin Lab Immunol. 1992;37:133–143. [PubMed] [Google Scholar]
- 29.Vingerhoets J, Vanham G, Kestens L, Gigase P. A convenient and economical freezing procedure for mononuclear cells. Cryobiology. 1995;32:105–108. doi: 10.1006/cryo.1995.1009. [DOI] [PubMed] [Google Scholar]
- 30.Wang S-Y, Hsu M-L, Tzeng C-H, Hsu H-C, Ho C-K. The influence of cryopreservation on cytokine production by human T lymphocytes. Cryobiology. 1998;37:22–29. doi: 10.1006/cryo.1998.2094. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg A, Betensky R A, Zhang L, Ray G. Effect of shipment, storage, anticoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin Diagn Lab Immunol. 1998;5:804–807. doi: 10.1128/cdli.5.6.804-807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zar J H. Biostatistical analysis. Englewood Cliffs, N.J: Prentice-Hall, Inc.; 1984. [Google Scholar]