Abstract
Objective:
To compare the efficacy and safety of two different dosage levels of olanzapine for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving high emetic risk chemotherapy.
Methods:
This study was a randomized, double-blind, controlled trial designed to show non-inferiority in the efficacy of olanzapine 5 mg compared to 10 mg in patients treated with high dose cisplatin or doxorubicin/cyclophosphamide. Non-inferiority was defined as a lower margin of the 95% confidence interval (95% CI) that not lower than the margin set at -25%.
Result:
A total of 140 patients were randomized to 5 mg group (n=70) or 10 mg group (n=70) of olanzapine. The complete response (CR) rate in the overall phase of olanzapine 5 and 10 mg was 58.6% v 62.9% (95%CI: -20.4, 11.8). The CR rate comparison between olanzapine 5 and 10 mg was 81.4% v 74.3% (95%CI: -6-6, 20.8) and 66.7% v 76.1% (95%CI: -23.5, 6.3) for the acute and delayed phase, respectively. No nausea rates in acute, delayed and overall phase were 70.0% v 68.6% (95%CI: -13.8, 16.6), 45.7% v 48.6% (95%CI: -19.4, 13.6) and 43.5% v 47.9% (95%CI: -19.2, 13.6). The rate of adverse events (AE) including somnolence were not different between the 5 and 10 mg groups.
Conclusion:
The two dosage levels of olanzapine were not different in terms of the efficacy and AE in the prophylaxis of CINV.
Key Words: Olanzapine, chemotherapy-induced nausea and vomiting, high emetic risk chemotherapy
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is one of the worst side effects for patients receiving chemotherapy. In the absence of an anti-emesis, more than 90 percent of patients receiving highly emetogenic chemotherapy (HEC) including high-dose cisplatin and the combination of doxorubicin/cyclophosphamide (AC) experience vomiting (Roila et al., 2016; National Comprehensive Cancer Network, 2020).
The mechanism of CINV involves multiple neurotransmitters and receptors in the gastrointestinal tract and central nervous system including dopamine with the dopamine receptor, 5-hydroxytryptamine (5-HT) with the 5-HT receptor, and substance P with the neurokinin-1 receptor. Several anti-emetic medications have been developed and approved for CINV prophylaxis and treatment including dexamethasone, 5-hydroxytryptamine type 3 receptor antagonist (5-HT3 RA), neurokinin-1 receptor antagonist (NK-1 RA), and most recently olanzapine (Hesketh, 2008; Rojas and Slusher, 2012).
Olanzapine is approved as an antipsychotic agent. Additionally, it exerts an antiemetic property by blocking major neurotransmitters related to vomiting induced by chemotherapy, particularly dopamine at dopaminergic (D2) receptor and 5-HT at 5-HT type 2c and 3 receptors (Navari, 2014). Previously, 10 mg of olanzapine plus triplet-regimen (dexamethasone, 5-HT3 RA and NK-1 RA) has been a standard of care for prevention of CINV in patients receiving HEC (Navari et al., 2016). However, this dosage of olanzapine has led to increased somnolence in many patients. Later studies have also suggested that 5mg of olanzapine plus triplet-regimen showed comparable efficacy with lower incidence of somnolence (Yanai et al., 2018; Hashimoto et al., 2020). Both 5 and 10 mg of olanzapine combined with triplet-regimen are recommended for the prevention of CINV in patients receiving HEC (National Comprehensive Cancer Network, 2020).
NK-1 RA has not always been used because of the low adherence to practice guidelines and reimbursement problems in some countries (Yu et al., 2015; Ithimakin et al., 2020; Aapro et al., 2021). Previous studies conducted in patients receiving HEC which omitted the use of NK-1 RA and added 10 mg of olanzapine to the doublet-regimen (dexamethasone and 5-HT3 RA) showed promising outcomes as an optional antiemetic regimen (Tienchaiananda et al., 2019; Vimolchalao et al., 2020). Results did show that 10 mg of olanzapine was associated with more sleepiness and undesirable appetite than placebo (Tienchaiananda et al., 2019; Vimolchalao et al., 2020).
The purpose of this study was to investigate non-inferiority in the antiemetic efficacy of 5 mg olanzapine compared to a 10 mg dose and to measure any changes in treatment side effects. To stay consistent with day-to-day practice in Thailand, olanzapine was combined with a doublet regimen (dexamethasone and ondansetron) in patients receiving HEC without NK-1 RA.
Materials and Methods
Eligibility Criteria
Criteria for study enrollment were patients 18-years and older with Eastern Oncology Cooperative Group (ECOG) performance status from 0 to 2 who were scheduled to receive the first cycle of either AC (doxorubicin 60 mg/m2 combined with cyclophosphamide 600 mg/m2) or cisplatin ≥ 70 mg/m2. Cisplatin was administered intravenously for one day and allowed to be given concurrently with other chemotherapy including fluorouracil, etoposide, or radiation. Patients receiving cisplatin were admitted in the hospital for 120 hours after administration for intravenous hydration and observation. All patients were chemotherapy-naïve, diagnosed with histologically-confirmed solid tumor and had adequate organ function (including normal bone marrow function, aminotransferase <100 U/L, total bilirubin <2 mg/dL, creatinine clearance >60 ml/min). The exclusion criteria included pregnant or breast-feeding, symptomatic brain metastasis, receiving abdominal radiotherapy, receiving anticonvulsants or fluoroquinolone antibiotic, previous 48-hour use of NK-1 RA, 5-HT3 receptor antagonists, dexamethasone, dopamine receptor antagonists, antihistamines, benzodiazepines, and antipsychotics. Patients with a medical history of heart disease, arrhythmia, uncontrolled heart failure or acute myocardial infarction in the past 6 months, uncontrolled diabetes or a history of uncontrolled diabetes were also excluded.
Treatment
Eligible patients were randomly assigned to receive either a daily dose of 5 mg oral olanzapine or 10 mg olanzapine for four consecutive days. Olanzapine was prescribed to start on the first day of chemotherapy (day 1) and to be taken after dinner for 4 days (day 1-4). All patients were administered dexamethasone 20 mg intravenously for 30 minutes before initial chemotherapy infusion and 8 mg orally on day 2 to 4. Ondansetron 8 mg was also given intravenously 30 minutes before chemotherapy infusion and another 8 mg orally in the morning on the day after chemotherapy. Metoclopramide 10 mg and/or ondansetron 8 mg were administered orally or intravenously as rescue medications.
Study Design
This randomized, double-blind (patients and physicians), active treatment-controlled trial aimed to determine the non-inferiority in the efficacy of olanzapine 5 mg and 10 mg combined with ondansetron and dexamethasone in HEC at Chonburi Cancer Hospital between June 2020 and February 2021. Eligible patients were randomized to each arm at a 1:1 ratio by computer-generated mixed blocks of 2, 4, 6, and 8 allocation schedules. Randomized patients were stratified by chemotherapy regimens receiving either cisplatin (≥70mg/m2) or AC regimen. All patients were followed for 5 days. The 5 mg and 10 mg tablets of olanzapine were encapsulated in indistinguishable sachets by a pharmacist who was blinded to the randomization sequence.
All patients gave written informed consent to participate in this study. This study was registered in the Thai Clinical Trials Registry (number TCTR20200708006).
Assessment
The primary outcome was complete response (CR) defined as no emesis and no use of rescue treatment in overall phase (0-120 hours after chemotherapy). Secondary outcomes were CR in acute phase (0-24 hours after chemotherapy), CR in delayed phase (24-120 hours after chemotherapy), and no nausea (visual analog scale equals 0) in acute, delayed and overall phases. Time to treatment failure was defined as time from receiving chemotherapy to time of the first vomiting or receipt of the first-dose of rescue treatment, whichever occurred first.
All participants were asked to fill out a record form on days 1 to 5 (0-120 hour after chemotherapy) daily. The record form consisted of a visual analog scale of nausea, time when vomiting, time of using rescue drugs, and somnolence score. On day 5, the participant was asked to record adverse events (AE) assessed by common toxicity criteria for adverse events version 5 (National Cancer Institute, 2017). The researcher telephoned or visited patients daily to remind them to complete the form daily. The record form was submitted either by mail, at the next hospital/physician visit or prior to discharge from the hospital.
Statistical Analysis
The primary objective of this study was to show non-inferiority of 5 mg of olanzapine to 10 mg of olanzapine of CR in overall phase. In the previous study (Navari et al., 2011; Tienchaiananda et al., 2019; Ithimakin et al., 2020; Vimonchalao et al., 2020), olanzapine 10 mg combined with doublet-regimen without NK-1 RA showed a CR ranging from 50 to 77 percent in the overall phase. The sample size was calculated based on the assumption that 5 mg and 10 mg of olanzapine group would achieve an overall phase CR of 63% and 68%, respectively. Based on intention to treat analysis, at least 70 patients per group were required to ensure 80% power to show non-inferiority between the groups at a margin of – 25 % and type-I error at 0.05.
All demographic data were reported using descriptive statistics including mean (SD) and 95% confidence intervals. Complete response and no nausea rates of all phases were analyzed by proportion differences and 95% CI. The episodes of no vomiting and the number of patients who did not use the rescue treatment in all phases were analyzed by performing Pearson’s chi-square test. Mean differences in the VAS nausea scores by groups were compared using the independent t-test. Time-to-treatment failure was performed using the Kaplan-Meier method. Efficacy analysis was done on an intention-to-treat basis. Two-sided p values were used throughout this study with a statistical threshold of p <.05. All statistical analyses were performed using SPSS version 26.0 (SPSS Statistics, IBM Corp. Armonk, NY, USA).
Results
Patients
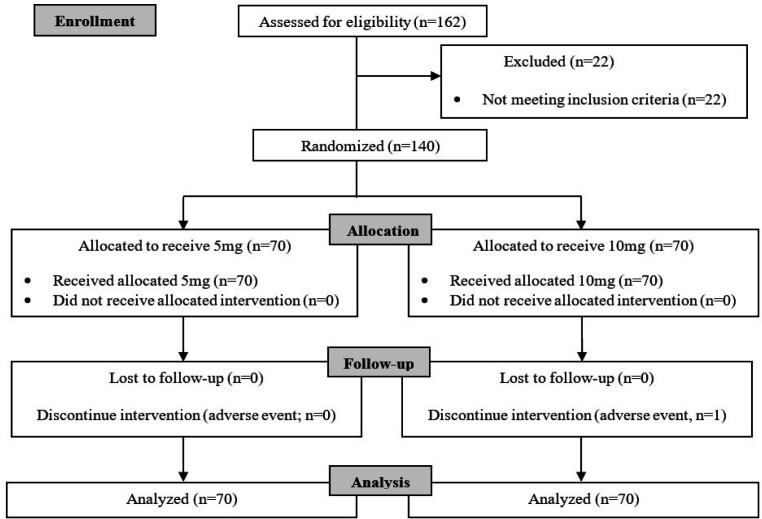
Between June 17, 2020 and Feb 9, 2021, 140 eligible participants were randomly assigned to receive olanzapine 5 mg (70 patients) or 10 mg (70 patients) (Figure 1).
Figure 1.
Treatment Flow of the Study
Baseline characteristics did not show any statistical differences between groups (Table 1). A majority of the participants (60%) were females diagnosed with breast cancer receiving AC. Mean dose of cisplatin in both groups was comparable.
Table 1.
Baseline Characteristics of 5 and 10 mg Olanzapine Groups
| Baseline Characteristic | Olanzapine group | p | |
|---|---|---|---|
| 5 mg (n=70) | 10 mg (n=70) | ||
| Age, years | |||
| Mean (SD) | 49 (11.5) | 49 (11.3) | 0.66 |
| Sex, number | |||
| Female/ Male | 47/23 | 47/23 | 1 |
| Body surface area | |||
| Mean (SD) | 1.58 (0.19) | 1.61 (0.16) | 0.28 |
| ECOG performance status, n | |||
| 0/1 | 52/18 | 53/17 | 0.84 |
| Alcohol use, n (%) | 0.18 | ||
| Current/Former | 25 (35.7) | 33 (47.1) | |
| Never | 45 (64.3) | 37 (52.9) | |
| Smoking status, n (%) | 0. 35 | ||
| Current/Former | 18 (25. 7) | 23 (32. 9) | |
| Never | 52 (74. 3) | 47 (67. 1) | |
| History of motion sickness, n (%) | 19 (27.1) | 19 (27.1) | 1 |
| History of morning sickness, n (%) | 17 (24.2) | 20 (28.5) | 0. 52 |
| Primary tumor, n (%) | 0.39 | ||
| Breast | 41 (58.6) | 43 (61.4) | |
| Head-neck | 25 (35.7) | 26 (37.1) | |
| Others | 4 (5.7) | 1 (1.4) | |
| Chemotherapy regimen, n (%) | 0.73 | ||
| AC | 41 (58.6) | 43 (61.4) | |
| Cisplatin | 29 (41.4) | 27 (38.6) | |
| Cisplatin dose, mg/m2 | |||
| Mean (SD) | 74.2 (2.8) | 75.4 (3.2) | 0.17 |
| Radiotherapy, n (%) | 4 (5.7) | 2 (2.9) | 0.39 |
Primary Outcome
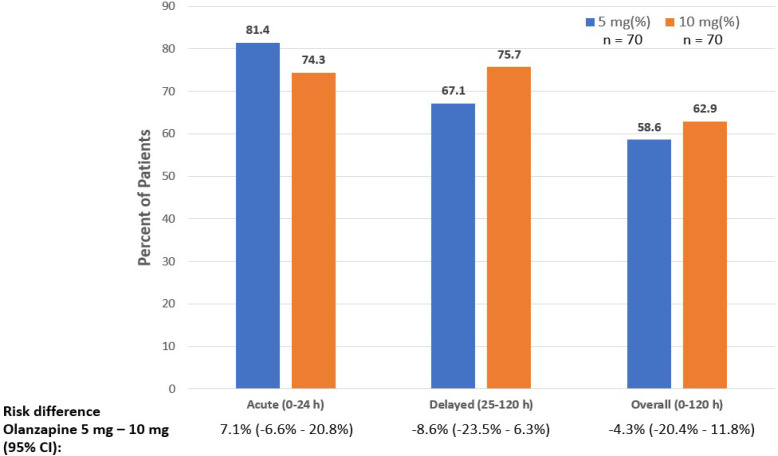
There was non-inferiority in the CR for overall phase of 5 mg group compared to 10 mg group of olanzapine (58.6% v 62.9%, 95%CI (-20.4, 11.8)) (Figure 2).
Figure 2.
Complete Response Rates Compared between 5 and 10 mg of Olanzapine
Secondary outcome
The CR of 5 mg group of olanzapine was non-inferior to 10 mg group of olanzapine for acute phase and delayed phase (Figure 2). No nausea rates of 5 mg olanzapine group showed non-inferiority to the 10 mg olanzapine group for the acute, delayed and overall phases (Table 2).
Table 2.
No Nausea Rates Compared between 5 and 10 mg of Olanzapine
| No nausea | Olanzapine Group | Risk Different (95% CI)" |
p | |
|---|---|---|---|---|
| 5 mg (n=70) | 10 mg (n=70) | |||
| % | % | |||
| Acute (0-24 h) | 70 | 68.6 | 1.4% (-13.8%, 16.6%) | 0.85 |
| Delayed (24-120 h) | 45.7 | 48.6 | -2.9% (-19.4%, 13.6%) | 0.73 |
| Overall (0-120 h) | 42.9 | 45.7 | -2.8% (-19.2%, 13.6%) | 0.73 |
Other Outcomes
The no rescue treatment rate was 71.4% in both 5 mg and 10 mg olanzapine group, p = 1.00. Mean number of rescue times was 3.35 (3.03) and 2.37 (1.57) in the 5 mg and 10 mg olanzapine groups, p = 0.41, respectively. The no vomiting rate of 69.6% in 5 mg was comparable to the rate of 77.1% in 10 mg group, p = 0.25. Mean number of vomiting times of 4.30 (5.59) was not significantly different to the 2.35 (1.76) in the 5 mg and 10 mg group, p = 0.39, respectively.
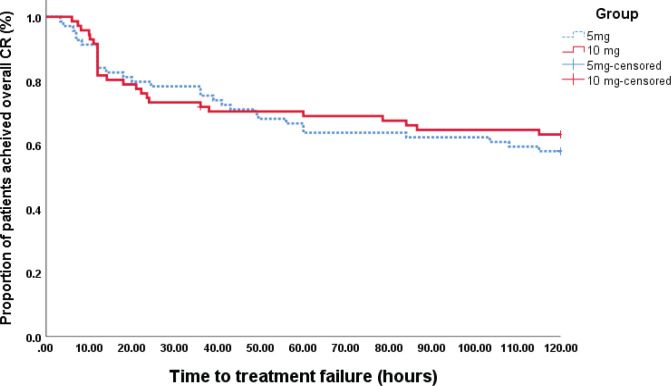
Mean time to treatment failure for olanzapine 5 mg was 84.81 min (46.6) which was not significantly different than the 10 mg result of 86.65 min (47.8) (p = 0. 77) (Figure 3).
Figure 3.
Time to Treatment Failure 5 mg and 10 mg of Olanzapine by Kaplan – Meier Method
Mean visual analog scale of nausea on days 1 to 5 comparing the 5 mg versus 10 mg group were: Day 1: 0.84 (1.80) v 1.46 (2.65), p = 0.35; Day 2: 1.46 (2.65) v 0.87 (1.73), p = 0.12; Day 3: 1.56 (2.49) v 0.95 (1.58), p = 0.08; Day 4: 1.00 (1.83) v 1.07 (1.77), p = 0.81; and Day 5: 0.86 (1.50) v 0.82 (1.42), p = 0.87 in 5 mg versus 10 mg group of olanzapine, respectively.
Adverse Events
No difference in AE was found between groups (Table 3). The most frequent grade 3 AE including acute kidney dysfunction in cisplatin regimen requiring hospitalization and febrile neutropenia in AC regimen showed no difference between groups. Grade 4 AE was found in two participants. One patient in the 5 mg group had severe hyponatremia (serum sodium 115 mg/deciliter) and the other patient in the 10 mg group had thrombocytopenia leading to gastrointestinal bleeding. One participant in the olanzapine 10 mg arm discontinued olanzapine due to severe muscle rigidity.
Table 3.
Comparison of Adverse Events between 5 and 10 mg Olanzapine Groups
| Adverse events | Olanzapine group | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 mg (n = 70), n (%) | 10 mg (n =70), n (%) | |||||||
| Grade | Grade | |||||||
| Any | 1 | 2 | ≥3 | Any | 1 | 2 | ≥3 | |
| Somnolence | 49 (70) | 40 (57) | 9 (13) | 0 | 39 (56) | 27 (39) | 12 (17) | 0 |
| Dysguesia | 44 (63) | 41 (59) | 3 (4) | 0 | 34 (49) | 28 (40) | 6 (9) | 0 |
| Dry mouth | 43 (61) | 43 (61) | 0 | 0 | 40 (57) | 37 (53) | 3 (4) | 0 |
| Hiccups | 40 (57) | 38 (54) | 2 (3) | 0 | 31 (44) | 30 (43) | 1 (1) | 0 |
| Anorexia | 40 (57) | 35 (50) | 5 (7) | 0 | 29 (41) | 21 (30) | 8 (11) | 0 |
| Nausea | 36 (51) | 30 (43) | 6 (9) | 0 | 32 (46) | 25 (36) | 7 (10) | 0 |
| Constipation | 35 (50) | 33 (47) | 2 (3) | 0 | 38 (54) | 36 (51) | 2 (3) | 0 |
| Insomnia | 35 (50) | 30 (43) | 4 (6) | 1 (1) | 30 (43) | 23 (33) | 6 (9) | 1 (1) |
| Vomiting | 18 (26) | 13 (19) | 5 (7) | 0 | 11 (16) | 9 (13) | 2 (3) | 0 |
| Diarrhea | 6 (9) | 5 (7) | 1 (1) | 0 | 8 (11) | 7 (10) | 1 (1) | 0 |
| AKI | 4 (6) | - | - | 4 (6) | 4 (6) | - | - | 4 (6) |
| Muscle rigidity | 3 (4) | 3 (4) | 0 | 0 | 5 (7) | 4 (6) | 0 | 1 (1) |
| FN | 2 (3) | - | - | 2 (3) | 3 (4) | - | - | 3 (4) |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 1 (1) |
| Hyponatremia | 1 (1) | 0 | 0 | 1 (1) | 0 | 0 | 0 | 0 |
Abbreviations: AKI, acute kidney injury; FN, febrile neutropenia
Mean appetite scores on days 0 to 5 comparing the 5 mg versus 10 mg group were not significantly different: Day 0 (baseline): 0.37 (0.97) v 0.92 (1.99), p = 0.05; Day 1: 1.15 (2.08) v 1.61 (2.32), p = 0.21; Day 2: 1.43 (2.38) v 1.44 (2.16), p = 0.98; Day 3: 1.64 (2.48) v 1.68 (2.51), p = 0.92; Day 4: 1.58 (2.34) v 1.86 (2.47), p = 0.48; and Day 5: 1.31 (2.08) v 2.08 (2.70), p = 0.06 in 5 mg versus 10 mg group of olanzapine, respectively.
Mean somnolence scores of both groups showed no difference from day 1 through day 5 (Table 4).
Table 4.
Somnolence Score Compared between 5 and 10 mg of Olanzapine
| Somnolence | Olanzapine group | P | |
|---|---|---|---|
| (mean VAS) | 5mg (n= 70) | 10mg (n=70) | |
| Day 0 (baseline) | 1.10 (1.73) | 1.54 (2.01) | 0.16 |
| Day 1 | 2.32 (2.57) | 3.32 (3.29) | 0.05 |
| Day 2 | 2.62 (2.84) | 2.76 (2.54) | 0.75 |
| Day 3 | 2.62 (2.66) | 2.69 (2.57) | 0.87 |
| Day 4 | 2.37 (2.43) | 2.43 (2.35) | 0.57 |
| Day 5 | 2.03 (1.94) | 1.94 (2.15) | 0.92 |
Abbreviations: VAS, visual analog scale
Discussion
This study was the first randomized, double-blind, controlled study which showed that olanzapine 5 mg combined with doublet-regimen (dexamethasone and 5-HT3 RA) showed non-inferiority in efficacy compared to olanzapine 10 mg combined with doublet-regimen for the prophylaxis of CINV in patients receiving HEC in every phase (acute, delayed and overall) for both CR and nausea prevention. The outcome of previous studies investigating olanzapine-containing regimen in HEC are summarized in Appendix (Table Suppl 1).
Doublet-regimen plus olanzapine 10 mg has been shown to significantly improve both CR and nausea prevention in patients receiving HEC when doublet-regimen with placebo was used as a control (Tienchaiananda et al., 2019; Vimolchalao et al., 2020). In addition, a recent systematic review which studied the addition of olanzapine to doublet-regimen for the prevention of CINV in high and moderate emetogenic chemotherapy indicated that the efficacy of olanzapine 5 mg was not different from 10 mg in subgroup analysis (Zhou et al., 2020).
Combined with triplet-regimen, olanzapine 10 mg significantly improved both CR and nausea prevention compared to placebo in patients receiving HEC, either high dose cisplatin or AC regimen (Navari et al., 2016; Yeo et al., 2020). However, olanzapine 10 mg led to more somnolence. Later studies were conducted using a lower dose of olanzapine. Hashimoto et al. showed that 5 mg of olanzapine significantly improved CR when comparing to placebo (Hashimoto et al., 2020). Yanai et al. also found that the efficacy of 5 mg and 10 mg olanzapine in controlling CINV showed no difference (Yanai et al., 2018).
Results from Yanai et al. and the present study indicated that olanzapine 5 and 10 mg had equivalent efficacy for the prophylaxis of CINV in patients receiving HEC, regardless of backbone regimen (triplet- or doublet-regimen). Therefore, the author suggested that 5mg of olanzapine should be used in a combined antiemetic regimen.
Regarding AEs, no difference was found between 5 and 10 mg of olanzapine and no new AEs in our study. One patient in the 10 mg group did discontinue olanzapine because of muscle rigidity. The somnolence pattern in patients receiving olanzapine as part of antiemesis in this study was similar to those of other studies (Navari et al., 2016; Yanai et al., 2018; Vimolchalao et al., 2020). The maximal score of somnolence occurred in the first two days after treatment began and then gradually decreased. In this study, there was no significant difference found in somnolence scores between the 5 and 10 mg olanzapine groups. This could be partly explained by both groups receiving the medication after dinner leading to peak effects such as sleepiness in which the drug reaches maximal concentration in 6 hours after oral administration (Yanai et al., 2018; Hashimoto et al., 2020; United State of America Food and Drug Administration, 2020).
A previous retrospective study reported that olanzapine 2.5 and 5 mg showed the same efficacy in the antiemetic outcome. Olanzapine 2.5 mg also had a lower incidence of somnolence compared to the 5 mg dose (Chiu et al., 2016). Additional study is required to assess the efficacy and safety of olanzapine 2.5 mg.
There were two key limitations in the present study. First, the study was conducted at a single center. Replication of the study at multiple centers would strengthen external validity. Secondly, the ability of the study to show outcome differences between groups might have been hampered by sample size especially comparison of events among the small rescue treatment subpopulation. A larger sample size with a more diverse population should be used in any further study.
In summary, olanzapine 5 mg plus dexamethasone and ondansetron showed non-inferiority in efficacy compared to olanzapine 10 mg in the prophylaxis of CINV in patients receiving high dose cisplatin (≥70mg/m2) and AC regimens. There was also no difference found in AE between the two dosages.
Author Contribution Statement
Conception and design: all authors; Provision of study material or patients: Naparat Othaganont; Collection and assemble of data: Pichayapa Pichaya, Pimonwan Promsuwan; Data analysis and interpretation: Sitthi Sukauichai, Chaninun Ketkaew; Manuscript writing: all authors; Final approval of manuscript: all authors; Accountable for all aspects of the work: all authors.
Acknowledgements
The authors gratefully thank Dr. Wanlop Jaidee (Burapha University, Chonburi, Thailand) for providing his suggestion on statistical analysis and Dr. Michael Ullman for providing edits to the manuscript. We appreciate and thank the patients who participated in this study as well as physicians, nurses, pharmacists, and other staff for their support.
Funding Statement
This research received no external funding.
Ethical Declaration
This study was approved by the Ethics Committee of Chonburi Cancer Hospital (number 14/2020, 12 June 2020) based on the Declaration of Helsinki and Good Clinical Practice.
Study Registration
This study was registered in the Thai Clinical Trials Registry (number TCTR20200708006).
Availability of data
The data of the study is available upon request.
Conflict of Interest
The authors declare no conflict of interest.
References
- Aapro M, Scotté F, Escobar Y, et al. Practice Patterns for Prevention of Chemotherapy-Induced Nausea and Vomiting and Antiemetic Guideline Adherence Based on Real-World Prescribing Data. Oncologist. 2021;25:1–10. doi: 10.1002/onco.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu L, Chiu N, Chow R, et al. Olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a retrospective study. Ann Palliat Med. 2016;5:172–8. doi: 10.21037/apm.2016.04.05. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:242–9. doi: 10.1016/S1470-2045(19)30678-3. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ. Chemotherapy-Induced Nausea and Vomiting. N Engl J Med. 2008;358:2482–94. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- Ithimakin S, Theeratrakul P, Laocharoenkiat A, et al. Randomized, double-blind, placebo-controlled study of aprepitant versus two dosages of olanzapine with ondansetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving high-emetogenic chemotherapy. Supp Care Cancer. 2020;28:5335–42. doi: 10.1007/s00520-020-05380-6. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 .
- National Comprehensive Cancer Network. Clinical Practice Guideline in Oncology version: Antiemesis. 2020. [Accessed July 25, 2020]. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf .
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9:188–95. doi: 10.1016/j.suponc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol. 2014;722:180–6. doi: 10.1016/j.ejphar.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134–42. doi: 10.1056/NEJMoa1515725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roila F, Molassiotis A, Herrstedt J, et al. MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27:S119–33. doi: 10.1093/annonc/mdw270. [DOI] [PubMed] [Google Scholar]
- Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT3 and tachykinin NK1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol. 2012;684:1–7. doi: 10.1016/j.ejphar.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Tienchaiananda P, Nipondhkit W, Maneenil K, et al. A randomized, double-blind, placebo-controlled study evaluating the efficacy of combination olanzapine, ondansetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving doxorubicin plus cyclophosphamide. Ann Palliat Med. 2019;8:372–80. doi: 10.21037/apm.2019.08.04. [DOI] [PubMed] [Google Scholar]
- United State of America Food and Drug Administration. Zyprexa: Labeling-Package Insert. 2020. [Accessed August 1, 2021]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020592s074,021086s048,021253s061lbl.pdf .
- Vimolchalao V, Sakdejayont S, Wongchanapai P, et al. The efficacy and safety of the addition of olanzapine to ondansetron and dexamethasone for prevention of chemotherapy- induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Int J Clin Oncol. 2020;25:396–402. doi: 10.1007/s10147-019-01570-3. [DOI] [PubMed] [Google Scholar]
- Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin. Oncol. 2018;23:382–8. doi: 10.1007/s10147-017-1200-4. [DOI] [PubMed] [Google Scholar]
- Yeo W, Lau TKH, Li L, et al. A randomized study of olanzapine-containing versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients. Breast J. 2020;50:30–8. doi: 10.1016/j.breast.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Burke TA, Chan A, et al. Antiemetic therapy in Asia Pacific countries for patients receiving moderately and highly emetogenic chemotherapy—a descriptive analysis of practice patterns, antiemetic quality of care, and use of antiemetic guidelines. Supp Care Cancer. 2015;23:273–82. doi: 10.1007/s00520-014-2372-3. [DOI] [PubMed] [Google Scholar]
- Zhou JG, Huang L, Jin SH, et al. Olanzapine combined with 5-hydroxytryptamine type 3 receptor antagonist (5-HT3 RA) plus dexamethasone for prevention and treatment of chemotherapy-induced nausea and vomiting in high and moderate emetogenic chemotherapy: a systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2020;5:e000621. doi: 10.1136/esmoopen-2019-000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the study is available upon request.