Abstract
Background and aim:
Prostate cancer is the second most common cancer among men that has affected their quality of life. This study aimed to find prostate tissue-specific genes using bioinformatics methods to specifically target prostate cells in case of metastasis to other tissues.
Materials and Methods:
In this study, after finding a specific gene (MSMB) that is highly expressed in cancer, the optimal promoter region of this gene was isolated and inserted in an expression vector. Then, this vector was transfected into two prostate cancer cell lines (DU145 and LNCaP) and three non-prostate cell lines (LX-2, MRC-5, and U87) using the PEI chemical method. The expression of this vector in these cells was examined using fluorescent microscopy and flow cytometry.
Results:
We observed that the expression of MSMB promoter in DU145 cell line has a much higher activity than the CMV promoter, which is a ubiquitous promoter. The MSMB promoter didn’t show any activity in cells other than that of prostate derived cell lines.
Conclusion:
MSMB gene promoter with specific expression and high efficiency in prostate tissue compared to CMV promoter can play an essential role in gene therapy of prostate cancer.
Key Words: Prostate cancer, MSMB gene, promoter, gene therapy
Introduction
Prostate cancer is the second most common malignancy in men worldwide (Bray et al., 2018; Ferlay et al., 2018). Prostate cancer is usually diagnosed in many cases by an increase in the plasma level of prostate-specific antigen (PSA> 4 ng / ml), a glycoprotein expressed by prostate tissue (Rawla, 2019). Currently, surgery and radiation therapy are known as topical prostate cancer treatment and follow them chemo and hormone therapy are used as systemic cure (Teo et al., 2019). Also, medicines such as immunotherapy using Sipuleucel-T drug development have been proposed (Mao et al., 2021).
In recent years, researchers have considered gene therapy as a preferred approach as more than 60% of clinical trials in the field of cancer treatment (Anguela and High, 2019). Scientists have always been looking for treatments that can target only tumor cells while reducing damage to normal cells. Targeted expression of genes, for example, using a gene promoter, is a vital gene therapy strategy against cancers (Chen et al., 2018).
Promoters are usually upstream of the transcription starting point in genes, where transcription factors bind to these sites. Accordingly, controlling the expression of genes in a tissue can be done in this part of the genome. Promoters that can be used as therapeutic targets in cancer fall into three categories: tissue-specific, cancer-specific, and tumor-specific (Montaño-Samaniego., 2020). Specific promoters can be used to specifically express toxic genes or genes encoding prodrug-activating enzymes (producing toxic compounds or inducing apoptosis) or genes related to tumor suppressors only in target cells (Malekshah et al., 2016). In this way, the off-target effect can be eliminated. Specific promoters provide a way to overcome the conventional problems associated with gene therapy (Dorer and Nettelbeck, 2009)
Cancer-specific promoters increase expression in variety of cancers (Liu et al., 2016). One of the advantages of this type of promoter is that they are on the shelf. The hTERT promoter has long been considered the best standard for targeting various cancer cells at the transcriptional level in multiple tissues, including the prostate (Jiang et al., 2017). Promoters such as COX2, RAD51, Survivin , and HER2 have also been shown to increase expression in many cancers (Garg et al., 2010; Zhang et al., 2010; Hine et al., 2012; Rama et al., 2015; Tabriz et al., 2021). However, the biggest drawback of these promoters was their high level of expression in normal mode. Tissue-specific promoters have also been used in gene therapy of various cancers. One of the biggest problems with these promoters is that in addition to tumor cells, they also damage normal prostate cells (Rama Ballesteros et al., 2020). However, many efforts are needed to use them as an accurate tool in the clinical treatment of cancer.
Microseminoprotein beta (MSMB) gene was one of the genes examined for specificity and increased expression in cancer compared to normal in the prostate (Sjöblom et al. 2016). The MSMB gene is located on chromosome 10 and encodes a Microseminoprotein beta protein. This protein is a regulatory protein belonging to an immunoglobulin-binding factor family which is secreted from prostate epithelial cells into semen. It has been suggested that it may have a protective role in suppressing prostate cancer, antifungal effects, sperm coverage, and apoptosis (Haiman et al., 2013). Also, it has even been cited as a valuable biomarker after hormone ablation therapy (Imasato et al., 2000).
This study aimed to identify and evaluate the MSMB gene promoter using various bioinformatics tools in order to use this promoter to manipulate prostate cancer cells.
Materials and Methods
The ethics committee approved the study protocol at Shiraz University of Medical Sciences (IR.SUMS.REC.1398.1115).
Bioinformatics analysis of MSMB gene regulatory region
To ensure MSMB gene specificity, bioinformatics data was analysed in different healthy tissues as well as isolated samples of prostate cancer. For the initial study of 2,000 upstream nucleotides, the transcription starting point was considered as the promoter region, and the intended analyses were performed in Nucleotide BLAST in order to confirm the similarity between the selected fragment and our target region. Then the nucleotide sequence obtained in the previous step was put up on the NEB Cutter site to investigate the cleavage sites of all enzymes on the desired nucleotide sequence. Special attention was paid to the enzymes that were supposed to cut the vector and nucleotide fragment that we proposed as a promoter, so that there would be no enzymatic cleavage site in the middle of the desired fragment. The next step was to predict the promoter region using bioinformatics databases.
Then, primers were designed for the region considered as the promoter. Since the pcDNA3 vector lacked the eGFP reporter gene, primers were designed for the eGFP gene to be cloned into pcDNA3.
Isolation of MSMB gene regulatory region by PCR
Blood DNA was extracted using the salting-out method according to the protocol (Suguna, et al., 2014). The target area on human DNA was isolated using the PCR technique and visualized on 1% agarose gel (Table 1). The length of the eGFP fragment for cloning in the pcDNA3 vector under the control of the CMV promoter was 742 bp and the length of the putative promoter region for the MSMB gene was 1216 bp. According to the manufacturer’s protocol, the desired DNA fragment was extracted from agarose gel using the Gene All gel extraction kit.
Table1.
Primers Sequences and PCR Conditions Related to Amplification of MSMB Gene
| Primers | Sequences | PCR Condition |
|---|---|---|
| MSMB Forward | 5'-GAAGATCTAAGCTGTGGCGAGGAGTGA-3' | Anneling:60; 95:15 min; 95:30 s; 60:60 s; 72:2 min |
| MSMB Reverse | 5'-CCCAAGCTTAGCAGGACTCCTTATAGACAGGT-3' |
Cloning of eGFP gene in pcDNA3 vector
First, the eGFP reporter gene had to be cloned in the MCS region of the pcDNA3 vector. To this purpose, the vector was cut with HindIII and XhoI restriction enzymes. The eGFP gene was also cleaved with HindIII and SalI enzymes. Since SalI and XhoI create compatible cohesive ends, the resulting components can join in a ligation reaction. After enzymatic cleavage of the vector and eGFP fragment, they were subjected to enzymatic ligation reaction according to the protocol (Sambrook and Russell, 2001).The new plasmid was transformed into competent DH5α bacteria, and it was cultured on LB agar containing the appropriate amount of Ampicillin. Finally, colony PCR was done to detect pcDNA3 + eGFP vector.
Cloning the MSMB gene in the pcDNA3 + eGFP vector
Before cloning the MSMB in the promoter region, the CMV promoter must had been removed from the pcDNA3 + eGFP vector, and replaced by the MSMB promoter, which was previously isolated from DNA by PCR. To do this, both the vector and the insert region (MSMB gene) were cleaved by BglII and HindIII restriction enzymes. The fragment which isolated from the new vector was 899 bp in length. Then, the MSMB gene was ligated into the vector (Figure 1), and the ligation product was transformed into susceptible bacteria. Moreover, some molecular tests such as colony PCR, PCR with suitable primers to isolate MSMB gene from plasmid pcDNA3 + eGFP + MSMB, and digestion tests were performed. After the confirmation, plasmids were extracted in high concentrations.
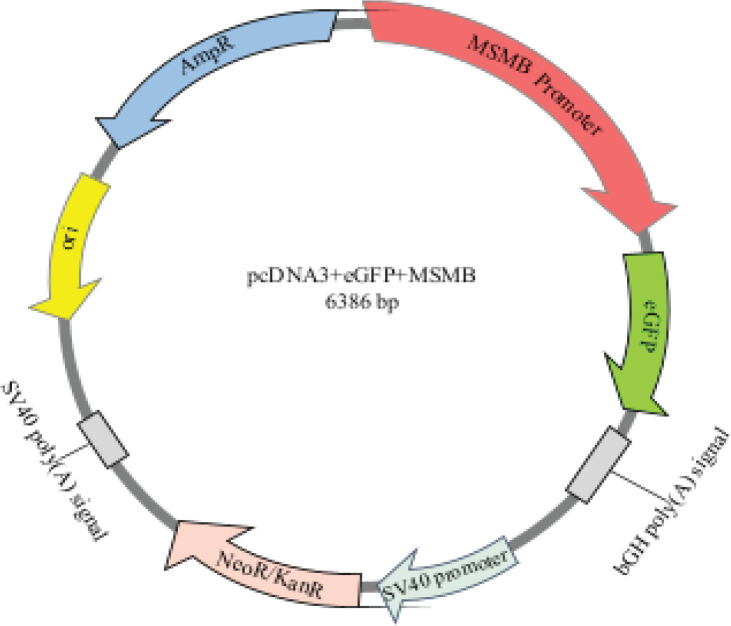
Figure 1.
pcDNA3+MSMB+eGFP Vector. Schematic representation of MSMB gene promoter inserted in the vector
Cell culture
In this study, LNCaP (androgen-sensitive human prostate cells) DU145 (derived from central nervous system metastasis), LX-2 (hepatic stellate cells), MRC-5 (derived from normal lung tissue), and U87 (human primary glioblastoma cell) cell lines were bought from the National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran). The culture medium of the cells was different: LNCaP and DU145 cell lines were grown in RPMI 1640 GlutaMAXTM medium (Biowest, Nuaillé, France) supplemented with 10% fetal bovine serum (GibcoTM, EU Approved South American) and 1% Pen-Strep (Biowest, Nuaillé, France). The culture medium of LX-2 cells was RPMI 1640 but FBS 5%. MRC-5 and U87 cells were grown in DMEM GlutaMAXTM medium (Biowest, Nuaillé, France) medium with FBS 10% and Pen-Strep 1%. The passage process was performed according to standard protocols, and finally, they were incubated under 5% CO2 condition.
Transfection and Flow cytometry
We employed Polyethylenimine (PEI) mediated chemical transfection method to insert the target plasmid into the cell. To ensure the efficiency of PEI and to find its proper dilution, for each cell, different concentrations of PEI, different concentrations of DNA, and the number of cells seeded in the 6-well plate separately. However, so as to prevent errors, each transfection was carried out in triplicate. The day before transfection, a suitable number of cells were seeded separately in 6-well plate.
After 48-72 hours, eGFP expression and transfection efficiency were checked using a fluorescent microscope. Finally, the flow cytometry technique was used to evaluate the number of cells that expressed eGFP using FACSCaliburTM flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and FlowJo™ vX software.
Results
Isolation of MSMB gene from human DNA
MSMB gene was amplified from human DNA by MSMBF/MSMBR primers and Pfu enzyme with high accuracy (Figure 2). This isolated fragment was used for cloning in the pcDNA3 vector.
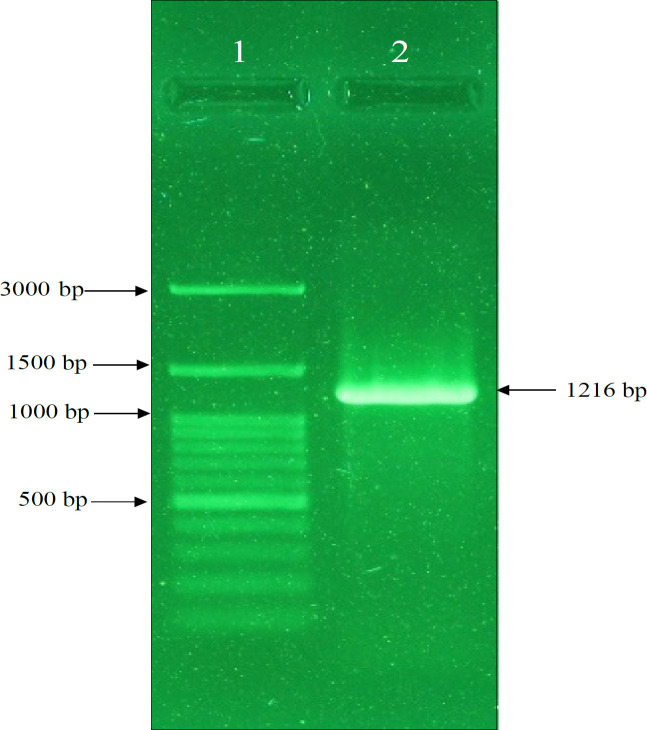
Figure 2.
Result of Agarose Gel Electrophoresis of MSMB Gene. Amplification of MSMB gene by pfu polymerase enzyme. Lane 1: DNA ladder, Lane 2: Promoter of MSMB gene with a length of 1216 bp
Construction of pcDNA3 + eGFP vector
The pcDNA3 vector was cleaved with HindIII and XhoI enzymes and the eGFP gene with HindIII and SalI enzymes (Figure 3). In the next step, the eGFP gene fragment is inserted into the pcDNA3 vector through a ligation reaction. Finally, after ligation and transformation of the ligation product, a colony PCR reaction was performed (Figure 4).
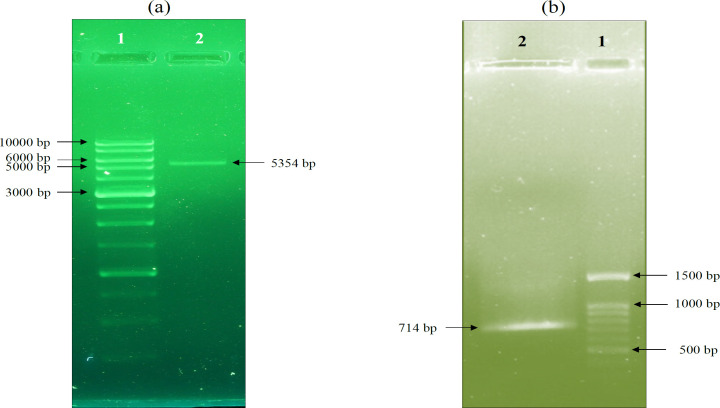
Figure 3.
Agarose Gel Electrophoresis of pcDNA3 Vector and eGFP Gene Digestions. a) The pcDNA3 vector was 5428 bp; after enzymatic digestion the 74 bp fragment was removed and the digested plasmid remained at 5354 bp, which is visible. b) The length of the eGFP gene fragment was 742 bp, which after enzymatic digestion was 714 bp. However, the isolated fragment is so small (28 bp) that it is not visible on gel electrophoresis. Lane 1 on “a” and “b” indicate the ladder
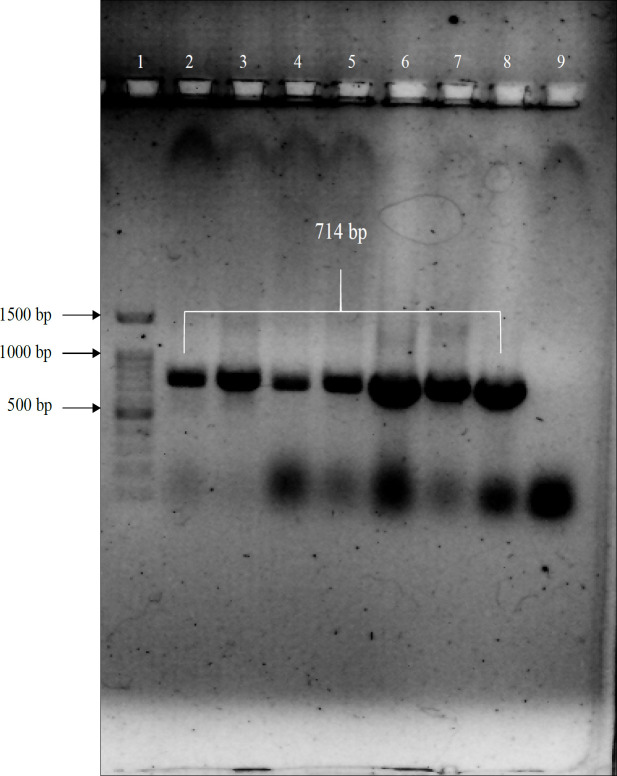
Figure 4.
Colony PCR Gel Electrophoresis. The colony PCR was performed on DH5α bacteria with eGFPF / eGFPR primers. The figure shows that lane 1 is a 1kb DNA ladder and lanes 2, 3, 4, 5, 6, 7, 8 are positive colony results
Construction of pcDNA3 + eGFP + MSMB vector
The MSMB gene promoter obtained in the previous steps was cleaved with HindIII and BglII enzymes (Figure 5). Next, the cleaved fragment was inserted into the pcDNA3 + eGFP vector. Then, the cloned plasmid was transformed into the Escherichia coli DH5α, and a colony PCR method was performed to confirm the positive colonies.
Figure 5.
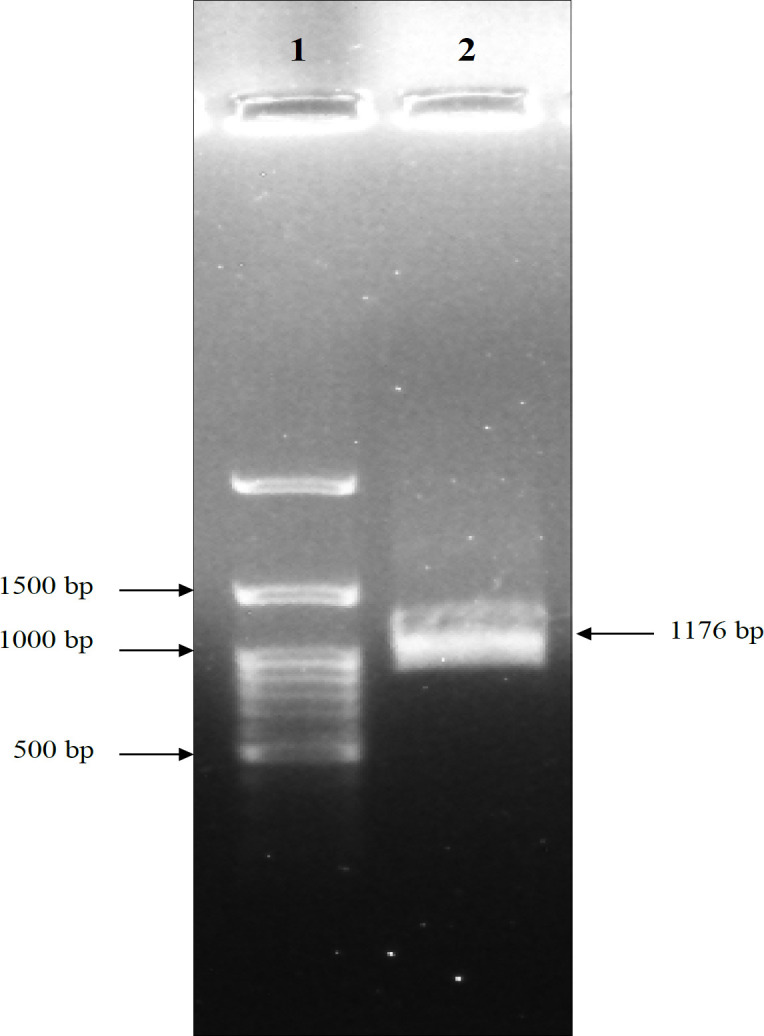
Result of Agarose Gel Electrophoresis of MSMB Gene Digestion. As shown in the figure, BglII and HindIII enzymes digested the MSMB gene, removing a 40 bp fragment rendering the gene size 1176 bp
Molecular analysis to confirm the pcDNA3 + eGFP + MSMB vector
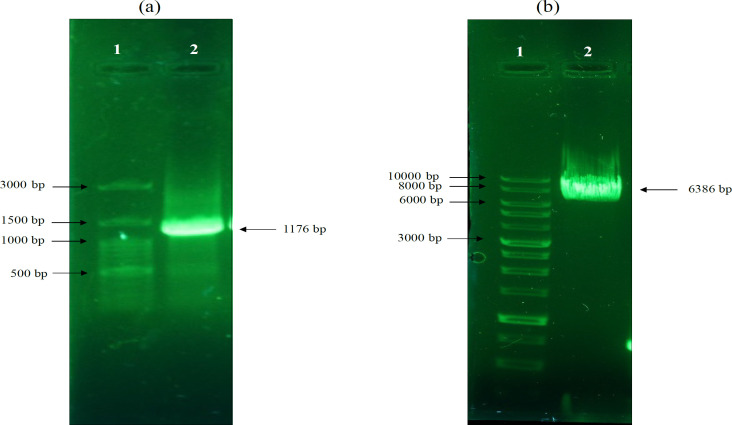
To further confirm the insertion of MSMB fragment into pcDNA3 + eGFP vector, PCR reaction was performed using MSMBF/MSMBR primer on purified pcDNA3+eGFP+MSMB vector. Additionally, to ensure that the extracted plasmids (pcDNA3+eGFP+MSMB) are the plasmids we are looking for, we performed an enzymatic cleavage on the plasmids with HindIII restriction enzyme. (Figure 6).
Figure 6.
Confirmation of Correct Cloning of pcDNA3+eGFP+MSMB Plasmid. a) PCR reaction was performed on purified pcDNA3 + eGFP + MSMB vector and 1176 bp band was observed. b) The enzymatic cleavage with hindIII showed that the pcDNA3+eGFP+MSMB gene construct (with a length 6386 bp) construct constructed correctly
Transfection of pcDNA3 + eGFP + MSMB plasmid into different cells
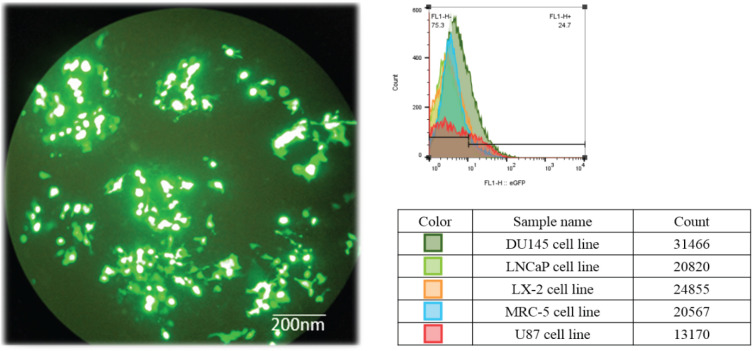
The pcDNA3 + eGFP + MSMB vector transfection was performed only in DU145 cell line (human prostate cancer cell line) and the other cell lines (LNCaP, Lx-2, MRC-5, and U87) did not have successful transfection. The pcDNA3 + eGFP + MSMB gene construct was transfected to DU145 cell line by polyethyleneimine (PEI), shown in Figure 7. Transfection efficiency was measured by counting eGFP expressing cells, which revealed the efficiency to be approximately 40% (36 hours after transfection). After transfection, a flow cytometry test was performed to isolate the cells containing the eGFP plasmid (Figure 7).
Figure 7.
Transfection and Flow Cytometry Results. a) Schematic illustration of DU145 cell transfection with pcDNA3 + eGFP + MSMB. b) After cell transfection, targeted cells that exhibit eGFP expression were sorted using FACSCaliburTM flow cytometer. Accordingly, the DU145 Cell line had the highest transfection rate with pcDNA3 + eGFP + MSMB
Discussion
Prostate cancer is one of the most important causes of cancer mortality among men (Igbokwe et al., 2021). Current treatments have several side effects and researchers are always looking for new therapies with most significant impact on tumor tissue (Anguela and High, 2019). To achieve this goal, gene therapy using specific tumor tissue promoters is one of the methods considered by researchers. Therefore, this study aimed to identify and evaluate the specificity and functional potency of the MSMB gene promoter for use in prostate cancer gene therapy. Few studies have been performed on the promoter region of the MSMB gene as a gene-directed in prostate cancer gene therapy. Therefore, it was decided to study and select a new promoter region based on bioinformatics tools to study the promoter of this gene.
MSMB gene expression is specific to prostate tissue, and its protein is secreted only from the epithelial cells of the prostate duct (Karunasinghe et al., 2014). Bergström (2018), in a study, showed that the synthesis of MSMB protein in benign prostate is suppressed, and this indicates the reason for the decrease in serum levels of MSMB protein in patients with prostate cancer (Bergström et al., 2018). Additionally, a study by Byrne (2019) found that men with high levels of circulating MSP (MSMB gene protein) had a lower risk of prostate cancer, as a result, MSP may play a protective role in prostate cancer (Byrne et al., 2019).
In our study, bioinformatics analysis showed that the expression of the MSMB gene decreased in cancer tissues compared to normal. However, despite this decrease in expression, it still had high expression. The MSMB gene usually is one of the most highly expressed genes in prostate tissue. Although cancer progression appears to reduce the expression of this gene, its expression has been higher than most prostate-specific genes. After final confirmation of the MSMB gene as one of the genes selected for the present study, we examined the promoter region of this gene. Also, Ochiai (1995) examined the promoter region of the MSMB gene. In their search, from 2860 bp upstream of the MSMB gene to 218 bp downstream of the transcription starting point, which included exon 1 and part of intron 1, was considered the promoter region (Ochiai et al., 1995). In the study of Lou (2012), it was shown that the smallest core promoter region of the MSMB gene is fragment between -27 to -236 from the transcription start point. This 210 bp region showed the highest transcriptional activity in all prostate cancer cell lines which tested in this study and concluded that this fragment is responsible for the action of the MSMB-based promoter (Lou et al., 2012).
After selecting the promoter region and cloning it in the pcDNA3 expression vector, related expression was studied in different cell lines. To evaluate the MSMB gene promoter, a gene construct containing pcDNA3 + eGFP + MSMB was created, and its effectiveness was measured in different cell lines. Our study showed that the expression of pcDNA3 + eGFP constructs under the control of MSMB promoter was 25% in the DU145 cell line and about 3% in the LNCaP cell line. Also, the expression of this construct in non-prostatic cell lines was 5% on average. The results showed that the selected 1176 bp region, which also includes part of 5`-UTR, has relatively good expression in DU145 cells line which introduces as an androgen-independent cell line. Accordingly, Gabril (2004) conducted a study to evaluate the promoter/enhancer region of the MSMB gene to target the prostate in the LNCaP cell line. Based on the results of this study, the MSMB gene promote/enhancer has the potential to target prostate-specific targeting and can be used in prostate cancer gene therapy (Gabril et al., 2004).
In conclusion, the results of this study showed that the MSMB gene promoter is a good choice for gene therapy and entry into clinical trials. Considering the results of this study and finding the need for new treatments for prostate cancer, we can use the positive results that the MSMB promoter had in DU145 cell line. However, further studies are needed to prove this hypothesis, and even more bioinformatics tools should be used to find genes specific to other cancers.
Author Contribution Statement
Maryam Darayee: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Bita Geramizadeh: Funding acquisition, Conceptualization, Resources, Supervision, Writing – original draft, Writing – review & editing. Seyed Mohammad Bagher Tabei: Funding acquisition, Resources, Writing – original draft, Writing – review & editing. Alireza Rezvani: Resources. Saeede Soleimanian: Investigation. Amir Rahimi: Funding acquisition, Conceptualization, Resources, Supervision, Writing – original draft, Writing – review & editing. Data Availability: All relevant data are within the paper.
Acknowledgements
The authors would like to thank the staff of the Transplant Research Center of Shiraz University of Medical Sciences for their support.
Funding
This work is based on the Doctor of Philosophy thesis in molecular medicine by Maryam Darayee (Project NO. 97-01-74-17956) supported by Shiraz University of Medical Sciences, Shiraz, Iran. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethics
This work was approved by the ethical committee of Shiraz University of Medical Sciences (NO: IR.SUMS.REC.1398.1115).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Anguela XM, High KA. Entering the modern era of gene therapy. Ann Rev Med. 2019;70:273–88. doi: 10.1146/annurev-med-012017-043332. [DOI] [PubMed] [Google Scholar]
- Anklesaria JH, Mhatre DR, Mahale SD. Structural and molecular biology of PSP94: Its significance in prostate pathophysiology. Front Biosci (Landmark edition) 2018;23:535–562. doi: 10.2741/4604. [DOI] [PubMed] [Google Scholar]
- Bergström SH, Järemo H, Nilsson M, et al. Prostate tumors downregulate microseminoprotein-beta (MSMB) in the surrounding benign prostate epithelium and this response is associated with tumor aggressiveness. Prostate. 2018;78:257–65. doi: 10.1002/pros.23466. [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Byrne KS, Appleby P, Key T, et al. The role of plasma microseminoprotein-beta in prostate cancer: an observational nested case–control and Mendelian randomization study in the European prospective investigation into cancer and nutrition. Ann Oncol. 2019;30:983–89. doi: 10.1093/annonc/mdz121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Yue D, Lei L, et al. Promoter-operating targeted expression of gene therapy in cancer: current stage and prospect. Mol Ther Nucleic Acids. 2018;11:508–514. doi: 10.1016/j.omtn.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61:554–71. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France: international agency for research on cancer ; 2018. pp. 1–6. [Google Scholar]
- Gabril M, Xuan J, Moussa M, et al. Characterization of initiation of angiogenesis in early stages of prostate adenocarcinoma development and progression in a transgenic murine model. Urology. 2004;64:1233–7. doi: 10.1016/j.urology.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Garg H, Salcedo R, Trinchieri G, Blumenthal R. Improved nonviral cancer suicide gene therapy using survivin promoter-driven mutant Bax. Cancer Gene Ther. 2010;17:155–63. doi: 10.1038/cgt.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman CA, Stram DO, Vickers AJ, et al. Levels of beta-microseminoprotein in blood and risk of prostate cancer in multiple populations. J Nat Cancer Instit. 2013;105:237–43. doi: 10.1093/jnci/djs486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine CM, Seluanov A, Gorbunova V. Rad51 promoter-targeted gene therapy is effective for in vivo visualization and treatment of cancer. Mol Ther. 2012;20:347–55. doi: 10.1038/mt.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbokwe M, Salako A, Badmus T, et al. Tissue Zinc Concentration in Prostate Cancer: Relationship with Prostate Specific Antigen and Gleason Score in a Cohort of Nigerian Men. Asian Pac J Cancer Biol. 2021;6:147–53. [Google Scholar]
- Imasato Y, Xuan JW, Sakai H, et al. PSP94 expression after androgen deprivation therapy: a comparative study with prostate specific antigen in benign prostate and prostate cancer. J Urol. 2000;164:1819–24. [PubMed] [Google Scholar]
- Jiang H, Guo S, Xiao D, et al. Arginine deiminase expressed in vivo, driven by human telomerase reverse transcriptase promoter, displays high hepatoma targeting and oncolytic efficiency. Oncotarget. 2017;8:37694. doi: 10.18632/oncotarget.17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunasinghe N, Bishop K, Murray P, et al. Role of β-microseminoprotein from prostate cancer initiation to recurrence: A mini-review. World J Clin Urol. 2014;3:20–30. [Google Scholar]
- Liu T, Yuan X, Xu D. Cancer-specific telomerase reverse transcriptase (TERT) promoter mutations: biological and clinical implications. Genes. 2016;7 doi: 10.3390/genes7070038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Li H, Yeager M, et al. Promoter variants in the MSMB gene associated with prostate cancer regulate MSMB/NCOA4 fusion transcripts. Hum Genet. 2012;131:1453–66. doi: 10.1007/s00439-012-1182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekshah OM, Chen X, Nomani A, Sarkar S, Hatefi A. Enzyme/prodrug systems for cancer gene therapy. Curr Pharmacol Rep. 2016;2:299–308. doi: 10.1007/s40495-016-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Hu M, Yang G, Gao E, Chen W. Current Status of Castration-Resistant Prostate Cancer Drug Therapy. Int J Surg Oncol. 2021;6:41–9. [Google Scholar]
- Montaño-Samaniego M, Bravo-Estupiñan DM, Méndez-Guerrero O, Alarcón-Hernández E, Ibáñez-Hernández M. Strategies for targeting gene therapy in cancer cells with tumor-specific promoters. Front Oncol. 2020;10:2671–89. doi: 10.3389/fonc.2020.605380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai Y, Inazawa J, Ueyama H, Ohkubo I. Human gene for β-microseminoprotein: its promoter structure and chromosomal localization. J Biochem. 1995;117:346–52. doi: 10.1093/jb/117.2.346. [DOI] [PubMed] [Google Scholar]
- Rama A, Aguilera A, Melguizo C, Caba O, Prados J. Tissue specific promoters in colorectal cancer. Dis Markers. 2015:2015. doi: 10.1155/2015/390161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama Ballesteros AR, Hernandez R, Perazzoli G, et al. Specific driving of the suicide E gene by the CEA promoter enhances the effects of paclitaxel in lung cancer. Cancer Gene Ther. 2020;27:657–68. doi: 10.1038/s41417-019-0137-3. [DOI] [PubMed] [Google Scholar]
- Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Molecular cloning: A laboratory manual, the third edition, Directional Cloning into Plasmid Vectors, Cold spring harbor laboratory press. New York: cold spring harbor; 2001. pp. 107–10. [Google Scholar]
- Sjöblom L, Saramäki O, Annala M, et al. Microseminoprotein-beta expression in different stages of prostate cancer. PLoS One. 2016;11:e0150241. doi: 10.1371/journal.pone.0150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suguna , S , Nandal D, Kamble S, Bharatha A, Kunkulol R. Genomic DNA isolation from human whole blood samples by non enzymatic salting out method. Int J Pharm Pharm Sci. 2014;6:198–99. [Google Scholar]
- Tabriz HM, Nazar E, Ghiasi M. Evaluation Expression COX-2 in Prostatic Carcinoma by PCR and Immunohistochemistry and Its Relationship with Gleason Score. Asian Pac J Cancer Biol. 2021;6:105–9. [Google Scholar]
- Teo MY, Rathkopf , DE , Kantoff P. Treatment of advanced prostate cancer. Ann Rev Med. 2019;70:479–99. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Jia W, Rennie PS. Bioengineered viral vectors for targeting and killing prostate cancer cells. Bioengineered Bugs. 2010;1:92–6. doi: 10.4161/bbug.1.2.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]