Abstract
Background:
The BNT162b2 mRNA COVID-19 vaccine has been administered to children and adolescents with cancer and hematologic diseases since they are at high risk of manifesting severe symptoms if they have COVID-19 infection but the adequate immune response after vaccination in these immunocompromised patients are questionable.
Objective:
To evaluate the immune response of children and adolescents with cancer and hematologic diseases after receiving 2 doses of the BNT162b2 mRNA COVID-19 vaccine.
Methods:
This is a prospective cohort study of patients with cancer and hematologic disease, who aged 12- 18 years old and received 2 doses the BNT162b2 vaccines at 4 weeks apart were enrolled. Immunogenicity was determined by measuring serum anti-SARS-CoV-2 immunoglobulin antibodies directed against the receptor binding domain (RBD) of S1 domain of the spike protein (Anti S-RBD), surrogated viral neutralization test (sVNT) of SARS-CoV-2 and Delta strain. Blood samples were collected and analyzed at 4 and 12 weeks after vaccination. The seroprotective rate was defined as sVNT ≥ 68%.
Results:
From Oct 2021 to Jan 2022, 43 children were enrolled, 21 were on-therapy and 22 were off-therapy. 25 were hematologic malignancy, 15 solid tumor and 3 hematologic diseases with immunosuppressive drugs. The GMT (95%CI) of a anti S-RBD IgG level at 4 weeks after vaccination were 56.05 (13.2,238.2) and 3633 (2689,4908) BAU/mL in on-therapy and off-therapy group, respectively, p<0.001. The sVNT (95%CI) of delta strain were 26% (5.85-73.55%) and 97.05% (96.0-97.4%) as the seroprotective level which were 33.3% in on-therapy group and 100% in off-therapy group (p<0.001). 14 children in on-therapy group need an additional dose.
Conclusion:
After complete vaccination, the seroprotective rate and antibody level in pediatric and adolescent patients with cancer and hematologic disease who receive immunosuppressive agents are quite low, compared with patients who had complete treatment. Additional dose of primary series should be offered.
Key Words: SARS-CoV-2 vaccine, COVID-19 vaccine, BNT162b2 mRNA COVID-19 vaccine, neutralizing antibody
Introduction
The COVID-19 pandemic has affected the global population especially patients with chronic disease since 2019 (Zhu et al., 2020). Children with cancer and hematologic diseases who received immunosuppressive drugs and thus are severely immunocompromised and are vulnerable and more prone to developing severe infections than healthy children (Andre et al., 2020; Boulad et al., 2020; De Rojas et al., 2020). Nowadays, beyond social distancing, masking, and washing hands, vaccines have been distributed as an effort to create immunity against SARS-CoV-2 infection and prevent further spread of the pandemic. These vaccines act by stimulating the recipient’s immune system to produce specific antibodies that bind to the spike protein of SARS-CoV-2 and block the virus’s ability to infect the host cells. The BNT162b2 mRNA COVID-19 vaccine was one of the mRNA vaccines developed and the first to be listed in WHO’s Emergency Use Listing (Danzetta et al., 2020).
In Thailand, the BNT162b2 mRNA COVID-19 vaccine has been administered to children and adolescents age ranging between 12-18 years old who are at risk for severe infections, and those with cancer and hematologic diseases who have received chemotherapy and immunosuppressive drugs.
Both chemotherapy and immunosuppressive drugs are known to be the standard treatment for the latter group. These agents however affect the patients’ cell-mediated and humoral immunity by making the immunity defect, thus resulting to the patients becoming vulnerable to severe infection. Despite this, recovery is noted 6-12 months after completion of treatment (Han et al., 2018; Soonie et al., 2007).
Direct application of mRNA into dendritic cells was shown to induce polyclonal CD4+ and CD8+ mediated antigen-specific T cell responses and the production of protective antibodies from B cell. Several recently published studies have emphasized mRNA vaccines’ failure to elicit protective immune responses in patients with malignant disease, especially in those currently receiving chemotherapy or immunosuppressive agents (Heine et al., 2021). Furthermore, previous reports showed that patients with hematologic malignancies already had poor responses to other vaccines, such as influenza, varicella, pneumococcal and hepatitis B (La Torre et al., 2016; Pullukcu et al., 2008; Mullane et al., 2019).
In patients with COVID-19 infection, the definitive antibody level for protective immunity is inconclusive until now (Wang et al., 2021; Lumley et al., 2021). The neutralizing antibody titer against the SARS-CoV-2 is a highly predictive indicator to measure the protective immune response to symptomatic infection (Abu-Raddad et al., 2021; Bernal et al., 2021). The gold standard for neutralizing antibody detection is the plaque reduction neutralization test (PRNT50). This, however, requires live pathogen and complex laboratory settings. Thus, the technique using surrogate virus neutralization test (sVNT), which detects total immunodominant neutralizing antibodies targeting the viral spike (S) protein receptor-binding domain (RBD), is generally used (Tan et al., 2020).
Total binding antibodies level (IgG, IgM and IgA) against the SARS-CoV-2 can be detected by simple laboratory techniques, including rapid test, Enzyme-Linked Immunosorbent Assay (ELISA), and Electrochemiluminescence Assay.
In Thailand, there was no study to evaluate the efficacy of BNT162b2 mRNA COVID-19 vaccines in pediatric cancer or other immunocompromised patients. The aim of this study was to determine the immune response in children and adolescents with cancer and hematologic diseases after they received 2 doses of the BNT162b2 mRNA COVID-19 vaccination. A comparison of the immune response is made between one group currently receiving chemotherapy or immunosuppressive agents with another group who has completed treatment and discontinued immunosuppressive agents for more than 6 months thus with immune function recovery to nearly normal (Han et al., 2018; Soonie et al., 2007). The comparison is done by using anti-SARS-CoV-2 total immunoglobulin G antibodies (anti S-RBD total Ig) and immunoglobulin G (IgG) directed against the receptor binding domain (RBD) of S1 domain of the spike protein (Anti S-RBD total IgG and IgG), SARS-CoV-2 surrogate viral neutralization test (sVNT) and variants of concern (VOCs) of Delta strain.
Materials and Methods
This prospective cohort study was conducted at King Chulalongkorn Memorial Hospital (KCMH), Bangkok, Thailand, in 2021. The study protocol was approved by the institutional Review Board, Faculty of Medicine, Chulalongkorn University. Written informed consent was obtained from the participants and participants’ guardians.
Study Population
Participants were patients, age 12-18 years old who were diagnosed with cancer and hematologic diseases at KCMH, and who received 2 doses of the BNT162b2 mRNA COVID-19 vaccine between August to September 2021. Some patients had ongoing chemotherapy or immunosuppressive therapy (on-therapy) and some who had completed the therapy (off-therapy). All patients were asked for history of COVID-19 infection and COVID-19 exposure risk before receiving vaccination and during this study. Nasopharyngeal swab for RT-PCR COVID-19 screening was performed to confirm that none had COVID-19 infection before vaccination and to rule out clinically suspected COVID-19 infection during the study.
The BNT162b2 mRNA COVID-19 vaccine (30 μg per dose) was administered intramuscularly at the deltoid region following the 2-dose regimen at 4 weeks apart.
Exclusion criteria included: 1) patients with history of and recent COVID-19 infection before or during the study, 2) patients who received other vaccination within 1 month, 3) patients who received intravenous immunoglobulin within 3 months.
Data collection and Methods of detection
Baseline demographics, including age, sex, diagnosis, current treatment, and blood count parameters during disease-specific follow-up (within 2 weeks of blood sample collection for antibody titer) especially white blood cells (WBC) count, were collected.
For all participants, 6-ml clotted blood was collected at 4 and 12 weeks (+/- 2 weeks) after second dose of the BNT162b2 mRNA COVID-19 vaccine.T
The immune response after vaccination was determined by measuring antibody titer against SARS-CoV-2 by serum anti S-RBD total Ig (U/ml), anti S-RBD IgG (BAU/ml), sVNT (%inhibition) of SARS-CoV-2 wild type and VOCs of Delta strain(%inhibition).
Method of detection
1.The SARS-CoV-2 Antibodies (Anti S-RBD total Ig, Anti S-RBD IgG)
The SARS-CoV-2 total antibodies were detected using the Elecsys® Anti-SARS-CoV-2 on Cobas e411 immunoassay analyzers (Roche Diagnostics, Rotkreuz, Switzerland), which is under the US-FDA EUAs (US Food and Drug Administration’s Emergency Use Authorization). The Elecsys® is an immunoassay for SARS-CoV-2 total antibodies against the RBD of S1 domain of spike protein and antibodies against the RBD of S1 domain of spike protein IgG subtype against SARS-CoV-2 wild type.
2.The SARS-CoV-2 Neutralizing Antibody (sVNT of SARS-CoV-2 wild type, VOCs of Delta strain)
The SARS-CoV-2 neutralizing antibody was detected by using the blocking technique of the cPassTM SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript), which is also under the US-FDA EUAs. The protein-protein interaction between Horseradish peroxidase (HRP) conjugated recombinant SARS-CoV-2 RBD fragment (HRP-RBD), and the human ACE2 receptor protein (hACE2) can be blocked by neutralizing antibodies against SARS-CoV-2 RBD. The neutralizing antibody level was detected as percent signal inhibition (% inhibition) following the manufacturer’s protocol.
The seroprotective rate was defined as sVNT ≥ 68% adopted from the US-FDA guidance for a high titer of the COVID-19 convalescent plasma (De Santis et al., 2021).
Patients who didn’t reach the seroprotective level of sVNT will receive the additional dose of The BNT162b2 mRNA COVID-19 vaccine and repeat antibody titers and sVNT at 4 weeks after additional dose.
Statistical analysis
Statistical analysis was performed by the SPSS software for Windows 21.0 (SPSS Inc., Chicago, IL). Categorical variables are presented as frequencies and percentages, and continuous variables are presented as median and interquartile range (IQR). The Mann-Whitney U test was used to compare the continuous variable between two groups, Chi-square test was used to compare the proportion between two groups and Wilcoxon signed ranks test was used to compare the continuous data within the same group.
Results
Characteristics of study population
Forty-three patients who received the 2-dose regimen of the BNT162b2 mRNA COVID-19 vaccine participated in this study. All of them were followed up at the pediatric hematology and oncology clinic, KCMH and screened for risk and symptoms of COVID infection regularly. None of the patients had COVID-19 infection before receiving the vaccine or at any time during this study. RT-PCR test was done to prove non-infection before they were enrolled into the study. Most of the patients are diagnosed with hematologic malignancy (leukemia and lymphoma) (n=25; 58.1%). Others are patients with solid tumor including brain tumor, neuroblastoma, soft tissue sarcoma and nasopharyngeal cancer (N=15; 34.9%) and hematologic disease including aplastic anemia, Fanconi anemia and chronic immune thrombocytopenia (ITP) who currently on immunosuppressive drugs (N=3; 6.8%). Characteristics of these 43 participants are given in Table 1.
Table 1.
Characteristics of All the Participants. (N=43)
| Characteristics | Total (N=43) | On-therapy group (N=21) | Off-therapy group (N=22) | P-value |
|---|---|---|---|---|
| Age (years); median (IQR) | 15 (13,16) | 15.0 (13,16) | 15.0 (14,17) | 0.452 |
| Sex; N (%) | ||||
| Female | 17 (39.5) | 7 (33.3) | 10 (45.5) | 0.329 |
| Male | 26 (60.5) | 14 (66.7) | 12 (54.5) | |
| Diagnosis; N (%) | ||||
| Hematologic disease on immunosuppressive drugs | 3 (6.8) | 3 (14.9) | 0 (0) | 0.398 |
| Hematologic malignancy | 25 (58.1) | 13 (61.9) | 12 (54.5) | |
| Solid tumor | 15 (34.9) | 5 (23.8) | 10 (45.5) | |
| Platelet count (/mm3); median (IQR) | 262000 (194000,312000) | 231000 (178000, 353000) | 268000 (215000,302000) | 0.622 |
| Hemoglobin (g/dl); median (IQR) | 13.4 (12.6,14.4) | 13.3 (11.3, 14.1) | 13.4 (13, 14.6) | 0.184 |
| Total white blood cell count (/mm3); median (IQR) | 5910 (4240,7310) | 4420 (3100, 7120) | 6100 (5130, 7660) | 0.032* |
| Absolute neutrophil count | 3085 (2320,3800) | 2900 (1900,3980) | 3330 (2530, 3700) | 0.391 |
| Absolute lymphocyte count | 2110 (1180,2740) | 1330 (710,2040) | 2590 (2120, 2850) | 0.001* |
| Interval between vaccine completion and the blood collection (day); median (IQR) | 31 (29,35) | 31 (29,35) | 32 (30,34) | 0.85 |
*P-value between on-therapy and off-therapy group corresponds to Mann-Whitney U and Chi-square test.
Participants’ blood samples were taken at 4 weeks and 12 weeks after vaccination. Because there is no definitive cut-off level on sVNT and antibody titer, the ≥ 68% inhibition according to the US-FDA was adopted to implicate seroprotective level.
The seroprotective rate is 68.2% for both SARS-CoV-2 wild type and VOCs of Delta strain. 14 patients did not have adequate immune response for both strains and all 14 accounted for 66.7% of patients (14/21) in the on-therapy group. On the other hand, the seroprotective rate of the patients in the off-therapy group is 100%.
Based on these results, data were analyzed and grouped into 2: 1) on-therapy group (currently on chemotherapies or immunosuppressive drugs), and 2) off-therapy group. All patients in off-therapy group had ended their treatment for more than 6 months. The median age of both groups was 15.0 years old. The on-therapy group included more males as compared with the off-therapy group. The median intervals between the second dose and the blood collection were 31 days for the on-therapy group and 32 days for off-therapy group. The baseline blood count parameters between the 2 groups are not statistically significant except for the total white blood cell count and absolute lymphocyte count. The characteristics of these 2 groups are summarized in Table 1.
The Immunogenicity
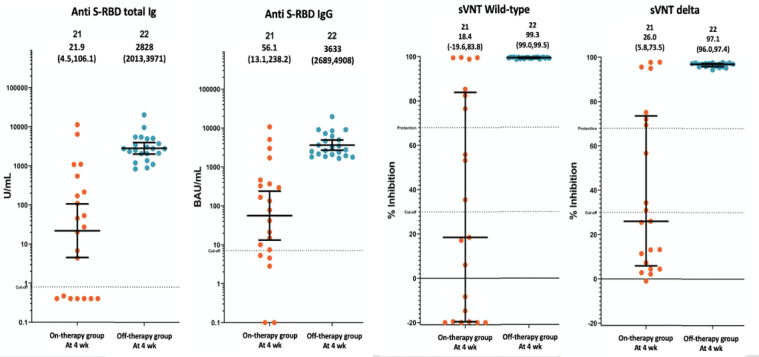
The results of all serologic parameters at 4 weeks after complete vaccination in the on-therapy group (N=21) was statistically significantly lower than off-therapy group (N=22) as shown in Table 2 and Figure 1.
Table 2.
The Immunogenicity at 4 Weeks after 2-dose of BNT162b2 mRNA COVID-19 (Pfizer) Vaccines
| Serologic parameters | On-therapy group (N=21) | Off-therapy group (N=22) | P-value* |
|---|---|---|---|
| Anti S-RBD total Ig (U/ml); GMT (95%CI) | 21.9 (4.5,106.1) | 2828 (2013,3971) | <0.001 |
| Anti S-RBD IgG (BAU/ml); GMT (95%CI) | 56.1 (13.1,238.2) | 3633 (2689,4908) | <0.001 |
| sVNT SARS-CoV-2 wild-type (%inhibition); median (IQR) | 18.4 (-19.6,83.8) | 99.3 (99.0,99.5) | <0.001 |
| sVNT SARS-CoV-2 delta (%inhibition); median (IQR) | 26 (5.8,73.5) | 97.1 (96.0,97.4) | <0.001 |
| Seroprotective rate#; N (%) | |||
| SARS-CoV-2 wild type | 7 (33.3) | 23 (100) | <0.001 |
| Delta strain | 7 (33.3) | 23 (100) | <0.001 |
*P-value corresponds to Mann-Whitney U and Chi-square test; #The seroprotective rate was defined as sVNT ≥ 68% adopted from the US-FDA guidance; S-RBD, Spike protein ribosomal-binding domain; sVNT, surrogated viral neutralization test
Figure 1.
Anti S-RBD total Ig, anti S-RBD IgG, sVNT SARS-CoV-2 wild type and sVNT delta strain at 4 weeks after vaccines of patients on-therapy group (N=21) and off-therapy group (N=22)
Because immunocompromised patients have higher risks for serious complications and morbidity if they have COVID-19 infection, all 14 patients who did not have adequate immune responses were sent for a booster dose of the BNT162b2 mRNA COVID-19 vaccine after the serologic results came out without waiting for the blood test at 12 weeks. We followed up their immune response by taking blood sample for the same parameter at 4 weeks after the booster dose, the findings of which will be discussed separately. For this reason, at 12 weeks after 2 doses of vaccination, the 14 patients who were sent for a booster dose were excluded in our data analysis because their antibody level and sVNT might be increased in response to the booster dose. There were 3 patients who refused the blood sampling at 12 weeks after vaccination: 1 in the on-therapy group and 2 in the off-therapy group. Hence, the population (N) in the on-therapy group and off-therapy group decreased from 21 to 6 and 22 to 20, respectively. Comparison of antibody levels between 4 and 12 weeks in both on-therapy and off-therapy groups was only done in patients who had data at 12 weeks as shown in Table 4.(Please insert table 4)
The SARS-CoV-2 total Antibodies (Anti S-RBD total Ig and IgG subtype)
At 4 weeks after complete vaccination, the geometric mean titer (GMT) of anti S-RBD total Ig (U/ml) and anti S-RBD IgG (BAU/ml) in the on-therapy group was statistically significantly lower than off-therapy group (21.9 vs 2,828; P<0.001 and 56.05 vs 36.33; P<0.001, respectively). At 12 weeks after vaccinations, the GMT (95%CI) of anti S-RBD total Ig (U/ml) was 1,075 (372,7814) for on-therapy group (N=6) and 2,733 (1988,3757) for off-therapy group (N=20) with no significant difference from the 4 weeks after vaccination. The GMT (95%CI) of anti S-RBD IgG (BAU/ml) in on-therapy and off-therapy groups were 775.5 (167.1,3599) and 1213 (911.3,1614), respectively, which had a statistically significant decrease only in the off-therapy group as shown in Table 3.
Table 3.
The Immunogenicity at 4 and 12 Weeks after 2-dose of The BNT162b2 mRNA COVID-19 (Pfizer) Vaccines and Comparison between 4 and 12 Weeks
| Serologic parameters | Timing (weeks) | On-therapy group (N=6) | Off-therapy group (N=20) |
|---|---|---|---|
| Anti S-RBD total Ig (U/ml); GMT (95%CI) | 4 | 729.3 (181.9,2925) | 2709 (1873,3919) |
| 12 | 1705 (372,7814) | 2733 (1988,3757) | |
| P-value within group* | 0.345 | 0.91 | |
| Anti S-RBD IgG (BAU/ml); GMT (95%CI) | 4 | 1063 (307.8,3673) | 3538 (2546,4916) |
| 12 | 775.5 (167.1,3599) | 1213 (911.3,1614) | |
| P-value within group* | 0.345 | <0.001* | |
| sVNT SARS-CoV-2 wild-type (%inhibition); median (IQR) | 4 | 92.1 (80.9,99.5) | 99.3 (99.0,99.5) |
| 12 | 96.2 (77.7,98.9) | 97.9 (94.9,98.6) | |
| P-value within group* | 0.843 | <0.001 | |
| sVNT SARS-CoV-2 delta (%inhibition); median (IQR) | 4 | 85.1 (71.3,96.1) | 96.9 (96.0,97.4) |
| 12 | 92.6 (70.4,97.2) | 96.5 (93.9,97.1) | |
| P-value within group* | 0.812 | <0.001 | |
| Seroprotective rate#; N (%) | |||
| SARS-CoV-2 wild type | 4 | 6 (100%) | 20 (100%) |
| 12 | 5 (83.3%) | 20 (100%) | |
| Seroprotective rate#; N (%) | |||
| Delta strain | 4 | 6 (100%) | 20 (100%) |
| 12 | 5 (83.3%) | 20 (100%) |
*P-value within group corresponds to Wilcoxon signed ranks test; #The seroprotective rate was defined as sVNT ≥ 68% adopted from the US-FDA guidance; S-RBD, Spike protein ribosomal-binding domain; sVNT, surrogated viral neutralization test
The seroprotective rate from surrogate neutralizing Antibody (sVNT of SARS-CoV-2 wild type and VOCs of Delta strain)
The seroprotective rate against SARS-CoV-2, both wild type and delta strains, at 4 weeks after vaccination in off-therapy group was 100% (22/22). The statistical significance was superior to the on-therapy group which was 33.3% (7/21) (P-value <0.001). 12 weeks after the second dose of BNT162b2 mRNA COVID-19 vaccine, one among the 6 patients in the on-therapy group that used to have sVNT ≥ 68% lost his sVNT seroprotective level in both wild type (sVNT 85.3 decrease to 41.8%) and delta strain (sVNT 75.1 decrease to 38.2%). Thus, the seroprotective rate in the on-therapy group was 83.3% (5/6). Further details on that patient showed that he had received intensive chemotherapy for his brain tumor during vaccination. On the other hand, all the patients in the off-therapy group were able to maintain their immune level (sVNT >68%) so the seroprotective rate at 12 weeks was 100% (22/22).
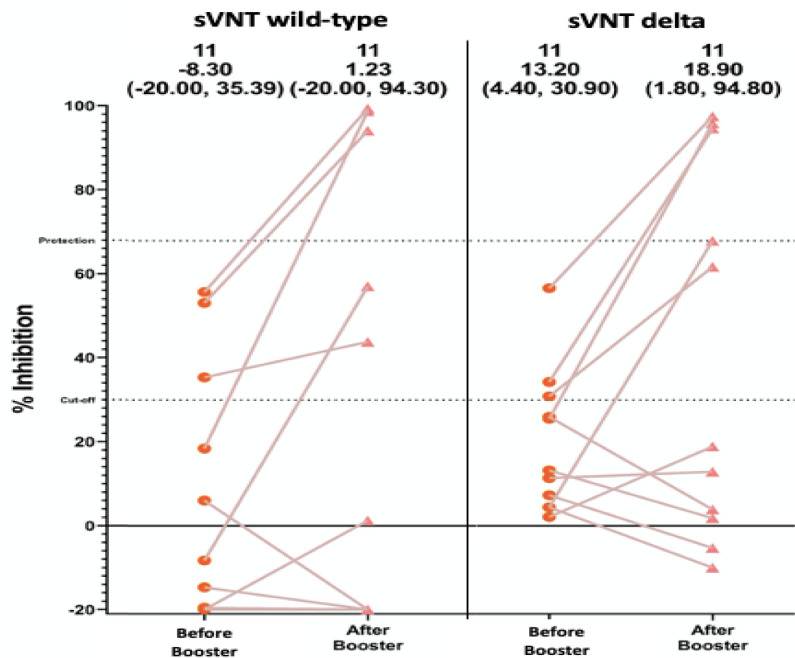
14 patients in the on-therapy group with sVNT<68% were sent for a third dose of vaccination to boost their immunity to COVID-19 infection. One of 14 patients refused to receive the booster dose and 2 of the patients who received the third dose refused to have blood sampling so only 11 patients were tested for sVNT wild type and VOCs delta strains at 4 weeks after the booster dose. Of the 11 patients, 3 (27.3%) had sVNT wild type ≥ 68% and 4 (36.4%) had sVNT VOC delta ≥ 68% as shown in Figure 2.
Figure 2.
sVNT SARS-CoV-2 wild type and sVNT delta strain before and after 3rd dose of BNT162b2 mRNA COVID-19 (Pfizer) vaccine (N=11)
Adverse effects and Clinical outcome
All study participants were approached via telephone call at the end of the study to inquire about the possibility of SARS-CoV-2 infection. At 12 weeks follow-up following administration of the second dose of BNT162b2 mRNA COVID-19 vaccine, none of the study subjects developed clinical disease. 41.9% of the participants (18/43) had local side effect after vaccination. None had serious side effect and everyone in on-therapy group were able to continue their treatments without any complication or interruption including those who received the third dose of BNT162b2 mRNA COVID-19 vaccine.
Discussion
This study shows the immune responses at 4 and 12 weeks after 2-doses of BNT162b2 mRNA COVID-19 vaccine in Thai pediatric and adolescents, age 12-18 years old, with cancer and hematologic disease and received immunosuppressive agents.
On recent studies, the seroconversion and seroprotective rate after 2 doses of mRNA COVID-19 vaccine in pediatric patients with cancer are varied, ranging from 47-90% (Malard et al.,2021; Thakkar et al., 2021; Agha et al., 2021). With our study, at 4 weeks after second dose, the seroprotective rate measured from both standard methods, sVNT of SARS-CoV-2 wild type and VOCs of delta-strain is 67.4% (29/43).
As shown in the result, all the patients who had inadequate immune response to SARS-CoV-2 are the patients with ongoing chemotherapy or immunosuppressive agent therapy. So, we classified the patients into 2 groups 1) on-therapy group and 2) off-therapy group. The data showed that the seroprotective rate was very low in the on-therapy group (sVNT 33.3% in both wild type and VOC delta strains). On the other hand, seroprotective rate in the off-therapy group is 100% in both sVNT wild type and VOC delta strain.
In addition, on comparison, the seroprotective rate for sVNT wild type and VOC delta strain for the on-therapy group is statistically lower than off-therapy group (33.3 vs 100%, p<0.001). With antibody level, the off-therapy group has higher anti S-RBD total Ig and anti S-RBD IgG levels than the on-therapy group (p<0.001). This might be due to the significantly lower total white blood cell count and absolute lymphocyte count in on-therapy group when compared to off-therapy group; in addition to the fact that chemotherapies and immunosuppressive agents can affect patient’s immune function. Recovery of the immune function was noted in the off-therapy group within 6-12 months after cessation of treatment (Han et al., 2018; Soonie et al., 2007).
We collected blood samples for immunologic testing at 12 weeks after 2nd dose of vaccine to determine the sustainability of immunogenicity. 14 seronegative patients and 3 patients that did not have serologic testing at 12 weeks were excluded; so, the total N was 26 with 6 patients in on-therapy group and 20 patients in off-therapy group. In Table 4, the GMT of anti S-RBD IgG level was significantly decreased in the off-therapy group when compared within the group (p<0.001). This was not observed in the on-therapy group probably because of the small sample size (N=6). The seroprotective rate (sVNTs) against SAR-CoV2 wild type and delta strains was not significantly decreased in both groups at 12 weeks after vaccination but there was one patient in the on-therapy group who had lost his seroprotective level (sVNT decreased to < 68% in both wild type and delta strain). Possible cause might be the intensive chemotherapy that he received during the study.
The number of the patients that had sVNT against SAR-CoV2 wild type and VOCs delta strain ≥ 68% after the third dose of BNT162b2 mRNA COVID-19 vaccine (additional dose) was 3 out of 11 (23.7%) and 4 out of 11 (36.4%), respectively. This meant that the additional dose of BNT162b2 mRNA COVID-19 vaccine was able to boost the immune response to seroprotective level in some, but not all, immunocompromised patients.
The Thai government’s initial policy was to give two doses of BNT162b2 mRNA COVID-19 vaccine to children and adolescence, age 12-18 years, including immunocompromised patients. From the result of this study, in line with CDC recommendation, a third dose of BNT162b2 mRNA COVID-19 vaccine should be considered in immunocompromised as an additional dose. And since a decline of the immunologic level in both immunocompromised (on-therapy) and immunocompetent (off-therapy) groups were noted at 12 weeks after completion of the 2-dose regimen, a booster dose of BNT162b2 mRNA COVID-19 vaccine may be required.
Moreover, according to other studies, the seroprotective level after 2 doses of BNT162b2 mRNA COVID-19 vaccine against the most recent variant of concern, Omicron strain, seem to be lower than against wild type and delta strain, which is insufficient to protect against omicron stain (Schmidt et al., 2021; Muik et al., 2022). Therefore, the booster dose of this vaccine might be considered.
Limitations of this study include a small sample size, no definitive cut-off level of sVNT and antibody titer so the 68% inhibition by the US-FDA was adopted to implicate the protective immune response. We also did not measure the cellular immune response. Finally, the protective immunity against SARS-CoV-2 Omicron strain is not determined in this study, thus, any further future study should be focused on the most recent variant of concern, SARS-CoV-2 Omicron strain.
In conclusion, the seroprotective rate (sVNT against SARS-COV-2 wild type and VOCs delta strains) and antibody level (anti S-RBD total Ig and anti S-RBD IgG) in pediatric and adolescent patients with cancer and hematologic disease who receive immunosuppressive agent are quite low compared with patients who have completed the treatment and could sustain their immunologic level just like the normal population. Therefore, the third dose (booster dose) of BNT162b2 mRNA COVID-19 vaccine should be considered in immunocompromised patients.
Author Contribution Statement
HP and SL designed the study. HP and CS collected data, analyzed, and reviewed the results. HP, CS, WJ and DS wrote and revised the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements
This work was not supported by any fund. This study was approved by the institute ethics committee from Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB no.812/64). The institutional review board chief committee is Emeritus Professor Tada Sueblinvong, MD.
We are very thankful to our laboratory staffs in the Division of Pediatric Hematology and Oncology and the Center of Excellence in Clinical Virology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University. And special thanks to Ritthideach Yorsaeng, Ph.D for his support and help in production of figure.
Availability of data
The datasets generated and/or analyzed during the current study are not publicly available.
Statement conflict of interest
The authors disclose no potential conflicts of interest.
References
- Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B and B 1 351 variants. N Engl J Med. 2021;385:187–9. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha M, Blake M, Chilleo C, et al. Suboptimal response to Coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking Era. Open Forum Infect Dis. 2021;8:353. doi: 10.1093/ofid/ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André N, Rouger-Gaudichon J, Brethon B, et al. COVID-19 in pediatric oncology from French pediatric oncology and hematology centers: high risk of severe forms. Pediatr Blood Canc. 2020;67:e28392. doi: 10.1002/pbc.28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal JL, Andrew N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B 1 617 2 (delta) variant. N Engl J Med. 2021;385:585–94. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulad F, Kamboj M, Bouvier N, et al. COVID-19 in children with cancer in New York city. JAMA Oncol. 2020;6:1459–60. doi: 10.1001/jamaoncol.2020.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzetta ML, Amato L, Cito F, et al. SARS-CoV2-RNA persistence in naso-pharyngeal swabs. Microorganisms. 2020;8:1124. doi: 10.3390/microorganisms8081124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rojas T, Pérez-Martinez A, Cela E, et al. COVID-19 infection in children and adolescents with cancer in Madrid. Pediatr Blood Canc. 2020;67:e28397. doi: 10.1002/pbc.28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis GC, Mendrone A, Langhi D Jr, et al. Suggested guidelines for convalescent plasma therapy for the treatment of COVID-19. Hematol Transfus Cell Ther. 2021;43:212–3. doi: 10.1016/j.htct.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Harmoney KM, Dokmeci E, et al. Dynamic re-immunization of off-treatment childhood cancer survivors: An implementation feasibility study. PLoS One. 2018;13:e0191804. doi: 10.1371/journal.pone.0191804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine A, Juranek S, Brossart P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Molecular Cancer. 2021;20:52. doi: 10.1186/s12943-021-01339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Torre G, Mannocci A, Colamesta V, et al. Influenza and pneumococcal vaccination in hematological malignancies: a systematic review of efficacy, effectiveness, and safety. Mediterr J Hematol Infect Dis. 2016;8:e3016044. doi: 10.4084/MJHID.2016.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–40. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malard F, Gaugler B, Gozlan J, et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021;11:1–8. doi: 10.1038/s41408-021-00534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A, Lui BG, Wallisch A-K, et al. Neutralization of SARS-COV-2 omicron pseudovirus by BNT162B2 vaccine-elicited human Sera. Science. 2022: 7591. doi: 10.1126/science.abn7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane KM, Morrison VA, Camacho LH, et al. Safety and efficacy of inactivated varicella zoster virus vaccine in immunocompromised patients with malignancies: a two-arm, randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2019;19:1001–12. doi: 10.1016/S1473-3099(19)30310-X. [DOI] [PubMed] [Google Scholar]
- Pullukcu H, Ertem E, Karaca Y, et al. Efficacy of accelerated hepatitis B vaccination program in patients being actively treated for hematologic malignancies. Int J Infect Dis. 2008;12:166–70. doi: 10.1016/j.ijid.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS-COV-2 omicron variant. N Engl J Med. 2021:2021. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soonie RP, Miguel O, Bernard JC, et al. Revaccination of children after completion of standard chemotherapy for acute leukemia. Clin Infect Dis. 2007;44:635–42. doi: 10.1086/511636. [DOI] [PubMed] [Google Scholar]
- Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–8. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- Thakkar A, Gonzalez-Lugo J, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–90. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kaperak C, Sato T, et al. COVID-19 reinfections: a rapid systematic review of case reports and case series. J Investig Med. 2021;69:1253–5. doi: 10.1136/jim-2021-001853. [DOI] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, et al. China novel coronavirus investigating and research team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available.