Abstract
Background and Purpose:
Rutin (RUT) is one of the phenolic compounds found in the invasive plant species, Carpobrotus edulis. Several studies have confirmed numerous pharmacological properties of RUT, including antioxidant, antidiabetic, anti-inflammatory, antimicrobial and anticancer activities. As a result, the goal of this work was to make RUT-loaded PCL-PEG and test its anti-cancer effects against the Skov3 human ovarian cancer cell line.
Materials and Methods:
The NPs were made using the W1/O/W2 process, and their physicochemical properties were assessed by FE-SEM, FTIR, and DLS. MTT assay were used to investigate the anti-proliferative characteristics of drug-loaded NPs. Real-time PCR was also utilized to examine the expression levels of apoptotic genes including caspase-8, -9, -3, and Bax, as well as anti-apoptotic genes like Bcl-2.
Results:
Cytotoxicity testing revealed that RUT-loaded PCL-PEG improved cytotoxicity in a dose- and time-dependent manner. In treated MDA-MB-231 cells with RUT-loaded PCL-PEG, there was a significant up-regulation of caspase-8, -9, -3, and Bax genes compared to treated cells with free RUT.
Conclusion:
Finally, RUT-loaded PCL-PEG NPs are recommended as ideal delivery nanocarriers for enhancing RUT’s anticancer characteristics for ovarian cancer treatment.
Key Words: Rutin, PCL-PEG nanoparticles, anticancer, Skov3 cells, apoptosis
Introduction
There are several malignancies known as the leading causes of mortality in the world (Pourgholi et al., 2021; Khazei et al., 2022). Cancer is an uncontrolled growth of cells that can attack or spread to other tissues and organs (Nikmanesh et al., 2020; Samadzadeh et al., 2021; Shahabi et al., 2021). Due to the asymptomatic nature of the illness and late diagnosis, ovarian cancer is a prevalent malignancy in women, with a high fatality rate. According to the American Cancer Society’s estimations for 2022, about 19,880 women will be diagnosed with ovarian cancer and about 12,810 women will die due to cancer in the U.S. (Siegel et al., 2022). Ovarian cancer ranks fifth in cancer mortality among women and the risk of developing ovarian cancer throughout the lifetime is around 1 in 75 women (Barani et al., 2021).
Surgery, chemotherapy, and radiation therapy are the three most common ovarian cancer treatment options, depending on the type and stage of the disease (Farajzadeh et al., 2018; Faramarzi et al., 2019). Despite development of modern treatment options and chemotherapeutic drugs, worries about relapse, deterioration of the quality of life, and major side effects of the treatment cannot be neglected (Amirsaadat et al., 2021). As a result, researchers have been looking for novel ways that are more efficient, less expensive, and cause less harm to patients.
Bioactive phytochemicals are natural substances that are secondary metabolites of therapeutic plants (Dadashpour et al., 2011). They have anti-inflammatory and anti-cancer properties (through cell cycle participation, apoptosis, angiogenesis, and metastasis), and high antioxidant content; they also increase immunity of the body. According to multiple researches, phytochemicals are used as chemo-preventive and chemotherapeutic agents for a variety of cancers.
The RUT is a symbolic flavonoid found in a variety of natural sources, including fruits, vegetables, and juices, and it has been studied for its medicinal potential (Hoai et al., 2020). The RUT has been shown to have antioxidant, antibacterial, anti-inflammatory, antidiabetic, and anticancer properties (Caparica et al., 2020). Furthermore, studies have shown that RUT can inhibit tumor development and induce cell cycle arrest and death in a variety of in vitro cell types (Chen et al., 2013; Caparica et al., 2020). Furthermore, the poor solubility of rutin reduces its bioavailability and makes it difficult to include into delivery methods (Sharma et al., 2013). The employment of a nanotechnology-based strategy that may enhance the chemo-preventive and chemotherapeutic effects is a viable alternative approach for overcoming these limitations and increasing anticancer efficacy (Javan et al., 2019).
Nanotechnology is a nanometer-scale technology of which the functional qualities are determined by factors other than its size (Nejati et al., 2020). Researchers have focused on the application of this technology for the regulated transmission and release of pharmaceuticals, particularly in cancer treatment (Adlravan et al., 2021; Amirazad et al., 2022; Dadashpour et al., 2022). The PCL is one of the most commonly used polymer nanoparticles (Nejati et al., 2020). It is a biodegradable polymer nanoparticle that has been approved by the FDA and the European Medicines Agency (EMA) as it converts to natural monomeric metabolites in the body (Mogheri et al., 2021; Mousazadeh et al., 2021). The RUT has a few limitations and disadvantages that decrease its biological function; however, these limitations are meaningfully remunerated by the advances in nanotechnology. These limitations include poor biological availability, short half-life, low solubility, low duration of stability in the bloodstream, and rapid metabolism and degradation (De Gaetano et al., 2021). As a result, encapsulating anti-cancer phytochemicals like RUT in biodegradable PCL-PEG nanoparticles might have several benefits over existing drug delivery strategies.
The Skov3 human ovarian cancer cell line was treated by free RUT and RUT encapsulated in the PCL-PEG NPs and the effect of these nanoparticles on the cytotoxicity properties was investigated.
Materials and Methods
Material
The National Center for Genetic and Biological Resources of Iran provided the skov3 ovarian cell line. Fetal Bovine Serum (FBS), Rutin, MTT powder, Roswell Park Memorial Institute (RPMI) 1640, Streptomycin/penicillin, dimethyl sulfoxide (DMSO), and dichloromethane (DCM) were procured from Sigma 96 Chemical (St. Louis, MO).
Methods
Synthesis of PLGA-PEG
The PCL-PEG was obtained by polymerizing PCL and DL-lactide in an open ringed fashion. In the polymerized melt, PEG4000 and PCL were copolymerized in the presence of Sn(Oct)2 as the catalyst. A mixture reaction was prepared using a 1:3 ratio of PCL to DL-lactide and 0.05% (W/W) Sn(Oct)2, which was heated to 175°C and stored for 4 hours.
Preparation of nanoparticles containing drug
The double emulsion approach was used to encapsulate RUT in PCL-PEG NPs. First, 200 mg of PCL-PEG NPs was dissolved in dichloromethane (DCM, 5 ml). To make a w/o/w emulsion, PVA (20 ml, 0.5% w/v) and simvastatin (20 mg) were added to the organic phase and emulsified with a sonicator probe at 10 W for 45 seconds. Next, the resulting emulsion was agitated at room temperature to evaporate the organic phase, and the remaining solution was centrifuged for 40 minutes at 15,000 rpm and freeze-dried to remove all residual solvents.
Characterization of NPs
The average size of the NPs was determined using Dynamic Light Scattering (DLS). The morphology of NPs was determined using field emission scanning electron microscopy (FE-SEM) (Philips, Eindhoven, The Netherlands). Fourier transform infrared (FTIR) spectroscopy was used to confirm the structure of SIM-loaded NPs (Perkin-Elmer 1725X, Waltham, MA, USA).
Entrapment efficiency
Through centrifuging the complex with distilled water at 5,000 rpm for 15 minutes at room temperature, the quantity of RUT encapsulated in the PCL-PEG NPs was measured. The absorbance of the supernatant solution was measured using UV-Visible spectrophotometry at 360 nm. The following formula was used to compute the entrapment efficiency:
Eq.1 EE= (Weight of drug in NPs)/(total Weight drug) × 100%
In vitro release test and determination of release kinetics
Freshly generated phosphate buffer saline (PBS, pH 7.4) was used to test RUT in vitro release platforms at 37°C ± 1°C. In a dialysis bag, 4 mg of RUT encapsulated in PCL-PEG NPs were disseminated in release medium and sealed with closures. With a steady rotation rate of 100 rpm, the dialysis bags were dipped into the receptor phase, which contained 80 mL of the release medium. To prevent evaporation of the release liquid, the receptor compartment was enclosed. The samples were taken at predefined intervals of the same volume from the new dissolving medium. Following that, the samples were evaluated using the confirmed HPLC procedure outlined earlier.
Cell culture
In the RPIM-1640, cells were cultured with 10% fetal bovine serum and 1% antibiotics (100 units penicillin and 100 mg/ml streptomycin). For cellular proliferation, the cultures were grown at 37°C in a humidified incubator with a 5% CO2 atmosphere until confluent.
Cell viability assay test
The MTT technique was used to study the inhibitory action of rutin in free and loaded PCL-PEG NPs on Skov-3 cells (Mazloum-Ardakani et al., 2019). Skov-3 cells were grown in 96-well plates (2 x 105 cells/ml) before being treated by rutin in free and PCL-PEG NPs for 24 hours at various doses. The medium was then filled with 100 µL of MTT solution with a final concentration of 0.5 mg/ml. The generated formazan salt was solubilized using DMSO (100 µl) after 4 hours of incubation and the optical density was measured at 540 nm employing a microplate reader (BioTek Instruments, USA).
Evaluating the level of gene expression
The expression of apoptotic genes was evaluated using the real-time PCR method. Rutin and rutin-loaded PCL-PEG NPs were administered to Skov-3 cells. The cells were removed from the bottom of the flask and centrifuged after 48 hours, and then processed for RNA extraction using the Trizol reagent following the manufacturer’s guidelines. The quality of the extracted RNA was determined using agarose gel electrophoresis. The first-strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania) was used to synthesize complementary DNA (cDNA) from RNA according to the manufacturer’s instruction. Each qPCR cycle included denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C (30 s). The amount of mRNA expression of a related gene in the treated cells was compared to that of the control cells (without treatment). As a reference, the Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) housekeeping gene was employed.
Statistical analysis
GraphPad Prism® version 8.0 software was used to do statistical analyses. For multiple comparisons, the one-way analysis of variance (ANOVA) was employed to investigate the differences between mean values, followed by LSD as a post hoc test (P ≤ 0.05).
Results
Characterization of RUT-loaded PCL PEG- NPs
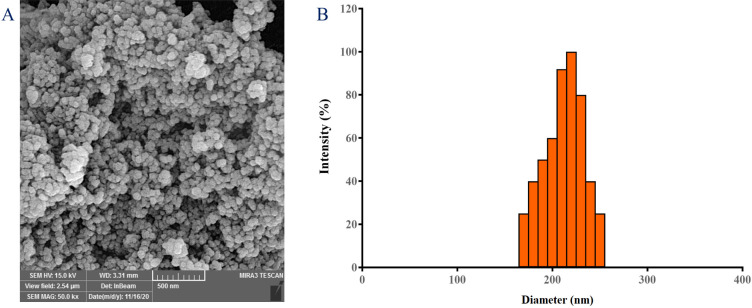
The FESEM analysis revealed spherical NPs with a smooth surface and an average diameter of 180 nm (Figure 1-A). Because of the tiny size, the NPs cannot be tracked and removed by the reticuloendothelial system. Furthermore, practically all cells are thought to preferentially absorb slightly positively charged NPs with a size of less than 400 nm (Xiao et al., 2015). As a result, it can be concluded that the size range of SIM-loaded PCL-PEG NPs is sufficient to achieve a long half-life time in the systemic circulation, as well as to passively target malignant cells. The zeta potential, which defines the surface charge of NPs, was -15.2±4.23 mV. The DLS data (Figure 1-B) confirmed the FESEM findings by showing particles with a mean size of 230.07 nm.
Figure 1.
Characterization of RUT-loaded PCL-PEG NPs. Dynamic light scattering (DLS) (A), and scanning electron microscopy (SEM) (B)
Figure 2 shows the FT-IR of RUT, PCL-PEG copolymers, and RUT-loaded PCL-PEG NPs. The vibrational bands at 3,365.95 cm-1 (phenolic O-H stretch), 2,925.82 cm-1 (asymmetric -CH2- stretch of alkanes) 2,852.89 cm-1 (symmetric -CH3 and -CH2- stretching of alkanes), 2,359.97 cm-1 and 2,342.40 cm-1 (CO2 asymmetrical stretching), and 1,635.67 cm-1 (C=O stretch) were detected in the FT-IR. These bands are distinct in the RUT-loaded PCL-PEG-PCL copolymer, and there is no major shifting, indicating that RUT is successfully incorporated.
Figure 2.
FTIR Spectra of RUT, PCL-PEG, and RUT-loaded PCL-PEG NPs
Drug loading and entrapment efficiency
To reduce the dosage, costs of production, and size for biodistribution, and quality control needs active drugs must be encapsulated (Singh et al., 2010). The range of drug encapsulation efficiencies (EE) was about 82.32 with a loading capacity of 14.3 ± 2.2%. The anticancer agent’s remarkable encapsulation efficiency appears to be due to RUT’s hydrophobic nature.
Drug release profile
The rate of RUT discharge from RUT-loaded PCL-PEG NPs was examined at pH 7.4 (Figure 3). The nanoparticulate drug release systems released RUT at a rate of about 75% for the first 200 hours. The RUT had an initial burst release followed by a gradual release rate during the first 7 hours. The RUT’s long-term release was most likely due to its encapsulation in the NP’s inner portion. As a result, copolymeric NPs may be thought of as highly selective and sensitive nano-systems for delivering hydrophobic medicines for a variety of therapeutic goals.
Figure 3.
RUT Drug Release Patterns from PCL-PEG NPs in PBS at pH 5 Over Time. The data is provided as a mean ± SD (n = 3)
Cell viability and MTT-based cytotoxicity assay
Skov3 cells were treated with different dosages of free and RUT-loaded PCL-PEG NPs for 24 hours to assess the inhibition of RUT-loaded PCL-PEG NPs on the growth of human ovarian cancer cells, and the MTT assay was used to detect the cytotoxic impact of RUT-loaded PCL-PEG NPs on the cells. In a concentration-dependent manner, the treatment of RUT-loaded PCL-PEG NPs resulted in a significant reduction of cell proliferation (Figure 4). The IC50 value of 20.23 ± 3.4 µg/ml was used to assess the inhibitory impact of skov3 cell proliferation. When compared to bulk rutin, rutin nanocrystals had at least 100 times higher capability to block the formation of malignant cells, according to Bohlouli et al. (Bohlouli et al., 2021).
Figure 4.
In vitro Cytotoxicity of Control, Free RUT, and RUT-loaded PCL-PEG NPs versus skov3 Cells as measured by MTT. The data is provided as a mean ± SD (n=3)
Real-time PCR
Real-time PCR was utilized to detect apoptosis-related gene expression in skov3 cell lines treated with pure and RUT-loaded PCL-PEG NPs, (Figure 5). The amount of caspase-8, -9, -3, and Bax gene expression alterations between the control and treated skov3 cells was assessed using the GAPDH and the region was normalized and computed using the 2-∆∆ct technique. Bcl-2 is an anti-apoptotic protein, whereas Bax is a cell death promoter (Dadashpour et al., 2018). The creation of a Bcl-2–Bax heterodimer triggers a survival signal in cells. Both of these proteins are transcriptional targets for the protein p53, which causes apoptosis in response to DNA damage (Aubrey et al., 2018). Studies have shown that the Bcl-2/Bax ratio impacts tumor growth and aggressiveness (McIlwain et al., 2013). Apoptotic genes such as caspase -8, -9, -3, and Bax were upregulated by all types of treatments. Compared to free chemotherapeutic molecules, nano-encapsulated versions of RUT in PCL-PEG NPs dramatically boosted apoptotic gene expression. Furthermore, the results demonstrated a statistically significant shift in the mRNA expression level of anti-apoptotic genes such as Bcl-2 in RUT-loaded PCL-PEG NPs treated cells. One study found that RUT nanocrystal-treated cells had a little increase in caspase-9 gene expression, albeit this was not significant (Bohlouli et al., 2021).
Figure 5.
The Expression Levels of Apoptotic Related Gems, Relative to the Reference Gene (GAPDH) in skov3 Cells Treated with free RUT and RUT-loaded PLC-PEG NPs
Discussion
In conclusion, the RUT, as a flavonol, has anti-cancer properties. Through the regulation of critical signaling proteins, it alteres the proliferation, migration, and survival of malignant cells. The low absorption of the drug, as well as the high dosage needed to treat cancer and the resulting adverse effects have limited its usage. The goal of this study was to develop an efficient carrier for RUT administration and investigate the effect of its encapsulated form on ovarian cancer cell inhibition and proliferation. FDA-approved copolymers were used to create nano-encapsulated RUT. The topography, shape, charge, size, and drug release profile of NPs were all acceptable. The RUT anticancer effects were increased in ovarian cancer cells. Our findings suggested that EUT’s therapeutic indices were improved thanks to its nanoformulation. Because of RUT’s intrinsic chemopreventive potential, NPs containing RUT might be a promising method for generating long-term, safe, and effective treatments for breast cancer.
Author Contribution Statement
Akram Firouzi-Amandi, Mahdieh Tarahomi and Hamed Rahmani-Youshanlouie: Methodology, Investigation, Data curation, Original draft preparation. Reza Mosaddeghi Heris and Davoud Jafari-gharabaghlou: Writing- Reviewing and Editing. Nosratollah Zarghami and Mehdi Dadashpour: Supervision, Conceptualization, funding acquisition, Reviewing and Editing.
Acknowledgements
The authors would like to thank to the Cancer Research Center, Semnan University of Medical Sciences, Semnan, Iran.
Availability of data and materials
The analyzed data during the current study are available from the corresponding authors on reasonable request.
Ethics approval and consent to participate
The Ethics Committee of Semnan Univercity of Medical Sciences has approved the current study (Code of Ethics: IR.SEMUMS.REC.1400.327).
Declaration of interest
The authors’ statement has no conflicts of interest in this article content.
References
- Adlravan E, Nejati K, Karimi MA, et al. Potential activity of free and PLGA/PEG nanoencapsulated nasturtium officinale extract in inducing cytotoxicity and apoptosis in human lung carcinoma A549 cells. J Drug Deliv Sci Technol. 2021;61:102256. [Google Scholar]
- Amirazad H, Dadashpour M, Zarghami N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J Biol Eng. 2022;16:1–18. doi: 10.1186/s13036-021-00282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirsaadat S, Jafari-Gharabaghlou D, Alijani S, et al. Metformin and Silibinin co-loaded PLGA-PEG nanoparticles for effective combination therapy against human breast cancer cells. J Drug Deliv Sci Technol. 2021;61:102107. [Google Scholar]
- Aubrey BJ, Kelly GL, Janic A, et al. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Different. 2018;25:104–13. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barani M, Bilal M, Sabir F, et al. Nanotechnology in ovarian cancer: Diagnosis and treatment. Life Sci. 2021;266:118914. doi: 10.1016/j.lfs.2020.118914. [DOI] [PubMed] [Google Scholar]
- Bohlouli S, Jafarmadar Gharehbagh F, Dalir Abdolahinia E, et al. Preparation, Characterization, and Evaluation of Rutin Nanocrystals as an Anticancer Agent against Head and Neck Squamous Cell Carcinoma Cell Line. J Nanomaterials. 2021:2021. [Google Scholar]
- Caparica R, Júlio A, Araújo MEM, et al. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules. 2020;10:233. doi: 10.3390/biom10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Miao Q, Geng M, et al. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci World J. 2013:2013. doi: 10.1155/2013/269165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadashpour M, Firouzi-Amandi A, Pourhassan-Moghaddam M, et al. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Materials Sci Eng C. 2018;92:902–12. doi: 10.1016/j.msec.2018.07.053. [DOI] [PubMed] [Google Scholar]
- Dadashpour M, Ganjibakhsh M, Mousazadeh H, et al. Increased Pro-Apoptotic and Anti-Proliferative Activities of Simvastatin Encapsulated PCL-PEG Nanoparticles on Human Breast Cancer Adenocarcinoma Cells. J Cluster Sci. 2022;2022:1–12. [Google Scholar]
- Dadashpour M, Rasooli I, Sefidkon F, et al. Lipid peroxidation inhibition, superoxide anion and nitric oxide radical scavenging properties of Thymus daenensis and Anethum graveolens essential oils. 2011: 109–20. [Google Scholar]
- De Gaetano F, Cristiano MC, Venuti V, et al. Rutin-loaded solid lipid nanoparticles: characterization and in vitro evaluation. Molecules. 2021;26:1039. doi: 10.3390/molecules26041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajzadeh R, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Nano-encapsulated metformin-curcumin in PLGA/PEG inhibits synergistically growth and hTERT gene expression in human breast cancer cells. Artificial Cells Nanomed Biotechnol. 2018;46:917–25. doi: 10.1080/21691401.2017.1347879. [DOI] [PubMed] [Google Scholar]
- Faramarzi L, Dadashpour M, Sadeghzadeh H, et al. Enhanced anti-proliferative and pro-apoptotic effects of metformin encapsulated PLGA-PEG nanoparticles on SKOV3 human ovarian carcinoma cells. Artificial Cells Nanomed Biotechnol. 2019;47:737–46. doi: 10.1080/21691401.2019.1573737. [DOI] [PubMed] [Google Scholar]
- Hoai TT, Yen PT, Dao TTB, et al. Evaluation of the Cytotoxic Effect of Rutin Prenanoemulsion in Lung and Colon Cancer Cell Lines. J Nanomaterials. 2020:2020. [Google Scholar]
- Javan N, Khadem Ansari MH, Dadashpour M, et al. Synergistic antiproliferative effects of co-nanoencapsulated curcumin and chrysin on mda-mb-231 breast cancer cells through upregulating mir-132 and mir-502c. Nutr Cancer. 2019;71:1201–13. doi: 10.1080/01635581.2019.1599968. [DOI] [PubMed] [Google Scholar]
- Khazei K, Jamali M, Sarhadi S, et al. Transcriptome profiling of curcumin-treated T47D human breast cancer cells by a system-based approach. Gene Rep. 2022;2022:101556. [Google Scholar]
- Mazloum-Ardakani M, Barazesh B, Moshtaghioun SM, et al. Designing and optimization of an electrochemical substitute for the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell viability assay. Sci Rep. 2019;9:14966. doi: 10.1038/s41598-019-51241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harbor Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogheri F, Jokar E, Afshin R, et al. Co-delivery of metformin and silibinin in dual-drug loaded nanoparticles synergistically improves chemotherapy in human non-small cell lung cancer A549 cells. J Drug Deliv Sci Technol. 2021;66:102752. [Google Scholar]
- Mousazadeh H, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J Controlled Release. 2021;330:1046–70. doi: 10.1016/j.jconrel.2020.11.011. [DOI] [PubMed] [Google Scholar]
- Nejati K, Mehdi D, Ghareghomi S, et al. GDNF gene-engineered adipose-derived stem cells seeded Emu oil-loaded electrospun nanofibers for axonal regeneration following spinal cord injury. J Drug Deliv Sci Technol. 2020;60:102095. [Google Scholar]
- Nikmanesh F, Sarhadi S, Dadashpour M, et al. Omics Integration Analysis Unravel the Landscape of Driving Mechanisms of Colorectal Cancer. Asian Pac J Cancer Prev. 2020;21:3539. doi: 10.31557/APJCP.2020.21.12.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgholi A, Dadashpour M, Mousapour A, et al. Anticancer Potential of Silibinin Loaded Polymeric Nanoparticles against Breast Cancer Cells: Insight into the Apoptotic Genes Targets. Asian Pac J Cancer Prev. 2021;22:2587. doi: 10.31557/APJCP.2021.22.8.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabi A, Naghili B, Ansarin K, et al. Let-7d and miR-185 Impede Epithelial-Mesenchymal Transition by Downregulating Rab25 in Breast Cancer. Asian Pac J Cancer Prev. 2021;22:305. doi: 10.31557/APJCP.2021.22.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Ali A, Ali J, et al. Rutin: therapeutic potential and recent advances in drug delivery. Exp Opinion Investigat Drugs. 2013;22:1063–79. doi: 10.1517/13543784.2013.805744. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022:2022. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- Singh M, Hemant K, Ram M, et al. Microencapsulation: A promising technique for controlled drug delivery. Res Pharmaceutical Sci. 2010;5:65. [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Si X, Han MK, et al. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J Materials Chem B. 2015;3:7724–33. doi: 10.1039/c5tb01245g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data during the current study are available from the corresponding authors on reasonable request.