Abstract
Objectives:
Aims were to investigate the prevalence and risk factors of venous thromboembolism (VTE) in gynecologic malignancy cases. Value of screening tool (Caprini) for prediction of VTE was also assessed.
Study design:
A retrospective study of gynecologic malignancy subjects who underwent major gynecological operation via exploratory laparotomy at Thammasat University Hospital, Pathum Thani, Thailand from January 2015 to December 2020. Participants were categorized into VTE and non-VTE groups. Caprini score, associated laboratory and clinical factors of both groups were evaluated.
Results:
A total of 392 subjects were recruited into the study. Prevalence of VTE was 7.4 (29/392) percent. VTE was diagnosed in subjects with endometrial, ovarian and cervical cancer at percentage of 7.8 (15/192), 7.9 (11/138) and 5.7 (3/53), respectively. Demographic characters of both groups were comparable. VTE group had significant more Caprini score, platelets count and platelet lymphocyte ratio (PLR) than non-VTE group. Modified Caprini score (2 multiply Caprini score plus 1 multiply PLR) was generated for better VTE prediction. Sensitivity and specificity of Caprini (≥5.5) and modified Caprini scores (≥22.8) were 72.4 vs 39.4, and 79.3 vs 52.1 percent, respectively.
Conclusion:
Prevalence of VTE among gynecologic malignancy cases was 7.4 percent. The modified Caprini score was an alternative VTE predictive tool. Cut-off point of modified Caprini score at equal or more than 22.8 was proposed.
Key Words: Gynecologic malignancy, venous thromboembolism, prevalence, prediction, caprini
Introduction
Venous thromboembolism (VTE) is a condition in which there is clotting in the deep venous system, resulting in deep vein thrombosis (DVT), pulmonary embolism (PE) and thrombosis in various organs. This condition consists of blood flow stasis, hypercoagulation and vessel wall injury. The presentations of DVT are swelling, redness and warmth of the affected area. Meanwhile, the presentations of pulmonary embolism are dyspnea, chest pain and other nonspecific symptoms. A diagnosis of venous thromboembolism is made by ultrasonography or computed tomography pulmonary angiogram (Clarke-Pearson et al., 2020). Currently, the treatment of VTE requires prolonged anticoagulation therapy. Therefore, American society of hematology (ASH) recommended universal preoperative VTE prophylaxis for all patients undergoing gynecologic cancer operation (Lyman et al., 2021). However, most gynecologic oncologists do not follow this recommendation because VTE is thought to have a low incidence (Stroud et al., 2014), especially in an Asian population. Regardless, the incidence of VTE among Asian population has been increasing, from 3.2 to 13.8-29.2 per one hundred thousand Asian people in recent studies (Lee et al., 2017; Hong, 2018; Zhang et al., 2019).
There are several VTE prediction tools derived mostly from Caucasian databases, such as the Caprini and Khorana scores (Pannucci et al., 2017 and Mulder et al., 2019). However, Asian population was not well-represented. Therefore, the prediction tool for VTE is still inconclusive. Gynecologic malignancy is one of the major conditions that contributes to the prevalence of VTE in Thailand, up to 1.3 per 100 cases (Oranratanaphan et al., 2015). The contributing factors to VTE in gynecological malignancy are the types of cancer, histology, advanced stages, age and body mass index (BMI) (Takasaki et al., 2019; Wang et al., 2020; Kahr et al., 2021).
To promote perioperative VTE prophylaxis for gynecologic operations, this study aimed to investigate the prevalence, associated risk factors and predictive scoring tool for VTE in gynecologic malignancy cases.
Materials and Methods
A retrospective cohort study was conducted at Thammasat University Hospital, Pathum Thani, Thailand from January 2015 to December 2020. This study was approved by Ethical Committee of Faculty Medicine, Thammasat University, Thailand, in 2021 (MTU-EC-OB-0-279/63).
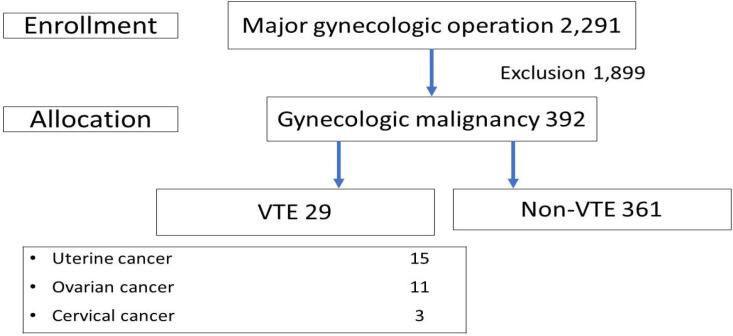
The participants were subjects who underwent major gynecologic operation via exploratory laparotomy with histopathology confirmed gynecologic malignancy during the study period (Figure 1). The exclusion were subjects who had overlapping gynecologic operations, non-gynecologic operations and/or benign or borderline tumors as final histology. The recruited subjects were divided into two groups (VTE and non-VTE). The VTE group consisted of gynecologic cancer subjects who developed VTE. The others were classified in the non-VTE group. Patients with clinical manifestations of VTE (swelling, redness, pain at lower extremities or acute dyspnea) were diagnosed by evidence of clot formation in the venous system detected by Doppler ultrasonography and/or computed tomography (CT). The cases diagnosed with VTE within 30 days after surgery were classified as perioperative VTE. Meanwhile, the subjects without clinical symptoms of VTE were observed and diagnosed by incidental findings from the imaging studies. All subjects were appointed at the tumor clinic for extensive metastasis surveillance in the first two years. VTE subjects followed up with hematologists and gynecologic oncologists for a long period of anticoagulation treatment.
Figure 1.
Flow Chart of the Study Selection. VTE, venous thromboembolism
The database was reviewed from an electronic hospital chart to collect the subjects’ information. The demographic characteristic consisted of age, body mass index (BMI), menopause status, parity, site of cancer, stage and histology. Preoperative investigations included hemogram, blood chemistry and applicable imaging studies. The clinical characteristics included the diagnosis, operation type and complication. VTE was a complication of interest. All participants were evaluated with Caprini score as a VTE prediction tool.
Statistical analysis was performed using a commercial statistical program (SPSS Corp, NY, USA). Demographic data of both groups were expressed by mean ± standard deviation (SD) or percentage with clinical application. The categorical characters were calculated using either chi-square or Fisher’s exact test. The logic model of scoring was used to find coefficients of variation between both groups by logistic regression analysis. The logic model was the modified Caprini formula generated according to a significant odds ratio variation. Receiver-operating characteristic (ROC) was calculated to determine appropriate cut-off point values. According to the Caprini score and data from the current study, the ROC curve was analyzed, generated and compared. The diagnostic performance of both models was evaluated by area under the curve (AUC), regarding the risk of VTE. Sensitivity, specificity, positive predictive value, negative predictive value and accuracy were calculated for the study population. A p-value less than 0.05 was considered statistically significant.
Results
A total of 2,291 subjects underwent major gynecologic operations via exploratory laparotomy. After exclusion, 392 subjects were recruited into the study. Twenty-nine subjects were diagnosed with VTE. Fifty-five cases underwent preoperative prophylaxis of VTE. Twelve percent (7/55) of cases were diagnosed with VTE after surgery. There were DVT, PE, combined DVT and PE and portal vein thrombosis cases at a percentage of 72.4, 6.8, 17.2 and 3.4, respectively as Figure 1. The remaining 363 subjects were in the non-VTE group.
Demographic characters namely age, BMI, menopause, parity and underlying disease were comparable as shown in Table 1. Among advanced cancer stage subjects, 44.8 (13/29) percent was in the VTE group compared to 23.9 (87/363) percent in the non-VTE group (p=0.013). Subjects with uterine cancer developed VTE at 7.8 (15/192) percent and 53.3 (8/15) percent of them had non-endometrioid histology. Among uterine cancer subjects without VTE, only one-fifth (38/177) had non-endometrioid histology type (p=0.005).
Table 1.
Demographic Character of Gynecologic Oncology Cases and VTE
| VTE (n=29) * |
Non VTE (n=363) * | p-value | |
|---|---|---|---|
| Age(year)** | 58.1±14.1 | 56.2±12.2 | 0.424 |
| BMI (kg/m2) ** | 24.2±4.5 | 25.1±5.5 | 0.384 |
| Menopause | 22 (75.8) | 246 (67.7) | 0.37 |
| Nulliparous | 10 (34.4) | 113 (31.1) | 0.71 |
| Underlying disease | |||
| Hypertension | 13 (44.8) | 129 (35.5) | 0.32 |
| Diabetes | 4 (13.8) | 52 (14.33) | 0.97 |
| Advanced stage | 13 (44.8) | 87 (23.9) | 0.013 |
| Operation time (min)** | 174.7±45.0 | 175.2±57.7 | 0.962 |
| Suboptimal | 7 (24.1) | 58 (16.0) | 0.256 |
| Node dissection | 25 (86.2) | 292 (80.04) | 0.447 |
| Uterine cancer | 15 (51.7) | 177 (48.7) | 0.76 |
| Non-endometrioid | 8 (53.3) | 38 (21.5) | 0.005 |
| Ovarian cancer | 11 (37.8) | 128 (35.2) | 0.77 |
| Clear cell | 5 (50) | 33 (25.7) | 0.09 |
| Cervical cancer | 3 (11.4) | 50 (13.77) | 0.603 |
| Caprini score | 6.4±1.6 | 5.7±0.97 | <0.05 |
| Hemoglobin(gm%) ** | 11.4±1.5 | 11.8±1.6 | 0.293 |
| WBC (cells/mm3) ** | 8,455±4767 | 8038±3375 | 0.537 |
| Platelets (cells/mm3) ** | 358.6±168.0 | 310.1±110 | 0.029 |
| Albumin(gm%) ** | 3.5±0.7 | 3.8±0.6 | 0.016 |
| PLR** | 23.2±35.2 | 15.2±14.7 | 0.016 |
*n (%), **mean ±standard deviation (SD), VTE, venous thromboembolism; n (%), number of patient (percent); BMI, body mass index; Advanced stage, FIGO stage 3-4; PLR, platelet lymphocyte ratio
The Caprini score in the VTE group was significantly higher than that of the non-VTE group (6.4±1.6, 5.7± 0.97, p<0.05). Platelet lymphocyte ratio (PLR) and platelet level among VTE group were more than those in the non-VTE group with a statistical significance. In contrast, the albumin in subjects with VTE had a significantly lower level than those in the non-VTE group (Table 1).
The significant variables in Table 1 were chosen by logistic regression. The selected model was used for odd ratio value and calculating multipliers as presented in Table 2. Logistic regression analysis was done to compare VTE and non-VTE groups to Caprini score (expo 1.9, 95%Cl 1.34–2.66) and PLR (expo 1.0, 95%Cl 1.00–1.04). To improve the detection rate of VTE, the modified Caprini formula was generated: 2 multiply Caprini score plus 1 multiply PLR.
Table 2.
The Variables in the Equation of Model
| B | S.E. | Wald | df | Sig. | Exp(B) | |
|---|---|---|---|---|---|---|
| Caprini score | 0.64 | 0.18 | 13.16 | 1 | 0 | 1.9 |
| PLR | 0.02 | 0.01 | 6.31 | 1 | 0.12 | 1 |
| Constant | -6.77 | 1.17 | 33.42 | 1 | 0 |
The modified Caprini score=2*Caprini + 1*PLR; PLR, platelet lymphocyte ratio
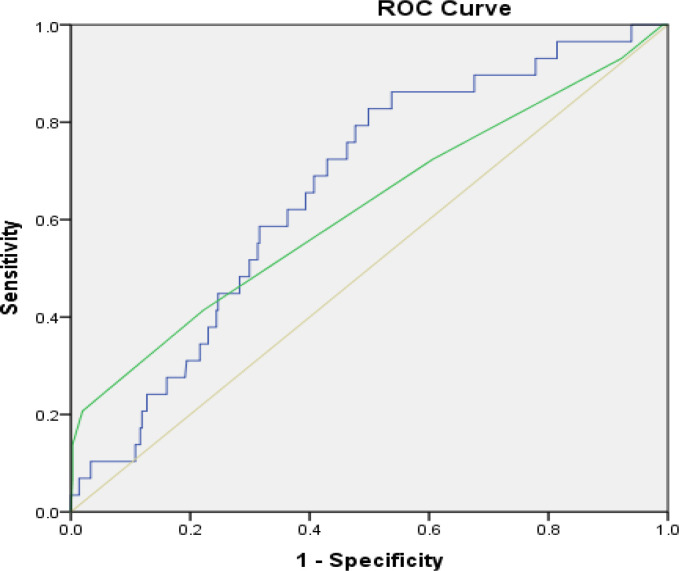
The ROC curve analysis was generated to find the best cut-off value. The cut-off point of Caprini and modified Caprini score to predict VTE were 5.5 and 22.8, respectively. The areas under the curve between Caprini and modified Caprini score were 0.62 (95%Cl 0.50–0.74) and 0.66 (95%Cl 0.57–0.75), respectively. The sensitivity and specificity of the Caprini score were 72.4 and 39.4 percent, respectively. Meanwhile, these of the modified Caprini score were 79.3 and 52.1 percent, respectively (Figure 2, Table 3).
Figure 2.
Comparison Curve to Predict Venous Thromboembolism between Modified Caprini and Caprini Score. ROC, receiver operator characteristic; 2*Caprini score + 1*PLR; modified Caprini score; PLR, platelet lymphocyte ratio
Table 3.
Comparison of Diagnostic Value for Caprini and Modified Caprini Score to Predict VTE
| Caprini (>5.5) | modified Caprini (>22.8) | |
|---|---|---|
| AUC | 0.62 | 0.66 |
| 95%Cl | 0.50-.74 | 0.57-.75 |
| Sensitivity (%) | 72.4 | 79.3 |
| Specificity (%) | 39.4 | 52.1 |
| PPV (%) | 8.0 | 11.7 |
| NPV (%) | 94.7 | 96.9 |
| Accuracy (%) | 42.1 | 54.1 |
ROC, receiver operating characteristic; AUC, area under curve; Cl, confidence level; VTE, venous thromboembolism; PPV, positive predictive value; NPV, negative predictive value
Discussion
The incidence of VTE varied due to under diagnosis, low awareness and differences in race (Lee et al., 2017; Wang et al., 2018). The prevalence of VTE found in subjects with gynecologic malignancy at Thammasat University Hospital during the data collection period was 7.4 (29/392) percent.
Compared to the study from Japan, Korea and Denmark, the prevalence of VTE in gynecologic cancer patients varied from 5.5 to 11 percent (Ohashi et al., 2020; Shin et al., 2021, Kahr et al., 2021). In year 2021, Shin’s and Kahr’s groups had reported the prevalence of VTE in ovarian cancer patients among Koreans and Danes as 9.6 (122/1268) and 11(551/4991) percent (Shin et al., 2021; Kahr et al., 2021). However, Ohashi et al., (2020) reported an incidence of VTE in Japanese patients with gynecologic malignancy at 5.5 (50/906) percent. The finding from the current study was in lieu with Ohashi’s (5.5 %), Shin’s (9.6%) and Kahr’s (11%) literature, the incidence from their work might be from the inclusion of both operative and non-operative case (Ohashi et al., 2020; Shin et al., 2021, Kahr et al., 2021). In contrast, Barber et al reported from the USA in 2016 that the incidence of VTE among gynecologic malignancy operations was 1.8 percent. The incidence was lower than the previously mentioned literature. Barber’s subjects came from gynecologic operations with ubiquitous preoperative VTE prevention (Barber et al., 2016).
According to an American study in 2016, nearly all cases of gynecologic cancer (97%) had a high risk of VTE when using Caprini score with a cut-off point of more or equal to five (≥ 5). Additionally, the Caprini score was limited in discriminating the risk of VTE among gynecologic cancer cases (Barber et al., 2016).
The prevalence of VTE among non-endometrioid uterine cancer subjects in the current study was similar to reports from Canada (Pin et al., 2020). This study found that half of the subjects with VTE had uterine cancer (15/29). Among uterine cancer cases with non-endometrioid histology, nearly one-fifth (8/46) developed VTE. In 2020, Pin et al also reported from Canada that 12.3 (15/122) percent of patients with non-endometrioid uterine cancer developed VTE (Pin et al., 2020).
Previous studies also reported that the aggressive cell types of ovarian and endometrial cancers, such as non-endometrioid, high-grade serous carcinoma and clear cell carcinoma increased the risk of VTE. Thrombocytosis in cancer could explain these findings (overexpression of the circulating interleukin-6 initiate of VTE) (Branchford et al., 2018; Azar et al., 2020; Pin et al., 2020). The incidence of VTE in ovarian cancer was 7.9 percent. Subjects who had ovarian cancer with clear cell histology were associated with an increased risk of VTE in Danish and Japanese reports (Takasaki et al., 2019; Kahr et al., 2021). In this study, half of all ovarian malignancies were clear cell carcinoma. However, prevalence of clear cell carcinoma in both VTE and non-VTE groups were comparable (p=0.09).
Findings from the current study indicated that the advanced stages, non-endometrioid uterine cancer, Caprini score, platelet count, albumin level and PLR were significant risk factors for VTE. Only the Caprini score and PLR were significant variables from multivariable analysis. Modified Caprini score was generated by adding the PLR. The increased sensitivity, specificity and positive predictive value (PPV) were accomplished by the modified Caprini formula (Figure 2, Table 3).
The cause of malignancy-associated venous thromboembolism is multifactorial (Ay et al., 2017). The inflammatory response of vascular disease biomarkers such as neutrophil lymphocyte ratio (NLR) and PLR were highly desired (Grilz et al., 2018; Farah et al., 2019; Xeu et al., 2021). Farah and Grilz reported that NLR and PLR were significantly elevated in VTE subjects (Farah et al., 2019; Grilz et al., 2018). Xue et al., (2021) from China, reported NLR and PLR to be capable predictive tools of thromboembolism. In the current study, NLR and PLR were higher in the VTE group than the non-VTE group. PLR showed a statistically significant difference between both groups. However, NLR did not reach a significant statistic.
Predictive Caprini score for high risk VTE from the other studies (Stroud et al., 2014; Barber et al., 2016; Wang et al., 2020) and the current study had cut-off points at 5 and more than 5.5, respectively. Caprini score used in the current population had sensitivity of 72.4 percent and specificity of 39.4 percent. Meanwhile, Caprini score in Stroud’s, Barber’s and Wang’s population had higher sensitivity (100%, 95.8% and 82.1%) when the same cut-off point was used. Regardless, wide range of specificity and PPV were identified at a percentage of 8/3/22.6 and 3/4.1/51.5, respectively. In 2017, a meta-analysis from the USA proposed a higher cut-off point (equal or more than 7) for providing an advantage to peri-operative surgery patients (Pannuci et al., 2017). Pannuci et al., (2017) also recommended that VTE prophylaxis should be administered when Caprini score is equal to or more than three points. The comparison of the current to previous literatures were summarized and presented in Table 4.
Table 4.
Comparison of Characteristic of Risk Assessment Model for VTE
| Stroud | Panacci | Barber | Shi | Wang | Current study | ||
|---|---|---|---|---|---|---|---|
| Year | 2014 | 2016 | 2016 | 2018 | 2020 | 2020 | |
| Country | USA | USA | USA | China | China | Thailand | |
| Number | 945 | 5,972 | 17,713 | 974 | 212 | 392 | |
| White (%) | 67.4 | 77.6 | |||||
| Age (years) | 51 | 62 | 66 | 58 | |||
| BMI (kg/m2) | 31.5 | 30.4 | 24.2 | ||||
| Prevalence (%) | 3.3 | 2.45 | 1.8 | 1.75 | 7.3 | ||
| Surgery | GO | OV | GO | GO | GO | GO | |
| CS | ≥ 5 | ≥ 7 | ≥ 5 | ≥ 7 | ≥ 5 | ≥ 5.5 | MCS ≥ 22.8 |
| Sensitivity (%) | 100 | 61.5 | 95.8 | 20 | 82.1 | 72.4 | 79.3 |
| Specificity (%) | 8 | 75.5 | 3 | 99 | 22.6 | 39.4 | 52.1 |
| PPV (%) | 3.6 | 5.8 | 4.1 | 50 | 51.5 | 8 | 11.7 |
| NPV (%) | 100 | 98.7 | 97.6 | 96 | 55.8 | 94.7 | 96.9 |
VTE, venous thromboembolism; BMI, body mass index(kg/m2); GO, gynecological cancer; OV, overall; CS, Caprini score; MCS, modified Caprini score (2 multiply Caprini score + 1 multiply PLR); PPV, positive predictive value; NPV, negative predictive value
VTE prophylaxis had not been routinely administered in the study institute even though universal VTE prophylaxis was recommended by ASH in year 2021 guidelines as possible (Lyman et al., 2021). If we could not follow the ASH Guideline Committee, VTE prophylaxis should be applied to gynecologic cancer cases with Caprini or modified Caprini score at equal to or more than 6 or 22.8, respectively. Interestingly, Shi et al., (2021) from China (2018) stated that the incidence of VTE in gynecologic cancer subjects who received VTE prophylaxis was 1.75(17/974) percent. The low VTE incidence in Shi’s study was a result of the universal VTE prophylaxis protocol. Additionally, the study from Canada by Bisch et al., (2020) concluded that preoperative pharmacologic prophylaxis decreased VTE prevalence by approximately 40 percent.
The current study increased the sensitivity and specificity for the prediction tool of VTE among gynecologic malignancy cases. We acknowledged the importance of identifying the better accurate predictive tool for VTE. In current study, Caprini score and PLR were utilized to generate a modified formula (2 multiply Caprini score plus 1 multiply PLR). The cut-off point of modified Caprini at 22.8 was proposed. The sensitivity was approximately 80 percent and specificity increased to more than half. Additionally, PPV had increased to 12 percent. This model had shown a larger ROC curve compared to that of the Caprini score (0.66 vs. 0.62). Therefore, the modified Caprini score was an alternative tool to better VTE prediction.
The detailed profile analysis of subjects who developed VTE was the study strength. The histology type of gynecologic malignancy was assessed and discussed. The limitations of this study included specific demographic data and a small population size, which do not allow the findings to be generalized to the global population. Moreover, some confounding factors had influenced the strength of associated risk factors. For valuable future research, morbidity and mortality, expense and cost-effectiveness and other biomarker laboratories to impact VTE events may be discussed. In conclusion, the current study showed the prevalence of VTE (7.4%) among gynecologic malignancy operation. Harmful situations resulting in morbidity and mortality may be induced in this group of patients. Thus, modified Caprini score increased predictive and diagnosis of venous thromboembolism. We recommended VTE prophylaxis when modified Caprini score was equal or more than 22.8.
Author Contribution Statement
None.
Acknowledgements
Thankfully, research fund of this study was supported by the Faculty of Medicine, Thammasat University, Pathumthani, Thailand.
Ethical approval
This work was approved by the Ethical Committee of Faculty Medicine, Thammasat University, Thailand in year 2021 (MTU-EC-OB-0-279/63).
Conflicts of interest
None.
References
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms and management. Thromb Haemost. 2017;117:219–30. doi: 10.1160/TH16-08-0615. [DOI] [PubMed] [Google Scholar]
- Azar WJ, Christie EL, Mitchell C, et al. Noncanonical IL6 Signaling-Mediated Activation of YAP Regulates Cell Migration and Invasion in Ovarian Clear Cell Cancer. Cancer Res. 2020;80:4960–71. doi: 10.1158/0008-5472.CAN-19-3044. [DOI] [PubMed] [Google Scholar]
- Barber EL, Clarke-Pearson DL. The limited utility of currently available venous thromboembolism risk assessment tools in gynecological oncology patients. Am J Obstet Gynecol. 2016;215:445.e1–9. doi: 10.1016/j.ajog.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisch S, Findley R, Ince C, et al. Efficacy of preoperative pharmacologic thromboprophylaxis on incidence of venous thromboembolism following major gynecologic and gynecologic oncology surgery: a systematic review and meta-analysis. Int J Gynecol Cancer. 2021;31:257–64. doi: 10.1136/ijgc-2020-001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchford BR, Carpenter SL. The Role of Inflammation in Venous Thromboembolism. Front Pediatr. 2018;6:142. doi: 10.3389/fped.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke-Pearson DL, Sullivan SA, Pierce SR, et al. Preoperative Evaluation and Postoperative Management. In: Berek JS, editor. Berek and Novak’s Gynecology. 2020. [Google Scholar]
- Farah R, Nseir W, Kagansky D, et al. The role of neutrophil-lymphocyte ratio, and mean platelet volume in detecting patients with acute venous thromboembolism. J Clin Lab Anal. 2020;34:e23010. doi: 10.1002/jcla.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilz E, Posch F, Königsbrügge O, et al. Association of Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio with the Risk of Thromboembolism and Mortality in Patients with Cancer. Thromb Haemost. 2018;118:1875–84. doi: 10.1055/s-0038-1673401. [DOI] [PubMed] [Google Scholar]
- Hong J, Lee JH, Yhim HY, et al. 2018) Incidence of venous thromboembolism in Korea from 2009 to 2013. PLoS One. 13:e0191897. doi: 10.1371/journal.pone.0191897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahr HS, Christiansen OB, Riddersholm SJ, et al. The timing of venous thromboembolism in ovarian cancer patients: A nationwide Danish cohort study. J Thromb Haemost. 2021;19:992–1000. doi: 10.1111/jth.15235. [DOI] [PubMed] [Google Scholar]
- Lee LH, Gallus A, Jindal R, et al. Incidence of Venous Thromboembolism in Asian Populations: A Systematic Review. Thromb Haemost. 2017;117:2243–60. doi: 10.1160/TH17-02-0134. [DOI] [PubMed] [Google Scholar]
- Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927–74. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder FI, Candeloro M, Kamphuisen PW, et al. The Khorana score for prediction of venous thromboembolism in cancer patients: a systematic review and meta-analysis. Haematologica. 2019;104:1277–87. doi: 10.3324/haematol.2018.209114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Ikeda M, Kunitoh H, et al. Venous thromboembolism in cancer patients: report of baseline data from the multicentre, prospective Cancer-VTE. Jpn J Clin Oncol. 2020;50:1246–53. doi: 10.1093/jjco/hyaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranratanaphan S, Termrungruanglert W, Khemapech N. Incidence and Clinical Characteristic of Venous Thromboembolism in Gynecologic Oncology Patients attending King Chulalongkorn Memorial Hospital over a 10 Year Period. Asian Pac J Cancer Prev. 2015;16:6705–9. doi: 10.7314/apjcp.2015.16.15.6705. [DOI] [PubMed] [Google Scholar]
- Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized Venous Thromboembolism Risk Stratification Using the 2005 Caprini Score to Identify the Benefits and Harms of Chemoprophylaxis in Surgical Patients: A Meta-analysis. Ann Surg. 2017;265:1094–103. doi: 10.1097/SLA.0000000000002126. [DOI] [PubMed] [Google Scholar]
- Pin S, Mateshaytis J, Ghosh S, et al. Risk factors for venous thromboembolism in endometrial cancer. Curr Oncol. 2020;27:198–203. doi: 10.3747/co.27.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Ye J, Zhuang X, et al. Application value of Caprini risk assessment model and elevated tumor-specific D-dimer level in predicting postoperative venous thromboembolism for patients undergoing surgery of gynecologic malignancies. J Obstet Gynaecol Res. 2019;45:657–64. doi: 10.1111/jog.13832. [DOI] [PubMed] [Google Scholar]
- Shin W, Lee S, Lim MC, et al. Incidence of venous thromboembolism after standard treatment in patients with epithelial ovarian cancer in Korea. Cancer Med. 2021;10:2045–53. doi: 10.1002/cam4.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud W, Whitworth JM, Miklic M, et al. Validation of a venous thromboembolism risk assessment model in gynecologic oncology. Gynecol Oncol. 2014;134:160–3. doi: 10.1016/j.ygyno.2014.04.051. [DOI] [PubMed] [Google Scholar]
- Takasaki K, Miyamoto M, Takano M, et al. Thrombotic events induce the worse prognosis in ovarian carcinomas and frequently develop in ovarian clear cell carcinoma. Int J Clin Oncol. 2019;24:1273–83. doi: 10.1007/s10147-019-01464-4. [DOI] [PubMed] [Google Scholar]
- Wang KL, Yap ES, Goto S, et al. The diagnosis and treatment of venous thromboembolism in Asian patients. Thromb J. 2018;16:4. doi: 10.1186/s12959-017-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang J, Bingbing Z, et al. Risk factors, risk assessment, and prognosis in patients with gynecological cancer and thromboembolism. J Int Med Res. 2020;48:300060519893173. doi: 10.1177/0300060519893173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Ma D, Jiang J, et al. Diagnostic and Prognostic Value of Immune/Inflammation Biomarkers for Venous Thromboembolism: Is It Reliable for Clinical Practice? J Inflamm Res. 2021;14:5059–77. doi: 10.2147/JIR.S327014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lei J, Shao X, et al. Trends in Hospitalization and In Hospital Mortality From VTE, 2007 to 2016, in China. Chest. 2019;155:342–53. doi: 10.1016/j.chest.2018.10.040. [DOI] [PubMed] [Google Scholar]